Abstract
Phoenixin-14 amide, herein referred to as phoenixin, is a newly identified peptide from the rat brain. Using a previously characterized rabbit polyclonal antiserum against phoenixin, enzyme-immunoassay detected a high level (>4.5 ng/g tissue) of phoenixin-immunoreactivity (irPNX) in the rat spinal cords. Immunohistochemical studies revealed irPNX in networks of cell processes in the superficial dorsal horn, spinal trigeminal tract and nucleus of the solitary tract; and in a population of dorsal root, trigeminal and nodose ganglion cells. The pattern of distribution of irPNX in the superficial layers of the dorsal horn was similar to that of substance P immunoreactivity (irSP). Double-labeling the dorsal root ganglion sections showed that irPNX and irSP express in different populations of ganglion cells. In awake mice, intrathecal injection of phoenixin (1 or 5 μg) did not significantly affect the tail flick latency as compared to that in animals injected with aCSF. Intrathecal administration of phoenixin (0.5, 1.25 or 2.5 μg) reduced significantly reduced the number of writhes elicited by intraperitoneal injection of acetic acid (0.6%, 0.3 ml/30g) as compared to that in mice injected with aCSF. While not affecting the tail flick latency, phoenixin antiserum (1:100) injected intrathecally10 min prior to intraperitoneal injection of acetic acid significantly increased the number of writhes as compared to mice pre-treated with normal rabbit serum. Intrathecal injection of non-amidated phoenixin (2.5 μg) did not significantly alter the number of writhes evoked by acetic acid. Our result shows that phoenixin is expressed in sensory neurons of the dorsal root, nodose and trigeminal ganglia, the amidated peptide is bioactive, and exogenously administered phoenixin may preferentially suppress visceral as opposed to thermal pain.
Keywords: spinal cord, sensory neurons, thermal pain, visceral pain
1. Introduction
Completion of the human genome sequencing project has uncovered more than 700 genes that belong to the G-protein coupled receptor (GPCR) superfamily. Approximately half of these genes encode sensory receptors; a large number of which are predicted to be the targets of odorants. Of the remaining 360 receptors, the natural ligand has been identified for approximately 210 receptors, leaving 150 so called orphan GPCRs with no known ligands (Wise et al., 2004; Wettschureck and Offermanns, 2005). Since the 80's, a concerted effort has been made to identify endogenous ligands acting on orphan GPCRs and vice versa. A number of experimental and methodological approaches, including a high throughput screening of small molecules and peptide ligands, reverse pharmacology, and use of bioinformatics to predict candidate ligands, have been developed to streamline the identification process. By utilizing the bioinformatics algorithm from information provided by the Genome Projects, we have identified several previously unrecognized, secreted, highly conserved neuropeptides; one of which being neuronstatin (Samson et al., 2008; Dun et al., 2010). Recent studies suggest that neuronstatin acts on the orphan GPR107 (Yosten et al., 2012).
Utilizing a similar strategy, two novel peptides phoenixin-14 amide, referred to herein as phoenixin, and phoenixin-20 amide were identified and isolated from the rat brain (Yosten et al., 2013). Phoenixin is identical among multiple species including human, rat, mouse, porcine, canine and Xenopus; whereas, phoenixin-20 amide differs in one amino acid between the human and porcine or canine sequence (Yosten et al., 2013). The precursor for phoenixin is an uncharacterized protein C4orf52, which contains a glycine residue that can undergo C-terminal amidation, and several conserved dibasic residues after glycine indicative of potential carboxypeptidase cleavage sites (Fricker, 2012). The most abundant peptide generated from C4orf52 is a 14 residue peptide, DVQPPGLKVWSDPF-amide, which we termed phoenixin-14 amide. An N-terminal extended peptide, phoenixin-20 amide (AGIVQEDVQ PPGLKVWSDPF-amide) is co-expressed with phoenixin in tissue samples such as the heart and hypothalamus (Yosten et al., 2013). Gene expression of C4orf52 has been shown in several human organs by the organism specific databases “GC04P025864” and “BioGPS gnf1h09115_at”. Serial Analysis of Gene Expression (SAGE) for C4orf52 also indicates that phoenixin precursor gene expression in the spinal cord is higher than that of several other tissues such as the brain, pancreas, spleen and intestine (see http://gene4.weizmann.ac.il/cgi-bin/cardsisp.pl?gene=c4orf52); a finding that is consistent with a comparative analysis of forty-five non-central nervous system tissues (Roth et al., 2006).
In our earlier study, phoenixin was chemically synthesized and antibody directed against the synthetic peptide was raised in rabbits (Yosten et al., 2013). The antibody was then applied to the development of an enzyme immunoassay (EIA) to quantify the amount of immunoreactive phoenixin (irPNX) in various tissues of the rat. A low level of peptides was detected in peripheral tissues, including the thymus, stomach, and spleen; the tissue with a high level of irPNX was that of hypothalamus (Yosten et al., 2013). Neuropeptides that are expressed in the brain can, with few exceptions, be expected in the spinal cord and/or peripheral neural tissues. The current study was undertaken to explore the occurrence, distribution and possible function of phoenixin in the rodent spinal cord.
2. Experimental procedures
2.1. Experimental Animals
Adult male ICR mice (Ace Animal Inc., Boyertown, PA), weighing 25-30g were used in immunohistochemical and behavioral studies; and male Sprague-Dawley rats, weighing 300-325g (Ace Animal Inc.) were used in EIA and immunohistochemical studies. Experimental protocols were reviewed and approved by the Temple University Institutional Animal Care and Use Committee, in accordance with the NIH Guide for the Care and Use of Laboratory Animals 1996. Animals were housed under a 12/12-h light/dark cycle with free access to food and water. Mice were transported to the behavioral testing room at least two hours prior to testing. Every effort was made to minimize the distress of the animals and to prevent their suffering.
