Abstract
While both epidemiological and experimental animal studies have demonstrated that perinatal exposure to polychlorinated biphenyls (PCBs) negatively impacts cognitive and psychomotor function, there remains considerable uncertainty regarding mechanisms by which PCBs cause these functional deficits. In vitro studies have shown that PCBs can trigger apoptosis in cultured neurons and suggest this effect is mediated in part by increased levels of reactive oxygen species (ROS). However, whether PCBs cause similar effects in vivo in the developing brain has yet to be reported. In this study, rat pups were exposed to the commercial PCB mixture Aroclor 1254 (A1254) at 0.1 or 1.0 mg/kg/d in the maternal diet throughout gestation and lactation. Apoptosis and oxidative damage were quantified in three brain regions within several days after birth and at weaning. Caspase-3 activity was significantly increased in the cortex, hippocampus and cerebellum of newborn but not weanling rats exposed to A1254 at 1.0 mg/kg/d in the maternal diet. The most prominent effect was observed in the cerebellum, and PCB-induced apoptosis in this brain region was confirmed by TUNEL. Western blotting revealed that developmental A1254 exposure also increased levels of 3-nitrotyrosine and 4-hydroxynonenal levels in the cerebellum of new-born rats, indicating increased oxidative damage of proteins and lipids, respectively. These findings provide the first in vivo data in support of the hypothesis that PCB-induced oxidative stress alters spatiotemporal profiles of apoptosis, and suggest that this is an important mechanism contributing to the developmental neurotoxicity of PCBs.
Keywords: Apoptosis, developmental neurotoxicity, neurodevelopment, oxidative stress, polychlorinated biphenyls
Introduction
Polychlorinated biphenyls (PCBs) are a structurally related group of stable, highly lipophilic chemicals with widespread distribution in the environment [1]. Despite the fact that production of these compounds ceased over three decades ago, PCBs persist in the environment and high residue levels are still detected in human tissues [2-4]. Epidemiological data indicate that PCBs negatively impact neuropsychological function in exposed children [5-7], and experimental animal studies confirm that developmental exposure to PCBs causes cognitive and psychomotor deficits [8].
It is widely postulated that these functional deficits reflect subtle perturbations of neuronal connectivity in the developing brain [9]; however, the cellular and molecular mechanisms by which PCBs interfere with neuronal connectivity remain an area of active investigation.
One neurodevelopmental event that is a critical determinant of neuronal connectivity is apoptosis. Apoptosis is essential to normal brain development [10,11], occurring in proliferative zones and in postmitotic cells in both the fetal and postnatal brain [12]. Disruption of either the timing or the magnitude of apoptosis in a given brain region can alter cell number and thus connectivity, causing deficits in higher-order function even in the absence of obvious pathology [11,13,14]. Apoptotic signaling pathways are triggered by various molecular cues, one of the more common being reactive oxygen species (ROS) [15]. PCBs have been shown to increase ROS in primary neuronal cell cultures [8,16-19] and in the brain of rats exposed as adults [2-22]. That PCB-induced ROS may be linked to increased neuronal apoptosis is suggested by reports that PCBs increase apoptosis in primary neuronal cell cultures [17,23,24], and this pro-apoptotic effect can be blocked by agents that decrease intracellular levels of ROS [17,23,25].
The data obtained using in vitro models strongly support the hypothesis that the developmental neurotoxicity of PCBs is mediated at least in part by increased neuronal apoptosis consequent to elevated levels of ROS. However, whether PCBs increase either apoptosis or ROS in the developing brain has not yet been reported. The goal of this study, therefore, was to quantify apoptosis and oxidative damage in various brain regions of rat pups exposed developmentally to PCBs. To approximate human exposures, rat pups were exposed throughout gestation and lactation via maternal consumption of Aroclor 1254 (A1254), a commercial PCB mixture that exhibits a congener profile similar to that found in human tissues including breast milk [26,27]. Our findings indicate that developmental exposure to A1254 causes a significant increase in apoptosis in the hippocampus, cortex and cerebellum at early developmental stages and this is coincident with increased expression of biomarkers of oxidative damage.
Materials and Methods
Animals and PCB exposures
Animals were treated humanely and with regard for alleviation of suffering. All protocols involving animals were approved by the Institutional Animal Care and Use Committees of the John Hopkins University (Baltimore, MD) and Oregon Health & Science University (Portland, OR) prior to the initiation of experimentation. Adult female rats were housed individually, except during breeding, in standard plastic cages with Alpha-Dri bedding (Shepherd Specialty Papers, Watertown, TN, USA) in a temperature-controlled (22 ± 2°C) room on a 12-hour reverse light–dark cycle. Food and water were provided ad libitum. Dams delivered litters of 10–12 pups on average (n = 5 dams per treatment group). Litters were culled to five males and five females at PND1 and pups were weaned on PND21.
