Abstract
The current study investigates the roles of orthopaedic biomaterial particles (Ti, PMMA, UHMWPE, Co-Cr) on the differentiation and functions of bone marrow stromal cells (BMSCs). Cells were isolated from femurs of BALB/c mice and cultured in complete osteoblast-induction medium in presence of micron-sized biomaterial particles at various doses. MTT assay and lactate dehydrogenase (LDH) assay were performed for cell proliferation and cytotoxicity. Differentiation and function of osteoblasts were evaluated by alkaline phosphatase (ALP), osteocalcin, RANKL, OSX, and Runx2 expressions. Murine IL-1, IL-6 and TNFα in culture media were determined by ELISA. Challenge with low doses of Ti, UHMWPE, or Co-Cr particles markedly promoted the bone marrow cell proliferation while high dose of Co-Cr significantly inhibited cell growth (p<0.05). Cells challenged with low dose of PMMA or UHMWPE particles (0.63 mg/ml) exhibited strong ALP activity, whereas Ti, and Co-Cr groups showed minimal effects (p<0.05). UHMWPE and Ti particles also promoted higher expression of pro-inflammatory cytokines. Real-time PCR data suggested that cells treated with low dose (0.5mg/ml) particles resulted in distinctly diminished RANKL expression compared to those exposed to high concentrated (3mg/ml) particles. In conclusion, various types of wear debris particles behaved differently in the differentiation, maturation, and functions of osteogenic cells; and the particulate debris-interacted bone marrow stromal cells may play an important role in the pathogenesis and process of the debris-associated aseptic prosthetic loosening.
Keywords: aseptic loosening, wear debris, osteoblast, bone marrow stromal cells, gene expression
INTRODUCTION
Over 500,000 total joint arthroplasties (TJA) are performed each year in USA to treat terminal stage of osteoarthritis, and the number of these orthopedic procedures continues to increase annually1. Despite the popularity and dramatic effectiveness, there is relatively high incidence of long term failure in both cemented and cement-less joint replacement. While failures due to infection, fracture and dislocation have become relatively rare with improved surgical techniques, osteolysis-associated aseptic prosthetic loosening has become a common cause of long-term failure following total joint replacement1–3. As no effective drug therapy is currently available to inhibit osteolysis, surgical revision is eventually required.
Investigations have suggested that particulate debris generated during wear plays a critical role in the pathogenesis of aseptic loosening (AL)4,5. Implants are impervious to mechanical loads and enzymatic destruction, so continuously generated wear particles disturb the biological environment resulting in the accumulation of phagocytes that ingest the foreign material. Repeated phagocytosis by macrophages and other inflammatory cells results in cellular activation and the release of inflammatory cytokines, including interleukin-1β, tumor necrosis factor-α, interleukin-6, and other mediators6,7. These cytokines contribute to the local osteoclastogenesis, periprosthetic osteolysis, and ultimately aseptic loosening. We have previously reported that gene transfers of human interleukin-1 receptor antagonist (IL-1Ra) effectively ameliorated orthopedic biomaterial-induced local inflammation in synovial-like murine air pouch membranes and human periprosthetic tissues implanted in mice8,9. Current studies have suggested that particulate wear debris initiate a complex pathology in periprosthetic tissues that includes the participation of bone marrow stromal cells, osteoblasts and haematogenous cells such as macrophages, monocytes, lymphocytes, which in turn lead to an unbalance of osteoclastogenesis regulators (RANKL and OPG)10–14.
While the monocyte/macrophage lineage cells play a critical and direct role in the differentiation of osteoclasts, it is important to consider whether (in addition to promoting osteoclast activity) wear debris also contribute to the implant loosening through direct inhibition of bone formation. Bone marrow stromal cells (BMSCs) including osteoblast progenitor cells are naturally present within the prosthesis site and in close contact with the implant; and may be critical contributors to the maintenance of bone homeostasis. Perturbation of BMSCs by implants and wear debris may affect the cells differentiation and functions, leading to the reduced osteogenesis. In addition_as major resident cells around prosthesis, BMSCs may also participate in the periprosthetic inflammation and wear debris associated osteoclastogenesis15,16. Numerous reporters have indicated that variant kinds of wear particle induce different cellular and molecular response at the periprosthetic site17. We have previously reported that different composition of particles exhibited variant cellular infiltration and inflammatory responses on in the air pouch model18,19. UHMWPE, PMMA debris and metal particles are commonly present in periprosthetic tissue retrieved from AL patients at surgical revisions20. The current study extends our previous findings to test the hypothesis that various types of orthopaedic biomaterials (Ti, PMMA, UHMWPE, Co-Cr) may distinctly influence the proliferation and differentiation of bone marrow osteoblastic progenitor cells and participate in the process of aseptic prosthetic loosening.
MATERIALS AND METHODS
BMSCs culture and induction
Bone marrow cells were flushed from bilateral femurs and tibiae of male BALB/c mice (6–8 weeks of age). Using density centrifugation over Histopaque®-1083 (Sigma-Aldrich, St. Louis, MO), mononuclear cells (MNCs) were isolated. The cells were cultured and purified by adherence method in DMEM culture medium supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Grand Island, NY), 100U/mL penicillin (Invitrogen, Grand Island, NY), 100 mg/mL streptomycin (Invitrogen, Grand Island, NY), and 2mM L-glutamine (Invitrogen, Grand Island, NY) at 37°C and 5% CO2 atmosphere21. After 24 hours, unattached cells in supernatant were moved. An average of 3×106 attached BMSCs-like cells were obtained from a male BALB/c mouse. For differentiation to osteoblasts, BMSCs were induced in DMEM culture medium supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Grand Island, NY), 10mM β-glycerol phosphate (Sigma-Aldrich, St. Louis, MO), 0.1mM L-ascorbic acid (Sigma-Aldrich, St. Louis, MO), and 10nM dexamethasone (Sigma-Aldrich, St. Louis, MO), 2 mM glutamine (Invitrogen, Grand Island, NY), 100 U/ml penicillin (Invitrogen, Grand Island, NY), 100 μg/ml streptomycin (Invitrogen, Grand Island, NY)22.
