Abstract
Background:
Quercetin has been distributed in a wide range of foods, but some of its known effects in vitro, are not proven in human studies. Therefore, the aim of this study was evaluation of the effects of quercetin intake on cardiovascular risk factors and inflammatory biomarkers in women with type 2 diabetes.
Methods:
This double-blind randomized clinical trial was carried out on 72 women for 10 weeks. Subjects were assigned to quercetin and placebo groups using a permutated block randomization of size two. Quercetin was given to participants as a 500 mg capsule daily. Biochemical variables were measured at baseline and at the end of the study, and changes were compared using appropriate statistical methods.
Results:
Compared with placebo, quercetin intake decreased systolic blood pressure significantly (−8.8 ± 9.3 vs. −3.5 ± 11.7, P = 0.04). Although changes in diastolic blood pressure between the groups was not significant (P = 0.19), high-density lipoprotein cholesterol (HDL-C) was significantly decreased in both groups while changes in total cholesterol, low-density lipoprotein cholesterol (LDL-C), triglycerides (TG) and ratio of TG/HDL-C and LDL-C/HDL-C were not significant between and within groups. Quercetin supplementation significantly reduced the serum concentration of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) (P = 0.01 and P < 0.0001, respectively); however, the mean changes in serum levels of IL-6, TNF-α, and high-sensitivity C-reactive protein were not significant between the groups.
Conclusions:
Quercetin supplementation reduced systolic blood pressure significantly but had no effect on other cardiovascular risk factors and inflammatory biomarkers. Considering the biological effects of quercetin in vitro, we need more studies with a stronger design and sample size with different doses of quercetin.
Keywords: Blood pressure, inflammatory biomarkers, lipids profile, quercetin
INTRODUCTION
Diabetes is one of the most prevalent diseases in all communities. The World Health Organization has estimated the number of diabetic patients to be 171 million people in 2000, and the projection for 2030 is 366 million people.[1,2] Diabetes is one of the most important risk factors for cardiovascular diseases (CVD); this process is done through an accelerated procedure of atherosclerosis.[3] CVD are the main causes of death in the world. High prevalence of CVD has been seen in developed countries,[4,5] and 38% of mortality occurs due to heart diseases in Iran.[6,7] An increased production of free radicals due to hyperglycemia is seen in type 2 diabetes.[8] Indeed, the balance between antioxidants and free radicals has been disturbed due to high levels of pro-oxidants in the body; therefore, complications of diabetes can be accelerated.[9] Likewise, inflammatory biomarkers values such as C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) are increased in diabetes.[10] The aim of many studies included pro-oxidants decreasing and antioxidants increasing, which could improve the oxidative stress and lipid peroxidation status in diabetic patients.[11,12,13,14]
It seems likely that adequate intake of food groups such as vegetables, grains, and fruits containing flavonoids and polyphenols compounds are related to the prevention and management of obesity, type 2 diabetes and heart diseases.[15,16] Recently, among flavonoids, quercetin has earned the attention of many researchers. Quercetin has been distributed in a wide range of foods[17] such as onions, apples, and tea.[18,19] In one study, intake of high levels of quercetin was associated with reduced incidence of type 2 diabetes.[20] In vitro studies showed different effects of quercetin as anti-inflammatory, antioxidant, anti-clotting, and vasodilatory properties.[21,22,23] But, human and animal studies did not have consistent results,[23,24] which may result from different physiology of species and also different levels of oxidative stress and inflammatory status. Also, the effects of quercetin supplementation were more visible in individuals with high levels of inflammation and oxidative stress.[25] On the other hand, in healthy subjects who had lower levels of oxidative stress and cytokine, intake of quercetin had no significant effect on TNF-α.[26] Regarding lipid profiles, in a cross-sectional study, consumption of quercetin compared with other flavonoids was inversely associated with low-density lipoprotein cholesterol (LDL-C).[27] But the results of human clinical trials and experimental animal studies in this field were conflicting.[22,28,29] To the best of our knowledge, there is no study to examine the effect of quercetin supplementation on the cardio metabolic status of people with type 2 diabetes. According to the conflicting effects of quercetin on inflammation and cardiovascular risk factors, and lack of studies on diabetic patients with high oxidative stress and related problems such as nephropathy, neuropathy and retinopathy,[30,31] we assumed that quercetin supplementation could improve the metabolic and inflammatory status in people with type 2 diabetes. Therefore, the aim of the present study was evaluation of the effects of quercetin intake on lipids profile, blood pressure, and inflammatory biomarkers in women with type 2 diabetes.
