Abstract
Objective. To evaluate the impact of comorbidities on achieving remission by examining changes in the clinical disease activity index (CDAI) in RA patients in the community-based Consortium of Rheumatology Researchers of North America (CORRONA) registry.
Methods. A subcohort of 1548 RA subjects with varying disease duration met the following inclusion criteria: started a DMARD/biologic agent, continued therapy ≥3 months, CDAI ≥2.8 at study entry and followed longitudinally from baseline to follow-up (mean time 7.46 months). Patients reported comorbidities according to a standardized list of 33 conditions. Entry characteristics were compared across age categories using one-way analysis of variance. Linear and logistic regression models were constructed to assess characteristics [e.g. age, disease duration, number of previous DMARDs/biologics, baseline modified health assessment questionnaire (MHAQ), baseline CDAI and number of comorbidities] associated with primary outcomes: change in CDAI (baseline to follow-up) and CDAI remission (yes/no).
Results. Although disease activity measures at entry were similar across age categories, older patients had more comorbidities, less improvement in CDAI/MHAQ and were less likely to attain remission at follow-up. However, after adjusting covariates an increasing number of patient-reported comorbidities and higher baseline CDAI (but not age) were consistently and independently associated with a lower likelihood of clinical improvement or remission (P < 0.001).
Conclusion. In this observational cohort of community RA patients an increasing number of patients reported comorbidities, independently correlated with less CDAI improvement over time. These results reaffirm that comorbidities may be an important factor in consideration of treat-to-target recommendations and aid in understanding achievable RA therapeutic goals.
Keywords: comorbidities, rheumatoid arthritis, age, remission
Introduction
The advent of biologic agents has increased expectations for therapeutic response in RA, and remission has been proposed as a reasonable and highly desirable target for treatment results in all patients. Recently there has been international interest in the development of treat-to-target guidelines to facilitate tighter control of RA disease activity [1]. Studies demonstrate that achieving remission and low disease activity improves function, limits disability and perhaps limits comorbidities commonly associated with RA, thus these targets are reasonable to guide treatment decisions. However, the risk–benefit ratio of striving to achieve such goals requires consideration as well. Incorporation of comorbidities, long disease duration, drug-related risks, shared physician–patient decision making and chronological age are clearly important factors that require adjustments of treatment target goals [1].
Comorbid medical conditions commonly coexist with RA and frequently require management with additional medications and monitoring. Ageing may add another layer of complexity in the management of the RA patient, especially to mitigate adverse events and drug toxicities [2–5]. Approximately one-third of all RA patients in the United States are older than 65 [6, 7], and there is the expectation that these numbers will increase.
Several studies in RA have analysed age and comorbidities cross-sectionally [8–12], suggesting that functional status and disease activity measures are worse in older RA patients with a greater number of comorbidities. In contrast, older and younger RA patients respond comparably to therapeutic interventions depending on disease duration in controlled clinical trials, where all subjects must satisfy the same entry criteria [13–15]. Nevertheless, physicians in clinical practice tend to treat older RA patients less aggressively [16–19].
In contrast to patients participating in clinical trials, many patients seen in rheumatology practice have multiple comorbidities and would have been excluded from such trials. It is important to know how this group of patients responds to therapeutic interventions in RA. We hypothesized that older patients and/or patients with more comorbidities are less likely to achieve clinical remission, regardless of disease duration, possibly associated with less aggressive treatment. This hypothesis was tested in a large, prospective observational study of RA patients, the Consortium of Rheumatology Researchers of North America (CORRONA), who were started on a DMARD or biologic agent.
Patients and methods
The CORRONA database is a prospective observational cohort and was assembled with the intention of facilitating cohort studies in rheumatological diseases (RA, PsA, undifferentiated arthritis) by accumulating longitudinal data representing community patients with rheumatic disease. This registry began in October 2001 and continues to recruit and follow patients from both academic and private practice sites. Details of this registry have been published previously [20–24]. All patients provided written informed consent prior to being enrolled in the cohort. In addition, the UCLA Institutional Review Board granted approval for performing this study.
Patient population
Data from the CORRONA registry from October 2001 to August 2007 was included in the database for these analyses. RA patients were from 76 different sites and >200 rheumatologists in the USA. At entry, patients completed a patient enrolment form, which includes information regarding medical and surgical history, family history, review of systems, medication use, assessment of pain and global arthritis activity on a visual analogue scale (VAS) and a modified health assessment questionnaire (MHAQ) [25]. Patients reported comorbidities according a standardized list of 33 comorbid conditions on the enrolment form. The treating rheumatologist also recorded detailed information on drug utilization, with any new starts or changes in dose at the time of registration and at each clinical encounter. Standard physician-derived measures include the 28 tender joint count (TJC), 28 swollen joint count (SJC) and physician global assessment (0–100 VAS). At follow-up visits, patients and physicians completed follow-up questionnaires of joint counts, VAS assessments, MHAQ and details reported elsewhere [20–24]. The gross number of patient-reported comorbidities was used in this cohort, as seen in other clinical studies [10, 11]. Published studies have shown high agreement between patient-reported and physician-reported comorbidities [26–29].
Prespecified cohort
For our analysis, a subset of patients was selected from the CORRONA RA cohort in order to test the hypotheses, and prior to the performance of any analyses. Within the CORRONA database there were a total 14 811 RA patients; however, a majority of patients did not meet the pre-specified cohort criteria. Among the 4937 patients who started a DMARD/biologic agent, there were a total of 1658 patients who did not modify their RA medication regimen during the baseline to follow-up period of at least 3 months. A total of 1548 RA patients met the criteria of ≥3 months of treatment with the new DMARD/biologic, without changing background RA treatment, and were not in remission at baseline (CDAI ≥ 2.8). These criteria were used to develop a stringent homogeneous subcohort.
