Abstract
Objective. To evaluate the effect of golimumab on haemoglobin levels in patients with RA, PsA or AS.
Methods. Secondary analysis was performed on integrated data from five randomized controlled studies: three RA, one PsA and one AS (2303 patients total). Golimumab 50 or 100 mg was injected s.c. every 4 weeks with or without MTX. Control groups received placebo injections plus MTX or background therapy. Patients with haemoglobin levels below the age- and sex-specific normal ranges were considered to have anaemia. Ferritin levels were used to distinguish anaemia of mixed aetiology (≥15 and <60 ng/ml) and anaemia of inflammation (≥60 ng/ml). Changes from baseline to weeks 14 and 24 in haemoglobin level were compared between treatment groups using an analysis of variance on the van der Waerden normal scores.
Results. At baseline, 21% of RA patients, 9% of PsA patients and 15% of AS patients had anaemia. Of these, 24%, 57% and 25%, respectively, had anaemia of inflammation. The median increase from baseline to week 14 in the haemoglobin level of anaemic patients was 0.3 g/dl in the control group and 0.9 g/dl in the golimumab group (P < 0.001). Haemoglobin levels improved within the subgroups of patients with anaemia of mixed aetiology (control, 0.4 g/dl vs golimumab, 0.7 g/dl) (P = 0.305) and with anaemia of inflammation (0.2 vs 1.4 g/dl, respectively) (P < 0.001).
Conclusion. Compared with the control group, patients receiving golimumab treatment had significantly improved haemoglobin levels, particularly among patients with anaemia of inflammation.
Keywords: rheumatoid arthritis, anaemia, anti-TNF-α agent, golimumab, psoriatic arthritis, ankylosing spondylitis
Introduction
The prevalence of anaemia in patients with RA ranges from 30% to 60%, although a paucity of published data exists [1, 2]. In a recent epidemiological study of patients with RA, Wolfe et al. [2] reported that lower haemoglobin levels were associated with increased disease activity as measured by the number of tender and swollen joints, ESR, CRP level, HAQ score and assessments of pain and fatigue. Anaemia independently contributes to physical disability in patients with RA [3].
Anaemia of chronic disease [4], also referred to as anaemia of inflammation, may occur in patients with acute or chronic immune activation and is associated with the production of proinflammatory cytokines including IL-1-beta, IL-6 and TNF-α [4, 5]. This type of anaemia is a function of disordered homeostasis. Reticuloendothelial system cells retain greater than normal amounts of iron; thus, less iron is readily available for erythroid progenitors as well as erythropoiesis [4]. Hepcidin, a hormone known to reduce iron absorption from the gastrointestinal tract, is most directly linked with IL-6 [6]. Circulating hepcidin levels are elevated in patients with active RA and thus may contribute to the development of anaemia in these patients; TNF inhibitors, through their inhibitory effects on IL-6, may indirectly inhibit hepcidin and thereby reverse this effect [7]. Treatment for anaemia of inflammation is directed at treating the underlying cause of inflammation.
The pathophysiology of anaemia in RA remains to be fully elucidated; however, the cytokine TNF-α, along with other proteins, has been associated with the development of anaemia in RA patients by its role in the inhibition of erythropoiesis [6, 8, 9]. In patients with RA, improvements in haemoglobin levels occur after treatment with infliximab, a biologic TNF-α inhibitor [5, 10, 11]. Here, we evaluated the effect of golimumab, a TNF-α inhibitor that is administered s.c. every 4 weeks, on haemoglobin levels in patients from five large, phase 3, randomized, placebo-controlled studies of rheumatic diseases including RA, PsA and AS.
Materials and methods
Patient data were obtained from five multicentre, double-blind, randomized, placebo-controlled studies of golimumab. The designs of each of these studies have been described in detail previously [12–16].
In GO-BEFORE [12], patients with active RA who had not previously received MTX were randomly assigned to receive placebo plus MTX, golimumab 100 mg plus placebo, golimumab 50 mg plus MTX or golimumab 100 mg plus MTX.
In GO-FORWARD [13], patients with active RA despite previous treatment with MTX were randomly assigned to receive placebo plus MTX, golimumab 100 mg plus placebo, golimumab 50 mg plus MTX or golimumab 100 mg plus MTX. Patients were required to be on a stable dose of MTX for ≥4 weeks prior to study drug administration. At week 16, all patients (except those in the 100 mg plus MTX group) who had <20% improvement in their tender and swollen joint counts entered early escape.
In GO-AFTER [14], patients with active RA who had previously received ≥1 TNF-α inhibitor were randomly assigned to receive placebo, golimumab 50 mg or golimumab 100 mg. At week 16, all patients (except those in the 100-mg group) who had <20% improvement in their tender and swollen joint counts entered early escape.
In GO-REVEAL [15], patients with active PsA were randomly assigned to receive placebo, golimumab 50 mg or golimumab 100 mg. At week 16, all patients (except those in the 100-mg group) with <10% improvement in their tender and swollen joint counts entered early escape.
In GO-RAISE [16], patients with active AS were randomly assigned to receive placebo, golimumab 50 mg or golimumab 100 mg. At week 16, all patients (except those in the 100-mg group) who had <20% improvement in total back pain and morning stiffness entered early escape.
All golimumab injections were administered every 4 weeks. Patients in GO-BEFORE and GO-FORWARD also received concomitant MTX according to the protocol and their assigned treatment group. Patients in the other studies continued concomitant DMARDs, including MTX, SSZ or HCQ, at stable doses if they were receiving them at baseline. In GO-AFTER, GO-REVEAL and GO-RAISE, concomitant MTX/DMARD therapy was not required. All studies excluded patients with haemoglobin levels <8.5 g/dl, creatinine levels >1.5 mg/dl and uncontrolled renal, hepatic, hematological, gastrointestinal, endocrine, pulmonary, cardiac, neurological, psychiatric or cerebral disease.
