Abstract
Background:
N-3 Fatty acids reduce the risk of cardiovascular disease. Previous studies have shown that they may reduce inflammation, oxidative stress, and fat mass in patients with type 2 diabetes, but the results are inconclusive, due, in part, to type of omega-3 fatty acids used. The aim of this study was to determine the effects of pure eicosapentaenoic (EPA) and docosahexaenoic acids (DHA), the two major omega-3 fatty acids, on inflammation, oxidative stress, and fat mass in patients with type 2 diabetes.
Methods:
Sixty patients with DM-II were randomly allocated to receive daily either ~1 gr EPA or ~1 gr DHA, or a canola oil as placebo for 12 weeks in a randomized triple-blind, placebo-controlled trial. Serum MDA, CRP, body weight, BMI, and fat mass were measured at baseline and after intervention.
Results:
Forty-five patients with a mean (±SD) age of 54.9 ± 8.2 years with BMI of 27.6 ± 4.1 kg/m2 and fasting blood glucose 96.0 ± 16.2 mg/dl completed the intervention. Neither EPA nor DHA had significant effects on serum FBS, C-reactive protein, body weight, BMI, and fat mass after intervention (P > 0.05). In addition, while MDA increased 18% in the placebo group (P = 0.009), it did not change in the EPA or DHA group (P > 0.05).
Conclusions:
Twelve weeks of supplementation with 1gr/d EPA or DHA prevent increasing oxidative stress without changing marker of inflammation. This study is the first report demonstrating that neither EPA nor DHA have effects on body fat mass in type 2 diabetic patients.
Keywords: Inflammation, omega 3 fatty acids, oxidative stress, type 2 diabetes mellitus
INTRODUCTION
Diabetes mellitus is a risk factor for coronary artery disease and cerebrovascular disease.[1] It seems that inflammation and oxidative stress play a role in the pathophysiology of type 2 diabetes.[2] Some cross-sectional studies have shown that insulin resistance and type 2 diabetes are associated with higher levels of C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) that are markers of subclinical systemic inflammation.[3] Adipose tissue may be an important mediator of this association, because some of these molecules are secreted by adipocytes.[4] The inhibition of signaling of the insulin receptor is a primary mechanism through which inflammation leads to insulin resistance.[5] As inflammation, oxidative stress, and obesity also play role in cardiovascular diseases,[6,7] therefore reduction of markers of inflammation and oxidative stress markers that are high in patients with diabetes can reduce cardiovascular diseases risk in this group of patients.
Previous studies have shown that omega-3 fatty acids may reduce inflammation, oxidative stress, and fat mass,[8,9] but the results are inconclusive, due, in part, to the type of omega-3 fatty acids used. The most important omega-3 fatty acids are eicosapentaenoic acid (EPA) (20:5) and docosahexaenoic acid (DHA) (22:6) which provoke different effects on cell function. For example, in Raji Cells (a kind of B-lymphocyte), DHA raised the expression of 7 genes, whereas EPA up-regulated 20 genes and down-regulated 1 gene.[10] In addition, to our knowledge, effects of pure EPA or DHA on body fat have not been assessed in previous studies. Therefore, we conducted the present study to determine the effects of pure EPA and DHA, the two major omega-3 fatty acids, on CRP and malondialdehyde (MDA) as markers of inflammation and oxidative stress, respectively, and fat mass in patients with type 2 diabetes.
METHODS
Patients
Sixty patients aged 30–65 years with type 2 diabetes mellitus (DM-II) were referred from Iranian Diabetes Society and Institute of Endocrinolgy and Metabolism, Firouzgar Hospital, Tehran University of Medical Sciences, Tehran, Iran. All subjects were taking oral hypoglycemic agents, and had a FBS <140 mg/dl, a systolic blood pressure <140 and a diastolic blood pressure <90 mm Hg and a body mass index (BMI) between 20 and 35 kg/m2. Their blood cholesterol and triglyceride levels were <200 mg/dl <150 mg/dl, respectively. They consumed ≤ 2 fish meals/wk, and had not taken fish oil supplements or non-steroidal anti-inflammatory drugs during three months before intervention. The subjects were excluded if they were taking insulin, had a recent heart disease; had significant liver, thyroid or renal disease, macroproteinuria, neuropathy; smoked; or had weight changes during three months before enrolment. The study protocol was approved by the Ethic Committee of Tehran University of Medical Sciences and written informed consent was obtained from the patients. This trial was registered in Iranian Registry of Clinical Trials (Irct ID: IRCT138812102394N4).
