Abstract
Objective. To describe the use of and response to biologic therapies commenced in adults with JIA.
Methods. Patients with arthritis onset <16 years were identified from the British Society for Rheumatology Biologics Register for rheumatoid arthritis (BSRBR-RA) and stratified into ILAR JIA subtypes. Patterns of biologic use and treatment persistence were explored, with disability levels (HAQ) and remission rates [28-Joint Disease Activity Score (DAS28)] evaluated at 6 and 12 months.
Results. Arthritis with an onset of <16 years was confirmed in 225 patients and the ILAR subtype was determined in 154 (68%). Only 58 (26%) patients had a diagnosis of JIA recorded in the BSRBR-RA. The median age at biologic commencement was 31 years [interquartile range (IQR) 23–39] and 76% were female. The biologic therapies were etanercept (49%), infliximab (28%), adalimumab (22%) and anakinra (1%). Fifty per cent of patients received more than one biologic during follow-up (2 agents, n = 64; ≥3 agents, n = 49). Treatment persistence at 1 year was 78% (95% CI 71%, 82%), falling to 42% (95% CI 34%, 49%) at 5 years. Both the HAQ and DAS28 improved significantly at 6 months, with 21% and 28% of patients in remission (DAS28 < 2.6) at 6 and 12 months, respectively.
Conclusion. This study describes patterns and identifies outcomes of biologic use in a national cohort of adults with JIA. With no national guidance currently available in this area, the choice of first biologic was inconsistent, although treatment outcomes were good. These data confirm that biologic therapies are an important treatment option in adults with active JIA in adulthood.
Keywords: anti-TNF therapy, disease activity, treatment outcome, juvenile idiopathic arthritis
Introduction
JIA is one of the most common chronic illnesses of childhood, with an incidence of 10/100 000 children-years in the UK [1]. JIA is not a disease confined to childhood, with more than one-third of patients continuing to have episodes of active inflammation during their adult years [2–4]. The evidence base for the optimal management of adults with JIA is lacking, in part due to the complexity of obtaining very long-term follow-up data in children with chronic illness.
The management of JIA has traditionally been modelled on the management of RA, with MTX and now anti-TNF medications forming the mainstay of therapy [5–9]. However, JIA is an umbrella term for a group of related childhood-onset arthritides, many of which are quite different from RA. Adults with JIA are a heterogeneous group with different clinical characteristics than adults with other inflammatory arthritides [2, 10]. Optimal management and outcomes of JIA may therefore differ from RA. As our understanding of the variation between the ILAR subtypes of JIA increases [11, 12], we can begin to hypothesize that the response to anti-TNF therapies may also differ according to subtype.
The use and benefit of anti-TNF therapies in adults with persistent JIA is poorly described. Therefore, using data from adults with JIA registered within the British Society for Rheumatology Biologics Register Rheumatoid Arthritis (BSRBR-RA), this study aims to describe (i) the distribution of ILAR subtypes among UK patients with JIA starting treatment with biologic therapies in adulthood, (ii) the pattern of biologic use in this population and (iii) the reasons for discontinuation of the primary biologic therapy and early treatment response data.
Methods
Study population
The BSRBR-RA is a prospective, national, longitudinal, observational study established in October 2001 [13]. The main aim of the study is to examine the long-term safety of biologic agents in patients with RA in the UK [14]. The BSRBR-RA recruited patients with RA starting etanercept between 2001 and 2005, adalimumab between 2003 and 2008 and infliximab between 2001 and 2007, with a target RA recruitment of 4000 patients starting each drug. Patients with other rheumatic diseases, including JIA, were eligible to be recruited at any time, with the exception of AS and PsA, for which recruitment stopped in 2006.
Ethics approval was obtained for the BSRBR-RA in December 2000 from the Multicentre Research Ethics Committee (MREC) for the North-West of England and all patients gave written informed consent to participate in the study.
Data collection
At the start of biologic therapy, clinical teams complete a baseline questionnaire that includes information on diagnosis, date of symptom onset, current disease activity [using the RA 28-joint Disease Activity Score (DAS28), a composite score including a 28 swollen joint count, 28 tender joint count, ESR and a patient global assessment] [15] and details of past and current DMARD and biologic use. Information on co-morbidities is collected at baseline. The patient completes an HAQ standardized for UK use [16]. Follow-up data are collected every 6 months for 3 years from the rheumatologist and patient, then annually from the rheumatologist thereafter. Physician-derived follow-up details include disease activity assessment (DAS28), anti-rheumatic drug details, including dates and reasons for cessation if appropriate, and adverse events. Patients are requested to complete an HAQ every 6 months for 3 years.
Subject selection and definition
All patients with a recorded onset of arthritis prior to their 16th year were identified from the register to ensure identification of all patients with JIA, including those labelled with a diagnosis other than JIA by the treating rheumatologist. In all cases the current treating rheumatologist was contacted and asked to provide further information to confirm a diagnosis of JIA and to establish an ILAR classification.
Data analysis
This analysis was limited to those individuals with a confirmed diagnosis of JIA (idiopathic arthritis lasting >6 weeks with onset prior to the 16th birthday) [11, 12]. Physician-derived data were analysed to determine the reasons for stopping the primary biologic. Kaplan–Meier survival curves were used to describe treatment persistence with the patient’s first anti-TNF therapy. Patients were censored at date of death, first missed dose or last follow-up if still on treatment, whichever came first. Temporary stops of <90 days (commonly for surgery or adverse events such as infection), followed by recommencement of the same anti-TNF therapy, were counted as continuous use of the drug.
Changes in disability were assessed at 6 and 12 months using changes in the HAQ score from baseline [17]. A minimal clinically important difference (MCID) was defined as an improvement in the HAQ score of at least 0.22 units [18]. Disease activity at 6 and 12 months following commencement of the primary biologic therapy was determined using the DAS28 [15]. Response was categorized using the European League Against Rheumatism (EULAR) response criteria into good, moderate or non-response based on changes in the DAS28 and final DAS28 achieved [19] and remission was defined as a DAS28 <2.6 [19, 20]. Non-parametric descriptive statistics were used to compare the response between groups and across time points. STATA 10.1 software was used for all statistical analyses (STATA Corporation, College Station, TX, USA).