Patients were defined as anaemic if their haemoglobin levels were below the age- and sex-specific normal range of the central laboratory (Quintiles Laboratories, Smyrna, GA, USA). Normal haemoglobin ranges for the central laboratory were 11.6–16.2 g/dl for women aged 65 years or younger, 11.0–16.1 g/dl for women aged 66 years or older, 13.0–17.5 g/dl for men aged 65 years or younger and 12.6–17.7 g/dl for men aged 66 years or older. Patients were excluded from the analysis if they received i.v. iron, recombinant human erythropoietin or a blood transfusion at any time through week 24. Patients in each study were allowed to continue stable doses of concomitant NSAIDs or corticosteroids (up to 10 mg/day prednisone equivalent) that they were receiving at study entry. None of the studies excluded NSAID or corticosteroid use.
We also categorized anaemia based on ferritin levels to separate patients whose low haemoglobin levels were the result of iron deficiency from those with anaemia of inflammation. Patients with anaemia and ferritin levels ≥60 ng/ml were considered to have anaemia of inflammation. Patients with anaemia and ferritin levels ≥15 and <60 ng/ml were also evaluated as a group that included patients with a mixture of iron deficiency and inflammatory anaemia (hereafter referred to as anaemia of mixed aetiology). This group excluded patients with very low iron levels (patients who had ferritin levels <15 ng/ml were considered to have pure iron deficiency anaemia). Patients with pure iron deficiency anaemia were included in the analyses of patients with anaemia, but excluded from the analysis of patients with anaemia of mixed aetiology and anaemia of inflammation. Analyses of patients with anaemia and patients with anaemia of inflammation were prespecified in the original study protocols and statistical analysis plans; analyses of patients with anaemia of mixed aetiology were post hoc.
The DAS28 (using CRP) [17–19] was used to assess disease activity in RA and PsA. The BASDAI [20] was used to assess disease activity in AS.
Statistical analysis
Changes from baseline in haemoglobin levels at week 14 (week 16 for GO-BEFORE) and at week 24 were analysed. For patients with missing haemoglobin values at week 14, the last non-missing haemoglobin value obtained prior to week 14 was used as the week-14 value. For patients who entered early escape at week 16, the week-24 haemoglobin value was replaced with the week-16 value, except in GO-BEFORE in which there was no early escape prior to week 24. Continuous variables were compared using analysis of variance on the van der Waerden normal scores. Medians and interquartile ranges were used to summarize the data because they were not normally distributed.
Results
Patient characteristics
A total of 2303 patients were included in this analysis. At baseline, approximately 21% of patients in the RA studies (320/1542), 9% of patients in the PsA study (37/405) and 15% of patients in the AS study (53/356) were anaemic (Table 1). The proportions of patients with anaemia were similar in the control and golimumab groups in the RA and AS studies but not in the PsA study, in which approximately 4% of patients in the placebo group and 11% of patients in the golimumab group were anaemic at baseline. Across all studies, 27% of anaemic patients had anaemia of inflammation, which represents 5% of the total study population.
Table 1.
Baseline disease characteristics for all patients, patients with anaemia, patients with anaemia of mixed aetiology and patients with anaemia of inflammation
| RA studiesa |
PsA study |
AS study |
||||
|---|---|---|---|---|---|---|
| Characteristics | Placebo | Golimumab | Placebo | Golimumab | Placebo | Golimumab |
| All patients, n | 448 | 1094 | 113 | 292 | 78 | 278 |
| Age, years | 52.0 (43.0–60.0) | 52.0 (43.0–59.0) | 47.0 (40.0–54.0) | 47.0 (38.5–55.0) | 41.0 (31.0–50.0) | 38.0 (29.0–46.0) |
| Female, n (%) | 375 (83.7) | 878 (80.3) | 44 (38.9) | 117 (40.1) | 23 (29.5) | 78 (28.1) |
| Disease duration, years | 4.6 (1.5–11.4) | 4.2 (1.1–10.1) | 5.1 (1.8–10.2) | 5.2 (1.9–10.4) | 7.3 (2.8–18.6) | 5.2 (1.5–12.3) |
