Abstract
Background:
Annual pilgrimage (Yatra) to the cave shrine of Shri Amarnath Ji’ is a holy ritual among the Hindu devotees of Lord Shiva. Located in the Himalayan Mountain Range (altitude 13,000 ft) in south Kashmir, the shrine is visited by thousands of devotees and altitude sickness is reportedly common.
Materials and Methods:
More than 600,000 pilgrims visited the cave shrine in 2011 and 2012 with 239 recorded deaths. Thirty one patients with suspected altitude sickness were referred from medical centers en-route the cave to Sher-i-Kashmir Institute of Medical Sciences, a tertiary-care center in capital Srinagar (5,000 ft). The clinical features and the response to treatment were recorded.
Results:
Thirty-one patients (all lowlanders, 19 male; age 18-60 years, median 41) had presented with acute onset breathlessness of 1-4 days (median 1.9 d) starting within 12-24 h of a rapid ascent; accompanied by cough (68%), headache (8%), dizziness and nausea (65%). Sixteen patients had associated encephalopathy. Clinical features on admission included tachypnea (n = 31), tachycardia (n = 23), bilateral chest rales (n = 29), cyanosis (n = 22) and grade 2-4 encephalopathy. Hypoxemia was demonstrable in 24 cases and bilateral infiltrates on radiologic imaging in 29. Ten patients had evidence of high-altitude cerebral edema. All patients were managed with oxygen, steroids, nifedipine, sildenafil and other supportive measures including invasive ventilation (n = 3). Three patients died due to multiorgan dysfunction.
Conclusions:
Altitude sickness is common among Amaranath Yatris from the plains and appropriate educational strategies should be invoked for prevention and prompt treatment.
KEY WORDS: Altitude illness, cerebral edema, mountain sickness, pilgrimage, pulmonary edema
INTRODUCTION
Even as excluding Antarctica only 2.5% of the world's land mass exists above 3,000 m, the heights continue to attract the tourists, trekkers, skiers, and mountaineers; many of whom are lowland or sea level dwellers.[1] Millions of visitors of all ages travel to high and even extreme altitudes every year. As a person ascends to higher altitudes, the air pressure falls and with it the partial pressure of O2 (PO2) in inhaled air, the arterial PaO2, and the O2 saturation of the blood. Travel to high altitude thus laces individuals at risk for a variety of complications related to the low ambient oxygen conditions. The most important of these complications include the relatively benign acute mountain sickness (AMS) and the potentially life-threatening high-altitude cerebral edema (HACE), and high-altitude pulmonary edema (HAPE). Although AMS is more frequent, HAPE is less common, the reported prevalence of AMS and HAPE at 4559 m being approximately 50% and 4%, respectively.[2] The prevalence of HAPE in trekkers to the Alps and mountaineers of Colorado ranges from 0.01% to 0.2%[3,4] whereas it is seen in up to 4% of those trekking rapidly up the Himalayas.[2]
The Himalayan Mountain Ranges apart from being regarded as the epitome of adventure in mountaineering are considered the mystical dwelling of the “Gods” from ancient times. A number of sacred pilgrimage sites in these ranges such as Kailash Mansarovar, the Holy Mountain in Tibet and the Char Dhams Kedarnath, Badrinath, Yamunotri, and Gangotri are visited by thousands of religious devotees to pay obeisance. The 130-ft high holy cave of “Baba Amarnath Ji,” situated at a height of 13,000 ft (3882 m) in the Northern Indian state of Jammu and Kashmir and believed to be the abode of Lord Shiva, is one such site of great reverence for the Hindus. The cave shrine is visited by thousands of devotees each year over a 6-week period starting from the last part of the month of June; the pilgrimage termed as “Amarnath Yatra.”[5] Many pilgrims develop altitude related ailments enroute requiring medical attention and sometimes these prove fatal. We herewith present our experience with 31 patients who were referred to our institution with various altitude related illnesses during the annual pilgrimages of 2011 and 2012.
