SUMMARY
Background
Non-alcoholic steatohepatitis (NASH) is a common cause of serum alanine aminotransferase (ALT) elevations and chronic liver disease, but it is unclear how well ALT elevations reflect the liver injury.
Aim
To assess how well changes in ALT elevations reflect improvements in liver histology in response to vitamin E therapy.
Methods
The vitamin E and placebo arms of the Pioglitazone vs. Vitamin E vs. Placebo in Non-alcoholic Steatohepatitis (PIVENS) trial were reassessed for associations among changes in ALT levels, body weight and liver histology. An ALT response was defined as a decrease to ≤40 U/L and by ≥30% of baseline. Liver biopsies taken before and after treatment were scored for non-alcoholic fatty liver disease activity (NAS) and fibrosis.
Results
ALT responses were more frequent among vitamin E (48%) than placebo (16%) recipients (P < 0.001). Among vitamin E recipients, ALT responses were associated with decreases in NAS (P < 0.001), but not fibrosis scores (P = 0.34), whereas among placebo recipients, ALT responses were associated with significant decreases in both (P < 0.05). Weight loss (≥2 kg) was also associated with ALT response (P < 0.001), improvements in NAS (P < 0.001) and fibrosis (P < 0.02), but vitamin E had an added effect both with and without weight loss. Weight gain (≥2 kg) was associated with lack of ALT response and worsening NAS and fibrosis scores in patients not on vitamin E.
Conclusions
Decrease of ALT levels to normal in patients with NASH is usually associated with improved histological activity. Management should stress the value of weight loss and strongly discourage weight gain. Vitamin E can improve both ALT levels and histology with and without weight loss. Clinical Trial Number: NCT00063622.
INTRODUCTION
Vitamin E is a naturally occurring fat-soluble vitamin that is believed to be an important antioxidant, capable of ameliorating the effects of free radicals produced intracellularly during normal metabolism as well as periods of oxidative stress.1 Clinically, overt vitamin E deficiency is rare, occurring largely in persons with malabsorption syndromes and associated with defects in immune function, neurological development and membrane fluidity.2–4 Vitamin E is present in many multivitamin preparations and by itself is promoted as beneficial in normal cellular functions and possibly important in prevention of cancer, cardiovascular disease and ageing. However, in several large, long-term studies, administration of vitamin E supplements was not associated with significant improvement in cardiovascular,5, 6 cancer6–8 or ageing-related9 outcomes. Indeed, a meta-analysis indicated that vitamin E supplementations may be associated with excess all-cause mortality.10
Recently, several small open-label studies11, 12 followed by two randomised controlled trials13, 14 have indicated a potential role for vitamin E supplementation in liver disease. In these studies, natural forms of vitamin E, given in a high, single daily dose, decreased serum aminotransferase levels and improved liver histology in a proportion of patients with non-alcoholic fatty liver disease (NAFLD) in its more severe form known as non-alcoholic steatohepatitis (NASH). In the Pioglitazone vs. Vitamin E vs. Placebo for the Treatment of Nondiabetic Patients with Non-alcoholic Steatohepatitis (PIVENS) trial, a 96-week course of natural vitamin E (RRR-α-tocopherol, 800 IU once daily) was associated with a marked decrease in mean serum alanine aminotransferase (ALT) levels within the first 6 months of treatment that was maintained for the entire period of therapy and followed by relapse once treatment was stopped.13 Liver biopsies taken before and at the end of the 96 weeks showed improvements in steatosis, inflammation and ballooning cell injury, the histological hall-marks of NASH. Indeed, central review of liver biopsies masked to treatment group indicated that 47% of vitamin E-treated compared to 21% of placebo-treated subjects no longer met histological criteria for the diagnosis of NASH by the end of treatment (P = 0.05). The mechanism(s) by which vitamin E improves disease activity in NASH, the optimal effective dose, the role of weight loss in the response and the best approach to monitoring patients for a clinical response (ALT levels, liver biopsy) remain(s) unclear. Secondary analyses of the PIVENS trial are directed at answering some of these questions.
This secondary analysis was carried out to assess whether there was an association between reduction in serum ALT levels and improvements in liver histology, to evaluate the utility of ALT levels as surrogate markers for a histological response, and to identify the effects of weight loss on improvements in ALT values and liver histology in both the vitamin E and placebo groups.
