Abstract
Introduction
Though being physically active has associated with a healthier ankle-brachial index (ABI) in observational studies, ABI usually does not change with exercise training in patients with peripheral artery disease (PAD). Less is known about the effect of exercise training on ABI in patients without PAD but at high risk due to the presence of type 2 diabetes (T2DM).
Methods
Participants (n=140) with uncomplicated T2DM, and without known cardiovascular disease or PAD, aged 40–65 years, were randomized to supervised aerobic and resistance training 3 times per week for 6 months or to a usual care control group. ABI was measured before and after the intervention.
Results
Baseline ABI was 1.02±0.02 in exercisers and 1.03±0.01 in controls (p=0.57). At 6 months, exercisers vs. controls improved ABI by 0.04±0.02 vs. −0.03±0.02 (p=0.001). This change was driven by an increase in ankle pressures (p<0.01) with no change in brachial pressures (p=0.747). In subgroup analysis, ABI increased in exercisers vs. controls among those with baseline ABI<1.0 (0.14±0.03 vs. 0.02±0.02, p=0.004), but not in those with a baseline ABI≥1.0 (p=0.085). The prevalence of ABI between 1.0–1.3 increased from 63% to 78% in exercisers and decreased from 62% to 53% in controls. Increased ABI correlated with decreased HbA1c, systolic and diastolic blood pressure, but the effect of exercise on ABI change remained significant after adjustment for these changes (β=0.061, p=0.004).
Conclusion
These data suggest a possible role for exercise training in the prevention or delay of PAD in T2DM, particularly among those starting with an ABI <1.0.
Keywords: exercise, peripheral artery disease, ankle-brachial index, type 2 diabetes
INTRODUCTION
An estimated 8.5 million (7.2%) adults over the age of 40 in the US have peripheral artery disease (PAD),(1) a condition of reduced blood flow to the lower limbs that is associated with functional limitations(2) and a two-fold increase in the risk of cardiovascular mortality.(3) The prevalence of PAD is 2–3 times higher in persons with vs. without type 2 diabetes (T2DM).(4) PAD develops earlier, progresses more quickly, and is often detected at later stages in T2DM, which is problematic since early detection leads to better prognosis.(5) These factors, along with the growing population burden of T2DM, underscore the need for a better understanding of methods for the prevention and treatment of PAD in this population.
PAD is commonly evaluated by the ankle-brachial index (ABI), which reflects blood flow in the legs relative to the upper limbs. Though an ABI<0.9 is the diagnostic cutpoint for PAD, cardiovascular mortality risk begins to increase at an ABI<1.0(6). Physical activity is associated with a decreased cardiovascular disease risk in populations with and without T2DM.(7, 8) This risk reduction likely occurs through several pathways including improved vascular function, lipid profile, and glycemic control. However, whether physical activity has a beneficial effect on ABI or PAD risk is less clear. Experimental studies in PAD patients with and without intermittent claudication have found that exercise training improves walking distance,(9, 10) but not ABI.(9) Conversely, observational studies have generally found that increased physical activity by self-report or fitness is associated with higher values for ABI in populations free from PAD.(11–15) Therefore, it is possible that exercise training could improve ABI in patients with low ABI (<1.0) but without prior symptoms or diagnosed PAD. However, no randomized trials have evaluated the effect of exercise training on ABI in such a population.
The present study was a post-hoc analysis of ABI in the trial, Sugar, Hypertension, and Physical Exercise (SHAPE2),(16) which randomized participants with T2DM and suboptimal untreated blood pressure (BP) or treated hypertension to either a 6-month supervised exercise intervention or a control group. Resting BP as the primary outcome. In the present report, we tested the hypothesis that exercise training would improve ABI. Additionally, we examined whether potential changes in ABI were associated with changes in other cardiovascular risk factors.
METHODS
Participants
Participants were recruited from the greater Baltimore area from 2004–2010, primarily through newspaper advertisements. Eligibility criteria included age 40–65 years and T2DM treated with diet or oral medications but not insulin. Insulin use was an exclusion because it generally represents a more complicated form of T2DM, requires more intense monitoring during exercise training, and may require modifications to the exercise prescription. T2DM was confirmed by a primary care provider, based on the 2003 ADA diagnostic criteria. Further, participants had to have either 1) suboptimal, untreated BP defined as SBP 120–159 mmHg or DBP 85–99 mmHg, which falls within the ranges of Pre- or Stage I hypertension as defined by the JNC VII Guidelines (17) or 2) current treatment for hypertension with SBP≤159 mmHg and DBP≤99mmHg with no lower limit for BP if being treated. Participants were also nonsmoking, sedentary (<90 minutes of exercise per week), had no history of heart disease or any condition that would limit the ability to participate in an exercise intervention. As part of the screening, we performed a maximal graded exercise stress test. Subjects with >1 mm ST-T wave depression, high-grade ventricular arrhythmias, or cardiac symptoms were excluded and referred to their health care provider for follow-up. Other exclusions included fasting glucose <400 mg/dL or glycosylated hemoglobin (HbA1c), a summary measure of glucose control over the past 3 months, ≥11%. Participants reporting a history of PAD were excluded from the study.