MATERIALS AND METHODS
The Amarnath Holy Cave standing at 3,888 m is located in a narrow gorge at the farther end of the Lidder valley in South Kashmir [Figure 1].[5] Two routes are available to the pilgrims, the longer route through Pahalgam (414 km from Jammu) [Figure 1] and the relatively short Baltal route (363 km from Jammu). More than 600,000 pilgrims (634,000 in 2011 and 621,000 in 2012) visited the holy cave in 2011 (June 25 to August 14, 2011) as well as in 2012 (June 30, 2012 to August 2, 2012). Two hundred and thirty-nine deaths (130 in 2012) were reported, chiefly from cardiac and pulmonary causes in the 2 years of pilgrimage. Many Yatris develop medical complications that are managed locally in medical camps erected specifically for the duration of the “Yatra.” The patients for the current study those referred from these medical camps to SKIMS, a 750-bedded tertiary care cum referral center, once the locally employed measures proved unsuccessful. We recorded the clinical presentations, the investigations profile and response to various treatments of the patients. The diagnosis of AMS, HAPE, and HACE was based on Lake Louise score.[6] Informed consent was obtained from all the participants and the study was approved by the Institute Ethics committee. The data were analyzed using SPSS software version 11.0 and values have been expressed as mean ± SD.
Figure 1.
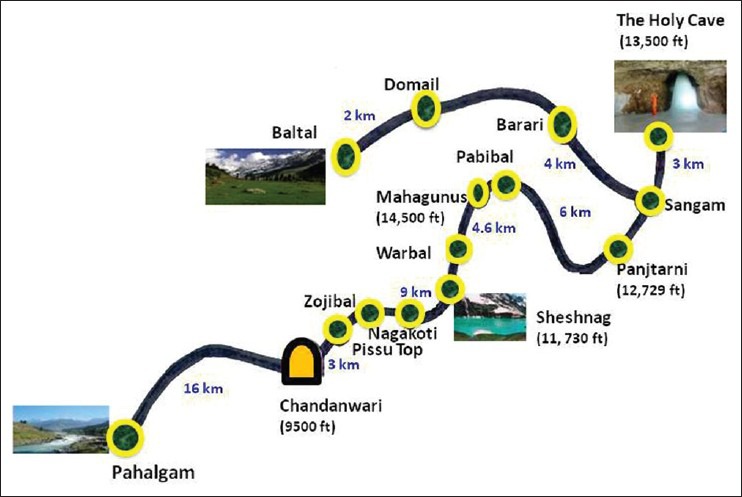
The route to the holy cave shrine of ‘shri Amarnath Ji’
RESULTS
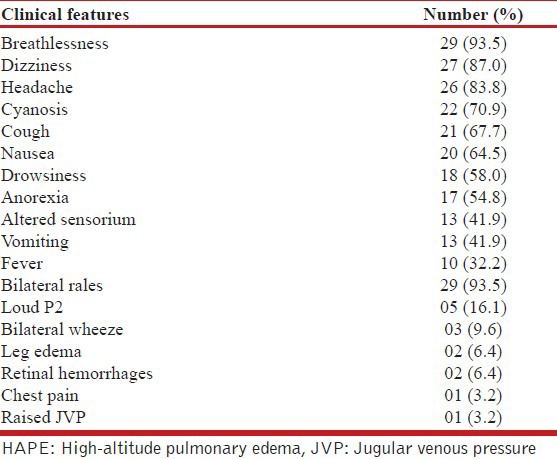
A total of thirty-one patients, suspected as having high altitude sickness were referred from medical centers enroute. The patients consisted of 19 male and 12 females with ages ranging from 18 years to 60 years (median 41 years) All the patients were lowlanders hailing from various plains of the country including Delhi, Punjab, Gujarat, Andhra Pradesh, West Bengal and Maharashtra. All patients satisfied the lake Louise criteria for HAPE with a median score of 7 (range of 4-14), whereas 10 had evidence of HACE in addition. Patients presented with acute onset breathlessness of 1-4 days (median 1.9 d) starting within 12-24 h of a rapid ascent, accompanied by cough (68%), headache (8%), dizziness, nausea (65%), and other symptoms as depicted in Table 1. Sixteen patients had associated encephalopathy. Underlying comorbidities included hypertension (n = 6), diabetes mellitus (n = 5), chronic alcoholism (n = 4) and chronic obstructive pulmonary disease (n = 1). All patients had been ferried to a lower altitude and given oxygen therapy and other measures (steroids, nifedipine, and diuretics) and were referred to SKIMS (located 125 km away from Baltal at an altitude of 5,000 ft) once the measures were not helpful. On admission, all were clinically tachypneic and 23 had tachycardia. Other clinical features included grade 2-4 encephalopathy (n = 16), cyanosis (n = 22), and bilateral chest rales (n = 29). Five patients had a loud P2 and jugular venous pressure was elevated in one patient [Table 1].
Table 1.