Dams were dosed with A1254 (lot #124-191; AccuStandard, New Haven, CT, USA) beginning 2 weeks prior to breeding and continuing throughout gestation and lactation until weaning. A1254 was diluted in corn oil and pipetted onto one-half of a Keebler® Golden Vanilla Wafer (Kellogg Company, Battle Creek, MI, USA). Control animals received wafers dosed with an equal volume (500μl) of vehicle. Doses were adjusted daily to account for changes in body weight of the dams. Dams were fed the wafers in a separate cage to prevent the pups from accessing the wafers and were watched carefully to ensure that the entire wafer was consumed (typically within 5 min).
Samples used in these studies were obtained from two different cohorts. In cohort 1, adult Long Evans rats were Current Neurobiology Volume 1 Issue 1 purchased from Charles River Laboratories (Hollister, CA, USA), dams were exposed to vehicle or A1254 at 1.0 mg/kg/d, and pups were euthanized on postnatal day 1 (PND1) or PND21 to obtain whole brains, which were flash frozen and stored at −80°C until homogenized for caspase-3 activity measurements. In cohort 2, adult Wistar rats were purchased from Charles River Laboratories, dams were exposed to vehicle or A1254 at 0.1 or 1.0 mg/kg/d and pups were euthanized at PND3. Two pups of each gender from each treatment group were perfused sequentially with saline and 4% paraformaldehyde (PFA), their brains dissected and stored in 4% PFA until further analyzed by TUNEL. An additional 10-15 pups from each treatment group were not perfused prior to harvesting their brains, which were rapidly dissected on ice to obtain specific brain regions, which were flash frozen and stored at −80°C until used for analyses of caspase 3 activity or oxidative damage. We have previously published data collected from these same cohorts demonstrating that these exposures did not negatively impact developmental outcomes or cause overt signs of intoxication in dams or pups as determined by lack of treatment-related changes in maternal weight gain during gestation, maternal body weight during lactation, length of gestation, litter size, and weight gain of offspring during lactation [28,29].
Caspase-3 assay
Caspase-3 activity was measured using the ApoAlert™ Caspase Fluorescent Assay Kit (BD Biosciences, Palo Alto, CA, USA), which is a fluorometric assay that detects formation of the fluorescent product 7-amino-4-trifluoromethyl coumarin (AFC) following hydrolysis of the non-fluorescent substrate DEVD-AFC by activated caspase-3. Briefly, frozen brain samples were quickly thawed, homogenized in lysis buffer provided in the kit, and cell lysates centrifuged at 20,800 × g for 10 min at 4°C to precipitate cellular debris. Supernatants were reacted with DEVD-AFC as described in the kit instructions and fluorescence detected using a SPECTRAmax plate reader (Molecular Device, Sunnyvale, CA, USA) equipped with a 400 nm excitation filter and 505 nm emission filter. To confirm that fluorescence in samples was in fact due to caspase-3 activity, negative control reactions were performed by adding a caspase-3 inhibitor (DEVD-CHO) to a subset of samples per the manufacturer’s directions.
Terminal deoxynucleotidyl transferase biotin-d-UTP nick end-labeling (TUNEL)
Apoptosis was quantified in the cerebellum of PND3 pups using the DeadEnd™ Colorimetric TUNEL System (Promega, Madison, WI, USA) per the manufacturer’s instructions. Parasagittal cryosections (12μm thickness) were cut from both hemispheres of fixed PND3 brains starting 1mm from the midline. Sections were coded so that individuals doing the morphometric analyses were blinded to experimental conditions. Two different visual fields (400X magnification) from each of 2 sections per animal in 4 animals per treatment group (n = 16 total fields per experimental group) were randomly chosen and cells with dark brown nuclei were counted as TUNEL-positive or apoptotic.
Quantification of 3-nitrotyrosine (3-NT) and 4-hydroxynonenal (4-HNE)
Levels of 3-NT and 4-HNE in the cerebellum were quantified by western blotting. Briefly, cerebella were homogenized in cold lysis buffer (20 mM HEPES, 150 mM NaCl, 1% TritonX-100, 10% glycerol, 1× complete protease inhibitor cocktail, pH 7.4) using a glass homogenizer. Lysates were centrifuged at 15,300 × g for 2 min and protein concentrations in the supernatants were determined using the BCA protein assay reagent (Thermo Scientific, Rockford, IL, USA). Samples (30 μg protein per lane) were separated by SDS–PAGE on 12% polyacrylamide gels then transferred to polyvinylidene difluoride membranes. After incubation in Licor blocking buffer (Licor Biosciences, Lincoln, NE, USA) diluted 1:1 in PBS, membranes were reacted overnight at 4°C with anti-bodies specific for α-tubulin (1:20,000; Sigma, St. Louis, MO), 4-HNE (1:3,000; Chemicon, Temecula, CA, USA) or 3-NT (1:3,000; Upstate Biotechnology, Lake Placid, NY, USA) diluted in Licor blocking buffer containing 0.1% Tween-20. After four 5 min washes in PBS with 0.1% Tween-20, membranes were reacted with infrared dye-conjugated secondary antibodies (1:2000, Rockland Immunochemicals, Gilbertsville, PA, USA) for 90 min at room temperature. Membranes were then washed and bands visualized and quantified using the Odyssey Infrared Imaging System (Licor Biosciences). Densitometric values obtained for bands recognized by antibodies specific for 3-NT and 4-HNE were normalized against densitometric values obtained for bands recognized by α-tubulin antibodies in the same sample.
