Abstract
Numerous factors impact patients’ health beyond traditional clinical characteristics. We evaluated the association of risk factors in kidney transplant patients’ communities with outcomes prior to transplantation. The primary exposure variable was a community risk score(range 0–40) derived from multiple databases and defined by factors including prevalence of comorbidities, access and quality of healthcare, self-reported physical and mental health and socioeconomic status for each US county. We merged data with the Scientific Registry of Transplant Recipients(SRTR) and utilized risk-adjusted models to evaluate effects of community risk for adult candidates listed 2004–2010(n=209,198). Patients in highest-risk communities were associated with increased mortality(Adjusted Hazard Ratio[AHR]=1.22,1.16–1.28), decreased likelihood of living donor transplantation(Adjusted Odds Ratio[AOR]=0.90,0.85–0.94), increased waitlist removal for health deterioration(AHR=1.36,1.22–1.51), decreased likelihood of preemptive-listing(AOR=0.85,0.81–0.88), increased likelihood of inactive listing(AOR=1.49,1.43–1.55) and increased likelihood of listing for expanded criteria donor kidneys(AHR=1.19,1.15–1.24). Associations persisted with adjustment for rural-urban location; furthermore the independent effects of rural-urban location were largely eliminated with adjustment for community risk. Average community risk varied widely by region and transplant center(median=21, range 5–37). Community risks are powerful factors associated with processes of care and outcomes for transplant candidates and may be important considerations for developing effective interventions and measuring quality of care of transplant centers.
Introduction
There are numerous factors that impact patient outcomes beyond traditional clinical characteristics. These factors include individual behavior, environmental conditions, genetic and biological factors not typically captured in databases, socioeconomic status, mental health and access to and quality of healthcare (1–7). Modifiable behavioral risk factors, including tobacco use, poor diet and inactivity, and alcohol consumption, explain a substantial proportion of deaths in the United States (8). In the context of kidney transplantation, there is evidence that individual behavior and socioeconomic status are important factors associated with patient outcomes (9–13). Understanding the etiology of patient outcomes is important in order to develop effective interventions and potentially tailoring treatment protocols to address individual patient needs.
In the past several years, the University of Wisconsin Population Health Institute in conjunction with the Robert Wood Johnson Foundation has developed a project entitled County Health Rankings and Roadmaps (14). The project includes publicly available data aggregated from national surveys and registries including The National Center for Chronic Disease Prevention and Health Promotion, the National Center for Health Statistics, the Behavioral Risk Factor Surveillance System, Medicare/Dartmouth Institute and the Census Bureau for virtually every county in the United States. The data characterize communities based on access and quality of care, prevalence of comorbid conditions, environmental hazards and behavioral attributes of individuals within counties. Based on these data, in a prior study, kidney transplant recipients from highest risk communities had 26% increased hazard for post-transplant mortality independent of known patient, donor and clinical risk factors (15). These results along with emerging evidence in a variety of healthcare contexts suggests that conditions in patients’ communities are highly influential factors associated with patient outcomes.
For this study, we hypothesized that community risk factors are significantly associated with patient prognoses prior to transplantation. Our primary aims were to evaluate whether both clinical outcomes, such as mortality on the waiting list, and processes of care, such as placement on the waiting list preemptively or as inactive status, would be significantly associated with the presence of risk factors in patients’ community. We sought to quantify these associations and also describe variability in the presence of risk factors by transplant center and region of the country.
Methods
Data sources
This study used data from the Scientific Registry of Transplant Recipients(SRTR). The SRTR data system includes data on all donors, wait-listed candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere (16). The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. In addition, the study utilized data from the County Health Rankings project (14). The information compiled in the County Health Rankings database is aggregated between 2004 and 2010; therefore, we included adult (age 18+) candidates listed for a solitary kidney transplant in the same period from the SRTR. We merged the two databases based on the zip code of the permanent residence of transplant candidates at the time of listing and the applicable county from the County Health Rankings database. Finally, in order to understand whether outcomes were independent of rural/urban location of candidates, we also merged data with the Rural-Urban Continuum codes developed by the Economic Research Service by the United States Department of Agriculture (17). These codes categorize the level of rural to urban status based on a 9-point ordinal scale ranging from “County in metro area with 1 million population or more” to “Non-metro county completely rural or less than 2,500 urban population.”
Statistical Analyses
The primary explanatory variable was a previously published community risk score that was based on ten factors attained from the County Health Rankings data(Table 1) (15). The score was calculated based on the quintile of each of these factors (0 points for levels in the first 20th percentile and 1 point for each subsequent quintile) present in patients’ residential county. Of note, points for median household income were inversely incorporated into the score (i.e. the highest income quintile received 0 points). The cumulative score ranged from 0–40 with 40 representing the highest possible risk (15). We displayed outcome measures based on every ten units of the risk score as well as evaluating the score as a continuous linear effect. The outcome measures for the present study were (a) wait list mortality (considered as intent-to-treat including patients de-listed, and censored at the time of transplantation or last follow up), (b) time to either living or deceased donor transplantation (censored at last follow up, wait list removal or death), (c) likelihood of living donor transplantation among candidates receiving a transplant, (d) time to wait list removal for health deterioration (censored at death, last follow up and date of transplant), (e) likelihood of preemptive listing (prior to dialysis initiation), (f) likelihood of initial listing for expanded criteria donors(ECD) and (g) likelihood of initial placement on the waiting list as inactive. In addition, we evaluated time to living and deceased donor transplantation separately censored at the other form of transplantation.
Table 1.
Distribution of Community Risk Factors in the Study Population (n=209,198)
| Community Risk Factor | 20th percentile | 40th percentile | 60th percentile | 80th percentile |
|---|---|---|---|---|
| Low Birth Weight (%) | 6.9 | 7.6 | 8.3 | 9.2 |
| Preventable Hospital Stay Rate | 55.8 | 65.3 | 72.6 | 83.9 |
| Smoking (%) | 14.7 | 17.5 | 20.1 | 23.1 |
| Obesity (%) | 22.5 | 25.5 | 27.8 | 30.0 |
| Physical Inactivity (%) | 19.5 | 22.6 | 24.9 | 28.0 |
| Poor Physical Health Days | 3.0 | 3.3 | 3.5 | 3.8 |
| Poor Mental Health Days | 2.9 | 3.2 | 3.5 | 3.7 |
| Potential Life Years Lost | 5618 | 6713 | 7531 | 8756 |
| Self-reported days in Fair or Poor Health | 12.6 | 14.6 | 17.3 | 19.5 |
| Annual Household Median Income (in $1000s) | 43.2 | 48.8 | 55.3 | 66.8 |
For time-dependent outcomes, we calculated rates (per 1000 patient years) and developed multivariable Cox proportional hazard models including risk factors at the time of listing in the SRTR. These included candidate age, race, gender, body mass index(BMI), panel reactive antibody(PRA)level, primary cause of End-Stage Renal Disease(ESRD), primary insurance type, prior transplantation, educational attainment and blood type. Missing values of covariates were categorized as an individual level in the models and as such no patients were excluded in the models on the basis of missing data.