METHODS
PIVENS trial
The PIVENS trial was a prospective, double-dummy, double-blind randomised trial of pioglitazone vs. vitamin E vs. placebo in 247 nondiabetic, adult patients with histologically defined non-alcoholic steatohepatitis. The aims, design and primary outcomes of this trial have been published.13, 15 Briefly, subjects underwent a preliminary medical evaluation, including liver biopsy, and those with histological features of NASH and a non-alcoholic fatty liver disease activity score of at least 4 (on a scale of 0–8) were randomised to receive (i) pioglitazone (30 mg) and vitamin E placebo; or (ii) vitamin E (800 IU) and pioglitazone placebo; or (iii) both placebos by mouth once daily for 96 weeks. All patients were followed with out-patient visits and routine blood tests at 3-month intervals and repeat liver biopsies after 96 weeks of treatment. Therapy was then stopped and subjects were followed for another 24 weeks on no treatment. The study was conducted between 2005 and 2009 at eight US medical centres and the data was managed by a central Data Coordinating Center (for locations, see Appendix S1). All patients gave written informed consent and all details of the trial were approved by local institutional review boards. In addition, the design, protocol, details and conduct of the trial were monitored by an independent data safety and monitoring board appointed by the NIDDK.
Reanalysis
This post hoc analysis focused on serum alanine aminotransferase (ALT) responses in patients who received vitamin E or placebo and the association of these changes with initial clinical features, changes in histological features and interactions with changes in body weight. Changes in ALT levels between baseline, week 24 and week 96 were analysed and an ALT response was defined as a decrease to a level of ≤40 U/L and by at least 30% from baseline. This definition was developed after inspection of the clinical course of patients with and without a histological response. The level of 40 U/L was chosen because it is commonly used by clinical laboratories as the upper limit of the normal range for ALT. A requirement of a 30% decrease was needed because an elevation in serum ALT levels was not required for enrolment, and some patients had normal or minimally elevated levels at the time of randomisation. This definition was tested post hoc and found to have the highest area under the receiver operating characteristic (AUROC) for predicting an improvement in NAS score among the vitamin E recipients in comparison to using 30 U/L as the upper limit of normal, with and without the requirement for a 30% decrease in ALT (Table S1). This definition also yielded a higher AUROC than a more complex, logistic regression algorithm with the addition of an ALT value of less than 60 U/L and a 50% decrease from baseline. Finally, substituting AST for ALT values did not increase the AUROC for predicting improvement in NAS scores (data not shown).
ALT responses were categorised into four types: (i) an early and sustained response, the ALT criteria being met at both weeks 24 and 96; (ii) a late response only, the ALT criteria being met at week 96, but not week 24; (iii) an early response only, the ALT criteria being met at week 24, but not week 96 (defined as breakthrough); and (iv) a nonresponse, the ALT criteria not being met at either time. The majority of patients were followed for at least 24 weeks after stopping therapy (to week 120). A relapse was defined as a loss of the ALT response at week 120 or the last available determination. For analysis of associations, the four response types were collapsed into two groups: ALT response (early and sustained response and late response only: i.e. ALT ≤40 U/L at week 96) and ALT nonresponse (early only and nonresponse: i.e. >40 U/L at week 96). Similarly, comparing pre-treatment and 96 week liver biopsies, histological responses were categorised into (i) improvement (a 2-point decrease in NAS score); (ii) worsening (a 2-point increase in NAS score); or (iii) no change (a −1, 0 or +1 change in NAS score). Finally, changes in body weight between baseline and week 96 were categorised into (i) weight gain (2 kg or more), weight loss (2 kg or more) or no change (<2 kg gain or loss).
All liver biopsies were scored for features of NAFLD and NASH using a standardised scoring system by the Pathology Committee of the NASH CRN.16 The NAFLD activity score (NAS) comprised the combination of scores given for steatosis (0–3+), lobular inflammation (0–3+) and ballooning degeneration (0–2 +). Fibrosis was scored on a 5-point system (0–4+), 3+ representing bridging fibrosis and 4+ cirrhosis. Liver biopsies were also assessed for presence of Mallory’s hyaline and other features typical of NAFLD. The length of biopsies was measured to assess adequacy. Details of the scoring system, including its intra- and interobserver variation, have been described.16
This reanalysis of the PIVENS trial was limited to patients treated with vitamin E or placebo who underwent repeat liver biopsy at 96 weeks. In addition, patients whose pre-treatment serum ALT levels were <30 U/L were excluded, as a further 30% decrease in ALT levels was considered unlikely to occur. Patients in the pioglitazone arm of the PIVENS trial were not included in this analysis because of differences in ALT and histological responses and because pioglitazone was associated with weight gain.13
Statistical analyses
Group comparisons used the t-test with unequal variance for continuous outcomes and the Chi-squared test (or Fisher’s exact test for small numbers) for categorical outcomes. Linear regression of change in histology (i.e. NAS or fibrosis) on change in weight did not vary by treatment group. Final regression models included baseline values of NAS or fibrosis, change in weight and an indicator variable for group. Sensitivity analyses using resistant regression did not change the interpretation of the results. P values were nominal. The data analysis for this paper was generated using SAS software 9.3 (SAS Institute Inc., Cary, NC, USA) and Stata software (StataCorp, 2011. Stata Statistical Software: Release 12. College Station, TX, USA: StataCorp LP).
