Abstract
Childhood socioeconomic status (SES) predicts executive function performance and measures of prefrontal cortical function, but little is known about its anatomical correlates. Structural MRI and demographic data from a sample of 283 healthy children from the NIH MRI Study of Normal Brain Development were used to investigate the relationship between SES and prefrontal cortical thickness. Specifically, we assessed the association between two principal measures of childhood SES, family income and parental education, and gray matter thickness in specific subregions of prefrontal cortex and on the asymmetry of these areas. After correcting for multiple comparisons and controlling for potentially confounding variables, parental education significantly predicted cortical thickness in the right anterior cingulate gyrus and left superior frontal gyrus. These results suggest that brain structure in frontal regions may provide a meaningful link between SES and cognitive function among healthy, typically developing children.
Keywords: socioeconomic status, SES, poverty, structural neuroimaging, prefrontal cortex, brain development
Children who grow up in poverty tend to have lower IQs and academic achievement scores and are less likely to develop basic reading and mathematics proficiency than their higher-SES counterparts (Brooks-Gunn & Duncan, 1997; Gottfried, Gottfried, Bathurst, Guerin, & Parramore, 2003; Sirin, 2005). These outcome measures, while clinically meaningful, reflect the combined influence of many specific neurocognitive systems. It is these underlying systems that mediate the association between SES and cognitive performance and provide possible targets for interventions designed to reduce SES disparities. The methods of cognitive neuroscience, such as neuropsychological testing and structural brain imaging, can help to identify specific neurocognitive systems that vary along socioeconomic gradients.
The goal of the present study is to investigate the relation between SES and prefrontal cortical thickness in healthy normal children. We focus on prefrontal cortex for three reasons. First, this brain region is essential for executive function, which is associated with academic success (Blair & Diamond, 2008; Ursache, Blair & Raver, 2011) and intelligence as measured by psychometric tests (Deary, Penke & Johnson, 2010). Second, the long developmental trajectory of prefrontal cortex (Casey, Giedd & Thomas, 2000; Gogtay et al., 2004), and its sensitivity to environmental factors including stress (McEwen & Gianaros, 2011), suggest that differences in the experiences of lower and higher SES children could impact prefrontal development. Third, and most directly relevant, many studies have found SES differences in executive function and in prefrontal activity.
In children ranging from infancy to adolescence, SES has been found to correlate with executive function as measured by many different tasks (Ardila, Rosselli, Matute & Guajardo, 2005; Lipina, Martelli, Vuelta & Colombo, 2005; Lipina, Martelli, Vuelta, Injoque-Ricle & Colombo, 2004; Mezzacappa, 2004; Sarsour et al., 2011) and as measured by latent executive function constructs derived from multiple executive function tasks (Blair et al., 2011; Hughes, Ensor, Wilson & Graham, 2010; Rhoades, Greenberg, Lanza & Blair, 2011; Wiebe et al., 2011). Furthermore, in studies where multiple neurocognitive systems have been assessed, executive function appears to be disproportionately affected by SES (Farah et al., 2006; Noble, McCandliss & Farah, 2007; Noble, Norman & Farah, 2005). Additionally, event-related potential (ERP) studies in children have demonstrated SES differences in measures of selective attention associated with prefrontal cortex (D’Anguilli, Herdman, Stapells & Hertzman, 2008; Kishiyama, Boyce, Jimenez, Perry & Knight, 2009; Stevens, Lauinger & Neville, 2009), and a functional magnetic resonance imaging (fMRI) study has shown SES differences in the degree to which prefrontal cortical areas are recruited during a nonverbal stimulus-response learning task (Sheridan, Sarsour, Jutte, D’Esposito & Boyce, 2012). Other behavioral and electrophysiological evidence regarding SES differences in executive function and prefrontal activity are reviewed by Hackman and Farah (2009), who find the vast majority, but not all, of the relevant published studies show that higher SES in children is accompanied by higher executive function and/or more mature or advantageous patterns of brain activity. Prior studies have quantified SES through parental education, total family income, family income-to-needs ratio and combinations of education and income measures.
Relatively few published studies report the effects of childhood SES on brain structure, and most have focused on regions other than prefrontal cortex. SES as measured by family income, but not parental education, has been found to predict hippocampal gray matter volume in a large sample of healthy children between the ages of 4 and 18 (Hanson, Chandra, Wolfe & Pollack, 2011). A separate study of 60 children yielded a similar result, with hippocampal volume predicted by family income-to-needs ratio and not parental education (Noble, Houston, Kan & Sowell, 2012). That study also found that amygdala volume was predicted by education but not by income-to-needs. Most relevant to prefrontal areas, this study also revealed an interaction between age and parental education (but not a main effect of parental education) in left perisylvian areas including the left inferior frontal gyrus. A marginally significant correlation between SES and inferior frontal gyrus gray matter volume was observed in a small sample of 5-year-old children (Raizada, Richards, Meltzoff & Kuhl, 2008), and another study of 10-year-old children found a positive correlation between SES and gray matter volume in a number of brain regions, including bilateral hippocampi, middle temporal gyri, left fusiform and right inferior occipito-temporal gyri (Jednoróg et al., 2012), as well as greater gyrification with higher SES in medial prefrontal regions.
In a large sample of typically developing children in the NIH MRI Study of Normal Brain Development, family income and parental education were not found to significantly predict whole-brain or gross regional volumes, including frontal lobe volume (Brain Development Cooperative Group, 2012; Lange, Froimowitz, Bigler, Lainhart & Brain Development Cooperative Group, 2010). This same data set was also analyzed by Andrew Beck, whose results are reported in an unpublished undergraduate thesis (2010), relating overall prefrontal gray matter volume to SES. Beck found a weak but statistically significant relation between family income and gray matter volume in this data set. Because a large body of literature suggests that subregions of prefrontal cortex are specialized for different aspects of executive function (Stuss & Knight, 2002), the current study extends prior work by investigating subregions of prefrontal cortex, rather than prefrontal cortex as a whole.
Unlike previous studies, which used volumetric measures of morphology, the current study used a measure of cortical thickness. Cortical thickness is defined in neuroimaging studies as the shortest distance between the white matter surface and pial gray matter surface. This quantitative measurement provides a direct index of cortical morphology that can be measured reliably using multiple approaches (Lerch & Evans, 2005). Furthermore, cortical thickness is a more specific measure of brain morphology than gray matter volume. Gray matter volume is a function of both cortical thickness and surface area, which are genetically and phenotypically independent (Winkler et al., 2010). Cortical thickness has been shown to be a meaningful index of brain development, showing developmental changes that may reflect the process of synaptic proliferation and pruning or the effect of myelination on the measurement of thickness (Giedd et al., 1999; Paus, 2005; Shaw et al., 2008; Sowell et al., 2004). Cortical thickness has also shown associations with cognitive ability (Porter, Collins, Muetzel, Lim & Luciana, 2011; Shaw et al., 2006) and behavior (Ducharme et al., 2012; Shaw et al., 2011) among healthy children.