Statistical Analysis
All data are presented as the mean ± SEM and analyzed using unpaired t-test or one-way ANOVA. If significant effects were identified in the ANOVA (p < 0.05), post-hoc analyses were performed using the Newman-Keuls Multiple Comparison Test.
Results
PCBs induce apoptosis in the developing brain
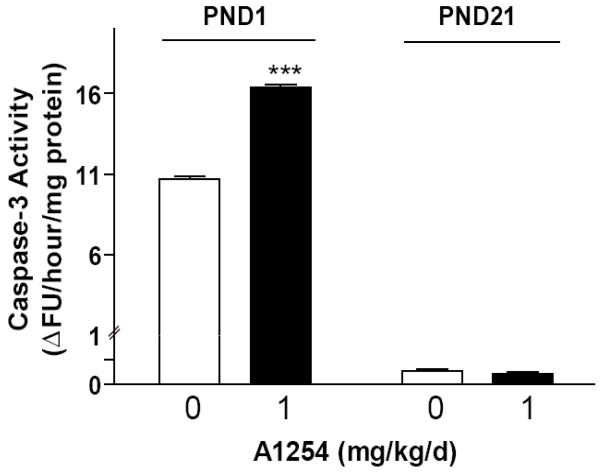
Caspase-3 is an active cell death protease involved in the execution phase of apoptosis, during which cells undergo morphological changes such as DNA fragmentation, chromatin condensation and apoptotic body formation [30]. Thus, as a first test of the hypothesis that PCBs induce apoptosis in the developing brain, we determined whether developmental exposure to environmentally relevant levels of PCBs increases caspase-3 activity in the whole brain, and if so, whether vulnerability varies as a function of developmental stage. In the normal developing rodent brain, apoptosis peaks in most brain regions on PND1 and then falls rapidly to background levels; however, in the cerebellum, apoptosis peaks again on PND21 [12]. Thus, we chose to examine caspase-3 activity at PND1 and PND21. Exposure to A1254 at 1 mg/kg/d in the maternal diet throughout gestation and lactation significantly increased caspase-3 activity in whole brain homogenates from PND1 rat pups relative to age-matched vehicle controls (Figure 1). By weaning at PND21, however, there was no difference in caspase-3 activity between vehicle and A1254-exposed pups (Figure 1).
Figure 1. Developmental exposure to PCBs increases caspase-3 activity in the brain of newborn rats.
Exposure to A1254 at 1 mg/kg/d in the maternal diet through-out gestation and lactation significantly increases caspase activity in the brain of newborn pups (PND1) but not in the brain of weanling pups (PND21). Data presented as the mean ± SEM (N = 6 per treatment group); ***p<0.001 (unpaired t-test).
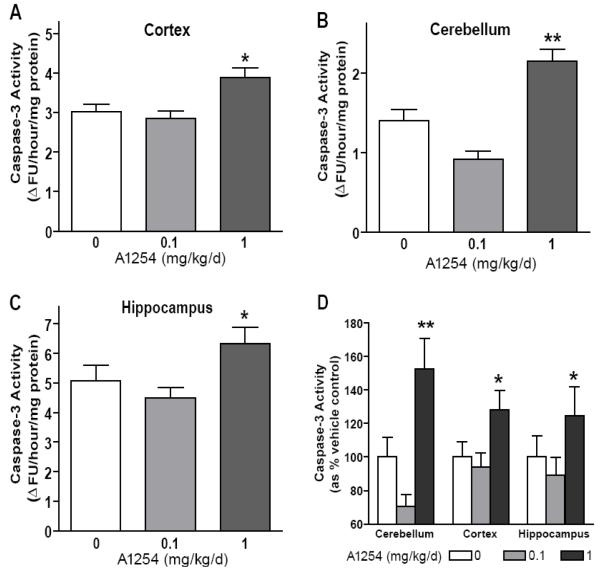
To further characterize the pro-apoptotic activity of PCBs, we examined the effects of two different doses of A1254 on caspase-3 activity in three different brain regions. Since learning, memory and psychomotor deficits occur in multiple species following exposure to PCBs (Schantz et al. 2003), we examined apoptosis in brain regions implicated as primary anatomic substrates of these functions, including the hippocampus [32], cortex [33,34] and cerebellum [35]. Relative to age-matched vehicle controls, developmental exposure to A1254 at 1.0, but not 0.1 mg/kg/day, increased caspase-3 activity in the cortex, cerebellum and hippocampus of PND3 rats (Figure 2A-C). The percent increase in caspase-3 activity in A1254-treated pups relative to brain-region matched vehicle controls was significantly more in the cerebellum relative to either the cortex or hippocampus (Figure 2D). Therefore, all further studies focused on this more vulnerable brain region.