METHODS
Subjects
In this study, participants were recruited among women with type 2 diabetes who were referred to the Endocrinology and Metabolism Research Center of Isfahan University of Medical Sciences. Patients who were eligible for the study were enrolled by the researcher after signing the informed consent. Inclusion criteria consisted of a history of diabetes for at least 3 years, age between 35 and 55 years, not smoking and addiction, lack of insulin, lack of severe heart diseases, stroke, severe liver and renal diseases, gastrointestinal disorders, thyroid dysfunction, rheumatoid arthritis, and infectious diseases. Exclusion criteria included changing the dose and type of medication during the study, pregnancy occurrence and less than 70% of compliance. At the beginning and end of intervention, clinical (height, weight, waist circumference, hip circumference, blood pressure) and biochemical assessments (TNF-α, high-sensitivity (hs)-CRP, serum lipids, IL-6) were performed. Sample size determination in the current research was performed based on the suggested approach by Campbell et al.[32] for comparing means between two group as follows: If the observations come from a normal distribution, then, with the anticipated standardized effect size between them, based on the results of Egert et al.'s study,[22] specified as Δ = 0.75, the sample size in each group for a two-sided test α = 0.05 and power 1−β =0.8 was 36.
The study was approved by the Ethical Committee of Isfahan University of Medical Sciences, Isfahan, Iran, and has been registered at the Iranian website (IRCT201011015062N1) for registration of clinical trials. In addition, sampling and implementation of study was performed in 2011.
Study design
This double-blind randomized clinical trial study was carried out for 10 weeks. A permutated block randomization method was used to randomly allocate subjects into the supplement and placebo groups. Quercetin (Solaray, USA) was given to participants as a dosage of 500 mg per day. Placebo capsules were similar in appearance to quercetin, and contained lactose, which was produced in the School of Pharmacy, Isfahan University of Medical Sciences. Supplements were given to individuals in two stages (in the 1st and 5th week) by someone except the researcher in the endocrinology center and compliance was measured by counting the remaining capsules at the end of the study and calculated by the formula:
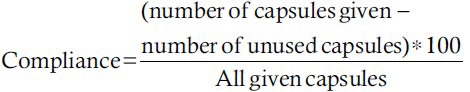
During the study, participants recorded any side-effects or observations and were asked not to change their usual diet and physical activity. Nutrient intake was collected through 1 day food record at the 1st, 5th, and last weeks of intervention, and was calculated with Modified N4 (version 1). Physical activity of the subjects was evaluated by the short International Physical Activity Questionnaire at the beginning and at the end of the study (www.ipaq.ki.se) and was expressed as metabolic equivalent-minutes per week (MET-min/week).[33]
Anthropometry and biochemical assessments
Anthropometric measurements were performed at the beginning and at the end of the study. Height was measured in a standing position without shoes by a stadiometer with the nearest 0.5 cm. Weight was determined with minimal clothing and without shoes by a Seca scale with a precision of 100 g. Waist circumference was measured in the middle bottom ribs and pelvic bones after a normal exhale. The most prominent part was considered to be the hip circumference measurement. Systolic and diastolic blood pressure (DBP) was measured with a sphygmomanometer (Rester) in a sitting position and after 5-10 min rest.[22]
In order to evaluate the biochemical variables, 12-h fasting blood samples were taken at the beginning and at the end of the intervention. Until blood clot formation, the samples were kept at room temperature for 1 h and were then centrifuged at 2000 rpm for 15 min to separate serums. Serums were maintained into specific batch at temperatures −70°C until the time of testing.