To systematically investigate the relationship between socioeconomic status and cortical thickness in frontal brain regions, the current study used SES measures to predict cortical thickness in 10 prefrontal regions of interest (ROIs) in healthy children from the first time point of data collection in the NIH MRI Study of Normal Brain Development, the same large data set referred to earlier (Evans, 2006; Brain Development Cooperative Group, 2012; Lange et al., 2010). Furthermore, due to literature suggesting that socioeconomic status may relate to lateralization development (see Boles, 2011 for a review), three frontal asymmetry measures were also used as outcomes measures of interest. Because of an existing report of an SES by age interaction in the left inferior frontal gyrus (Noble et al., 2012), an additional analysis investigated age as a possible moderator of SES effects on prefrontal cortical thickness. The predictive power of family income and parental education were assessed separately because of recent literature suggesting that different measures of SES have unique relationships with cognitive outcomes (Duncan & Magnuson, 2012) and structural phenotypes (Hanson et al., 2011; Noble et al., 2012).
Method
Participants
Data used in the preparation of this article were obtained from the NIH Pediatric MRI Data Repository created by the NIH MRI Study of Normal Brain Development (Evans, 2006; website: https://nihpd.crbs.ucsd.edu/nihpd/info/index.html), a public-access database designed to be a research tool for investigations of healthy brain and behavior development. This is a multisite, longitudinal study of typically developing children from ages newborn through young adulthood conducted by the Brain Development Cooperative Group and supported by the National Institute of Child Health and Human Development, the National Institute on Drug Abuse, the National Institute of Mental Health, and the National Institute of Neurological Disorders and Stroke (Contract #s N01-HD02-3343, N01MH9-0002, and N01-NS-9-2314, −2315, −2316, −2317, −2319 and −2321). A listing of the participating centers and a complete listing of the study investigators can be found at: https://nihpd.crbs.ucsd.edu/nihpd/info/participating_centers.html.
As part of Objective 1 of the study, structural MRI, behavioral and clinical measures were collected at three time points for 433 healthy children and adolescents between the ages of 4:6 and 18:3 years; the present analysis uses data from the first time point (release 4.0). The Institutional Review Board at the University of Pennsylvania also approved the analysis of these human-subjects data.
Participating children had been screened using rigorous demographic, prenatal history, physical, behavioral, family history, and neurological criteria (see Evans, 2006 for a full description of inclusionary and exclusionary criteria). Data collection occurred at 6 pediatric study centers in major urban areas and population-based sampling was used to obtain a demographically-representative sample (Evans, 2006).
A self-report measure of family income was obtained in 10 possible levels: 0–$5,000, $5,001–$10,000, $10,001–$15,000, $15,001–$25,000, $25,001–$35,000, $35,001–$50,000, $50,001–$75,000, $75,001–$100,000, $100,001–$150,000, and over $150,000. Parental education level was measured in six possible categories for each parent: less than high school, high school, some college, college, some graduate level, graduate level. Finally, race (White, African American/Black, Asian, American Indian/Alaskan Native, Native Hawaiian/Pacific Islander) and ethnicity (Hispanic or Latino, Not Hispanic or Latino) were reported for each parent.
Of the 431 children with behavioral data from the first time point, 283 children had available MRI data that met quality control standards as well as available data for all covariates used in analysis. Demographic data for the children used in analysis are summarized in Table 1. The subset of children used in analysis did not differ from the excluded children in sex (t (429) = −.79, p = .43, d = −.08), IQ (t (378) = −1.8, p = .07, d = −.21), parental education (t (427) = −.45, p = .43, d = −.05) or family income (t (429) = −.22, p = .83, d = −.02). However, the MRI sample had a significantly older age (t (429) = −8.96, p < .001, d = −.90) than the sample of children without MRI data or covariates. The mean age for children in the MRI sample was 11.47 years (SD = 3.50 years).
Table 1.
Demographic information for the sample of children used in analyses (N=283)
| Variable | n (%) | Mean (SD) |
|---|---|---|
| Age (in years) | 11.47 (3.50) | |
| Female | 151 (53.36) | |
| Family Income Category | ||
| <$5000 | 1 (0.35) | |
| $5,001–$10,000 | 2 (0.71) | |
| 10,001–$15,000 | 3 (1.06) | |
| 15,001–$25,000 | 7 (2.47) | |
| 25,001–$35,000 | 10 (3.53) | |
| 35,001–$50,000 | 47 (16.61) | |
| 50,001–$75,000 | 68 (24.03) | |
| 75,001–$100,000 | 77 (27.21) | |
| $100,001–$150,000 | 68 (24.03) | |
| over $150,000 | 0 (0) | |
| Family-size Adjusted Family Income | 76169.13 (33022.32) |
|
| Maternal education | ||
| Less than High School | 2 (0.71) | |
| High School | 43 (15.19) | |
| Some College | 78 (27.56) | |
| College | 94 (33.22) | |
| Some Graduate Level | 13 (4.59) | |
| Graduate Level | 53 (18.73) | |
| Paternal education | ||
| Less than High School | 7 (2.47) | |
| High School | 56 (19.79) | |
| Some College | 74 (26.15) | |
| College | 79 (27.92) | |
| Some Graduate Level | 10 (3.53) | |
| Graduate Level | 57 (20.14) | |
| Parental education | 7.53 (2.31) | |
| Parental education (square root transformed) |
2.71 (.43) | |
| Maternal race | ||
| White | 235 (83.04) | |
| African American/Black | 23 (8.13) | |
| Asian | 4 (1.41) | |
| American Indian/Alaskan Native | 1 (0.35) | |
| Native Hawaiian/Pacific Islander | 0 (0) | |
| Multiple races listed | 3 (1.06) | |
| Not provided | 17 (6.00) | |
| Maternal ethnicity | ||
| Not Hispanic or Latino | 264 (93.29) | |
| Hispanic or Latino | 19 (6.71) | |
| Paternal race | ||
| White | 222 (78.44) | |
| African American/Black | 24 (8.48) | |
| Asian | 5 (1.77) | |
| American Indian/Alaskan Native | 2 (0.71) | |
| Native Hawaiian/Pacific Islander | 2 (0.71) | |
| Multiple races listed | 8 (2.83) | |
| Not provided | 20 (7.07) | |
| Paternal ethnicity | ||
| Not Hispanic or Latino | 259 (91.52) | |
| Hispanic or Latino | 24 (8.48) | |
SES indicators
Family income was estimated as the midpoint of the reported income range (as summarized in Table 1) and was adjusted for household size based on adjustments used by the US Department of Housing and Urban Development (HUD) to define the highest income level at which a family qualifies for public assistance. Each parent's education level was assigned a value from 1–6 (Less than High School = 1, High School = 2, Some College = 3, College = 4, Some Graduate Level = 5, Graduate Level = 6). Maternal education level and paternal education level were summed for each child in order to create a parental education index with possible values from 2 to 12. The parental education variable was square-root transformed in order to reduce violations of normality assumptions.