We also included state of residence as an indicator variable in order to mitigate the effect of regional differences independent of risks within individual counties. We tested the proportionality assumption of the Cox models by examining the complementary log-log survival plots of the primary exposure variable. For dichotomous outcomes that were not time-dependent, we developed multivariable logistic models adjusted for the same list of covariates. As patients were clustered within counties, we included adjustment for dependency with the robust sandwich estimator in Cox models and utilized counties as a hierarchical random effect in logistic models. All analyses were conducted in SAS (version 9.2., Cary, N.C).
Results
Study Population and the Community Risk Score
There were 209,198 adult solitary kidney transplant candidates with available zip code information in the SRTR after excluding 279 patients without matching zip codes in the County Health Rankings database. The distribution of the ten community risk factors for the study population is depicted in Table 1. The average community risk score for candidates was 20.0(standard deviation =10.2). Table 2 displays the proportion of patients by community risk score stratified by candidate characteristics. As indicated, younger age, hypertension, higher BMI, African American race, public primary insurance and lower educational attainment were associated with residence in higher risk communities.
Table 2.
Distribution of Community Risk Score by Transplant Candidate Characteristics
| Candidate Characteristic | Level | N (%) | Community Risk Score (% of patients in each group) | p-value** | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 0–10 | 11–20 | 21–30 | 31–40 | ||||
|
| |||||||
| Age at listing | 18–34 | 27175 (13) | 12 | 13 | 13 | 14 | <0.001 |
| 35–54 | 89757 (43) | 41 | 42 | 44 | 44 | ||
| 55–64 | 60202 (29) | 29 | 29 | 29 | 29 | ||
| 65+ | 32064 (15) | 18 | 16 | 15 | 13 | ||
|
| |||||||
| Primary Cause of Kidney Failure | Glomerulonephritis | 41051 (20) | 22 | 20 | 19 | 19 | <0.001 |
| Diabetes | 62490 (30) | 30 | 29 | 31 | 29 | ||
| Polycystic Kidney Disease | 15818 (8) | 8 | 8 | 7 | 7 | ||
| Hypertension | 45065 (22) | 16 | 20 | 23 | 27 | ||
| Other/missing | 44771 (21) | 23 | 23 | 20 | 19 | ||
|
| |||||||
| Body mass index (kg/m2) | 13–19 | 10949 (5) | 6 | 5 | 5 | 4 | <0.001 |
| 20–24 | 54440 (26) | 29 | 27 | 25 | 23 | ||
| 25–29 | 67859 (32) | 33 | 33 | 32 | 32 | ||
| 30–34 | 45566 (22) | 19 | 21 | 22 | 24 | ||
| 35+ | 25018 (12) | 10 | 11 | 13 | 14 | ||
| missing | 5368 (3) | 3 | 2 | 3 | 3 | ||
|
| |||||||
| Race/ethnicity | Caucasian | 98460 (47) | 52 | 47 | 45 | 45 | <0.001 |
| African American | 62157 (30) | 17 | 27 | 31 | 45 | ||
| Other | 48581 (23) | 31 | 26 | 24 | 10 | ||
|
| |||||||
| Gender | Male | 125980 (60) | 61 | 61 | 60 | 59 | <0.001 |
| Female | 83218 (40) | 39 | 39 | 40 | 41 | ||
|
| |||||||
| Primary Payer | Private | 93372 (45) | 54 | 47 | 41 | 37 | <0.001 |
| Medicare | 94112 (45) | 35 | 43 | 48 | 53 | ||
| Medicaid | 15121 (7) | 8 | 7 | 7 | 7 | ||
| Other/missing | 6593 (3) | 3 | 3 | 3 | 3 | ||
|
| |||||||
| Panel Reactive Antibody Level % | 0 | 102042 (49) | 49 | 49 | 48 | 49 | <0.001 |
| 1–30 | 26877 (13) | 11 | 13 | 13 | 14 | ||
| 31–80 | 13538 (7) | 6 | 6 | 7 | 7 | ||
| 81–100 | 10228 (5) | 4 | 5 | 5 | 6 | ||
| Missing | 56513 (27) | 30 | 28 | 27 | 24 | ||
|
| |||||||
| Educational Attainment | Less than High School | 13266 (6) | 6 | 7 | 7 | 5 | <0.001 |
| High School | 82017 (39) | 33 | 36 | 41 | 48 | ||
| Some or Completed College | 73800 (35) | 39 | 38 | 33 | 31 | ||
| Graduate School | 12887 (6) | 9 | 7 | 5 | 4 | ||
| Missing | 27228 (13) | 13 | 12 | 14 | 13 | ||
|
| |||||||
| All patients | 209198 | 18 | 31 | 31 | 20 | ||
Score ranges between 0 and 40 with increasing score associated with higher risk communities
Chi-square test for association of average community risk score with candidate characteristics
Wait List Mortality
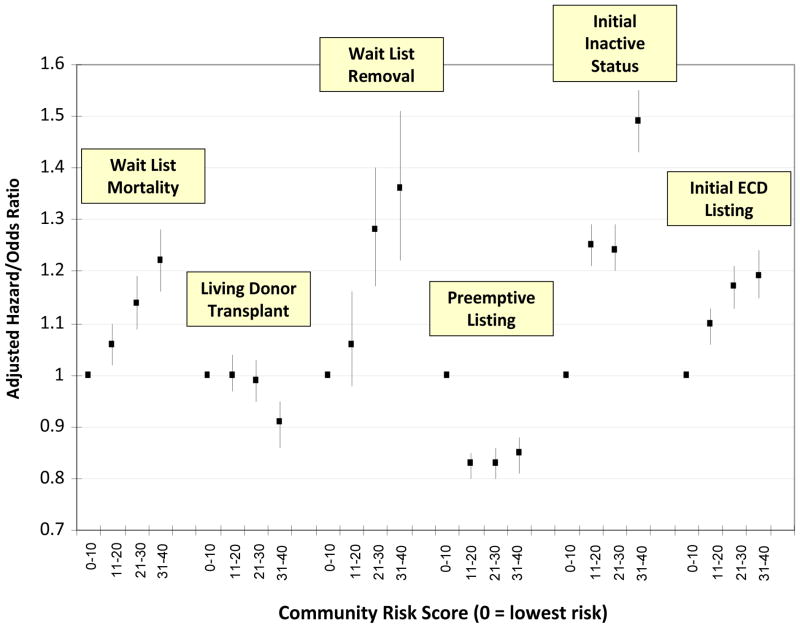
The median follow up time for candidates was 17.5 months (25th percentile=6.2 months, 75th percentile=36.2 months). The overall rate of wait list mortality was 5.1/1000 patient years which increased with increasing community risk(Table 3). Following multivariable adjustment, increasing community risk had a dose-dependent association with higher mortality including 22% increased hazard for candidates in highest risk communities(Figure 1). Other factors associated with increased mortality included older age, diabetes as primary cause of ESRD, low BMI, high PRA, patients on dialysis at the time of listing, low educational attainment, Medicare as primary insurance and Caucasian race (full model displayed in Supplementary Table 1). As a continuous variable, the community risk score was associated with a 6% elevated hazard for death(AHR=1.06, 95% C.I. 1.04–1.08) for each ten units of the risk score.