Results
Patient characteristics
Through January 2010, 495 patients were identified as having a possible onset of arthritis prior to their 16th birthday. The clinical teams returned 234 forms, with the diagnosis of JIA confirmed in 225 patients. The reporting clinicians provided sufficient information to further classify the ILAR subtype in 154/225 (68%) patients.
Just 26% of patients in this cohort were reported to have JIA by the treating clinical team on the baseline questionnaire (Table 1)—22% as the primary diagnosis and 4% as a secondary diagnosis (primary label RA). Most patients (78%) had another primary rheumatologic diagnosis {RA, n = 129; AS, n = 23; PsA, n = 16; other, n = 8, including 4 seronegative arthritides [confirmed ILAR subtype: 1 psoriatic JIA (JPsA), 2 enthesitis-related arthritides (ERA) and one with insufficient information to differentiate between the two], 1 inflammatory arthritis (ILAR subtype: JPsA) and 3 adult-onset Still’s disease (ILAR subtype: extended oligoarticular JIA(EO) and 2 unknown}.
Table 1.
Diagnostic label in the BSRBR-RA database
| ILAR subtype | SJIA | POJIA | RF− PJIA | RF+ PJIA | ERA | JPsA | UnJIA | Subtype unknown | Total |
|---|---|---|---|---|---|---|---|---|---|
| Number, % | 11 (5) | 38 (17) | 19 (8) | 36 (16) | 24 (11) | 26 (12) | 0 | 71 (31) | 225 |
| Primary diagnostic label in registry, n (%) | |||||||||
| JIA | 4 (36) | 11 (29) | 8 (42) | 3 (8) | 3 (12) | 4 (15) | 16 (23) | 49 (22) | |
| RA | 7 (64) | 26 (68) | 11 (58) | 33 (92) | 4 (17) | 6 (23) | 42 (59) | 129 (57) | |
| PsA | 0 | 0 | 0 | 0 | 0 | 13 (50) | 3 (4) | 16 (7) | |
| AS | 0 | 0 | 0 | 0 | 15 (62) | 1 (4) | 7 (10) | 23 (10) | |
| Other (not JIA) | 0 | 1 (3) | 0 | 0 | 2 (9) | 2 (8) | 3 (4) | 8 (4) | |
| JIA documented anywhere on baseline form, % | 4 (36) | 15 (39) | 10 (53) | 5 (14) | 3 (12.5) | 5 (19) | 16 (23) | 58 (26) | |
SJIA: systemic onset JIA; POJIA: persistent oligoarticular JIA; EO: extended oligoarticular JIA; RF− PJIA: RF-negative polyarticular JIA; RF+ PJIA: RF-positive polyarticular JIA; PJIA: psoriatic JIA; UnJIA: unclassifiable JIA.
Baseline characteristics and biologic prescribing patterns in the final study cohort are presented in Table 2. The median age of the cohort at commencement of the primary biologic was 31 years [interquartile range (IQR) 23–39], 76% female. The median disease duration since diagnosis was 21 years (IQR 12–30). The proportion of female patients differed significantly with the ILAR subtype (P < 0.001), with a higher proportion of males in the ERA and PsA subtypes. There was no significant difference in any other baseline characteristics across the ILAR subtypes.
Table 2.
Baseline characteristics and prescribing patterns at commencement of first biologic
| ILAR subtype | SJIA | OJIA | RF− PJIA | RF+ PJIA | ERA | JPsA | Subtype unknown | Total | P-value |
|---|---|---|---|---|---|---|---|---|---|
| Number | 11 (5) | 38 (17) | 19 (8) | 36 (16) | 24 (11) | 26 (12) | 71 (31) | 225 | |
| Age, yearsa | 26 (22–31) | 28.5 (23–37) | 29 (21–36) | 31 (22–42) | 34 (22–39) | 29 (25–39) | 36 (24–42) | 31 (23–39) | 0.38 |
| Female, % | 82 | 82 | 95 | 94 | 17 | 62 | 82 | 76 | <0.001 |
| Disease duration, years | 14 (8–22) | 20 (15–26) | 20 (11–24) | 16.5 (9–29) | 19 (8–28) | 19 (11–30) | 25 (15–31) | 21 (12–30) | 0.88 |
| Smoking status | |||||||||
| Never | 8 (73) | 27 (71) | 9 (47) | 25 (69) | 15 (63) | 11 (42) | 37 (52) | 132 (59) | 0.12 |
| Previously | 0 | 1 (2.5) | 5 (26) | 5 (14) | 2 (8) | 6 (23) | 16 (23) | 35 (16) | |
| Current | 2 (18) | 9 (24) | 4 (21) | 6 (17) | 7 (29) | 9 (35) | 18 (25) | 55 (24) | |
| Missing | 1 (9) | 1 (2.5) | 1 (5) | 0 | 0 | 0 | 0 | 3 (1) | |
| Co-morbiditiesb | |||||||||
| PUD | 1 (10) | 0 | 0 | 1 (3) | 2 (8) | 0 | 3 (4) | 7 (3) | 0.19 |
| Depression | 2 (20) | 5 (13) | 3 (16) | 3 (8) | 8 (33) | 6 (23) | 15 (21) | 42 (19) | 0.20 |
| Asthma | 0 | 3 (8) | 2 (11) | 5 (14) | 1 (4) | 2 (8) | 9 (13) | 22 (10) | 0.70 |
| Baseline DMARDs | |||||||||
| None | 3 (27) | 12 (31) | 9 (47) | 12 (33) | 6 (25) | 10 (38) | 25 (35) | 78 (35) | |
| MTX monotherapy | 7 (64) | 19 (50) | 6 (32) | 14 (39) | 12 (50) | 11 (42) | 29 (41) | 98 (44) | 0.89 |
| MTX combinationc | 1 (9) | 3 (8) | 1 (5) | 4 (11) | 4 (17) | 3 (12) | 11 (15) | 27 (12) | |
