Abstract
This chapter summarizes advances in the following areas: (1) dendritic cell (DC)-mediated simian immunodeficiency virus (SIV) transmission, (2) role of DCs in innate and adaptive immunity against SIV, and (3) approaches to harness DC function to induce anti-SIV responses. The nonhuman primate (NHP) model of human immunodeficiency virus (HIV) infection in rhesus macaques and other Asian NHP species is highly relevant to advance the understanding of virus–host interactions critical for transmission and disease pathogenesis. HIV infection is associated with changes in frequency, phenotype, and function of the two principal subsets of DCs, myeloid DCs and plasmacytoid DCs. DC biology during pathogenic SIV infection is strikingly similar to that observed in HIV-infected patients. The NHP models provide an opportunity to dissect the requirements for DC-driven SIV infection and to understand how SIV distorts the DC system to its advantage. Furthermore, the SIV model of mucosal transmission enables the study of the earliest events of infection at the portal of entry that cannot be studied in humans, and, importantly, the involvement of DCs. Nonpathogenic infection in African NHP hosts allows investigations into the role of DCs in disease control. Understanding how DCs are altered during SIV infection is critical to the design of therapeutic and preventative strategies against HIV.
6.1 Introduction
Human immunodeficiency virus (HIV) originated from simian immunodeficiency viruses (SIVs) that naturally infect African nonhuman primates (NHPs), such as the chimpanzee, African green monkeys (AGMs), and sooty mangabeys (SMs) (Heeney et al. 2006). SIVs closely parallel HIV in genomic organization, genetic sequence, and biological properties. SIV infection in natural hosts is generally nonpathogenic despite the high rate of viral replication. In contrast, experimental SIV infection of rhesus macaques (RMs) and other Asian NHP species results in a CD4+ T cell loss and animals typically develop AIDS-like immunodeficiency within 1–2 years (Desrosiers 1990).
As will be discussed herein, macaque DCs exhibit comparable phenotypes, functions, and in vivo distribution to human DCs. Thus, the macaque model of HIV infection is especially useful for examining the roles of DCs in the early events of transmission and pathogenesis. Animals can be challenged with SIV intravenously or mucosally, allowing to (1) dissect the earliest events of transmission and virus dissemination, (2) follow disease progression in treated and untreated settings, and (3) evaluate the efficacy of experimental vaccines or microbicides for their ability to prevent infection and/or disease progression. Infectious SIV–HIV hybrids (SHIVs) can be used to evaluate the activity of HIV-specific inhibitors.
6.2 Macaque DCs
Macaque DCs are found in lymph nodes (LNs), blood, and mucosal tissues (Pope et al. 1997; Hu et al. 1998, 1999; Ignatius et al. 1998, 2001; Coates et al. 2003; Lore 2004; Teleshova et al. 2004a, b; Chung et al. 2005; Brown et al. 2007; Diop et al. 2008; Malleret et al. 2008b; Brown and Barratt-Boyes 2009; Xu et al. 2010; Gujer et al. 2011). Myeloid DCs (mDCs) are defined in blood as HLA-DR+CD11c+CD123− cells lacking expression of the lineage markers (Lin) CD3, CD14, and CD20, whereas plasmacytoid DCs (pDCs) are identified as Lin−HLA-DR+CD11c−CD123+ cells. Generation of larger numbers of monocyte-derived DCs (moDCs) (O’Doherty et al. 1997) facilitated the execution of more extensive studies on the macaque DC biology and DC–SIV interplay.
Macaque DCs also require stimulation to differentiate into mature, potent immunostimulatory cells capable of inducing strong adaptive T cell responses (Mehlhop et al. 2002; Frank et al. 2003; Teleshova et al. 2004b). Activation of macaque moDCs or circulating DCs results in (1) up-regulation of CD25, CD40, CD80, CD83, CD86, CD208, CD205, and HLA-DR; (2) reduced endocytic activity; (3) increased production of cytokines and chemokines (e.g., IL-12, IFN-α, TNF-α); and (4) enhanced T cell stimulatory activity (Mehlhop et al. 2002; Coates et al. 2003; Teleshova et al. 2004a, b). Distinct features of mDCs vs. pDCs highlight their unique roles in coordinating these innate and adaptive events (Table 6.1).
Table 6.1.
Characteristics of NHP mDCs and pDCs
| DC subsets | Co-stimulatory molecules | TLR expression | Functions | SIV-induced maturation |
|---|---|---|---|---|
| mDCs | CD40mo | TLR3 | Endocytic | No changes in CD80 and CD86 expression |
| CD80low | TLR4 | Produce IL-12 | ||
| CD86mo | TLR7 | Stimulate T cells (most potently when mature) | ||
| TLR8 | ||||
| pDCs | CD40mo | TLR7 | Endocytic | Increased CD80 and CD86 expression |
| CD80low | TLR9 | High producers of IFN-α | ||
| CD86low | Produce TNF-α | IFN-α release |
Summarized from (Coates et al. 2003; Teleshova et al. 2004b; Ketloy et al. 2008; Brown et al. 2009; Gujer et al. 2011)
6.3 DC-Mediated SIV Transmission
6.3.1 DC–SIV Interactions
Just like HIV–human DC interplay, SIV capture by moDCs requires functional Env glycoproteins (Frank et al. 2002), involving CD4, CCRs, and C-type lectin receptors (CLRs, as well as yet unidentified CLRs) (Turville et al. 2001a, b) (Fig. 6.1). Both immature and mature moDCs are effective in capturing SIV (Mehlhop et al. 2002) and capable of clathrin-coated pit-mediated uptake of the virus (Frank et al. 2002). The cellular distribution of internalized SIV (and HIV) is dramatically different in immature and mature moDCs (Frank et al. 2002, 2003; Morcock et al. 2005). In immature cells, one to two virus particles are retained in vesicles at the cell periphery. This contrasts the perinuclear localization of multiple virions in large vesicular compartments in mature moDCs. Virus captured by moDCs does not colocalize with endolysosomal markers, suggesting that the virus alters classical endolysosomal processing machinery (Frank and Pope 2002; Turville et al. 2004). Entrapped virus (in immature and mature DCs) is mostly degraded within 24 h, coinciding with a decreased ability to transmit captured virus to T cells across DC–T cell synapses, while immature moDCs exhibit a second phase of increased transmissibility as they facilitate R5 HIV replication for transfer to T cells (Frank and Pope 2002; Turville et al. 2004) (Fig. 6.1). Virus transfer from DCs to T cells also involves multiple receptors including CD4, CCR5, and CD209 (Wu et al. 2002; Turville 2001a).