Trial design
Sixty participants were randomly allocated to receive daily either four EPA (980 mg) or DHA (964 mg) soft gels, or placebo for 12 weeks in a double blind placebo controlled trial. Each EPA soft gel contained 245 mg EPA, 30 mg DHA, and 2.5 mg mixed tocopherols, and one DHA soft gel consisted of 241 mg DHA, 33 mg EPA, and 2.5 mg mixed tocopherols. Soft gels of EPA, DHA, and placebo (consisting of canola oil) were supplied by Minami Nutrition, Belgium. There was only a negligible amount of EPA in DHA soft gels and DHA in EPA soft gels. Ten milliliters of fasting blood were taken at baseline and at the end of the intervention. Subjects were instructed not to change their usual diet, level of physical activity, or other lifestyle factors during the intervention period. To control confounding factors, food record, physical activity, and general questionnaires were completed at baseline and after intervention. Body weight, BMI, and fat mass were measured at baseline and after intervention. We used Seca Digital Scale for monitoring of weight. Body fat mass was measured using BIA (Biostat, Palmerston North, New Zealand) in the lied position after a patient had lied down for 5 minutes. Compliance was estimated by counting pills. Patients were considered compliant if they consumed more than 90% of the medication.
Food intake analysis
Subjects were given written and verbal instructions on how to keep diet records, with food weighed or measured. Dietary intake was monitored by the same dietitian throughout the study and subjects were asked to complete a 3-d diet record (2 weekdays and 1 weekend day) and a lifestyle questionnaire at baseline and at the end of the 12 week intervention. Nutrients intake were analyzed by using N-IV software.
Biochemical measurements
Plasma and serum were separated from whole blood and frozen at –70°C until analyzed. MDA was measured by chemical colorimetric method using Cayman, MI, USA. CV and sensitivity of MDA kit were 5.08 and 0.08 μM/L respectively. Serum concentrations of CRP were determined by enzyme-linked immunoassay using Diagnostic BbiochemCanada kit, Ontario, Canada. CV and sensitivity of CRP kit were 5.3 and 10 ng/mL, respectively.
Statistical analysis
Given a = 0.05, power = 0.2 and a final difference of 1 on the MDA between the groups, the sample size was calculated by using (Var=25) to be at least 14 in each group.[11] Data were analyzed by SPSS using 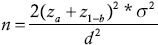 repeated measure ANCOVA adjusted for baseline values to determine effects of EPA and DHA compared to placebo. All data were tested for normality by using histograms and komogorov-smirnov statistics. There were no non-normal data either before or after the intervention. Differences between the three groups were considered significant when P < 0.05. All values have been reported as mean ± SD.
repeated measure ANCOVA adjusted for baseline values to determine effects of EPA and DHA compared to placebo. All data were tested for normality by using histograms and komogorov-smirnov statistics. There were no non-normal data either before or after the intervention. Differences between the three groups were considered significant when P < 0.05. All values have been reported as mean ± SD.