Table 3.
Unadjusted Rates and Proportions of Candidate Processes and Outcomes by Community Risk Score
| Rate/Proportion of Candidate Process/Outcome | Community Risk Score (95% Confidence Interval for Rates) | ||||
|---|---|---|---|---|---|
| 1–10 | 11–20 | 21–30 | 31–40 | p-value | |
| Wait List Mortality (per 1000 patient years) | 4.7 (4.6, 4.9) | 4.9 (4.7, 5.0) | 5.2 (5.1, 5.3) | 5.5 (5.3, 5.6) | <0.001 |
| Rate of Transplantation (per 1000 patient years) | 13.9 (13.7, 14.1) | 14.6 (14.4, 14.8) | 14.1 (13.9, 14.3) | 15.1 (14.8, 15.3) | <0.001 |
| Proportion of Transplant Recipients Receiving a Living Donor (%) | 40 | 35 | 32 | 26 | <0.001 |
| Rate of Wait List Removal for Health Deterioration (per 1000 patient years) | 0.9 (0.9, 1.0) | 1.1 (1.0, 1.1) | 1.3 (1.2, 1.4) | 1.4 (1.4, 1.5) | <0.001 |
| Preemptive Listing (%) | 32 | 26 | 24 | 24 | <0.001 |
| Initially Listed as Inactive Status (%) | 23 | 26 | 23 | 25 | <0.001 |
| Initially Listed on Expanded Criteria Donor List (%) | 40 | 46 | 51 | 50 | <0.001 |
Figure 1. Adjusted Relative Risks of Pre-transplant Outcomes based on Community Risk Level*.
* models adjusted for candidate age, race, gender, insurance status, educational attainment, dialysis status, panel reactive antibody level, body mass index, blood type, primary cause of end-stage renal disease, state of residence and prior transplant recipient. Error bars represent 95% confidence intervals.
Time to Transplantation
The rate of transplantation following listing was 14.4/1000 patient years which was higher among patients in higher risk communities(Table 3). However, following adjustment for patient characteristics, there was no significant association of community risk with time to transplantation. Relative to candidates in the lowest risk communities, the hazard for transplantation each subsequent community risk group was 1.06(95% C.I. 1.00–1.03), 0.98(95% C.I. 0.95–1.00) and 0.99(95% C.I. 0.96–1.02). Factors associated with more rapid time to transplantation included Caucasian race, candidates on the ECD list, younger age, candidates not initially placed on the inactive list, lower PRA, candidates not on dialysis at listing and moderate BMI. In addition, there were significant differences by blood type and state of residence. Adjusted results were consistent for time to deceased donor transplantation (censored at the time of living donor transplantation), however time to living donor transplantation was lower among patients in the highest risk communities relative to patients in the lowest risk communities(AHR=0.89, 0.85, 0.94).
Living Donor Transplantation
Among patients that received a transplant during the study period, 37% received a transplant from a living donor. This proportion was reduced with increasing community risk score including 26% of patients in the highest risk communities compared to 40% in the lowest risk communities(Table 3). This association was consistent in the multivariable model for the highest risk communities (Adjusted Odds Ratio [AOR]=0.87, 95% C.I. 0.82–0.93, relative to the lowest risk communities, Figure 1). Other factors associated with increased likelihood of living donor transplantation included Caucasians and initial status as inactive. Reduced likelihood of living donor transplantation was associated with increasing age, Medicare as primary insurance, less educational attainment, elevated PRA, diabetes as primary cause of ESRD and dialysis at the time of listing.
Wait List Removal for Health Deterioration
The rate of removal from the waiting list for health deterioration was 1.19/1000 patient years and rates significantly increased by community risk level(Table 3). The multivariable results were similar including a 36% increased adjusted hazard for de-listing associated with highest risk communities(AHR=1.36, 95% C.I. 1.22–1.51, Figure 1). Other factors associated with increased hazard for wait list removal included older age, diabetes as primary cause of ESRD, dialysis at the time of listing, lower educational attainment, Medicare as primary insurance, Caucasians and initially listing as inactive.
Preemptive Listing
Overall, 26% of the study population was placed on the waiting list preemptively. This proportion declined by increasing community risk score from 32% in the lowest risk communities to 24% in highest risk communities(Table 3). Results were consistent in the multivariable model; candidates in higher risk communities had reduced likelihood of preemptive listing(Figure 1). Factors associated with increased likelihood of preemptive listing included older age, Caucasians and initial listing as inactive. Factors associated with decreased likelihood of preemptive listing included diabetes as primary cause of ESRD, elevated PRA, lower educational attainment and Medicare as primary insurer.