Predictors of response
Change in CDAI (baseline to follow-up), change in CDAI disease activity category (baseline to follow-up) and CDAI remission at follow-up were the primary outcome measures used to evaluate disease activity. The baseline visit is defined as the visit when a change in therapy was instituted. CDAI is calculated by adding the sum of TJC and SJC as well as patient global and physician global assessment using a 10-cm VAS [CDAI = SJC28 + TJC28 + patient global (in cm) + physician global (in cm)]. Unlike the DAS28, it does not require an acute-phase reactant test result. Remission is defined as a score <2.8, mild disease activity between 2.8 and 10, moderate disease activity between 11 and 22 and severe disease activity >22 [30].
The following clinical and laboratory factors were considered as potential baseline predictors of treatment response: disease duration, age, number of patient-reported comorbidities, time from baseline to follow-up, gender, prednisone use, number of prior DMARDs, physician global assessment, SJC, TJC, ESR, baseline CDAI, baseline MHAQ, RF positivity, ethnicity and education. Patients were subdivided based on age categories as follows: <45, 45–65 and >65 years. DMARD/biologic agents were categorized into three groups: (i) HCQ, minocycline, AZA, cyclosporine (CYC), D-Pen or sulphasalazine (SSZ); (ii) MTX or LEF and (iii) TNF inhibitor or other biologic agent. HCQ and minocycline were combined with other DMARDs in group (i) due to the small numbers of patients who were started on these agents.
Statistics
Patients were categorized based on age as follows: <45, 45–65 and >65 years. Demographic and clinical characteristics at entry were compared across age categories using one-way analysis of variance (ANOVA) or χ2 tests as appropriate. Change scores of disease activity measures from baseline to follow-up were also compared across age groups using one-way ANOVA. The individual comorbidities (each with a weight of 1) were summed to calculate the total number of patient-reported comorbidities (range 0–33). For purposes of modeling, quintiles were reported to better describe the impact of increasing comorbid conditions on the outcome measures.
Multiple linear regression analyses were performed to assess the simultaneous effect of combinations of predictors on the change in CDAI. Multiple logistic regression models were created to evaluate predictors of CDAI remission and change in CDAI category. The components of CDAI (i.e. SJC, physician global, etc.) were not used in the models due to collinearity. Age, race, treatment variables, gender, disease duration, baseline MHAQ and baseline CDAI were included as covariates in each of the models. Interaction effects between patient-reported comorbidities with age categories, baseline CDAI categories and disease duration were also evaluated in the linear and logistic models.
Results
Baseline demographics and clinical measures
In the cohort the mean age was 58.1 (s.d. 13, range 19–91) years and the mean number of patient-reported comorbidities was 5.6 in the entire cohort (s.d. 3.3, range 0–22). Several demographic factors captured at registry entry were statistically different across the age groups (Table 1).
Table 1.
Baseline characteristics and details of DMARD/biologic started by age and comorbidities (n = 1548)
| Baseline characteristics | Age <45 years (n = 217), mean/% (s.d.) | Age 45–64 years (n = 823), mean/% (s.d.) | Age ≥ 65 years (n = 488), mean/% (s.d.) | P-value |
|---|---|---|---|---|
| Age, years | 36.9 (6.2) | 55.1 (5.6) | 72.6 (6) | |
| Disease duration, years | 5.6 (4.7) | 11.0 (9.4) | 14.1 (12.2) | <0.001 |
| RF/CCP ab, % | 74 | 74 | 77 | 0.60 |
| Gender, % female | 88.48 | 75.82 | 71.31 | <0.001 |
| Current smoker, % | 21.6 | 19.08 | 7.76 | <0.001 |
| BMI | 29.1 (7.7) | 30.3 (7.5) | 28.3 (6.4) | <0.001 |
| Race, % | 0.09 | |||
| White | 81.52 | 84.4 | 86.83 | |
| Other | 18.48 | 15.60 | 13.17 | |
| No. comorbidities (0–33) | 3.7 (2.6) | 5.5 (3.2) | 6.7 (3.4) | <0.001 |
| No. prior DMARDs | 2.1 (1.5) | 2.3 (1.7) | 1.83 (1.7) | <0.001 |
| Prednisone dose, % | <0.001 | |||
| No prednisone use | 64 | 62 | 55 | |
| 1–5 mg | 6 | 10 | 17 | |
| 5–10 mg | 15 | 18 | 20 | |
| ≥10 mg | 13 | 9 | 9 | |
| ESR | 23.9 (18) | 26.6 (22) | 34.4 (26.8) | <0.001 |
| Stiffness, min | 80 (124) | 100 (180) | 85.0 (174.8) | 0.13 |
| TJC 28 | 7.3 (7.2) | 6.4 (6.7) | 6.0 (6.4) | 0.16 |
| SJC 28 | 7.0 (6.8) | 7.2 (6.3) | 7.6 (6.3) | 0.01 |
| MD global VAS | 34.4(22.3) | 34.8 (21) | 35.6 (21.3) | 0.12 |
| Patient global VAS | 38.3 (23.1) | 42.1 (26) | 40.0 (26.7) | 0.25 |
| Pain VAS | 41.4 (24.6) | 43.1 (26) | 42.1 (26.5) | 0.051 |
| MHAQ | 0.44 (0.5) | 0.52 (0.5) | 0.44 (0.5) | <0.001 |
| CDAI | 21.5 (14) | 21.3 (13) | 21.1 (13) | 0.52 |
| CDAI mild, % | 23.0 | 21.5 | 24.4 | 0.80 |
| CDAI moderate, % | 36.9 | 37.4 | 35.0 | |
| CDAI severe, % | 40.1 | 41.1 | 40.6 |
| Details of DMARD/biologic started by age and comorbidities | ||||
|---|---|---|---|---|
| Age <45 years (n = 217), % | Age 45–64 years (n = 823), % | Age ≥65 years (n = 488), % | P-value | |
| DMARD started, % | 0.003 | |||
| HCQ, minocycline, AZA, CYCL, c-PEN, SSZ | 12.44 | 15.92 | 17.21 | |
| MTX, LEF | 28.11 | 32.93 | 38.93 | |
| Biologic agent | 59.45 | 51.15 | 43.85 | |
| Comorbidity 0–2 (n = 550), % | Comorbidity 3–5 (n = 612), % | Comorbidity >5 (n = 386), % | P-value | |
|---|---|---|---|---|
| DMARD started, % | 0.78 | |||
| HCQ, minocycline, AZA, CYCL, c-PEN, SSZ | 14.4 | 16.3 | 17.4 | |
| MTX, LEF | 34 | 34 | 33.4 | |
| Biologic agent | 51.6 | 49.7 | 49.2 |
Increased age (across the age groups) was significantly associated with an increased percentage of male gender patients, longer disease duration and greater number of comorbidities (P ≤ 0.001). The mean number of comorbidities in the oldest group was almost double that in the youngest group (mean 6.7 vs 3.7, P < 0.001). Disease duration was greater in the >65 age group compared with the <45 age group (mean 14.1 vs 5.6 years, P < 0.001).