| Other | 0 | 4 (11) | 3 (16) | 5 (14) | 2 (8) | 2 (8) | 6 (9) | 22 (9) | |
| Total number of DMARDs prior to first biologic | 3 (2–4) | 4 (3–5) | 3 (2–6) | 4 (2–6) | 2 (2–4.5) | 3 (2–5) | 4 (3–5) | 3 (2–5) | 0.54 |
| Primary biologic | |||||||||
| Etanercept | 6 (55) | 17 (45) | 14 (73) | 18 (50) | 12 (50) | 17 (65) | 26 (37) | 110 (49) | |
| Infliximab | 2 (18) | 13 (34) | 2 (11) | 8 (22) | 9 (37.5) | 6 (23) | 24 (34) | 64 (28) | 0.60 |
| Adalimumab | 3 (27) | 8 (21) | 3 (16) | 9 (25) | 3 (12.5) | 3 (12) | 21 (29) | 50 (22) | |
| Anakinra | 0 | 0 | 0 | 1 (3) | 0 | 0 | 0 | 1 (1) | |
| Total biologics | |||||||||
| 1 | 6 (55) | 18 (47) | 14 (74) | 18 (50) | 12 (50) | 17 (65) | 27 (38) | 112 (50) | 0.63 |
| 2 | 2 (18) | 12 (32) | 2 (10) | 9 (25) | 9 (38) | 6 (23) | 24 (34) | 64 (28) | |
| ≥3 | 3 (27) | 8 (21) | 3 (16) | 9 (25) | 3 (12) | 3 (12) | 20 (28) | 49 (22) | |
| Biologic therapy used ever | |||||||||
| Etanercept | 8 (73) | 27 (71) | 16 (84) | 23 (64) | 17 (71) | 19 (73) | 39 (55) | 110 (72) | |
| Infliximab | 3 (27) | 16 (42) | 5 (26) | 10 (28) | 9 (37.5) | 7 (27) | 30 (42) | 50 (32) | |
| Adalimumab | 5 (45) | 16 (42) | 8 (42) | 15 (42) | 7 (29) | 6 (23) | 33 (46) | 57 (37) | |
| Anakinra | 1 (9) | 1 (2.5) | 0 | 1 (3) | 0 | 0 | 1 (1) | 3 (2) | |
| Rituximab | 0 | 4 (11) | 1 (5) | 5 (14) | 1 (4) | 1 (4) | 2 (3) | 12 (8) | |
| Abatacept | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1) | 0 | |
| Tocilizumab | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1) | 0 | |
All values median (IQR) or n (%). SJIA: systemic-onset JIA; OJIA: oligoarticular JIA; RF− PJIA: RF-negative polyarticular JIA; RF+ PJIA: RF-positive polyarticular JIA; JPsA: psoriatic JIA; UnJIA: unclassifiable JIA; PUD: peptic ulcer disease. aMedian age in years at commencement of the first biologic. bNo patients were reported as having malignancy, tuberculosis or demyelinating disease. cMTX combination: MTX prescribed in combination with one or more other DMARDs.
Many (56%) patients received their biologic in combination with MTX (alone or with another DMARD) and 35% had discontinued all DMARDs at commencement of biologic therapy. The median number of previous DMARDs was 3 (IQR 2–5). The primary biologic therapy was etanercept in 49%, infliximab in 28%, adalimumab in 22% and anakinra in 1%. There was no significant difference in primary biologic therapy across the ILAR subtypes, although infliximab was more commonly prescribed for patients with oligoarticular JIA and ERA. Fifty per cent received more than one anti-TNF over the study period, with 22% receiving three or more anti-TNF therapies.
We were unable to establish the ILAR category for 71 subjects. Fifty-seven (80%) had a polyarticular disease course, but missing information such as RF titres (missing in 21/57) or the number of joints involved in the first 6 months (missing in 19/57) rendered the ILAR subtype indeterminable. The majority of this subgroup had a higher median age than patients with the ILAR subtype available [36 years (IQR 24–42) vs 29 years (IQR 22–38), P = 0.04] and longer median disease duration since diagnosis [25 years (IQR 15–31) vs 19 years (IQR 11–27), P = 0.0027].
Treatment persistence and reasons for treatment discontinuation
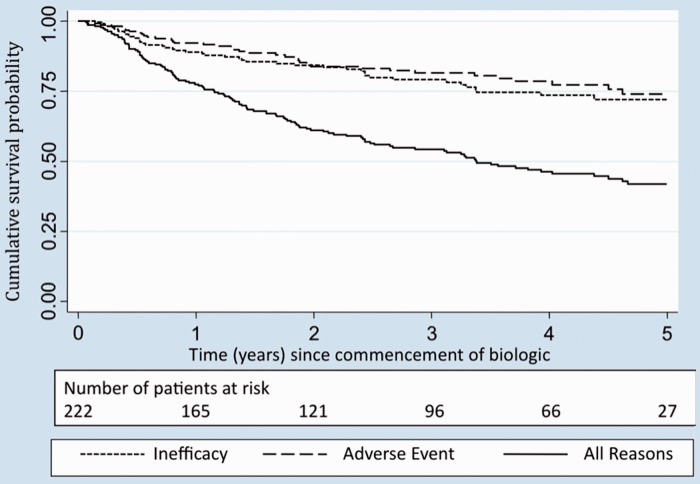
Consultant-derived follow-up data (including anti-rheumatic drug details) were available in 222/225 patients (99%). In total there were 590 person-years of observation (median person-years per patient 2.4 years, range 0.06–7.2 years). Overall, 114/222 (51%) patients discontinued the primary biologic therapy (Table 3), 45/114 (39%) for inefficacy and 38/114 (33%) for adverse events. No serious adverse events (fatal or otherwise life-threatening, resulting in unplanned or prolonged hospitalization, physically disabling or resulting in a birth defect) were reported as a reason for stopping biologic therapy. Reasons cited for stopping medication in the other category most commonly included pregnancy (n = 7) and planned surgery (n = 5). No patients were recorded as stopping the drug for remission. The probability of remaining on the primary biologic therapy was 78% (95% CI 71%, 82%) at 1 year (Fig. 1). This dropped to 42% (95% CI 34%, 49%) at 5 years, with a median drug survival of 3.3 years.
