Abstract
Poor marital quality has been linked repeatedly to spouses’ health problems, with alterations to physiological stress-response systems, such as the hypothalamic-pituitary-adrenocortical (HPA) axis, as one putative mechanism. This study assessed wives and husbands’ HPA axis (i.e., cortisol) reactivity to marital criticism during laboratory-based conflict discussions, in the context of marital aggression experienced during the previous year. Ninety-five couples provided one saliva sample prior to – and three samples following – a triadic family conflict discussion involving their teenage child. Marital criticism during the conflict discussion was related to heightened HPA reactivity for husbands only. For wives, an interaction emerged between criticism during the conflict and previous-year marital aggression: only those wives who had experienced high levels of marital aggression demonstrated a positive association between criticism and cortisol output. Husbands thus appeared to be more physiologically reactive to the in-the-moment critical behaviors, whereas wives’ responses to proximal conflict were related to previous and perhaps more chronic experiences of marital aggression. These findings shed light on ways in which within-couple processes during family conflicts involving children contribute to individual physiological functioning, enhancing our understanding of the role of family relationships in physical health outcomes.
Keywords: marital couples, conflict, cortisol, intimate partner aggression
Married individuals generally reap considerable health benefits from their relationship status (Schoenborn, 2004); however, poor marital quality can increase risk for negative physical and mental health outcomes (Whisman, 2007; Rohrbaugh, Shoham, & Coyne, 2006; Whisman & Uebelacker, 2012; Robles & Kiecolt-Glaser, 2003). Just as overall stress takes a toll physiologically (Chida & Hamer, 2008), increasing evidence indicates that marital stress is associated with impaired physiological functioning (Robles & Kiecolt-Glaser, 2003). Men generally derive more health benefits from marriage than do women, and men and women also tend to exhibit different profiles of physiological reactivity to relationship stress (Kiecolt-Glaser & Newton, 2001). Research has not fully explored which aspects of marital relationships are associated with differential physiological functioning or with downstream health outcomes for men and women.
The association between marriage and physiological functioning is complicated by the relative contributions of proximal stressors versus distal stressors to profiles of bodily response. Short-term activation of neuroendocrine response systems, such as the hypothalamic-pituitary-adrenocortical (HPA) axis, in response to stress has benefits for managing acute threats and is essential for survival. Yet frequent activation of the body’s response systems due to chronic stress ultimately can place the body under “allostatic load” a condition in which the body’s systems create new physiological set-points. This resetting can establish a persistently maladaptive style of physiological response to stress that ultimately weakens the body’s neuroendocrine and immune systems and accelerates disease processes (Korte, Koolhaas, Wingfield, & McEwen, 2004).
Marital conflict presents a unique blend of both acute (i.e., the eruption of an overt confrontation) and chronic (i.e., long-term patterns of unresolved hostility) challenges. The present study investigates the acute, proximal influence of spouses’ criticism as well as the more chronic, distal influence of physical or emotional marital aggression during the past year. Both types of marital conflict are investigated to provide a fuller assessment of immediate and historical marital discord on physiological functioning of both husbands and wives.
Acute, Proximal Marital Stress and Physiological Activity
Many spouses react physiologically to laboratory-based conflict discussions, which are designed to approximate naturalistic circumstances in which couples talk about relationship problems. Bidirectional associations between marital satisfaction and physiological indices have emerged in studies employing these procedures. Couples reporting lower marital satisfaction showed heightened neuroendocrine reactivity during conflict (Kiecolt-Glaser et al., 1997). In addition, high levels of observed neuroendocrine reactivity to in-lab conflict discussions predicted declines over time in marital satisfaction (Kiecolt-Glaser et al., 2003).
Negative conflict behaviors during laboratory-based discussions have been associated with indices of heightened arousal, including cardiovascular reactivity, i.e., blood pressure and heart rate (Robles & Kiecolt-Glaser, 2003; Newton & Sanford, 2003) and hormone release i.e., prolactin, ACTH, epinephrine, norepinephrine, and growth hormone (Malarkey et al., 1994). In the extant literature, wives sometimes have shown more physiological reactivity (i.e., higher amplitude responses) to negative behaviors than husbands (Heffner et al., 2006; Kiecolt-Glaser et al., 1996). However, under certain conditions, husbands show similar reactivity profiles, for example, when more invested in obtaining changes (Newton & Sanford, 2003) or when power is equitably distributed (Loving et al., 2004). Although marital conflict discussions typically have been limited to the two adult partners, including other people may alter power structures or spouses’ investment in obtaining change. Discussions in the present study involve a child, which may heighten socio-evaluative threat for both husbands and wives. Given these potential influences, we do not anticipate different physiological response profiles for men versus women.
Researchers’ laboratory inductions of marital discussions elicit wide-ranging levels of actual conflict behaviors, such as criticism and disparaging remarks. Criticism represents a negative behavior common during marital conflict, but it has not been assessed independently of other non-evaluative negative conflict behaviors (e.g., interrupting, negative affect, noncompliance; Malarkey et al., 1994) and high-intensity or aggressive conflict behaviors (e.g., overt hostility, dominance; Newton & Sanford, 2003). Assessing criticism independently tests the influence of common, mildly negative partner evaluations likely to characterize the disagreements of non-clinic-referred couples.
Chronic, Distal Marital Conflict as a Context for Physiological Reactivity to Acute, Proximal Marital Conflict
Although laboratory-based marital conflict discussions can be acute stressors, they also occur in the larger context of the couple’s ongoing history of marital conflict. Laboratory-based couple discussions have been used as an immediate stressor analogous to the typical research paradigm to assess HPA reactivity, the Trier Social Stress Test, which involves social evaluation by unknown judges and a mathematics challenge. Yet, unlike the novelty of the Trier Social Stress Test, laboratory-based couple discussions reproduce a familiar stressor. As such, the HPA reactivity evoked by conflict discussions may be related not only to proximal behavior within the conflict, but also to distal experiences of more chronic and perhaps more serious conflict.
Spouses with chronic marital aggression have shown increased reactivity to later marital conflict on certain indices of physiological arousal (e.g., heart rate; Gottman, Jacobson, Rushe, & Shortt, 1995, Jacobson et al., 1994); however, reactivity of the HPA axis has not been adequately assessed. The only study we found that assessed effects of marital aggression on cortisol reactivity (Feinberg, Jones, Granger, & Bontempo, 2011) found no association between previous aggression and post-conflict cortisol levels. This study, however, did not assess the nature or intensity of conflict actually occurring in the discussion.