RESULTS
Study subjects
Among patients randomised to receive vitamin E (n = 84) or placebo (n = 83) in the PIVENS trial, 15 did not have a repeat liver biopsy at 96 weeks, 2 had no ALT results during follow-up, and 11 had serum ALT levels <30 U/L at baseline and were excluded. The final cohort consisted of 71 vitamin E and 68 placebo-treated recipients. The baseline demographic, laboratory and histological features of these 139 patients were similar to those of the 28 patients who were excluded, except for higher mean ALT levels (87 vs. 63 U/L, P = 0.04) and higher mean triglycerides (174 vs. 126 mg/dL, P = 0.004), probably due in part to the exclusion of subjects with low initial ALT values (data not shown). The 139 patients for analysis included 80 women (58%) and 103 (74%) non-Hispanic Whites. The average age was 46 years (range 19–71 years) and average initial body weight was 96 kg (range 52–168 kg). Baseline serum ALT values ranged from 30 to 280 U/L (mean = 87 U/L) and initial NAS scores ranged from 2 to 8 (mean = 5.0). There were no differences in these baseline features between the vitamin E and placebo groups (Table 1).
Table 1.
Baseline characteristics
| Clinical feature | Vitamin E (n = 71) |
Placebo (n = 68) |
P value |
|---|---|---|---|
| Demographics | |||
| Age in years (mean ± s.d.) |
46 ± 12 | 45 ± 12 | 0.65 |
| Female sex | 59% | 56% | 0.73 |
| Race/ethnicity | |||
| Non-Hispanic White | 76% | 82% | 0.62 |
| Hispanic White | 9% | 5% | |
| Non-White | 15% | 13% | |
| Liver test results (mean ± s.d.) | |||
| ALT (U/L) | 90 ± 47 | 85 ± 49 | 0.55 |
| AST (U/L) | 61 ± 33 | 57 ± 31 | 0.43 |
| GGT (U/L) | 78 ± 27 | 83 ± 27 | 0.32 |
| Alkaline Phosphatase (U/L) |
78 ± 27 | 83 ± 27 | 0.27 |
| Bilirubin (mg/dL) | 0.75 ± 0.38 | 0.78 ± 0.36 | 0.65 |
| Albumin (g/dL) | 4.2 ± 0.3 | 4.2 ± 0.4 | 0.90 |
| Prothrombin time (sec) |
11.9 ± 2.8 | 11.7 ± 1.9 | 0.61 |
| Lipids and metabolic factors (mean ± s.d.) | |||
| Triglycerides (mg/dL) | 176 ± 207 | 171 ± 91 | 0.79 |
| Fasting glucose (mg/dL) |
96 ± 14 | 94 ± 14 | 0.47 |
| HOMA-IR (mg/dL × μU/mL/405) |
5.2 ± 4.0 | 5.4 ± 5.4 | 0.93 |
| Body mass index (kg/m2) |
34 ±7 | 34± 7 | 0.53 |
| Liver Histology (mean ± s.d.) | |||
| Total NAS | 5.1 ± 1.4 | 4.9 ± 1.3 | 0.22 |
| Steatosis | 1.9 ± 0.9 | 1.9 ± 0.8 | 0.66 |
| Lobular inflammation | 1.8 ± 0.7 | 1.6 ± 0.7 | 0.10 |
| Hepatocellular ballooning |
1.4 ± 0.7 | 1.3 ± 0.7 | 0.82 |
| Fibrosis stage | 1.5 ± 1.0 | 1.6 ± 1.1 | 0.77 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma glutamyl transpeptidase; HOMA-IR, homeostatic model assessment for insulin resistance; NAS, non-alcoholic fatty liver activity score.
ALT Responses
The serial mean ALT levels in the placebo and vitamin E-treated groups over the 96 weeks of therapy and subsequent 24 weeks of follow-up (to week 120) are shown in Figure 1. There was a marked decline in ALT levels in the vitamin E group beginning within the first month of treatment that was statistically significant in comparison to the placebo group by week 8 (P = 0.01). In vitamin E recipients, ALT levels declined by an average of 39 U/L by week 24. Thereafter, there was a slight further decline in mean ALT levels in both the placebo and vitamin E groups, so that by week 96 the mean decline was 41 U/L in vitamin E and 21 U/L in placebo recipients. After therapy was stopped at 96 weeks, mean ALT levels rose in the vitamin E, but not in the placebo group and the two treatment arms had virtually identical average ALT levels at week 120. These results were similar to those shown in the entire cohort in the primary publication of the PIVENS trial.13
Figure 1.