Image processing
Multi-spectral MRI data were collected on 1.5T scanners in six imaging centers in the U.S. Data from The American College of Radiology (ACR) phantom and a human phantom were used to normalize acquisition across scanners (Evans, 2006) and minimize inter-site variability in image quality. During image acquisition, data from The American College of Radiology (ACR) phantom and a human phantom were used to normalize acquisition across scanners (Evans, 2006). In the current study, we processed the T1-weighted MR images. Image processing was based on the open-source program Advanced Normalization Tools (ANTS; http://www.picsl.upenn.edu/ANTS/) and the associated pipelining framework PipeDream (sourceforge neuropipedream). ANTS was used to create a population averaged template. The template was initialized using data from 31 subjects who had 1mm isotropic T1-weighted images and were representative of the sample in terms of age, sex, scan site, and SES. In the final iteration of template building, all subjects were included. We combined multi-atlas labeling techniques (Heckemann, Hajnal, Aljabar, Rueckert & Hammers, 2006) with publicly available brain labeling data sets to perform brain masking (Shattuck et al., 2007), three tissue segmentation (http://www.nirep.org/) and cortical parcellation (Hammers et al., 2003) in the template. Each subject was then processed using Pipedream which uses the symmetric normalization methodology (Avants et al., 2011) to diffeomorphically normalize each subject to a template. The template segmentations were then propagated into subject space and used as priors for the Markov Random Field approach implemented in the ANTS tool Atropos, which has been validated on public datasets (Avants, Tustison, Wu, Cook & Gee, 2011). Cortical thickness was estimated using Diffeomorphic Registration Based Cortical Thickness (DiReCT; Das, Avants, Grossman & Gee, 2009). DiReCT uses diffeomorphic mapping within a prior-constrained estimate of the distance between the gray/white interface and the gray/cerebrospinal fluid interface to estimate cortical thickness (Das et al., 2009). Each cortical region of interest was defined by multiplying the subjects’ cortical segmentation by the region of interest label. The mean cortical thickness was then computed within the cortical ROI.
Regions of interest
The selection of regions of interest (ROIs) was guided by the literature reviewed earlier on neurocognitive SES disparities, as well as the literature on the effects of stress on brain development. Regions associated with executive functions found to differ as a function of childhood SES included left and right superior frontal gyri, left and right middle frontal gyri, left and right inferior frontal gyri, and left and right anterior cingulate gyri. Regions susceptible to stress also include the left and right anterior cingulate gyri and left and right orbitofrontal gyri.
In addition, three asymmetry measures were calculated as follows and treated as a priori measures of interest: left-minus-right superior frontal gyrus, left-minus-right middle frontal gyrus, left-minus-right inferior frontal gyrus.
Statistical approach
Analyses used hierarchical linear regression executed in Statistical Package for the Social Sciences to predict cortical thickness in each region of interest from parental education and family income. Using an approach similar to Noble et al. (2012), potentially confounding variables were entered in the first step of a hierarchical linear regression model, and SES variables (parental education and family income) were added in the second step of the model.
In the first step, ROI thickness was predicted from age (in days), sex, total brain volume, Full-Scale IQ (Wechsler, 1999), body mass index (BMI), and child/race ethnicity (dummy-coded as “Non-White” or “White”). While it has been argued that it is often unjustified to control for IQ in studies of neurocognitive outcomes (Dennis et al., 2009), previous studies with this dataset report associations between cortical thickness and IQ (Karama et al., 2009; Karama et al., 2011), suggesting that IQ should be considered a possible confounding variable. Body mass index, calculated as (Weight in kg)/(height in m)2, was used as a covariate because it has been shown to significantly predict structural measures, including whole-brain gray matter and white matter volume in the visit 1 NIHPD data (Brain Development Cooperative Group, 2012). The BMI variable was winsorized due to an extreme outlier. Child race/ethnicity was coded based on reported parental race/ethnicity, and only two categories were used in order to prevent the creation of categories with small numbers of children. Children in the “Non-White” group (n = 72) had a mean family income of $63,841.16 (SD = $34,481.63) and a mean parental education of 2.53 (SD = .39) on the square-root transformed scale. Children in the “White” group (n = 211) had a mean family income of $80,375.83 (SD = $31,503.54) and a mean parental education of 2.77 (SD = .42). Scan site was not used as a covariate because socioeconomic status was not evenly distributed across testing sites (parental education F (5, 277) = 4.80, p < .001; family income: F (5, 277) = 2.12, p = .06), so including scan site as a covariate would reduce SES variability.
Critically, in the next step, parental education and family income were added to the model. Change in model R2 and F statistics are reported along with regression coefficients. Finally, SES by age interaction terms were added to the model for each ROI. Bonferroni correction was used to correct for multiple comparisons of 13 regions of interest by setting the significance threshold at α = .0038 (e.g., .05/13), and results are reported using both uncorrected (p < .05) and corrected (p < .0038) alpha levels.
Results
The parental education and family income variables were significantly correlated with each other (r = .57, p < .001) and with full scale IQ (parental education r = .41, p < .001; family income r = .35, p < .001). Neither SES variable was significantly correlated with sex, age, BMI, or total brain volume (all p values > .07).
Without correcting for multiple comparisons, four prefrontal regions and one asymmetry measure showed significant relations with SES as measured by improved regression model fit when SES indices are added: Using a threshold of p < .05, a significant change in the model F statistic was found after adding parental education and family income to the model in the left anterior cingulate gyrus (Δ F (2, 274) = 3.20, p = .04, Δ R2 = .02), right anterior cingulate gyrus (Δ F (2, 274) = 8.10, p < .001, Δ R2 = .05), left superior frontal gyrus (Δ F (2, 274) = 6.09, p = .003, Δ R2 = .04), right superior frontal gyrus (Δ F (2, 274) = 4.09, p = .018, Δ R2 = .024), and superior frontal asymmetry measure (Δ F(2, 274) = 5.25, p = .006, Δ R2 = .04). Change in model R2 and regression coefficients for the SES variables for all ROIs are displayed in Table 2.
Table 2.
Change in R2 and change in F value for all ROIs after adding SES variables to the model.