Initial Inactive Status
Twenty-four percent of the study population was initially placed on the waiting list as inactive. This proportion moderately varied by community risk level including 25% of candidates in the highest risk level. In the multivariable models, candidates residing in the highest risk counties had 49% increased likelihood of listing as inactive(AOR=1.49,95% C.I. 1.43–1.55, Figure 1). Additional factors associated with increased likelihood of inactive status included diabetes as primary cause of ESRD, elevated BMI and Medicare as primary insurer. Factors associated with decreased likelihood of inactive status included dialysis at the time of listing, Caucasians and older age. There was also marked variation of inactive listing by state of residence (p<0.001).
Initial Listing for Expanded Criteria Donors
Overall, 47% of patients were initially placed on the ECD list. The proportion increased with community risk level from 40% in the lowest risk communities to 50% in the highest risk communities(Table 3). This association was significant in the multivariable model including 19% increased likelihood for patients in highest risk communities (AOR=1.19, 95% C.I. 1.15–1.24, Figure 1). Additional factors associated with ECD listing included older age, Caucasians, Medicare as primary payer, lower educational attainment, elevated PRA and diabetes as primary cause of ESRD.
Transplant Center and Regional Variability
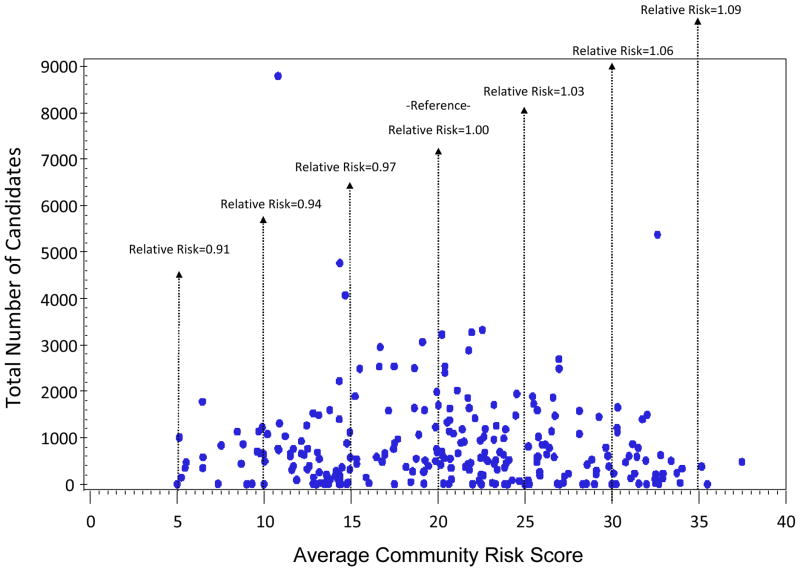
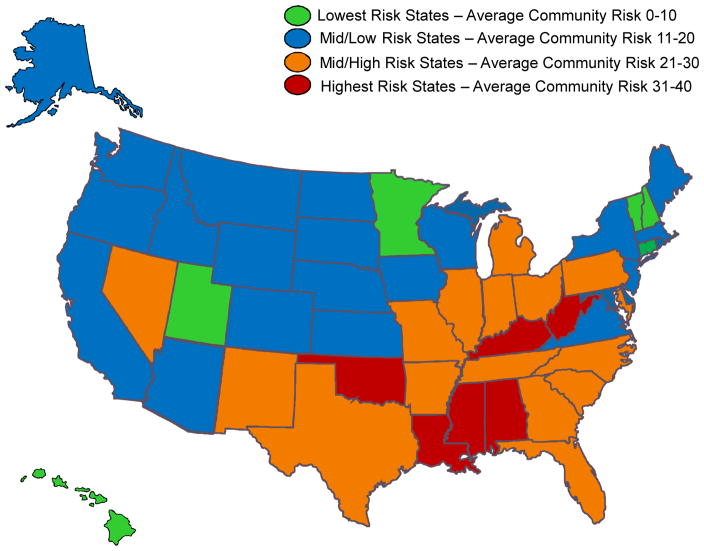
The average community risk score of candidates varied by both transplant center and state of residence. Figure 2 displays the distribution of the average community risk score of candidates listed at transplant centers (n=256) with the total number of candidates listed at the center over the study period. The median risk score by center was 21 and the 25th and 75th percentiles were 14 and 26 respectively. Ten percent of centers had an average community risk score greater than 31 and 10% of centers had an average risk score less than 11. There was no significant correlation between candidate volume and average community risk score (Spearman’s correlation=−0.06, p=0.34). As depicted in the figure, the estimated relative risk for wait list mortality among patients at centers with an average community risk of 35 was 9% higher than for patients at centers with average community risk equal to 20. In contrast, the estimated relative risk for mortality for patients at centers with an average community risk of 5 was 9% lower than for patients at centers with average community risk. The average community risk score by state of residence is displayed in Figure 3, West Virginia, Alabama, Mississippi, Oklahoma and Kentucky had the highest average risk. In contrast, Hawaii, Minnesota, New Hampshire, Connecticut, Utah and Vermont had the lowest average community risk among candidates.
Figure 2. Average Community Risk Score by Candidate Volume for US transplant centers (n=256)*.
* No significant correlation between number of candidates and average community risk score (Spearmans rho = −0.06, p=0.34); relative Risk defined as the adjusted hazard for wait list mortality for patients based on the average community risk at individual transplant centers. For example, patients at centers with an average community risk score of 35 have 9% excess risk as compared to a patients at centers with an average community risk score = 20, while patients at centers with an average community risk score of 5 have 9% reduced risk as compared to patients at centers with average community risk.
Figure 3.
Distribution of Average Community Risk Score by State of Residence
Community Risk and Rural-Urban Residence
In order to understand the independent impact of patients’ residence in rural-urban locations, all models were developed with additional adjustment for rural-urban continuum code. For these models, 1,296 patients were excluded from the study population as their county residence was not defined in the data with rural-urban designation. There was an association of the community risk score such that patients residing in the most urban classification(n=125,082) had the lowest average community risk(mean=17.7) while patients from the most rural counties(n=1225) had the highest community risk score (mean=26.4) with levels in-between generally following this pattern. In unadjusted models, as compared to candidates residing in the most urban counties, patients in the most rural counties had 24% increased wait list mortality(AHR=1.24, 95% C.I. 1.06–1.46) and overall rural-urban status was strongly associated with mortality (p<0.001).. However, in the multivariable model, adjusted for patient-level characteristics along with the county risk score, rural-urban status was no longer statistically significantly associated with wait list mortality(p=0.41) including no association with the most rural neighborhoods(AHR=1.02, 95% C.I. 0.87–1.20). Community risk remained strongly associated with wait list mortality in this model including a dose-response effect and a 20% increased hazard associated with the highest risk communities(AHR=1.20,95% C.I. 1.15–1.26). These effects and the relationships with other outcome and process measures are summarized in Table 4. All findings in these models remained consistent adjusted for clustering at the center (rather than county) level.