More generally, there is evidence that a history of recurring stresses can disrupt normal HPA reactions to acute stressors (e.g., Goldman-Mellor, Hamer, & Steptoe, 2012). Thus repeated exposure to marital aggression may alter spouses’ responses to later conflicts, though there are competing hypotheses for the direction of HPA disturbance. Distal, repeating marital aggression may increase sensitivity to later criticisms that occur in a laboratory discussion, i.e., hyper-responsiveness. Alternatively, repeated exposure to marital conflict may result in HPA habituation or attenuated cortisol reactivity. In this case, even if criticisms in the lab are viewed as threatening, repeated HPA activation over time may have compromised the body’s capacity to amass the anticipated biological stress reaction. Thus, based on a history of repeating marital conflict, the challenge of a new conflict discussion in the lab could produce either an exaggerated cortisol response or could fail to elicit a cortisol response, both of which are signs of HPA axis dysregulation (Repetti, Robles, & Reynolds, 2011; Taylor, Way, & Seeman, 2011).
Triadic Family Problem-Solving Discussions
To date, most studies of couples’ physiology have relied on discussions of marital/romantic relationship issues conducted only with the two partners. Although this focus is appropriate for newlyweds or childless couples, couple conflict interactions at later stages of the family life cycle are likely to also focus on parenting issues. In fact, parents of adolescents in one study reported that child issues were the most common source of their marital disagreements (Papp, Cummings, & Goeke-Morey, 2009). These conflict discussions often involve the children themselves, particularly when children reach adolescence, and adolescent youth can also play an important role in marital conflicts (Feinberg, Kan, & Hetherington, 2007). Despite the prevalence of conflict discussions involving both parents and an adolescent child in couples’ daily lives, little is known about couples’ physiological responses to these discussions. Importantly, the presence of an observing child imbues criticism between partners with an additional socio-evaluative aspect, which is an important feature of stressors that trigger cortisol responses (Dickerson & Kemeny, 2004).
Present Study
The present study extends existing knowledge of the physiology of marriage by assessing the influence of proximal as well as distal marital conflict on HPA axis reactivity. Couples and their adolescent child engaged in a 15-minute discussion of a high intensity family conflict. Outside observers subsequently coded the discussions for husband-to-wife criticisms and wife-to-husband criticisms. As a measure of HPA axis activity, salivary cortisol was collected prior to the discussion, immediately following the discussion, and at two follow up intervals. These four time points within the hour surrounding the discussion allowed for the assessment of total cortisol activity including baseline effects as well as discussion reactivity. Two hypotheses guided our analyses for the present study. First, regarding proximal conflict behavior, we expected that higher criticism from the partner – as an acute stressor – would be associated with heightened HPA reactivity to the conflict discussion. Second, we hypothesized that distal marital aggression would alter spouses’ HPA reactivity to criticism within the conflict discussion such that the combination of distal marital aggression and lab-based criticism would be associated with a distinct pattern of HPA activity. Given the mixed findings in past studies, we did not postulate whether such couples would experience flatter cortisol output or conversely higher output.
Methods
Participants
The present study used a community sample of couples (N = 95), recruited from the Greater Los Angeles into a longitudinal study examining effects of family conflict on family members’ well-being. Eligible couples met the following criteria at enrollment: co-resident for 3 or more years, had co-resident child aged 9-10, and were able to complete measures in English.
The current sample was drawn from a larger sample of 104 couples who participated in the fourth wave of data collection and provided saliva samples. Five couples could not be included due to video-recording equipment failure. Prior to the laboratory visit, we instructed families to refrain from drinking alcohol for 48 hours, smoking for 24 hours, and eating or drinking for one hour, to reduce common sources of noise in cortisol data (Kirschbaum & Hellhammer, 1992). Four couples provided unusable cortisol data due to both spouses’ conditions or factors known to affect cortisol (e.g., pregnancy, not sleeping the previous night). We scrutinized participants’ reports for medications or health conditions that may alter cortisol concentrations (e.g., Granger, Hibel, Fortunato, & Kapelewski, 2009) and dropped the cortisol data for six wives (diabetes [1], antidepressants [5]), and four husbands (diabetes [2], steroids [1], immunosuppressants [1]). Finally, cortisol data from three wives and one husband were dropped for systematically elevated cortisol (i.e., every sample > 3 SD above the mean). Thus, 81 couples provided complete cortisol data for both spouses; however, 95 couples provided at least one spouse’s cortisol data and behavioral data and were included in the current analyses.
Among included couples, wives’ and husbands’ mean ages were 45.6 (SD = 6.0, range = 33.4-59.0) and 48.2 (SD = 7.0, range = 32.8-71.9), respectively. Wives averaged 14.7 years of education (SD = 2.4, range = 7-20) and husbands 15.0 years (SD = 2.6, range = 8-20). In 20% of the couples, both partners self-identified as Hispanic/Latino ethnicity; in terms of race, couples were 54.7% Caucasian/White, 16.8% as African American/Black, 9.5% as Asian/Pacific Islander, and 18.9% multiple races within the couple. Approximately one-quarter (25.5%) reported an annual household income < $50,000, 22.4% $50,000 – 75,000, 21.2% 75,000 – 100,000, and 30.9% > $100,000. Couples had been married or cohabiting 15.8 years, on average (SD = 6.0; range = 3-31) and participated in this study with their child (M age = 15.3, SD = 0.7, range = 13.7-17.1).
Procedures
The university IRB approved all study procedures, and all three family members provided consent or assent. Study procedures were designed to evoke discussions of salient, important conflict areas among family members. Prior to the discussion, all family members participated in a standardized relaxation exercise: watching a 10-minute calming video with nature scenes and music in a dimly lit room. Next, each family member completed an inventory of common family issues to identify potential discussion topics. Experimenters conducted private interviews with each participant to prime them to develop and express their individual point of view. Experimenters then conferred, using the inventory and interviews to identify the three most conflictual topics across family members. These topics were presented to the family to guide the 15-minute family discussion. Families were instructed to: discuss the highest-conflict topic first, ensure that each individual expressed his/her point of view, and discuss the problem as they normally would (See Gordis, Margolin, Spies, Susman, & Granger, 2010 for further details). To directly assess the similarity of the conflict discussions to families’ discussions outside the laboratory, we administered a questionnaire immediately after the discussion on which participants rated: “How similar was this discussion to other family discussions you have had?” Based on a 0 (not at all similar) to 4 (very similar) response scale, husbands’ mean rating was 2.7 and wives’ was 2.9, indicating laboratory discussions moderately similar to at-home conflicts.
Saliva samples were collected at four time points surrounding the conflictual discussion. Baseline (T1) samples were collected immediately following the relaxation exercise and preceding any identification or discussion of conflict topics. Three saliva samples were collected after the conflict discussion with T1 + 40min immediately following the discussion and then the final two at 10-min intervals (T1 + 50min and T1 + 60min) while participants filled out questionnaires in quiet, private rooms.