Depicting the various clinical features seen in 31 patients with HAPE
The various investigations at admission are depicted in Table 2. Hypoxemia on admission was seen in 24 patients. Hypokaemia was demonstrable in 18 (58%) cases and 20 had a polymorphonuclear leucocytosis at presentation. Serum urea was elevated in 12 patients. Bilateral infiltrates were observed on radiographic imaging of the chest in 29 patients and unilateral pulmonary edema in 2 cases. High resolution computed tomography done in 10 patients showed bilateral patchy infiltrates in 5 patients with 2 patients having features of unilateral pulmonary edema being predominantly right sided in one and left sided in the other [Figures 2-4]. None of the patients had electrocardiographic evidence of myocardial infarction, and troponin-T and cardiac enzymes (CK and LDH) were normal in all. Ten patients had evidence of HACE with computed tomography of the head revealing diffuse effacement of cerebral sulci and compression of ventricles [Figure 5]. One patient had evidence of renal failure at admission. Bacterial cultures of blood and sputum were sterile. Nasopharyngeal swabs for Influenza A and B viruses tested negative on real-time reverse transcriptase polymerase chain reaction. Patients were managed with oxygen, steroids, nifedipine, and sildenafil and other supportive measures. Invasive ventilation was required in 3 cases. While 28 of the patients recovered with a median hospital stay of 4 days, 3 patients developed multi-organ dysfunction and succumbed to their illness. Autopsies were denied by attendants of all the deceased citing religious reasons.
Table 2.
Depicting the various laboratory parameters in 31 patients with HAPE
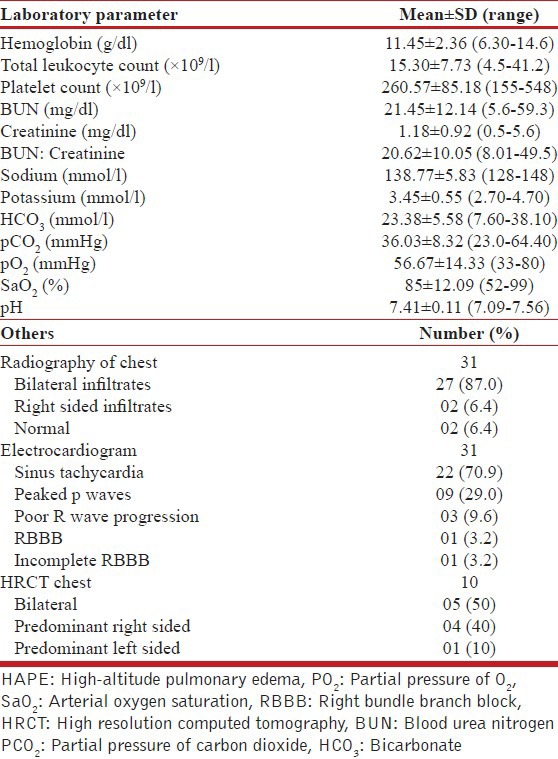
Figure 2.
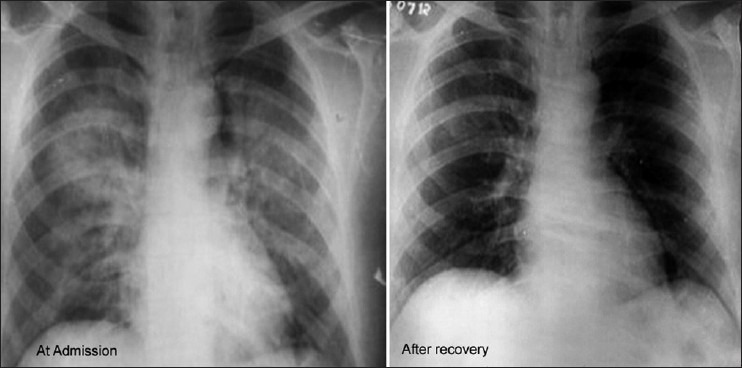
Chest radiograph in a young patient showing pulmonary edema at admission and after recovery
Figure 4.
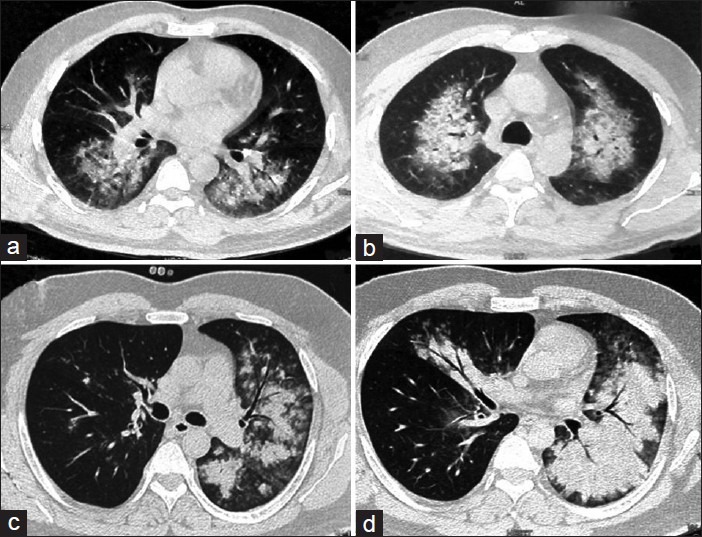
Computed tomography images showing evidence of bilateral alveolar edema (a and b) and predominant left sided edema (c and d)
Figure 5.