Intervention
Participants were randomized to either a supervised exercise intervention or a usual care control group. The exercise intervention consisted of 3 supervised sessions per week, based on guidelines for diabetes and hypertension.(18) Briefly, sessions consisted of a 10–15 minute warm-up, 45 minutes of aerobic exercise at a target heart rate between 60–90% of maximum heart rate, and a cool down. Each session also included 7 weight training exercises (lat-pull down, leg extension, leg curl, bench press, leg press, shoulder press, and seated mid-rowing) performed as 2 sets of 12–15 repetitions at 50% of 1-repetition maximum. Weight was increased when participants could easily complete 15 repetitions. If participants did not attend at least 62 sessions (80% compliance) over the 26 week period, an additional month was allowed for participants to reach at least 62 sessions.
During screening, all potential participants were given written exercise guidelines from the National Institute of Aging (http://www.nia.nih.gov/HealthInformation/Publications/ExerciseGuide) and information about the American Heart Association Diet. No participants received any further dietary advice and controls received no further physical activity advice or exercise intervention.
Assessments
Assessments were conducted by staff in a blinded manner at baseline and 6 months. ABI was derived from systolic BPs measured in the arms and legs after 10 minutes of rest in a supine position with arms and legs straight and at rest. Manual cuffs were used for all BP measurements and arm circumference was determined during screening to select the appropriate cuff size consistent with JNC7 recommendations.(17) The same cuff size was used for the lower leg and a straight wrapping technique was employed. Arm BPs were measured using a sphygmomanometer and a stethoscope whereas and leg BPs were measured with a sphygmomanometer and an 8 MHz Doppler (Parks Medical Electronics, Inc., Aloha, OR) to detect pulses. One measurement was made at each of the six sites in the following order: left arm, left ankle (dorsalis pedis, posterior tibialis), right arm, right ankle. Right ABI was calculated as the ratio of the higher right ankle pressures (dorsalis pedis or posterior tibialis) divided by the higher brachial pressure (right or left side) or, in the case where right and left brachial pressures differed by >10 mmHg, the average of the right and left brachial pressures. Left ABI was calculated in a similar way. The lower ratio of either side was considered the participant’s overall ABI.(19)
Cardiorespiratory fitness testing as described above was performed at the end the assessment so as not to affect vascular outcomes. Resting BP (separate from the ABI assessment) was measured in triplicate after 5 minutes of seated rest(17) using an automated device (Dinamap MPS Select; Johnson & Johnson, New Brunswick, NJ). The Johns Hopkins General Clinical Research Center Core Laboratory analyzed fasting blood samples using standard methods for lipids (Cholestech Corp.), insulin (Linco Research Inc.), glucose (Beckman Diagnostics), and HbA1c (Med. Computer Systems). The quantitative insulin sensitivity check index (QUICKI) was calculated to estimate insulin sensitivity using the formula QUICKI = 1/[log fasting insulin × log fasting glucose].
Statistical analysis
This report evaluated the effect of exercise training on ABI, a secondary outcome measured in the SHAPE2 clinical trial.(16) Independent t-tests evaluated differences in baseline characteristics and overall and side-specific ABIs, brachial pressures, and ankle pressures (baseline, 6-month, and change) across randomized groups. Mixed models evaluated main effects of group and time, and a group-by-time interaction with ABIs, brachial pressures, and ankle pressures as the dependent variables. ABI was categorized as normal, borderline, or low. Normal ABI (1.00–1.39) represents no apparent atherosclerotic limitation to blood flow in the legs. Borderline ABI (0.90 – 0.99) does not meet the diagnostic threshold for PAD, but is associated with an increased risk of cardiovascular mortality(6) and functional decline(20). Low ABI (<0.90) is the diagnostic criteria for PAD, is sensitive and specific to PAD diagnosed by angiography,(19) and is associated with an even higher cardiovascular risk.(6) No participants had an ABI>1.4 at either assessment visit. ABI category distributions were compared across groups at baseline and 6 months using a χ2 test. We report results using an intention-to-treat analysis, with missing ABI measurements (n=5 at baseline, n=29 at 6 months) imputed by chained equations (STATA ice command) using 10 imputations for each missing measurement generated from regression equations.
The primary analysis described above and two subsequent secondary analyses were then conducted only in subjects completing the study protocol. First, because we expected that participants with borderline or low ABI (<1.0) may be more likely to experience an increase in ABI in response to exercise training, we stratified participants into groups with baseline ABI <1.0 and ≥ 1.0. Second, we calculated Pearson’s correlations between changes in ABI with changes in traditional cardiovascular risk factors. Cardiovascular risk factor changes that were significantly correlated with changes in ABI were added to a regression model (both baseline and the change in that cardiovascular risk factor) to evaluate whether changes in ABI appeared to be mediated by exercise-induced changes in other cardiovascular risk factors. The type 1 error rate was set at α=0.05. Sample size (n=140) was based on BP, the primary outcome of the SHAPE2 trial.(16) Based on the standard deviation of change in ABI from our sample (SD=0.13) and the number of participants randomized (n=70 per group) or with complete data for analysis (49 exercisers, 60 controls), we had 80% to detect a difference in the change in ABI between intervention groups of 0.062 and 0.070, respectively.
