Abstract
Background:
Nephrotic syndrome (NS) is a major clinical concern in human health, especially in children. Despite of the etiology, the prediction of remission in different treatment regimens based on suitable biomarkers is under development. The goal of this evaluation was the demonstration of correlation between serum level of Neutrophil gelatinase associated lipocalin (NGAL) and cystatin-C with kidney function in patients with NS.
Methods:
During the period between September 2008 and December 2011, 52 patients admitted to St. Al Zahra University Hospital were selected for evaluation. The measured parameters consisted of NGAL, cystatin-C, creatinine, albumin, blood urea nitrogen, urine protein, glomerular filtration rate. Demographic data were collected and considered in comparisons. Comparison between variables and their correlations were examined.
Results:
Means of serum NGAL and cystatin-C were significantly higher in case than the control group, P < 0.05. The mean of serum NGAL in patients without remission and who achieved remission were 23.09 (standard deviation [SD] ±10.11) and 36.26 (SD ± 20.10) ng/ml respectively; P < 0.05. Serum NGAL levels had a correlation with the following factors: Systolic blood pressure, diastolic blood pressure (DBP), cystatin-C, remission. Linear regression analysis showed a significant correlation between cystatin-C and systolic and DBP.
Conclusions:
Based on the results, serum NGAL can be used as a prognostic marker for remission. In addition, NGAL and cystatin-C are biomarkers of kidney injury in NS.
Keywords: Children, cystatin-C, glomerular filtration rate, Nephrotic syndrome, Neutrophil gelatinase associated lipocalin
INTRODUCTION
Nephrotic syndrome (NS) is characterized by massive proteinuria, hypoalbuminemia, edema and hyperlipidemia with 2-3/100,000 annual incidences. Steroid has a main role in treatment, but patients who do not have appropriate response to steroid, have a greater chance to attain end stage renal disease (ESRD).[1,2] Resistant NS has been widely accepted as an increasing etiology of ESRD.[3,4] Therefore, detecting early stages of chronic kidney diseases (CKD) has greatest importance in treating patients at risk.[5,6,7] The available standard biochemical markers are not sensitive enough to determine high-risk patients.[8] However, a variety of factors including response to steroid, hypertension, extent of glomerular sclerosis and interstitial fibrosis have been accepted as prognostic markers of getting ESRD.[9,10] Recently, new biomarkers have been introduced to define patients at risk.[11,12,13] Among these biomarkers, Neutrophil gelatinase associated lipocalin (NGAL) and cystatin-C have greatest significance.[14,15,16,17,18,19,20] There is few data regarding the role of NGAL and cystatin-C in children with NS.[21] In this study, we evaluated serum NGAL and cystatin-C in both Steroid Sensitive Nephrotic syndrome (SSNS) and steroid resistant Nephrotic syndrome (SRNS) children. In addition, we evaluated any possible correlation between these two biomarkers and response to steroid.
METHODS
In this cross-sectional study, 104 participants aged 1-18 years were enrolled i.e., 52 cases and an equal number of control. Since normal value for these two parameters in normal children is varied widely, a control group was enrolled to compare the results. Cases were selected from children with idiopathic NS who had been hospitalized from September 2008 to December 2011 in the only referral Pediatric Nephrology Department, affiliated to Isfahan University of Medical Sciences, Isfahan, Iran. Histopathologic findings mostly consisted of minimal change disease and or focal segmental glomerular sclerosis (FSGS). The histopathologic diagnosis of FSGS was based on the following criteria: (i) A lesion affecting some of the glomeruli in the renal biopsy while others remain unaffected and (ii) the affected glomeruli having a portion that has undergone capillary collapse with obliteration of capillary lumina with or without adhesions.