Measures
Cortisol
Saliva samples were immediately frozen and later shipped in dry ice to Salimetrics, LLC to be assayed for concentrations of free salivary cortisol. Each saliva sample was assayed twice to establish reliability of measurement; analyses used the mean of these values. Analyses were repeated if any pair of results from a single sample differed by more than seven percent. Out of a total of 792 cortisol concentrations, we dropped 3 samples above four ug/dL (values this high may indicate sampling error) and winsorized 11 individual cortisol samples (across 7 spouses) to M + 3 SD to minimize the impact of outliers, as recommended by Granger et al. (2006). We used mean substitution to impute the value for one missing cortisol sample to allow for Area Under the Curve (AUC) calculations and analyses.
Following Pruessner and colleagues (Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003), we calculated Area Under the Curve with respect to Increase (AUCI) to evaluate cortisol reactivity. AUCI calculates the area under the cortisol concentration curve, accounting for time elapsed between measurements and subtracting the baseline cortisol concentration from subsequent measurements. Thus, the AUCI measure assessed reactivity specific to the experience of the conflict itself. For this reason, we used AUCI to evaluate the role of criticism. A related index of HPA activity is Area Under the Curve with respect to ground (AUCG), which includes baseline levels of cortisol. For hypothesis two, regarding the interaction between distal aggression and proximal conflict, both AUCG and AUCI were evaluated due to the possibility that a couple’s history may impact reactivity more generally.
Criticism
Thirteen trained coders, naïve to study aims and hypotheses, coded two types of critical marital behaviors: (a) circumscribed disapproval of the spouse’s ideas and suggestions (e.g., “That’s a ridiculous idea”) and (b) more global condemnation of the spouse’s character or personality (e.g., “You are so immature!”). Two coders independently rated each actor (wife-to-husband and husband-to-wife) on a scale ranging from 0 (not at all) to 3 (a lot) for the extent of criticism within each 3-minute interval to collect 5 ratings for each behavior across the discussion. Inter-rater reliabilities, measured through intraclass correlations, were .75 for wives’ criticism of ideas and .72 for wives’ criticism of person, and .89 and .85 for husbands’ criticism of ideas and of person, respectively. We used an average of the coders’ ratings in all analyses. Nineteen percent of spouses exhibited some criticism of the spouse’s character/personality, whereas 62% criticized the spouse’s ideas. The two types of criticism correlated at r = .49 for wives and r = .51 for husbands. We averaged ratings across the 5 intervals, and then summed the two criticism codes so that criticism had a possible range of 0-6 for each actor.
Previous-year marital aggression
Husbands and wives completed the Domestic Conflict Index (DCI; Margolin, John, & Foo, 1998), a 61-item questionnaire assessing the frequency of aggressive and non-aggressive conflict behaviors within the past year that includes many items from the Conflict Tactics Scale-2 (Straus, Hamby, Boney-McCoy, & Sugarman, 1996). Each partner reported on his or her own behaviors and those of the spouse. We examined 27 items assessing physically aggressive and emotionally aggressive behaviors. For each behavior, participants indicated the frequency range during the past year, using the following categories: zero per year (0), once yearly (1), 2-5 times per year (2), 6-12 times per year (3), 2-4 times per month (4), and more than once per week (5). We calculated scores by summing the maximum reported value for each partner on each item, to counteract family members’ tendencies to underreport aggressive behavior (Arias & Beach, 1987). Sixty percent of couples endorsed husband-to-wife aggression (physical or emotional) and 70% endorsed wife-to-husband aggression in the past year. The most frequently endorsed physical aggression items were “pushed, grabbed, or shoved” (9.5% of spouses) and “threw an object at” (5.3%); the most frequently endorsed emotional aggression items were “made threats to leave the relationship” (27.9%), and “insulted or shamed in front of others” (26.3%). One couple reported aggression levels > 3 SD above the sample mean for both husband and wife; we winsorized these values to the next highest reported value to decrease their influence on results (Rivest, 1994).
Analyses: Hierarchical Linear Models
We used Hierarchical Linear Modeling (HLM; Raudenbush, Bryk, Cheong, & Congdon, 2004) to test our hypotheses, in order to account for interdependence between spouses’ cortisol. Data were modeled at the dyadic level, utilizing a two-intercept model (Kenny, Kashy, & Cook, 2006); this allows for the simultaneous modeling of wives’ and husbands’ cortisol. All HLM results reported represent the final estimation of fixed effects, with robust standard errors. Two-level HLM models were used, with the Level 1 equation modeling the AUC outcome with husband and wife dummy codes as predictors. Time of day, criticism, and aggression were entered as Level-2 predictors. When testing our hypothesis that aggression would moderate the effect of criticism on cortisol, we included the relevant interaction term. Individual-level variables were group-mean centered (i.e., within husbands and within wives), and couple-level variables were grand-mean centered prior to entry in models. We first modeled aggression and criticism as couple-level effects, and then conducted follow-up analyses to assess influences of the spouse’s (i.e., partner effects) versus one’s own (actor effects) criticism on cortisol activity.
Results
Descriptives
Table 1 presents descriptive data on all study variables. Wives were significantly more critical than husbands during the discussion, t(94) = 2.02, p = .046. Husbands had significantly higher cortisol than wives at T1 + 50min. t(80) = 4.39, p < .001, T1 + 60min, t(79) = 3.67, p < .001, and overall higher cortisol activity AUCG, t(80) = 2.56, p = .012; spouses did not differ on AUCI. Husbands and wives also did not differ significantly on previous-year marital aggression.
Table 1. Descriptive Statistics for Previous-Year Marital Aggression, Discussion Criticism, and Cortisol.
| N | M | SD | Minimum | Maximum | |
|---|---|---|---|---|---|
| Wives | |||||
| Marital Aggression | 95 | 3.36 | 4.71 | 0.00 | 20.0 |
| Criticism | 95 | .39a | .52 | 0.00 | 2.50 |
| Cortisol T1 | 86 | .072 | .038 | .014 | .216 |
| Cortisol T1 + 40min | 86 | .072 | .046 | .010 | .243 |
| Cortisol T1 + 50min | 86 | .067 b | .034 | .001 | .180 |
| Cortisol T1 + 60min | 86 | .068c | .038 | .003 | .186 |
| Cortisol AUCG | 86 | 276.23 d | 140.60 | 73.59 | 843.03 |
| Cortisol AUCI | 86 | −3.51 | 89.12 | −193.17 | 309.74 |
| Husbands | |||||
| Marital Aggression | 95 | 3.03 | 5.77 | 0.00 | 34.0 |
| Criticism | 95 | .31a | .62 | 0.00 | 4.10 |
| Cortisol T1 | 90 | .083 | .050 | .007 | .248 |
| Cortisol T1 + 40min | 90 | .082 | .044 | .005 | .219 |
| Cortisol T1 + 50min | 90 | .093 b | .052 | .025 | .259 |
| Cortisol T1 + 60min | 89 | .088c | .047 | .021 | .240 |
| Cortisol AUCg | 90 | 325.88 d | 152.98 | 85.00 | 778.29 |
| Cortisol AUCi | 90 | 7.79 | 121.82 | −451.65 | 385.90 |
Note. Values for Cortisol reported in ug/dL, and reflect data cleaning (i.e., winsorization of outliers). Matching superscripts indicate significant differences between husbands and wives.