Fig. 6.1.
moDC–SIV interactions and possible modes of virus transmission to permissive CD4+ T cells. SIV internalized via CLRs (and other possible attachment receptors) is held in the periphery of immature moDCs vs. a deeper perinuclear location in mature moDCs. Captured virus rapidly moves to the contact points between the DC and CD4+ T cells and is transferred to the T cells, which amplifies infection. Entrapped virus is degraded in lysosomes within 24 h, ultimately reducing the infectious virus load in the DCs. R5 HIV infection of immature DCs and subsequent spread of newly produced viruses to T cells have also been demonstrated for human moDCs, but this has not been extensively studied with macaque moDCs
Macaque pDCs express high level of CCR5 (Reeves and Fultz 2007), but the percentage of macaque pDCs that express CXCR4 is highly variable (Reeves and Fultz 2007; Patterson et al. 2001). Comparable to human studies (Donaghy et al. 2003), pDCs were shown to be susceptible to in vivo infection with SIV, although lower levels of SIV gag DNA were detected in mDCs (Brown et al. 2009).
6.3.2 Importance of the DC–T Cell Milieu
Macaque DCs emigrating from organ cultures (skin, nasopharyngeal, and vaginal mucosa) form conjugates with T cells that support SIV replication (Pope et al. 1997; Ignatius et al. 1998, 2001; Hu et al. 1999). Blood- and skin-derived DCs from uninfected macaques similarly support high viral replication when mixed with T cells from blood, skin, spleen, or LNs and transmit virus to syngeneic and allogeneic T cells (Ignatius et al. 1998, 2001). In fact, separation of the subsets by cell sorting revealed that SIV replication predominantly occurs in the DC–T cell conjugate fraction (Ignatius et al. 1998). Trypsin treatment of SIV-loaded DCs did not affect their ability to transfer virus to T cells, supporting the idea that the transmitted virus is internalized (Ignatius et al. 1998). SIV replication in these mixtures proceeds in the absence of overt activation of the resting T cells and is augmented in the presence of antigen-responding T cells (Ignatius et al. 1998, 2001; Messmer et al. 2000). Of note, virus replication is equally robust when DCs or resting T cells introduce the virus to the CD4+ T cell–DC environment (Messmer et al. 2000).
Although SIV replication is comparable when mature macaque moDCs are cocultured with either naïve or memory T cells, immature DCs promote more robust replication in the presence of memory T cells (like the DCs and T cells located at the body surfaces) (Frank and Pope 2002). This supports early observations of vigorous replication of SIV in the DC–memory T cell mixtures obtained from the skin and mucosal surfaces (Pope et al. 1997; Hu et al. 1998; Ignatius et al. 1998, 2001). In agreement, extensive replication of SIV in CCR5+ memory T cells is reported within the gut in the DC–memory T cell locale (Veazey et al. 1998).
moDC–T cell contact triggers SIV movement to the DC–T cell synapse (Turville et al. 2004). Movement of tubular MHC II peptide-bearing compartments to contact points between DCs and peptide-specific T cells was reported (Boes et al. 2002; Chow et al. 2002), suggesting that virus may exploit this machinery to spread from DCs to CD4+ T cells, in particular to virus-specific T cells (Douek et al. 2002; Lore et al. 2005).
6.3.3 DCs and Mucosal SIV Transmission
6.3.3.1 Vaginal SIV Transmission
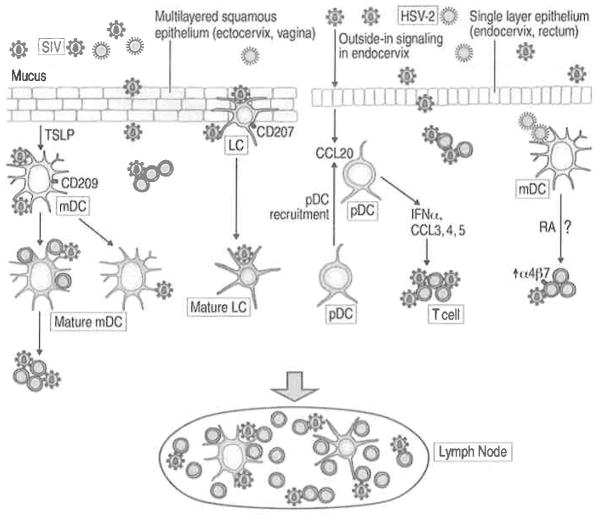
The first in vivo evidence that DCs may represent targets for SIV was obtained after looking for virus DNA in the mucosa following vaginal challenge of RMs with SIV (Spira et al. 1996). Virus was found to enter the vaginal mucosa within 60 min of exposure, infecting primarily intraepithelial DCs including Langerhans cells (LCs) (Hu et al. 2000). SlV-infected cells are detected in draining LNs within 18 h of intravaginal SIV exposure posing a challenge for the development of preventative strategies. Mucosal site-specific virus-induced events following vaginal SIVmac251 challenge have been reported (Li et al. 2009). The predominant foci of SIV-infected cells were identified in the endocervix and coincided with “outside-in” signaling events that include CCL20 production by endocervical epithelial cells and recruitment of CCR6+ pDCs that produce type I IFNs and chemokines attracting CCR5+ T cells (Li et al. 2009). Subsequent to pDC and T cell recruitment into the endocervix, clusters of infected cells appear in the transformation zone and in the vagina. Infection with SIV induces pro-inflammatory responses in the ectocervical and vaginal mucosa. Importantly, blocking virus-induced inflammation with vaginally administered glycerol monolaurate correlated with protection against SIV infection in vivo (Li et al. 2009). Another cytokine, thymic stromal lymphopoietin (TSLP), was suggested to play a role during mucosal SIV transmission based on its increased expression concurrent with an increase in viral replication in the vaginal mucosa within the first 2 weeks from vaginal SIV exposure (Fontenot et al. 2009). HIV induces human genital epithelial cells to produce TSLP (via NFκB), which activates DCs to promote HIV replication in the DC–T cell milieu (Fontenot et al. 2009).
DCs are sparse in the mucosa and the primary virus amplification occurs rather within resting memory T cells, the predominant available target cells (90%) (Miller et al. 2005; Zhang et al. 2004). These data support the theory that the delivery of virus by the few infected/virus-carrying DCs may represent a “quality” rather than “quantity” type of mechanism and that infected/carrying virus DCs may fuel infection locally and, after migrating, in the lymphoid tissue leading to systemic spread (Turville et al. 2006). The timing of DC-mediated transfer of SIV in the lymphoid tissue is supported by in vitro findings in moDCs (Turville et al. 2004). The potential contributions of DCs in vaginal SIV transmission are summarized in Fig. 6.2.