Control group was selected from age- and sex-matched children who had been referred to pediatric clinics for routine health examination. The demographic, clinical and laboratory data were recorded for all participants. These data for case group comprised of height, weight, age at the onset of disease, 24 h urinary protein excretion, serum albumin, blood urea nitrogen (BUN), serum creatinine, serum NGAL, serum cystatin-C, glomerular filtration rate (GFR) (based on both updated Schwartz equation and new cystatin-C based Schwartz formula)[22,23,24,25] systolic and diastolic blood pressure (DBP) and medications record. Data was updated regularly every 3-6 months, during the follow-up visit for each patient. Data sheet for the control group consisted of demographic data, BUN, serum albumin, serum creatinine, serum NGAL, serum cystatin-C, GFR and systolic and DBP.
Serum cyclosporine-A level was measured by ELISA method (Abnova-USA) in patients to follow its toxic concentration.
Serum creatinine and BUN were measured by enzymatic methods on an auto-analyzer instrument (Hitachi 917, Japan). Serum NGAL was measured by ELISA method (Bioproto Kit). Serum cystatin C was measured by particle-enhanced immunoturbidimetric method (Dako, Glostrup, Denmark).[26] A cystatin C level of lower than 1.38 mg/l is suggested as normal in the general population.[27,28]
To calculate GFR for each subject, we applied the following equations:

Definitions
CKD was defined as GFR lower than 90 ml/min/1.73 m2; Hypertension was defined as blood pressure higher than 95th percentile for age and height according to data from the task force report on high blood pressure in children and adolesents.[29] Response to treatment was classified as complete remission, partial remission and non-remission: Complete remission was defined as negative or trace proteinuria adjusted by body surface area <350 mg/m2/day. Partial remission was defined as a reduction in proteinuria, but still remaining in the supranormal range. Non-responder (SRNS) was considered as the inability to induce remission and/or partial remission within 4 weeks of daily steroid therapy following by three pulses of methylprednisolone. SSNS was defined as remission after 4-6 weeks of full dose of steroid therapy.[1,29]
Treatment protocols
All patients underwent similar initial treatment strategy. They were initially treated with oral prednisolon (60 mg/m2/day/4 to 6 weeks), with or without three consecutive doses of methyl prednisolone pulses (10-30 mg/kg/dose). The medication was then tapered in an alternate-day regimen over 6-9 months.
Patients were categorized into following subgroups: (1) subgroup A: those who were SSNS and remained steroid sensitive until the end of the study, (2) subgroup B: those who were SSNS and gradually turned to SRNS, (3) subgroup C: those who were SRNS and remained steroid resistant until the end of the study.
Patients who responded partially to prednisolon or demonstrated steroid side-effects received cyclophosphamide (2-3 mg/kg/day for 2 to 3 months). For those patients who did not respond to mentioned medications, cyclosporine-A (3-5 mg/kg/day) was commenced after performing kidney biopsy. In cases that did not respond to cyclosporine A in a 6 month course, mycophenolate mofetil (500-1000 mg/m2/day) was replaced. Angiotensin-converting enzyme inhibitor (ACEI) was added as an adjuvant therapy to control hypertension or proteinuria.
Statistical analysis
Statistical analyses were performed using the software (SPSS: An IBM Company, version 16.0,
IBM Corporation, Armonk, New York, USA). The categorized data were reported as frequencies and percentages. Continuous data were reported as mean and standard deviation.
Pearson correlation test was used to control the association between demographic parameters. Student t-test was performed to comparing the means between biochemical factors and GFR. Linear regression test was applied to examine the correlations between quantitative variables. P value of <0.05 was considered as significant.
Ethics
This study was approved by the Research and Ethics Committee of Isfahan University of Medical Sciences. Written informed consent from all parents and oral assent from children were obtained. This study was performed in accordance with the ethical standards of the Helsinki declaration.
RESULTS
Overall, 104 participants (52 cases and 52 controls) were enrolled. Male to female ratio in case group was 2/1. This ratio was similar in the control group.