Table 3.
Physician-reported reasons to stop primary biologic therapy
| Stop reason | Etanercept | Infliximab | Adalimumab | Anakinra | Total, n (%) |
|---|---|---|---|---|---|
| Still on drug | 66 | 19 | 22 | 1 | 108 (49) |
| Inefficiency | 17 | 18 | 10 | 0 | 45 (20) |
| Adverse event | 13 | 13 | 12 | 0 | 38 (17) |
| Other | 13 | 14 | 4 | 0 | 31 (14) |
| Total | 109 | 64 | 48 | 1 | 222 |
Fig. 1.
Kaplan–Meier curve illustrating survival time on the primary biologic.
Effect of biologic therapy on disease activity (DAS28)
The DAS28 was available for 91% of patients at baseline, with a median score of 6.3, with 84% >5.1 (Table 4), and no significant variation with the ILAR subtype (P = 0.62). The median DAS28 at 6 months was 3.8 (IQR 2.7–4.9), with little change at 12 months [3.7 (IQR 2.5–5.2)].
Table 4.
Effect of biologic therapy on disease activity
| ILAR subtype | SJIA | OJIA | RF− PJIA | RF+ PJIA | ERA | JPsA | Subtype unknown | Total | P |
|---|---|---|---|---|---|---|---|---|---|
| Number of patients | 11 | 38 | 19 | 36 | 24 | 26 | 71 | 225 | |
| Baseline DAS28a | 5.8 (4.4–7.0) | 6.3 (5.4–7.5) | 6.6 (5.3–7.0) | 6.4 (5.9–7.2) | 5.9 (4.9–6.7) | 6.3 (5.4–7.0) | 6.5 (5.7–7.1) | 6.3 (5.4–7.1) | 0.62 |
| 6-month DAS28b | 4.9 (2.6–5.6) | 3.7 (3.0–4.5) | 4.0 (3.0–5.3) | 4.0 (3.2–5.0) | 3.0 (1.6–4.8) | 3.3 (2.6–4.1) | 3.8 (2.6–5.2) | 3.8 (2.7–4.9) | 0.36 |
| 6-month EULAR response, % | |||||||||
| None | 37.5 | 22 | 18 | 22 | 10 | 25 | 20 | 21 | |
| Moderate | 37.5 | 47 | 47 | 59 | 30 | 37.5 | 43 | 46 | 0.58 |
| Good | 25 | 31 | 35 | 19 | 60 | 37.5 | 37 | 33 | |
| Percentage DAS28 remission, 6 months | 25 | 16 | 18 | 13 | 31 | 25 | 26 | 21 | 0.71 |
| 12-month DAS28c | 2.9 (1.9–5.1) | 4.0 (2.9–4.9) | 4.3 (2.5–5.0) | 4.5 (2.9–5.4) | 2.0 (1.2–4.7) | 2.6 (2.4–4.4) | 3.7 (2.8–5.6) | 3.7 (2.5–5.2) | 0.10 |
| 12-month EULAR response, % | |||||||||
| None | 28.5 | 11.5 | 27 | 30 | 11 | 28 | 18 | 22 | |
| Moderate | 28.5 | 61.5 | 33 | 47 | 33 | 33 | 54 | 46 | 0.48 |
| Good | 43 | 27 | 40 | 23 | 56 | 39 | 28 | 32 | |
| Percentage DAS28 remission, 12 months | 29 | 19 | 27 | 20 | 58 | 50 | 21 | 28 | 0.055 |
All values median (IQR) or n (%). SJIA: systemic onset JIA; OJIA: oligoarticular JIA; RF− PJIA: RF-negative polyarticular JIA; RF+ PJIA: RF-positive polyarticular JIA; PJIA: psoriatic JIA; UnJIA: unclassifiable JI. aNumber of patients with DAS28 available at baseline: 204/225 (91%). bNumber of patients with DAS28 available at 6 months: 172/225 (76%). cNumber of patients with DAS28 available at 12 months: 151/225 (67%).
Few patients (3/204, 1.5%) had a DAS28 compatible with remission (DAS28 < 2.6) at the start of treatment; 36/172 (21%) and 42/151 (28%) were in DAS28 remission at 6 and 12 months, respectively (Table 4). Overall, 4% patients had no swollen joints at baseline (based on a 28-joint count), increasing to 26% at 6 months and 33% at 1 year. Two per cent of patients had no tender joints at baseline, increasing to 18% at 6 months and 22% at 12 months. No patients had a patient global assessment of 0 cm at baseline, increasing to 13% at 6 months and 6% at 1 year. There was no significant difference in remission rates between ILAR subtypes of JIA at 6 or 12 months (P = 0.71 and P = 0.055, respectively), although more patients with ERA and JPsA achieved remission at 12 months, with a trend towards significance.
Effect of biologic therapy on disability (HAQ)
HAQ scores were available for 88% of patients at baseline, 61% at 6 months and 56% at 12 months (Table 5). The median baseline HAQ decreased significantly by 6 months (P < 0.001) and was sustained at 12 months (P < 0.001). No statistically significant difference was seen in baseline HAQ (P = 0.08) or HAQ improvement across the ILAR subtypes (P = 0.36 at 6 months and P = 0.39 at 12 months). A similar proportion of patients achieved a minimal clinically important difference (MCID > 0.22) in the HAQ at 6 and 12 months across the ILAR subtypes (P = 0.33 at 6 months and P = 0.38 at 12 months), although the numbers of patients were very small.