Older RA patients were less likely to start a biologic agent than younger patients (44% vs 60%, P = 0.003; Table 1). However, the distribution of the types of DMARD/biologic agents started did not significantly differ across three categories of the number of patient-reported comorbidities (0–2, 3–5 and >5 comorbidities; P = 0.78). In addition, older RA patients were more likely to be taking prednisone; approximately 45% in patients >65 years vs 36% in patients <45 years (P = 0.03).
Overall, in these patients who were starting a new DMARD/biologic agent, most disease activity measures were clinically similar across the age categories at baseline (Table 1). Although the swollen joint count was statistically significantly different across age categories, the difference across groups was minimal (mean 7.0 in the <45 years age group vs 7.6 in the ≥65 years age group, P = 0.01). ESR increased with age in this cohort as well. At baseline, CDAI, physician global VAS, patient global VAS, pain VAS, number of minutes of morning stiffness, fatigue and TJC28 were similar in the three age groups. The mean MHAQ was statistically significantly higher in the 45–64 years age category (mean 0.52), and age categories <45 and ≥65 years both had the same mean MHAQ of 0.44 (P < 0.001).
Patient-reported comorbidities
Supplementary data (available at Rheumatology Online) describes the frequency of each of the 33 patient-reported comorbid conditions in the RA cohort. Of the 33 reported conditions, hypertension (HTN), back problems, dry mouth/eyes, gastroesophogeal reflux disease (GERD), OA, anaemia, chronic obstructive pulmonary disease (COPD) and depression made up the highest frequencies, ranging from 26% to 38% for each. The conditions with a frequency <5% included myocardial infarction, gastrointestinal bleed, stroke, skin cancers, SLE, alcoholism, other mental disease and Parkinson’s disease.
Changes in disease activity measures over time
The duration of follow-up for this cohort ranged from 3.01 to 43.80 months, with the mean duration of follow-up being 7.46 months overall. These patients had been followed in CORRONA for varying periods of time prior to qualifying for the cohort analyses. CDAI remission at follow-up was significantly less likely in the older age group in comparison with the younger RA group (11% vs 20%, P = 0.01) (Table 2). The patients in the older age category also improved less overall than the patients in the younger group (−5.9 vs −8.6, P < 0.01). Patients were categorized into low, moderate and severe disease activity states based on their CDAI disease status at baseline and at follow-up. Patients improved if they moved to a lower disease activity category, worsened if they moved to a higher disease activity category and were unchanged if the disease activity category remained unchanged. Older patients were less likely to improve a CDAI category; 57% of patients in the <45 years age group improved a disease activity category compared with 44% in the ≥65 years age group (P = 0.045). MHAQ improvement was shown to have less improvement in the older age category than the other two (−0.09 vs −0.05, P = 0.02). Other disease activity measure changes were similar across the age categories. The time between baseline and follow-up assessments was similar across the age groups (Table 2).
Table 2.
Change scores from baseline to follow-up
| Age <45 years (n = 217), mean (s.d.) | Age 45–64 years (n = 823), mean (s.d.) | Age ≥65 years (n = 488), mean (s.d.) | P-value | |
|---|---|---|---|---|
| ESR | −7.2 (15.7) | −4.07 (16) | −6.82 (20.1) | 0.08 |
| TJC28 | −3.81 (6.9) | −2.64 (6.9) | −2.41 (7.0) | 0.001 |
| SJC28 | −2.61 (6.2) | −2.32 (5.5) | −2.01 (5.9) | 0.16 |
| Physician global VAS | −13.4 (22.4) | −12.9 (20.8) | −11.4 (21.2) | 0.15 |
| Patient global VAS | −8.34 (26.7) | −9.83 (27.8) | −7.39 (27.2) | 0.10 |
| MHAQ | −0.09 (0.33) | −0.09 (0.38) | −0.05 (0.38) | 0.02 |
| CDAI | −8.57 (14.2) | −7.22 (13.1) | −5.93 (13.5) | 0.003 |
| CDAI remission, % | 19.82 | 13 | 11.27 | 0.01 |
| CDAI category | 0.045 | |||
| Improve, % | 57 | 50.7 | 44.42 | |
| No change, % | 33.33 | 38.75 | 43.05 | |
| Worsen, % | 9.66 | 10.55 | 12.53 | |
| Time baseline to follow-up, months | 7.32 (3.6) | 7.35 (3.5) | 7.66 (3.9) | 0.08 |
Fig. 1A describes the unadjusted relationship among the number of patient-reported comorbid conditions (range 0–33) and the percentage of patients achieving CDAI remission (not adjusted for baseline factors). As the number of comorbidities decreases, the chance that an RA patient will achieve remission increases, as seen for increasing disease duration. Patients with more than eight comorbid conditions had <10% chance of attaining remission, compared with patients with zero to two comorbidities, who had a 22.5–26% chance. Fig. 1B depicts the unadjusted relationship between the patient’s age and the percentage of patients achieving CDAI remission as already described in Table 1. The figures demonstrate an inverse linear relationship between the number of comorbid conditions and the probability of attaining CDAI remission.