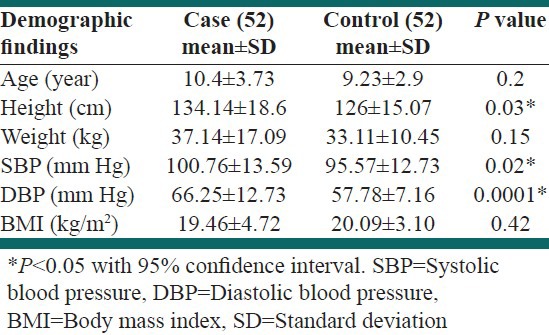
The means of age were not significantly different in both groups. The body mass index (BMI) was not different between groups, P = 0.42. Systolic blood pressure (SBP) was significantly higher in case than the control group; 100.76 ± 13.59 mmHg versus 95.57 ± 8.49 mmHg, respectively, P = 0.021. Means of DBP were 66.25 ± 12.73 mmHg in the case and 57.78 ± 7.16 mmHg in the control group, P < 0.0001. Demographic parameters including age, height, weight, SBP, DBP and BMI are pointed out in Table 1.
Table 1.
Clinical and demographic features of the participants
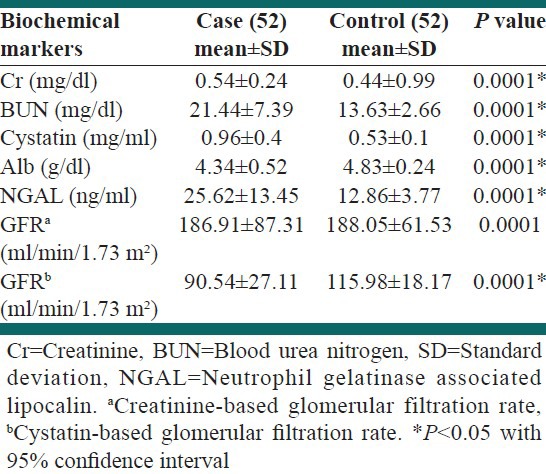
The mean for serum albumin, serum NGAL, serum cystatin-C, BUN, creatinine and GFR estimated by new Schwartz formula were significantly different in case from the control group [Table 2].
Table 2.
Laboratory findings of the participants
Patients’ medications were categorized to the following regimens including prednisolone (51.3%), prednisolone/cyclosporine-A (47.5%), prednisolone/cyclosporine-A/cellcept (9.6%) and one of the above regimen + ritoximab (7.8%).
Mean serum level of cyclosporine was 25.65 ± 22.54 and no one found in toxic level (>450 ng/ml).[30,31]
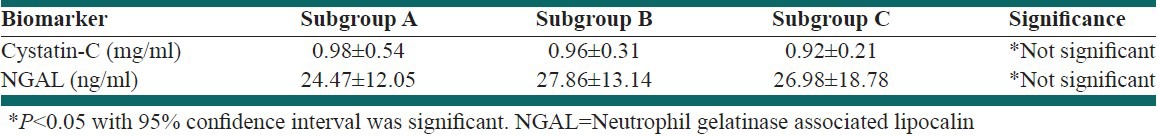
There was no difference in either NGAL or cystatin-C among three case-subgroups; P > 0.05 [Table 3].
Table 3.
Serum biomarkers levels in case subgroups
The means of serum NGAL in patients without remission and who achieved remission were 23.09 ± 10.11 and 36.26 ± 20.10 ng/ml respectively, P = 0.004.
Nonetheless, there was no significant difference between serum NGAL in patients with or without fibrosis; 27.39 ± 15.7 ng/ml and 22.80 ± 8.35 ng/ml respectively; P = 0.17.
Considering serum cystatin-C, no significant difference was shown between patients with and without fibrosis, 0.96 ± 0.41 mg/ml and 0.96 ± 0.38 mg/ml respectively; P = 0.99. In addition, there was not any significant difference between patients who achieved or did not achieve remission, 0.92 ± 0.31 mg/ml versus 1.1 ± 0.65 mg/ml respectively; P = 0.32.