Table 5.
Effect of biologic therapy on disability
| ILAR subtype | SJIA | OJIA | RF− PJIA | RF+ PJIA | ERA | JPsA | Subtype unknown | Total | P |
|---|---|---|---|---|---|---|---|---|---|
| Number of patients | 11 | 38 | 19 | 36 | 24 | 26 | 71 | 225 | |
| Baseline HAQa | 1.69 (1.25–2.13) | 2 (1.63–2.3) | 1.81 (1.25–2.25) | 2.06 (1.56–2.44) | 1.44 (0.63–1.75) | 2.0 (1.38–2.63) | 2.06 (1.5–2.5) | 2 (1.5–2.4) | 0.08 |
| 6-month HAQb | 1.75 (0.88–1.88) | 1.63 (0.75–2.13) | 1.5 (1.0–2.13) | 1.75 (1–2.5) | 1.13 (0.38–1.38) | 1.75 (1.5–2.0) | 1.69 (0.75–2.13) | 1.75 (0.88–1.88) | 0.18 |
| Change in HAQ at 6 months | 0.56 (0.25–0.81) | 0.38 (0–0.63) | 0.56 (0.25–0.75) | 0.13 (–0.13–0.5) | 0.31 (–0.06–0.63) | 0.38 (0.13–0.63) | 0.5 (0.13–0.88) | 0.38 (0–0.63) | 0.36 |
| Percentage MCID HAQ at 6 months | 75 | 62 | 88 | 42 | 63 | 67 | 65 | 63 | 0.33 |
| 12-month HAQc | 0.63 (0.25–1.5) | 1.56 (0.56–2.13) | 1.63 (1.25–1.88) | 2.06 (0.81–2.5) | 1.25 (0–1.38) | 1.63 (1.13–1.81) | 1.5 (0.75–2.13) | 1.5 (0.75–2.13) | 0.07 |
| Change in HAQ at 12 months | 0.44 (0–0.94) | 0.38 (0–0.63) | 0.38 (0.38–0.63) | 0.13 (0–0.38) | 0.31 (0.13–0.5) | 0.63 (0.25–0.75) | 0.5 (0.13–0.75) | 0.38 (0.13–0.75) | 0.39 |
| Percentage MCID HAQ at 12 months | 50 | 64 | 83 | 43 | 63 | 79 | 73 | 67 | 0.38 |
All values median (IQR) or n (%). SJIA: systemic onset JIA; OJIA: oligoarticular JIA; RF− PJIA: RF-negative polyarticular JIA; RF+ PJIA: RF-positive polyarticular JIA; PJIA: psoriatic JIA; UnJIA: unclassifiable JIA. aNumber of patients with HAQ available at baseline: 197/225 (88%). bNumber of patients with HAQ available at 6 months: 137/225 (61%). cNumber of patients with HAQ available at 12 months: 126/225 (56%).
Discussion
This study is the first to describe prescribing patterns of biologic therapies in UK adults with JIA, demonstrating their efficacy both in terms of reduced disease activity and reduced disability. It reports a number of interesting findings, including a relatively high frequency of switching between biologic therapies and relatively high remission rates for adults with JIA compared with RA patients in the same cohort. It also demonstrates that the adults included in this study had severe disease, indicated by the median baseline HAQ score of 2.0 and higher levels of depression than are estimated for the general adult population (19% vs an estimated 11%) [21].
Patients with RF+ JIA, ERA and JPsA are over-represented in this cohort in comparison to paediatric cohorts in general [22]. This is likely to be explained by patterns of remission in JIA; the probability of complete remission in JIA within 10 years is highest in oligoarticular and lowest in RF+ polyarticular disease [23, 24]. The ILAR subtype distribution of this cohort reflects these remission patterns, confirming the relatively poor prognoses of the RF+, ERA and JPsA subtypes. Although the mean age of this cohort is lower than the mean age of RA patients in the BSRBR-RA, the median disease duration prior to the first biologic is 5 years longer [25]. Although this may relate to the availability and licensing of anti-TNF therapies compared with the onset of the disease, it is also possible that clinicians found it more difficult to obtain funding permissions for biologic therapies for adults with JIA. At the time of this study, no specific UK treatment guidelines for anti-TNF therapies in adult patients with JIA had been published. Current published guidelines for polyarticular JIA in the UK extend from 4 to 17 years [26].
Just 26% of patients with a confirmed diagnosis of JIA were listed as having JIA on their baseline questionnaire, with the majority listed as having RA. Possible explanations include incomplete information at transfer to adult services and a potential perception among reporting clinicians that JIA patients are less likely to receive funding for biologic therapies, as there is no guidance/consensus for their use, with the consequence that an adult rheumatic disease diagnosis is listed in the medical record. Patients with RF+, ERA and JPsA are less likely to be labelled with JIA; implying difficulty with diagnostic labelling is maximal within these subtypes.
Many patients (42%) with 5 years of follow-up data available remain on the primary biologic therapy, similar to rates observed in patients with RA from this same population [27]. Although the numbers are small, this suggests that biologic therapies can be efficacious in the short to medium term. Many (50%) patients in this cohort were prescribed more than one biologic therapy. Although 33% discontinued the primary biologic for adverse events, 39% switched for inefficacy, indicating ongoing active disease on the primary biologic.
There was no clear pattern to the primary biologic therapy, with 49% receiving etanercept, 28% infliximab and 22% adalimumab. The BSRBR-RA cannot be used to comment on prescribing patterns over time due to the study design, which was a cohort study rather than an open register.