Bivariate Associations Among Measures
We examined intercorrelations among husbands’ and wives’ previous-year marital aggression, criticism, and cortisol measures and found associations for husbands’ and wives’ previous-year marital aggression (r = .59, p < .001) and discussion criticism (r = .65, p < .001). We also found significant correlations between husbands’ and wives’ cortisol levels, controlling for sampling time, for the T1 + 40min sample (immediately post-discussion, r = .35, p = .002), the T1 + 50min sample (r = .26, p = .02), and AUCG (r = .24, p = .04). Within-spouse cortisol samples were correlated (rs ranged from .29 to .82).
Wives’ previous-year aggression was modestly positively correlated with their own criticism during the discussion (r = .25, p = .02); however, husbands’ previous-year aggression and criticism were uncorrelated. Husbands’ criticism was associated with lower husband cortisol at T1 baseline (r = −.25, p = .02), and wives’ criticism was associated with lower husband cortisol at baseline (r = −.31, p = .003) and higher husband cortisol AUCI (r = .37, p < .001). Neither husbands’ nor wives criticism was associated with wives’ cortisol. None of the bivariate associations between spouses’ previous-year aggression and cortisol was significant. See Supplemental Table 1 for the full results.
Effects of Criticism and Aggression on Spouses’ Cortisol
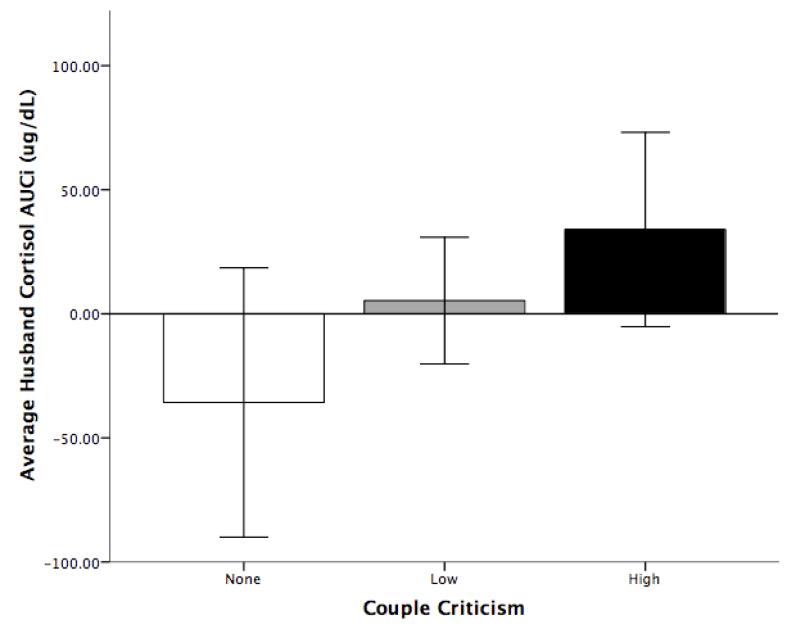
In testing the first hypothesis regarding the effects of criticism on AUCI, we first investigated overall bi-directional criticism (husband-to-wife plus wife-to-husband). Couple criticism was positively associated with husbands’ cortisol AUCI, coefficient = 27.20, t(170) = 2.36, p = .02. Figure 1 illustrates this effect by comparing husbands who had discussions with ’no criticism’, ’low criticism’ and ’high criticism’; low and high groups were based on a median split of those demonstrating any criticism. Husbands who did not experience criticism at all during their discussions evidenced a decline in cortisol from baseline to post-discussion samples, and thus had a negative AUCI value (consistent with the diurnal decline of cortisol). Both groups of husbands who experienced criticism showed increased cortisol following the discussion, but this increase was larger for husbands experiencing high criticism than those experiencing low criticism. We then investigated actor and partner effects. Husbands showed significant cortisol reactivity to criticism in both directions—when criticized by their wife, coefficient = 39.18, t(170) = 2.13, p = .03, and when they exhibited criticism to their wife, coefficient = 31.91, t(170) = 2.21, p = .03. In a final exploratory model in which spouses’ criticism scores were entered simultaneously, we did not find evidence that either the husband’s own criticism (coefficient = 16.67, t(168) = 0.50, p = .62, n.s.) nor their wives’ criticism (coefficient = 23.70, t(168) = 0.56, p = .57, n.s.) was uniquely associated with husbands’ cortisol reactivity. We found no significant effect for actor criticism, partner criticism, or couple-level criticism on wives’ cortisol AUCI. Thus, our first hypothesis, that criticism would be positively associated with cortisol reactivity, was supported for husbands only. See Supplemental Table 2 for full results.
Figure 1. Husbands’ Cortisol AUCI, by Couples’ Criticism Group.
Note. Low and high criticism groups were defined by a median split of nonzero criticism values.