Bivariate correlation coefficient test showed that SBP had significant positive correlations with BMI and cyclosporine levels; r = 0.75, P = 0.0001 and r = 0.33, P = 0.014 respectively. The same positive correlation was true for DBP.
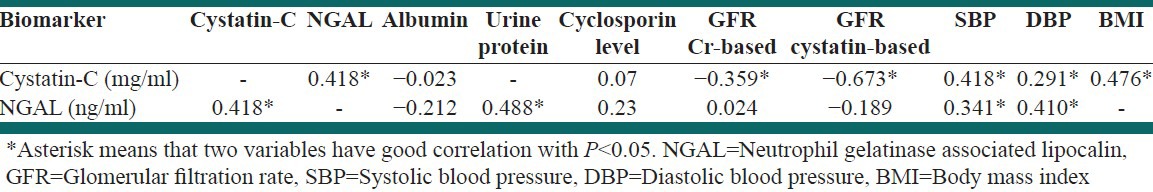
Applying bivariate correlation showed that serum cystatin-C level had significant positive correlation with the following factors; serum NGAL: r = 0.42, P = 0.002; SBP: r = 0.425, P = 0.002; DBP: r = 0.29, P = 0.03 [Table 4]. However, significant positive correlation was found between serum NGAL and the followings; SBP: r = 0.34, P = 0.013; DBP: r = 0.43, P = 0.001; remission: r = 0.39, P = 0.002 and cystatin-C as the above [Table 4]. Nevertheless, serum NGAL level had a negative correlation with GFR based cystatin-C: r = −0.358, P = 0.0001.
Table 4.
Correlation among biomarkers and biochemical parameters in case group
Regression analysis (linear method) demonstrated that serum NGAL had significant correlation with DBP and serum cystatin-C levels; P = 0.05 and P = 0.009 respectively.
Applying regression analysis showed that serum cystatin-C levels had significant correlation with the following factors; SBP: P = 0.008 and NGAL: P = 0.009.
DISCUSSION
NS as one of the most frequent glomerular disease in children may result in ESRD.[2] SRNS patients are at higher risk to develop ESRD. To determine the patients at risk, a variety of predisposing factors have been introduced, such as genetic mutations, response to steroid, severity of interstitial fibrosis and glomerular sclerosis.[1,8] Since diagnosis of steroid-resistant patients help physicians to choose the best modalities, finding new predictor biomarkers is mandatory.[32] In this study, we evaluated two novel biomarkers (NGAL, cystatin-C) in SSNS and SRNS.
Cystatin-C, a low molecular weight protein of cysteine protease family is produced by all nucleated cells and catabolized by proximal tubules.[17] Using a meta-analysis, Dharnidharka et al., showed that cystatin-C had superiority to creatinin in estimating GFR.[28] Cystatin-C has been widely accepted as a predictor biomarker in kidney and cardiovascular diseases.[5,33] In addition, serum cystatin-C has been introduced as a more sensitive biomarker than serum creatinin in predicting renal dysfunction in primary NS.[27,28] Indeed, this marker may reflect tubular injury in NS. It is demonstrated that urine cystatin-C levels was higher in NS with relapses.[28,34] Although, we did not assess the relapses, there was no significant difference between serum cystatin-C in patients with and without remission.
While GFR based serum cystatin-C was significantly lower in case comparing with the control group, it was not significantly different among sub-groups of case group. In addition, there was no significant difference in GFRs between those who achieved remission and those who did not.
We found that GFR based on either serum creatinin and or cystatin-C is higher in control than in case group. In addition, cystatin-C based GFR was lower than creatinin based GFR in case group. It asserted the previous claim that cystatin-C was a marker of early renal dysfunction.[27,28,35] However, we did not demonstrate any difference between cystatin-C levels in patients with or without fibrosis. Furthermore, cystatin-C could not differentiate between patients with and without remission.