RESULTS
Of 140 randomized participants, 114 (83%) completed the study and, of these, 109 had available ABI measurements at baseline and six months (Figure 1). Nineteen participants randomized to the exercise intervention (seventeen drop-outs, two withdrawn due to non-study related illness) vs. seven randomized to the control condition (five drop-outs, two withdrawn due to non-study related illness) did not complete the study protocol. Completers were similar to non-completers with respect to baseline characteristics listed in Table 1, with the exception of slightly better QUICKI (0.293 ± 0.023 vs. 0.283 ± 0.021, p=0.026).
Figure 1.
Flowchart of Study Participants in the Sugar Hypertension and Physical Exercise (SHAPE2) Trial
Table 1.
Baseline Characteristics by Randomized Group in the SHAPE2 Trial
| Overall | |||
|---|---|---|---|
| Exercisers (n=70) | Controls (n=70) | p-value | |
| Age | 57 ± 0.7 | 56 ± 0.8 | 0.097 |
| Gender | |||
| Male | 41 (59%) | 40 (57%) | |
| Female | 29 (41%) | 30 (43%) | 0.864 |
| Race | |||
| White | 39 (56%) | 42 (60%) | |
| Black | 30 (43%) | 25 (36%) | |
| Other | 1 (1%) | 3 (4%) | 0.457 |
| BMI, kg/m2 | 33.0 ± 0.6 | 33.6 ± 0.5 | 0.445 |
| SBP, mmHg | 127 ± 2 | 127 ± 2 | 0.902 |
| DBP, mmHg | 72 ± 1 | 71 ± 1 | 0.361 |
| Total cholesterol, mg/dL | 174 ± 5 | 185 ± 5 | 0.119 |
| HDL cholesterol, mg/dL | 50 ± 2 | 48 ± 2 | 0.417 |
| Triglycerides, mg/dL | 102 [78, 151] | 129 [90, 188] | 0.055 |
| HbA1c, % | 6.6 ± 0.2 | 6.7 ± 0.2 | 0.697 |
| QUICKI | 0.294 ± 0.003 | 0.286 ± 0.002 | 0.035 |
| ABI Category | |||
| Normal (1.00 – 1.39) | 42 (60%) | 46 (66%) | |
| Borderline (0.90 – 0.99) | 22 (31%) | 19 (27%) | |
| Low (<0.9) | 6 (9%) | 5 (7%) | 0.782 |
Data presented as mean ± SE, median [IQR], or n (%)
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; HDL, high density lipoprotein; QUICKI, quantitative insulin sensitivity check index; SBP, systolic blood pressure
In the intention-to-treat analysis, exercisers had slightly better insulin sensitivity by QUICKI but no other differences at baseline (Table 1). The distribution of normal, borderline, and low ABI at baseline was similar across groups. Eleven participants had an ABI<0.9. Though this cutpoint is diagnostic for PAD, these participants did not report a history of PAD diagnosis or symptoms of PAD during screening and no further diagnostic studies were conducted in these participants. Differences in participant characteristics between groups among completers and within low or high ABI groups can be found in the web appendix, Table 1.
Primary results from the SHAPE2 Trial
Primary results from the clinical trial providing data for the current analysis have been presented and discussed elsewhere.(16) In brief, the exercise training intervention failed to reduce BP, though exercisers increased cardiorespiratory and strength fitness, and reduced body fat percentage, weight, and HbA1c compared with controls.
Effect of the exercise intervention on ankle-brachial index
Overall ABI increased modestly in exercisers over the 6-month intervention while it decreased in controls (p-for-interaction=0.001, Table 2). The same pattern was observed when the right or left ABI were considered separately. Examination of the brachial and ankle pressures by intervention group and over time reveals that systolic brachial pressures did not change (p-for-interaction=0.441), but an increase in the ankle pressure among exercisers drove the overall increase in ABI in exercisers vs. controls. Results were nearly identical when analyses were conducted only among subjects who completed the protocol (web appendix, Table 2).
Table 2.