Fig. 6.2.
The role of DCs in mucosal SIV transmission. SIV can cross the multilayered squamous epithelium of the ectocervix/vagina and the columnar epithelium of the endocervix and rectum. Cell-free or cell-associated SIV (not shown) is trapped in mucus covering the epithelium. At mucosal surfaces during SIV exposure, DCs are proposed to be among the first target cells to encounter virus. These DCs include LCs in the epithelium and immature mDCs in the submucosa. CLRs (CD207, CD209) or other attachment factors expressed on immature DCs are capable of binding SIV and transferring virus in trans to CD4+ T cells locally in the mucosa or in the LNs. SIV carrying DCs can mature as the result of infection or cytokines/chemokines present in the mucosa during infection. Virus crosses the mucosal barrier to establish small founder population that expands using outside-in signaling identified in the endocervix and resulting in endocervical CCL20 production, subsequent recruitment of CCR6+ pDCs, CCR5+ target cell expressing chemokines, and production of IFN-α. SIV also induces the production of TSLP by vaginal epithelial cells that leads to DC maturation and fueling of DC-mediated transmission. In the rectal mucosa CD209+ cells and CD4+ T cells are among the first targets of SIV. HSV-2 is one of the main cofactors for HIV infection. HSV-2 can infect mDCs and increase the susceptibility of mucosal CD4+ T cells to SIV infection by RA-mediated induction of α4β7 on the T cells, as it was demonstrated in vitro
6.3.3.2 Rectal SIV Transmission
Knowledge of the role of DCs during rectal SIV transmission is limited. Like the HIV-binding CD209+ cells found in human rectal tissues (Gurney et al. 2005; Dieu et al. 1998), an abundance of CD209+ cells that efficiently bind SIV was reported in macaque rectal mucosa (Jameson et al. 2002) (Ribeiro Dos Santos et al. 2011). A low frequency of mDCs and pDCs within Lin−HLADR+ cells in macaque rectal tissues was also reported (Kwa et al. 2011). Rapid dissemination of SIV to draining LNs and peripheral blood (at 4 h post infection) after rectal challenge and lack of SIV antigen+ CD209+ cells in the mucosa suggest possible dissemination of virus by CD209+ cells (Ribeiro Dos Santos et al. 2011).
6.3.3.3 Oral/Tonsillar SIV Transmission
SIV can be transmitted via the oral route (Baba et al. 1996). Explosive SIV replication in the oral mucosal associated lymphoid tissue (MALT) following atraumatic application of virus on palatine and lingual tonsils suggests that MALT is a potential site for HIV transmission during oral sex, parturition, and breastfeeding (Stahl-Hennig et al. 1999). DCs are identified in the oral MALT in macaques (Pope et al. 1997). Tonsillar DC–T cell mixtures support SIV replication like other DC–T cell mixtures, with virus replication occurring predominantly in DC–T cell syncytia (Pope et al. 1997). Thus, the very different mucosal environments all house DCs and memory T cells, which likely play central roles in the onset and amplification of SIV infection.
6.3.4 DC Dynamics During Early/Acute and Chronic SIV
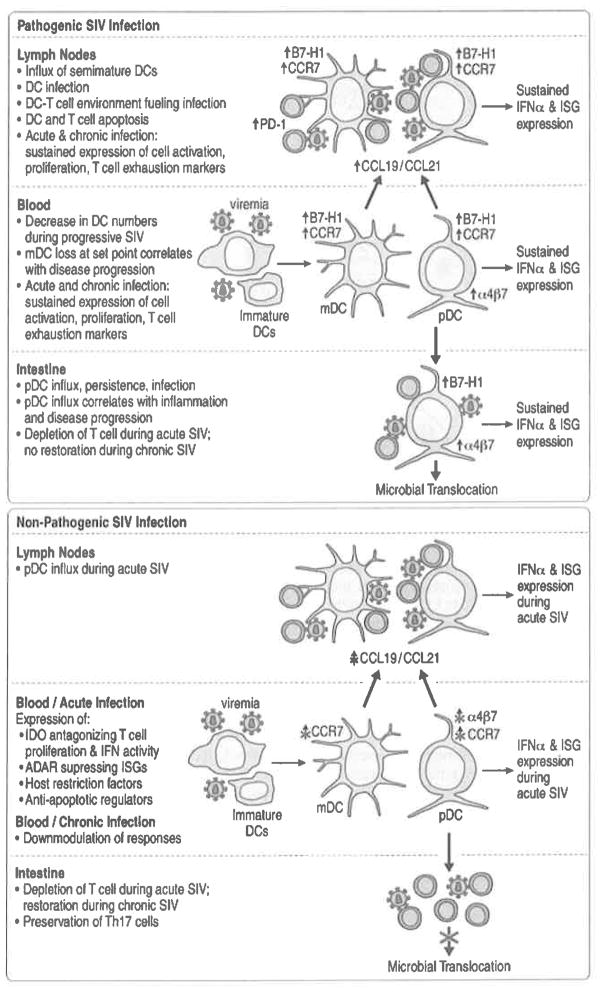
With DCs participating in the onset and spread of SIV infection, it is not surprising that DC biology is affected in infected individuals (Fig. 6.3). A decrease in pDC and mDC numbers in HIV-infected patients correlates with disease progression, suggesting that DC loss is related to the ability to control disease (Smed-Sorensen and Lore 2011). However, a substantial depletion of pDCs from blood during HIV-2 infection, which is significantly attenuated relative to HIV-1 infection, was reported (Cavaleiro et al. 2009). DC numbers and distribution are similarly affected during SIV infection (Barratt-Boyes and Wijewardana 2011; Reeves and Fultz 2007; Wijewardana et al. 2010; Malleret et al. 2008a), but the dynamics of these changes can be affected by the challenge virus (e.g., SIVmac239 vs. SHIV-89.6P) (Reeves and Fultz 2007).
Fig. 6.3.