| ROI | Model change when SES variables are added to the model |
Regression coefficients for SES variables | ||
|---|---|---|---|---|
| R2 change | F change (p) | SES variable | Beta (p) | |
| Left inferior frontal gyrus | 0.004 | 0.779 (.460) | ||
| Parental education | −.085 (.215) | |||
| Family income | .034 (.610) | |||
| Right inferior frontal gyrus | 0.005 | 0.803 (.449) | ||
| Parental education | −.065 (.338) | |||
| Family income | −.017 (.803) | |||
| Left middle frontal gyrus | 0.001 | 0.219 (.803) | ||
| Parental education | .038 (.568) | |||
| Family income | 7.34 * 10−4 (.991) | |||
| Right middle frontal gyrus | 9.38 * 10−5 | 0.017 (.983) | ||
| Parental education | .012 (.853) | |||
| Family income | −.007 (.918) | |||
| Left superior frontal gyrus | 0.036 | 6.094 (.003) | ||
| Parental education | .240 (6.09 * 10−4) | |||
| Family income | −.091 (.179) | |||
| Right superior frontal gyrus | 0.024 | 4.085 (.018) | ||
| Parental education | .196 (.005) | |||
| Family income | −.127 (.065) | |||
| Left anterior cingulate gyrus | 0.022 | 3.198 (.042) | ||
| Parental education | .187 (.013) | |||
| Family income | −.110 (.134) | |||
| Right anterior cingulate gyrus |
0.054 | 8.098 (3.83 * 10−4) | ||
| Parental education | .288 (1.24 * 10−4) | |||
| Family income | −.074 (.307) | |||
| Left orbitofrontal gyrus | 9.12 * 10−4 | 0.138(.871) | ||
| Parental education | −.022 (.769) | |||
| Family income | −.017 (.814) | |||
| Right orbitofrontal gyrus | 0.002 | 0.360 (.698) | ||
| Parental education | −.029 (.702) | |||
| Family income | −.034 (.638) | |||
| Inferior frontal asymmetry measure |
0.006 | 0.908 (.404) | ||
| Parental education | −.051 (.509) | |||
| Family income | .101 (.179) | |||
| Middle frontal asymmetry measure |
0.003 | 0.380 (.684) | ||
| Parental education | .049 (.522) | |||
| Family income | .015 (.841) | |||
| Superior frontal asymmetry measure |
0.036 | 5.253 (.006) | ||
| Parental education | .133 (.077) | |||
| Family income | .109 (.137) | |||
Note. Standardized regression coefficient and p values are also shown for parental education and family income when in the model simultaneous for each ROI.
After Bonferroni correction, two regions remained significantly related to SES: the right anterior cingulate gyrus (mean thickness = 2.37 mm; SD = .32) and left superior frontal gyrus (mean thickness = 2.66 mm, SD = .31), exceeding the corrected threshold of p < .0038. Results of the hierarchical regression for the right anterior cingulate gyrus and left superior frontal gyrus are shown in Table 3. In both cases, parental education significantly predicted the ROI while family income did not when the two SES variables were in the model simultaneously.
Table 3.
Change in R2, change in F and regression coefficients for hierarchical regressions for the right anterior cingulate gyrus and left superior frontal gyrus
| ROI: | Regression Step | R2 change | F change (p) | Beta (p) |
|---|---|---|---|---|
| Left superior frontal gyrus |
Model 1: | 0.164 | 8.997 (5.51 * 10−9) | |
| Age | −.191 (.003) | |||
| Sex (Female) | −.071 (.284) | |||
| Total brain volume |
−.214 (.002) | |||
| BMI | −.147 (.023) | |||
| IQ | .015 (.802) | |||
| Race (White) | −.122 (.036) | |||
| Model 2: | 0.036 | 6.094 (.003) | ||
| Age | −.179 (.005) | |||
| Sex (Female) | −.081 (.213) | |||
| Total brain volume |
−.210 (.002) | |||
| BMI | −.138 (.029) | |||
| IQ | −.044 (.479) | |||
| Race (White) | −.144 (.013) | |||
|
Parental education |
.240 (6.09 * 10−4) | |||
| Family income | −.091 (.179) | |||
| Right anterior cingulate gyrus |
Model 1: | 0.033 | 1.592 (.150) | |
| Age | −.016 (.822) | |||
| Sex (Female) | .021 (.767) | |||
| Total brain volume |
−.074 (.318) | |||
| BMI | −.116 (.093) | |||
| IQ | −.047 (.460) | |||
| Race (White) | −.051 (.416) | |||
| Model 2: | 0.054 | 8.098 (3.83 * 10−4) | ||
| Age | −.004 (.957) | |||
| Sex (Female) | .002 (.974) | |||
| Total brain Volume |
−.071 (.325) | |||
| BMI | −.104 (.125) | |||
| IQ | −.129 (.055) | |||
| Race (White) | −.081 (.187) | |||
|
Parental education |
.288 (1.24 * 10−4) | |||
| Family income | −.074 (.307) | |||
Note. SES variables were added simultaneously in Model 2. Significant SES effects (at Bonferroni-corrected threshold) are shown in bold.
BMI = body mass index.
To further investigate the differential ability of family income and parental education to predict cortical thickness in these ROIs, the model was repeated for the right anterior cingulate gyrus and left superior frontal gyrus using family income and parental education as independent predictors. In the right anterior cingulate gyrus, when controlling for age, sex, total brain volume, race, BMI, and IQ, parental education alone significantly predicted greater thickness (β = .25, p < .001) while family income alone did not predict thickness (β = .06, p = .32). Using the same model to predict thickness in the left superior frontal gyrus, parental education alone significantly predicted greater thickness (β = .19, p = .002), while family income alone did not predict thickness (β = .02, p = .69). Scatter plots of ROI thickness and parental education for the right anterior cingulate gyrus and left superior frontal gyrus are shown in Figures 1 and 2.
Figure 1. Scatterplot of right anterior cingulate gyrus thickness and parental education.
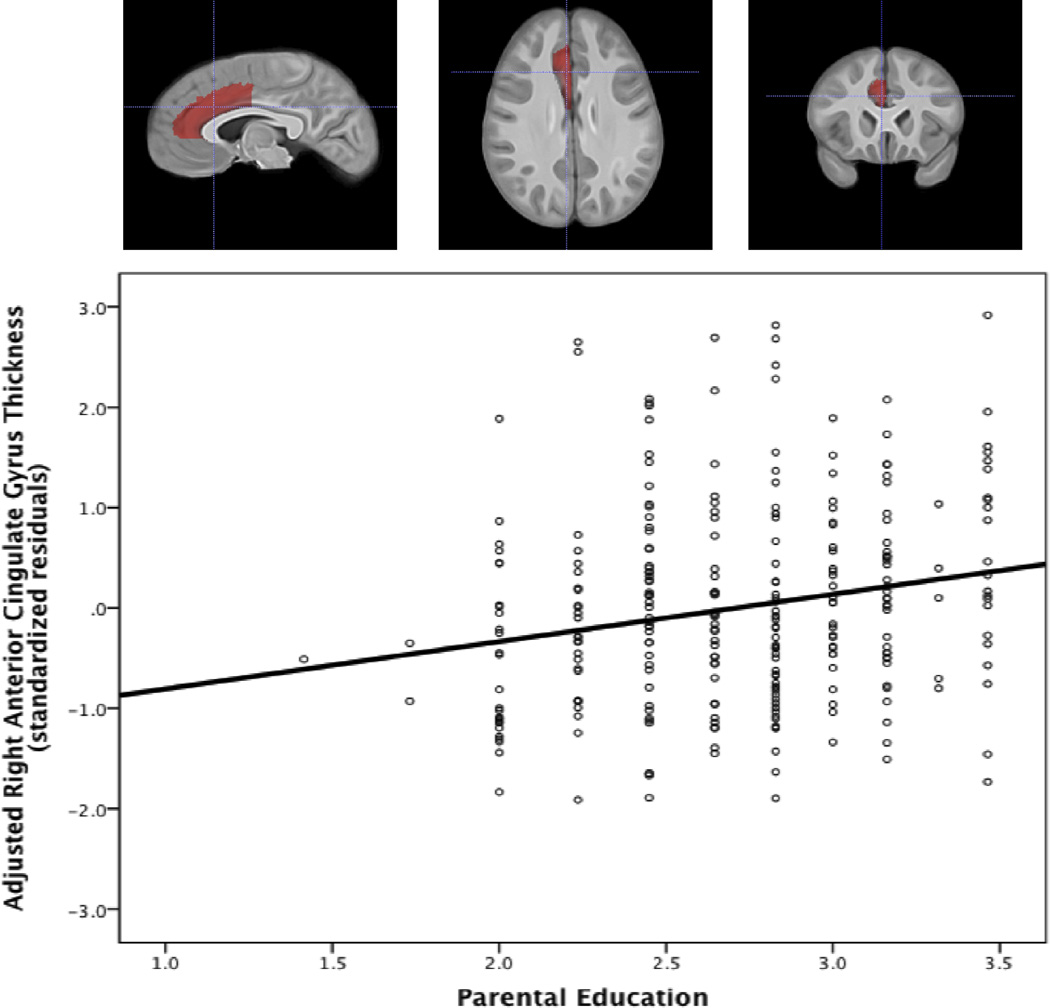
This scatterplot shows the association between the square-root transformed parental education variable and cortical thickness in the right anterior cingulate gyrus. Cortical thickness was adjusted for age, total brain volume, gender, IQ, BMI and race by using the standardized residuals from a model in which these variables predict thickness.