2.2. Isolation and identification of phoenixin from rat spinal cords
Rats (n=5) were anesthetized with 4% isofurane and decapitated. Spinal cords, with a total wet weight of 0.8 g, were mixed with 0.8 g of sillica beads (0.8 mm), divided into four polypropylene micro-centrifuge tubes, homogenized in 0.8 ml 5% acetic acid twice for each tube, and yielded a total of 4.8 ml homogenate fraction. After spinning the homogenates at 10,000xg for 20 minutes, the supernatant was removed and loaded into SDB-L cartridges (Phenomenex Inc., CA). After washing the cartridges with 4 volume of phosphate buffered saline (PBS), the binding substances were eluted by 60% isopropanol from SDB-L cartridges and lyophilized, reconstituted in PBS. Commercially available Bicinchoninic Acid (BCA) Protein Assay kit (Thermo Scientific) was used to quantify the protein content in each tissue homogenate. To identify the phoenixin peptide, the 60% isopropanol solution was further affinity purified using MagnaBind beads (Pierce/Thermo Scientific) conjugated to anti-phoenixin antiserum. Eluent from magnetic beads was directly applied to MALDI-TOF for the identification of phoenixin. In the final stage of verification of purified phoenixin, the bioinformatic predicted phoenixin peptide that had been synthesized and the purified phoenixin were processed under the same RP-HPLC separation conditions. A comparable molecular mass on MALDI-TOF and HPLC profiles of purified peptide and synthetic phoenixin confirmed the molecular identity of phoenixin.
2.3. Enzyme-immunoassay (EIA)
Rats (n=10) anesthetized with 4% isofurane were decapitated; spinal cords were removed and homogenized in 5% acetic acid buffer. After centrifugation, the large hydrophobic proteins of supernatant were excluded using Strata C18 columns (Phenomenex Co.), and eluents were collected and lyophilized. Samples were prepared and analyzed with phoenixin-14 amide or substance P EIA kit according to the manufacturer's instructions (Phoenix Pharmaceuticals, Burlingame, CA).
2.4. Immunohistochemistry
Animals anesthetized with 4% isofurane were intracardially perfused with PBS followed by 4% paraformaldehyde in PBS. Spinal cord, medulla oblongata, dorsal root ganglia, nodose ganglia, trigeminal ganglia and superior cervical ganglia were dissected, postfixed for 2 hr, and stored in 30% sucrose/PBS solution overnight. Tissues were processed for irPNX or irSP by the avidin-biotin complex (ABC) procedure or immunofluorescent method (Dun et al., 2010).
For the ABC method, tissues were first treated with 3% H2O2 to quench endogenous peroxidase, washed several times, blocked with 10% normal goat serum, and incubated in phoenixin-antiserum [1:1000 dilution; a rabbit polyclonal against phoenixin-14 amide; Phoenix Pharmaceuticals, Inc., Burlingame, CA]. The specificity of phoenixin antiserum has been previously characterized (Yosten et al., 2013). After thorough rinsing, sections were incubated in biotinylated anti-rabbit IgG (1:200 dilution; Vector Laboratories, Burlingame, CA) for 2 h and rinsed with PBS and incubated in avidin-biotin complex solution for 1.5 h (1:200 dilution; Vector Laboratories). After several washes in Tris-buffered saline, sections were developed in 0.05% diaminobenzidine/0.001% H2O2 solution and washed for at least 2 h with Tris-buffered saline. Sections were mounted on slides with 0.25% gel alcohol, air dried, dehydrated with absolute alcohol followed by xylene, and coverslipped with Permount. The procedure for immunolabeling with substance P antiserum, a guinea pig polyclonal (Novus Biologicals), was similar to that described for phoenixin.
In the case of immunofluorescent method, tissues were first blocked with normal goat serum (1:100 dilution in PBS, 0.5% bovine serum albumin, 0.4% Triton X-100), rinsed and then incubated with phoenixin antiserum (1:500 dilution) or substance P antiserum (1:500 dilution). After rinsing, sections were incubated in biotinylated anti-rabbit IgG or anti-guinea pig IgG (1:50 dilution, Vector Laboratories, Burlingame, CA) for 2 hr, rinsed with PBS and incubated in avidin fluorescein isothiocyanate (FITC, 1:50 dilution) for 5 hr. Sections were washed with PBS, mounted on subbed slides, covered with Citifluor mountant medium (Ted Pella Inc., Redding, CA) and coverslipped. Sections were examined under a Nikon Eclipse E600 fluorescence microscope or a confocal scanning laser microscope (Leica TCS SP5; Heidelberg, Germany) with excitation wavelength set to 488 nm for FITC.
With respect to double-labeling experiments, immunofluorescent techniques were applied (Dun et al., 2010). Sections were first incubated with phoenixin antiserum (1:500 dilution. a rabbit polyclonal) and then with substance P antiserum (1:500 dilution, a guinea pig polyclonal).Sections were incubated with appropriate secondary antiserum conjugated to either FITC or Texas Red, and examined under a confocal scanning laser microscope (Leica TCS SP5, Heidelberg, Germany) with excitation wavelengths set to 488 nm for FITC and 561 nm for Texas Red in the sequential mode.
In control studies, spinal cord or sensory ganglion sections were incubated in phoenixin antiserum or substance P antiserum, which was pre-absorbed, respectively, with the peptide phoenixin-14 amide (1 μg/ml) or substance P (1 μg/ml) overnight.
2.5. Tail flick latency test in mice
The tail flick test was implemented on a Model TF6 tail flick device (EMDIE Instrument Co., Maidens, VA) as described (Huang et al., 2010). Mice were lightly restrained in a soft cloth with their tail positioned in a grove above an aperture. Focused light was directed to the proximal 1/3 of the tail and the latency to flick away was measured. Two measurements made in each mouse one day before treatments were averaged as the basal tail flick latency, which is usually in the range of 2-6s. A cut-off limit of 10s was set in order to avoid tissue damage. In time-course or dose-response studies, tail flick latencies were recorded before and 10, 30, 60 min after intrathecal (i.t.) injection of aCSF, 1μg or 5 μg of phoenixin. Intrathecal injection was made to the L5 or L6 spinous process using a 25 μl Hamilton syringe coupled to a 30-gauge needle similar to that described (Hylden and Wilcox, 1980; Huang et al., 2010). The injection volume was kept constant at 5 μl for all solutions. Animals were used only once in all experiments.