| Number of swollen joints (0–68) | 13.0 (8.0–20.0) | 13.0 (8.0–20.0) | 10.0 (6.0–18.0) | 10.0 (6.0–15.0) | NA | NA |
| Number of tender joints (0–66) | 24.0 (14.0–37.0) | 25.0 (15.0–38.0) | 18.0 (11.0–30.0) | 18.5 (10.0–32.0) | NA | NA |
| Painb | 6.4 (4.8–7.9) | 6.6 (5.0–8.0) | 5.4 (3.1–7.5) | 5.7 (4.1–7.4) | 7.4 (6.0–8.6) | 7.4 (5.7–8.5) |
| Physical functionc | 1.5 (1.0–2.0) | 1.5 (1.0–2.0) | 1.0 (0.6–1.4) | 1.0 (0.5–1.5) | 4.9 (3.5–6.8) | 5.2 (3.2–6.9) |
| CRP, mg/dl | 1.0 (0.4–2.4) | 1.0 (0.4–2.9) | 0.6 (0.3–1.3) | 0.6 (0.3–1.6) | 1.2 (0.3–2.4) | 1.0 (0.4–2.5) |
| Haemoglobin, g/dl | 12.8 (11.8–13.7) | 12.8 (11.8–13.9) | 14.0 (13.1–14.7) | 13.9 (12.9–14.7) | 14.0 (12.7–14.5) | 13.9 (13.0–14.6) |
| Taking oral steroid, n (%) | 253 (56.5) | 627 (57.5) | 19 (16.8) | 46 (15.8) | 13 (16.7) | 44 (15.8) |
| Taking NSAIDs, n (%) | 341 (76.1) | 853 (78.3) | 88 (77.9) | 220 (75.3) | 72 (92.3) | 247 (88.8) |
| Taking MTXd, n (%) | 235 (47.5) | 514 (47.2) | 54 (47.8) | 140 (47.9) | 15 (19.2) | 57 (20.5) |
| Patients with anaemiae, n (%) | 91 (20.3) | 229 (20.9) | 4 (3.5) | 33 (11.3) | 14 (17.9) | 39 (14.0) |
| Age, years | 47.0 (38.0–57.0) | 48.0 (40.0–58.0) | 39.0 (31.5–46.5) | 42.0 (34.0–55.0) | 30.0 (23.0–41.0) | 36.0 (27.0–43.0) |
| Female, n (%) | 78 (85.7) | 194 (84.7) | 3 (75.0) | 14 (42.4) | 3 (21.4) | 11 (28.2) |
| Disease duration, years | 4.6 (1.6–13.2) | 3.5 (1.0–9.5) | 1.6 (0.7–5.1) | 5.6 (2.6–10.5) | 4.7 (1.0–6.3) | 7.8 (1.6–11.5) |
| Number of swollen joints (0–68) | 12.0 (9.0–21.0) | 15.0 (10.0–23.0) | 20.0 (14.5–23.0) | 12.0 (7.0–18.0) | NA | NA |
| Number of tender joints (0–66) | 23.0 (15.0–36.0) | 27.0 (16.0–42.0) | 30.5 (14.5–47.0) | 16.0 (12.0–37.0) | NA | NA |
| Painb | 6.3 (4.5–8.0) | 6.8 (5.2–8.2) | 6.2 (5.6–7.2) | 7.1 (5.4–8.4) | 6.7 (5.5–8.6) | 7.3 (5.4–8.9) |
| Physical functionc | 1.6 (1.3–2.0) | 1.8 (1.3–2.1) | 1.1 (0.9–1.4) | 1.4 (0.9–1.9) | 4.6 (4.1–7.3) | 5.6 (2.7–7.8) |
| CRP, mg/dl | 2.8 (1.2–5.8) | 3.1 (1.0–5.6) | 0.8 (0.4–1.9) | 2.4 (1.0–5.2) | 3.1 (1.8–6.0) | 2.8 (1.0–4.9) |
| Haemoglobin, g/dl | 11.0 (10.4–11.3) | 10.9 (10.1–11.3) | 11.0 (10.5–12.1) | 11.5 (10.8–12.4) | 12.3 (11.4–12.6) | 11.6 (11.0–12.3) |
| Taking oral steroid, n (%) | 54 (59.3) | 143 (62.4) | 0 (0.0) | 5 (15.2) | 4 (28.6) | 13 (33.3) |
| Taking NSAIDs, n (%) | 77 (84.6) | 192 (83.8) | 4 (100.0) | 30 (90.9) | 14 (100.0) | 36 (92.3) |
| Taking MTXd, n (%) | 49 (53.8) | 98 (42.8) | 3 (75.0) | 18 (54.5) | 4 (28.6) | 10 (25.6) |
| Patients with anaemia of mixed aetiologyf, n (%) | 19 (4.2) | 59 (5.4) | 2 (1.8) | 6 (2.1) | 2 (2.6) | 7 (2.5) |
| Age, years | 46.0 (41.0–58.0) | 47.0 (36.0–54.0) | 41.5 (34.0–49.0) | 52.0 (42.0–57.0) | 33.0 (25.0–41.0) | 36.0 (27.0–54.0) |
| Female, n (%) | 17 (89.5) | 56 (94.9) | 2 (100.0) | 4 (66.7) | 0 (0.0) | 3 (42.9) |
| Disease duration, years | 4.6 (1.4–14.3) | 3.7 (2.0–9.7) | 4.2 (0.7–7.6) | 5.8 (1.0–16.3) | 2.5 (0.1–4.8) | 9.6 (4.5–11.5) |
| Number of swollen joints (0–66) | 11.0 (7.0–25.0) | 17.0 (9.0–25.0) | 14.5 (11.0–18.0) | 23.0 (11.0–37.0) | NA | NA |
| Number of tender joints (0–68) | 26.0 (16.0–38.0) | 22.0 (16.0–42.0) | 28.0 (2.0–44.0) | 44.5 (26.0–59.0) | NA | NA |
| Painb | 6.4 (5.6–7.6) | 6.2 (4.9–7.8) | 7.2 (6.3–8.1) | 7.9 (6.9–8.6) | 8.5 (8.2–8.8) | 7.5 (5.4–9.1) |
| Physical functionc | 1.9 (1.5–2.3) | 1.5 (1.1–2.0) | 1.3 (1.0–1.6) | 1.7 (1.4–1.9) | 8.0 (7.3–8.7) | 7.6 (6.7–8.7) |
| CRP, mg/dl | 2.6 (1.4–7.1) | 2.8 (1.0–5.4) | 1.5 (0.3–2.6) | 1.3 (0.5–3.4) | 3.3 (1.5–5.1) | 3.1 (2.6–4.1) |
| Haemoglobin, g/dl | 11.0 (10.6–11.4) | 11.1 (10.3–11.3) | 10.5 (10.4–10.6) | 11.4 (11.1–12.4) | 11.6 (10.4–12.7) | 11.1 (10.8–12.2) |
| Taking oral steroids, n (%) | 8 (42.1) | 42 (71.2) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 2 (28.6) |
| Taking NSAIDS, n (%) | 16 (84.2) | 51 (86.4) | 2 (100.0) | 6 (100.0) | 2 (100.0) | 7 (100.0) |
| Taking MTXd, n (%) | 8 (42.1) | 21 (35.6) | 1 (50.0) | 4 (66.7) | 0 (0.0) | 0 (0.0) |
| Patients with anaemia of inflammationg, n (%) | 22 (4.9) | 54 (4.9) | 1 (0.9) | 20 (6.8) | 4 (5.1) | 9 (3.2) |
| Age, years | 55.5 (49.0–67.0) | 55.0 (48.0–61.0) | 44.0 (44.0–44.0) | 43.5 (35.5–55.0) | 44.0 (40.5–50.5) | 41.0 (33.0–51.0) |
| Female, n (%) | 17 (77.3) | 37 (68.5) | 0 (0.0) | 3 (15.0) | 0 (0.0) | 1 (11.1) |