Fig. 1.
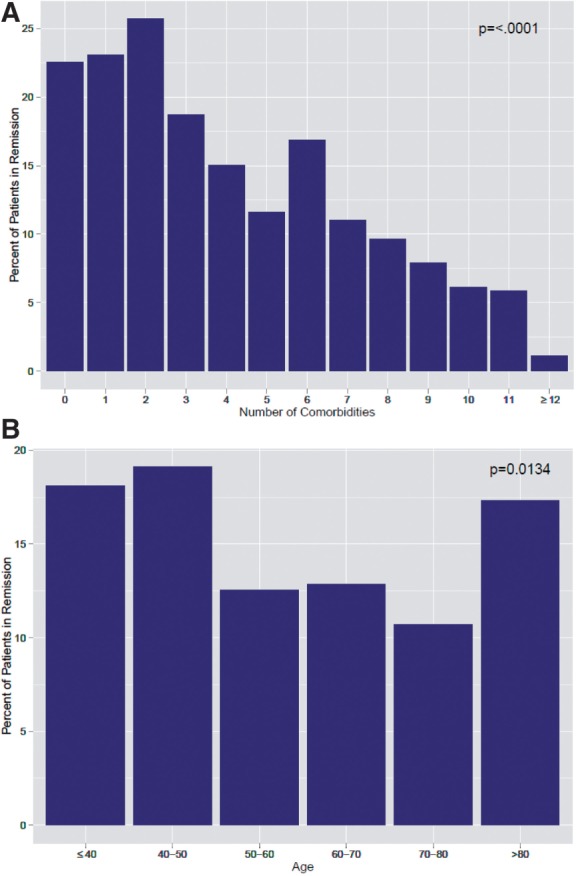
Unadjusted relationship percentage of patients achieving CDAI remission for the number of (A) patient-reported comorbidities and (B) patient age.
P-value represents unadjusted association (logistic regression) between remission and either (A) comorbidities or (B) age.
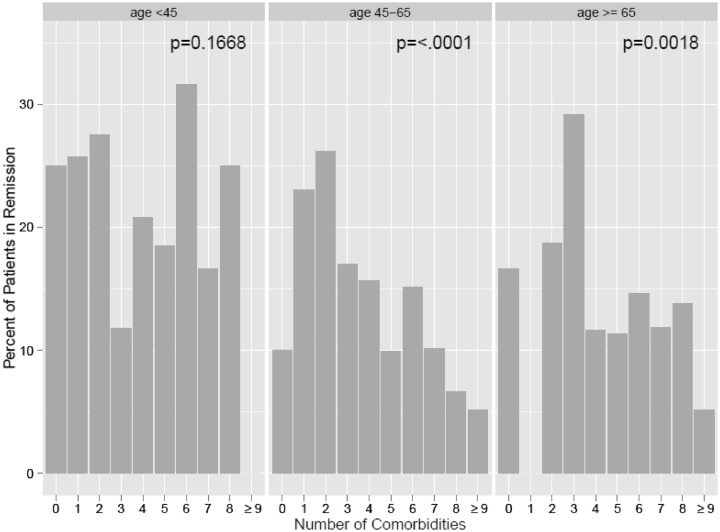
In addition, we evaluated the unadjusted probability of attaining CDAI remission as modified by interactions between the comorbidity measure and age. This was done to assess whether the effect of comorbidity is consistent across age and baseline CDAI disease activity categories. Fig. 2 illustrates the unadjusted relationship between CDAI remission and the number of comorbid conditions across the three different age categories. There was a statistically significant relationship between remission achievement and number of comorbidities for the age groups 45–65 and >65 years. When adjusted for other covariates, the significance seen in the figure disappeared [P = 0.93 for comorbidity × age (45–64 years) and P = 0.85 for comorbidity × age (≥65 years)]. For completeness, we evaluated the interaction effect of the number of patient-reported comorbidities with other variables known to influence remission rates, baseline CDAI and disease duration. Similarly we did not see statistically significant interaction effects (all P > 0.09).
Fig. 2.
Equal rates of remission based on the number of comorbidities and three age categories.
P-value represents the unadjusted association (logistic regression) between remission and comorbidities within each age strata.
Multivariate analyses
Patient-reported comorbidities and age were evaluated in the multivariate models as continuous and as categorical variables, yielding very similar results. Comorbidities were categorized as the closest approximation to quintiles across the cohort (see the Statistics section). These categories were incorporated in the models.
The results of the multivariate linear regression analysis for change in CDAI demonstrated that fewer comorbidities, white race and higher baseline CDAI were associated with a greater improvement in CDAI (Table 3). For example, when evaluating the change in CDAI from baseline to follow-up in the model, the improvement in CDAI was 3.89 units greater in patients with three or fewer comorbidities as compared with patients with nine or more comorbidities. Age, category of DMARD/biologic agent and prednisone use were not independently associated with the change in CDAI.
Table 3.
