Abstract
Primary neuronal cell culture is a powerful research tool for studies of cellular and molecular neurobiology, and the development of methods for manipulating DNA expression has provided new opportunities to exploit these in vitro models for mechanistic studies. However, because of the specialized equipment and training required to set up primary neuronal cell cultures of consistently high quality, and the need for multiple cultures to optimize transfection parameters for different experimental applications, this model system is often not practical for non-routine use. One solution is to collaborate with a laboratory that routinely cultures primary neurons, but currently this is not feasible if the collaborating laboratories are distant from each other. We describe a method that allows laboratories with the requisite tissue culture expertise to ship live primary cultures of transfected neuronal cells for subsequent experimentation in the receiving laboratory.
Keywords: Hibernate®, hippocampal neurons, neuronal cell culture, neuronal morphology, shipping, transfection, cell viability
A significant technical advance in neuroscience has been the development of methods for culturing and manipulating DNA expression in neurons [1]. For many applications, primary neuronal cell cultures are preferred over cell lines of neural lineage because cell lines do not form clearly distinguishable axons or dendrites and they do not make synapses. In contrast, primary neuronal cell cultures recapitulate the morphological, synaptic and neurochemical features of their in vivo counterparts [1].
Generating consistently high quality primary neuronal cell cultures can be challenging, especially for laboratories in which this is not a routine application. One challenge is dissection of the target neural tissue. The development of proprietary solutions that maintain brain tissue viability for prolonged periods of time after dissection (Hibernate®, BrainBits, Springfield, IL), has enabled shipment of fresh or frozen brain tissue. However, the viability of cultures derived from frozen tissues is significantly less than observed with neuronal cells plated immediately following dissection, and this does not alleviate challenges related to the culture and transfection of primary neurons.
An alternate solution is to collaborate with a laboratory that routinely cultures primary neurons, but if there is significant distance between the collaborating laboratories this is logistically impractical. This problem would be alleviated by the availability of a simple, reliable method for shipping live primary cultures of transfected neurons. Herein we describe an adaptation of the approach developed for shipping neural tissues by BrainBits (http://www.brainbitsllc.com) in which Hibernate® is used to preserve live cultures of transfected hippocampal neurons during shipment.
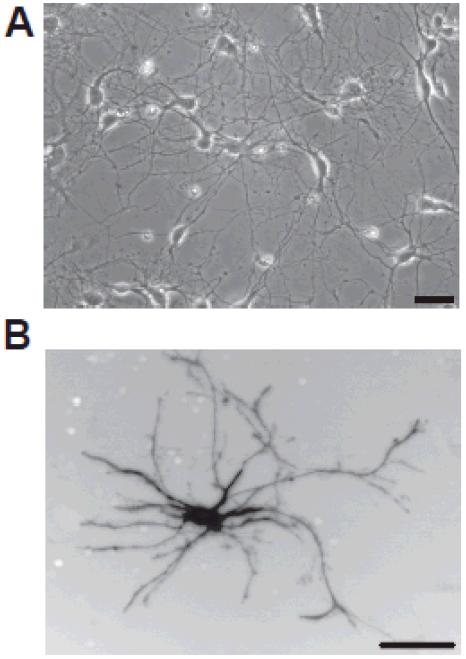
In initial studies, hippocampal neurons were dissociated from embryonic day 18 (E18) rat hippocampi as previously described [2]. A suspension of 1×106 neurons per ml was electroporated with the pmaxGFP vector (Lonza, Cologne, Germany) using an Amaxa Nucleofector (Amaxa Biosystems, Lonza) per the manufacturer’s directions. Following nucleofection, cells were plated at 3×105 cells per well in a 12-well tissue culture plate precoated with poly-D-lysine (100μg/ml; Sigma, St. Louis, MO) and laminin (6μg/ml; Invitrogen, Carlsbad, CA) and maintained in serum-free Neurobasal medium (Invitrogen, Carlsbad, CA) supplemented with B27 (Invitrogen) [3]. Cultures were incubated at 37°C in a humidified atmosphere of 5% CO2 for 24 hours before shipping. Prior to shipping, culture medium was aspirated from each well and immediately replaced with cold Hibernate E® supplemented with 2% B27 (Invitrogen). Wells were filled to maximal capacity to prevent exposure to air if plates were inverted, and wells were covered with PCR sealing film (MJ Research, Inc., Waltham, MA) prior to replacing the plate lid. Covered plates were wrapped tightly with Parafilm® (Pechiney Plastic Packaging, Chicago, IL) and packed in shipping containers surrounded with gel packs pre-cooled to 4°C. Additional packing material was included to minimize shifting of the plates during shipping across country. Upon receipt, the Hibernate® solution was replaced with Neurobasal medium supplemented with B27 pre-warmed to 37°C and incubated as described above. Approximately one-third of the culture medium was replaced every other day. As determined by phase contrast microscopy (Figure 1A) at 7 days in vitro (DIV), neurons look healthy with phase-dark cell bodies and an extensive network of processes. Fluorescence microscopy of a transfected neuron (Figure 1B) demonstrates that neurons elaborate an extensive dendritic arbor. These data indicate that shipping did not compromise neuronal cell viability. Contamination was not an issue even though antibiotics or fungicides were not added to the cultures.