Biomaterial particles
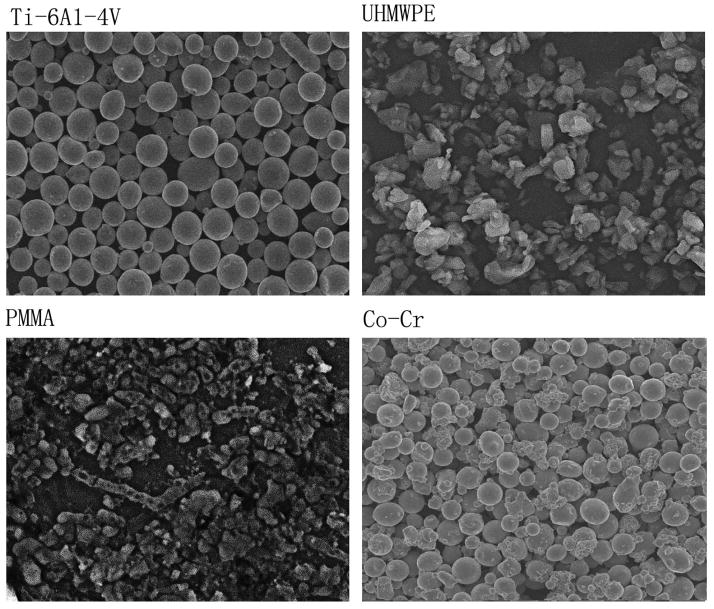
Four types of the particulate orthopaedic biomaterials were used to evaluate the bone marrow cell responses. The size distributions of the particles were determined by a S-2400 Hitachi scanning electron microscopy (SEM). Briefly, particles were dispersed on a 0.1 μm Isopore membrane filter, and dried for 24h. PMMA and UHMWPE samples were gold coated using a Fullam sputter coater prior to SEM imaging. Samples were imaged at 800x magnification to visualize particle size characteristics and particle concentration distribution, which was analyzed using the ImagePro+ software package (Media Cybernetics, Maryland). SEM imaging (Fig. 1) and particle analysis revealed that all materials were predominantly spherical in shape. Ultra high molecular weight polyethylene (UHMWPE) particles (the generous gift of Dr. John Cuckler, University of Alabama, Birmingham, AL) had a mean particle diameter of 3.6 μm, with diameters ranging from 0.8 to 23 μm. Cobalt–chromium alloy particles (mean particle size 5.7 μm, range 0.5 –20 μm) were the generous gift of Dr. Jack Parr (Wright Medical Inc., Memphis, TN). Titanium alloy particles (Ti–6Al–4V) (mean particle size 2.3 μm, size range 0.1–68 μm) were from the Zimmer Corporation (Warsaw, Indiana) and PMMA particles (mean diameter 4.0 μm, range 0.1–10 μm) were obtained from a commercial source (Polyscience, Warrington, PA). The metal and PMMA particles were washed in 70% ethanol solution and confirmed endotoxin-free using the Limulus assay (Endosafe; Charles Rivers, Charlestown, SC).
Figure 1.
Scanning electron microscopy (SEM) appearance of the particles used in experiments (800×).
Challenges of BMSCs with particles
BMSCs were seeded into 96-well flat bottomed culture plates, 24-well culture plates (Costar, Cambridge, MA), or 4-chamber culture slides, respectively, at a density of approximately 5×105 cells/ml in regular culture medium. After 24h, supernatant was removed and complete-osteoblast-induction medium was replaced. Each type of particles with different concentrations were directly added into the culture wells except for UHMWPE particles due to their floating nature. The UHMWPE particles were first mixed into 100% FBS before dispensing into the culture wells, and the particles were freshly prepared and added every time when medium changed. Conditioned medium from cultured BMSCs with different particles were collected for ELISA assay every 3 days. Cells in 96-well plate were used for cell proliferation determination, while the cells in 24-well plates were prepared for alkaline phosphatase (ALP) assay, lactate dehydrogenase (LDH) assay and mRNA expression analysis.
Assessment of cells proliferation and cytotoxicity
Cell proliferation in response to biomaterial challenge was evaluated by proliferation assay as detailed previously23,24. Aliquots (100μl) of 5×104 BMSCs were dispensed into each well of the 96-well plates in the presence of the biomaterial particles at 0.63, 1.25, 2.5 or 5 mg/ml. Cells cultured in osteoblast-induction medium without particles were provided as control group. MTT assay was performed to determine the mitochondrial activity by colorimetric reaction at 1, 3, 5, 7 days after incubation at 37°C and 5% CO2 atmosphere25. The plate was read at 590nm using a microplate photospectrometer (Molecular Devices, Menlo Park, CA), and cell responses were expressed as a proliferation index, which was calculated by normalization with the baseline proliferation (non-particle controls).
CytoTox 96® Non-Ratio Cytotoxicity Assay (Promega, Madison, WI) was used to measure dead/live cell ratio. Lactate dehydrogenase (LDH) activity in conditioned supernatant and lysed cells was respectively assayed by colorimetric reaction with Substrate Mix in the kit. The OD values were recorded spectrophotometrically at 490 nm and the proportion indicated the cell viability.
