The identification of pigmented tissue within the oral cavity may present a diagnostic dilemma for the clinician. The manifestation of mucosal pigment is variable and can range from focal to diffuse macular coloration or from a small nodular growth to a large mass.1 Is the pigmentation of physiologic origin? Is it pathologic in etiology? Does it represent a malignant process? These are questions that typically come to mind when a patient presents with oral mucosal pigmentation. The color, location, duration, distribution, and appearance of the pigmented lesion(s) may be of diagnostic importance. A thorough investigation of dental, medical, family and social histories, is also necessary to ensure accurate diagnosis. The presence of cutaneous pigmentation or other systemic signs and symptoms may be helpful in formulating a differential diagnosis. Clinical laboratory testing may also be beneficial. However, if the underlying cause of the pigmentation cannot be readily identified, a tissue biopsy is essential for definitive diagnosis.
A differential diagnosis for a clinically pigmented lesion may include an array of traumatic, reactive, neoplastic pathologies as well as pigmentation associated with systemic disease.1 Lesions or conditions of melanocytic, vascular, hematopoietic, and hemosiderotic origin may be given consideration. Genetic dysfunction may also manifest as oral pigmentation; Peutz-Jeghers syndrome is the prototypical example.1 Where appropriate, exogenous substances including chemical coloring agents, amalgam, graphite, drug metabolites and chromogenic bacteria could also be considered.1 To this end, relatively common causes of mucosal coloration, including petechiae, purpura, ecchymoses, hematomas, vascular tumors and exogenous substances are not considered true pigmented lesions. In contrast, melanin, which is synthesized by melanocytes, is a true pigment and usually imparts a brown, blue or black appearance to the mucosa.2
Melanin pigmentation may be focal, multifocal or diffuse in its presentation. Melanin is the pigment derivative of tyrosine and is synthesized by melanocytes, which typically reside in the basal cell layer of the epithelium.2 In the skin, melanin is thought to be cytoprotective against the damaging effects of sunlight. The role of melanocytes in oral epithelium remains unclear. Unless a patient is of a race or ethnicity in which mucocutaneous pigmentation is physiologic, melanocytes are uncommonly observed in routine oral mucosal biopsies. Thus, in a Caucasian patient, oral melanocytic pigmentation may not always be of any significant clinical consequence but it is always considered pathologic in origin.
Pathologic melanin production within the oral mucosa may be associated with an array of etiologies. The most concerning of these are malignant melanoma and various systemic disorders, including adrenal insufficiency and Cushing disease (discussed below). Importantly, the oral manifestations of these potentially life-threatening disorders can mimic an array of idiopathic, reactive and benign neoplastic lesions. Thus, an understanding of the various disorders and substances that can contribute to oral mucosal coloration is essential for the appropriate evaluation, diagnosis and management of the patient. This article focuses on oral pathologies of melanocytic origin.
REACTIVE MELANOCYTIC PIGMENTATION
Melanotic macule
Melanotic macules are the most common oral mucosal lesions of melanocytic origin.3 These small, solitary, well-circumscribed and often uniformly pigmented lesions develop most commonly in adult female patients. Any mucosal site may be affected but the lower lip, gingiva and palate are the most common areas. Localized trauma may be a potential etiology but this remains uncertain.
Melanotic macules very rarely, if ever, present larger than one centimeter in diameter. Although these are innocuous lesions, a biopsy is usually warranted for diagnosis since mucosal melanoma can mimic the appearance of a melanotic macule.1 Once a diagnosis is rendered, no additional treatment is required and the lesion typically will not recur. Since most melanotic macules do present on the lip, patients often request complete removal due to esthetic concerns.
Melanotic macules are caused by functional hyperactivity of the regional melanocytes ie there is increased melanin production. Histologically, this is evidenced by abundant melanin pigmentation within the basal epithelial cell layer with melanin incontinence in the superficial portions of the submucosa.3 Importantly, there is no increase in melanocyte number. If there is evidence of melanocytic hyperplasia, it should be diagnosed as such and the lesion would likely warrant complete surgical removal. It is possible that melanocytic hyperplasia may potentially herald the development of malignant melanoma.1,3
The differential diagnosis for melanotic macule is often limited to entities that may present as focal, macular clinical pigmentation. In addition to melanoma, a melanocytic nevus, amalgam tattoo, and focal ecchymosis are usually given consideration. Oral mucosal melanomas have no defining clinical characteristics. Thus, a biopsy of any persistent solitary pigmented lesion is always warranted.