Figure 2. Developmental PCB exposure dose-dependently increases caspase-3 activity in multiple brain regions.
Exposure to A1254 at 1.0 but not 0.1 mg/kg/d in the maternal diet throughout gestation and lactation increases caspase-3 activity in the cortex (A), cerebellum (B) and hippocampus (C) of rats at PND3. Comparison between brain regions of the percent increase in caspase-3 activity in a specific brain region among A1254-treated pups relative to caspase-3 activity in the same brain region among vehicle control pups indicates that developmental PCB exposure causes the greatest percentage increase in caspase activity in the cerebellum (D). Data presented as the mean ± SEM (N = 10-15 per treatment group); *p<0.05; **p<0.01 (One-way ANOVA with Newman-Keuls post hoc).
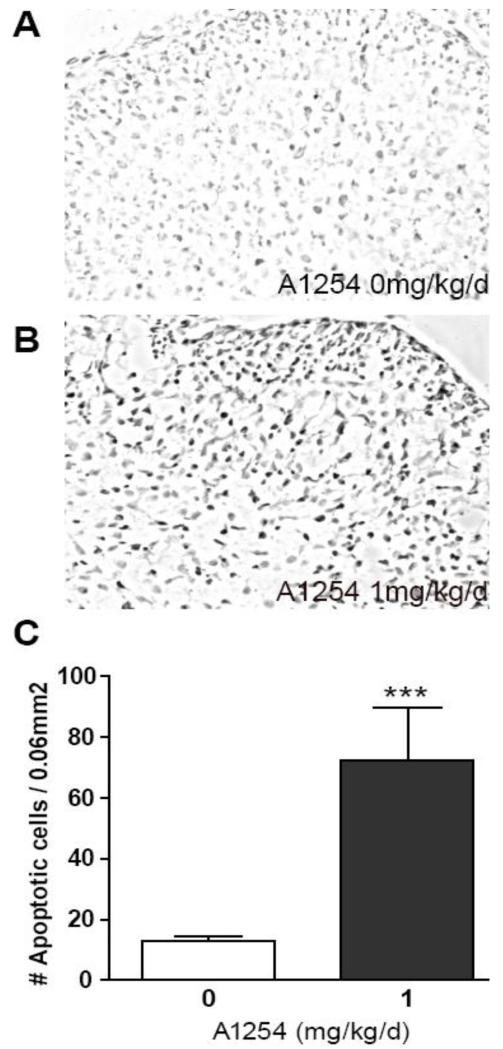
To corroborate findings obtained using caspase-3 activity as a biochemical marker of apoptosis; we assessed the effects of developmental A1254 exposure on apoptosis in the cerebellum of PND3 rats using TUNEL. TUNEL is a histological method for detecting DNA fragmentation, which is a characteristic feature of apoptosis that occurs downstream of caspase-3 activation [30,31]. Relative to age-matched vehicle controls, the number of TUNEL-positive cells was significantly increased in cerebellar sections obtained from pups exposed to A1254 at 1 mg/kg/d in the maternal diet (Figure 3).
Figure 3. Developmental PCB exposure increases cerebellar apoptosis as detected by TUNEL.
Representative photomicrographs of TUNEL staining in sections of cerebellum from PND3 rats exposed developmentally to vehicle (A) or A1254 at 1 mg/kg/d in the maternal diet (B). Quantification of the number of cells labeled by TUNEL, which detects DNA fragmentation, confirms significantly increased apoptosis in the cerebellum of A1254-exposed rats relative to vehicle controls (C). Data presented as the mean ± SEM (N = 16 fields per treatment group); ***p<0.001 (unpaired t-test).