| Disease duration, years | 8.6 (2.0–17.9) | 3.3 (1.0–9.4) | 0.6 (0.6–0.6) | 6.6 (5.0–13.4) | 7.8 (5.6–13.4) | 1.6 (0.6–10.0) |
| Number of swollen joints (0–66) | 12.5 (10.0–23.0) | 16.5 (11.0–24.0) | 22.0 (22.0–22.0) | 11.0 (7.5–16.0) | NA | NA |
| Number of tender joints (0–68) | 24.0 (20.0–38.0) | 31.0 (19.0–44.0) | 50.0 (50.0–50.0) | 15.5 (12.5–37.0) | NA | NA |
| Painb | 6.7 (4.4–8.6) | 6.8 (5.6–8.3) | 5.0 (5.0–5.0) | 7.1 (5.2–9.0) | 7.3 (4.5–9.2) | 6.1 (5.7–9.0) |
| Physical functionc | 1.8 (1.4–2.3) | 1.9 (1.5–2.3) | 1.1 (1.1–1.1) | 1.4 (0.9–1.8) | 5.3 (4.4–6.8) | 7.5 (6.4–7.9) |
| CRP, mg/dl | 5.6 (3.1–8.6) | 5.7 (3.0–9.8) | 0.4 (0.4–0.4) | 4.6 (1.5–6.3) | 4.5 (2.3–7.0) | 5.2 (2.8–5.4) |
| Haemoglobin, g/dl | 11.1 (10.1–11.3) | 11.1 (10.6–11.6) | 12.8 (12.8–12.8) | 12.4 (11.1–12.5) | 12.5 (12.3–12.8) | 11.7 (11.5–12.1) |
| Taking oral steroids, n (%) | 11 (50.0) | 28 (51.9) | 0 (0.0) | 3 (15.0) | 0 (0.0) | 0 (0.0) |
| Taking NSAIDs, n (%) | 17 (77.3) | 40 (74.1) | 1 (100.0) | 19 (95.0) | 4 (100.0) | 8 (88.9) |
| Taking MTXd, n (%) | 13 (59.1) | 30 (55.6) | 1 (100.0) | 10 (50.0) | 0 (0.0) | 0 (0.0) |
Values are presented as median (IQR) unless otherwise noted. Numbers of patients in the different anaemia categories may not add up to the total number of patients with anaemia as patients with ferritin <15 mg/ml were considered to have pure iron deficiency and were excluded. There were no missing haemoglobin data at baseline. aIncludes combined data from all three RA studies: GO-BEFORE, GO-FORWARD and GO-AFTER. Treatment groups are placebo with or without MTX and golimumab with or without MTX. bGeneral pain was assessed in the RA and PsA studies using a VAS (0–10 cm). Only night pain was assessed in the AS study (VAS, 0–10 cm). cPhysical function was measured in the RA and PsA studies using the HAQ (range 0–3) and in the AS study using the BASFI (range 0–10). dIt was suggested that patients taking MTX at baseline also receive supplemental folic acid. ePatients were considered to be anaemic if their haemoglobin levels were less than the age- and sex-specific normal range for the central laboratory. fPatients were considered to have anaemia of mixed aetiology if their haemoglobin levels were below the normal range for the central laboratory and their ferritin levels were ≥15 ng/ml and <60 ng/ml. gPatients were considered to have anaemia of inflammation if their haemoglobin levels were below the normal range for the central laboratory and their ferritin levels were ≥60 ng/ml. NA: not applicable.
Among all patients, median baseline haemoglobin levels were generally lower for patients in the golimumab group in the RA studies (12.8 g/dl) than for those in the PsA and AS studies (13.9 g/dl; Table 1). Also, the RA and PsA study cohorts tended to be older and the RA cohort was predominantly female (approximately 81%), whereas most patients in the PsA and AS studies were male (approximately 60% and 72%, respectively) (Table 1).
Baseline clinical characteristics for patients with anaemia were generally similar to those of the study population as a whole. However, patients with anaemia had higher median CRP levels than the overall population. Patients with anaemia of inflammation had the highest levels of CRP with the exception of those patients in the placebo group of the PsA study.
Change in haemoglobin levels from baseline
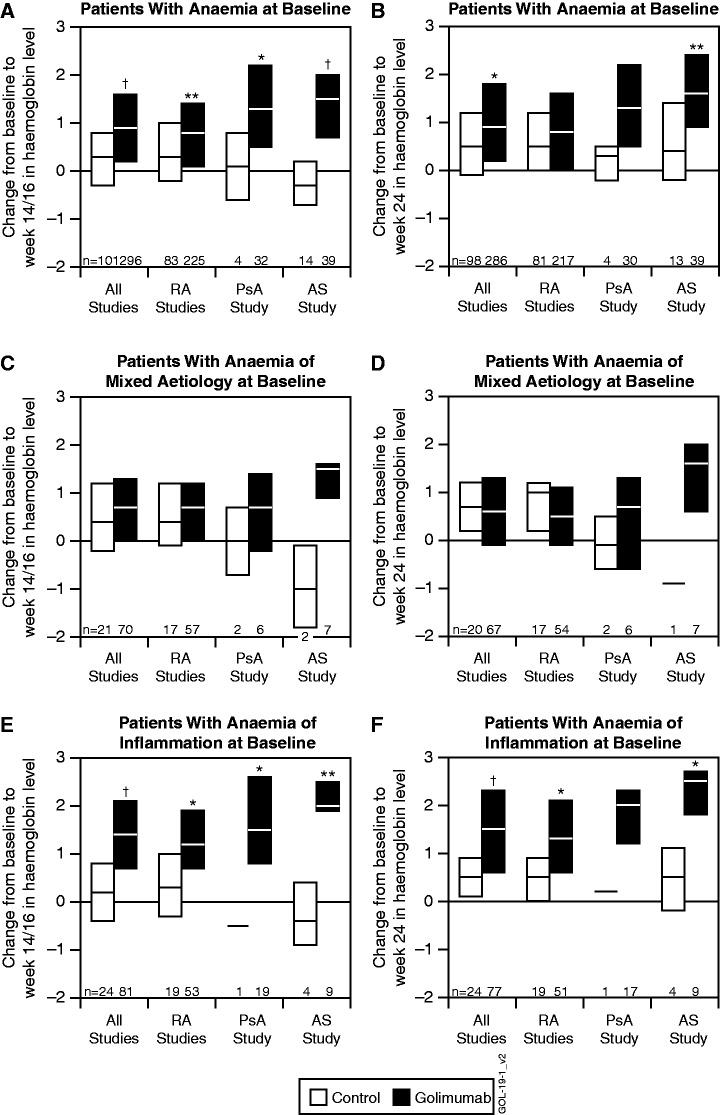
Among anaemic patients from all five studies, the median [interquartile range (IQR)] change from baseline to week 14/16 in haemoglobin level was 0.3 g/dl (−0.3–0.8 g/dl) in the control group and 0.9 g/dl (0.2–1.6 g/dl) in the golimumab group (P < 0.001) (Fig. 1A). Among patients with anaemia of mixed aetiology, the changes were 0.4 g/dl (−0.2–1.2 g/dl) in the control group and 0.7 g/dl (0.0–1.3 g/dl) in the golimumab-treated group (P = 0.305) (Fig. 1C). Among patients with anaemia of inflammation, the changes were 0.2 g/dl (−0.4–0.8 g/dl) in the control group and 1.4 g/dl (0.7–2.1 g/dl) in the golimumab-treated group (P < 0.001) (Fig. 1E). Changes from baseline to week 24 were similar to those at week 14 in all groups (Fig. 1B, D and F).