Oral melanoacanthoma
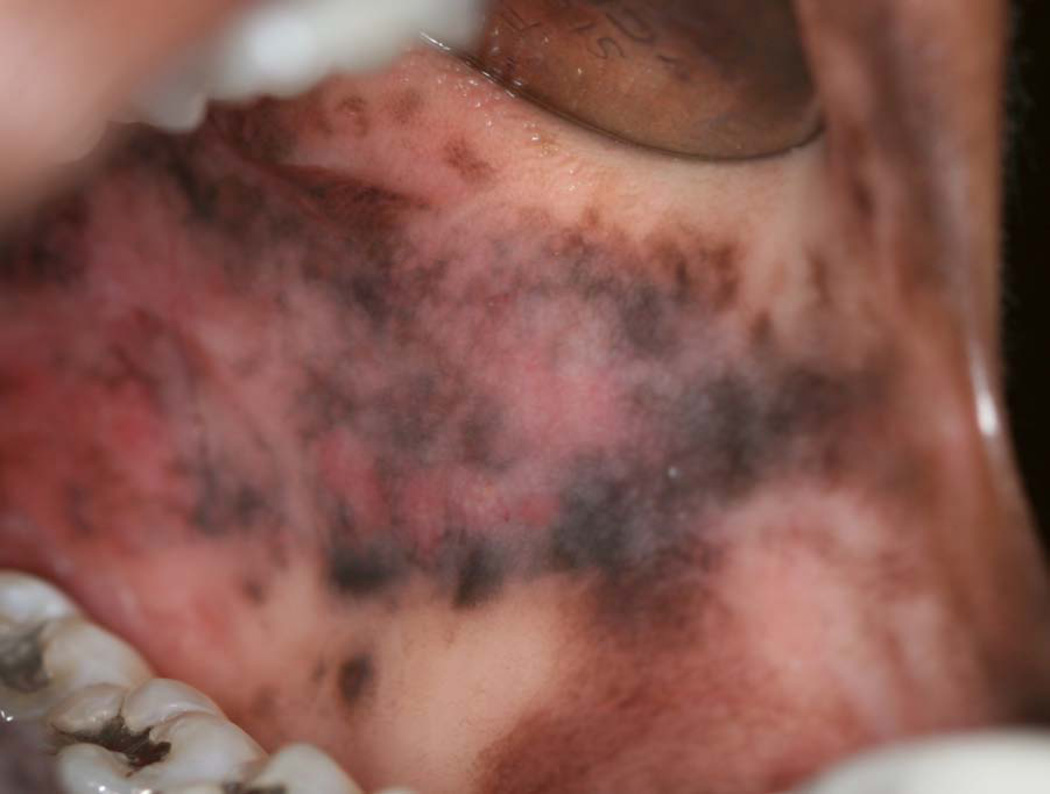
Oral melanoacanthoma is a relatively uncommon melanocytic lesion that may cause rapid, diffuse, and dark pigmentation of a large mucosal area.4 However, this is an innocuous pathology that is often self-limiting and may spontaneously resolve, with or without surgical intervention. Acute regional trauma or a history of chronic irritation may precede the development of the lesion.
Oral melanoacanthoma usually presents as an asymptomatic, ill-defined, rapidly enlarging, macular pigmentation, commonly observed in a black female patient. Although most lesions are heavily pigmented, the coloration may or may not be uniform. Any mucosal site may be affected, but buccal mucosal involvement is most common (Figure 1). Although typically solitary, rare patients may present with multifocal lesions.4
Figure 1.
Melanoacanthoma of the buccal mucosa. (Courtesy of Dr. Eric T. Stoopler, Philadelphia, PA.)
Oral melanoacanthoma is characterized by spongiotic epithelium containing dendritic, pigmented melanocytes extending through its full thickness.4 A mild inflammatory infiltrate composed of lymphocytes and occasional eosinophils is also seen.
Due to the ominous clinical presentation, melanoma is invariably considered in the differential diagnosis. Once a histologic diagnosis of oral melanoacanthoma has been established, no further treatment is required. The biopsy procedure itself may lead to spontaneous regression of the lesion.
Smoker’s melanosis
In addition to cancer and numerous other systemic complications, cigarette smoking can also induce oral mucosal pigmentation.5 Smoker’s melanosis is not considered a pre-neoplastic condition. Although the exact pathogenesis remains uncertain, melanin stimulation may represent a protective mucosal response to either the heat of the smoke or to an irritant within the cigarette. Females are most commonly affected. Smoker’s melanosis usually presents as diffuse but patchy and irregular pigmentation of the anterior facial maxillary and mandibular gingivae.5 Other mucosal sites are less commonly affected.
Histologically, the findings are non-specific in the form of abundant melanin within the basal cell layer with melanin incontinence. Similar histologic findings can be seen in melanotic macule, as well as in an array of other conditions that can present as diffuse pigmentation. A clinicopathologic correlation is required to make an accurate diagnosis of smoker’s melanosis. Importantly, melanoma can present as diffuse patchy pigmentation. Thus, if only one mucosal site is affected, melanoma should be considered in the differential diagnosis. Melanoma would not typically be considered in a patient with multifocal pigmentation affecting non-contiguous mucosal sites.