Discussion
Our in vivo data confirm that PCBs modulate apoptosis in the developing brain. Consistent with previous in vitro studies demonstrating that PCBs induce caspase-dependent cell death and DNA fragmentation in primary cerebellar granule cells [17,25] and hippocampal neurons [23], we observed that exposure to A1254 at 1.0 but not 0.1 mg/kg/d in the maternal diet throughout gestation and lactation significantly increased caspase-3 activity and the number of cells labeled by TUNEL in both the cerebellum and hippocampus. Previous in vitro studies of primary cortical neuronal cultures have yielded contradictory results: while one study reported that apoptosis was induced in primary cortical neuron cultures exposed to Aroclors 1248 and 1260 [24], two other studies suggested that this neuronal cell type is refractory to the pro-apoptotic effects of A1254 [23,40]. Our in vivo data, however, indicate that A1254 increased apoptosis in the cortex of developmentally exposed rat pups. The reason(s) underlying differential susceptibility to the proapoptotic effects of A1254 of cortical neurons exposed in vitro versus in vivo are not known. Plausible explanations include: 1) A1254 induces apoptosis in a subset of cells under-represented in primary cortical neuron cultures; 2) differences in the composition of the cellular milieu, particularly the ratio of neurons to glial and other non-neuronal cells; and 3) the influence of systemic factors. The latter two factors would be expected to contribute to differences in metabolic capacity between in vitro and in vivo models, the importance of which is suggested by a recent report that the hydroxylated metabolites of PCBs are equally or more potent than the parent compounds in inducing apoptosis in cultured cerebellar granule cells [17].
Our observation that developmental A1254 exposure increased caspase-3 activity in whole brain homogenates at PND1 but not at PND21 suggests that developmental age influences the effect of PCBs on apoptosis in the brain. Possible explanations include age-related changes in xenobiotic metabolism or in the expression and/or activity of molecular components that mediate the pro-apoptotic activity of PCBs. While the mechanisms underlying PCB-induced apoptosis in vivo have yet to be determined, in vitro studies have demonstrated that this pro-apoptotic activity can be blocked in cultured neurons by agents that decrease intracellular levels of ROS [17,23,25]. Our observations that A1254 exposures that increased apoptosis in the developing brain also significantly increased bio-markers of oxidative damage, specifically levels of 4-HNE and 3-NT, are supportive of a similar mechanism contributing to PCB-induced apoptosis in vivo. Establishing a causal relationship between PCB effects on ROS and apoptosis in the developing brain is the focus of future studies. If PCB-induced ROS are found to be causally related to the pro-apoptotic effect of PCBs, then developmental changes in antioxidant capacity [41-44] and/or the vulnerability of neurons to oxidative stress [45] could influence the critical window(s) of exposure for PCB-induced apoptosis.
The relevance of these studies to human health is suggested by several considerations. First, while these PCB exposure levels are higher than current background levels, they are about three-fold lower than those reported in children exposed prenatally to PCB-contaminated seafood [46]. Second, even seemingly modest increases in apoptosis in the developing brain are thought to interfere with normal neurodevelopment. For example, exposure of the developing rat brain to concentrations of NMDA antagonists associated with functional deficits increases the density of apoptotic neurons from background levels of 1-2 to 15-26% of the total neuronal density in layer II of parietal, frontal and cingulated cortices and to 12% in the late-rodorsal thalamus [47]. Similarly, mercury compounds increase the density of apoptotic neurons in embryonic brain cell aggregates from 5 to 15%, which correlates with a significant decrease in the overall size of the aggregate [48].
It is believed that removal of even a small number of postmitotic neurons during synaptogenesis can significantly alter patterns of connectivity [10,11], resulting in functional deficits in the absence of obvious pathology. Consistent with this proposal, separate studies of litter-mates of the pups used in the current study demonstrated that A1254 at 1 mg/kg/d in the maternal diet throughout gestation and lactation, the exposure paradigm that increased apoptosis, also interfered with dendritic growth and plasticity coincident with deficits in spatial learning and memory in the Morris water maze [29].
In summary, the findings reported herein provide the first in vivo data in support of the hypothesis that PCB-induced oxidative stress alters spatiotemporal profiles of apoptosis, and suggest that this may be an important mechanism contributing to the developmental neurotoxicity of PCBs.
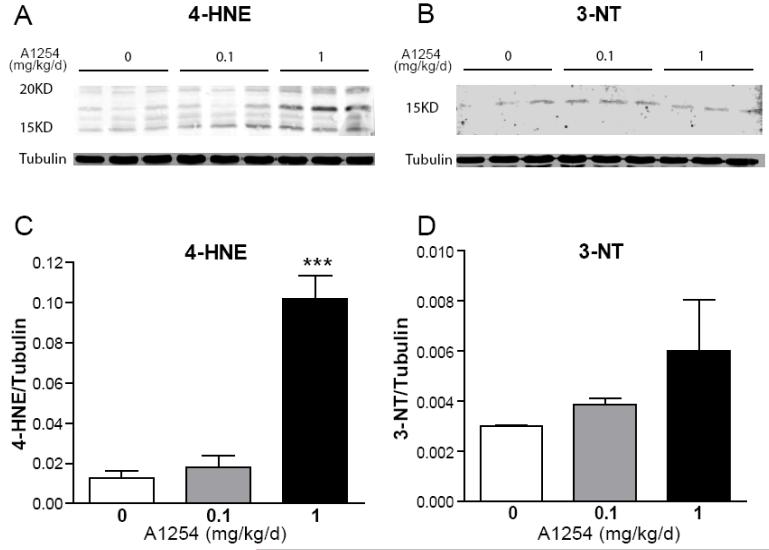
Figure 4. Developmental PCB exposure increases levels of oxidative stress biomarkers in the cerebellum.