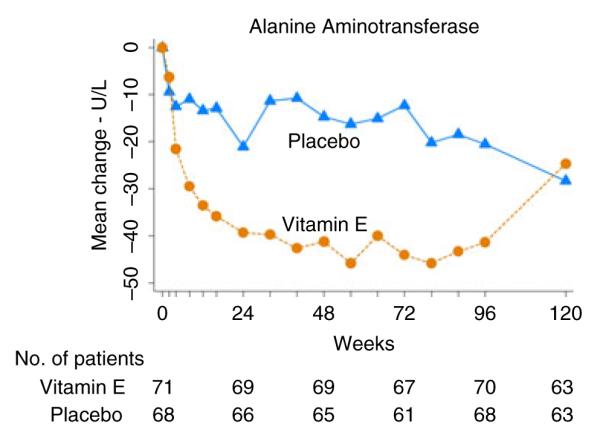
Mean changes in ALT levels from baseline by weeks after randomisation are shown separately for the placebo (n = 68) and vitamin E (n = 71) subjects in this reanalysis of the PIVENS trial.
For further analyses, an ALT response was defined as a decrease to ≤40 U/L and by ≥30% of baseline, and the pattern of response was categorised as either ‘early’ (at 24 weeks) or ‘late’ (at 96 weeks) and whether early and sustained or not (breakthrough). All categories of beneficial ALT responses were more common in vitamin E than placebo recipients, including an early and sustained response (24% vs. 6%), an early response, but subsequent breakthrough (15% vs. 1%) and a late response (24% vs. 10%). Conversely, only 37% of vitamin E recipients compared to 82% of placebo recipients were classified as having no ALT response at either time point.
Association of ALT and histological responses
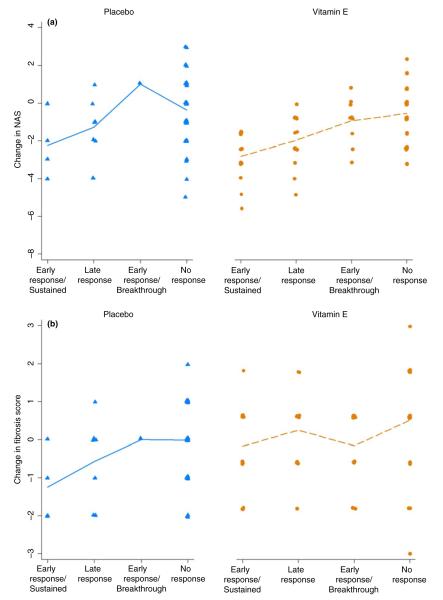
Changes in NAS score by type of ALT response and treatment group are shown in Figure 2a and changes in fibrosis scores in Figure 2b. Statistical analyses of the differences between groups are given in Table 2. Almost all patients (95%) who had an early and sustained ALT response also had a 2-point or greater improvement in NAS score regardless of whether they received vitamin E or placebo. Similarly, patients with a late ALT response (ALT ≤40 U/L at week 96) also were likely to have a histological response, although a 2-point or greater response was more likely to occur in the vitamin E (65%) than the placebo group (43%). In contrast, patients with an early response followed by breakthrough and those with no response were unlikely to have histological improvement, with little change in the average NAS scores. Changes in fibrosis scores showed a similar pattern among the four categories of ALT responses, but the differences were not statistically significant.
Figure 2.
Change in NAS and fibrosis scores between baseline and week 96 in patients receiving vitamin E or placebo by pattern of ALT response. (a) change in NAS scores. (b) changes in fibrosis scores. Patterns of ALT response are categorised into four groups: as early and sustained, a late response only, an early response only [breakthrough] and nonresponse. Each dot represents an individual patient; dots are plotted slightly above or below the line for clarity using 1% jittering; the line segments connect the means for each group.
Table 2.