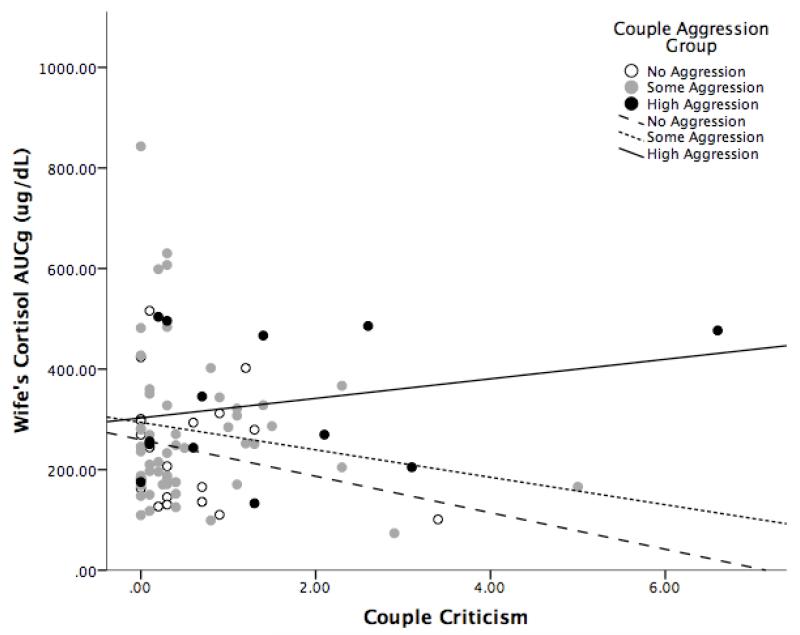
Our second hypothesis projected that distal couple aggression would moderate associations between proximal criticism behavior and spouses’ cortisol. We first modeled our effects at the couple level, which are shown in Table 2. The interaction between criticism and aggression was significant for wives’ AUCG, coefficient = 2.79, t(166) = 2.21, p = .03. Follow-up simple slopes analyses indicated that the association between couple’s criticism and wives’ AUCG was significant only at very high levels of previous-year aggression; for wives who had aggression at three standard deviations above the mean, the estimated coefficient for criticism was B = 65.2, t(166) = 2.15, p = .03. (Note: For wives who had experienced no aggression, the estimated coefficient for criticism was slightly but non-significantly negative, B = −29.6, t(166) = −1.60, p = .11.) To illustrate this effect, Figure 2 shows the association between couples’ criticism and wives’ cortisol AUCG values, grouped by previous-year aggression (with the high aggression group representing couples in the top 15%). At lower levels of aggression, wives’ cortisol AUCG is not linearly related to criticism during the discussion. At very high levels of previous-year aggression, however, wives’ AUCG is positively associated with criticism during the discussion. The interaction was not significant for wives’ AUCI, nor for husbands’ AUCG or AUCI.
Table 2. Summary of HLM Models of Criticism, Aggression, and Criticism × Aggression on Spouses’ Cortisol.
| AUCg | AUCi | |
|---|---|---|
| Fixed Effect | Coefficient (SE) | Coefficient (SE) |
| Husbands’ Intercept | 389.78 (103.80)** | 148.04 (112.20) |
| Time of Day | −4.57 (7.30) | −9.94 (8.07) |
| Couple Criticism | −43.08 (16.08)* | 26.20 (12.49)* |
| Couple Previous-Year Aggression | 0.84 (1.70) | −0.29 (1.09) |
| Criticism × Aggression | 1.23 (1.50) | 0.40 (1.14) |
| Wives’ Intercept | 355.89 (95.30)** | 131.71 (75.72) ☞ |
| Time of Day | −5.90 (6.60) | −9.38 (5.11) ☞ |
| Couple Criticism | −20.17(16.58) | −0.89 (11.00) |
| Couple Previous−Year Aggression | 0.86 (1.33) | −0.74 (0.83) |
| Criticism × Aggression | 2.79 (1.26)* | −0.57 (0.65) |
Note. Estimates of coefficients are of fixed effects, with robust standard errors.
p < .10
p <.05
p <.01. Approximate df = 166.
Figure 2. Association Between Couples’ Criticism and Wives’ Cortisol AUCG, by Aggression Group.
Note: No Aggression group n = 22, Some Aggression group n = 59, High Aggression group n =15. High Aggression group represents top 15% of marital aggression scores.
We conducted follow-up analyses to determine whether the interaction effect was specific to critical behaviors from husbands-to-wives or from wives-to-husbands. Although both husbands’ and wives’ criticism showed effects similar in direction and magnitude to the combined effect of spouses’ criticism described above, the interaction term was statistically significant for wives’ criticism, coefficient = 5.14, t (166) = 3.03, p = .003, but not husbands’ criticism, coefficient = 4.09, t (166) = 1.60, p = .11.
In addition, because the AUCG measure differs from the AUCI measure due to its inclusion of baseline (i.e., pre-discussion) cortisol levels, we ran a parallel model assessing baseline cortisol levels as the outcome. The results of this model were similar to the AUCG model in direction and pattern of significance, interaction term coefficient = .00087, t(166) = 2.34, p = .02. Thus, baseline effects played a role in the AUCG results, perhaps suggesting some impact of anticipation; however, the AUCG results were not due to baseline alone as there was no direct effect of aggression history on baseline cortisol levels.
Discussion
The current study links observed criticism within the marital dyad during a family conflict discussion to patterns of neuroendocrine reactivity in spouses. As anticipated, husbands who experienced more criticism (both as the one criticizing and the recipient) during family discussions evidenced higher cortisol reactivity (AUCI). As contrasted with husbands, although wives overall did not show a cortisol response associated with level of marital criticism, wives who experienced high total aggression within the past year did demonstrate an association between cortisol activity and marital criticism. In couples reporting high previous-year aggression, wives’ total cortisol output (AUCG) and baseline cortisol were positively linked to criticism during discussions. Thus, wives’ cortisol responses to proximal conflict behaviors were evident only when accounting for their levels of distal aggressive marital conflict.
Spouses’ Physiological Reactivity to Proximal Relationship Stress
Our selection and priming of a triadic family conflict as a stressor task is somewhat unique for a study of neuroendocrine responses. Notably, social stressors involving conflict discussions have failed to reliably produce increases in participants’ cortisol (Gunnar, Talge, & Herrera, 2009). Perhaps the primary differences between our study and previous studies is our readying of each family member for conflict and our direct measurement of the extent to which the stressor task was, indeed, creating a stressful situation. As Heyman (2001) notes, laboratory based interactions tend to underestimate the severity of conflict that occurs naturally during couples’ daily interactions. Thus, rather than assuming that family conflict discussions would be stressful for all spouses, we directly measured one of the most common, outwardly observable negative conflict behaviors (i.e., criticism) to index the severity of the discussion. This approach enabled us to find a link between the conflict stressor and husbands’ cortisol reactivity.
Wives in our study did not show the same general physiological sensitivity to criticism during the discussion. These findings contrast with some extant literature, which suggests that wives tend to be more reactive to negative marital behaviors than are husbands (e.g., Kiecolt-Glaser et al., 1996; Robles, Shaffer, Malarkey, & Kiecolt-Glaser, 2006). A possible explanation for the discrepancy between husbands’ and wives’ reactivity to partner criticism is that our conflict task was a three-person family discussion—with a child participating—as contrasted with two-person marital discussions in the previous literature. The child’s presence made our discussion simultaneously a parenting, co-parenting, and marital conflict task. Moreover, our procedures were designed to specifically elicit topics that were current conflicts for the entire family. After asking each family member to rate possible topics individually, we conducted private priming interviews with each family member, and then selected topics that reflected the most disagreement among family members. For example, when child concerns were targeted for discussion, there was often disagreement between parents about how to address the concern.