Fig. 1.
Median and IQR changes from baseline to week 14/16 and 24 in haemoglobin levels (g/dl).
For patients with anaemia at baseline (A and B), patients with anaemia of mixed aetiology (ferritin ≥15 ng/ml and <60 ng/ml) at baseline (C and D) and patients with anaemia of inflammation (ferritin ≥60 ng/ml) at baseline (E and F). Data for patients who received golimumab or placebo are shown for all studies, the three RA studies combined and the PsA and AS studies separately. *P < 0.05, **P < 0.01, †P < 0.001 using an analysis of variance on the van der Waerden normal scores.
Among patients with anaemia in the combined RA studies, those who received golimumab had statistically significantly greater improvement in haemoglobin levels between baseline and week 14 than those who received control treatment. Differences between the treatment groups were also evident in the PsA study at week 14 (Fig. 1A). Similar results were seen among patients with anaemia of inflammation (Fig. 1E and F). Among patients with anaemia of mixed aetiology, no statistically significant differences were observed between treatment groups (Fig. 1C and D).
Inflammation and changes from baseline in haemoglobin levels
We used CRP to evaluate the relationship between changes in haemoglobin levels and inflammation. Among patients who received golimumab, changes in haemoglobin from baseline to week 14 were significantly greater in patients with elevated CRP levels at baseline (>0.6 mg/dl) compared with patients with normal CRP levels at baseline (≤0.6 mg/dl) (Table 2). Although the differences were not always statistically significant, improvement was consistently greater among patients with CRP >0.6 mg/dl at baseline (Table 2). Among patients who received golimumab in all studies, there was a significant negative correlation between changes in haemoglobin and CRP from baseline to week 14 (−0.396, P < 0.001) and week 24 (−0.385, P < 0.001).
Table 2.
Changes from baseline to week 14/16 and 24 in haemoglobin in patients with anaemia, with or without elevated CRPa,b
| Change from baseline in haemoglobin levels, g/dl | Normal CRP at baseline (≤0.6 mg/dl) | Elevated CRP at baseline (>0.6 mg/dl) | P-value |
|---|---|---|---|
| Week 14c | |||
| Patients with anaemia | |||
| All studies | |||
| Placebo | |||
| n | 15 | 86 | |
| Median (IQR) | 0.0 (−0.30–0.50) | 0.30 (−0.30–0.90) | 0.266 |
| Golimumab | |||
| n | 55 | 241 | |
| Median (IQR) | 0.40 (−0.30–1.00) | 0.90 (0.30–1.70) | <0.001 |
| RA studies | |||
| Placebo | |||
| n | 13 | 70 | |
| Median (IQR) | 0.0 (−0.30–0.40) | 0.35 (−0.20–1.00) | 0.124 |
| Golimumab | |||
| n | 43 | 182 | |
| Median (IQR) | 0.30 (−0.30–1.00) | 0.90 (0.10–1.50) | 0.005 |
| Patients with anaemia of mixed aetiology | |||
| All studies | |||
| Placebo | |||
| n | 2 | 21 | |
| Median (IQR) | 0.25 (−0.20–0.70) | 0.40 (−0.20–1.20) | 0.817 |
| Golimumab | |||
| n | 11 | 60 | |
| Median (IQR) | 0.30 (−0.80–0.70) | 0.90 (−0.05–1.45) | 0.031 |
| RA studies | |||
| Placebo | |||
| n | 1 | 18 | |
| Median (IQR) | −0.20 (−0.20, −0.20) | 0.55 (−0.10–1.20) | 0.395 |
| Golimumab | |||
| n | 9 | 49 | |
| Median (IQR) | 0.03 (0.20–0.70) | 0.70 (−0.20–1.30) | 0.138 |
| Patients with anaemia of inflammationd | |||
| All studies | |||
| Placebo | |||
| n | 3 | 21 | |
| Median (IQR) | 0.00 (−0.50–0.50) | 0.20 (−0.40–0.80) | 0.613 |
| Golimumab | |||
| n | 7 | 74 | |
| Median (IQR) | 1.50 (−0.10–2.90) | 1.40 (0.70–2.10) | 0.823 |
| RA studies | |||
| Placebo | |||
| n | 2 | 17 | |
| Median (IQR) | 0.25 (0.00–0.50) | 0.30 (−0.30–1.00) | 0.874 |
| Golimumab | |||
| n | 5 | 48 | |
| Median (IQR) | 1.50 (1.00–1.60) | 1.20 (0.65–1.90) | 0.566 |
| Week 24 | |||
| Patients with anaemia | |||
| All studies | |||
| Placebo | |||
| n | 14 | 84 | |
| Median (IQR) | 0.15 (−0.20–0.50) | 0.50 (−0.05–1.20) | 0.193 |
| Golimumab | |||
| n | 55 | 231 | |
| Median (IQR) | 0.50 (−0.50–1.70) | 1.00 (0.40–1.80) | 0.006 |
| RA studies | |||
| Placebo | |||
| n | 12 | 69 | |
| Median (IQR) | 0.05 (−0.25–0.45) | 0.60 (0.00–1.20) | 0.158 |
| Golimumab | |||
| n | 43 | 174 | |
| Median (IQR) | 0.20 (−0.60–1.40) | 0.80 (0.20–1.60) | 0.017 |
| Patients with anaemia of mixed aetiology | |||
| All studies | |||
| Placebo | |||
| n | 2 | 21 | |
| Median (IQR) | 0.90 (0.50–1.30) | 0.50 (−0.20–1.20) | 0.517 |
| Golimumab | |||
| n | 11 | 61 | |
| Median (IQR) | 0.20 (−0.80–0.70) | 0.80 (0.00–1.60) | 0.040 |
| RA studies | |||
| Placebo | |||
| n | 1 | 18 | |
| Median (IQR) | 1.30 (1.30–1.30) | 0.70 (0.20–1.20) | 0.450 |
| Golimumab | |||
| n | 9 | 50 | |
| Median (IQR) | 0.20 (−0.80–0.40) | 0.70 (−0.10–1.20) | 0.062 |
| Patients with anaemia of inflammationd | |||
| All studies | |||
| Placebo | |||
| n | 3 | 21 | |
| Median (IQR) | 0.20 (0.00–0.20) | 0.60 (0.20–0.90) | 0.234 |
| Golimumab | |||
| n | 7 | 70 | |
| Median (IQR) | 1.80 (0.00–2.30) | 1.45 (0.70–2.30) | 0.492 |
| RA studies | |||
| Placebo | |||
| n | 2 | 17 | |
| Median (IQR) | 0.10 (0.00–0.20) | 0.60 (0.40–0.90) | 0.292 |
| Golimumab | |||
| n | 5 | 46 | |
| Median (IQR) | 0.60 (0.00–1.90) | 1.30 (0.60–2.10) | 0.391 |