DC frequency, distribution, and functionality during pathogenic and nonpathogenic SIV infection. Early and sustained changes in pDC and mDC numbers, phenotype, tissue distribution, and function are attributable to pathogenic SIV infection. SIV infection is associated with an activated/mature pDC phenotype and semi-mature mDC phenotype. SIV infection induces the regulatory molecule B7-H1, CCR7, and α4β7 integrin expression by blood DCs that leads to recruitment of cells to lymphoid tissues in a CCR7-dependent manner and to intestinal mucosa in an α4β7-dependent manner. The changes in DC phenotype and increased migration into lymphoid tissues and mucosa seen in pathogenic SIV infections do not occur in natural SIV hosts. Robust immune activation is evident in pathogenic and nonpathogenic SIV hosts. However, nonpathogenic hosts express factors antagonizing IFN activity and suppressing ISGs (i.e., tryptophan-depleting enzyme indoleamine 2,3 dioxygenase (IDO), an adenosine deaminase that suppresses ISG expression (ADAR)). Only nonpathogenic SIV hosts downmodulate their responses in the chronic phase of disease
Both mDCs and pDCs are lost from blood and lymphoid tissue in SIV-infected RMs with end-stage disease (Brown et al. 2007). Significant loss of mDCs in blood at virus set point is predictive of rapid progression to AIDS, whereas an increase in blood mDCs predicts long-term absence of disease. In stable (disease-free) and progressor animals, blood mDC numbers decreased within 2 weeks post infection; however, only progressor animals experienced irreversible mDC decrease within a 12-week follow-up, and stable animals had a significant increase during this period (Barratt-Boyes and Wijewardana 2011). mDC recruitment to LNs coincides with increased expression of the LN homing and recruitment molecules CCR7, CCL19, and CCL21. LN recruitment is offset by apoptosis in both acute and chronic infection (Wijewardana et al. 2010; Barratt-Boyes and Wijewardana 2011). Negligible SIV DNA is detected in LN mDCs suggesting that infection is not the major cause of the mDCs loss (Brown et al. 2009).
There is a dynamic pDC response in acute SIV infection with an initial increase in number of pDCs in blood followed by a decrease relative to baseline. pDC mobilization into blood and recruitment into the LNs coincide with peak viremia at 1–2 weeks post infection (Brown et al. 2009). Nevertheless, the absolute number of pDCs in both compartments drops, associated with widespread pDC activation, apoptosis, and infection. This parallels studies in humans showing that pDC loss is a better predictor of disease progression than CD4 counts (Soumelis et al. 2001). SIV DNA is detected in more than 4% of pDCs in LNs during acute infection, possibly resulting in pDC death (Brown et al. 2009). In acute SIV infection, pDCs acquire a mature phenotype, upregulating CD80, α4β7 integrin, and CCR7 (Brown et al. 2009; Kwa et al. 2011; Malleret et al. 2008a) and migrate into the colorectal mucosa and LNs. The migration of pDCs to the colorectal mucosa is α4β7 dependent and coincides with mucosal immune activation (Kwa et al. 2011). The induction of α4β7 during acute SIV infection is unique to pDCs, as mDCs do not upregulate this marker.
6.3.5 DC Interactions with Mucosal Pathogens Leading to Increased SIV Infection
Sexually transmitted infections (STIs), such as HSV-2, increase the risk of HIV acquisition (Freeman et al. 2006). Macaque studies found that HSV-2 pre-exposure increases the frequency of vaginal SHIV-RT infection (Crostarosa et al. 2009). In vitro studies have shown that macaque DCs are susceptible to HSV-2 infection, inducing apoptotic death of DCs; decreasing the expression of HLA-DR, CD40, CD80, CD83, and CD86; increasing the release of IL-6, TNF-α, CCL3, and CCL5 but not IL-12 or IFN-α; and reducing their ability to stimulate SlV-specific T cell responses in vitro (Peretti et al. 2005). Interestingly, rectal HSV-2 infection of macaques resulted in increased frequencies of α4β7-expressing T cells in tissues and blood, paralleling the retinoic acid (RA)-dependent increase in α4β7 expression and HIV replication seen in HSV-2-infected DC–T cell mixtures in vitro (Martinelli et al. 2011). These findings underscore the importance of developing NHP models of STIs to study the role of DCs in SIV transmission and the prevention of SIV in the context of a coinfection.
6.4 Innate and Adaptive Immunity
6.4.1 Dysregulation of DC Recognition
Macaque DCs are responsive to exogenous TLR3-, TLR7-, and TLR9-mediated stimulation (Mehlhop et al. 2002; Barratt-Boyes et al. 2010; Teleshova et al. 2004b). Upon exposure to retroviruses, both mDCs and pDCs can be activated by ligation of TLR7, which recognizes single-stranded RNA (ssRNA), initiating the antiviral type I IFN response and the expression of downstream IFN-stimulated genes (ISGs). Multiple studies during both acute and chronic SIV infection have detected cytokines and chemokines associated with an enhanced IFN response (Co et al. 2011; Sanghavi and Reinhart 2005; Hofmann-Lehmann et al. 2002; Durudas et al. 2009; Abel et al. 2002, 2005; Milush et al. 2007), suggesting the involvement of pDCs (the primary producers of IFN-α). Recent data implicate RM TLR7 polymorphisms in SIV set point viral load, pathogenesis, and survival (Siddiqui et al. 2011). The importance of two cytosolic receptors for viral RNA, RIG-I and MDA5, has been documented in human and in experimental SIV infection of macaques, demonstrating that the IFN response is directed primarily by MDA5 (Co et al. 2011). However, the cellular origin of the MDA5- and RIG-I-derived IFN in macaques has not been shown.
TLR3 recognizes double-stranded RNA (dsRNA) and is expressed by mDCs but not pDCs (Ketloy et al. 2008). Since the complex secondary structures formed by the SIV genome have been shown to mimic dsRNA, it is not surprising that dsRNA is detected during SIV infection (Co et al. 2011) or that increased expression of TLR3 mRNA has been observed in the LNs throughout the course of SIV infection (Sanghavi and Reinhart 2005). Stimulation of TLR3 leads to NFκB activation, which both promotes the transcription of antiviral cytokines and also enhances viral transcription from the LTR. In fact, treatment of susceptible cells with the synthetic dsRNA, poly(I:C), has been shown to block SIV replication while poly(I:C) is simultaneously capable of stimulating the viral LTR (Sanghavi and Reinhart 2005). The timing of poly(I:C) treatment of DCs (relative to HIV exposure) has been shown to have variable impact on DC-driven HIV infection (Trapp et al. 2009; de Jong et al. 2010) (Derby N. and Robbiani M., unpublished).
6.4.2 DC Host Restriction Factors
Many host restriction factors are expressed by DCs to protect against HIV and SIV, including the APOBEC family, SAMHD1, and tetherin. APOBEC3G (A3G) and to a lesser extent A3F protect DCs from immunodeficiency virus infection by deaminating minus-strand viral reverse transcripts, thereby generating defective or noninfectious virus (Mangeat et al. 2003). SIV Vif acts on A3G and A3F, targeting them for degradation (Yu et al. 2004). A3B and A3C restrict SIV (not HIV) by deamination-dependent and -independent mechanisms (Yu et al. 2004). The highest expression of A3G and A3F is found in animals with long-term non-progressing infection (Muszil et al. 2011). A3G mRNA was found predominantly in monocytes and DCs with only a small proportion in CD4+ T cells in the mesenteric LNs of macaques treated intracolorectally with poly(I:C) and IL-15, and this correlated with decreased viral load and improved prognosis following infection with SIVmac251 (Sui et al. 2010). Recent work showed that A3 A depletion increases the infectivity of SIV and, moreover, vpx-deficient SIV displays a severe infectivity defect in DCs, which is partially rescued by A3 A knockdown (Berger et al. 2011). This represents an interesting divergence of HIV and SIV evolution as only SIV expresses vpx.