2.6. Acetic acid-induced writhing in mice
Phoenixin (0.5, 1.25 and 2.5 μg) or aCSF was injected intrathecally 10 min before an intraperitoneal injection of acetic acid (0.6%, 0.3 ml/30 g body weight) to the mice. The number of writhes was counted between 5 and 15 min after the last injection (Collier et al., 1968; Huang et al., 2010). Animals were used only once.
2.7. Intrathecal administration of phoenixin antiserum or normal rabbit serum
Phoenixin antiserum (1:100 dilution) or normal rabbit serum in a volume of 5 μl was injected intrathecally 10 min prior to intraperitoneal injection of acetic acid or tail flick latency test. The number of writhes or tail flick latency was recorded as described above.
2.8. Materials
Phoenixin-14 amide antiserum, phoenixin-14 amide peptide, non-amidated phoenixin-14 peptide, phoenixin-20amide peptide, and EIA kits were from Phoenix Pharmaceuticals, Inc. (Burlingame, CA). Substance P antiserum was from Novus Biologicals. Unless otherwise stated, chemicals and reagents were from Sigma Aldrich Co. (St. Louis, MO).
2.9. Data analysis
Results are expressed as mean ± SEM of the measurements from 5 to 7 mice per group. For the tail flick test, the latency of tail flicks in each test period was recorded. For writhing test, the number of abdominal constrictions in each test period was recorded. Statistical analysis was performed with GraphPad Prism 4.0 software (GraphPad Software, San Diego, CA). Differences among multiple groups were determined by one or two way analysis of variance (ANOVA) followed by post-hoc Bonferroni test. Differences between two groups were determined by the Student's t-test. Statistical significance was set at P<0.05.
3. Results
3.1. Isolation and detection of phoenixin in rat spinal cords
Fig. 1A shows a mass spectrometry analysis of compounds isolated from rat spinal cords, where the major peak corresponded to the peptide phoenixin-14 amide; the quantity of phoenixin-20 amide was very low, nearly undetectable.
Fig.1.
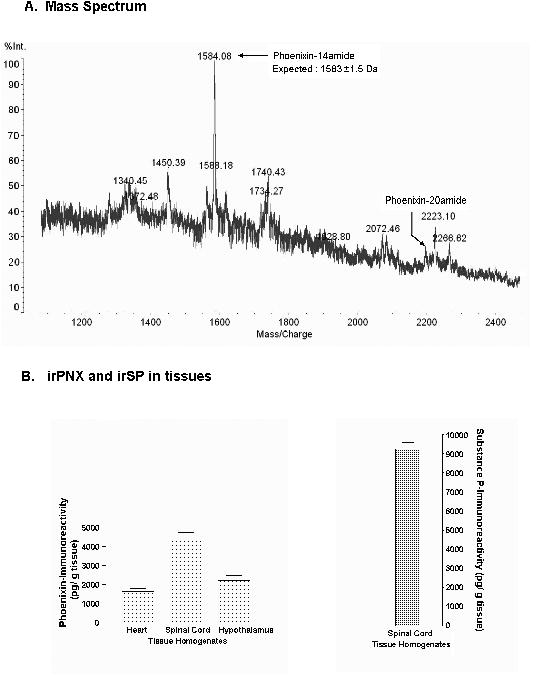
Isolation and identification of endogenous phoenixin in rat tissues. A, Mass spectrometry analysis of rat spinal cord extracts shows the major peak corresponds to the peptide phoenixin-14 amide; a small peak corresponding to oxidized phoenixin-20 amide (+16Da for Hydroxy-Trp) is also noted. B, measurement of irPNX and irSP in rat spinal cords and other tissue homogenates by EIA shows that rat spinal cords expressing the highest level of irPNX (4.5 ng/g tissues); rat hypothalamus and hearts yield about 2.5 ng/g and 1.7 ng/g tissues; irSP is about 9.22 ng/g tissues.
Among several tissues assayed by EIA, rat spinal cords expressed the highest (4.50 ±0.16 ng/g tissues) level of irPNX, and hypothalamus yielded the second highest level, averaging 2.5 ng/g tissues; rat hearts had approximately 1.7 ng/g tissues. For comparison, rat spinal cords contained 9.22 ± 0.16 ng/g tissues of substance P (Fig. 1B), a neuropeptide known to be present in the rat spinal cord (Nicoll et al., 1980).
3.2. Phoenixin immunoreactivity in spinal cords and medulla of rats and mice
To localize irPNX in the rat and mouse spinal cords, immunohistochemical studies were performed. Positively labeled cell bodies were not detected in the dorsal horn or ventral horn of the rats (n=7) or mice (n=3). Instead, networks of irPNX cell processes were conspicuously present in the superficial dorsal horn of all segments; i.e., cervical, thoracic, lumbar and sacral. A representative cervical, thoracic and lumbosacral section from rats, where irPNX punctuated cell processes were detected in laminae I and II, is illustrated in Fig. 2 A-E. Several strands of irPNX cell processes extended into deeper laminae (Fig. 2B and D). In addition, some lightly labeled cell processes/nerve endings were seen surrounding the central canal (Fig. 2C and E). This pattern of distribution of irPNX was noted in all spinal sections sampled, irrespective of rats or mice.
Fig. 2.
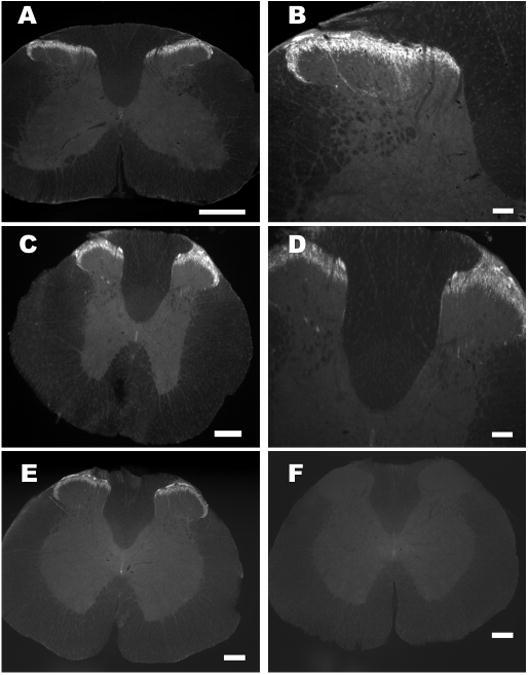
Fluorescence images of rat spinal segments labeled with phoenixin antiserum or phoenixin antiserum pre-absorbed with the peptide. A, C and E, cervical, thoracic and thoracolumbar spinal segment where irPNX is detected in superficial layers of the dorsal horn. B and D, a high magnification of A and C, where several strands of irPNX cell processes extend into the deeper laminae. F, a thoracolumbar segment labeled with phoenixin-antiserum pre-absorbed with the peptide (1 μg/ml); irPNX is not detected in this spinal section. Scale bar: A, C E and F, 250 μm; B and D, 100 μm.