ALP activity assay and Enzyme-linked immunosorbent assay (ELISA)
Cells in 24-well plate, co-cultured with two concentrations at 0.5 or 3 mg/ml of each particle, were harvested at 7 days. After lysed in CelLytic™ M (Sigma-Aldrich, St. Louis, MO) and centrifuged for 15 minutes at 12000 rpm, the supernatant was saved for protein assay. Cell lysates were assayed using the ALP Kit (Sigma-Aldrich, St. Louis, MO). Based on the colorimetric reaction with hydrolysis of p-nitrophenyl phosphate, ALP activity was determined by OD values read at 405 nm wavelength on a microplate spectrophotometer (Molecular Devices, Menlo Park, CA). Enzyme activity was normalized against protein concentration.
The levels of IL-1, IL-6 and TNFα in culture media after 3-day cell cultures were determined by ELISA as detailed previously26. All the antibody pairs were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The levels of each cytokine in the sample were calculated by regression analysis from a standard curve assayed on the same plate concomitantly.
Immunocytochemical (ICC) staining and image analysis
Aliquot (200 μl) of 1×105 BMSCs were seeded in 4-chamber slides and treated with each type of particle at 2 mg/ml. A control group without particle challenge was also included. Immunocytochemical staining was performed to detect osteocalcin as an indication of osteoblast phenotype at 8 days after treatments. The induced cells were fixed in 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO) and permeated with 0.1% Triton X-100 in PBS, then incubated in 3% blocking serum (ImmunoCruz™ goat ABC Staining System, Santa Cruz biotechnology, CA) for 1 hour at 37°C to minimize non-specific binding. Following the incubation with goat polyclonal antibody against mouse osteocalcin (2 μg/ml, Santa Cruz biotechnology, CA) for 60 minutes at room temperature, visualization was achieved using a biotinylated secondary antibody and avidin-peroxidase complex (ImmunoCruz™ goat ABC Staining System, Santa Cruz biotechnology, CA). Cells were then counterstained with Gill’s hematoxylin solution (Sigma-Aldrich, St. Louis, MO). Similarly, the osteoclastogenesis mediator RANKL expressing cells were visualized using the same ICC procedure against a polyclonal goat anti-mouse RANKL antibody (Santa Cruz biotechnology, CA). In negative controls of ICC staining, the primary antibodies were replaced with non-immune goat sera at the same concentration.
To reveal ALP expression in the cells, an ALP staining Kit (Sigma-Aldrich, St. Louis, MO) was used following the manufacture’s instruction. Briefly, alkaline-dye mixture was prepared to dissolve Fast Violet B capsule and Naphthol AS_MX Alkaline Phosphate in distilled water. Following fixation in citrate buffered acetone for 30 seconds; cells were incubated in alkaline-dye mixture for 30 minutes at 26°C and in Mayer’s Hematoxylin for 10 minutes (counterstain).
Digital images of ICC stained slides were captured and analyzed using the Image-Pro Plus software (version 7.0, Media Cybernetics, Silver Spring, MD. The level of expression and localization of these positive stains was evaluated in six different fields and expressed as positive cell number/mm2 or integrated optical density (IOD)8,9.
Real-time PCR
Real-time reverse transcriptase–polymerase chain reaction (RT-PCR) was performed to evaluate the influence of wear particles on osteoblastogenesis, with gene expression of Lrp5, NFAT, OSX, Runx2, RANKL, and OPG determined. Total RNA from cells homogenates was extracted using a commercial kit following the manufacturer’s instructions (Tel-Test, Friendswood, TX). The quantity and purity of the RNA and DNA were examined by spectrophotometric analysis at 260 and 280 nm wavelength. The cDNA was reverse transcribed from 0.5 μg of total RNA in a 20 μl reaction mixture containing 1×PCR buffer, 500 μM each of deoxynucleotide triphosphates, 0.5 units/μl of RNase inhibitor, 2.5 μM random hexamers, 5.5mM MgCl2, and 1.25 units/μl of RT (Perkin-Elmer Cetus, Norwalk, CT). The reaction mixture was incubated in a Veriti 96-well Thermal Cycler (Applied Biosystems) at 25°C for 10 minutes, 48°C for 25 minutes, followed by 95°C for 5 minutes. Real-time PCR was performed according to the manufacturer’s instructions. To standardize target gene level with respect to variability in quality of RNA and cDNA, we used GAPDH transcripts (a housekeeping gene) as an internal control. Reaction mixtures of 25 μl including 12.5 μl of 2×SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) and target gene primer pairs (at 400nM final concentration) and 2 μl cDNA was run in a MicroAmp optical 96-well reaction plate with MicroAmp optical caps for 35 cycles (denaturing at 94°C for 1 min, annealing at 60°C for 55 seconds, and elongation at 72°C for 60 seconds) in an StepOnePlus Real-Time PCR System (Applied Biosystems) and the fluorescent signals were recorded dynamically. The values of threshold cycle (Ct) at which a statistically significant increase in reporter signals (ΔRn) was first detected were used to calculate the relative quantification of target gene expression. With the Ct value of GAPDH as an internal control and the mean Ct value of target gene expression from non-particle (medium) control group as the calibrator sample (named 0% expression), the comparative quantification of gene expression of the samples from variant particles groups were calculated according to the formula given in the manufacture’s manual. To verify the correct amplification of the target gene, real time PCR products were visualized under UV light after electrophoresis on 1.8% agarose gels containing ethidium bromide8.
Statistical analysis
The results were expressed as arithmetic mean and standard deviation of six separate samples for experimental and control groups, while three independent experiments were performed with each particle challenge. Statistical analysis between groups was performed by single factor analysis of variance (ANOVA) test; with the Schafer formula for post hoc multiple comparisons (SPSS 17.0.1, Chicago, IL). A p-value of less than 0.05 was considered as significant difference.