Vpx is also important in the context of another barrier to HIV infection in myeloid cells, SAMHD1, which prevents HIV-1 infection in DCs by interfering with synthesis of viral cDNA (Hrecka et al. 2011; Laguette et al. 2011). It is involved in the regulation of the IFN pathway (Rice et al. 2009). In SIVsm, SIVmac, and HIV-2, SAMHD1 activity is overcome by Vpx, which targets SAMHD1 for proteasomal degradation (Sunseri et al. 2011; Laguette et al. 2011). Notably, there is no viral protein in HIV-1 that overcomes the action of SAMHD1, possibly suggesting a mechanism by which the virus failed to avert innate immunity. However, if vpx were present in the genome, a stronger immune response might be generated which could be detrimental to the virus. Thus, the lack of a viral antagonist might be the mechanism to avoid effective immune surveillance (Manel et al. 2010; Lim and Emerman 2011). The existence of SAMHD1 explains why HIV does not replicate efficiently in DCs, but so far, it has only been studied in moDCs and macrophages. Lower viremia, reduced viral replication, and slower disease progression have all been observed in macaques infected with vpx-defective SIVsm or SIVmac (Gibbs et al. 1995; Hirsch et al. 1998). No studies have been published to date on SIV replication in macaque DCs and the implications with respect to SAMHD1. It remains to be seen whether there is an association between SIV in natural hosts and the ability of the virus to replicate in DCs and elicit potentially effective antiviral immunity.
An important late restriction factor involved in the type I IFN response is tetherin (BST-2, CD317). Tetherin prevents viral release, maturation, and spreading of infection by tethering newly synthesized virions to the cell surface. Tetherin is constitutively expressed by B cells, activated T cells, and pDCs and is inducible on other cell types by type I IFNs. Identified by its activity against HIV-1 lacking vpu (Van Damme et al. 2008; Neil et al. 2008), tetherin is degraded by Vpu through recruitment of the ubiquitin ligase complex. However, most SIVs do not express vpu, and still SIV is capable of tetherin-mediated restriction. SIV Env and Nef can counter RM and SM (but not human) tetherin through non-ubiquitin-related mechanisms by downregulating protein expression from the cell surface into endosomes and possibly rerouting protein trafficking (Gupta et al. 2009; Jia et al. 2009; Zhang et al. 2009). Importantly, tetherin is not only an effector of the IFN response, but also a negative feedback regulator of pDC IFN production during inflammation. Tetherin binds to a receptor, immunoglobulin-like transcript 7 (ILT7), on human pDCs, signaling inhibition of IFN release (Cao et al. 2009). ILT7 is uniquely expressed by human pDCs, but a similar regulatory system may exist in NHPs. Such negative feedback mechanisms may also impact on IFN-related hyperimmune activation, another mechanism by which HIV and SIV overcome innate immune responses and promote viral replication.
6.4.3 SIV Dysregulation of Effective Immune Responses
Impaired DC function is a fundamental component of SIV infection leading to immune activation, which is considered the best independent correlate of disease pathogenesis in experimental pathogenic SIV. Many factors possibly play into sustaining immune activation during chronic infection including aberrant TLR signaling (Sanghavi and Reinhart 2005), microbial translocation, preferential infection of central memory instead of effector memory T cells, depleting the T cell pool (Brenchley et al. 2010), and T cell bystander activation resulting from production of proinflammatory cytokines.
Acute SIV infection increases expression of costimulatory molecules by pDCs but not by mDCs (Kwa et al. 2011). Recruited into the lymphoid tissue and intestinal mucosa, pDCs produce IFN-α and contribute to immune activation (Kwa et al. 2011) as highlighted earlier. Semi-mature mDCs with reduced expression of costimulatory molecules and/or CD83 are detected in the lymphoid tissues of SIV-infected macaques (Soderlund et al. 2004; Zimmer et al. 2002). Semi-mature mDCs potentially contribute to immune dysregulation since fully mature DCs are more adept at stimulating both CD4+ and CD8+ T cell responses (Frank et al. 2003). However, semi-mature mDCs are detected in vivo in animals with long-term stable infection (Wijewardana et al. 2010).
Dysregulated CD8+ T cell responses are another feature of pathogenic SIV infection characterized by the expression of PD-1, a marker of CD8+ T cell exhaustion. PD-1 is upregulated on SIV-specific CD8+ T cells in vitro and in vivo (Velu et al. 2007), and blocking PD-1 in chronically SIV-infected macaques increases the proportion and quality of SIV-specific CD8+ T cells, which is noteworthy considering that poly-functional CD8+ T cells are correlated with improved control of SIV (Hansen et al. 2009). During acute SIV infection, a ligand for PD-1 (B7-H1) is increased on mDCs (Xu et al. 2010) and pDCs (Barron et al. 2003; Wijewardana et al. 2010), and B7-H1 expression persists and correlates with PD-1 expression on T cells and impaired virus-specific T helper and CTL functions (Xu et al. 2010) (Fig. 6.3). Expression of both B7-H1 and PD-1 is stable in SIV controllers (Xu et al. 2010).
Immunosuppressive regulatory T cells (Tregs, CD4+CD25hiFoxP3+ T cells) and IL-17-producing Th17 cells are also prominently affected during SIV infection, and their dysregulation is affected by infection-induced DC dysregulation and hyperimmune activation. An increase in the proportion of CD8+ FoxP3-expressing Tregs has also been observed in the colorectal mucosa during pathogenic SIV infection, and this is associated with impaired antiviral responses and loss of viral control (Nigam et al. 2010). Th17 cells are also depleted during SIV infection. Instead of this resulting in suppressed inflammation, Th17 loss interferes with the control of gut pathogens (Raffatellu et al. 2008), particularly important given the increased gut permeability and microbial translocation that results from DC-mediated chronic immune activation. A related subset, the CD8+ Tc17 cells, are depleted in SIV-infected macaques with end-stage disease (Nigam et al. 2011).