aPatients were observed from baseline to weeks 14/16 and 24 and were considered to be anaemic if their haemoglobin levels were less than the age- and sex-specific normal range of the central laboratory. bPatients were considered to have anaemia of mixed aetiology if their haemoglobin levels were below the normal range for the central laboratory and their ferritin levels were ≥15 ng/ml and <60 ng/ml. cWeek 16 values were used for the GO-BEFORE RA study. dPatients were considered to have anaemia of inflammation if their haemoglobin levels were below the normal range for the central laboratory and their ferritin levels were ≥60 ng/ml. All P values are from analysis of variance on the van der Waerden scores comparing changes from baseline in haemoglobin levels in patients with normal CRP levels at baseline (≤0.6 mg/dl) vs those in patients with elevated CRP levels (>0.6 mg/dl).
Disease activity and changes from baseline in haemoglobin levels
Spearman correlation analyses were performed to assess the relationship between change from baseline in disease activity, as measured by DAS28 (using CRP) in RA and PsA and by BASDAI in AS, and change from baseline in haemoglobin levels at week 14 for the three anaemia populations (Table 3). In RA, the moderate statistically significant correlations were negative in direction and were observed consistently among patients in the golimumab-treated groups, but not among patients who received placebo. In the RA studies, the correlations were stronger for patients with anaemia of mixed aetiology and patients with anaemia of inflammation than for patients with anaemia. In the PsA and AS studies, sample size is small; thus there is insufficient information to draw a conclusion.
Table 3.
Spearman correlation between change from baseline at week 14a of haemoglobin and DAS28/BASDAI
| RA studies |
PsA study |
AS study |
||||
|---|---|---|---|---|---|---|
| Placebo | Golimumab | Placebo | Golimumab | Placebo | Golimumab | |
| Anaemia | 81 | 223 | 4 | 30 | 14 | 39 |
| DAS28/BASDAIb | −0.171 | −0.214 | 0.400 | −0.259 | −0.392 | −0.120 |
| P-value | 0.1276 | 0.0013 | 0.6000 | 0.1675 | −0.1656 | 0.4683 |
| Anaemia of mixed aetiology | 17 | 57 | 2 | 5 | 2 | 7 |
| DAS28/BASDAI | −0.134 | −0.462 | 1.000 | −0.500 | 1.000 | −0.414 |
| P-value | 0.6082 | <.0001 | — | 0.3910 | — | 0.3553 |
| Anaemia of inflammation | 19 | 52 | 1 | 18 | 4 | 9 |
| DAS28/BASDAI | −0.036 | −0.384 | NA | −0.649 | −0.600 | −0.134 |
| P-value | 0.8837 | 0.0049 | NA | 0.0035 | 0.4000 | 0.7302 |
aWeek 16 data were used for the GO-BEFORE RA study. bDAS28 (using CRP) was used in the RA and PsA studies. BASDAI was used in the AS study. NA: not applicable.
Discussion
Although the pathogenesis and incidence of anaemia may differ between RA, PsA and AS, studies have demonstrated that TNF inhibition is an effective treatment for all three diseases and may also improve comorbid conditions [21]. The current post hoc analysis demonstrates for the first time a significant impact of golimumab on haemoglobin levels in patients with anaemia who participated in one of five phase 3, randomized, placebo-controlled studies of golimumab in patients with RA, PsA or AS.
Because the number of patients with anaemia in each of the individual golimumab studies was small, we initially combined the data to evaluate the effect of golimumab on haemoglobin levels. The results indicate that anaemic patients who received golimumab had significantly greater improvement in haemoglobin levels than those who received placebo. Patients with anaemia of inflammation, in which anaemia is associated with sufficient iron levels (indicated by ferritin levels ≥60 ng/ml), showed greater improvement in haemoglobin levels than the population of anaemic patients as a whole. This is consistent with previously reported observations among anaemic RA patients treated with infliximab [11].
For the combined cohort of all five studies (three RA, one AS and one PsA), the improvement in haemoglobin levels was similar to that in the individual cohorts from the three RA studies and the AS study. The improvement in haemoglobin levels in the AS population following treatment (between placebo- and golimumab-treated patients) was particularly marked, despite the small number of evaluable patients. In the PsA study, the number of anaemic patients was not evenly distributed between placebo- and golimumab-treated groups at baseline. Because there were so few anaemic patients in the placebo group, the results from the PsA study should be interpreted with caution.
Approximately 20% of patients in the RA studies had anaemia at baseline, while smaller proportions of patients in the PsA and AS studies had anaemia (9% and 15%, respectively) at baseline. Differences in age and sex were observed among the patients with the various forms of inflammatory arthritis, with older patients in the RA and PsA cohorts, a predominantly female population in the RA cohort and a predominantly male population in the PsA and AS cohorts. These differences in baseline characteristics may account, in part, for variances in mean haemoglobin levels between the groups at baseline and thus for the observed differences after golimumab treatment. The prevalence of anaemia in all three of these studies was lower than the prevalence of anaemia reported in earlier studies [11]. This is consistent with the trend towards decreased baseline RA disease activity over time observed in another study. The same trend was observed in an analysis of baseline characteristics among RA patients enrolled in randomized clinical trials of TNF-α inhibitors that were conducted between 1993 and 2008 [22].