Multivariate linear model for change in CDAI (baseline to follow-up) and logistic model for CDAI remission
| Linear model for change in CDAI | ||
|---|---|---|
| β-coefficient (s.e.) | P-value | |
| Age, years | 0.02 (0.02) | 0.48 |
| 4 comorbiditiesa | 0.88 (0.98) | 0.37 |
| 5–6 comorbiditiesa | 0.74 (0.83) | 0.37 |
| 7–8 comorbiditiesa | 2.79 (0.95) | 0.003 |
| ≥9 comorbiditiesa | 3.89 (0.98) | <0.001 |
| Baseline CDAI | −0.68 (0.02) | <0.001 |
| Baseline MHAQ | 0.93 (0.67) | 0.17 |
| Female | 0.99 (0.71) | 0.16 |
| White | −2.27 (0.81) | 0.005 |
| RA duration, years | 0.02 (0.03) | 0.60 |
| MTX, LEFb | 1.23 (0.87) | 0.16 |
| Biologicsb | −0.32 (0.84) | 0.70 |
| Prednisone | −0.56 (0.6) | 0.35 |
| Logistic model for CDAI remission (≤2.8) | ||
|---|---|---|
| OR (95% CI) | P-value | |
| Age, years | 1 (0.99, 1.01) | 0.10 |
| 4 comorbiditiesa | 0.82 (0.49, 1.37) | 0.45 |
| 5–6 comorbiditiesa | 0.73 (0.47, 1.15) | 0.17 |
| 7–8 comorbiditiesa | 0.63 (0.36, 1.11) | 0.11 |
| ≥9 comorbiditiesa | 0.29 (0.13, 0.62) | 0.001 |
| Baseline CDAI | 0.97 (0.95, 0.98) | <0.001 |
| Baseline MHAQ | 0.76 (0.48, 1.19) | 0.23 |
| Female | 0.7 (0.48, 1.03) | 0.07 |
| White | 1.31 (0.8, 2.16) | 0.29 |
| RA duration, years | 0.94 (0.92, 0.97) | <0.001 |
| MTX, LEFb | 1.03 (0.62, 1.71) | 0.90 |
| Biologicsb | 1.4 (0.87, 2.27) | 0.17 |
| Prednisone | 0.79 (0.55, 1.13) | 0.19 |
| Logistic model for improvement of CDAI category | ||
|---|---|---|
| OR (95% CI) | P-value | |
| Age, years | 0.99 (0.98, 1) | 0.25 |
| 4 comorbiditiesa | 0.8 (0.55, 1.19) | 0.27 |
| 5–6 comorbiditiesa | 0.85 (0.62, 1.19) | 0.35 |
| 7–8 comorbiditiesa | 0.64 (0.44, 0.94) | 0.02 |
| ≥9 comorbiditiesa | 0.62 (0.42, 0.92) | 0.02 |
| Baseline CDAI | 1.03 (1.02, 1.04) | <0.001 |
| Baseline MHAQ | 0.85 (0.65, 1.12) | 0.25 |
| Female | 0.71 (0.54, 0.95) | 0.02 |
| White | 1.21 (0.88, 1.67) | 0.24 |
| RA duration, years | 1 (0.98, 1.01) | 0.54 |
| MTX, LEFb | 0.94 (0.67, 1.32) | 0.72 |
| Biologicsb | 1.3 (0.93, 1.81) | 0.12 |
| Prednisone | 1.25 (0.99, 1.59) | 0.07 |
aIn reference to ≤3 comorbidities. bIn reference to other DMARDs (i.e. plaquinel, SSZ, etc.).
The multivariate logistic analysis for likelihood of attaining CDAI remission demonstrated that patients with fewer comorbidities, shorter disease duration and higher CDAI baseline scores were more likely to achieve remission (Table 3). For patients with nine or more comorbidities, the odds of achieving remission decreased by 71% when compared with patients with three or fewer comorbidities (P = 0.001).
Another logistic multivariate analysis was performed for improvement in CDAI category (i.e. improving from severe to moderate disease activity, moderate to low, etc., Table 3). Fewer comorbidities and baseline CDAI were significantly associated with improvement in CDAI category. Interestingly, the number of comorbidities did not appear to influence whether a biologic was started. Nevertheless, patients who had seven to eight comorbidities and nine or more comorbidities had a respective 36% (P = 0.02) and 38% (P = 0.02) decreased odds of improving a disease activity category.
MHAQ was not significantly associated with any of the three outcomes of interest after adjusting for other covariates (Table 3), despite evidence that baseline and change in MHAQ was different across the age groups (Tables 1 and 2). Overall, baseline CDAI and the number of patient-reported comorbidities (not age) were consistently significantly related to all three outcomes of interest: change in CDAI, CDAI remission and improvement of CDAI category.
Discussion
This prospective analysis of data for a large cohort of patients initiating a new treatment in the CORRONA registry provides a detailed evaluation of the relationship between age and comorbidities on response to therapy after the start of a new DMARD/biologic agent. Overall, older RA patients who tended to have longer disease duration had less improvement in CDAI and were less likely to attain CDAI remission, but age was not a significant independent predictor of response in the multiple regression models. In contrast, the number of patient-reported comorbidities and baseline CDAI were a consistent, statistically significant predictor of response (CDAI remission, change in CDAI and improvement in CDAI category). Regression models demonstrated a dose effect for the comorbidity categories association to the response outcome measures. As an aside, the number of comorbidities did not appear to influence treatment decision making, and our results confirm other reports that rheumatologists may be age-biased in their decision to initiate a biologic (Table 1) [16–19].
The exact pathophysiology by which comorbidities associate with response to treatment is unclear. Perhaps comorbid conditions directly alter the measures we use to assess RA outcomes. For example, concomitant fibromyalgia has been shown to potentially confound RA response measures [31, 32]. Other commonly patient-reported comorbidities that could conceivably influence response measures include OA, back problems and depression.
Our observations in these analyses suggest that in treat-to-target guidelines aimed at tighter control of RA disease activity, rheumatologists should also consider comorbidities as an important aspect of therapeutic management, as patients with more comorbidities appear less likely to achieve remission. However, specific methods for doing so have yet to be characterized. The results of this study provide evidence that research in this area is needed.