Representative western blots (A,B) and corresponding densitometric analyses (C,D) of 4-hydroxynonenal (4-HNE, A,C) and 3-nitrotyrosine (3-NT, B,D) in homogenates of whole cerebellum from PND3 rats. Exposure to A1254 at 1.0 but not 0.1 mg/kg/d in the maternal diet throughout gestation and lactation increases 4-HNE, a biomarker of oxidized lipids and 3-NT, a biomarker of oxidized proteins, with the former effect reaching statistical significance. Data presented as the mean ± SEM (N = 4 per treatment group); ***p<0.001 (One-way ANOVA with Newman-Keuls post hoc).
Acknowledgements
This work was supported by the United States National Institutes of Health (grant #ES014901 awarded to PJL).
References
- 1.Hornbuckle KC, Carlson DL, Swackhamer DL, Baker JE, Eisenreich SJ. Polychlorinated Biphenyls in the Great Lakes. In: Hites RA, editor. The Handbook of Environmental Chemistry: Persistent Organic Pollutants in the Great Lakes. Springer-Verlag; Berlin: 2006. pp. 13–70. [Google Scholar]
- 2.DeCaprio AP, Johnson GW, Tarbell AM, Carpenter DO, Chiarenzelli JR, Morse GS, Santiago-Rivera AL-Schymura MJ. Polychlorinated biphenyl (PCB) exposure assessment by multivariate statistical analysis of serum congener profiles in an adult Native American population. Environ Res. 2005;98(3):284–302. doi: 10.1016/j.envres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Humphrey HE, Gardiner JC, Pandya JR, Sweeney AM, Gasior DM, McCaffrey RJ, Schantz SL. PCB congener profile in the serum of humans consuming Great Lakes fish. Environ Health Perspect. 2000;108(2):167–172. doi: 10.1289/ehp.00108167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park JS, Linderholm L, Charles MJ, Athanasiadou M, Petrik J, Kocan A, Drobna B, Trnovec T, Bergman A, Hertz-Picciotto I. Polychlorinated biphenyls and their hydroxylated metabolites (OH-PCBS) in pregnant women from eastern Slovakia. Environ Health Perspect. 2007;115(1):20–27. doi: 10.1289/ehp.8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carpenter DO. Polychlorinated biphenyls (PCBs): routes of exposure and effects on human health. Rev Environ Health. 2006;21(1):1–23. doi: 10.1515/reveh.2006.21.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Korrick SA, Sagiv SK. Polychlorinated biphenyls, organochlorine pesticides and neurodevelopment. Curr Opin Pediatr. 2008;20(2):198–204. doi: 10.1097/MOP.0b013e3282f6a4e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schantz SL, Widholm JJ, Rice DC. Effects of PCB exposure on neuropsychological function in children. Environ Health Perspect. 2003;111(3):357–576. doi: 10.1289/ehp.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mariussen E, Fonnum F. Neurochemical targets and behavioral effects of organohalogen compounds: an update. Crit Rev Toxicol. 2006;36(3):253–89. doi: 10.1080/10408440500534164. [DOI] [PubMed] [Google Scholar]
- 9.Pessah IN, Cherednichenko G, Lein PJ. Minding the calcium store: Ryanodine receptor activation as a convergent mechanism of PCB toxicity. Pharmacol Ther. 2009 doi: 10.1016/j.pharmthera.2009.10.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dikranian K, Ishimaru MJ, Tenkova T, Labruyere J, Qin YQ, Ikonomidou C, Olney JW. Apoptosis in the in vivo mammalian forebrain. Neurobiol Dis. 2001;8(3):359–379. doi: 10.1006/nbdi.2001.0411. [DOI] [PubMed] [Google Scholar]
- 11.Martin LJ. Neuronal cell death in nervous system development, disease, and injury (Review) Int J Mol Med. 2001;7(5):455–78. [PubMed] [Google Scholar]
- 12.White LD, Barone S., Jr. Qualitative and quantitative estimates of apoptosis from birth to senescence in the rat brain. Cell Death Differ. 2001;8(4):345–356. doi: 10.1038/sj.cdd.4400816. [DOI] [PubMed] [Google Scholar]
- 13.Barone S, Jr., Das KP, Lassiter TL, White LD. Vulnerable processes of nervous system development: a review of markers and methods. Neurotoxicology. 2000;21(1-2):15–36. [PubMed] [Google Scholar]
- 14.Sastry PS, Rao KS. Apoptosis and the nervous system. J Neurochem. 2000;74(1):1–20. doi: 10.1046/j.1471-4159.2000.0740001.x. [DOI] [PubMed] [Google Scholar]