Figure 2. Scatterplot of left superior frontal gyrus thickness and parental education.
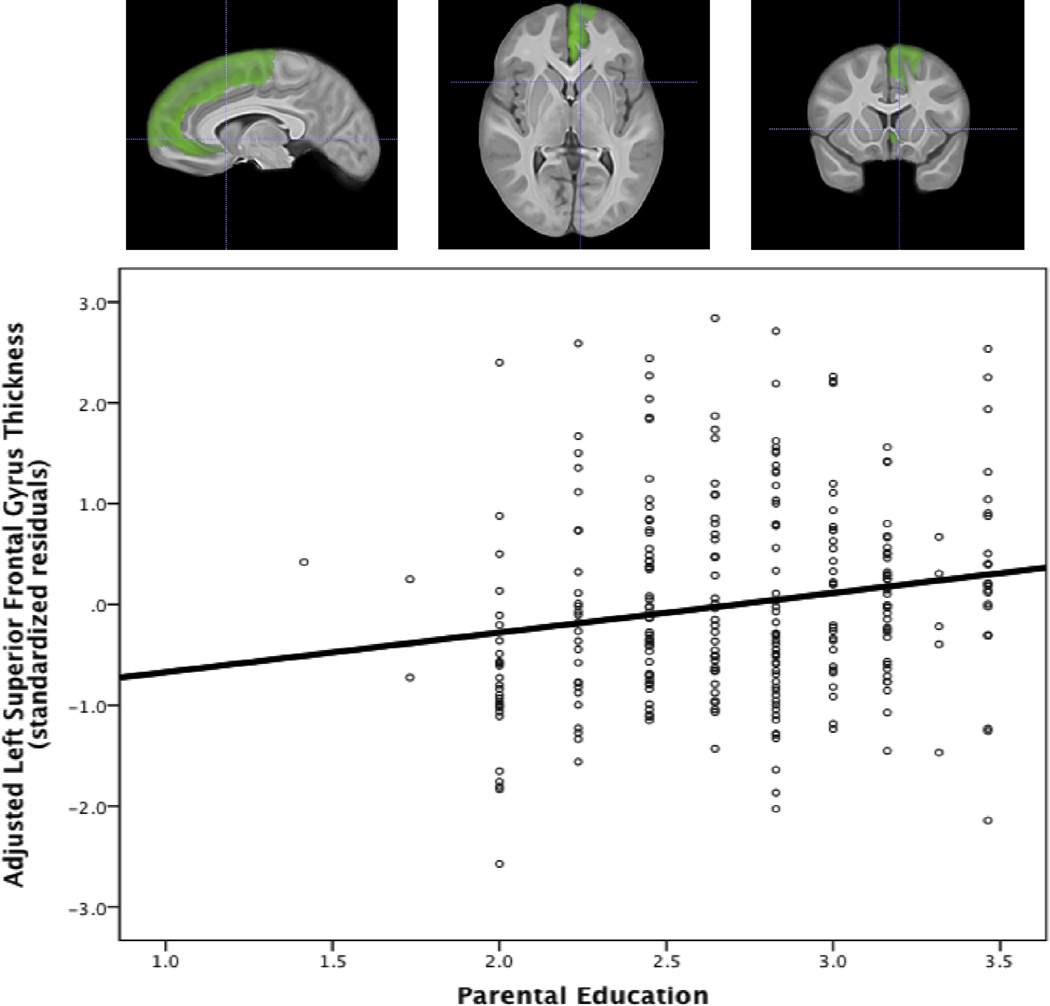
This scatterplot shows the association between the square-root transformed parental education variable and thickness in the left superior frontal gyrus. Cortical thickness was adjusted for age, total brain volume, gender, IQ, BMI and race by using the standardized residuals from a model in which these variables predict thickness.
When a parental education x age interaction was added to the model, model fit improved only in the left orbitofrontal gyrus (Δ R2 = .02, Δ F (1, 273)= 5.46, p = .02) and right orbitofrontal gyrus (Δ R2 = .02, Δ F (1, 273) = 6.07, p = .02). These effects were significant at the uncorrected alpha level of .05, but did not survive Bonferroni correction.
Discussion
Within this large sample of healthy children, parental education predicted increased cortical thickness in the left superior frontal gyrus and right anterior cingulate gyrus, using a conservative threshold for statistical significance. A measure of superior frontal asymmetry also showed SES differences, although they did not survive stringent correction for multiple comparisons. While SES differences in behavioral measures of executive function and ERP measures of prefrontal cortical function have previously been documented, this study provides novel structural evidence for SES differences in selective regions of the prefrontal cortex. These findings add to the emerging literature suggesting that SES relates to structural brain variation, with other studies of healthy children reporting main effects of SES in the hippocampus (Hanson et al., 2011; Noble et al., 2012) and amygdala (Noble et al., 2012). However, unlike previous studies, the current analyses did not show an SES main effect or age interaction in the left inferior frontal gyrus, which may reflect differences between cortical thickness and volumetric measures. The association between SES and thickness in the right anterior cingulate gyrus is interesting in light of a previous publication from this dataset reporting an association between relatively thin right anterior cingulate cortex and higher scores on the Aggressive Behavior scale of the Child Behavior Checklist (Ducharme et al., 2011). Longitudinal studies investigating the environmental and behavioral correlates of right ACC structure will be important to disentangle the relationship between environmental factors, brain development, and behavioral regulation ability.