Several published studies have described a cross-sectional relationship between the comorbidities and a reduced probability of remission [8–10]. A large multinational cross-sectional study of 5848 RA patients demonstrated that the number of comorbidities is independently associated with CDAI [odds ratio (OR) 0.75, CI 0.68, 0.83] [10]. Burmester et al. [8] evaluated 6610 active RA patients who were treated with adalimumab for 3 months for predictors of achieving remission. Patients with one or no comorbidity had an OR of 0.86 of attaining DAS28 remission compared with those with more than one. Krishnan et al. [33] studied a random sample of 1530 Finnish patients in the general population and demonstrated that age and comorbidities correlated with pain and patient global VAS. In a later article, Sokka et al. [9] suggested that only 15% of non-RA patients >50 years of age met ACR remission criteria. This suggests that even in a non-RA population, a population where remission should be easier to attain, comorbidities may play a substantial role in negatively impacting the ability to achieve remission.
In addition, several studies have examined the relationship between comorbidities and worsening functional disability [34–38]. Functional disability is an important outcome measure in RA. In 1991 Verbrugge et al. [38] evaluated chronic conditions in general in RA and showed a strong relationship between the number of comorbidities and functional disability in a cross-sectional and longitudinal cohort. In a more recent study, Radner et al. [36] evaluated the impact comorbidities have on the components of the HAQ in a cross section of 380 RA patients; they suggest that comorbidities may partly account for a portion of irreversible disability. Michaud et al. [39] recently reported that age and comorbidities were independently associated with the loss of functional status in RA in a cohort of 18 485 patients in the US National Data Bank for Rheumatic Diseases. Interestingly, our analyses demonstrated that after adjustment of factors known to impact treatment response in RA, MHAQ was not significantly associated with CDAI response, yet patient-reported comorbidities remained consistently associated with various formulations of CDAI response. Clinical trials often exclude older RA patients with multiple comorbid conditions. Our study is distinctive in its prospective quantitation of the influence of comorbidities on therapeutic response in a large community-based RA cohort of patients who started a new DMARD or biologic agent. Several comorbidity indices exist, including the Charlson Comorbidity Index (CCI), Index of Coexistent Disease (ICED), Chronic Disease Score (CDS), Composite Comorbidity Index (CompCI) and Gross Number of Comorbidities [40–43]. The CCI is used to estimate survival and thus is not appropriate for this study, where response to treatment in terms of disease activity is of interest, not mortality. A recent article describes the CCI’s association with RA mortality [44]. In addition, Wolfe et al. [45] assessed the prevalence of comorbidities in four rheumatic diseases and the impact of the CompCI. The ICED and CDS are most useful when prescriptions are obtained from electronic pharmacy databases; the information needed to calculate the CCI, ICED and CDS was not captured in the CORRONA database. The evaluation of the gross number of patient-reported comorbidities may be questioned due to possibilities of misclassification [46]. This is a limitation of the study; however, there are some data to support that patient-reported histories are often as accurate as histories recorded by physicians [26–29]. In addition, this specific list of the association of patient-reported comorbidites with response to RA treatment has yet to be validated in another cohort. Lastly, the specific patient-reported comorbidities do not include some comorbidites such as kidney disease and some extra-articular manifestations.
Additional limitations include the fact that the CORRONA database was not specifically designed for the purpose of this study and the results should be corroborated in additional cohorts. In order to overcome this, we identified a cohort of patients in the database to address our hypotheses. Notably, the results described from this cohort cannot be applied to an early RA cohort nor to RA patients with higher disease activity often seen in European registries. The reasons for these features have been reviewed, but they are representative of patients using biologic drugs in the USA, where approximately 45% of patients with RA are receiving biologic agents [47]. Nevertheless, the cohort that we studied had moderate to severe baseline disease activity (mean CDAI 21), probably because they were selected based on initiating a new treatment. In addition, this study evaluated RA patients who remained on the drug for at least 3 months and evaluated the patients after an average of 7 months, emphasizing early response. Despite these limitations, the data provide useful observations that have clinical relevance, especially as they represent results in a largely community-based cohort.
In conclusion, this large prospective cohort of RA patients demonstrates that increasing numbers of comorbidities decrease the likelihood of CDAI improvement and CDAI remission following the institution of a new DMARD/biologic agent. The patient’s chronological age was not independently associated with the outcome measures examined in this study cohort. Nevertheless, the decision to start a biologic agent was based to a greater extent on the RA patient’s age than on the number of patient-reported comorbidities. These results suggest that older patients with few comorbid conditions are likely to respond better to new treatments than younger patients with multiple comorbidities and should not be deprived of aggressive therapies only because of age. It is of course prudent to keep in mind the risks and benefits for an individual patient as well. Further studies are needed to gain a better understanding of which specific comorbidities influence response to therapy and factors that influence physicians’ treatment decisions in RA.
Rheumatology key messages.
RA patients with a greater number of comorbidities are less likely to achieve therapeutic response.
Age is not independently associated with RA therapeutic response.
RA treatment decisions may be age-biased rather than based on the number of comorbid conditions.
Supplementary data
Supplementary data are available at Rheumatology Online.
Acknowledgements
The authors thank Janet Elashoff, PhD, for her editorial comments and insightful discussions. The ACR/REF/ASP Junior Career Development Award in Geriatric Medicine was awarded to V.K.R. The National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under award number NIAMS K23 AR057818-02 helped to support V.K.R. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The New Investigator Award from the Scleroderma Foundation and the National Institutes of Health Award (NIAMS K23 AR053858-04) helped to support D.K.’s time.
Disclosure statement: D.E.F is a consultant for and received honoraria and research grants/research support from Abbvie, Actelion, Amgen, BMS, Gilead, GSK, NIH, Novartis, Pfizer, Roche/Genentech, UCB, Biogen Idec and Janssen. J.M.K. is a consultant for Abbvie, Amgen, BMS, Genetech, Pfizer, UCB and Vertex and has received grants from Abbvie, Amgen, BMS, Genetech and Pfizer. All other authors have declared no conflicts of interest.