With respect to the medulla, irPNX cell processes were detected in the superficial layers of the spinal trigeminal tract; some of which extended into the medial nucleus of the solitary tract (Sol M) and central nucleus of the solitary tract (SolC) (Fig. 3A-C). Some of the trigeminal irPNX cell processes extended dorsally toward the nucleus of ambiguus (nAmb) (Fig. 3D).
Fig. 3.
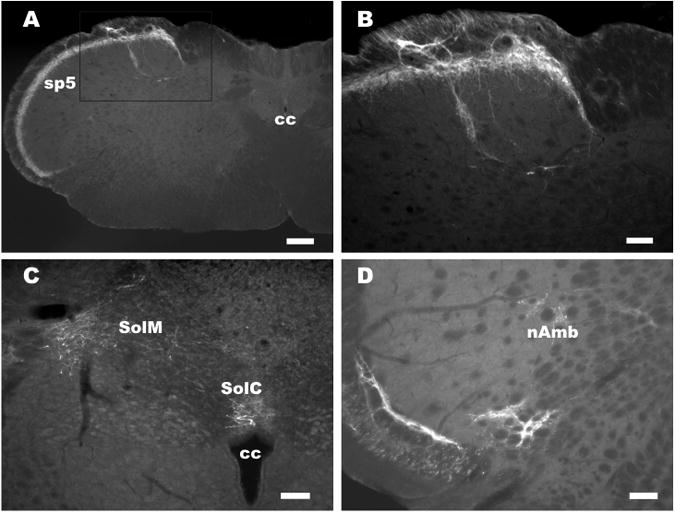
Phoenixin immunoreactivity in the rat medulla. A, a segment of medulla where irPNX is present in cell processes occupying the superficial layer of the spinal trigeminal tract. B, a higher magnification of boxed area in panel A, where some of the irPNX cell processes extend toward the nucleus of the solitary tract. C, irPNX cell processes are detected in the medial nucleus of the solitary tract (SolM) and central nucleus of the solitary tract (SolC), and above the central canal (cc). D, irPNX cell processes from the spinal trigeminal tract (sp5) project towards the nucleus of ambiguus (nAmb). Scale bar: A, 250 μm, B, C, and D, 100 μm.
For control experiments, spinal cord or medullary sections were incubated in phoenixin antiserum pre-absorbed with phoenixin-14 amide (1 μg/ml) overnight. Immunoreactivity was abolished in the dorsal horn of all control sections examined; a representative control section is illustrated in Fig. 2F. Similarly, medullary sections processed with phoenixin antiserum pre-absorbed with the peptide showed no irPNX (not shown). Collectively, control experiments indicate that the phoenixin antiserum used here was specific.
Substance P (SP) is a well documented sensory neuropeptide expressed in the spinal cord (Nicoll et al., 1980). The expressional pattern of irPNX was compared with that of irSP in the rat spinal cords. A dense network of irPNX was present in the superficial layers of the dorsal horn, where irPNX cell bodies were not detected (Fig. 4 A-C). Similar to the irPNX (Fig. 4 A-C), the superficial layers of the dorsal horn expressed a dense network of irSP cell processes (Fig. 4 D & E). Unlike the restricted expression of irPNX to the superficial layers, irSP cell processes were distributed to nearly the entire grey matter; with numerous cell processes in the lateral horn and ventral horn of the thoracic segment (Fig. 4D to F), which most probably are descending fibers from neurons in the ventral medulla (Helke et al., 1982).
Fig. 4.
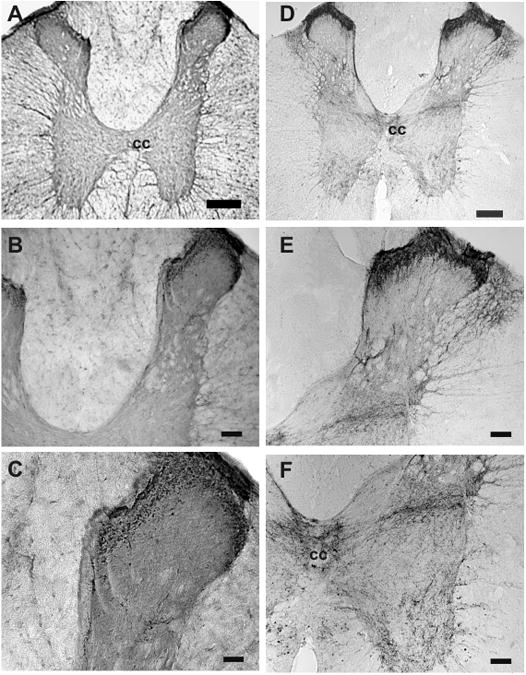
Distribution of phoenixin- and substance P- immunoreactivity in the rat spinal cord. A, irPNX cell processes are distributed almost exclusively to the superficial layers of the dorsal horn of a thoracic segment; the lateral and ventral horn are practically devoid of irPNX cell processes. B and C are higher magnifications showing varicose cell processes in the superficial layer. D, a thoracic spinal section where dense irSP cell processes are detected in the superficial layers, deeper laminae, lateral horn and ventral horn. E and F, numerous irSP cell processes are seen in the deeper laminae, lateral horn, central cancel (cc) and the ventral horn. Calibration bar: A and D, 250 μm; B, E and F, 100 μm; C, 50 μm.