Inflammation-associated hyperpigmentation
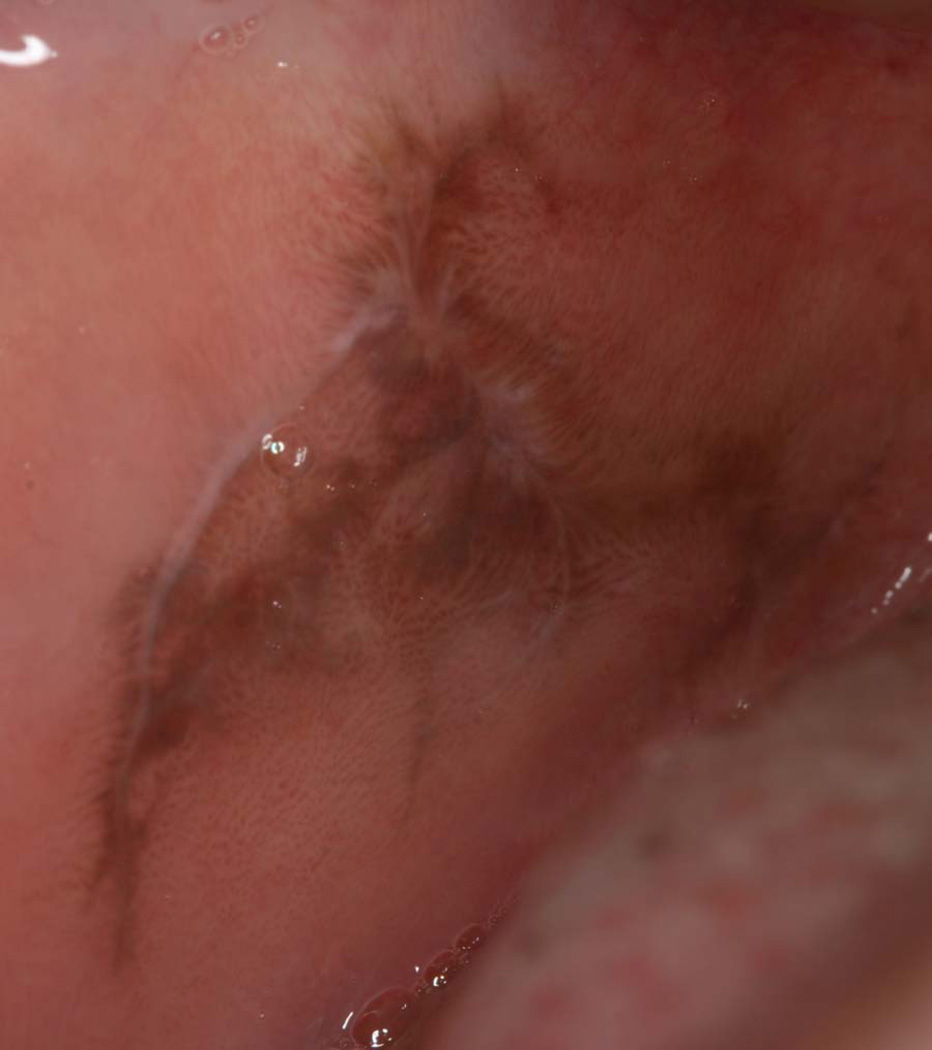
Inflammation-associated hyperpigmentation most commonly develops in dark-complexioned individuals.6 When it occurs in the skin, the pigmentation usually develops in an area previously subjected to trauma or inflammation such as in acne-prone areas of the face. In the oral cavity, this form of reactive pigmentation is most commonly observed in patients with clinical evidence of lichenoid inflammation.7 The pigment may be focal or diffuse and patchy, but is often regional to the lichenoid lesion (Figure 2). The coloration is often light brown. However, in rare instances, the pigmentation may become so dark that it obscures the underlying lichenoid pathology. In most cases, the clinical manifestation of the lichenoid lesion is usually what prompts the biopsy. In addition to the typical lichenoid histologic features, there is melanin pigmentation in the basal cell layer with melanin incontinence. Once a histologic diagnosis is rendered, treatment is aimed at resolution of the lichenoid inflammation, if the patient is symptomatic. The pigmentation may or may not disappear following treatment.
Figure 2.
Inflammation-induced hyperpigmentation associated with a lichenoid lesion of the buccal mucosa. (Courtesy of Dr. Eric T. Stoopler, Philadelphia, PA.)
Drug-induced melanosis
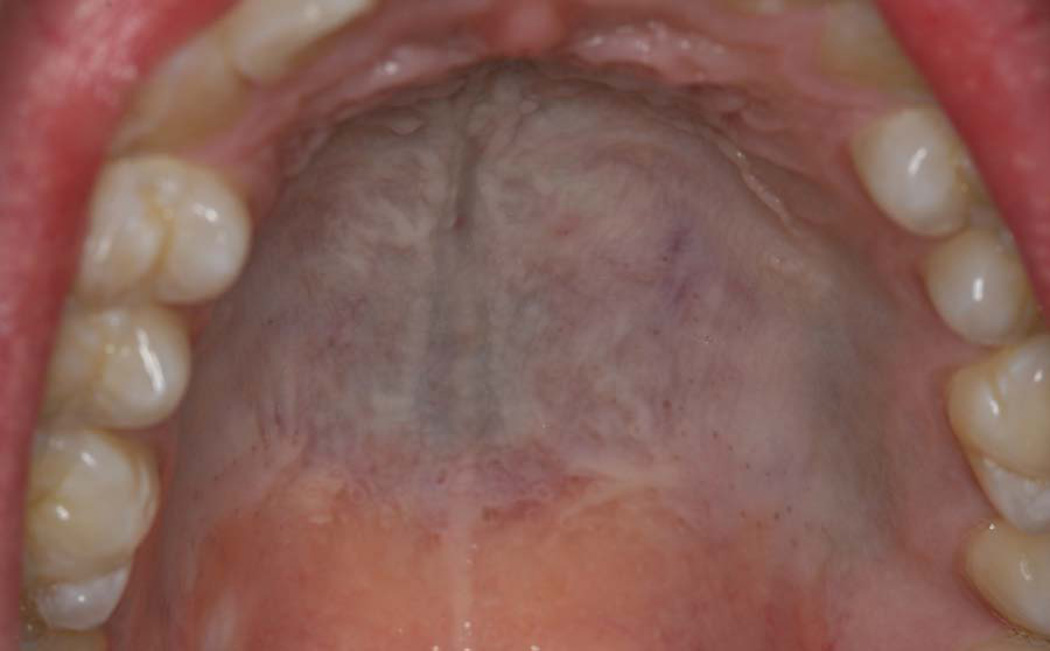
Medications may induce mucocutaneous coloration via a variety of different mechanisms.8 In some instances, drug metabolites may be incorporated or deposited into the regional tissues. While the mucosa may appear clinically pigmented, this is not true pigmentation. Drugs of the tetracycline family are representative examples of this phenomenon (Figure 3). In contrast, a host of medications, including antimalarial drugs, phenothiazines, oral contraceptives and various cytotoxic medications, induce true mucocutaneous pigmentation via induction of melanin.8 The mechanisms by which drugs induce melanin synthesis remain unclear. However, the mechanisms may vary between different drug classes. Some drugs, including chloroquine and chlorpromazine, have been shown to physically bind melanin.9 This results in retention of the drug within melanocytes, which may contribute to the increased pigmentation. Alternatively, it is possible that the drugs or specific drug metabolites may stimulate melanin synthesis.