6.4.4 Pathogenic vs. Nonpathogenic SIV Infection
6.4.4.1 Attenuated Virus Infection
In vivo, SIV lacking nef (SIVΔnef) results in highly attenuated infection (Kestler et al. 1991) and also provides a “vaccine effect,” protecting against wild-type SIV challenge (Daniel et al. 1992). Nef is important for SIV replication in immature DC–T cell mixtures (Messmer et al. 2000, 2002). When immature moDCs or T cells were loaded with SIV prior to coculture, expression of Nef was shown to be critical for SIV replication in the DC–T cell mixtures while mature DCs overcame the need for Nef in this setting (Messmer et al. 2000, 2002). Interestingly, if moDCs were activated after capturing SIVΔnef, the impaired replication in the cocultures was not rescued (Messmer et al. 2002). This indicates that maturing during migration to the LNs DCs that bear SIVΔnef would induce strong immune responses in the absence of overwhelming infection (Messmer et al. 2000). Notably, introduction of nef (via an adenovirus vector) or strong T cell activation (via SEB) rescues SIVΔnef replication in the immature DC–T cell milieu (Messmer et al. 2002). Nef was demonstrated to induce a specific activation program in DCs characterized by cytokine and chemokine production (without changing DC membrane phenotype) (Messmer et al. 2002), suggesting mechanisms underlying the nef-dependent replication in the immature DC–T cell environment. The attenuated replication of SIVΔnef was similarly apparent when virus-loaded immature DCs or T cells were injected subcutaneously into macaques and surprisingly resulted in greater macrophage infection compared to cell-free virus (predominantly T cell infections) (Ignatius et al. 2002), underscoring how cell-associated virus is infectious and that it might influence the earliest stages of cell-cell spread.
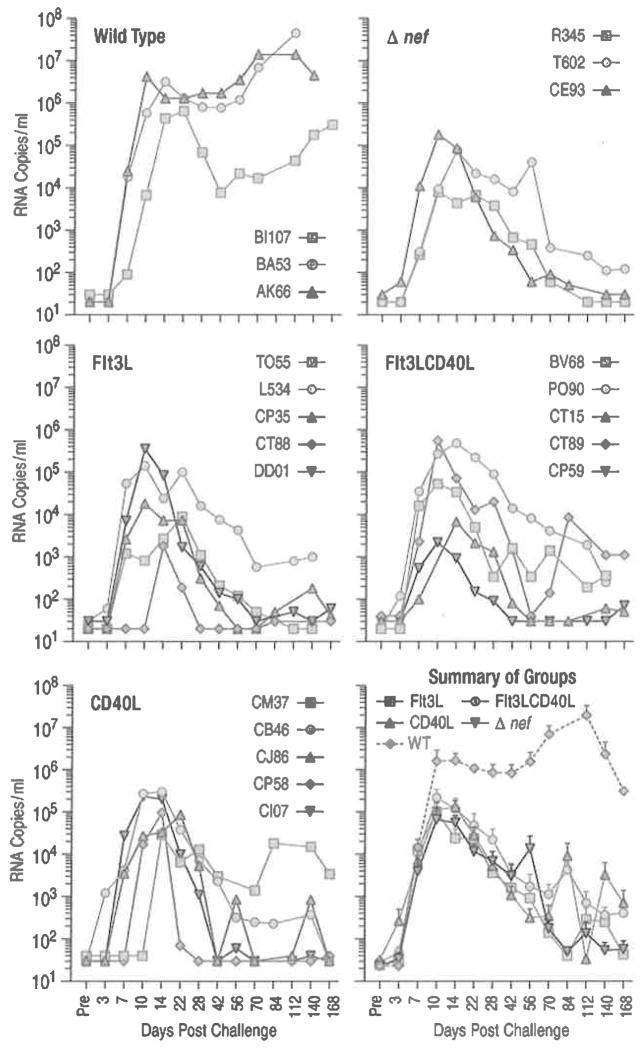
Exploring whether increased number of circulating (immature vs. mature) DCs would impact SIVΔnef replication in vivo, we exploited the use of Flt3L treatment to mobilize (Coates et al. 2003; Teleshova et al. 2004a; Reeves et al. 2009) and CD40L (Mehlhop et al. 2002; Coates et al. 2003; Teleshova et al. 2004a, b) to activate macaque DCs. Increased number of circulating DCs (>tenfold more Lin−HLA-DR+ cells) did not alter the fate of SIVmac239Δnef applied to the tonsils (Fig. 6.4; Peretti S. and Robbiani M., unpublished). This likely reflects the predominance of larger number of immature or suboptimally activated DCs that could not rescue the attenuated replication of the SIVmac239Δnef in vivo, in contrast to the in vitro rescue with fully matured DCs.
Fig. 6.4.
Increased number of circulating immature or partially matured DCs does not change the attenuated course of SIVmac239Δnef infection. Naive Indian RMs received Flt3L alone, Flt3L and CD40L, or CD40L alone (five animals per treatment group). Flt3L was administered for 7 days (100 μg/kg/day human Flt3L). To activate the cells, 0.6 mg/kg human CD40L trimer was given daily for 3 days after the Flt3L treatment. Another group of animals received only CD40L treatment. Animals were challenged with 2,000 TCID50 of SIVmac239Δnef across the palatine/lingual tonsils at the peak of DC mobilization 4 days post FLt3L treatment (Teleshova et al. 2004a) (or 1 day after CD40L). Control groups of untreated animals (three animals each) were challenged with 2,000 TCID50 of SIVmac239Δnef or wild-type SlVmac239 across the tonsils. Individual plasma viral loads (SIV RNA copies/ml of plasma) are shown for each animal in the different groups over time and the mean SIV RNA copies/ml (±SEM) are shown for each group in the summary plot. DC numbers in the blood were measured by four color flow cytometry. In the Flt3L-treated group the percentage of Lin−HLA-DR+ cells increased from 3.0%±0.5 to 33.1%±4.1 while in the Flt3LCD40L-treated group this number increased from 2.2% ±0.5 to 22.6%±6.2 (Mean±SEM). The percentage of Lin−HLA-DR+ cells in the CD40L-treated group did not change after treatment. The total number of Lin−HLA-DR+ (DC), Lin−HLA-DR+CD11c+CD123− (mDC), Lin−HLA-DR+CD11c−CD123+ (pDC), and Lin−HLA-DR+CD11c−CD123− (double negative) subsets increased by 16.3-, 1.8-, 3.8-, and 29.5-fold, respectively, for the Flt3L-treated group and by 12.3-, 2.8-, 3.1-, and 21.6-fold, respectively, for the Flt3LCD40L-treated group (each number being the fold increase (Post- divided by Pretreatment) of the average of the five animals within each group). The mobilized DCs were functional as evidenced by IL-12 production in response to CD40L stimulation in vitro, which was increased after Flt3L treatment as previously reported (Teleshova et al. 2004a). Of note, CD40L activation in vivo appeared only partially effective at best. In the CD40L-treated animals CD80 and CD86 expression increased by only 1.49- and 2.15-fold on mDCs and 1.34- and 1.98-fold on pDCs (respectively), while the levels of the FLt3L/CD40L-treated animals were basically comparable to those of the Flt3L-treated animals (other than CD86 on pDCs in the Flt3L-treated animals which increased by 1.6-fold). The limited effect of CD40L in the FLt3L-treated animals is likely due to the dose of CD40L being insufficient to activate the increased number of DCs following mobilization and/or the fact that the activated DCs migrated to the CD40L injection site (from the blood)
6.4.4.2 SIV in Natural Hosts
SIV infections of natural hosts result in life-long nonpathogenic infection. More than 40 SIVs naturally infect African NHPs with most studies conducted in SMs, AGMs, and mandrills (Hirsch et al. 1989; Marx et al. 1991). Multiple mechanisms underlying nonpathogenic SIV infections were proposed including lack of chronic immune activation, reduced infection of central memory CD4+ T cells, preserved or enhanced immune regeneration, absence of microbial translocation, and ability to mediate T helper function by CD3+CD4−CD8− T cells (Bosinger et al. 2011). There is no pDC depletion in the blood in nonpathogenic models of SIV infection (Mandl et al. 2008; Campillo-Gimenez et al. 2010).