The subset of patients with anaemia had higher CRP levels at baseline than the overall study population. This result is consistent with epidemiological data from Wolfe and Michaud [2], which indicate that the CRP level is the strongest predictor of anaemia in patients with RA. Our data indicate that the CRP level may also be a predictor of anaemia for patients with PsA or AS. Patients with elevated CRP levels at baseline who received golimumab exhibited greater improvements in haemoglobin levels at weeks 14 and 24 than those with normal CRP levels. The improvement in haemoglobin levels with treatment also correlated with an improvement in disease activity.
Identifying a specific cause of anaemia in patients with inflammatory diseases is difficult because haemoglobin levels can be affected by a variety of factors, including vitamin B12 deficiency, folic acid deficiency, NSAID-induced blood loss resulting from gastritis, iron deficiency from other causes and anaemia of inflammation [23]. In addition, the majority of RA patients are women, and anaemia is common among premenopausal women because they have menstrual bleeding. Thus, patients with inflammatory disease may have a mixture of iron deficiency anaemia and anaemia of inflammation.
The effect of inflammatory cytokines on ferritin levels confounds the diagnosis of anaemia of inflammation in the setting of active inflammatory disease [24], as ferritin is an acute phase reactant. Recent evidence suggests that hepcidin may be a better marker of anaemia of inflammation [25] and could aid in differentiating this form of anaemia from iron deficiency anaemia in the setting of inflammation. We did not measure hepcidin in the clinical trials analysed in this study. Instead, we attempted to focus on anaemia of inflammation by excluding patients with low ferritin levels who presumably would have had a significant component of iron deficiency. Patients with anaemia caused by iron deficiency would not be expected to exhibit increased haemoglobin levels after anti-TNF-α therapy. However, iron deficiency potentially caused by NSAID-induced gastrointestinal blood loss cannot be excluded in these patient groups. Patients who experience a reduction in disease activity after treatment with a TNF-α inhibitor may decrease NSAID use [26], which would lessen possible gastrointestinal blood loss.
This pooled analysis of data from five randomized, placebo-controlled studies of golimumab in patients with rheumatic diseases shows that golimumab improved haemoglobin levels in patients with anaemia, particularly in those patients with elevated CRP at baseline.
Rheumatology key messages.
Patients with RA, PsA and AS may have anaemia caused by inflammation, iron deficiency or both.
Golimumab improves haemoglobin levels, particularly among RA patients with anaemia of inflammation.
Acknowledgements
We thank the patients, investigators and study personnel who participated in the golimumab studies contributing data to this analysis. We also thank Scott Newcomer, MS, of ViroPharma Incorporated formerly of Janssen Biotech Inc., Mary Whitman, PhD, and Kirsten Schuck of Janssen Biotech Inc. for assisting with the manuscript preparation. Authors employed by the study sponsor were involved in the conception of the study design, collection, analysis and interpretation of the data. Medical writers employed by the study sponsor assisted with the preparation of the manuscript. All authors agreed to submit the manuscript.
Funding: This research was supported by Janssen Research & Development, LLC, Spring House, PA, and by Schering-Plough Research Institute, Inc.
Disclosure statement: S.X. is an employee of Johnson & Johnson at the time of this study and owns stock and/or stock options in Johnson & Johnson. M.C.W. is a co-investigator on two AstraZeneca-sponsored RA clinical trials. A.K. has received grants/research support and honoraria from Janssen. J.K. has received consultant fees from Amgen Inc.; Baxter Healthcare Corporation; Bristol-Myers Squibb Company; Celgene Corp.; Crescendo BioScience; fourteen22 Inc.; Genentech Inc.; Hospira, Inc.; Horizon Pharma, Inc.; Janssen Biotech, Inc; medac pharma Inc.; Molecular Partners A.G.; PanGenetics, B.V.; Pfizer Inc.; Roche Laboratories, Inc.; Savient Pharmaceuticals, Inc.; and UCB, Inc. He has received research grants paid to his employer from Abbott Laboratories; Ardea Biosciences, Eli Lilly and Co.; Fidia Farmaceutici SpA; Roche Laboratories, Inc. and Sanofi-aventis. D.E.F. has received grant/research support from AbbVie, Actelion, Amgen, BMS, Gilead, GSK, NIH, Novartis, Pfizer, Roche/Genentech and UCB. He is a consultant for AbbVie, Actelion, Amgen, BMS, Biogen Idec, Janssen, Gilead, GSK, NIH, Novartis, Pfizer, Roche/Genentech and UCB and a member of a speaker’s Bureau for AbbVie, Actelion and UCB (CME only). He has also received honoraria from AbbVie, Actelion, Amgen, BMS, Biogen Idec, Janssen, Gilead, NIH and Roche/Genentech. E.K. has received research funding from Abbott Laboratories; Amgen Inc.; AstraZeneca Pharmaceuticals LP; Bristol-Myers Squibb Company; Janssen; F. Hoffman-LaRoche Inc.; Genzyme; Merck; Novartis Pharmaceuticals; Pfizer Pharmaceuticals; and UCB. He has consultancy agreements/advisory board membership with Abbott Laboratories; AstraZeneca Pharmaceuticals; Biotest; Bristol-Myers Squibb Company; Janssen; F. Hoffman-LaRoche Inc.; Genentech Inc.; Merck; Nycomed; Pfizer Pharmaceuticals; and UCB. He has speaker honoraria agreements with Abbott Laboratories; Bristol-Myers Squibb Company; F. Hoffman-LaRoche Inc.; Merck; Pfizer Pharmaceuticals and UCB. F.T.M. is a member of an Abbott speakers’ bureau. B.H. is a full-time employee of Janssen Research & Development LLC and owns stock/stock options in Johnson & Johnson. M.K.D. was an employee of Johnson & Johnson at the time of this study and owns stock and/or stock options in Johnson & Johnson. M.U.R. was an employee of Janssen R&D at the time this work was done and owns Johnson & Johnson stocks. A.D. has received research grants, honoraria for serving on advisory boards, speaking and consulting for Abbott and UCB; research grants from Novartis, Janssen and Amgen and speaking fees from Pfizer. E.C.H. is an employee of Janssen R&D, LLC, which is a subsidiary of Johnson & Johnson, and owns stock/stock options in Johnson & Johnson. J.H.M. has declared no conflicts of interest.