Figure 3.
Tetracycline-induced melanosis of the palate. (Courtesy of Dr. Eric T. Stoopler, Philadelphia, PA.)
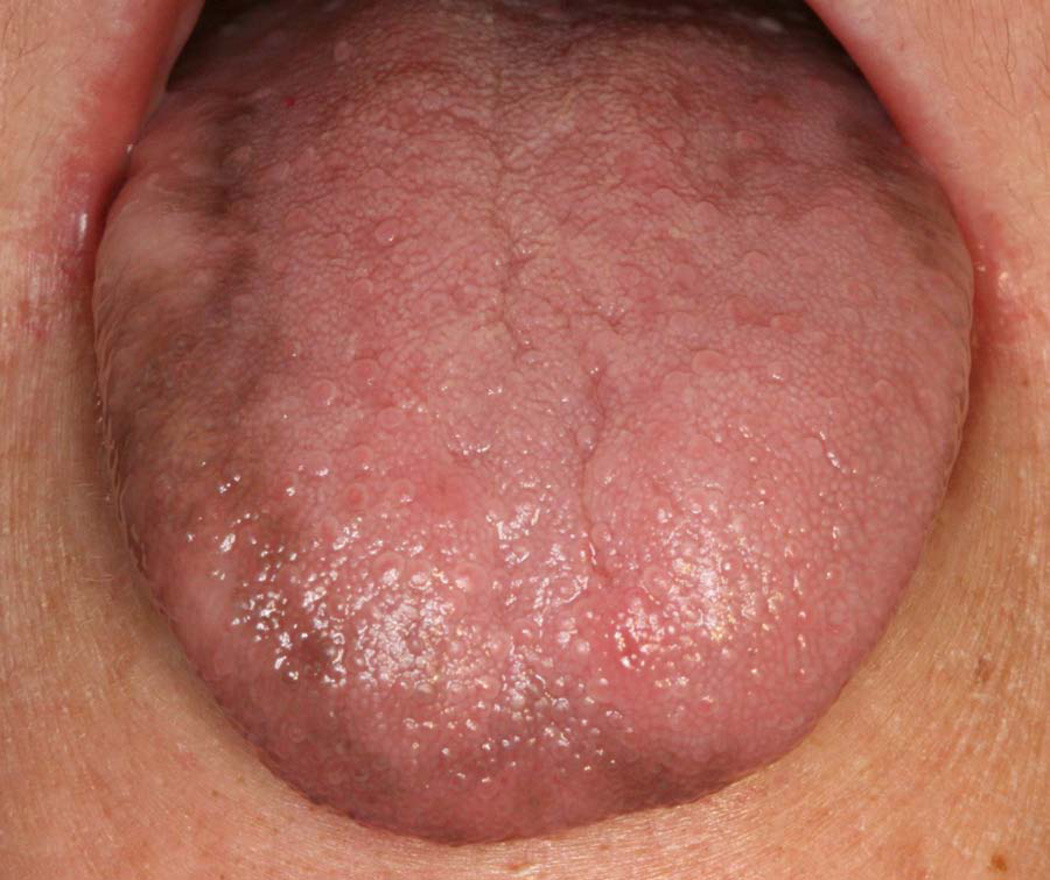
Drug-induced pigmentation may be diffuse but localized to one mucosal region, or it can be multifocal and involve multiple surfaces (Figure 4). The pigmentation is macular and may or may not be uniformly colored. Some drugs may be associated with a specific pattern of pigmentation. For example, hydroxychloroquine typically triggers pigmentation of the palatal mucosa.10
Figure 4.
Chemotherapy-induced pigmentation of the right dorsal tongue. (Courtesy of Dr. Eric T. Stoopler, Philadelphia, PA.)
A differential diagnosis includes other causes of diffuse mucosal pigmentation. A good clinical history and understanding about known drug side-effects may help in achieving an appropriate diagnosis. If the melanosis can be temporally associated with use of a specific medication that is known to induce pigmentation, then no further intervention is typically warranted. The discoloration may fade within several months after the medication is discontinued.8 Laboratory testing may be necessary to rule out an underlying endocrinopathy.
As with all other pigmented lesions, a biopsy is warranted if a diagnosis cannot be appropriately rendered. The histologic changes mimic those of melanotic macule; there is abundant melanin accumulation within the basal cell region with melanin incontinence. Thus, a clinico-pathologic correlation is often necessary to ensure accurate diagnosis.
NEOPLASTIC CAUSES OF MELANOCYTIC PIGMENTATION
Melanocytic nevus
Melanocytic nevi are a diverse group of benign tumors that arise as a consequence of melanocytic growth and proliferation.11 These lesions are uncommonly identified within the mucosa; the skin is much more commonly affected. The list of morphologically distinct nevi continues to expand. Among these, the intramucosal nevus is most frequently observed within the oral cavity; the blue nevus is the second most common.12 Less frequently observed are compound nevi and junctional nevi. Rare reports of oral melanocytic nevi exhibiting unique histologic patterns have also been described.13,14 Nonetheless, irrespective of the exact nevus subtype, they almost all present with similar clinical features.