RESULTS
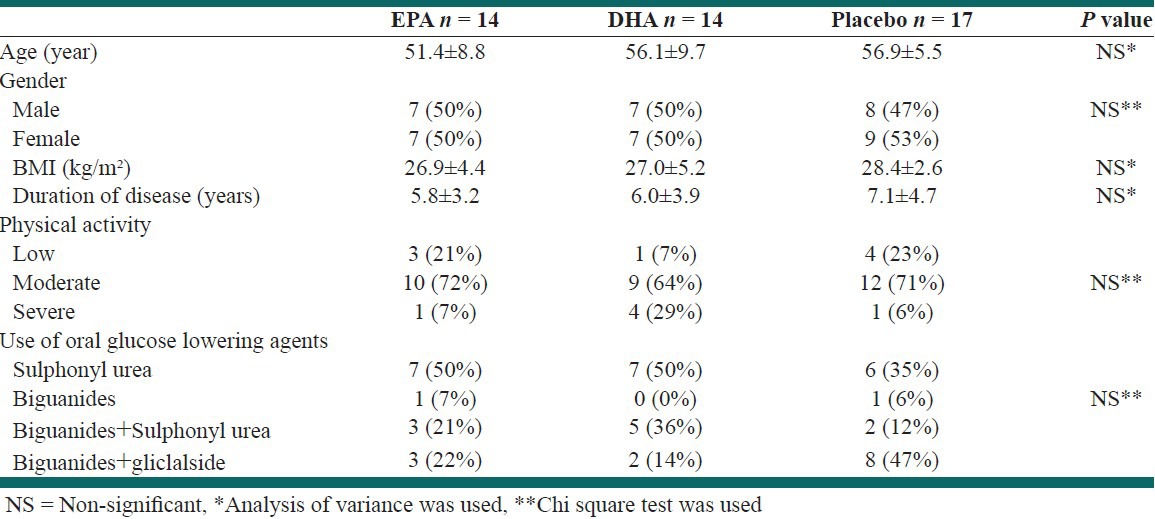
Forty-five subjects completed the 12 weeks intervention and had compliance with treatment. There were 14 subjects in the EPA group, 14 in the DHA group, and 17 in the placebo group. Withdrawals from the study were due to swallowing four capsules per day and intestinal side effects of them in EPA and DHA groups, and personal reasons in the placebo group. Baseline characteristics of patients are shown in Table 1. At baseline, there was no significant difference in age, gender, BMI, duration of disease, physical activity, or use of oral hypoglycemic drugs among the three groups. In addition, level of physical activity did not change significantly after intervention in any of the groups (P > 0.05).
Table 1.
Baseline characteristics of patients
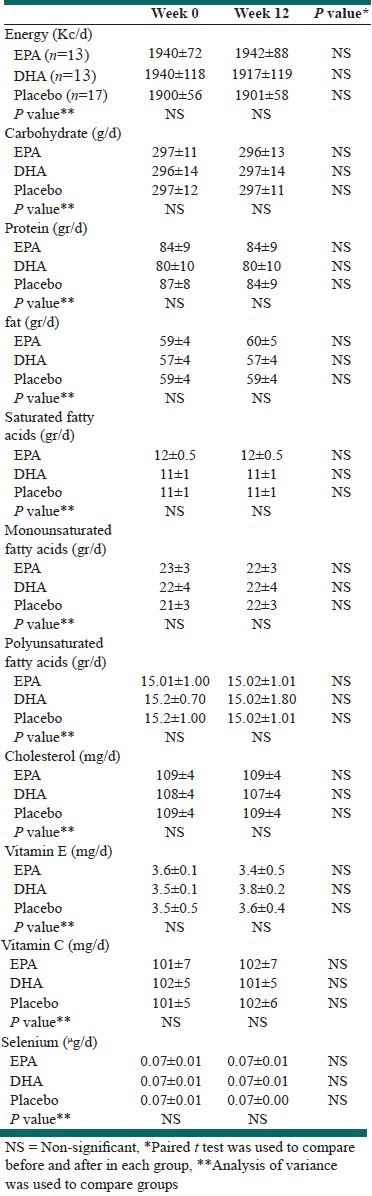
Diet records showed that there were no significant differences among the groups at baseline and end of the intervention in total energy, macronutrients, and micronutrients [Table 2].
Table 2.
Daily energy and nutrient intake in the EPA, DHA, and Placebo Groups
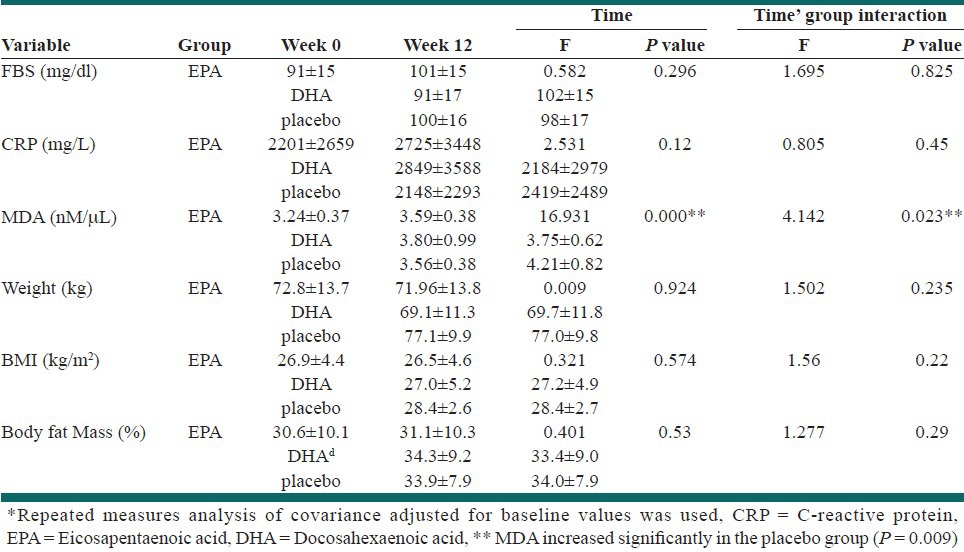
The mean values for serum FBS, MDA, CRP, and anthropometric measures are shown in Table 3. Repeated measures of analysis of covariance (ANCOVA), adjusted for baseline values, showed that there were no significant changes in FBS, serum CRP, body weight, BMI, and fat mass after intervention (P > 0.05). In addition, while MDA increased in the placebo group, it did not change in the EPA or DHA group (P > 0.05).