Although, proteinuria might effect on urine cystatin-C levels, hypoalbuminemia had no effect on serum cystatin-C.[36,37] In our study, applying regression analysis did not show any correlation between serum albumin and serum cystatin-C levels. Furthermore, in accordance with the previous studies, we did not find any correlation between corticosteroid consumption and serum cystatin-C levels.[38]
NGAL has been introduced as a new risk marker of acute kidney injury and a predictive marker of CKDs.[32] Increased urine NGAL levels in FSGS patients comparing with the control group have been shown.[26,39,40] In addition, in diseases related to proximal and distal tubules and glomerular filtration barrier, NGAL levels may be useful for observing the response to treatment.[41,42]
In a recent study on NS, Bennett et al., showed that that median NGAL was significantly higher in SRNS than both SSNS and healthy controls.[21] Consistent with Bennett et al.'s study, the higher amount of serum NGAL was detected in SRNS comparing with both SSNS and control group [Table 3].
Urine NGAL has been demonstrated as an early marker of tubulointerestitial impairment in CKDs.[43] Animal studies revealed that serum NGAL had a higher level in rats with kidney fibrosis. However, prescribing ACEI dramatically decreased serum NGAL levels.[44]
In this study, comparing serum NGAL levels between patients with and without fibrosis showed that serum NGAL was not significantly different in both groups. Although, regression analysis demonstrated a significant correlation between serum NGAL and remission (P = 0.037), no correlation was found between serum NGAL levels and severity of fibrosis. Nonetheless, consuming ACEIs by most of our patients may interfere with interpreting the results.
Increased serum level of NGAL has been described in chronic renovascular hypertension perhaps a result of kidney injury or ischemia.[45] Blumczynski et al., showed that in children with primary hypertension and kidney injury had higher levels of serum NGAL.[46]
In concert with the above studies, we reported that serum NGAL levels had a positive correlation with systolic and diastolic hypertension.
Whilst toxic levels of cyclosporine, A may increase serum NGAL, no patient in our study had toxic cyclosporine level.[31] Therefore, we did not find any correlation between toxic levels of cyclosporine-A and serum NGAL levels.
Our study revealed that serum NGAL had only a significant correlation with remission (P = 0.004). Therefore, higher serum NGAL levels may be a risk factor for diagnosis patients who did not achieved remission.
Urine NGAL levels had significant correlation with urine protein and inverse correlation with GFR.[32] Nishida et al., showed significant inverse correlations between both serum and urinary NGAL levels with GFR.[47] In consistent with these studies, we demonstrated that serum NGAL level had a positive correlation with serum cystatin-C and negative correlation with GFR based cystatin-C. Furthermore, we found that serum NGAL had no correlation with serum albumin.
It is suggested to evaluate a large number of patients categorized based on various underlying NS.
CONCLUSION
Serum NGAL is a novel biomarker that can predict the remission in NS patients. In addition, serum cystatin-C and GFR based cystatin-C might be used as markers of early prediction of kidney injury in NS. Considering the obtained data, it seems the major application of this study may be lead to shortening course of disease and hospitalization period as well as a reduction of cost of treatment and patient family stress.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Pais P, Avner E. Nephrotic syndrome. In: Kleigman R, editor. Text Book of Nelson. 19th ed. Philadelphia: Elsevir; 2011. pp. 1801–06. [Google Scholar]