ALT responses and changes in histological features
| Vitamin E |
Placebo |
|||||
|---|---|---|---|---|---|---|
| Histological feature | ALT R* (n = 34) | ALT NR* (n = 37) | P value | ALT R* (n = 11) | NR* (n = 57) | P value |
| Mean change in steatosis score | −1.1 | −0.4 | <0.001 | −0.7 | −0.0 | <0.001 |
| Mean change in inflammation score | −1.1 | −0.3 | <0.001 | −0.5 | −0.2 | 0.14 |
| Mean change in Ballooning score | −0.8 | −0.2 | 0.01 | −0.5 | −0.2 | 0.42 |
| Mean change in NAS† score | −3.0 | −0.8 | <0.001 | −1.6 | −0.4 | 0.03 |
| Mean change in Fibrosis score | −0.5 | −0.2 | 0.34 | −0.8 | −0.0 | 0.04 |
| Decrease in NAS by ≥2 points | 82% | 32% | <0.001 | 55% | 25% | 0.07 |
| Resolution of NASH‡ | 44% | 22% | 0.07 | 45% | 14% | 0.03 |
| Mean change in Weight (kg) | −0.9 | 1.8 | 0.03 | −3.7 | 1.8 | 0.01 |
ALT R = response defined as decrease to 40 U/L or less and by 30% or more from baseline. ALT NR = lack of ALT response.
Total non-alcoholic fatty liver activity score (NAS), comprising the sum of scores for steatosis, inflammation and ballooning cell injury.
Resolution of histological features that fulfil the criteria for diagnosis of non-alcoholic steatohepatitis (NASH).
Associations between ALT responses and quantitative changes in histological features are given in Table 2. In these analyses, patients were grouped as having an end-of-treatment ALT response (early and sustained or a late response) or a nonresponse (early response with breakthrough or nonresponse) as well as by treatment arm. Thus, patients with an end-of-treatment ALT response had improvements in all of the features of the NAS score, which overall was highly significant (P < 0.0001). A similar association was also found in the placebo-treated patients, but the differences were less striking, perhaps because of the fewer numbers of placebo subjects with ALT responses. Overall, a similar proportion of patients with an ALT response were found to have histological resolution of NASH in both the vitamin E (44%) and placebo groups (45%). There was also a decrease in fibrosis scores in the ALT responders, but the change was not significantly more than in nonresponders in the vitamin E group and was only marginally significant in the placebo group. Finally, there was a modest association between ALT responses and weight loss, which was most evident in the placebo recipients. This association was analysed further focusing upon weight change and histological improvement.
Weight change
Changes in body weight over the 96 weeks of treatment in the PIVENS trial were similar in the vitamin E and placebo groups. Thus, there was an overall average 0.7 kg increase in body weight (vitamin E = +0.5 kg, placebo = +0.9 kg) during the 96 weeks of treatment. Importantly, however, there was great variability in weight change among the 139 subjects, ranging from a gain of 14 kg to a loss of 15 kg. By categories, 22% of patients had weight loss (≥2 kg), 36% minimal or no change and 42% weight gain (≥2 kg).
Average changes in NAS and fibrosis scores in the vitamin E and placebo-treated patients are shown by category of weight change in Table 3. In both treatment groups, patients who lost weight were more likely to have improvements in NAS scores than those with no change or weight gain. However, improvements in NAS scores were greater among the vitamin E-treated patients irrespective of weight change. There was no evidence that the proportion of ALT responders varied by weight change between the vitamin E and placebo patients, indicating independent effects of both weight change and vitamin E (P = 0.29 from test of interaction). In contrast, there were minimal improvements in fibrosis scores (mean change = −0.3, 95% CI −0.1 to −0.4) and no diferences between the vitamin E and placebo groups (P = 0.21). In the placebo group, patients who gained weight were more likely to have worsening of NAS scores and fibrosis, an effect not seen in the vitamin E-treated patients. The difference in change in fibrosis score between placebo recipients who gained weight (+0.2) and those who had no change (−0.3) weight or lost (−0.7) was signi ficant (P = 0.005).
Table 3.
Relationship between weight changes and changes in histology
| Vitamin E |
Placebo |
|||||||
|---|---|---|---|---|---|---|---|---|
| Group | Weight change (kg) |
Weight change (kg) |
||||||
| Change in histology score | Loss (≥2) n = 17 |
No change n = 27 |
Gain (≥2) n = 27 |
P value | Loss (≥2) n = 14 |
No Change n = 23 |
Gain (≥2) n = 31 |
P value |
| Steatosis | −1.1 | −0.8 | −0.4 | 0.08 | −0.5 | −0.3 | +0.1 | 0.05 |
| Inflammation | −1.1 | −0.7 | −0.4 | 0.04 | −0.4 | −0.6 | +0.2 | 0.002 |
| Ballooning | −0.7 | −0.7 | −0.2 | 0.08 | −0.9 | −0.3 | +0.2 | <0.001 |
| Total NAS* | −2.8 | −2.2 | −1.0 | 0.007 | −1.9 | −1.1 | +0.5 | <0.001 |
| Fibrosis | −0.4 | −0.4 | −0.2 | 0.70 | −0.7 | −0.3 | +0.2 | 0.003 |
| Resolution of NASH† | 41% | 37% | 22% | 0.42 | 50% | 17% | 6% | 0.003 |
| Decrease in NAS by ≥ 2 | 71% | 65% | 37% | 0.05 | 64% | 39% | 6% | <0.001 |
| ALT Response | 59% | 48% | 41% | 0.52 | 43% | 13% | 6% | 0.01 |
Total non-alcoholic fatty liver activity score (NAS), comprising the sum of scores for steatosis, inflammation and ballooning cell injury.