Figure 1.
Primary cultures of dissociated hippocampal neurons shipped across the continental USA within 24 hours of being dissociated, transfected and plated can survive and differentiate for at least 7 days in vitro (DIV). Hippocampal neurons dissociated from the hippocampi of embryonic day 18 (E18) rat pups and transfected with a plasmid encoding GFP were plated onto plastic plates precoated with poly-D-lysine and laminin, shipped 24 hours later by overnight express delivery from Oregon to Kentucky and then maintained in culture for an additional 5 days. (A) Phase contrast micrograph of a culture at 7 DIV. (B) Fluorescent micrograph of a GFP positive neuron in this same culture. Bar = 50μm
In a second set of experiments we examined whether the impact of shipping on neuronal cell viability was influenced by the age of the animal at time of dissection, the age of the culture at the time of shipping, the substrate or the method of transfection. Hippocampi were dissected from P1 rats, dissociated and plated at 1.5 × 105 cells per well in a 24-well tissue culture plate on glass coverslips precoated with poly-D-lysine and laminin and maintained in Neurobasal medium supplemented with B27 [4]. A subset of these cultures were shipped at 2 DIV and maintained upon receipt as described above. A subset of cultures were transfected with plasmid encoding microtubule-associated protein 2 conjugated to enhanced GFP (MAP2-eGFP) at 4 DIV using Lipofectamine 2000 (Invitrogen) as previously described [4]. One plate of transfected cultures were shipped 24 hours post-transfection (5 DIV). All transfected cultures were imaged at 7 DIV to visualize GFP-positive cells; untransfected cultures were fixed in 4% paraformaldehyde at 7 DIV and immunostained for MAP2 as previously described [2].
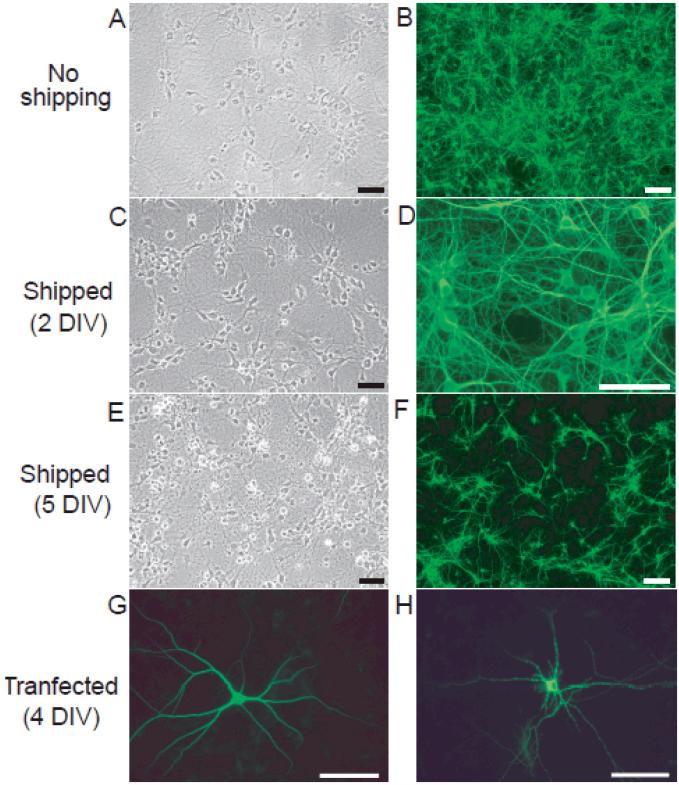
As observed with cultures derived from E18 rats, shipping cultures derived from P1 rats 24 hours after plating (2 DIV) did not compromise neuronal cell viability (Figure 2, panels A-D). Comparison of cultures that were not shipped (Figure 2A, B) to cultures shipped at 2 DIV (Figure 2C, D) by phase-contrast (Figure 2A, C) and fluorescent (Figure 2B, D) microscopy revealed no significant differences in cell density, cell morphology or MAP-2 immunoreactivity at 7 DIV. In contrast, shipping cultures at 5 DIV significantly decreases neuronal cell viability as indicated by an increased number of phase-bright neurons and degeneration of neurites (Figure 2, compare panels E and A). There is also a significant decrease in the density of MAP2-positive neurons in cultures shipped at 5 DIV (Figure 2F) relative to cultures that were not shipped (Figure 2B). Consistent with these signs of decreased viability, transfected neurons in cultures shipped at 5 DIV exhibit a “beaded” appearance along the length of their dendrites (Figure 2, compare panels G and H).