- 2.Vivarelli M, Moscaritolo E, Tsalkidis A, Massella L, Emma F. Time for initial response to steroids is a major prognostic factor in idiopathic nephrotic syndrome. J Pediatr. 2010;156:965–71. doi: 10.1016/j.jpeds.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 3.Wong CS, Furth SL. Epidemiology of renal disease in children. In: Kher K, editor. Clinical Pediatric Nephrology. 2nd ed. London England: Informa UKK Ltd; 2007. pp. 63–70. [Google Scholar]
- 4.Mitsnefes MM, Kathman TS, Mishra J, Kartal J, Khoury PR, Nickolas TL, et al. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in children with chronic kidney disease. Pediatr Nephrol. 2007;22:101–8. doi: 10.1007/s00467-006-0244-x. [DOI] [PubMed] [Google Scholar]
- 5.Villa P, Jiménez M, Soriano MC, Manzanares J, Casasnovas P. Serum cystatin C concentration as a marker of acute renal dysfunction in critically ill patients. Crit Care. 2005;9:R139–43. doi: 10.1186/cc3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen MT, Devarajan P. Biomarkers for the early detection of acute kidney injury. Pediatr Nephrol. 2008;23:47–9. doi: 10.1007/s00467-007-0470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mak RH. Acute kidney injury in children: The dawn of a new era. Pediatr Nephrol. 2008;23:2147–9. doi: 10.1007/s00467-008-1014-8. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki M, Wiers KM, Klein-Gitelman MS, Haines KA, Olson J, Onel KB, et al. Neutrophil gelatinase-associated lipocalin as a biomarker of disease activity in pediatric lupus nephritis. Pediatr Nephrol. 2008;23:403–12. doi: 10.1007/s00467-007-0685-x. [DOI] [PubMed] [Google Scholar]
- 9.Al-Ismaili Z, Palijan A, Zappitelli M. Biomarkers of acute kidney injury in children: Discovery, evaluation, and clinical application. Pediatr Nephrol. 2011;26:29–40. doi: 10.1007/s00467-010-1576-0. [DOI] [PubMed] [Google Scholar]
- 10.Bramham K, Mistry HD, Poston L, Chappell LC, Thompson AJ. The non-invasive biopsy – Will urinary proteomics make the renal tissue biopsy redundant? QJM. 2009;102:523–38. doi: 10.1093/qjmed/hcp071. [DOI] [PubMed] [Google Scholar]
- 11.Bagshaw SM, Bellomo R, Devarajan P, Johnson C, Karvellas CJ, Kutsiogiannis DJ, et al. Review article: Acute kidney injury in critical illness. Can J Anaesth. 2010;57:985–98. doi: 10.1007/s12630-010-9375-4. [DOI] [PubMed] [Google Scholar]
- 12.Tesch GH. Review: Serum and urine biomarkers of kidney disease: A pathophysiological perspective. Nephrology (Carlton) 2010;15:609–16. doi: 10.1111/j.1440-1797.2010.01361.x. [DOI] [PubMed] [Google Scholar]
- 13.Ohlsson S. Prabhakar S, editor. Urinary biomarkers in glomerulonephritis. An Update on Glomerullopathies-Etiology and Pathogenesis: In Tech. 2011. [June Second 2013]. pp. 269–76. Accessible at www.intechopen.com/download/pdf/19476 .
- 14.Schmidt-Ott KM. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury – Where do we stand today? Nephrol Dial Transplant. 2011;26:762–4. doi: 10.1093/ndt/gfr006. [DOI] [PubMed] [Google Scholar]
- 15.Soleimani MJ, Zargar Shoushtari MA, Shahrokh H, Habib Akhyari H, Kaffash Nayyeri R, Fereshtehnejad SM, et al. Comparison study of the diagnostic values of serum cystatin C and creatinine in the assessment of renal function in the early follow up of renal transplant patients. RJMS. 2009;63:79–86. [Google Scholar]