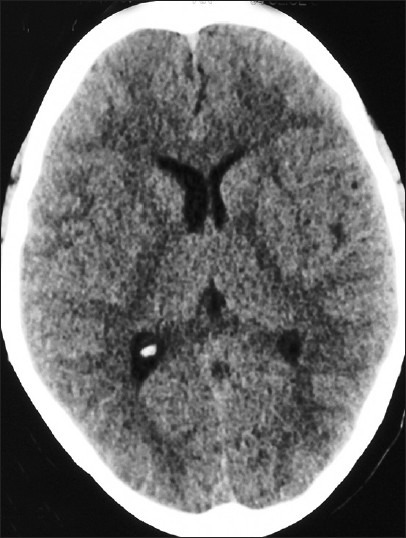
Computed tomography of the head revealing diffuse effacement of cerebral sulci and compression of ventricles. In a case with high altitude cerebral edema
Figure 3.
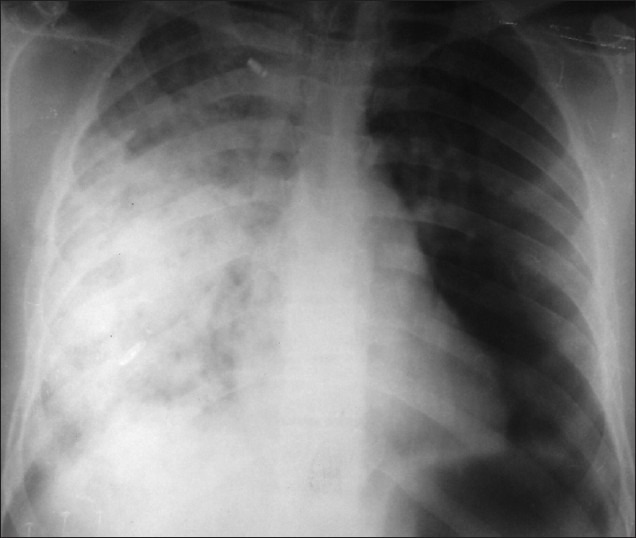
Unilateral right-sided pulmonary edema
DISCUSSION
Our case series highlights the fact that poorly acclimatized people from the plains are at high risk of developing various manifestations of high altitude illness during the holy pilgrimage to the “Amarnath” cave shrine. Even as non-traumatic surgical and hyperglycemic emergencies in the Yatris have been reported earlier,[7,8] and one case of HAPE has been reported,[9] ours is the first documented case series of altitude related illness in the pilgrim trekkers to this major Hindu religious site.
Two major reported determinants of altitude illness include the altitude attained and the rate of ascent. The altitude of the cave (> 13,000 ft) classifies it as a high altitude site as per the classification of the altitudes (high altitude > 2,500 m, very high altitude 255-5800 m and extreme altitude > 5,800 m).[10] The incidence of AMS has been reported to be 3.1% at 6,600 ft[11] as against high incidences of up to 53% at 15,044 ft.[2] The incidence of HAPE, however, is much less being up to 4% in trekkers in the Himalayas and climbers in the Alps ascending at a rate of greater than 600 m/day.[12] The incidence of HAPE increases from 2.5% to 15.5% when an altitude of 5500 m is reached by airlift[13] as opposed to trekking over 4-6 days.[14,15] Although none of our patients had been air-ferried to the cave, the rate of ascent in our patients was rapid as all of them had trekked along the quickly scalable Baltal route that allows lesstime for the trekkers to acclimatize. There were more males in our study as against previous reports of women outnumbering males.[16] Men are generally more eager to undertake the journey faster and consequently attempt a more rapid ascent.