Table 4.
Association of Rural-Urban Status with Candidate Processes and Outcomes
| Candidate Process/Outcome | Unadjusted Hazard for Increasing Rural Status* | Unadjusted Hazard for Increasing Community Risk Score (per 10 units) | Adjusted Hazard for Increasing Rural Status* | Adjusted Hazard for Increasing Community Risk Score (per 10 |
|---|---|---|---|---|
| Wait List Mortality | 1.03 (1.02, 1.04)† | 1.05 (1.03, 1.07)† | 1.00 (0.99, 1.00) | 1.06 (1.04, 1.07) † |
| Rate of Transplantation | 1.04 (1.04, 1.05) † | 1.00 (1.00, 1.01)† | 1.00 (0.99.1.01) | 1.00 (0.99, 1.01) |
| Likelihood of Living Donor Transplantation | 0.98 (0.97, 0.98) † | 0.80 (0.79, 0.81) † | 1.00 (0.99, 1.01) | 0.90 (0.89, 0.92) † |
| Wait List Removal for Health Deterioration | 1.09 (1.08,1.11) † | 1.16 (1.13, 1.19) † | 1.02 (0.99, 1.03) | 1.10 (1.07, 1.14) † |
| Likelihood of Preemptive Placement on the Waiting List | 0.98 (0.98, 0.99) † | 0.87 (0.86, 0.88) † | 0.98 (0.98, 0.99) † | 0.96 (0.95, 0.97) † |
| Likelihood of Initial Placement on the Waiting List as Inactive | 1.00 (0.99, 1.00) | 1.02 (1.01, 1.03) † | 1.00 (0.99, 1.01) | 1.15 (1.13, 1.17) † |
| Likelihood of Initial Placement on the Waiting List for Expanded Criteria Donors | 1.00 (0.99, 1.00) | 1.15 (1.14, 1.16) | 0.98 (0.97, 0.99) † | 1.07 (1.05, 1.08) † |
p<0.05
Rural status measured on ordinal scale with 1 = most urban and 9=most rural; adjusted models include candidate age, race, gender, insurance status, educational attainment, dialysis status, panel reactive antibody level, body mass index, blood type, primary cause of end-stage renal disease, state of residence and prior transplant recipient as covariates in addition to both rural-urban location and community risk.
Discussion
The principal findings of the study indicate that kidney transplant candidate processes and outcomes are independently associated with risk factors in patients’ community. Increased levels of community risks are strongly associated with higher wait list mortality, wait list removal rates, initial placement on the ECD list and initial inactive status. In addition, higher level of community risk is associated with decreased likelihood of preemptive listing and living donor transplantation. Cumulatively, the findings suggest that factors associated with patients’ communities indicative of health conditions, access to care, socioeconomic status and environmental conditions, have a prominent role in delivery of care and outcomes for kidney transplant patients. These associations may reflect both environmental conditions and underlying characteristics of patients from communities and are important to understand as a significant source of patient outcomes and for the development of effective interventions and personalized treatment protocols.
There is existing evidence that patients’ behavior, socioeconomic condition and comorbid conditions that are not routinely codified are associated with outcomes for transplant patients (10–12;18–22). However, it is striking that independent of numerous individual clinical and demographic characteristics and markers of individual socioeconomic status (e.g. educational attainment and insurance), risk associated with patients’ county of residence have such a profound and consistent association with processes of care and outcomes. There are several potential mechanisms that may explain these associations. Higher risk communities have higher prevalence of comorbid conditions and the associations characterized in this study may reflect underlying illness of patients that are not routinely collected. In addition, higher risk communities are partially defined by poorer access to care which may suggest that patients have historically received less frequent or lower quality care prior to or during the wait listing period. The specific conditions and risks in the environment in which patients reside may also influence outcomes and processes independent of individual characteristics. In fact, a recently published randomized trial demonstrated that individuals given vouchers to move to higher income communities had significant health benefits without any additional intervention (23). Social networking has been identified as an influential factor for transplant patients and may also be related to lower rates of living donation and preemptive listing in higher risk communities (24). This study cannot distinguish between sources of risks as related to individuals or direct environmental impact, but does formally demonstrate that knowledge of the candidates’ residence is an important tool for classifying patients’ risks and potentially for developing individualized care and policy. The nature of this individualized care likely varies depending on the specific process or outcome, but may include more vigilant monitoring of patients from higher risk communities, enhanced levels of education and/or medical and psychosocial evaluation, use of telehealth or at-home visits or community-based interventions to identify patients earlier in the end-organ disease process in higher risk communities.
Candidates from highest risk communities had over 20% excess mortality hazard on the waiting list and over 30% hazard of wait list removal relative to candidates in lower risk communities. This may suggest that patients in higher risk communities could benefit from specialized care including more rigorous medical screening during their initial candidacy assessment and more frequent re-evaluations following listing (25). Results also indicated that patients from higher risk communities were less likely to list prior to initiation of dialysis (26–28). This may reflect the level of pre-ESRD care in patients’ communities, individuals’ health literacy or marginal medical contraindications which could delay placement on the wait list. Thus, continued efforts for developing successful individual and community-based interventions may be particularly important for candidates residing in higher risk communities (29–32). One related question that results raise is whether protective effects of preemptive transplantation are partially explained by social, environmental or behavioral factors as opposed to solely the deleterious effects of dialysis given that patients from higher risk communities are more likely to both initiate dialysis early and have reduced pre- and post-transplant outcomes (33).
Approximately one-quarter of the study population were initially wait-listed as inactive, a practice which has grown over time (34;35). The association of community risk with more frequent initial inactive status may suggest that patients from higher risk communities are more likely to have marginal contraindications for transplant and require additional health, psychosocial or financial screening relative to patients from lower risk communities (36). This also may reflect differences in quality and access to healthcare in patients’ community, in particular that patients from higher risk communities are less facile navigating steps to receiving a transplant (37). Moreover, patients from higher risk communities are more likely to initially list for ECD kidneys. These donor kidneys are defined by relative increased risks of graft loss, but may often be available more rapidly to candidates (38;39). The association of ECD listing and community risk may indicate that patients from higher risk communities have increased comorbidity or risks associated with dialysis exposure and thus more suitable for a more rapid transplant, but the direct impact of ECD listing on candidate and recipient outcomes based on community risk level requires further study. Results may also reflect differences in consent processes for patients with different social and environmental conditions as well as variations in practices between centers that treat patients from higher risk communities (40;41).