Resolution of histological features that fulfil the criteria for diagnosis of non-alcoholic steatohepatitis (NASH).
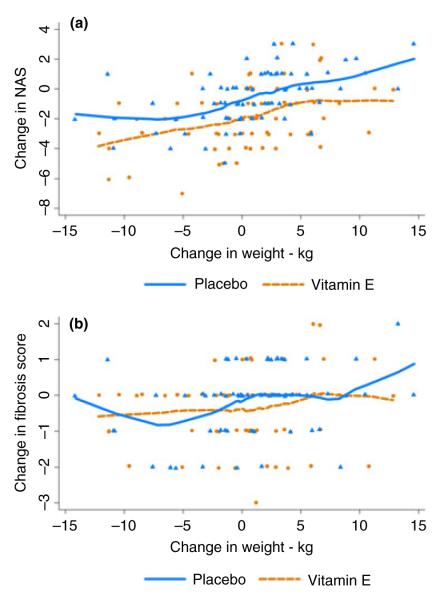
The individual changes in histological scores by weight change and treatment group in all 139 patients are shown for NAS and fibrosis scores separately in Figure 3a and 3b. There was a constant difference between the vitamin E and placebo patients in change in NAS regardless of change in weight, indicating an independent effect of vitamin E on disease activity irrespective of weight change (P = 0.44 from test of interaction of weight change by treatment group by linear regression of change in NAS score). In contrast, there appeared to be little effect of vitamin E on change in fibrosis scores, except in blunting the effects of weight gain.
Figure 3.
Change in individual NAS and fibrosis scores between baseline and week 96 in patients receiving vitamin E or placebo by amount of weight change during therapy (a) changes in NAS. (b) changes in fibrosis scores. Each dot represents an individual patient; dots are plotted slightly above or below the line for clarity using 1% jittering; the locally weighted scatterplot smoothing (lowess) curves are present for each group.
Relapse in ALT response
At the end of 96 weeks of therapy, patients were instructed to stop the study medications and were followed for another 24 weeks. After stopping therapy, 15 of 40 patients with an end-of-treatment ALT response who had ALT values at 120 weeks lost the response, including 13 of 31 (42%) vitamin E and 2 of 9 (22%) placebo recipients.
DISCUSSION
Non-alcoholic fatty liver disease has become the most common cause of liver test abnormalities adults in the Western world and is estimated to affect up to 30% of the adult population.17, 18, 19 This condition is also increasing in frequency among children and adolescents.20 NAFLD is often benign and nonprogressive and associated with few, if any, symptoms. On the other hand, a proportion of patients with NAFLD develop progressive liver disease that can result in cirrhosis, end-stage liver disease, need for liver transplantation, hepatocellular carcinoma and death.21–25 Patients with progressive disease usually have NASH on liver biopsy with the typical findings of centrizonal (zone 3) cellular injury (also called ballooning degeneration), inflammation, and fibrosis in addition to steatosis.22 The separation of simple steatosis from NASH non-invasively can be difficult and is not predicted by serum aminotransferase levels, thus leading to the need for liver biopsy to decide upon interventions.26, 27 These findings suggest that serum ALT and AST are unreliable markers for the degree of activity or severity, stage or degree of fibrosis and ultimate progression of NASH to end-stage liver disease.
In this secondary analysis of the PIVENS trial, however, serum ALT levels, if elevated at baseline, were found to have reasonable reliability in detecting improvement in the underlying liver disease, at least as assessed by liver biopsy and a validated histological scoring system. Over 80% of patients treated with vitamin E whose serum ALT levels fell to 40 U/L or less and by at least 30% of baseline had histological improvement. Importantly, none of these patients had evidence of worsening. In contrast, patients who did not achieve this degree of improvement in serum liver biochemical tests were less likely to show histological improvement and a significant proportion worsened.