However, an anecdotal risk of uveitis flare has been described with etanercept, so it is possible that infliximab or adalimumab were chosen in patients with uveitis [28]. This is supported by the increased frequency of infliximab as the primary biologic in the oligoarticular subtype. The presence of uveitis could not be ascertained in this cohort since uveitis is not recorded in the BSRBR-RA dataset. Anakinra was prescribed to one subject with RF+ JIA. The use of anakinra in polyarticular JIA has been described, although there is no good evidence for its efficacy [29]. There is good evidence for the efficacy of anakinra in systemic-onset JIA, but anakinra was not used for any patients with this subtype [30].
Remission rates at 6 and 12 months were determined through application of the RA DAS28 and EULAR response criteria [15, 19]. Very little is known about the validity of outcome measures in adults with JIA. No composite disease activity score has been validated for use in adults with JIA, including the recently introduced Juvenile Arthritis Disease Activity Score (JADAS) [31]. The DAS28 is likely to reflect disease activity most accurately in polyarticular patterns of disease, but has been criticized in adult psoriatic arthritis [32]. For similar reasons, the DAS28 may not be an effective measure of overall disease activity in a number of the ILAR subtypes. However, since composite disease activity scores should predict outcomes more accurately than their individual components, a decision was made to apply the DAS28 to this cohort of adults with JIA, accepting the potential limitations of this tool. This cohort has very long-standing disease (median disease duration 19 years), significantly longer than that observed in RA cohorts [33]. Despite this, the response to biologic therapy is very good, with remission rates (21%) better than those observed in RA patients (9%) and similar to those in patients with psoriatic arthritis (27.5%) within the same population [25, 34]. The heterogeneity of JIA makes it difficult to draw meaningful comparisons between patients with JIA and more homogeneous groups of patients with adult inflammatory diseases.
The HAQ is a robust measure of disability used in the assessment of functional ability in previous studies of adults with JIA [2, 10, 24, 35–39]. The median HAQ at baseline, 6 and 12 months in this population does not vary with the ILAR subtype of JIA, suggesting a similar threshold of disability corresponding to commencement of biologic therapy within the different ILAR subtypes.
The median HAQ decreases significantly from baseline to 6 months and baseline to 12 months and many patients exceed the MCID in the HAQ. Since there is no validated MCID for adults with JIA, the definition of MCID derived from populations of adults with RA has been applied to this cohort. Disability levels in childhood JIA have been shown to drop dramatically following commencement of etanercept [5, 6]. Longer-term follow-up of larger cohorts of adults with JIA on biologic therapies would help establish the clinical relevance of the changes noted.
This study has a number of limitations. Details regarding confirmation and subtype of JIA were collected retrospectively. We could only confirm or refute the diagnosis of JIA in 47% of our cohort due to physician non-response. The reasons for this were not known but resulted in the exclusion of up to 261 further cases, thus potentially introducing a degree of selection bias into our data.
Compared with the large sample size within the BSRBR-RA cohort, the sample size of this cohort is small. Although this may reflect a low absolute number of adults with JIA starting biologics in the UK, registration of adults with JIA commencing biologic treatment with the BSRBR-RA is not mandatory. Therefore this study may not have captured all adults with JIA in the UK commencing biologic therapies in adult life. HAQ scores were not available at follow-up in >40% of patients, introducing the possibility of selection bias. This level is slightly higher than that reported in the overall BSRBR-RA cohort, recently estimated at 30% (reference my moderate disease activity paper from 2009 - PMID:19706737), and therefore further bias may have been introduced.
We are aware that many adults with JIA will have commenced biologic therapies in childhood, rendering this cohort unrepresentative of all adults with JIA currently on biologics within the UK. However, in the absence of robust long-term follow-up of paediatric JIA cohorts, this is the largest cohort of adults with JIA commencing biologic therapies reported to date and these data are important, emphasizing the need for long-term surveillance in this heterogeneous condition.
The Juvenile arthritis MTX/Biologics long-term Observation (JuMBO) study recently published data on 346 adults with JIA commenced on etanercept during childhood [40]. Almost half (45%) of the cohort remained on etanercept alone in adult life (median follow-up period 6.5 years, IQR 4.2–8.4 years), with a median of 3.5 biologic and non-biologic DMARDS other than etanercept (range 1–13) over the course of the disease [40]. In comparison, 42% of our cohort of patients commencing biologics in adulthood remained on the primary biologic at 5 years, with 50% receiving more than one and 22% receiving multiple anti-TNF therapies. This study provides further evidence of the importance of biologic therapies in adults with JIA and is the first to document the efficacy of biologic therapies commenced in JIA patients during adulthood.
All 225 patients fulfilled diagnostic criteria for JIA, although the ILAR subtype could not be determined in 71/225 patients (32%). There are significant differences in baseline demographics between these 71 patients and the final study cohort. Many of those excluded fulfilled criteria for more than one ILAR subtype, but missing information meant they could not be classified as undifferentiated JIA. The ILAR classification system can be difficult to apply to adults with JIA and the missing information on many patients in this study highlights the difficulties associated with retrospective data collection. Important information such as the number of joints involved in the first 6 months can be lost over time.
The inability to classify 32% of the patients in this cohort may reflect incomplete clinical information at transfer from paediatric to adult services. This phenomenon is well documented [41], highlighting the need for long-term follow-up of large prospective cohorts of children with JIA such as the Childhood Arthritis Prospective Study (CAPS) [22]. It is extremely important that safety and efficacy studies for biological agents in JIA extend from childhood into the adult years, with existing paediatric registries supported to facilitate long-term follow-up.
In summary, this study increases the body of evidence for persistence of severe active JIA into adult life, adds further information about the demographics of adults with active JIA and demonstrates that biologic therapies are integral to the management of this group. It highlights how little is known about the management of adult JIA and the factors influencing the choice of therapy. Anecdotally, the choice of biologic therapy is influenced by patient choice, adherence history, clinical features that may be particular to JIA (such as flare of uveitis or systemic features), access to treatment centres and funding. There is no consensus about optimal care or biologic use in adult JIA, although the results of this study suggest response after 5 years is good, with similar drug survival to that seen in RA and perhaps higher rates of remission.