We did not have a prediction concerning the direction of the relationship between SES and prefrontal cortical thickness. By late childhood, development generally consists of thinning in these areas (Shaw, 2008) which might lead to an expectation of thinner cortex for more advantaged children, opposite what we found. Noble et al's findings are not directly relevant, as they concern grey matter volume, not thickness, in different prefrontal regions. Their data showed a trend toward a negative relation between parental education and volume at younger ages and a positive relation at older ages.
One interesting and unexpected finding was the fact that parental education and family income, while highly correlated, showed strong differences in their ability to predict cortical thickness in frontal regions of interest. Parental education, but not family income, significantly predicted thickness in the right anterior cingulate gyrus and left superior frontal gyrus. The strong difference between the predictive ability of parental education and family income provides support for the argument that SES indicators capture different aspects of environmental and genetic variation and should be treated separately (Bravemen et al., 2005; Duncan & Magnuson, 2012) but the mechanism for differences between parental education and family income is unclear. This difference may simply reflect differences in the sensitivity of the education and income scales in this dataset, or it may reflect meaningful differences in the genetic or environmental factors associated with these SES measures. One might expect that parental education would relate most closely to cognitive stimulation in the home environment (e.g., Hoff-Ginsberg & Tardif, 1995), while family income might be an important predictor of environmental stress exposure (e.g., Evans & English, 2002). However, little empirical work has addressed the ways in which parental education and family income are differentially associated with environmental factors. Studies investigating differential associations between SES measures and environmental factors are therefore needed to identify specific pathways through which the socioeconomic environment influences child development.
Observed SES differences are particularly striking given that children in this sample met rigorous exclusionary and inclusionary criteria (Evans, 2006), and low SES children were excluded based on these criteria at higher rates (Waber et al., 2007). While studies with this healthy sample of children provide important evidence that SES differences exist even among healthy, high-performing children, future studies may improve external validity by using more representative samples of low SES children.
The observational nature of this study is another important limitation, and results cannot be used to infer the direction of causality. Cortical thickness in frontal regions has been shown to be moderately heritable (Joshi et al., 2011; Winkler et al., 2010) though heritability measures of cognitive (Harden, Turkheimer & Loehlin, 2007; Tucker-Drob, Rhemtulla, Harden, Turkheimer & Fask, 2011; Turkheimer, Haley, Waldron, D’Onofrio & Gottesman, 2003) and structural brain (Chiang et al., 2011) measures have been found to be reduced in low-SES populations. Socioeconomic status is a distal measure that is associated with both genetic and environmental differences (Hackman, Farah & Meaney, 2010), but genetic or proximal environmental factors were not measured in this study, and reported associations between SES and cortical thickness likely reflect combined genetic and environmental influences. Future research on the structural correlates of SES will benefit from including measures of more proximal environmental factors (e.g. stress, cognitive stimulation) and examining the extent to which they mediate the relationship between SES and brain structure. Early work (Rao et al., 2010) demonstrating associations between specific aspects of the home environment and brain structure suggests that this may be a promising approach. Stress, which is associated both with SES (Cohen, Doyle & Baum, 2006; Evans & English, 2002; Lupien, King, Meaney & McEwen, 2001) and differences in prefrontal brain morphology (Cerquieria, Mailliet, Almeida, Jay & Sousa, 2007; Hanson et al., 2012, Lupien, McEwen, Gunnar & Heim, 2009; McEwen & Gianaros, 2011), may be another proximal environmental factor that provides a link between SES and prefrontal structure.
It is important to note that the identification of structural correlates of SES does not in any way imply that these SES differences are innate or unchangeable. Indeed, an emerging body of research demonstrates that structural brain measures (Draganski & May, 2008; Ilg et al., 2008; Keller & Just, 2009; Mackey, Whitaker & Bunge, 2012; Rosenzweig, 2003), including cortical thickness (Haier, Karama, Leyba & Jung, 2009) can be changed by environmental experience. It is our hope that identifying specific structural phenotypes that vary with socioeconomic status will lead to a better understanding of the mechanisms contributing to SES-disparities in health and achievement, and ultimately, will be used to design more effective policies and interventions that reduce these disparities.
Acknowledgements
This research was supported by NIH grants HD055689, DA022807, EB006266, DA014129 and NS045839.
Footnotes
Disclaimer
This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH
References
- Ardila A, Rosselli M, Matute E, Guajardo S. The influence of the parents’ educational level on the development of executive functions. Developmental Neuropsychology. 2005;28(1):539–560. doi: 10.1207/s15326942dn2801_5. [DOI] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage. 2011;54(3):2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Wu J, Cook PA, Gee JC. An open source multivariate framework for n-tissue segmentation with evaluation on public data. Neuroinformatics. 2011;9(4):381–400. doi: 10.1007/s12021-011-9109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AK. Does childhood prefrontal cortex volume vary with socioeconomic status? Unpublished manuscript, Stanford University; Stanford, CA: 2010. [Google Scholar]
- Blair C, Diamond A. Biological processes in prevention and intervention: The promotion of self-regulation as a means of preventing school failure. Development and Psychopathology. 2008;20(3):899–911. doi: 10.1017/S0954579408000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Granger DA, Willoughby M, Mills-Koonce R, Cox M, Greenberg MT, Kivlighan KT, Fortunato CK. Salivary cortisol mediates effects of poverty and parenting on executive functions in early childhood. Child Development. 2011;82(6):1970–1984. doi: 10.1111/j.1467-8624.2011.01643.x. the FLP Investigators. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles DB. Socioeconomic status, a forgotten variable in lateralization development. Brain and Cognition. 2011;76(1):52–57. doi: 10.1016/j.bandc.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Brain Development Cooperative Group. Total and regional brain volumes in a population-based normative sample from 4 to 18 years: the NIH MRI study of normal brain development. Cerebral Cortex. 2012;22(1):1–12. doi: 10.1093/cercor/bhr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, Posner S. Socioeconomic status in health research: one size does not fit all. Journal of the American Medical Association. 2005;294(22):2879–2888. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J, Duncan G. The effects of poverty on children. The Future of Children. 1997;7(2):55–71. [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54(1–3):241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Mailliet F, Almeida OFX, Jay TM, Sousa N. The prefrontal cortex as a key target of the maladaptive response to stress. The Journal of Neuroscience. 2007;27(11):2781–2787. doi: 10.1523/JNEUROSCI.4372-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang MC, McMahon KL, de Zubicaray GI, Martin NG, Hickie I, Toga AW, Wright MJ, Thompson PM. Genetics of white matter development: a DTI study of 705 twins and their siblings aged 12 to 29. NeuroImage. 2011;54(3):2308–2317. doi: 10.1016/j.neuroimage.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Baum A. Socioeconomic status is associated with stress hormones. Psychosomatic Medicine. 2006;68(3):414–420. doi: 10.1097/01.psy.0000221236.37158.b9. [DOI] [PubMed] [Google Scholar]