RESULTS
Cell characterization
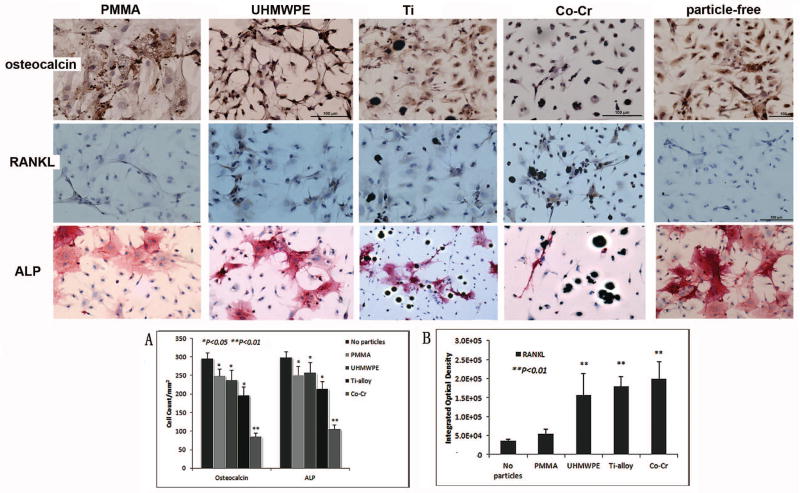
Cell colonies of primary passage were detected in complete osteoblast-induction medium. As differentiation and proliferation proceeded, the cells gradually exhibited osteoblasts-like spindle or polygonal morphology in osteoblastogenic medium. Addition of various types of particles resulted in phagocytosis by the cells during the cell cultures (Fig. 2). The osteoblast-like cell populations were confirmed with positive specific marker labeling. Osteocalcin expression was visualized in majority of the conditioned cells (76.3 ± 5.3%; Fig. 6), and the positive staining for ALP was observed in 85.3 ± 3.9% cells (Fig. 6).
Figure 2.
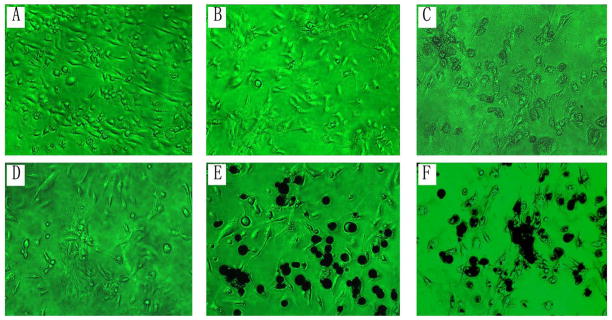
Morphology of BMSCs under the variant treatments at 4-day cultures (×200): (A) Cells in DMEM culture medium; (B) Cells in complete-osteoblast-induction medium without particles; (C–F) Co-culturing cells with biomaterial particles: (C) PMMA, (D) UHMWPE, (E) Titanium, and (F) Co-Cr.
Figure 6.
Immunocytochemical stains against osteocalcin, RANKL and ALP on particle-interacted (2mg/ml) or particle-free control cells (×200). Osteocalcin and RANKL were stained dark brown and the ALP+ cells stained in pink. Panel (A) summarizes the quantification of osteocalcin+ and ALP+ cells among groups, and panel (B) illustrates the RANKL expression determined by integrated optical density of ICC staining using a computerized image analysis system. (*P<0.05, **P<0.01)
Effects of particle exposure on cell proliferation and cytotoxicity
The proliferation assay indicated that Ti and UHMWPE significantly stimulated the proliferation of BMSCs at both high and low particle concentrations, and there was no marked difference between the various doses (Fig. 3A). Co-Cr particles also promoted cell proliferation except at the highest concentration (5mg/ml). In contrast, PMMA did not stimulate cell proliferation effect even at low doses (0.63mg/ml). In addition, cells challenged with the particles appeared to exhibit certain cytotoxic effect at high concentration (3mg/ml), although UHMWPE, Ti, or Co-Cr particle at low dose (0.5mg/ml) did not show significant difference when compared with the non-challenged control group (Fig. 3B).
Figure 3.
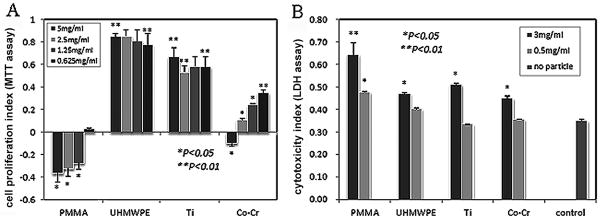
(A) MTT assay to estimate the viability and proliferation of BMSCs treated by various concentrated particulate PMMA, UHMWPE, Ti and Co-Cr. Stimulation indexes for each response were calculated by comparison with baseline proliferation (particle-free controls); (B) Release of lactate dehydrogenase (LDH) in conditioned medium as an indicator of cell damage, which was normalized to LDH levels in cell lysate to obtain the cytotoxicity index for the BMSCs at 7-day cultures.
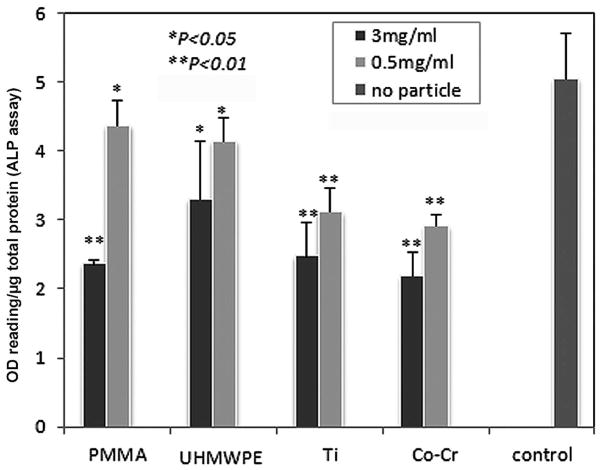
ALP activity
Figure 4 summarized the ALP activities of the BMSCs challenged by Ti–6Al–4V, PMMA, UHMWPE, or Co-Cr particles at two concentrations (3mg/ml or 0.5mg/ml). All types of biomaterial particles exhibited a varied degree of negative regulation on ALP activity, especially at the high dose (3mg/ml). However, it appeared that low dose treatment of PMMA, UHMWPE particles (0.5mg/ml) in complete induction medium revealed significantly stronger ALP activity than Ti and Co-Cr groups that only showed minimal ALP activities (Fig 4).