Twenty of our patients had an elevated leukocyte count. Elevated leukocyte count can either be a manifestation of the altitude illness or a part of accompanying infection. The high leukocyte count in AMS could be attributed to the generalized inflammatory process that has been identified with increased inflammatory markers such as leukotrienes, interleukin 6 and C-reactive proteins (CRP).[17,18] However, a possibility of accompanying infection must always be borne in mind in order to institute correct therapy. We did use antibiotics in patients whose leukocyte counts exceeded 20 × 109/l. It would be worthwhile to study markers of inflammation like the procalcitonin and CRP to determine the contribution of infection to the clinical and laboratory picture.
Twelve of our patients had azotemia with elevated blood urea nitrogen: Creatinine ratio. This could be attributed to the reduced plasma volume in these patients. An elevated heart rate (mean HR 100.4 + 15.8) could also be an indirect evidence of dehydration.[19] An interesting finding in 20 of our patients was the presence of hypokalemia [Table 2]. Hypokalemia can be attributed to the bicarbonate diuresis, which occurs due to hypoxia.[20] However, it could be partly contributed to by the diuretics, beta-2 agonists, and steroids that were received by the patients en-route. Hypokalemia can contribute to the respiratory muscle weakness of the patients and an eventual worsening of the respiratory failure. Since the facilities for electrolyte estimation are not available en-route to the cave, this could be potentially treatable factor of worsening respiratory function in the pilgrims and would argue for the availability of such facilities.
Immediate decent to a lower altitude is the first step in the management of altitude sickness. A decent of 1000 meters can be life-saving.[10,21] Descent and supplementary oxygen (to keep SpO2 above 90%) are the treatments of choice. Unfortunately, many of our patients had continued their ascent even after the appearance of symptoms of AMS, despite warnings and advice by the treating medics. This constitutes a serious issue that requires address. A belief in divine healing must not be allowed to interfere with medical management of symptomatic individuals. Pharmacological agents that help in the management of HAPE include acetazolamide, nifedipine, dexamethasone, and phosphodiesterase-5 inhibitors like sildenafil.[10,22,23,24,25] Hyperbaric oxygen therapy wherever available has also been shown to be of considerable value for the treatment of AMS where immediate descent is not possible;[26] however it was not used on any of our patients.
High-altitude illness is best prevented by a gradual ascent to promote acclimatization. It has been suggested that once above an altitude of 2500 m, the altitude at which one sleeps should not be increased by more than 600 m in 24 h and that an extra day should be added for acclimatization for every increase of 600-1200 min this altitude.[26] All of our patients had ascended rapidly, despite having been appraised of deleterious effects of a rapid ascent in preparatory camps, and consequently had a poor acclimatization to the altitude. Majority of the pilgrims believe that the divine journey is itself protective for disease and consequently do not seek any preventive strategies. Many pilgrims attribute the symptoms of AMS to attainment of “state of trance” due to the divine intervention and hence refuse any interventions to this believed state of heightened spirituality. The normalization of the symptoms after the visit to the cave is never attributed to the descent, but is mostly attributed to divine healing and adds to the obstinacy of the pilgrims for refusing treatment upon the onset of symptoms. Pharmacological agents that can be used to prevent the development of mountain sickness include acetazolamide, dexamethasone, nifedipine, and phospodiesterase inhibitors such as sildenafil and tadalafil.[22,23,27,28,29,30] Salmeterol[31] and more recently ibuprofen have also been was found to be effective in reducing the incidence of AMS.[32]
Pathophysiologically, AMS, HAPE, and HACE are thought to represent the effects of hypoxia on central nervous system and the pulmonary system. HAPE is thought to be as a result of altitude related hypoxia induced, inhomogeneous vasoconstriction of the pulmonary vasculature,[33,34] which leads to areas that are subjected to high pressure and flow, overdistention of pulmonary capillaries, and injury to the blood–gas barrier. The combination of high flow at higher pressure results in pressures that exceed the structural and dynamic capacity of the blood-gas barrier to maintain normal alveolar fluid balance.[35] This phenomenon causes extravasation of fluid, plasma proteins, and blood cells into the interstitial and alveolar spaces.[36] An impaired clearance of fluid filtered into the alveoli may also be involved in the pathophysiology of HAPE.[35] It has also been speculated that free oxygen radicals play a role in the pathogenesis and antioxidants may thus play a role in the treatment.[37] Pulmonary inflammation may trigger, potentiate or exacerbate the formation and degree of edema.[38] Decreased bioavailability of nitric oxide has also been postulated to explain the elevated pulmonary artery pressure.[39] Hypoxic damage postulated to cause HACE can either cytotoxic (intracellular, due to cellular swelling),[40] vasogenic (extracellular, due to blood brain barrier leakage),[41] or a combination of the two.[42] Hypoxia elicits neurohumoral and hemodynamic responses in both the brain and the lungs, that result in overperfusion of microvascular beds, elevated hydrostatic capillary pressure, capillary leakage, and consequent edema.