Baseline, 6-month, and Change in Ankle-Brachial Index (ABI) and Pressures in Exercisers (EX, n=70) vs. Controls (CONT, n=70) in SHAPE2
| Baseline | 6 months | Change | Group | Time | Group × Time |
||
|---|---|---|---|---|---|---|---|
| Overall ABI* | EX | 1.02 ± 0.01 | 1.07 ± 0.02† | 0.04 ± 0.02† | 0.597 | 0.047 | 0.001 |
| CONT | 1.03 ± 0.01 | 1.00 ± 0.01† | −0.03 ± 0.02† | ||||
| Right ABI | EX | 1.05 ± 0.01 | 1.09 ± 0.02† | 0.03 ± 0.02† | 0.721 | 0.052 | 0.005 |
| CONT | 1.06 ± 0.01 | 1.03 ± 0.01† | −0.03 ± 0.02† | ||||
| Left ABI | EX | 1.05 ± 0.01 | 1.10 ± 0.02† | 0.05 ± 0.02† | 0.452 | 0.004 | 0.035 |
| CONT | 1.05 ± 0.01 | 1.02 ± 0.01† | −0.03 ± 0.01† | ||||
| Systolic Brachial Pressure** | EX | 127 ± 2 | 126 ± 2 | −1 ± 2 | 0.793 | 0.716 | 0.441 |
| CONT | 126 ± 2 | 125 ± 2 | −1 ± 2 | ||||
| Right Ankle Pressure*** | EX | 133 ± 2 | 136 ± 2† | 3 ± 3† | 0.999 | 0.022 | 0.006 |
| CONT | 133 ± 2 | 128 ± 2† | −5 ± 2† | ||||
| Left Ankle Pressure*** | EX | 133 ± 3 | 138 ± 3† | 5 ± 3† | 0.782 | 0.042 | 0.002 |
| CONT | 133 ± 2 | 128 ± 2† | −5 ± 2† |
Data displayed as mean ± SE
Lower of the right and left ABIs
Higher of the right and left brachial pressures or average if difference between measures was >10 mmHg
Higher of the posterior tibialis and dorsalis pedis pressures
p<0.05 comparing EX vs. CONT within column
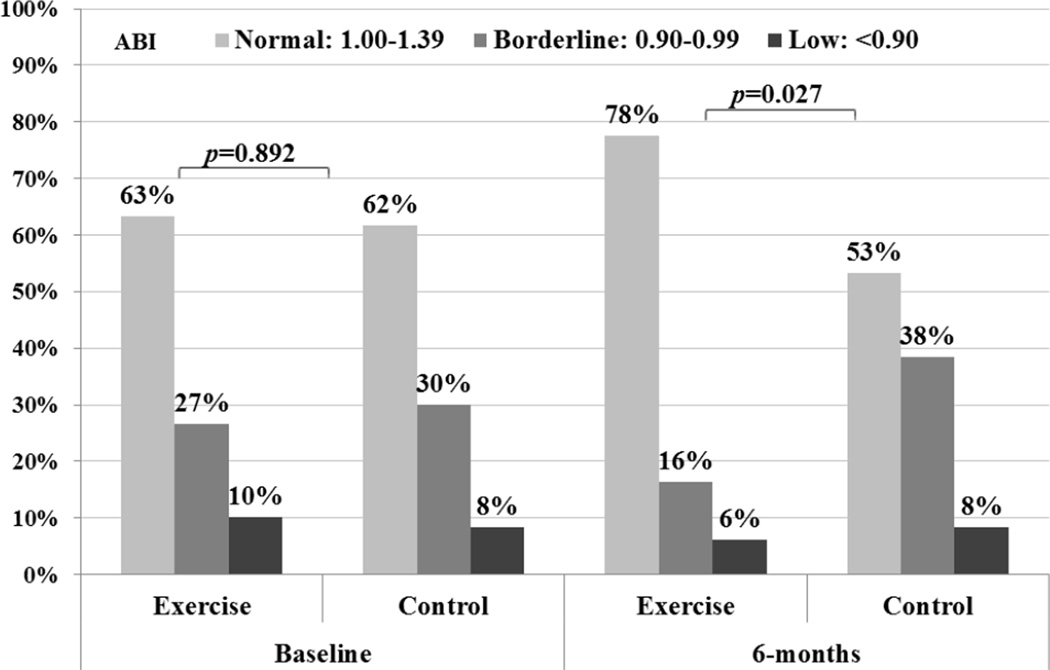
Among exercisers who completed the protocol, the distributions of participants categorized as normal, borderline, or low for overall ABI at baseline were similar (p=0.892) but differed significantly at 6 months (p=0.027). At 6-months, the proportion of exercisers with normal ABI had increased by 15%, while the proportion of controls with normal ABI had decreased by 9%. Furthermore, the proportion of exercisers with borderline ABI decreased by 11%, while the proportion of controls with borderline ABI increased by 8%. (Figure 2)
Figure 2.
Baseline and 6-month Ankle Brachial Index Category in Exercisers (n=49) and Controls (n=60) in the SHAPE2 Study.
Results stratified by baseline ankle-brachial index
Among participants with ABI ≥1.0 at baseline who completed the study, we observed a significant decrease in overall ABI over time, but this effect was not statistically different across intervention groups (p-for-interaction=0.085, Table 3). Results for the right ABI were similar to the overall ABI, though exercisers maintained a higher left ABI while the controls decreased ABI over the course of the study (p-for-interaction=0.047). When brachial and leg pressures were examined individually, the pattern emerges where systolic brachial pressures are unchanged but ankle pressures are sustained in exercisers and decline in controls.
Table 3.