- 15.Haddad JJ. Redox and oxidant-mediated regulation of apoptosis signaling pathways: immuno-pharmaco-redox conception of oxidative siege versus cell death commitment. Int Immunopharmacol. 2004;4(4):475–93. doi: 10.1016/j.intimp.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Fonnum F, Mariussen E, Reistad T. Molecular mechanisms involved in the toxic effects of polychlorinated biphenyls (PCBs) and brominated flame retardants (BFRs) J Toxicol Environ Health A. 2006;69(1-2):21–35. doi: 10.1080/15287390500259020. [DOI] [PubMed] [Google Scholar]
- 17.Dreiem A, Rykken S, Lehmler HJ, Robertson LW, Fonnum F. Hydroxylated polychlorinated biphenyls increase reactive oxygen species formation and induce cell death in cultured cerebellar granule cells. Toxicol Appl Pharmacol. 2009;240(2):306–313. doi: 10.1016/j.taap.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee DW, Gelein RM, Opanashuk LA. Heme-oxygenase-1 promotes polychlorinated biphenyl mixture aroclor 1254-induced oxidative stress and dopaminergic cell injury. Toxicol Sci. 2006;90(1):159–167. doi: 10.1093/toxsci/kfj052. [DOI] [PubMed] [Google Scholar]
- 19.Lee DW, Opanashuk LA. Polychlorinated biphenyl mixture aroclor 1254-induced oxidative stress plays a role in dopaminergic cell injury. Neurotoxicology. 2004;25(6):925–939. doi: 10.1016/j.neuro.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Venkataraman P, Krishnamoorthy G, Vengatesh G, Srinivasan N, Aruldhas MM, Arunakaran J. Protective role of melatonin on PCB (Aroclor 1,254) induced oxidative stress and changes in acetylcholine esterase and membrane bound ATPases in cerebellum, cerebral cortex and hippocampus of adult rat brain. Int J Dev Neurosci. 2008;26(6):585–591. doi: 10.1016/j.ijdevneu.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Venkataraman P, Muthuvel R, Krishnamoorthy G, Arunkumar A, Sridhar M, Srinivasan N, Balasubramanian K, Aruldhas MM, Arunakaran J. PCB (Aroclor 1254) enhances oxidative damage in rat brain regions: protective role of ascorbic acid. Neurotoxicology. 2007;28(3):490–498. doi: 10.1016/j.neuro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Muthuvel R, Venkataraman P, Krishnamoorthy G, Gunadharini DN, Kanagaraj P, Jone Stanley A, Srinivasan N, Balasubramanian K, Aruldhas MM, Arunakaran J. Antioxidant effect of ascorbic acid on PCB (Aroclor 1254) induced oxidative stress in hypothalamus of albino rats. Clin Chim Acta. 2006;365(1-2):297–303. doi: 10.1016/j.cca.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Howard AS, Fitzpatrick R, Pessah I, Kostyniak P, Lein PJ. Polychlorinated biphenyls induce caspase-dependent cell death in cultured embryonic rat hippocampal but not cortical neurons via activation of the ryanodine receptor. Toxicol Appl Pharmacol. 2003;190(1):72–86. doi: 10.1016/s0041-008x(03)00156-x. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez-Alonso JA, Lopez-Aparicio P, Recio MN, Perez-Albarsanz MA. Polychlorinated biphenyl mixtures (Aroclors) induce apoptosis via Bcl-2, Bax and caspase-3 proteins in neuronal cell cultures. Toxicol Lett. 2004;153(3):311–26. doi: 10.1016/j.toxlet.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Mariussen E, Myhre O, Reistad T, Fonnum F. The polychlorinated biphenyl mixture aroclor 1254 induces death of rat cerebellar granule cells: the involvement of the N-methyl-D-aspartate receptor and reactive oxygen species. Toxicol Appl Pharmacol. 2002;179(3):137–44. doi: 10.1006/taap.2002.9353. [DOI] [PubMed] [Google Scholar]
- 26.Hansen LG. The Ortho Side of PCBs: Occurrence and Disposition. Kluwer Academic Publishers; Boston: 1999. p. 269. [Google Scholar]
- 27.Kodavanti PR, Kannan N, Yamashita N, Derr-Yellin EC, Ward TR, Burgin DE, Tilson HA, Birnbaum LS. Differential effects of two lots of aroclor 1254: congener-specific analysis and neurochemical end points. Environ Health Perspect. 2001;109(11):1153–1161. doi: 10.1289/ehp.011091153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dziennis S, Yang D, Cheng J, Anderson KA, Alkayed NJ, Hurn PD, Lein PJ. Developmental exposure to polychlorinated biphenyls influences stroke outcome in adult rats. Environ Health Perspect. 