Another interesting finding of the study was the relationship between the presence of community risk factors and rural-urban location of patients. Multiple studies have evaluated the association of rural-urban status with transplant patient outcomes with mixed results, some findings suggesting lower quality and reduced outcomes of patients in rural settings and others indicated equivocal outcomes (42–45). Our study demonstrated that there was a significant association between a higher prevalence of risk factors in rural communities and in unadjusted models, reduced outcomes and different processes of care for patients in rural settings. However, when accounting for community risks and patient-level factors, the effects of rural-urban status was almost completely eradicated and the significant association of community risks with outcomes was maintained. Cumulatively, these findings seem to suggest that rural-urban status as generally defined may only be a gross measure of patient risks with not a direct causal factor that explains outcomes. Rather, the prevalence of community risks, which may be present in either rural or urban settings, is a much more sensitive and salient measure of patients prognosis prior to kidney transplantation.
One of the other important considerations of the study findings is the potential impact of community risk factors on quality assessment of transplant centers. There is marked variation of the prevalence of community risk factors at different centers. As community risks are not accounted for in performance evaluations, centers that treat patients from higher risk communities may be more likely to be identified with poor outcomes or processes of care, independent of true quality of care. For example, SRTR report cards depict rates of wait list mortality, wait list removal and preemptive transplants for each center which, based on the present findings, may be influenced by the risk factors in the community of patients that are treated at each center (46). Ultimately, the community risks identified in this study may be important to consider (or directly account for) in quality assessments in order to encourage treatment of patients from higher risk communities rather than dissuade treatment of patients over concern of quality oversight (47–50).
There are several important limitations of the study to consider for interpretation of the findings. First, the primary explanatory variable of the study describes risk factors at the county-level. In many cases, these risks may be highly variable within counties and the effect of within-county level variation may be substantial (51). However, this additional source of variability would generally dilute effects described in this study and the associations would likely be stronger if data were available on a more granular level. We did not investigate the specific contribution of each county risk factor on the outcomes for this study due to the scope of the analysis, but it should be acknowledged that each specific community risk factor may have different associations with the outcomes evaluated. In addition, it is notable that although community risk factors characterize outcomes and health factors for entire county populations, transplant patients are included in the denominator. Thus, although transplant patients certainly represent a vast minority of patients for county metrics, there is a minor degree of dependency with the measures. We evaluated the associations in this study strictly among kidney transplant candidates, but they may be salient and important to measure in other end-organ disease groups. It should also be noted that it is an ecological fallacy to attribute risks defined by a broad geographic region to all individuals in that region as thus acknowledge that there is clear heterogeneity within individual counties. Finally, an important limitation of the study is that, consistent with many observational studies, we are only able to assess associations which should not be interpreted as cause and effect relationships.
In summary, community risk factors are significantly associated with processes and outcomes for candidates of kidney transplantation in the United States. Community risks are likely a proxy for both individual and environmental conditions but are an important factor explaining transplant patient prognosis. Further understanding of the specific nature of these associations and the causal pathway are important to develop effective interventions that may require prospective studies. Healthcare for transplant patients requires multi-faceted approaches and consideration of community risks in treatment decisions may provide important opportunities to improve patient outcomes.
Acknowledgments
Funding for this study was partially supported by NIH/NIDDK (R01 DK085185, JDS, JRR), NIH/NIMH (P60MD00265, JDS, JDT, ARS) and NIH/National Center for Research Resources (RR024989, ARS). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or other funding entities.
Footnotes
The data reported here have been supplied by the Minneapolis Medical Research Foundation as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation n and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government.
The authors report no conflict of interest for this study.
The study was approved by the Cleveland Clinic Institutional Review Board.
Reference List
- 1.Anderson RT, Sorlie P, Backlund E, Johnson N, Kaplan GA. Mortality effects of community socioeconomic status. Epidemiology. 1997;8:42–47. doi: 10.1097/00001648-199701000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Fiscella K, Franks P. Poverty or income inequality as predictor of mortality: longitudinal cohort study. BMJ. 1997;314:1724–1727. doi: 10.1136/bmj.314.7096.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freedman VA, Grafova IB, Rogowski J. Neighborhoods and chronic disease onset in later life. Am J Public Health. 2011;101:79–86. doi: 10.2105/AJPH.2009.178640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galea S, Tracy M, Hoggatt KJ, Dimaggio C, Karpati A. Estimated deaths attributable to social factors in the United States. Am J Public Health. 2011;101:1456–1465. doi: 10.2105/AJPH.2010.300086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lantz PM, House JS, Lepkowski JM, Williams DR, Mero RP, Chen J. Socioeconomic factors, health behaviors, and mortality: results from a nationally representative prospective study of US adults. JAMA. 1998;279:1703–1708. doi: 10.1001/jama.279.21.1703. [DOI] [PubMed] [Google Scholar]