Figure 4.
ALP activity assay of cell lysate. The OD values of ALP activity were normalized against the total protein concentration in the sample and expressed as arbitrary unit. The control sample was from cells lysate without particle treatment (*p<0.05, **p<0.01 compared to the control).
Cytokine levels in conditioned media
The levels of murine IL-1, IL-6 and TNFα in culture media after 3-day cell cultures were summarized in Figure 5. BMSCs with UHMWPE or Co-Cr particles resulted in a distinct increase in the pro-inflammatory cytokine expressions to the culture media.
Figure 5.
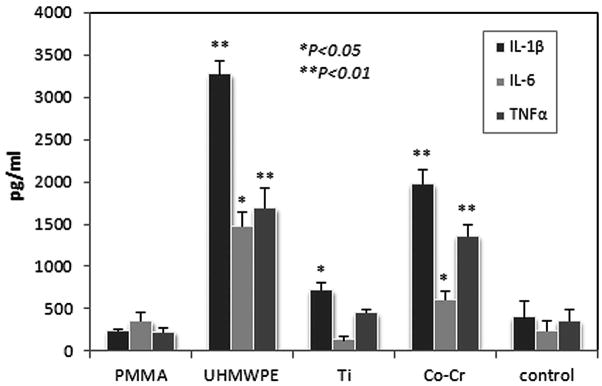
ELISA to assess the levels of IL-1β, IL-6 and TNFα in the culture media following Ti, HPE, or Co-Cr challenges. The control values were from culture medium without particles (*p<0.05, **p<0.01 compared to the control).
Cytology and Immunocytochemistry analysis
Hematoxylin and eosin staining demonstrated that the bone marrow cells were differentiated into osteoblast-like phenotype cells, appearing spindle or elongated morphology accompanied with extracellular matrix biosynthesis, spherical and larger nuclei22,27. Following different treatments, variant behaviors of proliferation, morphological characteristics, and matrix generation were observed in BMSCs cultured on slides (Fig. 6). Quantification of osteocalcin+ and ALP+ cells (Fig 6A) suggested that all types of particle interactions decreased the BMSCs differentiation to the osteoblast-like phenotype compared to complete-induction medium control group. Among them, Co-Cr particles exhibited the strongest inhibition (p<0.01).
As for the RANKL expression within the cells, the image analysis indicated that UHMWPE, Ti-alloy, and Co-Cr significantly promoted its expression, whereas PMMA challenge resulted in a minimal influence on the RANKL expression (Fig 6B).
Real-time PCR examination
To gain insights of the molecular basis of the biomaterial particle effects on BMSCs, we profiled changes in multi-gene expression of challenged BMSCs by real-time PCR technique. No marked variations in RunX2 and Lrp5 production were observed among the variant types of particles either at high (3mg/ml) or low (0.5mg/ml) doses (Fig. 7). However, BMSCs challenged with low concentrated (0.5mg/ml) particles exhibited a higher OSX and NFATc1 expression than at high concentration (3mg/ml), suggesting that OSX and NFATc1 may contribute to the osteogenesis from BMSCs with particles stimulation, especially with Ti–6Al–4V or Co-Cr particles stimulation that resulted in significantly higher OSX and NFATc1 generation than UHMWPE and PMMA.
Figure 7.
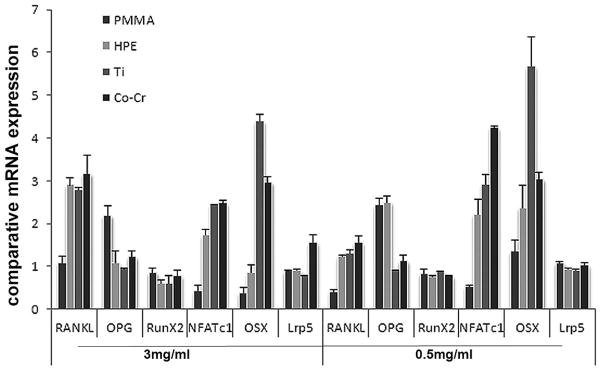
RT-PCR to compare the gene expression profiles among BMSCs groups following variant particles challenges (0.5 or 3mg/ml). The data is expressed as comparative gene copies against readings from no-particle challenged control group (see Materials and methods).
OPG expression with UHMWPE or PMMA particles treatment exhibited a distinctly higher level than Ti–6Al–4V and Co-Cr particles, which might suggest the OPG involvement in osteogenesis following challenge by UHMWPE or PMMA particles. Cells treated with variant particles at low dose (0.5mg/ml) exhibited distinctly reduced RANKL generation compared with stimulation using high concentration (3mg/ml), as expected by the pivotal effect of RANKL on osteolysis10,28. Specifically, PMMA treated BMSCs showed the lowest RANKL expression, which was consistent with the immunocytochemistry results, suggesting that PMMA particles may exert a weak effect on osteolysis mediated by BMSCs.