3.3. Phoenixin immunoreactivity in sensory ganglia
Neurons in the dorsal root, trigeminal, and nodose ganglion project their axons to innervate superficial layers of the spinal cord, spinal trigeminal tract and nucleus of the solitary tract. Accordingly, a population of ganglionic neurons expressed irPNX of varying intensities in all three types of sensory ganglion examined (Fig. 5A-C). Measurements of the diameter of irPNX neurons were performed using the image analysis software (ImageJ 1.41o, Wayne Rasband, NIH) (Huang et al., 2010). The diameter of irPNX cells with a clearly visible nucleus was measured from 100 randomly selected ganglion cells from five DRG sections. Fig. 6 illustrates a cell diameter-frequency distribution histogram. The majority of irPNX neurons, irrespective of dorsal root, trigeminal or nodose ganglia, fall between 25 μm and 40 μm, which are considered as medium sized ganglion cells. To further support the idea that irPNX is expressed in sensory neurons, sections of rat superior cervical ganglion, which is a collection of sympathetic postganglionic neurons, were processed for irPNX. Positively labeled neural elements were not detected in any of the superior cervical ganglion sections examined (not shown; n=4).
Fig. 5.
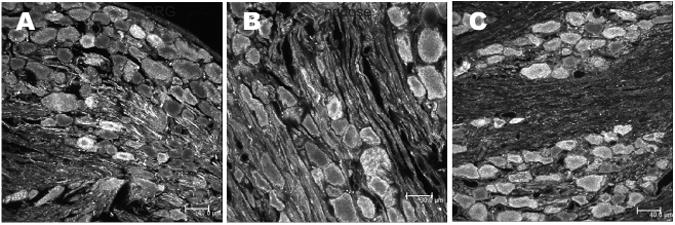
Phoenixin immunoreactivity in neurons of the rat sensory ganglia. A, B and C, a section of dorsal root, trigeminal and nodose ganglion where some of the ganglion cells are irPNX. Scale bar: A and C, 40 μm and B, 30 μm.
Fig.6.
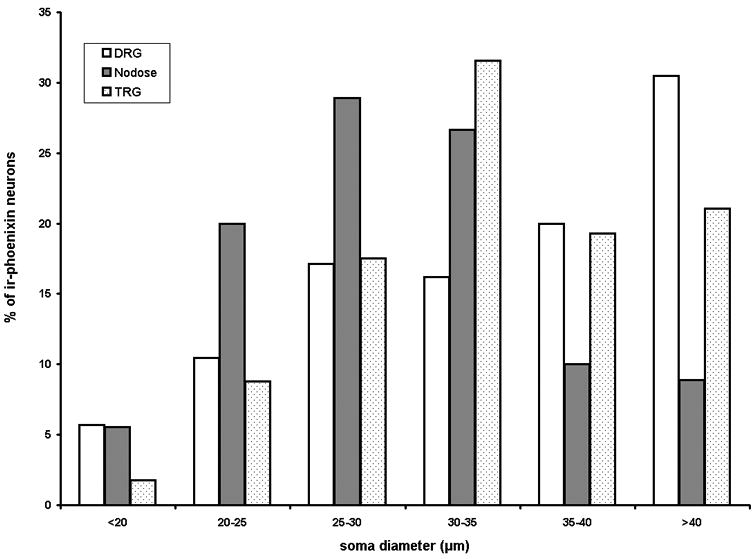
Histogram of percent of phoenixin-immunoreactive ganglion cells against cell diameters. The diameter of the majority of ganglion cells is between 25 and 40 μm.
In addition, double-labeling studies were carried out to address the question whether irPNX is expressed in irSP dorsal root ganglion cells. Ten dorsal root ganglion sections were double-labeled with substance P- and phoenixin-antiserum. irSP was not detected in any of the 100 irPNX dorsal ganglion cells randomly selected from 10 sections (not shown). The lack of co-expression of irSP and irPNX suggests that they represent two separate populations of dorsal root ganglion cells.
3.4. Tail flick latency test in mice
As irPNX is abundantly expressed in dorsal root and trigeminal ganglion cells and their cell processes, we evaluated the hypothesis that phoenixin may modify nociception in a mouse model of thermal pain. In the tail flick latency test, phoenixin (1 or 5 μg) was injected intrathecally to the awake mice, and the tail flick latency was measured 10, 30 and 60 min post-injection. Two-way ANOVA shows that the tail flick latency following intrathecal injection of phoenixin (1 or 5 μg) (n=7) was not significantly changed as compared to that following intrathecal injection of aCSF 10, 30 or 60 min post-injection (Fig. 7, n=7).
Fig.7.
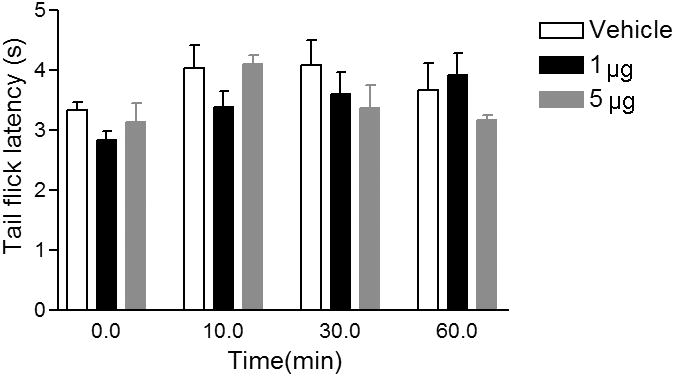
Tail flick latency not significantly affected by intrathecal administration of phoenixin in mice. Intrathecal injection of phoenixin 1 or 5 μg did not significantly change the tail flick latency as compared to that of mice injected with aCSF; p>0.05. Data were collected from 5 to 7 mice per group.