Baseline, 6-month, and Change in Ankle-Brachial Index (ABI) and Pressures in Exercisers (EX) vs. Controls (CONT) Stratified by Baseline ABI in SHAPE2
| Baseline | 6 months | Change | Group | Time | Group × Time | ||
|---|---|---|---|---|---|---|---|
| Baseline ABI≥1.0 (EX n=31 ; CONT n=37) | |||||||
| Overall ABI* | EX | 1.09 ± 0.01 | 1.07 ± 0.01† | −0.02 ± 0.02 | 0.824 | <0.001 | 0.085 |
| CONT | 1.09 ± 0.01 | 1.02 ± 0.02† | −0.06 ± 0.02 | ||||
| Right ABI | EX | 1.12 ± 0.02 | 1.10 ± 0.02† | −0.02 ± 0.02 | 0.268 | 0.017 | 0.414 |
| CONT | 1.10 ± 0.01 | 1.05 ± 0.02† | −0.05 ± 0.02 | ||||
| Left ABI | EX | 1.11 ± 0.02 | 1.11 ± 0.02† | 0.00 ± 0.02 | 0.447 | 0.004 | 0.047 |
| CONT | 1.09 ± 0.01 | 1.04 ± 0.02† | −0.05 ± 0.02 | ||||
| Systolic Blood Pressure** | EX | 124 ± 3 | 128 ± 2 | 4 ± 3 | 0.750 | 0.773 | 0.322 |
| CONT | 129 ± 2 | 127 ± 2 | −2 ± 3 | ||||
| Right Ankle Pressure*** | EX | 139 ± 4 | 141 ± 3† | 1 ± 3† | 0.901 | 0.004 | 0.027 |
| CONT | 141 ± 2 | 133 ± 3† | −8 ± 2† | ||||
| Left Ankle Pressure*** | EX | 138 ± 3 | 142 ± 3† | 4 ± 3† | 0.764 | 0.001 | 0.001 |
| CONT | 140 ± 2 | 131 ± 3† | −9 ± 2† | ||||
| Baseline ABI < 1.0 (EX n=18 ; CONT n=23 ) | |||||||
| Overall ABI* | EX | 0.92 ± 0.02 | 1.06 ± 0.03† | 0.14 ± 0.03† | 0.619 | 0.324 | 0.001 |
| CONT | 0.93 ± 0.01 | 0.96 ± 0.02† | 0.02 ± 0.02† | ||||
| Right ABI | EX | 0.97 ± 0.02 | 1.08 ± 0.043 | 0.11 ± 0.03† | 0.672 | 0.788 | 0.008 |
| CONT | 0.98 ± 0.02 | 0.99 ± 0.02† | 0.01 ± 0.02† | ||||
| Left ABI | EX | 0.97 ± 0.03 | 1.09 ± 0.03† | 0.12 ± 0.03† | 0.964 | 0.365 | 0.006 |
| CONT | 0.97 ± 0.02 | 0.99 ± 0.02† | 0.02 ± 0.02† | ||||
| Systolic Blood Pressure** | EX | 129 ± 4 | 123 ± 4 | −6 ± 5 | 0.432 | 0.832 | 0.300 |
| CONT | 124 ± 2 | 124 ± 3 | 0 ± 2 | ||||
| Right Ankle Pressure*** | EX | 126 ± 5 | 132 ± 5 | 7 ± 5 | 0.765 | 0.983 | 0.176 |
| CONT | 123 ± 3 | 123 ± 3 | 0 ± 3 | ||||
| Left Ankle Pressure*** | EX | 127 ± 7 | 134 ± 5 | 7 ± 5 | 0.640 | 0.602 | 0.299 |
| 121 ± 4 | 124 ± 4 | 2 ± 2 |
Data displayed as mean ± SE
Lower of the right and left ABIs
Higher of the right and left brachial pressures or average if difference between measures was >10 mmHg
Higher of the posterior tibialis and dorsalis pedis pressures
p<0.05 comparing EX vs. CONT within column
In participants with borderline or low ABI at baseline (<1.0), we observed greater overall ABI improvements in exercisers vs. controls (+0.14 vs. +0.02, p=0.001, Table 3), indicating an intervention effect. Results were similar for the right and left ABI examined individually. In this group, ankle pressures increased in exercisers while systolic blood pressure decreased, but these effects were not statistically significant by intervention group when considered individually.
Evaluation of mediation by changes in other cardiovascular risk factors
We found correlations between increased ABI and decreased HbA1c (r=−0.21, p=0.030), SBP (r=−0.24, p=0.013), and DBP (r=−0.21, p=0.032). Note that SBP and DBP were measured separately using methods described above and not as part of the ABI assessment. No correlations were observed between changes in ABI and changes in BMI, total cholesterol, HDL cholesterol, triglycerides, or QUICKI (web appendix, Table 3). In regression models with change in ABI as the dependent variable after adjustment for baseline ABI, SBP, DBP, and HbA1c and changes in SBP, DBP, and HbA1c, the effect of exercise on ABI change remained robust (β = 0.061 comparing exercisers to controls, p=0.004). This result implies that the effect of the exercise intervention on ABI is not fully explained by changes in other cardiovascular risk factors such as BP and HbA1c.
DISCUSSION
This is the first study to report that 6 months of exercise training improved ABI among participants with T2DM but without a prior diagnosis or symptoms of PAD. These findings have important clinical implications because these individuals are at increased risk for developing PAD, as evidenced by the decline in ABI observed over just 6 months in the control group. Moreover, the greater improvement observed among exercisers with ABI<1.0 at baseline relative to controls suggests that T2DM patients with borderline or low ABI may be able to attenuate or even reverse further declines in ABI, which adds further support for prescribing exercise in these patients.