- D’Anguilli A, Herdman A, Stapells D, Hertzman C. Children’s event-related potentials of auditory selective attention vary with their socioeconomic status. Neuropsychology. 2008;22(3):293–300. doi: 10.1037/0894-4105.22.3.293. [DOI] [PubMed] [Google Scholar]
- Das SR, Avants BB, Grossman M, Gee JC. Registration based cortical thickness measurement. NeuroImage. 2009;45(3):867–879. doi: 10.1016/j.neuroimage.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Penke L, Johnson W. The neuroscience of human intelligence differences. Nature Reviews Neuroscience. 2010;11(3):201–211. doi: 10.1038/nrn2793. [DOI] [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society. 2009;15(3):331–343. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, May A. Training-induced structural changes in the adult human brain. Behavioural Brain Research. 2008;192(1):137–142. doi: 10.1016/j.bbr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Ducharme S, Hudziak JJ, Botteron KN, Albaugh MD, Nguyen T-V, Karama S, Evans AC. Decreased regional cortical thickness and thinning rate are associated with inattention symptoms in healthy children. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(1):18–27. doi: 10.1016/j.jaac.2011.09.022. & the Brain Development Cooperative Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme S, Hudziak JJ, Botteron KN, Ganjavi H, Lepage C, Collins DL, Albaugh MD, Evans AC, Karama S. Right anterior cingulate cortical thickness and bilateral striatal volume correlate with child behavior checklist aggressive behavior scores in healthy children. Biological Psychiatry. 2011;70(3):283–290. doi: 10.1016/j.biopsych.2011.03.015. the Brain Development Cooperative Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GJ, Magnuson K. Socioeconomic status and cognitive functioning: moving from correlation to causation. Wiley Interdisciplinary Reviews: Cognitive Science. 2012;3(3):377–386. doi: 10.1002/wcs.1176. [DOI] [PubMed] [Google Scholar]
- Evans AC. The NIH MRI study of normal brain development. NeuroImage. 2006;30(1):184–202. doi: 10.1016/j.neuroimage.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Evans GW, English K. The environment of poverty: multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Development. 2002;73(4):1238–1248. doi: 10.1111/1467-8624.00469. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Shera DM, Savage JH, Betancourt L, Giannetta JM, Brodsky NL, Malmud EK, Hurt H. Childhood poverty: specific associations with neurocognitive development. Brain Research. 2006;1110(1):166–174. doi: 10.1016/j.brainres.2006.06.072. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried AW, Gottfried AE, Bathurst K, Guerin DW, Parramore MM. Socioeconomic status in children’s development and family environment: infancy through adolescence. In: Bornstein MH, Bradley RH, editors. Socioeconomic status, parenting and child development. Mahwah, NJ: Lawrence Erlbaum Associates; 2003. pp. 189–207. [Google Scholar]
- Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends in Cognitive Sciences. 2009;13(2):65–73. doi: 10.1016/j.tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nature Reviews Neuroscience. 2010;11:651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haier RJ, Karama S, Leyba L, Jung RE. MRI assessment of cortical thickness and functional activity changes in adolescent girls following three months of practice on a visual-spatial task. BMC Research Notes. 2009;2:174. doi: 10.1186/1756-0500-2-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammers A, Allom R, Koepp MJ, Free SL, Myers R, Lemieux L, Mitchell TN, Brooks DJ, Duncan JS. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Human Brain Mapping. 2003;19(14):224–247. doi: 10.1002/hbm.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Chandra A, Wolfe BL, Pollak SD. Association between income and the hippocampus. PloS One. 2011;6(5):e18712. doi: 10.1371/journal.pone.0018712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Chung MK, Avants BB, Rudolph KD, Shirtcliff EA, Gee JC, Davidson RJ, Pollak SD. Structural variations in prefrontal cortex mediate the relationship between early childhood stress and spatial working memory. The Journal of Neuroscience. 2012;32(23):7917–7925. doi: 10.1523/JNEUROSCI.0307-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden KP, Turkheimer E, Loehlin JC. Genotype by environment interaction in adolescents’ cognitive aptitude. Behaviorial Genetics. 2007;37(2):273–283. doi: 10.1007/s10519-006-9113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckemann RA, Hajnal JV, Aljabar P, Rueckert D, Hammers A. Automatic anatomical brain MRI segmentation combining label propagation and decision fusion. NeuroImage. 2006;33(1):115–126. doi: 10.1016/j.neuroimage.2006.05.061. [DOI] [PubMed] [Google Scholar]
- Hoff-Ginsberg, Tardif . Socioeconomic status and parenting. In: Bornstein MH, editor. Handbook of Parenting. 1st. Mahwah, NJ: Erlbaum; 1995. pp. 161–188. [Google Scholar]
- Hughes C, Ensor R, Wilson A, Graham A. Tracking executive function across the transition to school: a latent variable approach. Developmental Neuropsychology. 2010;35(1):20–36. doi: 10.1080/87565640903325691. [DOI] [PubMed] [Google Scholar]
- Ilg R, Wohlschläger AM, Gaser C, Liebau Y, Dauner R, Wöller A, Zimmer C, Zihl J, Mühlau M. Gray matter increase induced by practice correlates with task-specific activation: a combined functional and morphometric magnetic resonance imaging study. The Journal of Neuroscience. 2008;28(16):4210–4215. doi: 10.1523/JNEUROSCI.5722-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jednoróg K, Altarelli I, Monzalvo K, Fluss J, Dubois J, Billard C, Dehaene-Lambertz G, Ramus F. The influence of socioeconomic status on children’s brain structure. PLoS One. 7(8):e42486. doi: 10.1371/journal.pone.0042486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi AA, Leporé N, Joshi SH, Lee AD, Barysheva M, Stein JL, McMahon KL, Johnson K, de Zubicaray GI, Martin NG, Wright MJ, Toga AW, Thompson PM. The contribution of genes to cortical thickness and volume. Neuroreport. 2011;22(3):101–105. doi: 10.1097/WNR.0b013e3283424c84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karama S, Ad-Dab’bagh Y, Haier RJ, Deary IJ, Lyttelton OC, Lepage C, Evans AC. Positive association between cognitive ability and cortical thickness in a representative US sample of healthy 6 to 18 year-olds. Intelligence. 2009;37(2):145–155. doi: 10.1016/j.intell.2008.09.006. the Brain Development Cooperative Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karama S, Colom R, Johnson W, Deary IJ, Haier R, Waber DP, Ganjavi H, Jung R, Evans AC. Cortical thickness correlates of specific cognitive performance accounted for by the general factor of intelligence in healthy children aged 6 to 18. NeuroImage. 2011;55(4):1443–1453. doi: 10.1016/j.neuroimage.2011.01.016. the Brain Development Cooperative Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller TA, Just MA. Altering cortical connectivity: remediation-induced changes in the white matter of poor readers. Neuron. 2009;64(5):624–631. doi: 10.1016/j.neuron.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishiyama MM, Boyce WT, Jimenez AM, Perry LM, Knight RT. Socioeconomic disparities affect prefrontal function in children. Journal of Cognitive Neuroscience. 2009;21(6):1106–1115. doi: 10.1162/jocn.2009.21101. [DOI] [PubMed] [Google Scholar]