- 6.Waitzman NJ, Smith KR. Separate but lethal: the effects of economic segregation on mortality in metropolitan America. Milbank Q. 1998;76:341–73. 304. doi: 10.1111/1468-0009.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waitzman NJ, Smith KR, Stroup A. The direct and indirect effects of metropolitan area inequality on mortality. A hierarchical analysis. Ann N Y Acad Sci. 1999;896:347–349. doi: 10.1111/j.1749-6632.1999.tb08137.x. [DOI] [PubMed] [Google Scholar]
- 8.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 9.Garg J, Karim M, Tang H, Sandhu GS, DeSilva R, Rodrigue JR, Pavlakis M, Hanto DW, Baird BC, Goldfarb-Rumyantzev AS. Social adaptability index predicts kidney transplant outcome: a single-center retrospective analysis. Nephrol Dial Transplant. 2012;27:1239–1245. doi: 10.1093/ndt/gfr445. [DOI] [PubMed] [Google Scholar]
- 10.Goldfarb-Rumyantzev AS, Koford JK, Baird BC, Chelamcharla M, Habib AN, Wang BJ, Lin SJ, Shihab F, Isaacs RB. Role of socioeconomic status in kidney transplant outcome. Clin J Am Soc Nephrol. 2006;1:313–322. doi: 10.2215/CJN.00630805. [DOI] [PubMed] [Google Scholar]
- 11.Kasiske BL, Klinger D. Cigarette smoking in renal transplant recipients. J Am Soc Nephrol. 2000;11:753–759. doi: 10.1681/ASN.V114753. [DOI] [PubMed] [Google Scholar]
- 12.Sung RS, Althoen M, Howell TA, Ojo AO, Merion RM. Excess risk of renal allograft loss associated with cigarette smoking. Transplantation. 2001;71:1752–1757. doi: 10.1097/00007890-200106270-00009. [DOI] [PubMed] [Google Scholar]
- 13.Woodward RS, Page TF, Soares R, Schnitzler MA, Lentine KL, Brennan DC. Income-related disparities in kidney transplant graft failures are eliminated by Medicare’s immunosuppression coverage. Am J Transplant. 2008;8:2636–2646. doi: 10.1111/j.1600-6143.2008.02422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.University of Wisconsin Population Health Institute. County Health Rankings. 2011. [article online] [Google Scholar]
- 15.Schold JD, Buccini LD, Kattan MW, Goldfarb DA, Flechner SM, Srinivas TR, Poggio ED, Fatica R, Kayler LK, Sehgal AR. The association of community health indicators with outcomes for kidney transplant recipients in the United States. Arch Surg. 2012;147:520–526. doi: 10.1001/archsurg.2011.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levine GN, McCullough KP, Rodgers AM, Dickinson DM, Ashby VB, Schaubel DE. Analytical methods and database design: implications for transplant researchers, 2005. Am J Transplant. 2006;6:1228–1242. doi: 10.1111/j.1600-6143.2006.01277.x. [DOI] [PubMed] [Google Scholar]
- 17.USDA Economic Research Service. Rural-Urban Continuum Codes. Jul 5, 2012. 4-2-2013. Ref Type. Video Recording. [Google Scholar]
- 18.Axelrod DA, Dzebisashvili N, Schnitzler MA, Salvalaggio PR, Segev DL, Gentry SE, Tuttle-Newhall J, Lentine KL. The interplay of socioeconomic status, distance to center, and interdonor service area travel on kidney transplant access and outcomes. Clin J Am Soc Nephrol. 2010;5:2276–2288. doi: 10.2215/CJN.04940610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellison MD, Edwards LB, Edwards EB, Barker CF. Geographic differences in access to transplantation in the United States. Transplantation. 2003;76:1389–1394. doi: 10.1097/01.TP.0000090332.30050.BA. [DOI] [PubMed] [Google Scholar]
- 20.Gueye AS, Chelamcharla M, Baird BC, Nguyen C, Tang H, Barenbaum AL, Koford JK, Shihab F, Goldfarb-Rumyantzev AS. The association between recipient alcohol dependency and long-term graft and recipient survival. Nephrol Dial Transplant. 2007;22:891–898. doi: 10.1093/ndt/gfl689. [DOI] [PubMed] [Google Scholar]
- 21.Kasiske BL, Snyder JJ, Skeans MA, Tuomari AV, Maclean JR, Israni AK. The geography of kidney transplantation in the United States. Am J Transplant. 2008;8:647–657. doi: 10.1111/j.1600-6143.2007.02130.x. [DOI] [PubMed] [Google Scholar]
- 22.Prakash S, Rodriguez RA, Austin PC, Saskin R, Fernandez A, Moist LM, O’Hare AM. Racial composition of residential areas associates with access to pre-ESRD nephrology care. J Am Soc Nephrol. 2010;21:1192–1199. doi: 10.1681/ASN.2009101008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ludwig J, Sanbonmatsu L, Gennetian L, Adam E, Duncan GJ, Katz LF, Kessler RC, Kling JR, Lindau ST, Whitaker RC, McDade TW. Neighborhoods, obesity, and diabetes--a randomized social experiment. N Engl J Med. 2011;365:1509–1519. doi: 10.1056/NEJMsa1103216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ladin K, Hanto DW. Understanding disparities in transplantation: do social networks provide the missing clue? Am J Transplant. 2010;10:472–476. doi: 10.1111/j.1600-6143.2009.02963.x. [DOI] [PubMed] [Google Scholar]
- 25.Gaston RS, Danovitch GM, Adams PL, Wynn JJ, Merion RM, Deierhoi MH, Metzger RA, Cecka JM, Harmon WE, Leichtman AB, Spital A, Blumberg E, Herzog CA, Wolfe RA, Tyan DB, Roberts J, Rohrer R, Port FK, Delmonico FL. The report of a national conference on the wait list for kidney transplantation. Am J Transplant. 2003;3:775–785. doi: 10.1034/j.1600-6143.2003.00146.x. [DOI] [PubMed] [Google Scholar]
- 26.Fissell RB, Srinivas T, Fatica R, Nally J, Navaneethan S, Poggio E, Goldfarb D, Schold J. Preemptive renal transplant candidate survival, access to care, and renal function at listing. Nephrol Dial Transplant. 2012;27:3321–3329. doi: 10.1093/ndt/gfs012. [DOI] [PubMed] [Google Scholar]
- 27.Mange KC, Joffe MM, Feldman HI. Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. N Engl J Med. 2001;344:726–731. doi: 10.1056/NEJM200103083441004. [DOI] [PubMed] [Google Scholar]
- 28.Meier-Kriesche HU, Schold JD. The impact of pretransplant dialysis on outcomes in renal transplantation. Semin Dial. 2005;18:499–504. doi: 10.1111/j.1525-139X.2005.00096.x. [DOI] [PubMed] [Google Scholar]