Rheumatology key messages.
Biologic therapies are integral to the management of adults with severe JIA, reducing disease activity and disability.
There is no consensus about the optimal management of JIA in adulthood and the factors influencing the choice of therapy.
Prescribing patterns of biologic therapies in adults with JIA are variable.
Acknowledgements
The authors acknowledge the enthusiastic collaboration of all consultant rheumatologists and their specialist nurses in the UK in providing the data. In addition, the authors acknowledge support from the BSR Executive, the members of the BSR Registers and Research Committee and the BSRBR-RA Project Team in London for their active role in enabling the register to undertake its tasks. The authors also acknowledge the seminal role of the BSR Clinical Affairs Committee for establishing national biological guidelines and recommendations for such a register.
The BSRBR-RA is a UK-wide national project to investigate the safety of biologic agents in routine medical practice. D.P.M.S. and K.H. are principal investigators on the BSRBR-RA. The register is funded by restricted income from UK pharmaceutical companies, presently Abbott Laboratories, Swedish Orphan Biovitrum, Merck, Pfizer, Roche and UCB Pharma. This income finances a wholly separate contract between the BSR and the University of Manchester. The principal investigators and their team have full academic freedom and are able to work independently of pharmaceutical industry influence. All decisions concerning analyses, interpretation and publication are made autonomously of any industry contribution. F.M. is funded by a Barbara Ansell Fellowship from Arthritis Research UK.
Disclosure statement: H.E.F. has received honoraria, educational bursaries and competitive grant awards from Pfizer. All other authors have declared no conflicts of interest.
References
- 1.Symmons DP, Jones M, Osborne J, et al. Pediatric rheumatology in the United Kingdom: data from the British Pediatric Rheumatology Group National Diagnostic Register. J Rheumatol. 1996;23:1975–80. [PubMed] [Google Scholar]
- 2.Foster HE, Marshall N, Myers A, et al. Outcome in adults with juvenile idiopathic arthritis: a quality of life study. Arthritis Rheum. 2003;48:767–75. doi: 10.1002/art.10863. [DOI] [PubMed] [Google Scholar]
- 3.Minden K. Adult outcomes of patients with juvenile idiopathic arthritis. Horm Res. 2009;72(Suppl 1):20–5. doi: 10.1159/000229759. [DOI] [PubMed] [Google Scholar]
- 4.Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007;369:767–78. doi: 10.1016/S0140-6736(07)60363-8. [DOI] [PubMed] [Google Scholar]
- 5.Lovell DJ, Giannini EH, Reiff A, et al. Etanercept in children with polyarticular juvenile rheumatoid arthritis. Pediatric Rheumatology Collaborative Study Group. N Engl J Med. 2000;342:763–9. doi: 10.1056/NEJM200003163421103. [DOI] [PubMed] [Google Scholar]
- 6.Lovell DJ, Reiff A, Ilowite NT, et al. Safety and efficacy of up to eight years of continuous etanercept therapy in patients with juvenile rheumatoid arthritis. Arthritis Rheum. 2008;58:1496–504. doi: 10.1002/art.23427. [DOI] [PubMed] [Google Scholar]
- 7.Lovell DJ, Ruperto N, Goodman S, et al. Adalimumab with or without methotrexate in juvenile rheumatoid arthritis. N Engl J Med. 2008;359:810–20. doi: 10.1056/NEJMoa0706290. [DOI] [PubMed] [Google Scholar]
- 8.Ruperto N, Lovell DJ, Cuttica R, et al. A randomized, placebo-controlled trial of infliximab plus methotrexate for the treatment of polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum. 2007;56:3096–106. doi: 10.1002/art.22838. [DOI] [PubMed] [Google Scholar]
- 9.Southwood TR, Foster HE, Davidson JE, et al. Duration of etanercept treatment and reasons for discontinuation in a cohort of juvenile idiopathic arthritis patients. Rheumatology. 2011;50:189–95. doi: 10.1093/rheumatology/keq308. [DOI] [PubMed] [Google Scholar]
- 10.Minden K, Niewerth M, Listing J, et al. Long-term outcome in patients with juvenile idiopathic arthritis. Arthritis Rheum. 2002;46:2392–401. doi: 10.1002/art.10444. [DOI] [PubMed] [Google Scholar]
- 11.Petty RE, Southwood TR, Baum J, et al. Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J Rheumatol. 1998;25:1991–4. [PubMed] [Google Scholar]
- 12.Petty RE, Southwood TR, Manners P, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–2. [PubMed] [Google Scholar]
- 13.Watson K, Symmons D, Griffiths I, et al. The British Society for Rheumatology biologics register. Ann Rheum Dis. 2005;64(Suppl 4):iv42–3. doi: 10.1136/ard.2005.042499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silman A, Symmons D, Scott DG, et al. British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2003;62(Suppl 2):ii28–9. doi: 10.1136/ard.62.suppl_2.ii28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prevoo ML, van ’t Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 16.Kirwan JR, Reeback JS. Stanford Health Assessment Questionnaire modified to assess disability in British patients with rheumatoid arthritis. Br J Rheumatol. 1986;25:206–9. doi: 10.1093/rheumatology/25.2.206. [DOI] [PubMed] [Google Scholar]
- 17.Fries JF, Spitz PW, Young DY. The dimensions of health outcomes: the health assessment questionnaire, disability and pain scales. J Rheumatol. 1982;9:789–93. [PubMed] [Google Scholar]
- 18.Goldsmith CH, Boers M, Bombardier C, et al. Criteria for clinically important changes in outcomes: development, scoring and evaluation of rheumatoid arthritis patient and trial profiles. OMERACT Committee. J Rheumatol. 1993;20:561–5. [PubMed] [Google Scholar]