3.5. Writhing test in mice
A lack of significant effect of phoenixin in tail flick latency test led us to evaluate the response to phoenixin in an alternative; i.e., the acetic acid, pain model (Deleo et al., 1991; La et al., 2003; Chuang et al., 2004; Marcil et al., 2006; Huang et al., 2010; Kapural et al., 2010). Intrathecal administration of phoenixin decreased dose-dependently the number of writhes elicited by intraperitoneal injection of acetic acid as compared to that in mice injected intrathecally with aCSF. At the dose of 0.5 μg, phoenixin reduced the number of writhes when compared to animals injected with aCSF; the difference was not statistically significant (n=5; P>0.05; Fig. 8). At the doses of 1.25 μg and 2.5 μg, phoenixin significantly decreased the number of abdominal constrictions to 31 ± 3.97 (n=7; p<0.05) and 10 ± 4.52 per 10 min (n=5; P<0.01), respectively; the number of abdominal constrictions was 50 ± 3.22 per 10 min in mice injected with aCSF (n=5; Fig.8). Intrathecal administration of phoenixin non-amidated-14 peptide at the same doses; i.e., 0.5, 1.25 and 2.5 μg, failed to significantly change the number of writhes elicited by intraperitoneal injection of acetic acid in any of the mice tested (n=5 per group; not shown).
Fig.8.
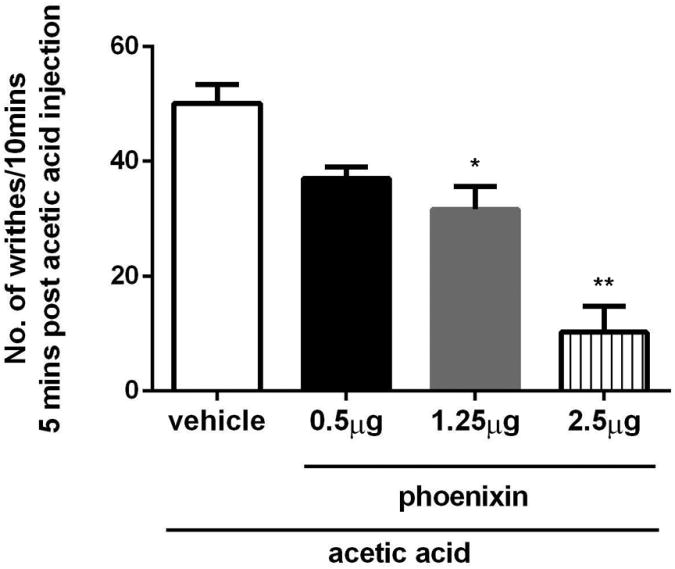
Phoenixin suppresses writhing induced by intraperitoneal injection of acetic acid in mice. Phoenixin at the doses of 1.25 μg and 2.5 μg significantly reduced the number of writhes as compared to that of aCSF injected mice; data were collected from 5 to 7 mice per group 5 to 15 min post-injection of acetic acid. * statistically significant, p<0.05; ** statistically significant, p<0.01.
In the next series of experiments, the effects of phoenixin antiserum (1:100 dilution) pretreatment 10 min prior to writhing test or tail flick latency test were assessed. Intrathecal administration of phoenixin antiserum significantly increased the number of writhes induced by acidic acid injections to 75 ± 4.47 per 10 min post-injection (n=7; p<0.05) as compared to 51 ± 5.77 (n=7) per 10 min post injection of normal rabbit serum. The tail flick latency was not significantly changed by intrathecal injection of phoenixin antiserum or normal rabbit serum (4.4 ± 0.32 sec vs 3.8 ± 0.46 sec; n=6, p>0.05).
4. Discussion
Two peptides of 14 and 20 residues termed phoenixin-14 amide and phoenixin-20 amide are isolated and identified in the rat brain (Yosten et al., 2013). Using a similar strategy and approaches, phoenixin-14 amide was also detected in the rat/mouse spinal cords and sensory ganglia. Because only a small quantity of phoenixin-20 amide was detected in rat spinal cords, phoenixin-14 amide was the focus of this study. Among several tissues/organs measured, EIA showed that the rat spinal cord contains the highest amount of irPNX, even higher than that detected in the rat hypothalamus (Yosten et al., 2013). In the rat brain, irPNX is expressed in neurons distributed to several brain regions including the hypothalamus, substantia nigra, Edinger-Westphal nucleus, pituitary gland, and nucleus of the solitary tract (Yosten et al., 2013). In this study, irPNX is expressed in cell processes distributed to the superficial layers of the dorsal horn, spinal trigeminal tract, and nucleus of the solitary tract; these fibers are mainly derived from sensory neurons in the dorsal root, trigeminal and nodose ganglia. In addition, some of the trigeminal irPNX cell processes turn dorsally toward the nucleus of ambiguus, which contains parasympathetic premotor neurons innervating the heart (Cheng and Powley, 2000), suggesting the peptide may engage in sensory-cardiac reflexes. The finding that a population of neurons in the dorsal root, trigeminal and nodose ganglion expresses irPNX is consistent with the sensory nature of irPNX. Superior cervical ganglion cells, which are sympathetic postganglionic neurons, exhibit little or no irPNX, further support the sensory nature of irPNX neurons in the peripheral nervous system.
Substance P is a well recognized sensory peptide expressed in the superficial layers of the dorsal horn and dorsal root ganglion cells involved in nociception (Nicoll et al., 1980). For comparative purposes, EIA showed that irSP is higher than irPNX (9.22 vs 4.50 ng/g tissues) in the rat spinal cord. This finding is consistent with the immunohistochemical observation that irPNX is mainly confined to the superficial layers of the dorsal horn; whereas, irSP is, in addition to the superficial layers, distributed to nearly the entire gray matter.
A functional role of phoenixin in sensory processing within the spinal cord is not known. Several well characterized neuropeptides such as substance P, calcitonin gene-related peptide, amylin, and pituitary adenylate cyclase activating polypeptide, are expressed primarily in small to medium sized sensory neurons (Dun et al., 1996; Plourde et al., 1997; Michael and Priestley, 1999; Huang et al., 2010). With respect to irPNX neurons, their cell diameter seems to fall between medium (25 to 40μm) and large (>40 μm), with a small percentage of cells <25 μm in diameter. It is generally recognized that nociception, such as thermal, mechanical or neuropathic pain, is transmitted mainly via primary afferent neurons of small diameter to the superficial layers of the dorsal horn (Julius and Basbaum, 2001). Our finding that irPNX is expressed in a heterogeneous group of sensory ganglion cells with cell sizes ranging from small to large, suggests that the peptide may subserve diverse sensory modalities.