How might our strategy for eliciting family conflict have resulted in greater reactivity for men than women? A possible explanation concerns relative power and comfort with this discussion task. Previous research found that spouses with lower relative power in their relationships show greater physiological reactivity to relationship stress (Loving et al., 2004). Perhaps women feel more empowered when problem solving and making decisions about child issues than when solving marital disputes. It is also possible that mothers are simply more accustomed to conflict and problem-solving in the ’family’ realm or in the presence of their child whereas fathers find these discussions more novel, and thus more stressful. Novelty of discussion topic has been related to increased cortisol reactivity in dating partners (Loving, Gleason, & Pope, 2009). Powers and colleagues (Powers, Pietromonaco, Gunlicks, & Sayer, 2006) suggested that women may have more impact on their partners’ physiological reactivity when women serve as facilitators of social interactions; again, this might be more likely in a conversation involving a child. Moreover, marital criticism in the presence of the child may function as a threat to husbands’ agency or achievement. Threats in general have been linked to enhanced reactivity across acute stressor tasks (Denson, Spanovic, & Miller, 2009), and there is some suggestion that achievement stress is linked to enhanced reactivity specifically for men (Stroud et al., 2002).
Spouses’ Physiological Reactivity to Distal Relationship Stress
Evidence regarding the effects of marital aggression on the physiology of men and women is limited. Although Feinberg et al. (2011) found no main effects of aggression history on cortisol reactivity for men or women, they did find baseline and recovery effects. We extend those findings by showing that, for women, aggression history is a relevant context for understanding links between proximal criticism and cortisol activation. Criticism was associated with enhanced overall cortisol output, which includes baseline measurements, for wives who had experienced the highest level of aggression during the previous year.
Based on our results, it seems that a history of bidirectional marital aggression is more salient for wives than for husbands when they approach future conflicts. Wives who have recently experienced high levels of marital aggression display a stronger link between physiological arousal and marital criticism than wives who have not. These wives thus appear to carry their marital conflict history with them as they approach later conflict, and they may be predisposed to physiologically anticipate and react to marital discussions that feature criticism. Our finding that baseline levels of cortisol are linked to marital criticism, among wives with a recent history of high marital aggression, suggests that these wives may be correctly anticipating that their discussions will involve more negativity. Interestingly, this finding is not strictly based on anticipation as there is no direct association between aggression history and baseline cortisol. Rather, wives in the high aggressive group who then go on to exhibit and receive more criticism, exhibit both an anticipatory cortisol response and a larger cortisol response overall (AUCG).
Importantly, we found that a history of marital aggression appears to prime wives’ physiology to be as strongly entwined with their criticism of the husband as with the husband’s criticism of them. To fully explain these physiological findings, we would need more psychological data on the impact and interpretation of criticism. For example, are women in highly aggressive marriages sensitized to view criticism as the harbinger of more negative interaction patterns? Does the criticism expressed or received in this controlled lab setting remind them of previous serious altercations or worry them about future fights? Do some of these women allow themselves to express more serious criticism than usual due to the safety of the protected laboratory setting? Similarly, how do we explain the absence of a link between criticisms and cortisol response in women from less aggressive relationships? Have these women learned that criticisms do not have particularly negative consequences? Future studies should assess these possibilities directly by investigating (a) whether wives’ experience of marital aggression is linked to their appraisals and expectations prior to engaging in a laboratory conflict, and (b) whether such perceptions are linked to their sensitivity to behaviors during conflict.
This study also shines light on the unique reactions of women who experience very high levels of aggression – a very small percentage of women in this community sample. With low levels of conflict often being adaptive, perhaps evidence of allostatic load only emerges for women who experience particularly destructive forms of intimate partner aggression. Further examination of the aggression reported by these couples revealed elevated frequency of emotionally aggressive behaviors and, for a majority of these couples, incidents of physical aggression (primarily slapping, pushing/grabbing/shoving, and destroying or throwing objects). Thus, it is possible that only this small percent of women have experienced relationship-related trauma. As previous data show, traumatic experiences have been linked to alterations in acute stress reactivity (Young, Tolman, Witkowski, & Kaplan, 2004). Future studies with couples should further examine HPA reactivity to ongoing conflict in women who experience severe relationship aggression as the meaning and physiological implications of everyday conflict may be quite different for these women.
Limitations and Future Directions
A few limitations to the generalizability of our findings deserve note. We assessed family processes of risk in a community sample that was not recruited based on known risks, such as couples seeking treatment or being mandated for treatment. Despite the many advantages of a community sample for this research, a disadvantage is that only a small number of couples in our sample reported very high levels of marital aggression. Thus, we did not have the statistical power to conduct more nuanced examinations of our finding relating proximal and distal marital experiences to wives’ cortisol. We were not able to determine, for example, whether wives’ responses were influenced by which spouse had perpetuated the aggression or by the type of criticism experienced during conflict (i.e., criticism of ideas or dismissal of the person). In addition, without a direct comparison of couple-only discussions, we do not know whether the presence of the child may have affected the nature, frequency, or impact of spouses’ criticisms. Thus, although our findings suggest the importance of assessing marital behaviors in multiple family contexts, we cannot directly generalize to dyadic marital conflicts.
Our use of salivary cortisol as a marker of psychosocial stress imposes certain limits on the interpretation of our results. Due to the 15-20 minute latency between stressor onset and cortisol response, cortisol cannot be used to assess moment-to-moment responses to discrete events in the environment. We took care to time our samples to capture responses to the entire discussion (i.e., our first post-discussion sample roughly indexed the beginning of the discussion and the final sample corresponded to the discussion’s end). Still, we cannot link increases in cortisol directly to spouses’ critical remarks. Another limitation of our study is that we did not account for other, non-marital sources of chronic stress that may have affected spouses’ cortisol responses. This is important because marital problems are linked not only to stressful processes within marriage, but also to stressful contextual factors (see Karney & Bradbury, 2005).
Future studies can extend our research in several ways. First, as noted above, our study did not include measures of spouses’ appraisals of marital criticism. Assessing these mediating psychological variables may clarify the nature of the associations between marital aggression and HPA responses to conflict behaviors. Next, future studies should incorporate direct measurement of indices of immune functioning and health status. Finally, future research should include other measures of physiological functioning, such as assessing acute reactivity in different stress response systems (e.g., electrodermal activity, cardiovascular reactivity), as well as measuring dysregulation of diurnal patterns of cortisol and other biomarkers of stress. Regarding future clinical applications, it is important that clinicians assess criticism in common family interactions as well as probe for a history of episodes of marital aggression. Interventions should offer partners strategies to engage in family discussions about ordinary conflicts, as well as high-intensity conflicts that may pull for coercive or aggressive tactics. Addressing how couples interact during family discussions (i.e., in the presence of children), aside from being ecologically valid, may allow clinicians to disrupt behavior that impacts spouses’ physiology.