Identifying people at risk for development of altitude illness and recommending appropriate preventive strategies would be very helpful in reducing the incidence of the AMS in Amarnath Yatris. In a recent Chinese study where AMS occurred in 56% of the 11,182 workers, rapid ascent to an altitude above 3500 m, sea-level or low land newcomers, age under 25 years, heavy physical exertion, obesity, and arterial oxygen saturation (SaO2) below 80% were found to be independent risk factors.[43] However, in another recent study, oximetry after rapid arrival to 4260 m was not predictive of AMS or summit success in a rapid ascent to 5640 m,[44] even though some investigators suggest routine use of oximtery at lower altitudes as a simple and specific indicator to identify those with a higher chance of development of mountain sickness.[45] Patients with metabolic syndrome should also be carefully monitored for early signs of AMS.[46]
The Yatris agreed to have concealed their ailments while seeking medical certificates from their practitioners in their intense desire to undertake the pilgrimage and there was a general lack of awareness of the challenges posed by the journey. These indicate a knowledge deficit, which needs to be adequately addressed through intensive educational efforts aimed at better preparing the Yatris for the holy trek.
Our study is limited by the fact that the patients include only the severe cases and the full characterization of the clinical syndrome without any selection bias would only be possible by on site recruitment of symptomatic Yatris.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Rennie D. See Nuptse and die. Lancet. 1976;2:1177–9. [PubMed] [Google Scholar]
- 2.Maggiorini M, Bühler B, Walter M, Oelz O. Prevalence of acute mountain sickness in the Swiss Alps. BMJ. 1990;301:853–5. doi: 10.1136/bmj.301.6756.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sophocles AM., Jr High-altitude pulmonary edema in Vail, Colorado, 1975-1982. West J Med. 1986;144:569–73. [PMC free article] [PubMed] [Google Scholar]
- 4.Hochstrasser J, Nanzer A, Oelz O. Altitude edema in the Swiss Alps. Observations on the incidence and clinical course in 50 patients 1980-1984. Schweiz Med Wochenschr. 1986;116:866–73. [PubMed] [Google Scholar]
- 5.Darshan of Shri Him Shiv Lingam in Shri Amarnath Ji Holy Cave (Gufa) [Last accessed 2012 Sep 03]. Available from: http://www.amarnathyatra.org .
- 6.Maggiorini M, Müller A, Hofstetter D, Bärtsch P, Oelz O. Assessment of acute mountain sickness by different score protocols in the Swiss Alps. Aviat Space Environ Med. 1998;69:1186–92. [PubMed] [Google Scholar]
- 7.Mir IS, Mir M, Ahmed M. Profile of non traumatic surgical disorders found in the pilgrims/trekkers travelling to Shri Amarnath Ji cave. Indian J Med Res. 2008;128:740–3. [PubMed] [Google Scholar]
- 8.Ganie MA, Koul S, Razvi HA, Laway BA, Zargar AH. Hyperglycemic emergencies in Indian patients with diabetes mellitus on pilgrimage to Amarnathji yatra. Indian J Endocrinol Metab. 2012;16(Suppl 1):S87–90. doi: 10.4103/2230-8210.94267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh J, Gorea RK, Agarrwal AD. Victimology of high altitude pilgrimage. J Punjab Acad Forensic Med Toxicol. 2005;5:972–8. [Google Scholar]
- 10.Taylor AT. High-altitude illnesses: Physiology, risk factors, prevention, and treatment. RMMJ. 2011;2:e0022. doi: 10.5041/RMMJ.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roeggia G, Roeggia M, Wagner A. Acute mountain sickness at moderate altitudes. Ann Intern Med. 1993;119:633–4. doi: 10.7326/0003-4819-119-7_part_1-199310010-00016. [DOI] [PubMed] [Google Scholar]
- 12.Hackett PH, Rennie D. Rales, peripheral edema, retinal hemorrhage and acute mountain sickness. Am J Med. 1979;67:214–8. doi: 10.1016/0002-9343(79)90393-0. [DOI] [PubMed] [Google Scholar]