Oral nevi are usually identified in patients over the age of thirty. The lesion is typically asymptomatic and may present as a small, solitary, brown or blue, well-circumscribed nodule or macule. Some nevi may not exhibit any evidence of clinical pigmentation.15 Any mucosal site may be affected, but the hard palate, buccal and labial mucosae and gingival are most typical.
In rare cases, oral nevi may be congenital or a manifestation of an unusual genetic disorder. More commonly, melanocytic nevi are acquired lesions, with genetic and environmental factors likely playing a role in their development. While cutaneous melanocytic nevi frequently exhibit somatic, activating mutations in the BRAF or NRAS oncogenes, it remains unclear if similar mutations are also implicated in the pathogenesis of oral mucosal nevi.16,17
Nevus cells are morphologically and biologically distinct from the melanocytes that are found within the basal layer of the oral epithelium. Nevus cells tend to be round, ovoid or spindled-shaped while the basal layer melanocytes are dendritic in appearance. Intramucosal, compound and junctional nevi likely share a common pathogenesis. It is theorized that over time, the junctional nevus evolves into a compound nevus and eventually into an intramucosal nevus. The histologic difference between these three variants relates to the location of the nevus cells within the tissues.
Blue nevi are not derived from the basal layer melanocytes.18 The lesional cells usually reside deep in the connective tissue giving the lesion a bluish tint. Several histologic variants of blue nevus exist but the most prevalent is the ‘common’ blue nevus.13,18 The ‘cellular’ blue nevus has also been reported within the oral cavity.18 Unlike the common blue nevus, cellular blue nevi may behave more aggressively and exhibit a greater rate of recurrence.
Since the differential diagnosis of focally pigmented lesions includes malignant melanoma, biopsy is required for accurate diagnosis of an oral melanocytic nevus. While malignant transformation of cutaneous blue nevi has been reported, this phenomenon has not been reliably documented for oral blue nevi. Nonetheless, conservative surgical excision is the treatment of choice for these oral lesions.
Malignant melanoma
Malignant melanoma is a cancer arising from malignant melanocytes.19 Melanoma is the most deadly primary skin cancer. These cancers exhibit even poorer prognosis when they occur in mucosal sites, including within the oral cavity. The occurrence of oral mucosal melanoma is the primary reason why all focally pigmented lesions and most diffusely pigmented lesions require a biopsy for diagnosis.
Primary oral mucosal melanomas make up less than 1% of total melanoma cases.20 These cancers typically occur over the age of 50 years and males may be more commonly affected. Oral melanoma can occur in any racial and ethnic group, but the highest incidence appears to be in Japanese patients.21 The most commonly affected sites include the hard palate and maxillary gingiva.
Oral melanoma can present as a macule, a plaque or a mass. It can be well-circumscribed or irregular, and focally or diffusely pigmented, and even lacking pigment (amelanotic). Occasional tumors may exhibit multifocal pigmentation due to the presence of melanotic and amelanotic areas within the same lesion. Diffuse but contiguous mucosal pigmentation should elicit more concern for a possible melanoma than would diffuse but non-contiguous pigmentation. Other non-specific signs and symptoms of cancer may include ulceration, pain, paresthesia or anesthesia, tooth mobility or spontaneous exfoliation, root resorption, and/or bone loss.19 In some cases, the patients may be asymptomatic. It is apparent that mucosal melanomas have no distinctive appearance. Since the differential diagnosis may be rather extensive, biopsy of any persistent solitary pigmented lesion is essentially mandatory.
Microscopically, oral mucosal melanomas are usually characterized by malignant melanocytes that are often observed within the connective tissue. Extension of the malignant cells into the epithelium (Pagetoid spread) may also be seen. Unlike cutaneous melanomas for which a series of histologic parameters, including a measure of tumor thickness, can be used to reliably predict prognosis, no such parameters reliably exist for oral melanoma.21 Once diagnosed, the next clinical challenge is to determine if the lesion represents a primary malignancy or represents a metastasis from a distant site. A reliable determination of the tumor’s primary anatomic site is critical since it will dictate tumor staging and guide therapy.
Unlike cutaneous melanoma where exposure to sunlight and specific gene mutations are known to play important roles in pathogenesis, the etiology and molecular mechanisms that give rise of oral mucosal melanoma remain poorly characterized.22 Activating BRAF and NRAS mutations are prevalent in a subset of cutaneous melanomas. Identifying BRAF-mutant tumors is particularly important because BRAF kinase inhibitors, including vemurafenib are currently being used in melanoma treatment, and often with favorable results.22 Since BRAF mutations are very rarely identified in mucosal melanoma, similar chemotherapeutic approaches are not useful. Accordingly, surgical resection remains the primary mode of therapy.22,23 Adjuvant radiation therapy and chemotherapy may be required for some patients. Since these tumors are very aggressive, high local tumor burden and regional lymph node metastases are often identified at the time of initial diagnosis. Studies have demonstrated 5-year survival rates of 15–40%.20–22
MULTI-FOCAL / DIFFUSE PIGMENTATION
Physiologic pigmentation is the most common cause of multi-focal or diffuse oral mucosal pigmentation. However, several pathologic sources may induce similar patterns of pigmentation. In addition to drug-induced pigmentation and smoking-induced melanosis described earlier, a differential diagnosis of diffuse or multi-focal mucosal hyperpigmentation may include endocrinopathies such as hypoadrenocorticism and Cushing disease, genetic dysfunction (Peutz-Jeghers syndrome) and idiopathic etiology (Laugier-Hunziker pigmentation). Other systemic associations have also been reported including HIV infection, Graves disease, primary biliary cirrhosis and vitamin B12 insufficiency.24 However the actual mechanisms by which these latter conditions trigger melanosis remain unclear and are excluded from this discussion.