References
- 1.Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69:631–7. doi: 10.1136/ard.2009.123919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doran MF, Crowson CS, Pond GR, et al. Predictors of infection in rheumatoid arthritis. Arthritis Rheum. 2002;46:2294–300. doi: 10.1002/art.10529. [DOI] [PubMed] [Google Scholar]
- 3.Doran MF, Crowson CS, Pond GR, et al. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287–93. doi: 10.1002/art.10524. [DOI] [PubMed] [Google Scholar]
- 4.Weyand CM, Goronzy JJ. Premature immunosenescence in rheumatoid arthritis. J Rheumatol. 2002;29:1141–6. [PubMed] [Google Scholar]
- 5.Weyand CM, Fulbright JW, Goronzy JJ. Immunosenescence, autoimmunity, and rheumatoid arthritis. Exp Gerontol. 2003;38:833–41. doi: 10.1016/s0531-5565(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 6.Doran MF, Pond GR, Crowson CS, et al. Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty-year period. Arthritis Rheum. 2002;46:625–31. doi: 10.1002/art.509. [DOI] [PubMed] [Google Scholar]
- 7.Rasch EK, Hirsch R, Paulose-Ram R, et al. Prevalence of rheumatoid arthritis in persons 60 years of age and older in the United States: effect of different methods of case classification. Arthritis Rheum. 2003;48:917–26. doi: 10.1002/art.10897. [DOI] [PubMed] [Google Scholar]
- 8.Burmester GR, Ferraccioli G, Flipo RM, et al. Clinical remission and/or minimal disease activity in patients receiving adalimumab treatment in a multinational, open-label, twelve-week study. Arthritis Rheum. 2008;59:32–41. doi: 10.1002/art.23247. [DOI] [PubMed] [Google Scholar]
- 9.Sokka T, Makinen H, Hannonen P, et al. Most people over age 50 in the general population do not meet ACR remission criteria or OMERACT minimal disease activity criteria for rheumatoid arthritis. Rheumatology. 2007;46:1020–3. doi: 10.1093/rheumatology/kem051. [DOI] [PubMed] [Google Scholar]
- 10.Sokka T, Hetland ML, Makinen H, et al. Remission and rheumatoid arthritis: data on patients receiving usual care in twenty-four countries. Arthritis Rheum. 2008;58:2642–51. doi: 10.1002/art.23794. [DOI] [PubMed] [Google Scholar]
- 11.Treharne GJ, Douglas KM, Iwaszko J, et al. Polypharmacy among people with rheumatoid arthritis: the role of age, disease duration and comorbidity. Musculoskeletal Care. 2007;5:175–90. doi: 10.1002/msc.112. [DOI] [PubMed] [Google Scholar]
- 12.Westhoff G, Weber C, Zink A. [Comorbidity in rheumatoid arthritis of early onset. Effects on outcome parameters] Z Rheumatol. 2006;65:487–4, 496. doi: 10.1007/s00393-006-0102-z. [DOI] [PubMed] [Google Scholar]
- 13.Fleischmann R, Baumgartner SW, Weisman MH, et al. Long term safety of etanercept in elderly subjects with rheumatic diseases. Ann Rheum Dis. 2006;65:379–84. doi: 10.1136/ard.2005.035287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleischmann R, Iqbal I. Risk: benefit profile of etanercept in elderly patients with rheumatoid arthritis, ankylosing spondylitis or psoriatic arthritis. Drugs Aging. 2007;24:239–54. doi: 10.2165/00002512-200724030-00005. [DOI] [PubMed] [Google Scholar]
- 15.Fleischmann RM, Baumgartner SW, Tindall EA, et al. Response to etanercept (Enbrel) in elderly patients with rheumatoid arthritis: a retrospective analysis of clinical trial results. J Rheumatol. 2003;30:691–6. [PubMed] [Google Scholar]
- 16.Fraenkel L, Rabidou N, Dhar R. Are rheumatologists' treatment decisions influenced by patients' age? Rheumatology. 2006;45:1555–7. doi: 10.1093/rheumatology/kel144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kievit W, van HL, van RP, Fraenkel L. Factors that influence rheumatologists' decisions to escalate care in rheumatoid arthritis: results from a choice-based conjoint analysis. Arthritis Care Res. 2010;62:842–7. doi: 10.1002/acr.20123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogasawara M, Tamura N, Onuma S, et al. Observational cross-sectional study revealing less aggressive treatment in Japanese elderly than nonelderly patients with rheumatoid arthritis. J Clin Rheumatol. 2010;16:370–4. doi: 10.1097/RHU.0b013e3181fe8b37. [DOI] [PubMed] [Google Scholar]
- 19.Tutuncu Z, Reed G, Kremer J, et al. Do patients with older-onset rheumatoid arthritis receive less aggressive treatment? Ann Rheum Dis. 2006;65:1226–9. doi: 10.1136/ard.2005.051144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furst DE, Chang H, Greenberg JD, et al. Prevalence of low hemoglobin levels and associations with other disease parameters in rheumatoid arthritis patients: evidence from the CORRONA registry. Clin Exp Rheumatol. 2009;27:560–6. [PubMed] [Google Scholar]
- 21.Greenberg JD, Kremer JM, Curtis JR, et al. Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:576–82. doi: 10.1136/ard.2010.129916. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg JD, Reed G, Decktor D, et al. A comparative effectiveness study of adalimumab, etanercept and infliximab in biologically naive and switched rheumatoid arthritis patients: results from the US CORRONA registry. Ann Rheum Dis. 2012;71:1134–42. doi: 10.1136/annrheumdis-2011-150573. [DOI] [PubMed] [Google Scholar]
- 23.Ranganath VK, Paulus HE, Onofrei A, et al. Functional improvement after patients with rheumatoid arthritis start a new disease modifying antirheumatic drug (DMARD) associated with frequent changes in DMARD: the CORRONA database. J Rheumatol. 2008;35:1966–71. [PMC free article] [PubMed] [Google Scholar]