Lipid profile (total cholesterol, HDL-C, LDL-C, triglycerides [TG]) was measured using the quantitative detection kit and photometric method (Pars Azmoon Inc., Iran) with fresh samples. Serum levels of TNF-α, hs-CRP, and IL-6 were determined by enzyme-linked immunosorbent assay (ELISA). Measurement of TNF-α were performed by the human TNF-α ELISA kit (Boster Inc., USA), measurement of serum IL-6 was done with human IL-6 ELISA kit (Boster Inc., USA) and serum hs-CRP was measured by the human hs-CRP ELISA kit (Pars Azmoon Inc., Iran). In addition, sampling and implementation of study was performed in 2011.
Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) software version 16.0 (SPSS Inc., Chicago, IL, USA). Normality of the studied variables was assessed using histograms and Kolmogorov-Smirnov test. All variables were normally distributed except hs-CRP and IL-6. Therefore, logarithmic transformation was used for normalization. Quantitative variables were presented as mean ± SD and qualitative variables as number (percent). Within and between group comparisons were assessed using the paired T-test and the independent samples, respectively.
RESULTS
Mean (±SD) of age and weight were 46.4 (±4.5) years and 74.7 (±1.3) kg, respectively. From 72 participants, 10 subjects were excluded (four due to gastrointestinal problems, three due to medication changes and three because of personal reasons), and only 62 patients completed the study [Figure 1]. There were no significant differences between groups in terms of weight, body mass index, and waist circumference. Compliance was 92.5% and 94% in the quercetin and placebo groups, respectively. There was no significant difference between groups in compliance (P = 0.39).
Figure 1.
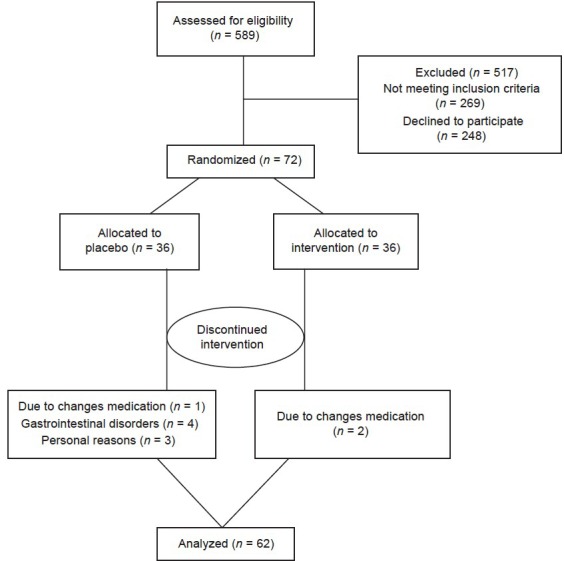
Patient flow diagram
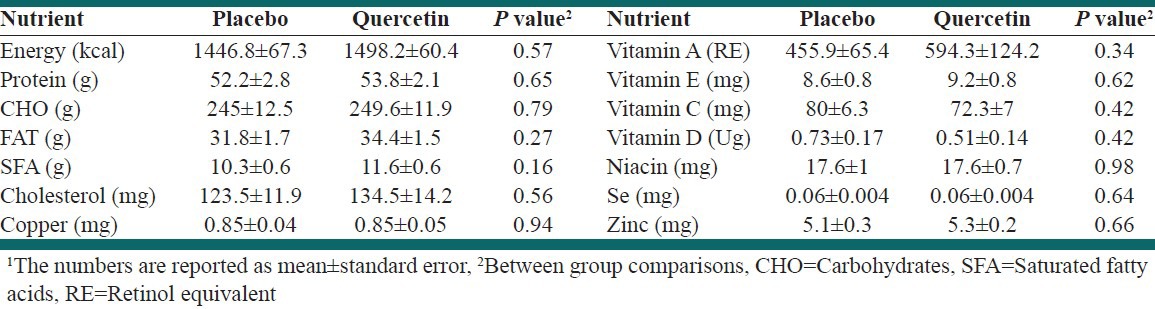
Three-day dietary records’ assessment showed that there were no significant differences between and within groups. Caloric, macronutrients, vitamin A, vitamin E, vitamin C, vitamin D, beta-carotene, selenium, copper, zinc, niacin, saturated fatty acids, and cholesterol were compared between groups using repeated measures analysis of variance, and there were no statistically significant differences [Table 1]. Physical activity was calculated as MET-min/week and there was no statistically significant difference between the groups (P = 0.8).