While a biopsy may be warranted to ensure the source of the pigment is not melanoma, a microscopic diagnosis is insufficient for diagnosis of the aforementioned conditions. This is because the histologic findings are non-specific and mimic those seen in melanotic macule, druginduced pigment and smoker’s melanosis. Therefore, a clinic-pathologic correlation accompanied by a thorough history and review of systems, physical exam, and additional laboratory testing, are often necessary to ensure accurate diagnosis.
Hypoadrenocorticism (adrenal insufficiency, Addison disease)
Hypoadrenocorticism is a potentially life-threatening disease that may be associated with a variety of etiologies.25 Adrenal destruction or impairment may be caused by trauma, autoimmune disease, infectious agents, neoplasia, genetic disease, certain medications and iatrogenic causes. Irrespective of the mechanism, the adrenal dysfunction results in a decrease in endogenous corticosteroid levels.
The adrenal gland is linked to the pituitary gland via a critical molecular feedback mechanism. As corticosteroid levels decrease, the anterior pituitary gland is naturally stimulated to increase synthesis and secretion of adrenocorticotropic hormone (ACTH).25 ACTH then acts on the adrenal cortex to stimulate corticosteroid production. This feedback loop then causes the pituitary to slow ACTH secretion. However, if low corticosteroid levels persist, there is a persistent ACTH secretion. The alpha-melanocyte-stimulating hormone (α-MSH) gene is an alternatively spliced gene originating from the same host gene that harbors ACTH. Thus, as serum ACTH levels rise, there is a concomitant increase in α-MSH secretion. In addition to its many functions, α-MSH is thought to directly stimulate melanocytes. This induces the cells to increase melanin production. Clinically, this manifests as diffuse mucocutaneous pigmentation. In the oral cavity, this usually presents as diffuse but patchy melanosis of multiple mucosal surfaces.
Mucocutaneous pigmentation is often one of the earliest clinical manifestations of hypoadrenocorticism. Hypoadrenocorticism is also associated with an array of other systemic signs and symptoms. These may include hypotension, easy bruising, fatigue, mood swings, depression, and weakness. Hyponatremia and hyperkalemia are frequent laboratory findings associated with adrenal insufficiency.25 Thus, if hypoadrenocorticism is included in the differential diagnosis, laboratory testing, including the evaluation of serum cortisol and electrolyte levels, is mandatory to ensure accurate diagnosis. Ultimately, a diagnosis of hypoadrenocorticism-associated pigmentation requires a clinico-pathologic correlation. With appropriate steroid replacement therapy, the pigmentation and other signs and symptoms will typically resolve.
Cushing disease
Cushing disease is caused by a primary activating pituitary pathology (usually neoplastic) that leads to continuous secretion of ACTH and α-MSH into the blood stream.26 However, since the pathology resides in the pituitary, there is no negative feedback loop. While diffuse mucocutaneous pigmentation may be one of the first signs of the disorder, affected individuals will also manifest an array of complications associated with pathologically elevated serum corticosteroid levels.
It is important to note that Cushing disease is not equivalent to Cushing syndrome (hyperadrenocorticism).26 While Cushing disease may be a cause of Cushing syndrome, not all forms of Cushing syndrome are caused by a primary pituitary disease. Cushing syndrome results from prolonged exposure to high concentrations of endogenous or exogenous corticosteroids.27 Iatrogenic causes are the most prevalent cause Cushing syndrome. A primary hyperfunctional adrenal pathology, or neoplasms, including small cell carcinomas, may also lead to aberrantly high levels of corticosteroids. In these latter instances, diffuse pigmentation would not be a clinical complication since ACTH / α-MSH expression would be either unaffected or even suppressed. However, if ACTH / α-MSH are pathologically increased via extra-pituitary mechanisms, including through aberrant expression by neoplasms in other sites, then the pigment may manifest.27
Patients with Cushing disease may present with a wide array of systemic complications resulting from excessive ACTH / α-MSH secretion, increased corticosteroid levels, and likely high levels of other hormones, including growth hormone, being released from the pituitary neoplasm. Weight gain, hypertension, diabetes mellitus, osteoporosis, dyslipidemia and the “moon facies” and are among the most common signs of disease.28 The irregular pattern of the oral pigmentation is similar to that seen in patients with other forms of diffuse or multi-focal hyperpigmentation.
Laboratory testing, including the evaluation of serum cortisol and ACTH levels, is mandatory to ensure accurate diagnosis. Like hypoadrenocorticism, a diagnosis of Cushing disease-associated pigmentation requires a clinico-pathologic correlation. The pigmentation will eventually resolve provided the underlying source of the disease is appropriately treated.
Peutz-Jeghers syndrome
Peutz-Jeghers syndrome is a rare genetic disease associated with germline mutations in the STK11/LKB1 tumor suppressor gene.29 This autosomal dominant disorder is characterized by intestinal polyposis and increased susceptibility to an array of different cancer types. A highly distinctive pattern of labial, perioral and acral macular pigmentation represents one of the earliest clinical manifestations of this disease (Figure 5). The macular pigmentation usually mimics dark freckling but without the reliance of sun exposure to increase or diminish color intensity.29 Pigmentation may also occur inside the mouth, but this is not common.30 The lip and perioral pigmentation are highly distinctive, although not pathognomonic for this disease (see Laugier-Hunziker pigmentation).31
Figure 5.