- 13.Singh I, Kapila CC, Khanna PK, Nanda RB, Rao BD. High-altitude pulmonary oedema. Lancet. 1965;1:229–34. doi: 10.1016/s0140-6736(65)91520-5. [DOI] [PubMed] [Google Scholar]
- 14.Hackett PH, Rennie D, Levine HD. The incidence, importance, and prophylaxis of acute mountain sickness. Lancet. 1976;2:1149–55. doi: 10.1016/s0140-6736(76)91677-9. [DOI] [PubMed] [Google Scholar]
- 15.Schneider M, Bernasch D, Weymann J, Holle R, Bartsch P. Acute mountain sickness: Influence of susceptibility, preexposure, and ascent rate. Med Sci Sports Exerc. 2002;34:1886–91. doi: 10.1097/00005768-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Kayser B. Acute mountain sickness in western tourists around the thorong pass (5,400 m) in Nepal. J Wilderness Med. 1991;2:110–7. [Google Scholar]
- 17.Kaminsky DA, Jones K, Schoene RB, Voelkel NF. Urinary leukotriene E4 levels in high-altitude pulmonary edema. A possible role for inflammation. Chest. 1996;110:939–45. doi: 10.1378/chest.110.4.939. [DOI] [PubMed] [Google Scholar]
- 18.Hartmann G, Tschöp M, Fischer R, Bidlingmaier C, Riepl R, Tschöp K, et al. High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine. 2000;12:246–52. doi: 10.1006/cyto.1999.0533. [DOI] [PubMed] [Google Scholar]
- 19.Shah MB, Braude D, Crandall CS, Kwack H, Rabinowitz L, Cumbo TA, et al. Changes in metabolic and hematologic laboratory values with ascent to altitude and the development of acute mountain sickness in Nepalese pilgrims. Wilderness Environ Med. 2006;17:171–7. doi: 10.1580/pr43-04. [DOI] [PubMed] [Google Scholar]
- 20.Gledhill N, Beirne GJ, Dempsey JA. Renal response to short-term hypocapnia in man. Kidney Int. 1975;8:376–84. doi: 10.1038/ki.1975.130. [DOI] [PubMed] [Google Scholar]
- 21.Hackett PH, Roach RC. High altitude cerebral edema. High Alt Med Biol. 2004;5:136–46. doi: 10.1089/1527029041352054. [DOI] [PubMed] [Google Scholar]
- 22.Leaf DE, Goldfarb DS. Mechanisms of action of acetazolamide in the prophylaxis and treatment of acute mountain sickness. J Appl Physiol. 2007;102:1313–22. doi: 10.1152/japplphysiol.01572.2005. [DOI] [PubMed] [Google Scholar]
- 23.Oelz O, Maggiorini M, Ritter M, Noti C, Waber U, Vock P, et al. Prevention and treatment of high altitude pulmonary edema by a calcium channel blocker. Int J Sports Med. 1992;13(Suppl 1):S65–8. doi: 10.1055/s-2007-1024598. [DOI] [PubMed] [Google Scholar]
- 24.Levine BD, Yoshimura K, Kobayashi T, Fukushima M, Shibamoto T, Ueda G. Dexamethasone in the treatment of acute mountain sickness. N Engl J Med. 1989;321:1707–13. doi: 10.1056/NEJM198912213212504. [DOI] [PubMed] [Google Scholar]
- 25.Ricart A, Maristany J, Fort N, Leal C, Pagés T, Viscor G. Effects of sildenafil on the human response to acute hypoxia and exercise. High Alt Med Biol. 2005;6:43–9. doi: 10.1089/ham.2005.6.43. [DOI] [PubMed] [Google Scholar]
- 26.Freeman K, Shalit M, Stroh G. Use of the Gamow Bag by EMT-basic park rangers for treatment of high-altitude pulmonary edema and high-altitude cerebral edema. Wilderness Environ Med. 2004;15:198–201. doi: 10.1580/1080-6032(2004)15[198:uotgbb]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 27.Hackett PH, Roach RC. High-altitude illness. N Engl J Med. 2001;345:107–14. doi: 10.1056/NEJM200107123450206. [DOI] [PubMed] [Google Scholar]
- 28.Ried LD, Carter KA, Ellsworth A. Acetazolamide or dexamethasone for prevention of acute mountain sickness: A meta-analysis. J Wilderness Med. 1994;5:34–48. [Google Scholar]
- 29.Bärtsch P, Maggiorini M, Ritter M, Noti C, Vock P, Oelz O. Prevention of high-altitude pulmonary edema by nifedipine. N Engl J Med. 1991;325:1284–9. doi: 10.1056/NEJM199110313251805. [DOI] [PubMed] [Google Scholar]