Table 1.
Results of between group comparison in terms of nutrient intake1
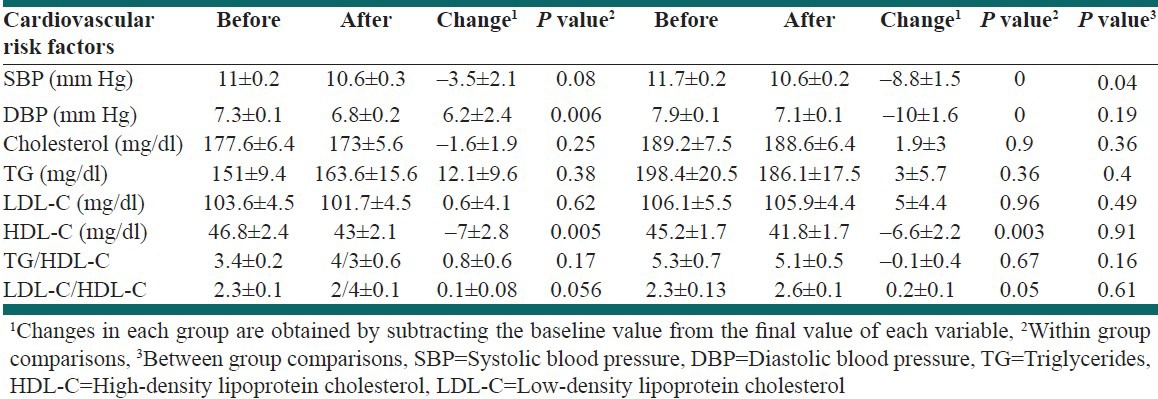
Results of within and between groups comparisons in terms of cardiovascular risk factors are shown in Table 2. As compared with placebo, quercetin intake significantly decreased the systolic blood pressure (−8.8 ± 9.3 vs. −3.5 ± 11.7, P = 0.04). DBP was significantly reduced by both quercetin and placebo supplementation, but there were no significant differences between the groups (P = 0.19). Changes in serum levels of cholesterol, LDL-C, TG, and the ratio of TG/HDL-C and LDL-C/HDL-C were not significant between and within groups. Both quercetin and placebo intake significantly declined HDL-C, but there was no significant difference between the groups.
Table 2.
Results of within and between group comparison in terms of cardiovascular risk factors
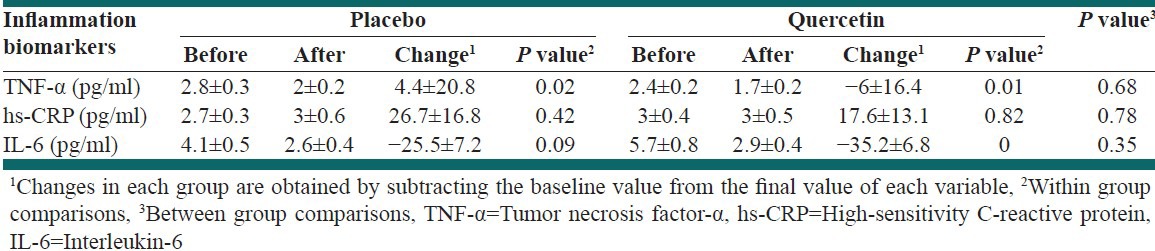
Quercetin supplementation and placebo significantly reduced serum concentrations of TNF-α, but we did not see a significant difference between groups. Quercetin consumption significantly decreased the serum concentrations of IL-6, but mean changes between groups were not significant. Quercetin intake did not significantly affect the serum levels of hs-CRP between groups and within groups [Table 3].
Table 3.