The possibility that innate responses to SIV infection in natural SIV hosts are less pronounced was suggested based on the observation that SIV-infected pDCs from natural hosts produce less IFN-α in response to TLR stimulation ex vivo than do SIV-infected pDCs from experimental hosts (Mandl et al. 2008; Diop et al. 2008). Recent studies demonstrated that the acute phase of both pathogenic and nonpathogenic infections is associated with a robust innate immune response to the virus (Bosinger et al. 2011). However, a different pattern of innate immune responses and immune activation in SIV infection in natural hosts points to DCs and innate immunity as fundamental players in driving pathogenesis of experimental SIV infection and probably HIV infection of humans as well (Pereira and Ansari 2009). While in natural hosts immune activation is observed during acute infection, particularly production of IFN-α and expression of ISGs (Manches and Bhardwaj 2009; Bosinger et al. 2009; Jacquelin et al. 2009), it does not become chronic leading to loss of mucosal barrier integrity followed by microbial translocation as it does during experimental infection (Brenchley et al. 2006). Instead, immune activation in the periphery and in the mucosa resolves during the acute-to-chronic phase transition, preserving the mucosal barrier and preventing microbial translocation (Manches and Bhardwaj 2009; Bosinger et al. 2009; Jacquelin et al. 2009; Mir et al. 2011; Kaur et al. 2008). During acute infection of natural hosts, an anti-inflammatory cytokine profile emerges characterized by an increased frequency of Tregs and immunosuppressive molecules including TGF-β and IL-10 (Kornfeld et al. 2005) suggesting their involvement in the maintenance of a non-inflamed state. The resolution of immune activation also correlates with induction of PD-1 on CD8+ T cells (Estes et al. 2008), which may serve a protective immunoregulatory role though implicated in pathogenesis in experimental infection. Preservation of the Th17 subset in natural infection may also be important for protection against microbial translocation, consistent with its loss during experimental infection being associated with loss of mucosal integrity. While both SIVmac and SIVsm infections in RMs enhanced α4β7 expression on blood pDCs and recruited pDCs to the intestinal mucosa, SIVsm infection of SMs did not (Kwa et al. 2011). DC frequency, distribution, and functionality during pathogenic and nonpathogenic SIV infection are summarized in Fig. 6.3.
6.5 Harnessing DC Function to Induce Anti-SIV Responses
6.5.1 DC-Targeted Preventative and Therapeutic Vaccines
Strong virus-specific CD4+ T helper responses and sustained CTL responses are crucial to control infection and DC-targeted approaches are being explored to most effectively induce these responses (Picker et al. 2012). Using Δnef/wild-type infected RMs as a source of primed T cells, mature moDCs pulsed with aldrithiol-2 (AT-2)-inactivated SIV were shown to activate SIV-specific CD4+ and CD8+ T cells in vitro (Mehlhop et al. 2002; Frank et al. 2003). Similarly, DCs transfected with SIV gag mRNA stimulate robust recall CD8+ T cell responses, with greater CD4+ T cell responses when antigen is targeted to the lysosome (Melhem et al. 2007). These data suggested that antigen-loaded moDCs have the potential to activate primed T cells, supporting the strategy to explore their potential as a therapeutic vaccine. Subcutaneous injection of autologous AT-2 SIV-loaded moDCs into infected RMs resulted in the reduction of SIV RNA and DNA, which correlated with SIV-specific humoral and cellular responses (Lu et al. 2003). Similar success was then observed in chronically infected people immunized with AT-2 HIV-loaded DCs (Lu et al. 2004). Thus, inactivated whole virus-pulsed DCs represent a promising strategy in therapeutic vaccination to help control virus replication and disease progression.
There have also been several studies examining the potential of antigen-loaded DCs as preventative vaccines. Intradermal injection of autologous antigen-loaded DCs elicited SIV-specific T and B cell responses and, although animals were not protected from infection upon oral challenge, the set point viremia in the vaccinated animals was at least 1 log lower than that seen in non-vaccinated animals (Wagner et al. 2002). Similarly, animals immunized with DCs loaded with an envelope peptide cocktail vaccine mounted antigen-specific T cell responses and ultimately reduced plasma viremia following virus challenge with SHIV89.6P (Nehete et al. 2005). In an attempt to better target antigens to appropriately activated DCs in order to activate more potent responses, novel strategies involving two types of DC receptors are being explored: receptors mediating antigen uptake (e.g., CD205) and TLRs. Immunization of RMs with CD205-targeted HIV gag with poly(I:C) as an adjuvant resulted in multifunctional Th1 responses and better induction and recall of CD8 immunity, as compared to non-targeted gag immunization (Flynn et al. 2011).
Another approach has been to use recombinant vectors to infect DCs and load them with antigens endogenously. moDCs infected with recombinant canarypox virus or adenovirus (AdV) vectors can stimulate virus-specific T cell responses in vivo (Villamide-Herrera et al. 2004; Brown et al. 2003). AdV is suggested to be highly efficient in transducing DCs (Brown et al. 2003). AdV-based vaccines administered directly to monkeys induced robust T cell responses and protected against SHIV challenge (Shiver et al. 2002). However, the STEP vaccine trial using three separate replication-defective Ad5 vectors encoding a conserved HIV gag, pol, or nef gene (Merck 023/HVTN 502) was halted as the vaccine was ineffective (Buchbinder et al. 2008). Studies addressing potential reasons for the failure of this vaccine are being done (Benlahrech et al. 2009; Patterson 2011).