DISCUSSION
Implant wear is an unavoidable event in TJA patients due to the normal articulation of the prosthesis; and wear debris associated chronic periprosthetic inflammation and bone resorption appear to play critical roles in pathogenesis of aseptic loosening4,5. It is widely accepted that a range of cell types originated from hematopoietic stem cells and bone marrow stem cells are within the periprosthetic tissue (monocytes/macrophages, lymphocytes, fibroblasts, osteoclasts, osteoblasts, etc), which participate in and contribuate to the local inflammation and the implant instability11–14. Bone is a dynamic tissue with a well-balanced homeostasis preserved by both formation and resorption of bone, it is important to consider whether, in addition to promoting osteoclast activity, wear debris may also contribute to osteolysis directly through osteoblast/BMSCs mediated periprosthetic inflammation and inhibited bone formation. Compared with osteoclast recruitment and activation, insufficient attention has been paid to the possible involvement in osteolysis by osteoblasts, the primary cell type responsible for bone formation29. The major factors that contribute to osteolysis are related to the amount, composition, accumulation rate, exposure time, and antigenicity of the wear particulates17,19. We have previously reported that variations in the material composition and shapes of the particulate stimuli resulted in different tissue responses, and that combinations of different biomaterials may elicit synergy in the level of the inflammatory response18,26. Effects of wear debris on bone marrow stromal cells, pre-osteoblast cells or mature osteoblasts have been discussed in previous studies, however, only one type of wear particles was involved in most of these studies respectively. The current study extended these observations, simultaneously examined the respective effects of variant clinical biomaterials on osteogenesis and other functions of BMSCs. In this study, we have examined the effect of variant types of wear particles on the differentiation and functions of BMSCs, including cell proliferation, osteoblast markers, inflammatory cytokines, and osteoclastogenesis regulators.
BMSCs, generally considered the major osteoprogenitor cell type and located in bone marrow stroma in high numbers29, are thus likely to be critically affected during particle-mediated aseptic loosening process. During the life course of the joint prosthesis, particularly in the case of poor initial implant fixation with excessive micromotion and constant accumulation of wear particles over time, bone marrow cells are continuously exposed to high concentrated particulate debris throughout the stage of bone repair or reconstruction. It may cause disturbance or disruption of normal BMSCs osteogenic differentiation process and subsequently result in a diminished population of functional osteoblasts, thus compromising osseointegration at the bone-implant interface. It is reported that while the clinical etiology of osteolysis seems to derive from wear, aseptic loosening without osteolysis also occurs30. While osseointegration around the implants happens initially, its ability to remain well-fixation may be hampered by the effects of wear particles on BMSCs. Several studies have shown particulate suppression of bone formation using BMSCs11,31,32. Under osteogenic conditions, exposure of human MSCs to sub-micron size titanium particles suppressed bone sialoprotein (BSP) gene expression, reduced collagen type I and BSP production, decreased cellular proliferation and viability, and inhibited matrix mineralization31. Our study demonstrated that all types of biomaterial particles exhibited a varied degree of negative regulations on the ability of MSCs to bony remodeling required for osseointegration, especially at higher concentration. It appeared that Ti and Co-Cr particles possessed more severe inhibitory influence on osteogenesis than PMMA and UHMWPE particles.
Pro-inflammatory cytokines are strongly implicated in wear particle mediated osteolysis30. Wear debris associated cytokine activation of osteoclasts and macrophages has been demonstrated33. The current study evaluated whether pro-inflammatory cytokines were involved in the particle-associated BMSC osteogenic differentiation. It is apparent that particle-stimulated osteoblastic cells contributed to the regulation of many pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α. In this study, BMSCs with UHMWPE or Co-Cr particles resulted in a distinct increase in IL-1β and TNFα expressions. Evidences have suggested that IL-1β and TNFα are potent mediators of bone resorption, activating osteoclast or precursor cells, and also providing activation signals to lymphocytes. In turn, the lymphocyte derived IL-2, IL-6, or γ-IFN could influence osteoclastic activity and bone remodeling34.
In addition, our data demonstrated a significantly elevated gene expression of RANKL in particle-challenged osteoblasts. As we have previously demonstrated, altered RANKL/OPG ratio plays a crucial role in the differentiation of ostoclasts from macrophage or osteoclast precursors35. In a certain sense, the balance between the expression of the stimulator of osteoclastogenesis, RANKL, and the inhibitor OPG dictates the quantity of bone resorption and the kinetics of remodeling10,28,35. Thus wear debris particle-challenged osteoblasts may contribute to the periprosthetic osteolysis through RANKL mediated osteoclast maturation and activation. Our study demonstrated a significantly elevated gene expression of RANKL in particle-challenged osteoblasts, which may be the key regulator for cell-cell communication and osteoclastogenesis. This observation is consistent with previous findings of increased RANKL levels as a result of exposure to Ti particles36.
The limitations of this in vitro study may include the use of synthetic biomaterial particles in a mean size range around 2.3 – 5.7μm, depending on different particles. One may argue that many studies have used much smaller particles (mostly metal particles) which might be more clincially relevant for new generations of prosthesis implants. In fact, particulate debris size from different types of biomaterials and specific articulating bearings in patients vary in a wide range. Substantial amount of larger particles are readily retrieved around loosed prosthetic implants. The particles used in this study appear within the size range that reported from literature37 and have resulted in meaningful and important preliminary findings. Further studies of in vitro and in vivo evaluations of different size of particles including those retrieved from patient periprosthetic tissues are warranted to better understand molecular mechanisms of wear debris associated aseptic loosening.
CONCLUSIONS
The data from this study revealed that BMSCs challenged by various orthopaedic particulate stimuli have resulted in different behaviors in cell proliferation, osteogenic effect, and pro-inflammatory response. Each type of particle exhibited varying degrees of suppressive effects on osteogenesis from BMSCs through the regulation of multiple gene expression associated with osteogenesis or osteoblast differentiation. Particle-stimulated BMSCs also respectively expressed a series of pro-inflammatory cytokine genes, which could facilitate the peri-prosthesis inflammation and osteoclasts activation, and ultimately result in osteolysis and bone resorption. Significantly, osteoblastic cells stimulated by wear particles expressed higher level of RANKL, the key cytokine regulator of osteoclast differentiation and activation, which suggested the pivotal role of osteoblasts in regulation of osteoclastogenesis. In conclusion, it appears that wear debris particle-challenged BMSCs contribute to the periprosthetic osteolysis through both osteoclast activation and impaired osteoblast functions.