Results of within and between group comparison in terms of inflammation biomarkers
DISCUSSION
In the present study, we investigated the effect of quercetin supplementation on blood pressure, lipids profile, and inflammatory biomarkers (TNF-α, hs-CRP, IL-6) in women with type 2 diabetes. We observed that 10 weeks’ of supplementation with quercetin resulted in reducing the systolic blood pressure significantly, while it had no significant effect on the other variables.
Blood pressure
To the best of our knowledge, there are only three human studies that examined the effect of quercetin supplementation on blood pressure.[22,28,34] Conquer et al.[28] examined the effect of quercetin supplementation on blood pressure in healthy subjects; these researchers reported that 1000 mg/d quercetin for 28 days could not have a significant effect on systolic and DBP. In contrast to this study, Edwards et al.[34] found that supplementation with 730 mg quercetin/d for 4 weeks in pre-hypertensives and stage 1 hypertensive individuals reduced systolic and DBPs only in stage 1 hypertensive subjects significantly; but without significant differences between two groups. In order to show the blood pressure-lowering effect of quercetin, it seems likely that we need a degree of hypertension. Egert et al.[22] have recently shown that 150 mg/d quercetin for 6 weeks in people with metabolic syndrome compared with the placebo group significantly decreased the systolic and DBPs.
So far, there is not study to examine the effect of quercetin in people with type 2 diabetes. Our data showed that quercetin supplementation for 10 weeks significantly reduced the systolic and DBPs in the supplement group, but, in comparison between groups, significant difference was observed only in systolic blood pressure (P = 0/49). This reduction in systolic blood pressure (−8/8) was related to 14% reduction in mortality of stroke and 9% decrease in deaths from cardiovascular disease.[35] Our outcome is worthwhile because, in association with mortality from cardiovascular diseases, systolic blood pressure is a higher serious risk factor than DBP.[36] Because of high oxidative stress in people with type 2 diabetes, vessels are affected and endothelial dysfunction can led to hypertension and atherosclerosis. A way in which flavonoids can lower the risk of heart disease is improvement of endothelium-dependent vasodilation.[37] DBP reductions in the placebo group were possibly due to changes in the level of physical activity or psychological placebo effect.
Inflammation
The anti-inflammatory effects of quercetin are clearly seen in in vitro studies.[21] This property was different in human and animal studies,[23,26,22] which may result from the different physiology of species and also different levels of oxidative stress and inflammatory status. Quercetin prevented generation of inflammatory cytokine TNF-α in cell cultures. This effect is linked to modulation of the NF-kB pathway.[38]
In the present study, quercetin intake significantly decreased the serum concentration of IL-6 and TNF-α. This finding was consistent with the results of Rivera et al.,[23] which showed that a higher dosage of quercetin (10 mg/kg) in obese rats who had higher levels of cytokines could reduce the visceral production of TNF-α and increase adiponectin. While in another study, receiving 150 mg quercetin for 6 weeks in obese and overweight subjects compared with placebo had no significant effect on the serum levels of TNF-α and hs-CRP.[22]
Decreased bioavailability of quercetin and small numbers of subjects were probable reasons of our results in inflammatory mediators. Also, animal studies have shown that the anti-inflammatory effect of quercetin is a dose-dependent effect.[23] According to the short half-life of quercetin, if the supplements were divided into several doses with a lesser amount, quercetin would have a better effect. But, these patients receive several medications and quercetin intake as several capsules with less value for them was not acceptable. Of course, biochemical changes of quercetin after absorption could not be ignored.[39,40] Also, contrary to our expectation, TNF-α had been decreased in the placebo group, which may have occurred due to the psychological effect of placebo intake.
Lipids profile
In human studies with different dosages of quercetin, no significant changes in blood lipids (HDL-C, LDL-C, TG) were seen.[28,34] Only in one study in 2009 did quercetin consumption result in a significant decrease in HDL-C levels compared with placebo.[22] Also, in the present study, in both the placebo and the supplemented groups, HDL-C decreased significantly, but this decrease was higher in the placebo group and there was no significant difference between the two groups.