This reanalysis of the PIVENS trial also showed the important and confounding effects of weight change on serum ALT levels as well as histological features of disease. Indeed, in patients who lost weight (using a cut point of only 2 kg over a 2-year period), the proportion of patients with histological improvement was similar in vitamin E and placebo groups (71% vs. 64%), although the quantitative degree of improvement in NAS score was greater with vitamin E than placebo (−2.8 vs. −1.9 points). Perhaps more strikingly, patients who gained weight were less likely to improve with vitamin E therapy, and those receiving placebo were likely to worsen. Weight gain was indeed associated with significant worsening of hepatic fibrosis in the placebo-treated patients. In contrast, patients who received placebo and who lost or did not gain weight and those who received vitamin E were unlikely to demonstrate progression of fibrosis scores over the 2-year period of this study whether or not ALT levels improved. These results provide strong and evidence-based support for dietary recommendations in patients with NASH. A first priority should be weight loss. But, perhaps more important is strict avoidance of further weight gain, which is closely associated with worsening of fibrosis, a surrogate, but convincing marker for disease progression.
The demonstration that vitamin E resulted in decreases in ALT levels and histological improvements in NASH was shown in the initial publication of the PIVENS trial 13 and was reinforced by several features in this study. First, there was a rapid and statistically significant decline in ALT levels in the vitamin E-, but not placebo-treated patients. The proportion of patients with an ALT response was three or more times higher in the vitamin E than placebo group, both at 24 weeks (39% vs. 7%) and 96 weeks (48% vs. 16%). Improvements in ALT levels were associated with improvements in histology scores and this was most evident in the vitamin E-treated subjects. Finally, discontinuation of vitamin E was followed by a prompt relapse and loss of the ALT response in 42% of patients.
Shortcomings of this post hoc analysis include the small sample size of the controlled study, which was adequate to assess the primary endpoint, but was somewhat limited for extensive secondary analyses. In addition, the definitions of ALT and histological responses were made a priori with only limited analysis of the dataset. However, both definitions are based upon clinical understanding of NAFLD and were easy to apply. The numbers of patients in the study were too few to attempt to generate statistically based algorithms for defining an ALT or histological responses; however, in preliminary analyses, modification of the criteria, such as dropping the requirement for a 30% decline in ALT from baseline, decreasing the criteria for an ALT response from ≤40 to ≤30 U/L, and using AST rather than ALT values did not improve the area under the curve in predicting histological responses (Table S1). Finally, this study was carried out in nondiabetic patients and the findings may not be applicable to patients with diabetes, who typically have more severe disease. Furthermore, only half of patients (48%) responded to vitamin E therapy and most relapsed when it was stopped.
In summary, these results show that vitamin E therapy is associated with improvements in serum aminotransferase levels, and that decreases of ALT values into the normal range (to ≤40 U/L and by 30% of baseline) are usually associated with histological improvement in disease activity (steatosis, inflammation, cell injury). Weight loss can also achieve these endpoints, but importantly weight gain has definite negative implications for the natural history and outcome of this common and increasingly important form of liver disease. The effects of vitamin E and weight loss on both ALT and histological responses were separate and independent, so that even patients who lost weight benefitted from vitamin E therapy.
Supplementary Material
ACKNOWLEDGEMENTS
RL serves as a consultant to Gilead, Corgenix and Daiichi Sankyo Inc and has received research grant support from Daiichi Sankyo Inc. AJS serves as a consultant to Abbott, Genfit, Ikaria, Merck, Norgene, Salix and Roche and has research grant support from Bayer-Onxy, Exhalenz, Genentech, Gilead, Gore, Ikaria, Intercept and Salix and receives royalties from Uptodate.
Declaration of funding interests: The NASH CRN is a multicentre prospective study of the natural history and treatment of non-alcoholic steatohepatitis funded as a cooperative agreement by the National Institute of Diabetes and Digestive and Kidney Diseases (Grant numbers: U01DK061718, U01DK061728, U01DK061731, U01DK061732, U01DK061734, U01DK061737, U01DK0 61738, U01DK061730, U01DK061713). In conduct of the NASH CRN studies, several clinical centres use support from General Clinical Research Centers or Clinical and Translational Science Awards (Grant numbers: UL1RR024989, UL1RR025761, M01RR00188, UL1RR 024131, UL1RR025014, UL1RR031990, UL1RR025741, UL1RR029887, UL1RR24156, UL1RR025055, UL1RR 031980). Further support is provided by the National Institute of Child Health and Human Development (NICHD) and by the intramural programme of the National Cancer (NCI). The PIVENS trial was conducted by the NASH CRN and supported in part by Collaborative Research and Development Agreement (CRADA) between NIDDK and Takeda Pharmaceuticals North America (for pioglitazone and placebo tablets and for additional financial support) and a Clinical Trial Agreement between NIDDK and Pharmavite (for vitamin E and placebo capsules). Full membership of the NASH CRN is listed in the Table S1.
Footnotes
AUTHORSHIP Guarantor of the article: Jay H. Hoofnagle.