Acknowledgments
This work was supported by a research grant from National Institutes of Health (5R03AR054929, S.-Y.Y.) and research funding from Orthopaedic Research Institute, Via Christi Health.
References
- 1.Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. Journal of Bone and Joint Surgery. 2005;87(7):1487–97. doi: 10.2106/JBJS.D.02441. [DOI] [PubMed] [Google Scholar]
- 2.Bozic KJ, Kurtz SM, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45–51. doi: 10.1007/s11999-009-0945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. Journal of Bone and Joint Surgery. 2009;91(1):128–33. doi: 10.2106/JBJS.H.00155. [DOI] [PubMed] [Google Scholar]
- 4.Kim KJ, Chiba J, Rubash HE. In vivo and in vitro analysis of membranes from hip prostheses inserted without cement. Journal of Bone and Joint Surgery. 1994;76(2):172–80. doi: 10.2106/00004623-199402000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Goldring SR, Schiller AL, Roelke M, Rourke CM, O’Neil DA, Harris WH. The synovial-like membrane at the bone-cement interface in loose total hip replacements and its proposed role in bone lysis. Journal of Bone and Joint Surgery. 1983;65(5):575–84. [PubMed] [Google Scholar]
- 6.Hundric-Haspl Z, Pecina M, Haspl M, Tomicic M, Jukic I. Plasma cytokines as markers of aseptic prosthesis loosening. Clin Orthop Relat Res. 2006;453:299–304. doi: 10.1097/01.blo.0000229365.57985.96. [DOI] [PubMed] [Google Scholar]
- 7.Stea S, Visentin M, Granchi D, Ciapetti G, Donati ME, Sudanese A, Zanotti C, Toni A. Cytokines and osteolysis around total hip prostheses. Cytokine. 2000;12(10):1575–9. doi: 10.1006/cyto.2000.0753. [DOI] [PubMed] [Google Scholar]
- 8.Yang SY, Wu B, Mayton L, Mukherjee P, Robbins PD, Evans CH, Wooley PH. Protective effects of IL-1Ra or vIL-10 gene transfer on a murine model of wear debris-induced osteolysis. Gene Therapy. 2004;11(5):483–91. doi: 10.1038/sj.gt.3302192. [DOI] [PubMed] [Google Scholar]
- 9.Yang SY, Nasser S, Markel DC, Robbins PD, Wooley PH. Human periprosthetic tissues implanted in severe combined immunodeficient mice respond to gene transfer of a cytokine inhibitor. Journal of Bone and Joint Surgery. 2005;87(5):1088–97. doi: 10.2106/JBJS.D.02052. [DOI] [PubMed] [Google Scholar]
- 10.Mandelin J, Li TF, Liljestrom M, Kroon ME, Hanemaaijer R, Santavirta S, Konttinen YT. Imbalance of RANKL/RANK/OPG system in interface tissue in loosening of total hip replacement. Journal of Bone and Joint Surgery British Volume. 2003;85(8):1196–201. doi: 10.1302/0301-620x.85b8.13311. [DOI] [PubMed] [Google Scholar]
- 11.Vermes C, Chandrasekaran R, Jacobs JJ, Galante JO, Roebuck KA, Glant TT. The effects of particulate wear debris, cytokines, and growth factors on the functions of MG-63 osteoblasts. Journal of Bone and Joint Surgery. 2001;83-A(2):201–11. doi: 10.2106/00004623-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Lassus J, Salo J, Jiranek WA, Santavirta S, Nevalainen J, Matucci-Cerinic M, Horak P, Konttinen Y. Macrophage activation results in bone resorption. Clin Orthop Relat Res. 1998;(352):7–15. [PubMed] [Google Scholar]
- 13.Koreny T, Tunyogi-Csapo M, Gal I, Vermes C, Jacobs JJ, Glant TT. The role of fibroblasts and fibroblast-derived factors in periprosthetic osteolysis. Arthritis and Rheumatism. 2006;54(10):3221–32. doi: 10.1002/art.22134. [DOI] [PubMed] [Google Scholar]
- 14.Sethi RK, Neavyn MJ, Rubash HE, Shanbhag AS. Macrophage response to cross-linked and conventional UHMWPE. Biomaterials. 2003;24(15):2561–73. doi: 10.1016/s0142-9612(03)00056-5. [DOI] [PubMed] [Google Scholar]
- 15.Okafor CC, Haleem-Smith H, Laqueriere P, Manner PA, Tuan RS. Particulate endocytosis mediates biological responses of human mesenchymal stem cells to titanium wear debris. Journal of Orthopaedic Research. 2006;24(3):461–73. doi: 10.1002/jor.20075. [DOI] [PubMed] [Google Scholar]
- 16.Chiu R, Smith KE, Ma GK, Ma T, Smith RL, Goodman SB. Polymethylmethacrylate particles impair osteoprogenitor viability and expression of osteogenic transcription factors Runx2, osterix, and Dlx5. Journal of Orthopaedic Research. 2010;28(5):571–7. doi: 10.1002/jor.21035. [DOI] [PubMed] [Google Scholar]
- 17.Bauer TW. Particles and periimplant bone resorption. Clin Orthop Relat Res. 2002;(405):138–43. doi: 10.1097/00003086-200212000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Wooley PH, Morren R, Andary J, Sud S, Yang SY, Mayton L, Markel D, Sieving A, Nasser S. Inflammatory responses to orthopaedic biomaterials in the murine air pouch. Biomaterials. 2002;23(2):517–26. doi: 10.1016/s0142-9612(01)00134-x. [DOI] [PubMed] [Google Scholar]
- 19.Ren W, Yang SY, Fang HW, Hsu S, Wooley PH. Distinct gene expression of receptor activator of nuclear factor-kappaB and rank ligand in the inflammatory response to variant morphologies of UHMWPE particles. Biomaterials. 2003;24(26):4819–26. doi: 10.1016/s0142-9612(03)00384-3. [DOI] [PubMed] [Google Scholar]