Acetic acid challenge is proposed to be a model of inflammatory or visceral pain (Collier et al., 1968; La et al., 2003; Marcil et al., 2006). It is noteworthy that visceral afferents terminate in superficial laminae (I and II) of the dorsal horn, the neck of the dorsal horn in lamina V as well as areas surrounding the central canal; whereas somatic afferents terminate throughout the spinal dorsal horn; i.e., laminae I-VI (Cervero and Connell, 1984). The topographic distribution of irPNX cell processes to the superficial laminae I and II, with few cell processes extending to the deeper laminae and central canal, is consistent with the proposal that irPNX neurons are likely to be engaged in visceral pain sensation. Recently, amylin, a 27-amino acid peptide, was found to be expressed in sensory neurons of the dorsal root, nodose and trigeminal ganglia (Huang et al., 2010). Further, pharmacological studies demonstrated that amylin upon intrathecal administration reduced significantly the number of writhes elicited by intraperitoneal injection of acetic acid, but failed to modify the tail flick latency (Huang et al., 2010), suggesting that phoenixin and amylin may preferentially modify visceral as compared to thermal pain. More importantly, two reports show that the region of gene C4orf52, which encodes the phoenixin precursor, was deleted in patients who exhibited partial epilepsy with pericentral spikes (PEPS), which are characterized by a variety of seizure types including hemiclonic, hemitonic, generalized clonic-tonic, complex partial seizures as well as stereotype episodes of epigastric pain (Kinton et al., 2002; Limviphuvadh et al., 2010). As the deletion of C4orf52 may be a contributing factor to epigastric pain phenotype in PEPS patients, endogenously released phoenixin may suppress visceral pain.
Phoenixin antagonists are currently not available. For this reason, phoenixin antiserum was used as a tool to evaluate a possible release of endogenous phoenixin following acetic acid challenge; normal rabbit serum served as a control. Pre-treatment of the mice with phoenixin antiserum significantly increased the number of writhes as compared to that in mice pre-treated with normal rabbit serum, implying phoenixin is liberated endogenously following acetic acid challenge and that phoenixin antiserum neutralizes, at least partly, the response by endogenously released phoenixin.
Our pharmacological studies show that phoenixn by intrathecal injections failed to alter significantly the tail flick latency; the latter being an index of thermal pain threshold. On the other hand, the same doses of phoenixin by intrathecal injections significantly reduced the number of writhes caused by intraperitoneal injections of acetic acid. Same doses of non-amidated phoenixin injected intrathecally failed to significantly affect the number of writhes induced by acetic acid, indicating that the amidated phoenixin is bioactive. The observation that exogenously administered phoenixin reduces writhes elicited by acetic acid is in line with the proposal that deletion of the gene that encodes phoenixin may unmask epigastric pain phenotype (Limviphuvadh et al., 2010). The sensory effect of phoenixin may not be limited to nociception, as future studies using different experimental models are likely to unveil a diverse role of phoenixin in sensory processing.
The receptor(s) that interacts with phoenixin has not yet been identified. Generally, GPCRs on the cell surface are molecular targets of peptides. Given the large number of GPCRs that have not been paired with their natural ligands, phoenixin may produce its biological activity via interacting with a heretofore unidentified GPCR.
5. Conclusion
Mass spectrometry and EIA detect a peptide of 14 residues, termed phoenixin-14amide, in the rodent spinal cord. Immunohistochemistry shows that irPNX is present in a population of mostly medium sized sensory ganglion cells including dorsal root, trigeminal and nodose, and in cell processes distributed to the superficial layers of the dorsal horn. Intrathecal administration of phoenixin in the dose of 1 or 5 μg failed to modify the tail flick latency in awake mice; whereas, phoenixin at the dose of 1.25 μg and 2.5 μg significantly reduced the number of writhes in mice injected intraperitoneally with acetic acid. As a corollary, phoenixin may preferentially suppress visceral pain as opposed to thermal pain.
Highlights.
Phoenixin, a novel peptide, is detected in superficial layers of the rodent dorsal horn
A population of dorsal root, trigeminal and nodose ganglion cells express phoenixin
Phoenixin by intrathecal injection reduces the number of writhing elicited by acetic acid
Acknowledgments
This study was supported by NIH Grants NS18713 and HL51314 from the Department of Health and Human Services. Ying Zhang was supported by YunNan Provincial United Fund (No. 2012FB013). We thank Vi Le (Phoenix Pharmaceuticals, Inc.) for her assistance in the EIA measurements.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- BCA
bicinchoninic acid protein assay
- EIA
enzyme-immunoassay
- FITC
fluorescein isothiocyanate
- GPCR
G-protein coupled receptor
- PNX
phoenixin-14 amide
- irPNX
phoenixin immunoreactivity
- PEPS
pericentral spikes
- PBS
phosphate buffered saline
Footnotes
Declare of interest: Authors declare no conflict of interest
Author contribution statement: SLD, RML, XFH,YZ, JJL, JKC and NJD designed and performed the experiments. RML, SLD, XFH, YZ, JKC, JJL and NJD analyzed the data, and RML and NJD prepared the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cervero F, Connell LA. Distribution of somatic and visceral primary afferent fibres within the thoracic spinal cord of the cat. J Comp Neurol. 1984;230:88–98. doi: 10.1002/cne.902300108. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Powley TL. Nucleus ambiguus projections to cardiac ganglia of rat atria: an anterograde tracing study. J Comp Neurol. 2000;424:588–606. [PubMed] [Google Scholar]