We are unaware of any other randomized trials reporting an improvement in ABI with exercise training. A Cochrane Review of exercise therapy for intermittent claudication concluded that exercise training had no effect on ABI in seven studies with a pooled effect of −0.01 (95% confidence interval: −0.5, 0.4).(9) However, comparison to our findings is limited because many of these studies were small, had heterogeneous interventions (e.g. medicated or surgical control groups), and most importantly, were conducted in patient populations with claudication symptoms. Presence of claudication symptoms could be indicative of established and more limiting atherosclerosis in the extremities.(9) It is possible that exercise training can be more effective for improving ABI earlier in the atherosclerotic process, before PAD is diagnosed by symptoms or a more profound reduction of blood flow in the legs. This dovetails with the finding that ABI improved most in participants with low and borderline ABI. These levels are suggestive of early PAD but perhaps not irreversible disease.
The results from our prospective randomized trial are comparable to cross-sectional reports that have examined associations between physical activity or fitness and ABI in populations with a spectrum of ABIs and with average ABI in the normal range. In a baseline analysis of more than 5,000 participants with T2DM from the Look AHEAD Study, ABI was higher across quintiles of fitness in men (p-for-trend=0.008) with a borderline significant trend in women (p-for-trend=0.055).(21) In the Cardiovascular Health Study among older adults, PAD as diagnosed by ABI<0.9 was more prevalent going from low to medium to high exercise intensity categories in both genders.(22) Though these studies cannot evaluate temporality, they are consistent with our findings.
Less clear is whether exercise is associated with a decreased incidence of PAD, with major limitations of the literature being self-report of physical activity and heterogeneous ABI assessment methods. To our knowledge, the effect of physical activity on PAD risk has only been reported in a population with T2DM in the Atherosclerosis Risk in Communities Study.(12) Of sport, work, and leisure-time physical activity indices assessed by self-report, only work physical activity was associated with incident PAD (low vs. high RR = 1.45, 95% confidence interval 1.08–1.96), though this association did not persist in multivariable models. In more general populations, there is limited observational evidence that physical activity may reduce incidence of asymptomatic PAD(11, 13) and physical activity has been associated with higher ABI.(11, 14, 15) However, uncertainty remains because several long-term follow-up studies have found no association between physical activity and PAD or lower ABI.(13, 15, 23) In light of the prospective effects of exercise training from SHAPE2, observational research with objective physical activity assessment could help clarify the potential role of physical activity in the primary prevention of PAD, as suggested herein.
It was not surprising that decreases in BP and HbA1c were associated with increased ABI in our participants. In the U.K. Prospective Diabetes Study, higher HbA1c and BP were both associated with increased risk of PAD in more than 5000 participants with T2DM and free from PAD at baseline.(24, 25) However, we report a novel finding that exercise-induced changes in ABI appeared to be independent from concurrent changes in BP and HbA1c.
Despite the well-documented effect of exercise training on lipids(26) and the known association between lipids and PAD(4, 5), we did not find that changes in ABI were related to changes in lipids in this analysis. This finding perhaps results from the healthier levels at baseline, over 50% of the study population using statins(16) and the failure of the intervention to affect lipids.(27) Evaluating associations between changes in ABI and lipids resulting from exercise in a population with hyperlipidemia is an area for future investigation, though based on our results, an improvement in lipids does not appear to be necessary for an improvement in ABI.
The results in Table 3 could be influenced by regression to the mean. For example, in participants with ABI≥1.0 at baseline, the decline in ABI among controls might result from regression to the mean, while in exercisers this regression to the mean could have been countered by the effect of the exercise intervention. The opposite is true for those with ABI<1.0 at baseline, where ABI might increase in all participants due to regression to the mean, though the greater increase in exercisers again suggests an intervention effect. Thus, differences between groups and statistics comparing groups should be valid based on the randomized design, but the actual magnitude of effects in any one subgroup formed by baseline values may be influenced by statistical regression.
One possible explanation for our findings is that SHAPE2 participants were able to exercise at a higher intensity or for a longer continuous duration than symptomatic PAD patients, and this greater intensity and/or continuous duration were needed to improve peripheral blood flow. It is well documented that lower ABI is associated with reduced physical function (e.g. walking speed, distance walked in 6 minutes), and this relationship exists with or without PAD symptoms.(2) The SHAPE2 participants had, on average, normal ABI and thus were likely to have better physical function and greater capacity for exercise training, which may explain why we saw an improvement in ABI with exercise while previous studies have not.
Considering that an increase in ankle pressure seemed to bring about the improved ABI among exercisers, another potential pathway to increased ABI could be increased collateral blood flow in the legs resulting from exercise training.(28, 29) Because ABI has not been shown to improve with exercise in PAD in humans, this mechanism was not thought to explain the magnitude of exercise-related improvements in physical function in these patients, potentially because of more advanced atherosclerosis on all levels of the vascular tree.(29) However, the SHAPE2 participants were distinct from populations with manifest PAD in that they did not have cardiovascular disease and possibily had less severe atherosclerosis in the peripheral vessels. These healthier or less affected vessels could have had more potential to improve collateral blood flow, which might have allowed a low ABI to be more reversible. A third possibility is that exercise training has been shown to improve endothelial function in populations with T2DM and hypertension,(30, 31) and better vascular function could improve peripheral blood flow. Though we did not find that the exercise intervention affected flow-mediated dilation in the brachial artery in the SHAPE2 Study,(27) we do not discount that improvement in vascular function in the legs could also explain our findings.