Table 3.
Mean and standard deviation of MDA and CRP and Anthropometric measures in Groups*
DISCUSSION
This study showed that 12 weeks consumption of 1g EPA or DHA have no effect on FBS which is supported by reports of other studies.[12,13,14,15,16] However, in Woodman study, intake of 4 g/d EPA or DHA caused a significant increase in fasting blood sugar that may be related to the dose of n-3 fatty acids. The differences in oral diabetic medication, degrees of obesity, and insulin resistance may also affect insulin sensitivity and blood sugar.
We found that although MDA increased significantly in the placebo group, its level did not change in EPA or DHA groups. Although dietary intake and physical activity did not change during intervention in any of the groups, MDA levels increased in the placebo group. Nevertheless, it seems that 1 g EPA or DHA can prevent increases in serum MDA in patients with DM-II. This finding is consistent with Nyby's et al. who showed that fish oil prevent increases in 8-isoprostant, a marker of lipid peroxidation, in male rats placed on diets containing 60% fructose.[17] Antioxidant effects of omega-3 fatty acids have been shown in other studies.[18] For example, Kesuvulul et al. showed a decrease in MDA after 2 month of supplementation with fish oil containing 1080 mg EPA + 720 mg in patients with DM-II13. Also, Mori et al. have shown that 4 g/d EPA or DHA can result in 20% and 19% fall in urinary F2-isoprostane excretion following 6 weeks of intervention.[19] On the other hand, in another trial fish oil did not change markers of oxidative stress compared to olive oil after 12 weeks in patients with DM-II.[20] The oxidative stress in DM is greatly increased due to prolonged exposure to glycaemia and impairment of the oxidant/antioxidant balance. The MDA levels were significantly correlated to DM and ECSOD.[21,22]
Several mechanisms have been proposed for antioxidant properties of omega-3 fatty acids including exerting anti-inflammatory effects, stimulation of antioxidant enzymes, and inhibition of the phospholipase A2. Furthermore, assembly of n-3 fatty acids in membrane lipids and lipoproteins makes double bonds less available for free radical attack.[13,19]
Neither EPA nor DHA decreased CRP in the present study. This is consistent with some previous studies which showed that fish oil or omega-3 fatty acids did not change CRP in patients with DM-II. For example, Mori's et al. showed that 4 g EPA or DHA did not decrease CRP compared to placebo after 6 weeks.[19] However, another type of omega-3 fatty acid, alpha linolenic acid (ALA), plus a diet rich in polyunsaturated fatty acids reduced CRP in hypercholesterolemic subjects.[23] Also, Plat showed a decrease in CRP when obese subjects consumed 1.1 g fish oil plus a weight loss diet.[24] It seems that there is a genetic basis for different CRP responses to diet and CRP gene polymorphism influences CRP levels.[23]
To our knowledge, effects of pure EPA and DHA on adipose tissue are assessed in the present study for the first time. The results showed that one gram EPA or DHA had no significant effect on body weight, BMI, or body fat mass compared to placebo, which is consistent with some previous studies which showed that EPA, DHA or fish oil did not change body weight in patients with DM-II compared to olive oil.[15,25] However, Kabir et al. showed that fish oil could reduce fat mass compared to paraffin, as a placebo, without a significant change in body weight in obese subjects with diabetes.[26] Canola oil, used as a placebo in the present study, may have some effects on fat mass.