- Chuang YC, Yoshimura N, Huang CC, Chiang PH, Chancellor MB. Intravesical botulinum toxin administration produces analgesia against acetic acid induced bladder pain responses in rats. J Urol. 2004;172:1529–1532. doi: 10.1097/01.ju.0000137844.77524.97. [DOI] [PubMed] [Google Scholar]
- Collier HO, Dinneen LC, Johnson CA, Schneider C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol Chemother. 1968;32:295–310. doi: 10.1111/j.1476-5381.1968.tb00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo JA, Coombs DW, McCarthy LE. Differential c-fos-like protein expression in mechanically versus chemically induced visceral nociception. Brain Res Mol Brain Res. 1991;1:167–170. doi: 10.1016/0169-328x(91)90118-h. [DOI] [PubMed] [Google Scholar]
- Dun NJ, Miyazaki T, Tang H, Dun EC. Pituitary adenylate cyclase activating polypeptide immunoreactivity in the rat spinal cord and medulla: implication of sensory and autonomic functions. Neurosci. 1996;73:677–686. doi: 10.1016/0306-4522(96)00057-7. [DOI] [PubMed] [Google Scholar]
- Dun SL, Brailoiu GC, Tica AA, Yang J, Chang JK, Brailoiu E, Dun NJ. Neuronostatin is co-expressed with somatostatin and mobilizes calcium in cultured rat hypothalamic neurons. Neurosci. 2010;166:455–463. doi: 10.1016/j.neuroscience.2009.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker LD. Neuropeptides and Other Bioactive Peptides: From Discovery to Function. Chapter 3. Morgan & Claypool Life Sciences; 2012. 5 Carboxypeptidase E″; pp. 43–46. ISBN 1-61504-521-X. [DOI] [Google Scholar]
- Helke CJ, Neil JJ, Massari VJ, Loewy AD. Substance P neurons project from the ventral medulla to the intermediolateral cell column and ventral horn in the rat. Brain Res. 1982;243:147–152. doi: 10.1016/0006-8993(82)91128-3. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Kellerth JO, Ndsson G, Pernow B. Substance P localization in the central nervous system and in some primary sensory neurons. Science. 1975;190:889–890. doi: 10.1126/science.242075. [DOI] [PubMed] [Google Scholar]
- Huang XF, Yang J, Chang JK, Dun NJ. Amylin suppresses acetic acid-induced visceral pain and spinal c-fos expression in the mouse. Neurosci. 2010;165:1429–1438. doi: 10.1016/j.neuroscience.2009.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Kapural L, Nagem H, Tlucek H, Sessler DI. Spinal cord stimulation for chronic visceral abdominal pain. Pain Med. 2010;11:347–355. doi: 10.1111/j.1526-4637.2009.00785.x. [DOI] [PubMed] [Google Scholar]
- Kinton L, Johnson MR, Smith SJ, Farrel F, Stevens J, Rance JB, Claudino AM, Duncan JS, Davis MB, Wood NW, Sander JW. Partial epilepsy with pericentral spikes: a new familial epilepsy syndrome with evidence for linkage to chromosome 4p15. Ann Neurol. 2002;51:740–749. doi: 10.1002/ana.10221. [DOI] [PubMed] [Google Scholar]
- La JH, Kim TW, Sung TS, Kang JW, Kim HJ, Yang IS. Visceral hypersensitivity and altered colonic motility after subsidence of inflammation in a rat model of colitis. World J Gastroenterol. 2003;9:2791–2795. doi: 10.3748/wjg.v9.i12.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limviphuvadh V, Chua LL, Eisenhaber F, Adhikari S, Maurer-Stroh S. Is LGI2 the candidate gene for partial epilepsy with pericentral spikes? J Bioinform Comput Biol. 2010;8:117–127. doi: 10.1142/s0219720010004550. [DOI] [PubMed] [Google Scholar]
- Marcil J, Walczak JS, Guindon J, Ngoc AH, Lu S, Beaulieu P. Antinociceptive effects of tetrodotoxin (TTX) in rodents. Br J Anaesth. 2006;96:761–768. doi: 10.1093/bja/ael096. [DOI] [PubMed] [Google Scholar]
- Michael GJ, Priestley JV. (1999). Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J Neurosci. 1999;19:1844–1854. doi: 10.1523/JNEUROSCI.19-05-01844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Schenker C, Leeman SE. Substance P as a transmitter candidate. Ann Rev Neurosci. 1980;3:227–268. doi: 10.1146/annurev.ne.03.030180.001303. [DOI] [PubMed] [Google Scholar]
- Plourde V, St-Pierre S, Quirion R. Calcitonin gene-related peptide in viscerosensitive response to colorectal distension in rats. Am J Physiol. 1997;273:G191–G196. doi: 10.1152/ajpgi.1997.273.1.G191. [DOI] [PubMed] [Google Scholar]
- Roth RB, Hevezi P, Lee J, Willhite D, Lechner SM, Foster AC, Zlotnik A. Gene expression analyses reveal molecular relationships among 20 regions of the human CNS. Neurogenetics. 2006;7:67–80. doi: 10.1007/s10048-006-0032-6. [DOI] [PubMed] [Google Scholar]
- Samson WK, Zhang JV, Avsian-Kretchmer O, Cui K, Yosten GL, Klein C, Lyu R, Wang YX, Chen XQ, Yang J, Price CJ, Hoyda TD, Ferguson AV, Yuan XB, Chang JK, Hsueh AJ. Neuronostatin encoded by the somatostatin gene regulates neuronal, cardiovascular, and metabolic functions. J Biol Chem. 2008;283:31949–31959. doi: 10.1074/jbc.M804784200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev. 2005;85:1159–1204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- Wise A, Jupe SC, Rees S. The identification of ligands at orphan G-protein coupled receptors. Annu Rev Pharmacol Toxicol. 2004;44:43–66. doi: 10.1146/annurev.pharmtox.44.101802.121419. [DOI] [PubMed] [Google Scholar]
- Yosten GL, Lyu RM, Hsueh AJW, Avsian-Kretchmer O, Chang JK, Tullock CW, Dun SL, Dun NJ, Samson WK. A novel reproductive hormone, phoenixin. J Neuroendocrinol. 2013;25:206–215. doi: 10.1111/j.1365-2826.2012.02381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosten GL, Redlinger LJ, Samson WK. Evidence for an interaction of neuronostatin with the orphan G protein-coupled receptor, GPR107. Am J Physiol Regul Integr Comp Physiol. 2012;303:R941–R949. doi: 10.1152/ajpregu.00336.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