Author contributions: Study concept and design: JHH, MVN, JT. Acquisition of data: KVK, RL, AJS, BANT. Analysis and interpretation of data: MVN, JHH, JT, DEK, JMC, KVK, RL, SJS, BANT. Drafting of manuscript: JHH. Critical revision: JHH, MVN, JT, DEK, JMC, KVK, RL, SJS, BANT. Statistical Analysis: MVN, JT. Obtained funding: JT, KVK, RL, SJS, BANT. Technical or material support: not applicable. Study supervision: JT and JHH. All authors approved the final version of the manuscript.
SUPPORTING INFORMATION Additional Supporting Information may be found in the online version of this article:
Table S1. Prediction of a 2-point decrease in NAS score at 96 weeks based upon ALT levels at 96 weeks: 6 definitions of an ALT response (sustained or late).
Appendix S1. Non-alcoholic Steatohepatitis Clinical Research Network membership.
Declaration of personal interests: JHH, MLVN, DEK, JMC, KVK, RL, BANT and JT have no conflicts of interest to report.
REFERENCES
- 1.Traber MG, Stevens JF. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic Biol Med. 2011;51:1000–13. doi: 10.1016/j.freeradbiomed.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kowdley KV, Mason JB, Meydani SN, Cornwall S, Grand RJ. Vitamin E deficiency and impaired cellular immunity related to intestinal fat malabsorption. Gastroenterology. 1992;102:2139–42. doi: 10.1016/0016-5085(92)90344-x. [DOI] [PubMed] [Google Scholar]
- 3.Tanyel MC, Mancano LD. Neurologic findings in vitamin E deficiency. Am Fam Physician. 1997;55:197–201. [PubMed] [Google Scholar]
- 4.Sokol RJ, Balistreri WF, Hoofnagle JH, Jones EA. Vitamin E deficiency in adults with chronic liver disease. Am J Clin Nutr. 1985;41:66–72. doi: 10.1093/ajcn/41.1.66. [DOI] [PubMed] [Google Scholar]
- 5.Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 6.Lonn E, Bosch J, Yusuf S, et al. HOPE and HOPE-TOO Trial Investigators. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293:1338–47. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 7.Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaziano JM, Glynn RJ, Christen WG, et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2009;301:52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sano M, Ernesto C, Thomas RG, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N Engl J Med. 1997;336:1216–22. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 10.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 11.Lavine JE. Vitamin E treatment of nonalcoholic steatohepatitis in children: a pilot study. J Pediatr. 2000;136:734–8. [PubMed] [Google Scholar]
- 12.Sanyal AJ, Mofrad PS, Contos MJ, et al. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2004;2:1107–15. doi: 10.1016/s1542-3565(04)00457-4. [DOI] [PubMed] [Google Scholar]
- 13.Sanyal AJ, Chalasani N, Kowdley KV, et al. NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavine JE, Schwimmer JB, Van Natta ML, et al. Nonalcoholic Steatohepatitis Clinical Research Network Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659–68. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chalasani NP, Sanyal AJ, Kowdley KV, et al. NASH CRN Research Group Pioglitazone versus vitamin E versus placebo for the treatment of non-diabetic patients with non-alcoholic steatohepatitis: PIVENS trial design. Contemp Clin Trials. 2009;30:88–96. doi: 10.1016/j.cct.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleiner DE, Brunt EM, Van Natta M, et al. Nonalcoholic Steatohepatitis Clinical Research Network Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 17.Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–10. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- 18.Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–31. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 19.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–85. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 20.Barshop NJ, Sirlin CB, Schwimmer JB, Lavine JE. Review article: epidemiology, pathogenesis and potential treatments of paediatric non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2008;28:13–24. doi: 10.1111/j.1365-2036.2008.03703.x. [DOI] [PubMed] [Google Scholar]
- 21.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–21. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 22.Rafiq N, Bai C, Fang Y, et al. Long-term follow up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7:234–8. doi: 10.1016/j.cgh.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Ekstedt M, Franzén LE, Mathiesen UL, et al. Long term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–73. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 24.Söodenberg C, Stål P, Askling J, et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595–602. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- 25.Charlton M, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249–53. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 26.Neuschwander-Tetri BA, Clark JM, Bass NM, et al. NASH Clinical Research Network Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology. 2010;52:913–24. doi: 10.1002/hep.23784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong VW-S, Wong GL-H, Tsang SW-C, et al. Metabolic and histological features of non-alcoholic fatty liver disease patients with different serum alanine aminotransferase levels. Aliment Pharmacol Ther. 2009;29:387–96. doi: 10.1111/j.1365-2036.2008.03896.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.