Although the present study had some limitations including small power for differentiating between EPA and DHA effects and use of canola oil as a placebo, it showed that pure EPA or DHA alone have favorable effect on MDA.
In conclusion, 12 weeks of supplementation with 1 g/d EPA or DHA has favorable effects on MDA, but it has no statistically significant effect on FBS, CRP, body weight, BMI, or fat mass. Further studies measuring other indices of oxidative stress and inflammation are needed.
Footnotes
Source of Support: Nil
Conflict of Interest: Authors have no conflict of interest
REFERENCES
- 1.Torp-Pedersen C, Rask-Madsen C, Gustafsson I, Gustafsson F, Kober L. Diabetes mellitus and cardiovascular risk: Just another risk factor? Eur Heart J Suppl. 2003;5(suppl F):F26–32. [Google Scholar]
- 2.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Rekeneire N, Peila R, Ding J, Colbert LH, Visser M, Shorr RI, et al. Diabetes, hyperglycemia, and inflammation in older individuals. Diabetes Care. 2006;29:1902–8. doi: 10.2337/dc05-2327. [DOI] [PubMed] [Google Scholar]
- 4.Kraja AT, Province MA, Arnett D, Wagenknecht L, Tang W, Hopkins PN, et al. Do inflammation and procoagulation biomarkers contribute to the metabolic syndrome cluster. Nutr Metab. 2007;21:28. doi: 10.1186/1743-7075-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–9. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazzone T, Chait A, Plutzky J. Cardiovascular disease risk in type 2 diabetes mellitus: Insights from mechanistic studies. Lancet. 2008;371:1800–9. doi: 10.1016/S0140-6736(08)60768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goycheva PGV PB. Oxidative stress and its compplications in diabetes mellitus. Trakia J Sci. 2006;4:1–8. [Google Scholar]
- 8.Nettleton JA, Katz R. n-3 long-chain polyunsaturated fatty acids in type 2 diabetes: A review. J Am Diet Assoc. 2005;105:428–40. doi: 10.1016/j.jada.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 9.Barre DE. The role of consumption of alpha-linolenic, eicosapentaenoic and docosahexaenoic acids in human metabolic syndrome and type 2 diabetes-a mini-review. J Oleo Sci. 2007;56:319–25. doi: 10.5650/jos.56.319. [DOI] [PubMed] [Google Scholar]
- 10.Verlengia R, Gorjão R, Kanunfre CC, Bordin S, De Lima TM, Fernandes Martins E, et al. Effects of EPA and DHA on proliferation, cytokine production, and gene expression in Raji cells. Lipids. 2004;39:857–64. doi: 10.1007/s11745-004-1307-2. [DOI] [PubMed] [Google Scholar]
- 11.Sarbolouki SH, Djalali M, Dorosty AR, Djazayery SA, Eshraghian MR, Ebadi SAR, et al. Effects of EPA and vitamin E on serum enzymatic antioxidants and peroxidation indices in patients with type II diabetes mellitus. Iranian J Public Health. 2010;39:82–91. [PMC free article] [PubMed] [Google Scholar]
- 12.Pooya Sh DM, Djazayery A, Saedisomeolia A, Eshraghian MR, Toorang F. The efficacy of omega-3 fatty acid supplementation on plasma homocysteine and malondialdehyde levels of type 2 diabetic patients. Nutr Metab Cardiovasc Dis. 2010;20:326–31. doi: 10.1016/j.numecd.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Kesavulu MM, Kameswararao B, Apparao C, Kumar EG, Harinarayan CV. Effect of n-3 fatty acids on lipid peroxidation and antioxidant enzyme status in type 2 diabetic patients. Diabetes Metab. 2002;28:20–6. [PubMed] [Google Scholar]
- 14.Puhakainen I, Ahola I, Yki-Jarvinen H. Dietary supplementation with n-3 fatty acids increases gluconeogenesis from glycerol but not hepatic glucose production in patients with non- insulin-dependent diabetes mellitus. Am J Clin Nutr. 1995;61:121–6. doi: 10.1093/ajcn/61.1.121. [DOI] [PubMed] [Google Scholar]