Strengths of this study include the relatively large sample size, the randomized design, a relevant population at high risk for the development of PAD, and a supervised exercise intervention of 6-months duration. A limitation of this study is that it began prior to the 2012 publication of the American Heart Association’s Scientific Statement on the Measurement and Interpretation of ABI.(19) Differences in our measurement protocol from these recommendations included the use of a stethoscope rather than a Doppler at the brachial site, failure to repeat the first brachial pressure, and a different sequence of measurement. Moreover, it is now recommended that ABI assessments be repeated, however that was not the standard at the time of our study. While our methods may have resulted in some measurement error, we suspect this error would be nondifferential, which would have the conservative effect of attenuating the observed results. Another limitation of this study is the high attrition rate, not surprisingly greater in exercisers. However, our findings were robust in intention-to-treat analyses and completers’ analyses. If anything, participants remaining in the trial were healthier with less room to improve, and this would be expected to attenuate the results we observed.
Our findings have two important clinical implications. First, exercise training improved ABI and in individuals with uncomplicated T2DM with ABI <1.0, a level that is associated with increased cardiovascular morbidity and mortality. Though exercise training is a cornerstone treatment for glycemic control and cardiovascular disease risk reduction in T2DM, the additional benefit of increased ABI provides further support for prescribing exercise in these patients. Secondly, this effect was independent from changes in BP, lipids, and glycemia. Future research should investigate pathways through which exercise training improves blood flow in the lower legs, including the potential for improved collateral blood flow.
Supplementary Material
Highlights.
We studied the effect of exercise on ankle-brachial index (ABI) in type 2 diabetes
ABI increased in exercisers vs. controls over 6 months
An increase in ankle blood pressure explained the observed change in ABI
The increase in ABI was stronger in patients with lower baseline values (ABI<1.0)
The change in ABI was independent of changes in blood pressure or glycemia
Acknowledgements
Grants
This work was supported by grants from the National Institute for Diabetes, Digestive, and Kidney Disorders (R01 DK062368-04, 02/02/04 – 12/31/10), and Grant Number UL1 RR 025005 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Bethany Barone Gibbs, Email: bbarone@pitt.edu.
Devon A. Dobrosielski, Email: ddobrosielski@towson.edu.
Andrew D. Althouse, Email: ada25@pitt.edu.
Kerry J. Stewart, Email: kstewart@jhmi.edu.
REFERENCES
- 1.Allison MA, Ho E, Denenberg JO, Langer RD, Newman AB, Fabsitz RR, et al. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med. 2007 Apr;32(4):328–333. doi: 10.1016/j.amepre.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 2.McDermott MM, Greenland P, Liu K, Guralnik JM, Celic L, Criqui MH, et al. The ankle brachial index is associated with leg function and physical activity: the Walking and Leg Circulation Study. Ann Intern Med. 2002 Jun 18;136(12):873–883. doi: 10.7326/0003-4819-136-12-200206180-00008. [DOI] [PubMed] [Google Scholar]
- 3.Heald CL, Fowkes FG, Murray GD, Price JF. Risk of mortality and cardiovascular disease associated with the ankle-brachial index: Systematic review. Atherosclerosis. 2006 Nov;189(1):61–69. doi: 10.1016/j.atherosclerosis.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004 Aug 10;110(6):738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 5.Jude EB, Eleftheriadou I, Tentolouris N. Peripheral arterial disease in diabetes--a review. Diabet Med. 2010 Jan;27(1):4–14. doi: 10.1111/j.1464-5491.2009.02866.x. [DOI] [PubMed] [Google Scholar]
- 6.O'Hare AM, Katz R, Shlipak MG, Cushman M, Newman AB. Mortality and cardiovascular risk across the ankle-arm index spectrum: results from the Cardiovascular Health Study. Circulation. 2006 Jan 24;113(3):388–393. doi: 10.1161/CIRCULATIONAHA.105.570903. [DOI] [PubMed] [Google Scholar]
- 7.Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010 Dec;33(12):e147–e167. doi: 10.2337/dc10-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006 Mar 14;174(6):801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watson L, Ellis B, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2008;(4):CD000990. doi: 10.1002/14651858.CD000990.pub2. [DOI] [PubMed] [Google Scholar]
- 10.McDermott MM, Ades P, Guralnik JM, Dyer A, Ferrucci L, Liu K, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA. 2009 Jan 14;301(2):165–174. doi: 10.1001/jama.2008.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson AM, Sadrzadeh-Rafie AH, Myers J, Assimes T, Nead KT, Higgins M, et al. Low lifetime recreational activity is a risk factor for peripheral arterial disease. J Vasc Surg. 2011 Aug;54(2):427–432. 32, e1–e4. doi: 10.1016/j.jvs.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wattanakit K, Folsom AR, Selvin E, Weatherley BD, Pankow JS, Brancati FL, et al. Risk factors for peripheral arterial disease incidence in persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 2005 Jun;180(2):389–397. doi: 10.1016/j.atherosclerosis.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 13.Hooi JD, Kester AD, Stoffers HE, Overdijk MM, van Ree JW, Knottnerus JA. Incidence of and risk factors for asymptomatic peripheral arterial occlusive disease: a longitudinal study. Am J Epidemiol. 2001 Apr 1;153(7):666–672. doi: 10.1093/aje/153.7.666. [DOI] [PubMed] [Google Scholar]