- 24.Sokolove J, Strand V, Greenberg JD, et al. Risk of elevated liver enzymes associated with TNF inhibitor utilisation in patients with rheumatoid arthritis. Ann Rheum Dis. 2010;69:1612–7. doi: 10.1136/ard.2009.112136. [DOI] [PubMed] [Google Scholar]
- 25.Pincus T, Summey JA, Soraci SA, Jr, et al. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26:1346–53. doi: 10.1002/art.1780261107. [DOI] [PubMed] [Google Scholar]
- 26.Bayliss EA, Ellis JL, Steiner JF. Subjective assessments of comorbidity correlate with quality of life health outcomes: initial validation of a comorbidity assessment instrument. Health Qual Life Outcomes. 2005;3:51. doi: 10.1186/1477-7525-3-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Margolis KL, Lihong Q, Brzyski R, et al. Validity of diabetes self-reports in the Women's Health Initiative: comparison with medication inventories and fasting glucose measurements. Clin Trials. 2008;5:240–7. doi: 10.1177/1740774508091749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukerji SS, Duffy SA, Fowler KE, et al. Comorbidities in head and neck cancer: agreement between self-report and chart review. Otolaryngol Head Neck Surg. 2007;136:536–42. doi: 10.1016/j.otohns.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 29.Wada K, Yatsuya H, Ouyang P, et al. Self-reported medical history was generally accurate among Japanese workplace population. J Clin Epidemiol. 2009;62:306–13. doi: 10.1016/j.jclinepi.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Aletaha D, Ward MM, Machold KP, et al. Remission and active disease in rheumatoid arthritis: defining criteria for disease activity states. Arthritis Rheum. 2005;52:2625–36. doi: 10.1002/art.21235. [DOI] [PubMed] [Google Scholar]
- 31.Atzeni F, Cazzola M, Benucci M, et al. Chronic widespread pain in the spectrum of rheumatological diseases. Best Pract Res Clin Rheumatol. 2007;25:165–71. doi: 10.1016/j.berh.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Toms J, Soukup T, Bradna P, et al. Disease activity composite indices in patients with rheumatoid arthritis and concomitant fibromyalgia. J Rheumatol. 2010;37:468. doi: 10.3899/jrheum.090805. [DOI] [PubMed] [Google Scholar]
- 33.Krishnan E, Hakkinen A, Sokka T, et al. Impact of age and comorbidities on the criteria for remission and response in rheumatoid arthritis. Ann Rheum Dis. 2005;64:1350–2. doi: 10.1136/ard.2005.037903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michaud K, Wolfe F. Patterns of comorbidity in rheumatic disorders [abstract] Arthritis Rheum. 2007;54:S339. [Google Scholar]
- 35.Radner H, Smolen JS, Aletaha D. Impact of comorbidity on physical function in patients with rheumatoid arthritis. Ann Rheum Dis. 2010;69:536–41. doi: 10.1136/ard.2009.118430. [DOI] [PubMed] [Google Scholar]
- 36.Radner H, Smolen JS, Aletaha D. Comorbidity affects all domains of physical function and quality of life in patients with rheumatoid arthritis. Rheumatology. 2011;50:381–8. doi: 10.1093/rheumatology/keq334. [DOI] [PubMed] [Google Scholar]
- 37.Verbrugge LM, Lepkowski JM, Imanaka Y. Comorbidity and its impact on disability. Milbank Q. 1989;67:450–84. [PubMed] [Google Scholar]
- 38.Verbrugge LM, Lepkowski JM, Konkol LL. Levels of disability among U.S. adults with arthritis. J Gerontol. 1991;46:S71–S83. doi: 10.1093/geronj/46.2.s71. [DOI] [PubMed] [Google Scholar]
- 39.Michaud K, Wallenstein G, Wolfe F. Treatment and nontreatment predictors of health assessment questionnaire disability progression in rheumatoid arthritis: a longitudinal study of 18,485 patients. Arthritis Care Res. 2011;63:366–72. doi: 10.1002/acr.20405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 41.Greenfield S, Apolone G, McNeil BJ, et al. The importance of co-existent disease in the occurrence of postoperative complications and one-year recovery in patients undergoing total hip replacement. Comorbidity and outcomes after hip replacement. Med Care. 1993;31:141–54. doi: 10.1097/00005650-199302000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Michaud K, Wolfe F. Comorbidities in rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007;21:885–906. doi: 10.1016/j.berh.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Von KM, Wagner EH, Saunders K. A chronic disease score from automated pharmacy data. J Clin Epidemiol. 1992;45:197–203. doi: 10.1016/0895-4356(92)90016-g. [DOI] [PubMed] [Google Scholar]
- 44.Gabriel SE, Crowson CS, O'Fallon WM. A comparison of two comorbidity instruments in arthritis. J Clin Epidemiol. 1999;52:1137–42. doi: 10.1016/s0895-4356(99)00124-9. [DOI] [PubMed] [Google Scholar]
- 45.Wolfe F, Michaud K, Li T, et al. Chronic conditions and health problems in rheumatic diseases: comparisons with rheumatoid arthritis, noninflammatory rheumatic disorders, systemic lupus erythematosus, and fibromyalgia. J Rheumatol. 2010;37:305–15. doi: 10.3899/jrheum.090781. [DOI] [PubMed] [Google Scholar]
- 46.Hudson M, Bernatsky S, Taillefer S, et al. Patients with systemic autoimmune diseases could not distinguish comorbidities from their index disease. J Clin Epidemiol. 2008;61:654–62. doi: 10.1016/j.jclinepi.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 47.Kremer JM, Greenberg J. Interpreting registry-derived drug studies: does societal context matter? Arthritis Rheum. 2009;60:3155–7. doi: 10.1002/art.24880. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.