In animal studies, serum levels of TG and TC were decreased after quercetin consumption, but this effect was seen in animals with higher blood lipids levels.[29] Also, in this study, we saw a reduction in TG levels in the supplemented group, but these changes were not significant. However, the following issue should be kept in mind – high levels of blood lipids in our study population were controlled by medication (during the intervention period, the amount of drugs was fixed), and probably due to the normal levels of blood lipids, we did not see a hypolipidemic effect of quercetin. Regarding the fact that dyslipidemia is a common disorder in diabetes, and that the participant had a 3-year history of diabetes, progression of dyslipidemia during intervention such as decreased HDL-C can be attributed to disease progressive and disturb the metabolic status of these patients. In addition, this small reduction in HDL-c levels was not associated with increased TG/HDL-C and LDL-C/HDL-C ratio. Perhaps, this change resulted from the physiological and clinical status of diabetic patients.
Among the strengths of this study, we can point to the population study (women with type 2 diabetes). According to gender, the collaboration of women is better than men, and they cooperated with the researcher better. Also, contrary to developed countries, the prevalence of diabetes among Iranian women is more than that among men.[41] Limitations of the present study were the high cost of the research project, the impossibility of compliance assessment by using measurement of plasma quercetin concentrations before and after study and lack of appropriate tools for assessment and analysis of dietary intakes in Iran.
CONCLUSIONS
Quercetin supplementation could cause a significant reduction in systolic blood pressure but had no effect on other cardiovascular risk factors and inflammatory biomarkers. Considering the biological effects of quercetin in vitro, we need more studies with a stronger design and sample size with different doses of quercetin. Because dietary approaches to stop hypertension (DASH) diet includes high amounts of fruits and vegetables, it is a good source of antioxidants such as quercetin and fiber. Also, a study reported that the DASH diet was associated with a lower risk of type 2 diabetes. Therefore, for future studies regarding the possible beneficial effects of diets rich in quercetin, such as the DASH dietary pattern among diabetes, is recommended.[42]
ACKNOWLEDGEMENTS
The authors are grateful the sincere cooperation of the participants and the personnel the Center for Endocrinology and Metabolism Research Center of Isfahan University of Medical Sciences.
Footnotes
Source of Support: The Isfahan University of Medical Sciences provided the financial support for this study
Conflict of Interest: None declared.
REFERENCES
- 1.Aghamollaie T, Eftekhar H, Shojaeizadeh D, Mohammad K, Nakhjavani M, Ghafrani F. Effect of a health education programe on behavior, HbA1C and health-related quality of patient. Acta Med Iran. 2004;43:89–94. [Google Scholar]
- 2.Hajianfar H, Bahonar A, Entezari MH, Askari G, Yazdani M. Lipid profiles and serum visfatin concentrations in patients with type II diabetes in comparison with healthy controls. Int J Prev Med. 2012;3:326–31. [PMC free article] [PubMed] [Google Scholar]
- 3.Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22:233–40. doi: 10.2337/diacare.22.2.233. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Geneva: 2002. Diet, physical activity and health. Fifty-fifth World Health Assembly. The Word Health Report 2001. Mental health: new understanding new hope. [Google Scholar]
- 5.Inter Health Steering Committee. Demonstration projects for the integrated prevention and control of non-communicable disease inter health program: epidemiological background and rationale. World Health Stat Q. 1991;44:489–504. [PubMed] [Google Scholar]