- 16.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–60. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 17.Hojs R, Bevc S, Ekart R, Gorenjak M, Puklavec L. Serum cystatin C as an endogenous marker of renal function in patients with mild to moderate impairment of kidney function. Nephrol Dial Transplant. 2006;21:1855–62. doi: 10.1093/ndt/gfl073. [DOI] [PubMed] [Google Scholar]
- 18.Mishra J, Mori K, Ma Q, Kelly C, Yang J, Mitsnefes M, et al. Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2004;15:3073–82. doi: 10.1097/01.ASN.0000145013.44578.45. [DOI] [PubMed] [Google Scholar]
- 19.Devarajan P. NGAL and emerging for kidney injury. Emerging biomarkers of acute kidney injury. Contrib Nephro. 2007;156:203–12. doi: 10.1159/000102085. [DOI] [PubMed] [Google Scholar]
- 20.Dent LC, Ma Q, Dastrala S, Bennett M, Mitsnefes MM, Barasch J, et al. Plasma neutrophil gelatinase-associated lipocalin predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: A prospective uncontrolled cohort study. Crit Care. 2007;11:R127. doi: 10.1186/cc6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett MR, Piyaphanee N, Czech K, Mitsnefes M, Devarajan P. NGAL distinguishes steroid sensitivity in idiopathic nephrotic syndrome. Pediatr Nephrol. 2012;27:807–12. doi: 10.1007/s00467-011-2075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–37. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staples A, LeBlond R, Watkins S, Wong C, Brandt J. Validation of the revised Schwartz estimating equation in a predominantly non-CKD population. Pediatr Nephrol. 2010;25:2321–6. doi: 10.1007/s00467-010-1598-7. [DOI] [PubMed] [Google Scholar]
- 24.Gheissari A, Taheri D, Mozafarpour S, Beigy H, Samanianpoor P, Merrikhi A, et al. The expression of cytoskeletal proteins in kidney specimens of children with primary focal segmental glomerulosclerosis. Indian J Nephrol. 2012;22:444–50. doi: 10.4103/0971-4065.106037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hofstra JM, Willems JL, Wetzels JF. Estimated glomerular filtration rate in the nephrotic syndrome. Nephrol Dial Transplant. 2011;26:550–6. doi: 10.1093/ndt/gfq443. [DOI] [PubMed] [Google Scholar]
- 26.Roja JR, Pérez M, Hurtado A, Asato C. Factors predicting for renal survival in primary focal segmental glomerulosclerosis. Nefrologia. 2008;28:439–46. [PubMed] [Google Scholar]
- 27.Zaffanello M, Franchini M, Fanos V. Is serum cystatin-C a suitable marker of renal function in children? Ann Clin Lab Sci. 2007;37:233–40. [PubMed] [Google Scholar]
- 28.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: A meta-analysis. Am J Kidney Dis. 2002;40:221–6. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 29.Ruf RG, Lichtenberger A, Karle SM, Haas JP, Anacleto FE, Schultheiss M, et al. Patients with mutations in NPHS2 (podocin) do not respond to standard steroid treatment of nephrotic syndrome. J Am Soc Nephrol. 2004;15:722–32. doi: 10.1097/01.asn.0000113552.59155.72. [DOI] [PubMed] [Google Scholar]
- 30.Flippin MS, Canter CE, Balzer DT. Increased morbidity and high variability of cyclosporine levels in pediatric heart transplant recipients. J Heart Lung Transplant. 2000;19:343–9. doi: 10.1016/s1053-2498(00)00061-9. [DOI] [PubMed] [Google Scholar]
- 31.Wasilewska A, Zoch-Zwierz W, Taranta-Janusz K, Michaluk-Skutnik J. Neutrophil gelatinase-associated lipocalin (NGAL): A new marker of cyclosporine nephrotoxicity? Pediatr Nephrol. 2010;25:889–97. doi: 10.1007/s00467-009-1397-1. [DOI] [PubMed] [Google Scholar]
- 32.Ležaić V. Serum and urine biomarkers determination and their significance in diagnosis of kidney disease. J Med Biochem. 2010;29:288–97. [Google Scholar]
- 33.Koenig W, Twardella D, Brenner H, Rothenbacher D. Plasma concentrations of cystatin C in patients with coronary heart disease and risk for secondary cardiovascular events: More than simply a marker of glomerular filtration rate. Clin Chem. 2005;51:321–7. doi: 10.1373/clinchem.2004.041889. [DOI] [PubMed] [Google Scholar]