2008;116(4):474–480. doi: 10.1289/ehp.10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang D, Kim KH, Phimister A, Bachstetter AD, Ward TR, Stackman RW, Mervis RF, Wisniewski AB, Klein SL, Kodavanti PR, Anderson KA, Wayman G, Pessah IN, Lein PJ. Developmental exposure to polychlorinated biphenyls interferes with experience-dependent dendritic plasticity and ryanodine receptor expression in weanling rats. Environ Health Perspect. 2009;117(3):426–435. doi: 10.1289/ehp.11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6(2):99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 31.Zou H, Li Y, Liu X, Wang X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274(17):11549–11556. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]
- 32.Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99(2):195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 33.Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychon Bull Rev. 2002;9(4):637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- 34.Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283(5408):1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- 35.Hernandez-Muela S, Mulas F, Mattos L. The contribution of the cerebellum to cognitive processes. Rev Neurol. 2005;40(Suppl 1):S57–64. [PubMed] [Google Scholar]
- 36.Beckman JS, Carson M, Smith CD, Koppenol WH. ALS, SOD and peroxynitrite. Nature. 1993;364(6438):584. doi: 10.1038/364584a0. [DOI] [PubMed] [Google Scholar]
- 37.Crow JP, Beckman JS. Reactions between nitric oxide, superoxide, and peroxynitrite: footprints of peroxynitrite in vivo. Adv Pharmacol. 1995;34:17–43. doi: 10.1016/s1054-3589(08)61079-0. [DOI] [PubMed] [Google Scholar]
- 38.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11(1):81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 39.Uchida K, Szweda LI, Chae HZ, Stadtman ER. Immunochemical detection of 4-hydroxynonenal protein adducts in oxidized hepatocytes. Proc Natl Acad Sci U S A. 1993;90(18):8742–8746. doi: 10.1073/pnas.90.18.8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inglefield JR, Mundy WR, Shafer TJ. Inositol 1,4,5-triphosphate receptor-sensitive Ca(2+) release, store-operated Ca(2+) entry, and cAMP responsive element binding protein phosphorylation in developing cortical cells following exposure to polychlorinated biphenyls. J Pharmacol Exp Ther. 2001;297(2):762–773. [PubMed] [Google Scholar]
- 41.Cao G, Giovanoni M, Prior RL. Antioxidant capacity in different tissues of young and old rats. Proc Soc Exp Biol Med. 1996;211(4):359–365. doi: 10.3181/00379727-211-43981. [DOI] [PubMed] [Google Scholar]
- 42.Cao G, Giovanoni M, Prior RL. Antioxidant capacity decreases during growth but not aging in rat serum and brain. Arch Gerontol Geriatr. 1996;22(1):27–37. doi: 10.1016/0167-4943(95)00674-5. [DOI] [PubMed] [Google Scholar]
- 43.Erden-Inal M, Sunal E, Kanbak G. Age-related changes in the glutathione redox system. Cell Biochem Funct. 2002;20(1):61–66. doi: 10.1002/cbf.937. [DOI] [PubMed] [Google Scholar]
- 44.Ono H, Sakamoto A, Sakura N. Plasma total glutathione concentrations in healthy pediatric and adult subjects. Clin Chim Acta. 2001;312(1-2):227–229. doi: 10.1016/s0009-8981(01)00596-4. [DOI] [PubMed] [Google Scholar]
- 45.Perry SW, Norman JP, Litzburg A, Gelbard HA. Anti-oxidants are required during the early critical period, but not later, for neuronal survival. J Neurosci Res. 2004;78(4):485–492. doi: 10.1002/jnr.20272. [DOI] [PubMed] [Google Scholar]
- 46.Grandjean P, Weihe P, Burse VW, Needham LL, Storr-Hansen E, Heinzow B, Debes F, Murata K, Simonsen H, Ellefsen P, Budtz-Jorgensen E, Keiding N, White RF. Neurobehavioral deficits associated with PCB in 7-year-old children prenatally exposed to seafood neurotoxicants. Neurotoxicol Teratol. 2001;23(4):305–317. doi: 10.1016/s0892-0362(01)00155-6. [DOI] [PubMed] [Google Scholar]
- 47.Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283(5398):70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 48.Monnet-Tschudi F. Induction of apoptosis by mercury compounds depends on maturation and is not associated with microglial activation. J Neurosci Res. 1998;53(3):361–367. doi: 10.1002/(SICI)1097-4547(19980801)53:3<361::AID-JNR10>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]