Figure 2. Neuronal viability decreases with increased age of primary hippocampal cultures at the time of shipping.
Phase contrast (A, C, E) and fluorescent (B, D, F, G and H) microphotographs of primary cultures of dissociated hippocampal neurons at 7DIV. All cultures were set up from the same dissection of postnatal day 1 (P1) rat hippocampi. A subset of cultures was transfected with MAP2-eGFP at 4 DIV (G, H); remaining cultures were immunostained for MAP2 at 7 DIV (B, D, F). Relative to control cultures that were not shipped (A, B), shipping cultures at 2 DIV (C, D) had minimal impact on neuronal cell viability whereas shipping cultures at 5 DIV (E, F) significantly decreased neuronal cell health. Comparison of transfected neurons in cultures that were not shipped (G) versus those in cultures shipped at 5 DIV confirms decreased viability in the latter. Bar = 50μm.
These data indicate the feasibility of shipping viable primary cultures of dissociated hippocampal neurons. This method is effective with hippocampal neurons derived from either embryonic or early postnatal rats, plated on either tissue culture plastic or glass coverslips and transfected using either nucleofection or lipid-mediated delivery systems. One factor that does influence the viability of cultures is the age of the culture at the time of shipping. Success is optimal when cultures are shipped prior to 5 DIV. This simple protocol will enable laboratories with the requisite tissue culture expertise to ship live primary cultures of transfected neuronal cells thereby facilitating collaborations across disciplines.
Acknowledgements
This work was supported by grants from the NIH (NS046649 and ES014901 to PJL and NS045103 to DAA) and the COBRE program of the NCRR (P20RR20171 to DAA). This paper is subject to the NIH Public Access Policy.
Footnotes
Competing Interests
The authors declare no competing interests.
References
- 1.Banker GA, Goslin K. Culturing Nerve Cells. The MIT Press; Cambridge, MA: 1998. [Google Scholar]
- 2.Lein PJ, Guo X, Shi GX, Moholt-Siebert M, Bruun DD, Andres DA. The novel GTPase Rit differentially regulates axonal and dendritic growth. J Neurosci. 2007;27:4725–4736. doi: 10.1523/JNEUROSCI.5633-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 4.Wayman GA, Impey S, Marks D, Saneyoshi T, Grant WF, Derkach V, et al. Activity-dependent dendritic arborization mediated by CaM151 kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron. 2006;50:897–909. doi: 10.1016/j.neuron.2006.05.008. [DOI] [PubMed] [Google Scholar]