- 15.Woodman RJ, Mori TA, Burke V, Puddey IB, Watts GF, Beilin LJ. Effects of purified eicosapentaenoic and docosahexaenoic acids on glycemic control, blood pressure, and serum lipids in type 2 diabetic patients with treated hypertension. Am J Clin Nutr. 2002;76:1007–15. doi: 10.1093/ajcn/76.5.1007. [DOI] [PubMed] [Google Scholar]
- 16.Shidfar F, Keshavarz A, Hosseyni S, Ameri A, Yarahmadi S. Effects of omega-3 fatty acids on serum lipids, apolipoproteins and malondialehyde in typ 2 diabetes patients. East Mediterr Health J. 2008;14:305–13. [PubMed] [Google Scholar]
- 17.Nyby MD, Matsumoto K, Yamamoto K, Abedi K, Eslami P, Hernandez G, et al. Dietary fish oil prevents vascular dysfunction and oxidative stress in hyperinsulinemic rats. Am J Hypertens. 2005;18:213–9. doi: 10.1016/j.amjhyper.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 18.Shidfar F, Keshavarz A, Jallali M, Miri R, Eshraghian M. Comparison of the effects of simultaneous administration of vitamin C and omega-3 fatty acids on lipoproteins, apo A-1, apo B, and malondialdehyde in hyperlipidemic patients. Int J Vitam Nutr Res. 2003;73:163–70. doi: 10.1024/0300-9831.73.3.163. [DOI] [PubMed] [Google Scholar]
- 19.Mori TA, Woodman RJ, Burke V, Puddey IB, Croft KD, Beilin LJ. Effect of eicosapentaenoic acid and docosahexaenoic acid on oxidative stress and inflammatory markers in treated-hypertensive type 2 diabetic subjects. Free Radic Biol Med. 2003;35:772–81. doi: 10.1016/s0891-5849(03)00407-6. [DOI] [PubMed] [Google Scholar]
- 20.Wong CY, Yiu KH, Li SW, Lee S, Tam S, Lau CP, et al. Fish-oil supplement has neutral effects on vascular and metabolic function but improves renal function in patients with type 2 diabetes mellitus. Diabet Med. 2010;27:54–60. doi: 10.1111/j.1464-5491.2009.02869.x. [DOI] [PubMed] [Google Scholar]
- 21.Nakhjavani M, Esteghamati A, Nowroozi S, Asgarani F, Rashidi A, Khalilzadeh O. Type 2 diabetes mellitus duration: An independent predictor of serum malondialdehyde levels. Singapore Med J. 2010;51:582–5. [PubMed] [Google Scholar]
- 22.Soliman GZ. Blood lipid peroxidation (superoxide dismutase, malondialdehyde, glutathione) levels in Egyptian type 2 diabetic patients. Singapore Med J. 2008;49:129–36. [PubMed] [Google Scholar]
- 23.Zhao G, Etherton TD, Martin KR, West SG, Gillies PJ, Kris-Etherton PM. Dietary α-linolenic acid reduces inflammatory and lipid cardiovascular risk factors in hypercholesterolemic men and women. J Nutr. 2004;134:2991–7. doi: 10.1093/jn/134.11.2991. [DOI] [PubMed] [Google Scholar]
- 24.Plat J, Jellema A, Ramakers J, Mensink RP. Weight loss, but not fish oil consumption, improves fasting and postprandial serum lipids, markers of endothelial function, and inflammatory signatures in moderately obese men. J Nutr. 2007;137:2635–40. doi: 10.1093/jn/137.12.2635. [DOI] [PubMed] [Google Scholar]
- 25.Mostad IL, Bjerve KS, Bjorgaas MR, Lydersen S, Grill V. Effects of n-3 fatty acids in subjects with type 2 diabetes: Reduction of insulin sensitivity and time-dependent alteration from carbohydrate to fat oxidation. Am J Clin Nutr. 2006;84:540–50. doi: 10.1093/ajcn/84.3.540. [DOI] [PubMed] [Google Scholar]
- 26.Kabir M, Skurnik G, Naour N, Pechtner V, Meugnier E, Rome S, et al. Treatment for 2 mo with n-3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: A randomized controlled study. Am J Clin Nutr. 2007;86:1670–9. doi: 10.1093/ajcn/86.5.1670. [DOI] [PubMed] [Google Scholar]