Conclusions
Despite considerable recognition of the emotional ramifications of marital conflict, the physical consequences are just beginning to be understood. This study incorporates the knowledge that current marital conflict always occurs within the context of ongoing prior marital events. The results contribute to a growing body of literature suggesting that within-couple processes contribute to individual physiological functioning. The extent to which the physiological reactions are adaptive or maladaptive is still to be determined. Yet our results lend some credence to potential health consequences if couples lack strategies to alleviate unremitting sources of couple conflict and fail to resolve new conflicts. It is recommended that therapeutic couples interventions address both husbands and wives’ sensitivities to different dimensions of their conflictual interactions. Emphasizing ongoing conflict behavior as well as aggression history can detect and disrupt communication behaviors that ultimately may confer health risk.
Supplementary Material
Acknowledgments
This research was supported by the NIH Eunice Kennedy Shriver National Institute of Child Health and Human Development [Grant R01 HD046807 awarded to Margolin], and by the NIH National Institute of Mental Health [Grant NRSA F31 MH094035 awarded to Rodriguez]. We gratefully acknowledge the contributions of Michelle Ramos, Esti Iturralde, Darby Saxbe, and Lauren Spies Shapiro and the entire team of coders.
References
- Arias I, Beach SRH. Validity of self-reports of marital violence. Journal of Family Violence. 1987;2:139–149. doi:10.1007/BF00977038. [Google Scholar]
- Chida Y, Hamer M. Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: A quantitative review of 30 years of investigations. Psychological Bulletin. 2008;134:829–885. doi: 10.1037/a0013342. doi:10.1037/a0013342. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. doi:10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Feinberg ME, Jones DE, Granger DA, Bontempo D. Relation of intimate partner violence to salivary cortisol among couples expecting a first child. Aggressive Behavior. 2011;37:492–502. doi: 10.1002/ab.20406. doi:10.1002/ab.20406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg ME, Kan ML, Hetherington EM. The longitudinal influence of coparenting conflict on parental negativity and adolescent maladjustment. Journal of Marriage and Family. 2007;69:687–702. doi: 10.1111/j.1741-3737.2007.00400.x. doi:10.1111/j.1741-3737.2007.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Mellor S, Hamer M, Steptoe A. Early-life stress and recurrent psychological distress over the lifecourse predict divergent cortisol reactivity patterns in adulthood. Psychoneuroendocrinology. 2012;37:1755–1768. doi: 10.1016/j.psyneuen.2012.03.010. doi:10.1016/j.psyneuen.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Gordis EB, Margolin G, Spies LA, Susman EJ, Granger DA. Interparental aggression and parent-adolescent salivary alpha amylase symmetry. Physiology and Behavior. 2010;100:225–233. doi: 10.1016/j.physbeh.2010.01.006. doi:10.1016/j.physbeh.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottman JM, Jacobson NS, Rushe RH, Shortt JW. The relationship between heart rate reactivity, emotionally aggressive behavior, and general violence in batterers. Journal of Family Psychology. 1995;9:227–248. doi:10.1037/0893-3200.9.3.227. [Google Scholar]
- Granger DA, Hibel LC, Fortunato CK, Kapelewski CH. Medication effects on salivary cortisol: Tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology. 2009;34:1437–1448. doi: 10.1016/j.psyneuen.2009.06.017. doi:10.1016/j.psyneuen.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, Blair C, El-Sheikh M, Mize J, Lisonbee JA, Buckhalt JA. Integrating the measurement of salivary α-amylase into studies of child health, development, and social relationships. Journal of Social and Personal Relationships. 2006;23(2):267–290. doi: 10.1177/0265407506062479. [Google Scholar]
- Heffner KL, Loving TJ, Kiecolt-Glaser JK, Himawan LK, Glaser R, Malarkey WB. Older spouses’ cortisol responses to marital conflict: Associations with demand/withdraw communication patterns. Journal of Behavioral Medicine. 2006;29:317–325. doi: 10.1007/s10865-006-9058-3. doi:10.1007/s10865-006-9058-3. [DOI] [PubMed] [Google Scholar]
- Heyman RE. Observation of couple conflicts: Clinical assessment applications, stubborn truths, and shaky foundations. Psychological Assessment. 2001;13:5–35. doi: 10.1037//1040-3590.13.1.5. doi:10.1037/1040-3590.13.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson NS, Gottman JM, Waltz J, Rushe R, Babcock J, Holtzworth-Munroe A. Affect, verbal, content, and psychophysiology in the arguments of couples with a violent husband. Journal of Consulting and Clinical Psychology. 1994;62:982–988. doi: 10.1037//0022-006x.62.5.982. doi:10.1037/0022-006X.62.5.982. [DOI] [PubMed] [Google Scholar]
- Karney BR, Bradbury TN. Contextual influences on marriage: Implications for policy and intervention. Current Directions in Psychological Science. 2005;14:171–174. doi:10.1111/j.0963-7214.2005.00358.x. [Google Scholar]
- Kenny DA, Kashy DA, Cook WL. Dyadic data analysis. Guilford; New York: 2006. [Google Scholar]
- Kiecolt-Glaser JK, Bane C, Glaser R, Malarkey WB. Love, marriage, and divorce: Newlywed’s hormones foreshadow relationship changes. Journal of Consulting and Clinical Psychology. 2003;71:176–188. doi: 10.1037//0022-006x.71.1.176. doi:10.1037/0022-006X.71.1.176. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R, Cacioppo JT, MacCallum RC, Snydersmith M, Kim C, Malarkey WB. Marital conflict in older adults: Endocrinological and immunological correlates. Psychosomatic Medicine. 1997;59:339–349. doi: 10.1097/00006842-199707000-00001. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Newton TL. Marriage and health: His and hers. Psychological Bulletin. 2001;127:472–503. doi: 10.1037/0033-2909.127.4.472. doi:10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Newton T, Cacioppo JT, MacCallum RC, Glaser R, Malarkey WB. Marital conflict and endocrine function: Are men really more physiologically affected than women? Journal of Consulting and Clinical Psychology. 1996;64:324–332. doi: 10.1037//0022-006x.64.2.324. doi:10.1037/0022-006X.64.2.324. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Methodological aspects of salivary cortisol measurement. In: Kirschbaum C, Read GF, Hellhammer D, editors. Assessment of Hormones and Drugs in Saliva in Biobehavioral Research. Hogrefe and Huber; Seattle: 1992. pp. 19–32. [Google Scholar]