- Lange N, Froimowitz MP, Bigler ED, Lainhart JE. Associations between IQ, total and regional brain volumes and demography in a large normative sample of healthy children and adolescents. Developmental Neuropsychology. 2010;35(3):296–317. doi: 10.1080/87565641003696833. & the Brain Development Cooperative Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch JP, Evans AC. Cortical thickness analysis examined through power analysis and a population simulation. NeuroImage. 2005;24(1):163–173. doi: 10.1016/j.neuroimage.2004.07.045. [DOI] [PubMed] [Google Scholar]
- Lipina SJ, Martelli MI, Vuelta B, Colombo JA. Performance on the A-not-B task of Argentinian infants from unsatisfied and satisfied basic needs homes. International Journal of Psychology. 2005;39:49–60. [Google Scholar]
- Lipina SJ, Martelli MI, Vuelta BL, Injoque-Ricle I, Colombo JA. Poverty and executive performance in preschool pupils from Buenos Aires city (Republica Argentina) Interdisciplinaria. 2004;21(2):153–193. [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Development and Psychopathology. 2001;13(3):653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Mackey AP, Whitaker KJ, Bunge SA. Experience-dependent plasticity in white matter microstructure: reasoning training alters structural connectivity. Frontiers in Neuroanatomy. 2012;6:1–9. doi: 10.3389/fnana.2012.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annual Review of Medicine. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzacappa E. Alerting, orienting, and executive attention: developmental properties and sociodemographic correlates in an epidemiological sample of young, urban children. Child Development. 2004;75(5):1373–1386. doi: 10.1111/j.1467-8624.2004.00746.x. [DOI] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Kan E, Sowell ER. Neural correlates of socioeconomic status in the developing human brain. Developmental Science. 2012;15(4):516–527. doi: 10.1111/j.1467-7687.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, McCandliss BD, Farah MJ. Socioeconomic gradients predict individual differences in neurocognitive abilities. Developmental Science. 2007;10(4):464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- Noble KG, Norman MF, Farah MJ. Neurocognitive correlates of socioeconomic status in kindergarten children. Developmental Science. 2005;8(1):74–87. doi: 10.1111/j.1467-7687.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends in Cognitive Sciences. 2005;9(2):60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Porter JN, Collins PF, Muetzel RL, Lim KO, Luciana M. Associations between cortical thickness and verbal fluency in childhood, adolescence, and young adulthood. NeuroImage. 2011;55(4):1865–1877. doi: 10.1016/j.neuroimage.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizada RDS, Richards TL, Meltzoff A, Kuhl PK. Socioeconomic status predicts hemispheric specialisation of the left inferior frontal gyrus in young children. NeuroImage. 2008;40(3):1392–1401. doi: 10.1016/j.neuroimage.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao H, Betancourt L, Giannetta JM, Brodsky NL, Korczykowski M, Avants BB, Gee JC, Wang J, Hurt H, Detre JA, Farah MJ. Early parental care is important for hippocampal maturation: Evidence from brain morphology in humans. NeuroImage. 2010;49(1):1144–1150. doi: 10.1016/j.neuroimage.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades BL, Greenberg MT, Lanza ST, Blair C. Demographic and familial predictors of early executive function development: contribution of a person-centered perspective. Journal of Experimental Child Psychology. 2011;108(3):638–662. doi: 10.1016/j.jecp.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig MR. Effects of differential experience on the brain and behavior. Developmental Neuropsychology. 2003;24(2–3):523–540. doi: 10.1080/87565641.2003.9651909. [DOI] [PubMed] [Google Scholar]
- Sarsour K, Sheridan M, Jutte D, Nuru-Jeter A, Hinshaw S, Boyce WT. Family socioeconomic status and child executive functions: the roles of language, home environment, and single parenthood. Journal of the International Neuropsychological Society. 2011;17(1):120–132. doi: 10.1017/S1355617710001335. [DOI] [PubMed] [Google Scholar]
- Shattuck DW, Mirza M, Adisetiyo V, Hojatkashani C, Salamon G, Narr KL, Poldrack RA, Bilder RM, Toga AW. Construction of a 3D probabilistic atlas of human cortical structures. NeuroImage. 2007;39(3):1064–1080. doi: 10.1016/j.neuroimage.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Gilliam M, Liverpool M, Weddle C, Malek M, Sharp W, Greenstein D, Evans A, Rapoport J, Giedd J. Cortical development in typically developing children with symptoms of hyperactivity and impulsivity: support for a dimensional view of attention deficit hyperactivity disorder. American Journal of Psychiatry. 2011;168(2):143–151. doi: 10.1176/appi.ajp.2010.10030385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Japoport J, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440(7084):676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. The Journal of Neuroscience. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, Sarsour K, Jutte D, D’Esposito M, Boyce WT. The impact of social disparity on prefrontal function in childhood. PloS One. 2012;7(4):e35744. doi: 10.1371/journal.pone.0035744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirin SR. Socioeconomic status and academic achievement: a meta-analytic review of research. Review of Educational Research. 2005;75(3):417–453. [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. The Journal of Neuroscience. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C, Lauinger B, Neville H. Differences in the neural mechanisms of selective attention in children from different socioeconomic backgrounds: an event-related brain potential study. Developmental Science. 2009;12(4):634–646. doi: 10.1111/j.1467-7687.2009.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Knight RT. Principles of frontal lobe function. 1st. New York: Oxford University Press; 2002. [Google Scholar]
- Tucker-Drob EM, Rhemtulla M, Harden KP, Turkheimer E, Fask D. Emergence of a gene x socioeconomic status interaction on infant mental ability between 10 months and 2 years. Psychological Science. 2011;22(1):125–133. doi: 10.1177/0956797610392926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkheimer E, Haley A, Waldron M, D’Onofrio B, Gottesman II. Socioeconomic status modifies heritability of IQ in young children. Psychological Science. 2003;14(6):623–628. doi: 10.1046/j.0956-7976.2003.psci_1475.x. [DOI] [PubMed] [Google Scholar]
- Ursache A, Blair C, Raver CC. The promotion of self-regulation as a means of enhancing school readiness and early achievement in children at risk for school failure. Child Development Perspectives. 2011;6(2):1–7. doi: 10.1111/j.1750-8606.2011.00209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waber DP, De Moor C, Forbes PW, Almli CR, Botteron KN, Leonard G, Milovan D, Paus T, Rumsey J. The NIH MRI study of normal brain development: performance of a population based sample of healthy children aged 6 to 18 years on a neuropsychological battery. Journal of the International Neuropsychological Society. 2007;13(5):729–746. doi: 10.1017/S1355617707070841. The Brain Development Cooperative Group. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. New York: Psychological Corporation; 1999. [Google Scholar]
- Wiebe SA, Sheffield T, Nelson JM, Clark CAC, Chevalier N, Espy KA. The structure of executive function in 3-year-olds. Journal of Experimental Child Psychology. 2011;108(3):436–452. doi: 10.1016/j.jecp.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, Duggirala R, Glahn DC. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. NeuroImage. 2010;53(3):1135–1146. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]