References
- 1.Wilson A, Yu HT, Goodnough LT, et al. Prevalence and outcomes of anemia in rheumatoid arthritis: a systematic review of the literature. Am J Med. 2004;116(Suppl 7A):50S–57S. doi: 10.1016/j.amjmed.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe F, Michaud K. Anemia and renal function in patients with rheumatoid arthritis. J Rheumatol. 2006;33:1516–22. [PubMed] [Google Scholar]
- 3.Han C, Rahman MU, Doyle MK, et al. Association of anemia and physical disability among patients with rheumatoid arthritis. J Rheumatol. 2007;34:2177–82. [PubMed] [Google Scholar]
- 4.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–23. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 5.Papadaki HA, Kritikos HD, Valatas V, et al. Anemia of chronic disease in rheumatoid arthritis is associated with increased apoptosis of bone marrow erythroid cells: improvement following anti-tumor necrosis factor-α antibody therapy. Blood. 2002;100:474–82. doi: 10.1182/blood-2002-01-0136. [DOI] [PubMed] [Google Scholar]
- 6.Roy CN, Andrews NC. Anemia of inflammation: the hepcidin link. Curr Opin Hematol. 2005;12:107–11. doi: 10.1097/00062752-200503000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–6. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vreugdenhil G, Löwenberg B, van Eijk HG, et al. Tumor necrosis factor alpha is associated with disease activity and the degree of anemia in patients with rheumatoid arthritis. Eur J Clin Invest. 1992;22:488–93. doi: 10.1111/j.1365-2362.1992.tb01495.x. [DOI] [PubMed] [Google Scholar]
- 9.Jongen-Lavrencic M, Peeters HR, Wognum A, et al. Elevated levels of inflammatory cytokines in bone marrow of patients with rheumatoid arthritis and anemia of chronic disease. J Rheumatol. 1997;24:1504–9. [PubMed] [Google Scholar]
- 10.Davis D, Charles PJ, Potter A, et al. Anaemia of chronic disease in rheumatoid arthritis: in vivo effects of tumour necrosis factor α blockade. Br J Rheumatol. 1997;36:950–6. doi: 10.1093/rheumatology/36.9.950. [DOI] [PubMed] [Google Scholar]
- 11.Doyle MK, Rahman MU, Han C, et al. Treatment with infliximab plus methotrexate improves anemia in patients with rheumatoid arthritis independent of improvement in other clinical outcome measures—a pooled analysis from three large, multicenter, double-blind, randomized clinical trials. Semin Arthritis Rheum. 2008;39:123–31. doi: 10.1016/j.semarthrit.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Emery P, Fleischmann RM, Moreland LW, et al. Golimumab, a human anti-tumor necrosis factor α monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum. 2009;60:2272–83. doi: 10.1002/art.24638. [DOI] [PubMed] [Google Scholar]
- 13.Keystone EC, Genovese MC, Klareskog L, et al. Golimumab, a human antibody to tumour necrosis factor α given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD Study. Ann Rheum Dis. 2009;68:789–96. doi: 10.1136/ard.2008.099010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smolen JS, Kay J, Doyle MK, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet. 2009;374:210–21. doi: 10.1016/S0140-6736(09)60506-7. [DOI] [PubMed] [Google Scholar]
- 15.Kavanaugh A, McInnes I, Mease P, et al. Golimumab, a new human tumor necrosis factor α antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: twenty-four-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum. 2009;60:976–86. doi: 10.1002/art.24403. [DOI] [PubMed] [Google Scholar]
- 16.Inman RD, Davis JC, Jr, van der Heijde D, et al. Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum. 2008;58:3402–12. doi: 10.1002/art.23969. [DOI] [PubMed] [Google Scholar]
- 17.Prevoo ML, van ’t Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 18.Prevoo ML, van Gestel AM, van ‘t Hof MA, et al. Remission in a prospective study of patients with rheumatoid arthritis. American Rheumatism Association preliminary remission criteria in relation to the disease activity score. Br J Rheumatol. 1996;35:1101–5. doi: 10.1093/rheumatology/35.11.1101. [DOI] [PubMed] [Google Scholar]
- 19.van Gestel AM, Haagsma CJ, van Riel PL. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum. 1998;41:1845–50. doi: 10.1002/1529-0131(199810)41:10<1845::AID-ART17>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 20.Garrett S, Jenkinson T, Kennedy LG, et al. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol. 1994;21:2286–91. [PubMed] [Google Scholar]
- 21.Shanahan JC, St Clair W. Tumor necrosis factor-alpha blockade: a novel therapy for rheumatic disease. Clin Immunol. 2002;103(3 Pt 1):231–42. doi: 10.1006/clim.2002.5191. [DOI] [PubMed] [Google Scholar]
- 22.Rahman MU, Buchanan J, Doyle MK, et al. Changes in patient characteristics in anti-tumor necrosis factor clinical trials for rheumatoid arthritis: results of an analysis of the literature over the past 16 years. Ann Rheum Dis. 2011;70:1631–40. doi: 10.1136/ard.2010.146043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vreugdenhil G, Wognum AW, van Eijk HG, et al. Anaemia in rheumatoid arthritis: the role of iron, vitamin B12, and folic acid deficiency, and erythropoietin responsiveness. Ann Rheum Dis. 1990;49:93–8. doi: 10.1136/ard.49.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zandman-Goddard G, Shoenfeld Y. Ferritin in autoimmune diseases. Autoimmun Rev. 2007;6:457–63. doi: 10.1016/j.autrev.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 25.Demirag MD, Haznedaroglu S, Sancak B, et al. Circulating hepcidin in the crossroads of anemia and inflammation associated with rheumatoid arthritis. Intern Med. 2009;48:421–6. doi: 10.2169/internalmedicine.48.1578. [DOI] [PubMed] [Google Scholar]
- 26.Braun J, Brandt J, Listing J, et al. Long-term efficacy and safety of infliximab in the treatment of ankylosing spondylitis: an open, observational, extension study of a three-month, randomized, placebo-controlled trial. Arthritis Rheum. 2003;48:2224–33. doi: 10.1002/art.11104. [DOI] [PubMed] [Google Scholar]