- 6.Naghavi M. Mortality in ten provinces of Iran. Tehran: Deputy for Health, Ministry of Health and Medical Education. 2002:53–106. [Google Scholar]
- 7.Mazloom Z, Hejazi N, Ekramzadeh M, Zare H. Anthropometric measurements and hypertension in cardiovascular disease patients. In: Proceeding of 10th Iranian Nutrition Congress, Tehran, Iran, October. 2008:184. [Google Scholar]
- 8.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 9.Hsueh WA, Law RE. Cardiovascular risk continuum: Implications of insulin resistance and diabetes. Am J Med. 1998;105:4S–14. doi: 10.1016/s0002-9343(98)00205-8. [DOI] [PubMed] [Google Scholar]
- 10.Devaraj S, Dasu MR, Jialal I. Diabetes is a proinflammatory state: A translational perspective. Expert Rev Endocrinol Metab. 2010;5:19–28. doi: 10.1586/eem.09.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dengel DR, Pratley RE, Hagberg JM, Rogus EM, Goldberg AP. Distinct effects of aerobic exercise training and weight loss on glucose homeostasis in obese sedentary men. J Appl Physiol. 1996;81:318–25. doi: 10.1152/jappl.1996.81.1.318. [DOI] [PubMed] [Google Scholar]
- 12.Scott JA, King GL. Oxidative stress and antioxidant treatment in diabetes. Ann N Y Acad Sci. 2004;1031:204–13. doi: 10.1196/annals.1331.020. [DOI] [PubMed] [Google Scholar]
- 13.Chowienczyk PJ, Brett SE, Gopaul NK, Meeking D, Marchetti M, Russell-Jones DL, et al. Oral treatment with an antioxidant (raxofelast) reduces oxidative stress and improves endothelial function in men with type II diabetes. Diabetologia. 2000;43:974–7. doi: 10.1007/s001250051478. [DOI] [PubMed] [Google Scholar]
- 14.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 15.van Dam RM, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Dietary patterns and risk for type 2 diabetes mellitus in U.S. men. Ann Intern Med. 2002;136:201–9. doi: 10.7326/0003-4819-136-3-200202050-00008. [DOI] [PubMed] [Google Scholar]
- 16.Estruch R, Martínez-González MA, Corella D, Salas-Salvadó J, Ruiz-Gutiérrez V, Covas MI, et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: A randomized trial. Ann Intern Med. 2006;145:1–11. doi: 10.7326/0003-4819-145-1-200607040-00004. [DOI] [PubMed] [Google Scholar]
- 17.Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet. 1993;342:1007–11. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 18.Morand C, Crespy V, Manach C, Besson C, Demigné C, Rémésy C. Plasma metabolites of quercetin and their antioxidant properties. Am J Physiol. 1998;275:R212–9. doi: 10.1152/ajpregu.1998.275.1.R212. [DOI] [PubMed] [Google Scholar]
- 19.Hollman PC, van Trijp JM, Mengelers MJ, de Vries JH, Katan MB. Bioavailability of the dietary antioxidant flavonol quercetin in man. Cancer Lett. 1997;114:139–40. doi: 10.1016/s0304-3835(97)04644-2. [DOI] [PubMed] [Google Scholar]
- 20.Knekt P, Kumpulainen J, Järvinen R, Rissanen H, Heliövaara M, Reunanen A, et al. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr. 2002;76:560–8. doi: 10.1093/ajcn/76.3.560. [DOI] [PubMed] [Google Scholar]
- 21.Erdman JW, Jr, Balentine D, Arab L, Beecher G, Dwyer JT, Folts J, et al. J Nutr. Vol. 137. Washington, DC: 2007. Flavonoids and heart health. In: Proceedings of the ILSI North America Flavonoids Workshop, May 31-June 1, 2005; pp. 718S–37. [DOI] [PubMed] [Google Scholar]
- 22.Egert S, Bosy-Westphal A, Seiberl J, Kürbitz C, Settler U, Plachta-Danielzik S, et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: A double-blinded, placebo-controlled cross-over study. Br J Nutr. 2009;102:1065–74. doi: 10.1017/S0007114509359127. [DOI] [PubMed] [Google Scholar]
- 23.Rivera L, Morón R, Sánchez M, Zarzuelo A, Galisteo M. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity (Silver Spring) 2008;16:2081–7. doi: 10.1038/oby.2008.315. [DOI] [PubMed] [Google Scholar]