- 14.Engstrom G, Ogren M, Hedblad B, Wollmer P, Janzon L. Asymptomatic leg atherosclerosis is reduced by regular physical activity. Longitudinal results from the cohort "men born in 1914". Eur J Vasc Endovasc Surg. 2001 Jun;21(6):502–507. doi: 10.1053/ejvs.2001.1359. [DOI] [PubMed] [Google Scholar]
- 15.Housley E, Leng GC, Donnan PT, Fowkes FG. Physical activity and risk of peripheral arterial disease in the general population: Edinburgh Artery Study. J Epidemiol Community Health. 1993 Dec;47(6):475–480. doi: 10.1136/jech.47.6.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobrosielski D, Barone Gibbs B, Ouyang P, Bonekamp S, Clark JM, Wang N, et al. Effect of Exercise on Blood Pressure in Type 2 Diabetes: A Randomized Controlled Trial. Journal of General Internal Medicine. 2012 doi: 10.1007/s11606-012-2103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003 May 21;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 18.Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA. American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc. 2004 Mar;36(3):533–553. doi: 10.1249/01.mss.0000115224.88514.3a. [DOI] [PubMed] [Google Scholar]
- 19.Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012 Dec 11;126(24):2890–2909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 20.Vanhees L, Rauch B, Piepoli M, van Buuren F, Takken T, Borjesson M, et al. Importance of characteristics and modalities of physical activity and exercise in the management of cardiovascular health in individuals with cardiovascular disease (Part III) Eur J Prev Cardiol. 2012 Dec;19(6):1333–1356. doi: 10.1177/2047487312437063. [DOI] [PubMed] [Google Scholar]
- 21.Wing RR, Jakicic J, Neiberg R, Lang W, Blair SN, Cooper L, et al. Fitness, fatness, and cardiovascular risk factors in type 2 diabetes: look ahead study. Med Sci Sports Exerc. 2007 Dec;39(12):2107–2116. doi: 10.1249/mss.0b013e31815614cb. [DOI] [PubMed] [Google Scholar]
- 22.Siscovick DS, Fried L, Mittelmark M, Rutan G, Bild D, O'Leary DH. Exercise intensity and subclinical cardiovascular disease in the elderly. The Cardiovascular Health Study. Am J Epidemiol. 1997 Jun 1;145(11):977–986. doi: 10.1093/oxfordjournals.aje.a009066. [DOI] [PubMed] [Google Scholar]
- 23.Kannel WB, Sorlie P. Some health benefits of physical activity. The Framingham Study. Arch Intern Med. 1979 Aug;139(8):857–861. [PubMed] [Google Scholar]
- 24.Adler AI, Stevens RJ, Neil A, Stratton IM, Boulton AJM, Holman RR, et al. UKPDS 59: Hyperglycemia and other potentially modifiable risk factors for peripheral vascular disease in type 2 diabetes. Diabetes Care. 2002 May;25(5):894–899. doi: 10.2337/diacare.25.5.894. [DOI] [PubMed] [Google Scholar]
- 25.Holman RR, Paul SK, Bethel MA, Neil HA, Matthews DR. Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med. 2008 Oct 9;359(15):1565–1576. doi: 10.1056/NEJMoa0806359. [DOI] [PubMed] [Google Scholar]
- 26.Vanhees L, Geladas N, Hansen D, Kouidi E, Niebauer J, Reiner Z, et al. Importance of characteristics and modalities of physical activity and exercise in the management of cardiovascular health in individuals with cardiovascular risk factors: recommendations from the EACPR. Part II. Eur J Prev Cardiol. 2012 Oct;19(5):1005–1033. doi: 10.1177/1741826711430926. [DOI] [PubMed] [Google Scholar]
- 27.Gibbs BB, Dobrosielski DA, Bonekamp S, Stewart KJ, Clark JM. A randomized trial of exercise for blood pressure reduction in type 2 diabetes: Effect on flow-mediated dilation and circulating biomarkers of endothelial function. Atherosclerosis. 2012 Oct;224(2):446–453. doi: 10.1016/j.atherosclerosis.2012.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green DJ. Exercise training as vascular medicine: direct impacts on the vasculature in humans. Exerc Sport Sci Rev. 2009 Oct;37(4):196–202. doi: 10.1097/JES.0b013e3181b7b6e3. [DOI] [PubMed] [Google Scholar]
- 29.Hamburg NM, Balady GJ. Exercise rehabilitation in peripheral artery disease: functional impact and mechanisms of benefits. Circulation. 2011 Jan 4;123(1):87–97. doi: 10.1161/CIRCULATIONAHA.109.881888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maiorana A, O'Driscoll G, Cheetham C, Dembo L, Stanton K, Goodman C, et al. The effect of combined aerobic and resistance exercise training on vascular function in type 2 diabetes. J Am Coll Cardiol. 2001 Sep;38(3):860–866. doi: 10.1016/s0735-1097(01)01439-5. [DOI] [PubMed] [Google Scholar]
- 31.Moriguchi J, Itoh H, Harada S, Takeda K, Hatta T, Nakata T, et al. Low frequency regular exercise improves flow-mediated dilatation of subjects with mild hypertension. Hypertens Res. 2005 Apr;28(4):315–321. doi: 10.1291/hypres.28.315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.