Typical pattern of pigmentation representative of Peutz Jeghers syndrome. (Courtesy of Dr. Eric T. Stoopler, Philadelphia, PA.)
Peutz-Jeghers disease should be given strong consideration as a potential diagnosis in a young child or adolescent who presents with diffuse labial and perioral pigmentation. An appropriate family history may increase the index of suspicion. A complaint of rectal bleeding may warrant referral to a gastroenterologist for evaluation. Therapy in these patients is directed at alleviating the gastrointestinal complications and cancer surveillance. The pigmentation will persist throughout these patients’ lives and will not resolve without therapeutic intervention. Laser therapy may be beneficial to diminish the esthetic concerns.
Laugier-Hunziker pigmentation
Laugier-Hunziker pigmentation is typically characterized by multi-focal pigmentation of the labial and buccal mucosae.31,32 Pigmentation of other mucosal surfaces within the mouth, perioral skin and other anatomic areas, including the esophagus, genitalia and conjunctiva may also be seen.31 The majority of affected patients also present with melanotic streaks involving the fingernails; there is no associated nail dystrophy. In rare cases, acral skin surfaces may also manifest the pigmentary changes.
This idiopathic pigmentation is usually identified in adult patients of either sex. It remains unclear if there is any distinct racial predilection. Patients usually present with multiple, small, discrete, irregular darkly pigmented macules. When the pigment occurs on the labial and acral surfaces, it may mimic the freckling that is often seen in Peutz-Jeghers syndrome.31
When encountered with a patient with multi-focal pigmentation involving multiple mucosal and cutaneous surfaces, it is critical to confirm a lack of other systemic signs or symptoms, including rectal bleeding, hypotension, hypertension, fatigue, easy bruising, and mood swings. Thorough medical, social and family histories are also required to ensure accurate empirical diagnosis. Laugier-Hunziker pigmentation is a diagnosis of exclusion and only rendered once all other potential sources for the pigmentation are eliminated from consideration.31 The primary complaint is usually one of esthetics. Laser therapy may be beneficial, but most cases require no treatment.
SUMMARY
Oral pigmentation may be focal, multifocal or diffuse. The lesions may be blue, purple, brown, gray, or black. They may be macular or tumefactive. Importantly, some are localized harmless accumulations of melanin, hemosiderin, or exogenous metal; others are harbingers of systemic or genetic disease, and some can be associated with life-threatening medical conditions that require immediate intervention. The differential diagnosis for any pigmented lesion can be quite extensive, and can include examples of endogenous and exogenous pigmentation. Although biopsy is a helpful and necessary aid in the diagnosis of focally pigmented lesions, with diffuse presentations lesions will require a thorough history and laboratory studies in order to establish a definitive diagnosis.
Key points.
Pigmented lesions within the oral cavity may present a diagnostic dilemma for the clinician.
A differential diagnosis for a pigmented lesion may include traumatic, reactive, neoplastic and systemic pathologies.
Mucosal melanomas have no distinctive appearance.
A clinico-pathologic correlation is often required to ensure accurate diagnosis of systemic causes of diffuse pigmentation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lenane P, Powell FC. Oral pigmentation. J Eur Acad Dermatol Venereol. 2000;14:448–465. doi: 10.1046/j.1468-3083.2000.00143.x. [DOI] [PubMed] [Google Scholar]
- 2.Westerhof W. The discovery of the human melanocyte. Pigment Cell Res. 2006;19:183–193. doi: 10.1111/j.1600-0749.2006.00313.x. [DOI] [PubMed] [Google Scholar]
- 3.Kaugars GE, Heise AP, Riley WT, Abbey LM, Svirsky JA. Oral melanotic macules. A review of 353 cases. Oral Surg Oral Med Oral Pathol. 1993;76:59–61. doi: 10.1016/0030-4220(93)90295-f. [DOI] [PubMed] [Google Scholar]
- 4.Fornatora ML, Reich RF, Haber S, Solomon F, Freedman PD. Oral melanoacanthoma: a report of 10 cases, review of the literature, and immunohistochemical analysis for HMB-45 reactivity. Am J Dermatopathol. 2003;25:12–15. doi: 10.1097/00000372-200302000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Taybos G. Oral changes associated with tobacco use. Am J Med Sci. 2003;326:179–182. doi: 10.1097/00000441-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Nordlund JJ. Postinflammatory hyperpigmentation. Dermatol Clin. 1988;6:185–192. [PubMed] [Google Scholar]
- 7.Laskaris GC, Papavasiliou SS, Bovopoulou OD, Nicolis GD. Lichen planus pigmentosus of the oral mucosa: a rare clinical variety. Dermatologica. 1981;162:61–63. doi: 10.1159/000250234. [DOI] [PubMed] [Google Scholar]
- 8.Dereure O. Drug-induced skin pigmentation. Epidemiology, diagnosis and treatment. Am J Clin Dermatol. 2001;2:253–262. doi: 10.2165/00128071-200102040-00006. [DOI] [PubMed] [Google Scholar]