- 24.Pinho RA, Araújo MC, Melo Ghisi GL, Benetti M. Coronary heart disease, physical exercise and oxidative stress. Arq Bras Cardiol. 2010;94:515–21. doi: 10.1590/s0066-782x2010000400018. [DOI] [PubMed] [Google Scholar]
- 25.Boots AW, Drent M, de Boer VC, Bast A, Haenen G. Quercetin reduces markers of oxidative stress and inflammation in sarcoidosis. Clin Nutr. 2011;30:506–12. doi: 10.1016/j.clnu.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Boots AW, Wilms LC, Swennen EL, Kleinjans JC, Bast A, Haenen GR. In vitro and ex vivo anti-inflammatory activity of quercetin in healthy volunteers. Nutrition. 2008;24:703–10. doi: 10.1016/j.nut.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 27.Arai Y, Watanabe S, Kimira M, Shimoi K, Mochizuki R, Kinae N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J Nutr. 2000;130:2243–50. doi: 10.1093/jn/130.9.2243. [DOI] [PubMed] [Google Scholar]
- 28.Conquer JA, Maiani G, Azzini E, Raguzzini A, Holub BJ. Supplementation with quercetin markedly increases plasma quercetin concentration without effect on selected risk factors for heart disease in healthy subjects. J Nutr. 1998;128:593–7. doi: 10.1093/jn/128.3.593. [DOI] [PubMed] [Google Scholar]
- 29.Juźwiak S, Wójcicki J, Mokrzycki K, Marchlewicz M, Białecka M, Wenda-Rózewicka L, et al. Effect of quercetin on experimental hyperlipidemia and atherosclerosis in rabbits. Pharmacol Rep. 2005;57:604–9. [PubMed] [Google Scholar]
- 30.Kehrer JP. Free radicals as mediators of tissue injury and disease. Crit Rev Toxicol. 1993;23:21–48. doi: 10.3109/10408449309104073. [DOI] [PubMed] [Google Scholar]
- 31.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–12. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 32.Campbell MJ, Donner A, Klar N. Developments in cluster randomized trials and Statistics in Medicine. Stat Med. 2007;26:2–19. doi: 10.1002/sim.2731. [DOI] [PubMed] [Google Scholar]
- 33.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 34.Edwards RL, Lyon T, Litwin SE, Rabovsky A, Symons JD, Jalili T. Quercetin reduces blood pressure in hypertensive subjects. J Nutr. 2007;137:2405–11. doi: 10.1093/jn/137.11.2405. [DOI] [PubMed] [Google Scholar]
- 35.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 36.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 37.Hooper L, Kroon PA, Rimm EB, Cohn JS, Harvey I, Le Cornu KA, et al. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials. Am J Clin Nutr. 2008;88:38–50. doi: 10.1093/ajcn/88.1.38. [DOI] [PubMed] [Google Scholar]
- 38.Nair MP, Mahajan S, Reynolds JL, Aalinkeel R, Nair H, Schwartz SA, et al. The flavonoid quercetin inhibits proinflammatory cytokine (tumor necrosis factor alpha) gene expression in normal peripheral blood mononuclear cells via modulation of the NF-kappa beta system. Clin Vaccine Immunol. 2006;13:319–28. doi: 10.1128/CVI.13.3.319-328.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–42. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 40.Askari G, Ghiasvand R, Paknahad Z, Karimian J, Rabiee K, Sharifirad G, et al. The effects of quercetin supplementation on body composition, exercise performance and muscle damage indices in athletes. Int J Prev Med. 2013;4:21–6. [PMC free article] [PubMed] [Google Scholar]
- 41.Esteghamati A, Gouya MM, Abbasi M, Delavari A, Alikhani S, Alaedini F, et al. Prevalence of diabetes and impaired fasting glucose in the adult population of Iran: National Survey of Risk Factors for Non-Communicable Diseases of Iran. Diabetes Care. 2008;31:96–8. doi: 10.2337/dc07-0959. [DOI] [PubMed] [Google Scholar]
- 42.Azadbakht L, Surkan PJ, Esmaillzadeh A, Willett WC. The dietary approaches to stop hypertension eating plan affects C-reactive protein, coagulation abnormalities, and hepatic function tests among type 2 diabetic patients. J Nutr. 2011;141:1083–8. doi: 10.3945/jn.110.136739. [DOI] [PMC free article] [PubMed] [Google Scholar]