- 29.Ephraim PL, Powe NR, Rabb H, Ameling J, Auguste P, Lewis-Boyer L, Greer RC, Crews DC, Purnell TS, Jaar BG, Depasquale N, Boulware LE. The providing resources to enhance African American patients’ readiness to make decisions about kidney disease (PREPARED) study: protocol of a randomized controlled trial. BMC Nephrol. 2012;13:135. doi: 10.1186/1471-2369-13-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodrigue JR, Cornell DL, Lin JK, Kaplan B, Howard RJ. Increasing live donor kidney transplantation: a randomized controlled trial of a home-based educational intervention. Am J Transplant. 2007;7:394–401. doi: 10.1111/j.1600-6143.2006.01623.x. [DOI] [PubMed] [Google Scholar]
- 31.Rodrigue JR, Pavlakis M, Egbuna O, Paek M, Waterman AD, Mandelbrot DA. The “House Calls” trial: a randomized controlled trial to reduce racial disparities in live donor kidney transplantation: rationale and design. Contemp Clin Trials. 2012;33:811–818. doi: 10.1016/j.cct.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thornton JD, ejandro-Rodriguez M, Leon JB, Albert JM, Baldeon EL, De Jesus LM, Gallardo A, Hossain S, Perez EA, Martin JY, Lasalvia S, Wong KA, Allen MD, Robinson M, Heald C, Bowen G, Sehgal AR. Effect of an iPod video intervention on consent to donate organs: a randomized trial. Ann Intern Med. 2012;156:483–490. doi: 10.1059/0003-4819-156-7-201204030-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schold JD, Sehgal AR, Srinivas TR, Poggio ED, Navaneethan SD, Kaplan B. Marked variation of the association of ESRD duration before and after wait listing on kidney transplant outcomes. Am J Transplant. 2010;10:2008–2016. doi: 10.1111/j.1600-6143.2010.03213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schold J, Srinivas TR, Sehgal AR, Meier-Kriesche HU. Half of kidney transplant candidates who are older than 60 years now placed on the waiting list will die before receiving a deceased-donor transplant. Clin J Am Soc Nephrol. 2009;4:1239–1245. doi: 10.2215/CJN.01280209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delmonico FL, McBride MA. Analysis of the wait list and deaths among candidates waiting for a kidney transplant. Transplantation. 2008;86:1678–1683. doi: 10.1097/TP.0b013e31818fe694. [DOI] [PubMed] [Google Scholar]
- 36.Patzer RE, Amaral S, Wasse H, Volkova N, Kleinbaum D, McClellan WM. Neighborhood poverty and racial disparities in kidney transplant waitlisting. J Am Soc Nephrol. 2009;20:1333–1340. doi: 10.1681/ASN.2008030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patzer RE, Perryman JP, Schrager JD, Pastan S, Amaral S, Gazmararian JA, Klein M, Kutner N, McClellan WM. The role of race and poverty on steps to kidney transplantation in the southeastern United States. Am J Transplant. 2012;12:358–368. doi: 10.1111/j.1600-6143.2011.03927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schnitzler MA, Whiting JF, Brennan DC, Lin G, Chapman W, Lowell J, Boxerman S, Hardinger KL, Kalo Z. The expanded criteria donor dilemma in cadaveric renal transplantation. Transplantation. 2003;75:1940–1945. doi: 10.1097/01.TP.0000076381.16276.1B. [DOI] [PubMed] [Google Scholar]
- 39.Schold JD, Harman JS, Chumbler NR, Duncan RP, Meier-Kriesche HU. The pivotal impact of center characteristics on survival of candidates listed for deceased donor kidney transplantation. Med Care. 2009;47:146–153. doi: 10.1097/MLR.0b013e31818475c9. [DOI] [PubMed] [Google Scholar]
- 40.Grams ME, Womer KL, Ugarte RM, Desai NM, Montgomery RA, Segev DL. Listing for expanded criteria donor kidneys in older adults and those with predicted benefit. Am J Transplant. 2010;10:802–809. doi: 10.1111/j.1600-6143.2010.03020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schold JD, Howard RJ, Scicchitano MJ, Meier-Kriesche HU. The expanded criteria donor policy: an evaluation of program objectives and indirect ramifications. Am J Transplant. 2006;6:1689–1695. doi: 10.1111/j.1600-6143.2006.01390.x. [DOI] [PubMed] [Google Scholar]
- 42.Axelrod DA, Guidinger MK, Finlayson S, Schaubel DE, Goodman DC, Chobanian M, Merion RM. Rates of solid-organ wait-listing, transplantation, and survival among residents of rural and urban areas. JAMA. 2008;299:202–207. doi: 10.1001/jama.2007.50. [DOI] [PubMed] [Google Scholar]
- 43.Maripuri S, Ikizler TA, Cavanaugh KL. Prevalence of Pre-End-Stage Renal Disease Care and Associated Outcomes among Urban, Micropolitan, and Rural Dialysis Patients. Am J Nephrol. 2013;37:274–280. doi: 10.1159/000348377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Hare AM, Johansen KL, Rodriguez RA. Dialysis and kidney transplantation among patients living in rural areas of the United States. Kidney Int. 2006;69:343–349. doi: 10.1038/sj.ki.5000044. [DOI] [PubMed] [Google Scholar]
- 45.Tonelli M, Klarenbach S, Rose C, Wiebe N, Gill J. Access to kidney transplantation among remote- and rural-dwelling patients with kidney failure in the United States. JAMA. 2009;301:1681–1690. doi: 10.1001/jama.2009.545. [DOI] [PubMed] [Google Scholar]
- 46.Dickinson DM, Dykstra DM, Levine GN, Li S, Welch JC, Webb RL. Transplant data: sources, collection and research considerations, 2004. Am J Transplant. 2005;5:850–861. doi: 10.1111/j.1600-6135.2005.00840.x. [DOI] [PubMed] [Google Scholar]
- 47.Howard RJ, Cornell DL, Schold JD. CMS oversight, OPOs and transplant centers and the law of unintended consequences. Clin Transplant. 2009;23:778–783. doi: 10.1111/j.1399-0012.2009.01157.x. [DOI] [PubMed] [Google Scholar]
- 48.Schold JD, Buccini LD, Srinivas TR, Srinivas RT, Poggio ED, Flechner SM, Soria C, Segev DL, Fung J, Goldfarb DA. The association of center performance evaluations and kidney transplant volume in the United States. Am J Transplant. 2013;13:67–75. doi: 10.1111/j.1600-6143.2012.04345.x. [DOI] [PubMed] [Google Scholar]
- 49.Weinhandl ED, Snyder JJ, Israni AK, Kasiske BL. Effect of comorbidity adjustment on CMS criteria for kidney transplant center performance. Am J Transplant. 2009;9:506–516. doi: 10.1111/j.1600-6143.2008.02527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Werner RM, Asch DA, Polsky D. Racial profiling: the unintended consequences of coronary artery bypass graft report cards. Circulation. 2005;111:1257–1263. doi: 10.1161/01.CIR.0000157729.59754.09. [DOI] [PubMed] [Google Scholar]
- 51.Krieger N. Choosing area based socioeconomic measures to monitor social inequalities in low birth weight and childhood lead poisoning: The Public Health Disparities Geocoding Project (US) 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]