- 34.Tkaczyk M, Nowicki M, Lukamowicz J. Increased cystatin C concentration in urine of nephrotic children. Pediatr Nephrol. 2004;19:1278–80. doi: 10.1007/s00467-004-1566-1. [DOI] [PubMed] [Google Scholar]
- 35.Cordeiro VF, Pinheiro DC, Silva GB, Jr, Lima JW, Mota RM, Libório AB, et al. Comparative study of cystatin C and serum creatinine in the estimative of glomerular filtration rate in children. Clin Chim Acta. 2008;391:46–50. doi: 10.1016/j.cca.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Nejat M, Hill JV, Pickering JW, Edelstein CL, Devarajan P, Endre ZH. Albuminuria increases cystatin C excretion: Implications for urinary biomarkers. Nephrol Dial Transplant. 2012;27(Suppl 3):iii96–103. doi: 10.1093/ndt/gfr222. [DOI] [PubMed] [Google Scholar]
- 37.Obrenovi R, Petrovi D, Majki N, Trbojevi J, Stojimirovi B. Influence of proteinuria on cystatin-C serum concentration in patients with primary glomerulonephritis. Jugoslov Med Biohem. 2006;25:21–5. [Google Scholar]
- 38.Bökenkamp A, van Wijk JA, Lentze MJ, Stoffel-Wagner B. Effect of corticosteroid therapy on serum cystatin C and beta2-microglobulin concentrations. Clin Chem. 2002;48:1123–6. [PubMed] [Google Scholar]
- 39.Youssef DM, El-Shal AA. Urine neutrophil gelatinase-associated lipocalin and kidney injury in children with focal segmental glomerulosclerosis. Iran J Kidney Dis. 2012;6:355–60. [PubMed] [Google Scholar]
- 40.Abeyagunawardena AS, Sebire NJ, Risdon RA, Dillon MJ, Rees L, Van’t Hoff W, et al. Predictors of long-term outcome of children with idiopathic focal segmental glomerulosclerosis. Pediatr Nephrol. 2007;22:215–21. doi: 10.1007/s00467-006-0264-6. [DOI] [PubMed] [Google Scholar]
- 41.Bolignano D, Lacquaniti A, Coppolino G, Donato V, Campo S, Fazio MR, et al. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:337–44. doi: 10.2215/CJN.03530708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuwabara T, Mori K, Mukoyama M, Kasahara M, Yokoi H, Saito Y, et al. Urinary neutrophil gelatinase-associated lipocalin levels reflect damage to glomeruli, proximal tubules, and distal nephrons. Kidney Int. 2009;75:285–94. doi: 10.1038/ki.2008.499. [DOI] [PubMed] [Google Scholar]
- 43.Nickolas TL, Forster CS, Sise ME, Barasch N, Valle DS, Viltard M, et al. NGAL (Lcn2) monomer is associated with tubulointerstitial damage in chronic kidney disease. Kidney Int. 2012;82:718–22. doi: 10.1038/ki.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang H, Zhu Y, Jia N, Zhang HT, Zheng J, Zhu WP, et al. Expression and significance of neutrophil gelatinase-associated lipocalin in renal interstitial fibrosis in rats. Zhonghua Yi Xue Za Zhi. 2012;92:2565–9. [PubMed] [Google Scholar]
- 45.Eirin A, Gloviczki ML, Tang H, Rule AD, Woollard JR, Lerman A, et al. Chronic renovascular hypertension is associated with elevated levels of neutrophil gelatinase-associated lipocalin. Nephrol Dial Transplant. 2012;27:4153–61. doi: 10.1093/ndt/gfs370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blumczynski A, Sołtysiak J, Lipkowska K, Silska M, Poprawska A, Musielak A, et al. Hypertensive nephropathy in children-do we diagnose early enough? Blood Press. 2012;21:233–9. doi: 10.3109/08037051.2012.666393. [DOI] [PubMed] [Google Scholar]
- 47.Nishida M, Kawakatsu H, Okumura Y, Hamaoka K. Serum and urinary neutrophil gelatinase-associated lipocalin levels in children with chronic renal diseases. Pediatr Int. 2010;52:563–8. doi: 10.1111/j.1442-200X.2010.03067.x. [DOI] [PubMed] [Google Scholar]