- 30.Maggiorini M, Brunner-La Rocca HP, Peth S, Fischler M, Böhm T, Bernheim A, et al. Both tadalafil and dexamethasone may reduce the incidence of high-altitude pulmonary edema: A randomized trial. Ann Intern Med. 2006;145:497–506. doi: 10.7326/0003-4819-145-7-200610030-00007. [DOI] [PubMed] [Google Scholar]
- 31.Basnyat B. Salmeterol for the prevention of high-altitude pulmonary edema. N Engl J Med. 2002;347:1282–5. [PubMed] [Google Scholar]
- 32.Lipman GS, Kanaan NC, Holck PS, Constance BB, Gertsch JH PAINS Group. Ibuprofen prevents altitude illness: A randomized controlled trial for prevention of altitude illness with nonsteroidal anti-inflammatories. Ann Emerg Med. 2012;59:484–90. doi: 10.1016/j.annemergmed.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 33.Maggiorini M, Mélot C, Pierre S, Pfeiffer F, Greve I, Sartori C, et al. High-altitude pulmonary edema is initially caused by an increase in capillary pressure. Circulation. 2001;103:2078–83. doi: 10.1161/01.cir.103.16.2078. [DOI] [PubMed] [Google Scholar]
- 34.Hopkins SR, Garg J, Bolar DS, Balouch J, Levin DL. Pulmonary blood flow heterogeneity during hypoxia and high-altitude pulmonary edema. Am J Respir Crit Care Med. 2005;171:83–7. doi: 10.1164/rccm.200406-707OC. [DOI] [PubMed] [Google Scholar]
- 35.Bärtsch P, Mairbäurl H, Maggiorini M, Swenson ER. Physiological aspects of high-altitude pulmonary edema. J Appl Physiol. 2005;98:1101–10. doi: 10.1152/japplphysiol.01167.2004. [DOI] [PubMed] [Google Scholar]
- 36.Swenson ER, Maggiorini M, Mongovin S, Gibbs JS, Greve I, Mairbäurl H, et al. Pathogenesis of high-altitude pulmonary edema: Inflammation is not an etiologic factor. JAMA. 2002;287:2228–35. doi: 10.1001/jama.287.17.2228. [DOI] [PubMed] [Google Scholar]
- 37.Roche E, Romero-Alvira D. Role of oxygen free radicals in altitude-related disorders. Med Hypotheses. 1994;42:105–9. doi: 10.1016/0306-9877(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 38.Shapiro DA, Rom WN. Injuries due to physical agents. Curr Opin Crit Care. 1995;1:242–5. [Google Scholar]
- 39.Duplain H, Sartori C, Lepori M, Egli M, Allemann Y, Nicod P, et al. Exhaled nitric oxide in high-altitude pulmonary edema: Role in the regulation of pulmonary vascular tone and evidence for a role against inflammation. Am J Respir Crit Care Med. 2000;162:221–4. doi: 10.1164/ajrccm.162.1.9908039. [DOI] [PubMed] [Google Scholar]
- 40.Houston CS, Dickinson J. Cerebral form of high-altitude illness. Lancet. 1975;2:758–61. doi: 10.1016/s0140-6736(75)90735-7. [DOI] [PubMed] [Google Scholar]
- 41.Lassen NA, Harper AM. Letter: High-altitude cerebral oedema. Lancet. 1975;2:1154. doi: 10.1016/s0140-6736(75)91053-3. [DOI] [PubMed] [Google Scholar]
- 42.Wohns RN. High altitude cerebral edema: A pathophysiological review. Crit Care Med. 1981;9:880–2. doi: 10.1097/00003246-198112000-00017. [DOI] [PubMed] [Google Scholar]
- 43.Wu TY, Ding SQ, Liu JL, Jia JH, Chai ZC, Dai RC. Who are more at risk for acute mountain sickness: A prospective study in Qinghai-Tibet railroad construction workers on Mt. Tanggula. Chin Med J (Engl) 2012;125:1393–400. [PubMed] [Google Scholar]
- 44.Wagner DR, Knott JR, Fry JP. Oximetry fails to predict acute mountain sickness or summit success during a rapid ascent to 5640 meters. Wilderness Environ Med. 2012;23:114–21. doi: 10.1016/j.wem.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 45.Roach RC, Greene ER, Schoene RB, Hackett PH. Arterial oxygen saturation for prediction of acute mountain sickness. Aviat Space Environ Med. 1998;69:1182–5. [PubMed] [Google Scholar]
- 46.Strapazzon G, Semplicini A. High-altitude cerebral effects: Risks and mechanisms. Lancet Neurol. 2009;8:604. doi: 10.1016/S1474-4422(09)70159-0. [DOI] [PubMed] [Google Scholar]