- 19.van Gestel AM, van ‘t Hof MA, van Rijswijk MH, et al. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organisation/International League Against Rheumatism Criteria. Arthritis Rheum. 1996;39:34–40. doi: 10.1002/art.1780390105. [DOI] [PubMed] [Google Scholar]
- 20.Fransen J, Creemers MC, van Riel PL. Remission in rheumatoid arthritis: agreement of the disease activity score (DAS28) with the ARA preliminary remission criteria. Rheumatology. 2004;43:1252–5. doi: 10.1093/rheumatology/keh297. [DOI] [PubMed] [Google Scholar]
- 21.Singleton N, Bumpstead R, O’Brien M, et al. Psychiatric morbidity among adults living in private households 2000. London: National Statistics; 2001. [DOI] [PubMed] [Google Scholar]
- 22.Adib N, Hyrich K, Thornton J, et al. Association between duration of symptoms and severity of disease at first presentation to paediatric rheumatology: results from the Childhood Arthritis Prospective Study. Rheumatology. 2008;47:991–5. doi: 10.1093/rheumatology/ken085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minden K, Kiessling U, Listing J, et al. Prognosis of patients with juvenile chronic arthritis and juvenile spondyloarthropathy. J Rheumatol. 2000;27:2256–63. [PubMed] [Google Scholar]
- 24.Oen K, Malleson PN, Cabral DA, et al. Disease course and outcome of juvenile rheumatoid arthritis in a multicenter cohort. J Rheumatol. 2002;29:1989–99. [PubMed] [Google Scholar]
- 25.Hyrich KL, Watson KD, Silman AJ, et al. Predictors of response to anti-TNF-alpha therapy among patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Rheumatology. 2006;45:1558–65. doi: 10.1093/rheumatology/kel149. [DOI] [PubMed] [Google Scholar]
- 26. National Institute for Clinical Excellence. Guidance on the use of etanercept for the treatment of juvenile idiopathic arthritis. Technology appraisal guidance no. 35. London: National Institute for Clinical Excellence, 2002.
- 27.Soliman MM, Ashcroft DM, Watson KD, et al. Impact of concomitant use of DMARDs on the persistence with anti-TNF therapies in patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2011;70:583–9. doi: 10.1136/ard.2010.139774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taban M, Dupps WJ, Mandell B, et al. Etanercept (enbrel)-associated inflammatory eye disease: case report and review of the literature. Ocul Immunol Inflamm. 2006;14:145–50. doi: 10.1080/09273940600659393. [DOI] [PubMed] [Google Scholar]
- 29.Ilowite N, Porras O, Reiff A, et al. Anakinra in the treatment of polyarticular-course juvenile rheumatoid arthritis: safety and preliminary efficacy results of a randomized multicenter study. Clin Rheumatol. 2009;28:129–37. doi: 10.1007/s10067-008-0995-9. [DOI] [PubMed] [Google Scholar]
- 30.Nigrovic PA, Mannion M, Prince FH, et al. Anakinra as first-line disease-modifying therapy in systemic juvenile idiopathic arthritis: report of forty-six patients from an international multicenter series. Arthritis Rheum. 2011;63:545–55. doi: 10.1002/art.30128. [DOI] [PubMed] [Google Scholar]
- 31.Consolaro A, Ruperto N, Bazso A, et al. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Rheum. 2009;61:658–66. doi: 10.1002/art.24516. [DOI] [PubMed] [Google Scholar]
- 32.Leeb BF, Andel I, Sautner J, et al. The Disease Activity Score in 28 joints in rheumatoid arthritis and psoriatic arthritis patients. Arthritis Rheum. 2007;57:256–60. doi: 10.1002/art.22531. [DOI] [PubMed] [Google Scholar]
- 33.Hyrich K, Symmons D, Watson K, et al. Baseline comorbidity levels in biologic and standard DMARD treated patients with rheumatoid arthritis: results from a national patient register. Ann Rheum Dis. 2006;65:895–8. doi: 10.1136/ard.2005.043158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saad AA, Ashcroft DM, Watson KD, et al. Efficacy and safety of anti-TNF therapies in psoriatic arthritis: an observational study from the British Society for Rheumatology Biologics Register. Rheumatology. 2010;49:697–705. doi: 10.1093/rheumatology/kep423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fantini F, Gerloni V, Gattinara M, et al. Remission in juvenile chronic arthritis: a cohort study of 683 consecutive cases with a mean 10 year followup. J Rheumatol. 2003;30:579–84. [PubMed] [Google Scholar]
- 36.Packham JC, Hall MA. Long-term follow-up of 246 adults with juvenile idiopathic arthritis: functional outcome. Rheumatology. 2002;41:1428–35. doi: 10.1093/rheumatology/41.12.1428. [DOI] [PubMed] [Google Scholar]
- 37.Flato B, Lien G, Smerdel A, et al. Prognostic factors in juvenile rheumatoid arthritis: a case-control study revealing early predictors and outcome after 14.9 years. J Rheumatol. 2003;30:386–93. [PubMed] [Google Scholar]
- 38.Arkela-Kautiainen M, Haapasaari J, Kautiainen H, et al. Favourable social functioning and health related quality of life of patients with JIA in early adulthood. Ann Rheum Dis. 2005;64:875–80. doi: 10.1136/ard.2004.026591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerhardt CA, McGoron KD, Vannatta K, et al. Educational and occupational outcomes among young adults with juvenile idiopathic arthritis. Arthritis Rheum. 2008;59:1385–91. doi: 10.1002/art.24100. [DOI] [PubMed] [Google Scholar]
- 40.Minden K, Niewerth M, Zink A, et al. Long-term outcome of patients with JIA treated with etanercept, results of the biologic register JuMBO. Rheumatology. 2012;51:1407–15. doi: 10.1093/rheumatology/kes019. [DOI] [PubMed] [Google Scholar]
- 41.McDonagh JE. Transition of care from paediatric to adult rheumatology. Arch Dis Child. 2007;92:802–7. doi: 10.1136/adc.2006.103796. [DOI] [PMC free article] [PubMed] [Google Scholar]