- Korte SM, Koolhaas JM, Wingfield JC, McEwen BS. The Darwinian concept of stress: Benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neuroscience and Biobehavioral Reviews. 2004;29:3–38. doi: 10.1016/j.neubiorev.2004.08.009. doi: 10.1016/j.neubiorev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: A review. Biological Psychology. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. doi:10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Loving TJ, Gleason MEJ, Pope MT. Transition novelty moderates daters’ cortisol responses when talking about marriage. Personal Relationships. 2009;16:187–203. doi:10.1111/j.1475-6811.2009.01218.x. [Google Scholar]
- Loving TJ, Heffner KL, Kiecolt-Glaser JK, Glaser R, Malarkey WB. Stress hormone changes and marital conflict: Spouses’ relative power makes a difference. Journal of Marriage and Family. 2004;66:595–612. doi:10.1111/j.0022-2445.2004.00040.x. [Google Scholar]
- Malarkey WB, Kiecolt-Glaser JK, Pearl D, Glaser R. Hostile behavior during marital conflict alters pituitary and adrenal hormones. Psychosomatic Medicine. 1994;56:41–51. doi: 10.1097/00006842-199401000-00006. [DOI] [PubMed] [Google Scholar]
- Margolin G, John RS, Foo L. Interactive and unique risk factors for husbands’ emotional and physical abuse of their wives. Journal of Family Violence. 1998;13:315–344. doi:10.1023/A:1022880518367. [Google Scholar]
- Matud MP. Gender differences in stress and coping styles. Personality and Individual Differences. 2004;37:1401–1415. doi:10.1016/j.paid.2004.01.010. [Google Scholar]
- Michaud K, Matheson K, Kelly O, Anisman H. Impact of stressors in a natural context on release of cortisol in healthy adult humans: A meta-analysis. Stress. 2008;11:177–197. doi: 10.1080/10253890701727874. doi:10.1080/10253890701727874. [DOI] [PubMed] [Google Scholar]
- Newton TL, Sanford JM. Conflict structure moderates associations between cardiovascular reactivity and negative marital interaction. Health Psychology. 2003;22:270–278. doi: 10.1037/0278-6133.22.3.270. doi:10.1037/0278-6133.22.3.270. [DOI] [PubMed] [Google Scholar]
- Papp LM, Cummings EM, Goeke-Morey MC. For richer, for poorer: Money as a topic of marital conflict in the home. Family Relations. 2009;58:91–103. doi: 10.1111/j.1741-3729.2008.00537.x. doi:10.1111/j.1741-3729.2008.00537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. doi:10.1016/S0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS, Cheong Y, Congdon RT. HLM 6: Hierarchical linear and nonlinear modeling. Scientific Software International; Lincolnwood, IL: 2004. [Google Scholar]
- Repetti RL, Robles TF, Reynolds B. Allostatic processes in the family. Development and Psychopathology. 2011;23:921–938. doi: 10.1017/S095457941100040X. doi:10.1017/S095457941100040X. [DOI] [PubMed] [Google Scholar]
- Rivest L-P. Statistical properties of Winsorized means for skewed distributions. Biometrika. 1994;81:373–383. doi:10.1093/biomet/81.2.373. [Google Scholar]
- Robles TF, Kiecolt-Glaser JK. The physiology of marriage: pathways to health. Physiology and Behavior. 2003;79:409–416. doi: 10.1016/s0031-9384(03)00160-4. doi:10.1016/S0031-9384(03)00160-4. [DOI] [PubMed] [Google Scholar]
- Robles TF, Shaffer VA, Malarkey WB, Kiecolt-Glaser JK. Positive behaviors during marital conflict: Influences on stress hormones. Journal of Social and Personal Relationships. 2006;23:305–325. doi:10.1177/0265407506062482. [Google Scholar]
- Rohrbaugh MJ, Shoham V, Coyne JC. Effect of marital quality on eight-year survival of patients with heart failure. American Journal of Cardiology. 2006;98:1069–1072. doi: 10.1016/j.amjcard.2006.05.034. doi:10.1016/j.amjcard.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Schoenborn CA. Marital status and health: United States, 1999-2002. U.S. National Center for Health Statistics. Advance Data from Vital and Health Statistics. 2004:1–32. [PubMed] [Google Scholar]
- Smith TW, Gallo LC, Goble L, Ngu LQ, Stark KA. Agency, communion, and cardiovascular reactivity during marital interaction. Health Psychology. 1998;17:537–545. doi: 10.1037//0278-6133.17.6.537. doi:10.1037/0278-6133.17.6.537. [DOI] [PubMed] [Google Scholar]
- Straus MA, Hamby SL, Boney-McCoy S, Sugarman DB. The revised Conflict Tactics Scales (CTS2): Development and preliminary psychometric data. Journal of Family Issues. 1996;17:283–316. doi:10.1177/019251396017003001. [Google Scholar]
- Taylor SE, Way BM, Seeman TE. Early adversity and adult health outcomes. Development and Psychopathology. 2011;23:939–954. doi: 10.1017/S0954579411000411. doi:10.1017/S0954579411000411. [DOI] [PubMed] [Google Scholar]
- Whisman MA. Marital distress and DSM-IV psychiatric disorders in a population-based national survey. Journal of Abnormal Psychology. 2007;116:638–643. doi: 10.1037/0021-843X.116.3.638. doi:10.1037/0021-843X.116.3.638. [DOI] [PubMed] [Google Scholar]
- Whisman MA, Beach SRH, Snyder DK. Is marital discord taxonic and can taxonic status be assessed reliably? Results from a national, representative sample of married couples. Journal of Consulting and Clinical Psychology. 2008;76:745–755. doi: 10.1037/0022-006X.76.5.745. doi:10.1037/0022-006X.76.5.745. [DOI] [PubMed] [Google Scholar]
- Whisman MA, Uebelacker LA. A longitudinal investigation of marital adjustment as a risk factor for metabolic syndrome. Health Psychology. 2012;31:80–86. doi: 10.1037/a0025671. doi:10.1037/a0025671. [DOI] [PubMed] [Google Scholar]
- Young EA, Tolman R, Witkowski K, Kaplan G. Salivary cortisol and posttraumatic stress disorder in a low-income community sample of women. Biological Psychiatry. 2004;55:621–626. doi: 10.1016/j.biopsych.2003.09.009. doi:10.1016/j.biopsych.2003.09.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.