- 20.Margevicius KJ, Bauer TW, McMahon JT, Brown SA, Merritt K. Isolation and characterization of debris in membranes around total joint prostheses. Journal of Bone and Joint Surgery. 1994;76(11):1664–75. doi: 10.2106/00004623-199411000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Wang L, Fatahi R, Kronenberg M, Kalajzic I, Rowe D, Li Y, Maye P. Isolation of murine bone marrow derived mesenchymal stem cells using Twist2 Cre transgenic mice. Bone. 2010;47(5):916–25. doi: 10.1016/j.bone.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu H, VandeVord PJ, Mao L, Matthew HW, Wooley PH, Yang SY. Improved tissue-engineered bone regeneration by endothelial cell mediated vascularization. Biomaterials. 2009;30(4):508–17. doi: 10.1016/j.biomaterials.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 23.Schaefer CJ, Whalen JD, Knapp T, Wooley PH. The influence of silicone implantation on type II collagen-induced arthritis in mice. Arthritis and Rheumatism. 1997;40(6):1064–72. doi: 10.1002/art.1780400611. [DOI] [PubMed] [Google Scholar]
- 24.Yang SY, Zhang K, Bai L, Song Z, Yu H, McQueen DA, Wooley PH. Polymethylmethacrylate and titanium alloy particles activate peripheral monocytes during periprosthetic inflammation and osteolysis. Journal of Orthopaedic Research. 2011;29(5):781–6. doi: 10.1002/jor.21287. [DOI] [PubMed] [Google Scholar]
- 25.Kasugai S, Hasegawa N, Ogura H. A simple in vito cytotoxicity test using the MTT (3-(4,5)-dimethylthiazol–2-yl)-2,5-diphenyl tetrazolium bromide) colorimetric assay: analysis of eugenol toxicity on dental pulp cells (RPC-C2A) Japanese Journal of Pharmacology. 1990;52(1):95–100. doi: 10.1254/jjp.52.95. [DOI] [PubMed] [Google Scholar]
- 26.Yang SY, Ren W, Park Y, Sieving A, Hsu S, Nasser S, Wooley PH. Diverse cellular and apoptotic responses to variant shapes of UHMWPE particles in a murine model of inflammation. Biomaterials. 2002;23(17):3535–43. doi: 10.1016/s0142-9612(02)00032-7. [DOI] [PubMed] [Google Scholar]
- 27.Zinger O, Anselme K, Denzer A, Habersetzer P, Wieland M, Jeanfils J, Hardouin P, Landolt D. Time-dependent morphology and adhesion of osteoblastic cells on titanium model surfaces featuring scale-resolved topography. Biomaterials. 2004;25(14):2695–711. doi: 10.1016/j.biomaterials.2003.09.111. [DOI] [PubMed] [Google Scholar]
- 28.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289(5484):1504–8. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 29.Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2003;5(1):32–45. doi: 10.1186/ar614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abu-Amer Y, Darwech I, Clohisy JC. Aseptic loosening of total joint replacements: mechanisms underlying osteolysis and potential therapies. Arthritis Res Ther. 2007;9 (Suppl 1):S6. doi: 10.1186/ar2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang ML, Nesti LJ, Tuli R, Lazatin J, Danielson KG, Sharkey PF, Tuan RS. Titanium particles suppress expression of osteoblastic phenotype in human mesenchymal stem cells. Journal of Orthopaedic Research. 2002;20(6):1175–84. doi: 10.1016/S0736-0266(02)00076-1. [DOI] [PubMed] [Google Scholar]
- 32.Vermes C, Roebuck KA, Chandrasekaran R, Dobai JG, Jacobs JJ, Glant TT. Particulate wear debris activates protein tyrosine kinases and nuclear factor kappaB, which down-regulates type I collagen synthesis in human osteoblasts. Journal of Bone and Mineral Research. 2000;15(9):1756–65. doi: 10.1359/jbmr.2000.15.9.1756. [DOI] [PubMed] [Google Scholar]
- 33.Rader CP, Sterner T, Jakob F, Schutze N, Eulert J. Cytokine response of human macrophage-like cells after contact with polyethylene and pure titanium particles. J Arthroplasty. 1999;14(7):840–8. doi: 10.1016/s0883-5403(99)90035-9. [DOI] [PubMed] [Google Scholar]
- 34.Manolagas SC, Jilka RL, Girasole G, Passeri G, Bellido T. Estrogen, cytokines, and the control of osteoclast formation and bone resorption in vitro and in vivo. Osteoporosis International. 1993;3 (Suppl 1):114–6. doi: 10.1007/BF01621882. [DOI] [PubMed] [Google Scholar]
- 35.Zhang T, Yu H, Gong W, Zhang L, Jia T, Wooley PH, Yang SY. The effect of osteoprotegerin gene modification on wear debris-induced osteolysis in a murine model of knee prosthesis failure. Biomaterials. 2009;30(30):6102–8. doi: 10.1016/j.biomaterials.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Granchi D, Amato I, Battistelli L, Ciapetti G, Pagani S, Avnet S, Baldini N, Giunti A. Molecular basis of osteoclastogenesis induced by osteoblasts exposed to wear particles. Biomaterials. 2005;26(15):2371–9. doi: 10.1016/j.biomaterials.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 37.Schmiedberg SK, Jones LC, Chang DH, Hungerford DS, Frondoza CG. Extraction and characterization of metallic wear debris from total joint arthroplasty. Biomed Sci Instrum. 2007;43:104–9. [PubMed] [Google Scholar]