- 9.Mars U, Larsson BS. Pheomelanin as a binding site for drugs and chemicals. Pigment Cell Res. 1999;12:266–274. doi: 10.1111/j.1600-0749.1999.tb00760.x. [DOI] [PubMed] [Google Scholar]
- 10.Kleinegger CL, Hammond HL, Finkelstein MW. Oral mucosal hyperpigmentation secondary to antimalarial drug therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:189–194. doi: 10.1067/moe.2000.106340. [DOI] [PubMed] [Google Scholar]
- 11.Krengel S. Nevogenesis--new thoughts regarding a classical problem. Am J Dermatopathol. 2005;27:456–465. doi: 10.1097/01.dad.0000175532.27368.3f. [DOI] [PubMed] [Google Scholar]
- 12.Buchner A, Leider AS, Carpenter WM, Littner MM. Melanocytic nevi of the oral mucosa--a clinicopathologic study of 60 new cases. Refuat Hashinayim. 1990;8:3–8. [PubMed] [Google Scholar]
- 13.Pinto A, Raghavendra S, Lee R, Derossi S, Alawi F. Epithelioid blue nevus of the oral mucosa: a rare histologic variant. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:429–436. doi: 10.1016/s1079-2104(03)00319-6. [DOI] [PubMed] [Google Scholar]
- 14.Damm DD, White DK, Lyu PE, Puno P. Balloon cell nevus of the oral mucosa. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:755–757. doi: 10.1016/j.tripleo.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Buchner A, Hansen LS. Pigmented nevi of the oral mucosa: a clinicopathologic study of 36 new cases and review of 155 cases from the literature. Part II: Analysis of 191 cases. Oral Surg Oral Med Oral Pathol. 1987;63:676–682. doi: 10.1016/0030-4220(87)90370-7. [DOI] [PubMed] [Google Scholar]
- 16.Gill M, Celebi JT. B-RAF and melanocytic neoplasia. J Am Acad Dermatol. 2005;53:108–114. doi: 10.1016/j.jaad.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Cohen Y, Goldenberg-Cohen N, Akrish S, et al. BRAF and GNAQ mutations in melanocytic tumors of the oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:778–784. doi: 10.1016/j.oooo.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Harvell JD, White WL. Persistent and recurrent blue nevi. Am J Dermatopathol. 1999;21:506–517. doi: 10.1097/00000372-199912000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Mohan M, Sukhadia VY, Pai D, Bhat S. Oral malignant melanoma: systematic review of literature and report of two cases. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012 doi: 10.1016/j.oooo.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 20.Mendenhall WM, Amdur RJ, Hinerman RW, Werning JW, Villaret DB, Mendenhall NP. Head and neck mucosal melanoma. Am J Clin Oncol. 2005;28:626–630. doi: 10.1097/01.coc.0000170805.14058.d3. [DOI] [PubMed] [Google Scholar]
- 21.McLean N, Tighiouart M, Muller S. Primary mucosal melanoma of the head and neck. Comparison of clinical presentation and histopathologic features of oral and sinonasal melanoma. Oral Oncol. 2008;44:1039–1046. doi: 10.1016/j.oraloncology.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Postow MA, Hamid O, Carvajal RD. Mucosal melanoma: pathogenesis, clinical behavior, and management. Curr Oncol Rep. 2012;14:441–448. doi: 10.1007/s11912-012-0244-x. [DOI] [PubMed] [Google Scholar]
- 23.Chan RC, Chan JY, Wei WI. Mucosal melanoma of the head and neck: 32-year experience in a tertiary referral hospital. Laryngoscope. 2012;122:2749–2753. doi: 10.1002/lary.23625. [DOI] [PubMed] [Google Scholar]
- 24.Alawi F. Pigmented lesions of the oral mucosa. In: Greenberg MS, Glick M, Ship JA, editors. Burket’s Oral Medicine, Eleventh Edition. Hamilton: BC Decker, Inc; 2008. [Google Scholar]
- 25.Shah SS, Oh CH, Coffin SE, Yan AC. Addisonian pigmentation of the oral mucosa. Cutis. 2005;76:97–99. [PubMed] [Google Scholar]
- 26.Castinetti F, Morange I, Conte-Devolx B, Brue T. Cushing's disease. Orphanet J Rare Dis. 2012;7:41. doi: 10.1186/1750-1172-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newell-Price J, Bertagna X, Grossman AB, Nieman LK. Cushing's syndrome. Lancet. 2006;367:1605–1617. doi: 10.1016/S0140-6736(06)68699-6. [DOI] [PubMed] [Google Scholar]
- 28.Feelders RA, Pulgar SJ, Kempel A, Pereira AM. The burden of Cushing's disease: clinical and health-related quality of life aspects. Eur J Endocrinol. 2012;167:311–326. doi: 10.1530/EJE-11-1095. [DOI] [PubMed] [Google Scholar]
- 29.Shah KR, Boland CR, Patel M, Thrash B, Menter A. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68:189–221. doi: 10.1016/j.jaad.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 30.Higham P, Alawi F, Stoopler ET. Medical management update: Peutz Jeghers syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:5–11. doi: 10.1016/j.tripleo.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 31.Lampe AK, Hampton PJ, Woodford-Richens K, Tomlinson I, Lawrence CM, Douglas FS. Laugier-Hunziker syndrome: an important differential diagnosis for Peutz-Jeghers syndrome. J Med Genet. 2003;40:e77. doi: 10.1136/jmg.40.6.e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mignogna MD, Lo ML, Ruoppo E, Errico M, Amato M, Satriano RA. Oral manifestations of idiopathic lenticular mucocutaneous pigmentation (Laugier-Hunziker syndrome): a clinical, histopathological and ultrastructural review of 12 cases. Oral Dis. 1999;5:80–86. doi: 10.1111/j.1601-0825.1999.tb00068.x. [DOI] [PubMed] [Google Scholar]