Strategies to increase the number of circulating DCs and make them more accessible for vaccine antigens are also being tested. Flt3L treatment of macaques led to mobilization of immature and functional DCs in blood and LNs (Teleshova et al. 2004a). Treatment of SIV- and SHIV-infected macaques with Flt3L resulted in mDC activation and mobilization in the absence of any changes to viral load or CD4+ T cells, suggesting that FLt3L could be added to mDC-targeted vaccines without the risk of creating virus-permissive environment (Reeves et al. 2009). Vaccines would then need to target these mobilized DCs properly (perhaps through CD205 and/or other DC-specific markers) to ensure efficient presentation to the immune system.
6.5.2 Mucosal Vaccination
Since DCs reside in the mucosal surfaces, it is tempting to explore whether they can be harnessed to improve vaccine efficacy. One way to explore this was pursued by exploiting the defective replication of SIVmac239Δnef in the tonsillar DC–T cell milieu. Atraumatic application of attenuated SIVmac239Δnef vaccine to the tonsils in macaques protects against tonsillar or rectal challenge with infectious SIVmac251 (Tenner-Racz et al. 2004; Stahl-Hennig et al. 2007), but this was comparable to the protection afforded by intravenous SIVΔnef infection.
Another strategy is to use inactivated viruses to target mucosal DCs, like that used in the autologous moDC vaccinations (above). AT-2 SIV (that interacts authentically with target cells and activates CD4+ and CD8+ T cells when presented by DCs) (Frank et al. 2003) was used to target the DCs within the tonsillar tissues and tonsillar vaccination of SIV-infected macaques on ART with AT-2 SIV adjuvanted with polyICLC (clinical grade poly(I:C), but not CpG-C ISS-ODNs) resulted in suppressed viremia once ART was removed (Vagenas et al. 2010). The results with respect to CpG-C ISS-ODNs were surprising since prior work indicated that CpG-C ISS-ODN in combination with AT-2 SIV activated pDCs, stimulated IFN-α production, and boosted SIV-specific T cell responses in vitro (Teleshova et al. 2004b). Interestingly, TLR9 expression was shown to decrease during acute SIV infection despite the absence of TLR9 ligands from the virus (Sanghavi and Reinhart 2005) suggesting that noninvasive mucosally applied therapeutic vaccines augmented with polyICLC show promise for controlling HIV/SIV replication.
6.6 Conclusion and Future Directions
Considerable progress has been made to define the role of DCs in SIV transmission and pathogenesis suggesting paradoxical functionality of DCs during infection, with the bulk of the NHP DC–SIV biology mirroring that of the human DC–HIV interplay. It remains unclear how beneficial and detrimental DC responses mounted during SIV are tipped toward the propagation of infection rather than the induction of effective SIV-specific responses. The hallmark of pathogenic SIV infection is aversion and dysregulation of DC-mediated responses that start very early during SIV infection and lead to extensive innate immune responses. In contrast to nonpathogenic SIV infection, these responses are not resolved during the transition from acute to chronic infection. This lack of immune resolution in the pathogenesis of HIV is supported by data in the viremic non-progressors. Although DCs are dys-regulated by SIV, proper stimulation may block infection of DCs and transmission to T cells. To this end, there are opportunities to improve anti-SIV/HIV immunity by therapeutic strategies based on providing DCs with appropriate antigen and adjuvants. The best choice of stimulus to induce appropriate DC maturation for induction of immune responses remains to be determined. There are opportunities for successful interventions to prevent transmission in the initial stage of infection where there is the greatest viral vulnerability at the portal of entry. In conclusion, significant progress has been made in recent years, but many important questions on the role of DCs in SIV infection remain to be answered.
Acknowledgments
N.T. is supported by the Swedish Ministry of Foreign Affairs, and the USAID Cooperative Agreement GPO-00-04-00019-00. M.R. is supported by NIH grants R37 AI040877, R01s DE018293, and AI084133, the Swedish Ministry of Foreign Affairs, and the USAID Cooperative Agreement GPO-00-04-00019-00. M.R. is a 2002 Elizabeth Glaser Scientist.
References
- Abel K, Alegria-Hartman MJ, Rothaeusler K, Marthas M, Miller CJ. The relationship between simian immunodeficiency virus RNA levels and the mRNA levels of alpha/beta interferons (IFN-alpha/beta) and IFN-alpha/beta-inducible Mx in lymphoid tissues of rhesus macaques during acute and chronic infection. J Virol. 2002;76(16):8433–8445. doi: 10.1128/JVI.76.16.8433-8445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel K, Rocke DM, Chohan B, Fritts L, Miller CJ. Temporal and anatomic relationship between virus replication and cytokine gene expression after vaginal simian immunodeficiency virus infection. J Virol. 2005;79(19):12164–12172. doi: 10.1128/JVI.79.19.12164-12172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba TW, Trichel AM, An L, Liska V, Martin LN, Murphey-Corb M, Ruprecht RM. Infection and AIDS in adult macaques after nontraumatic oral exposure to cell-free SIV. Science. 1996;272(5267):1486–1489. doi: 10.1126/science.272.5267.1486. [DOI] [PubMed] [Google Scholar]
- Barratt-Boyes SM, Wijewardana V. A divergent myeloid dendritic cell response at virus set-point predicts disease outcome in SIV-infected rhesus macaques. J Med Primatol. 2011;40(4):206–213. doi: 10.1111/j.1600-0684.2011.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt-Boyes SM, Wijewardana V, Brown KN. In acute pathogenic SIV infection plasma-cytoid dendritic cells are depleted from blood and lymph nodes despite mobilization. J Med Primatol. 2010;39(4):235–242. doi: 10.1111/j.1600-0684.2010.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron MA, Blyveis N, Palmer BE, MaWhinney S, Wilson CC. Influence of plasma viremia on defects in number and immunophenotype of blood dendritic cell subsets in human immunodeficiency virus 1-infected individuals. J Infect Dis. 2003;187(1):26–37. doi: 10.1086/345957. [DOI] [PubMed] [Google Scholar]
- Benlahrech A, Harris J, Meiser A, Papagatsias T, Hornig J, Hayes P, Lieber A, Athanasopoulos T, Bachy V, Csomor E, Daniels R, Fisher K, Gotch F, Seymour L, Logan K, Barbagallo R, Klavinskis L, Dickson G, Patterson S. Adenovirus vector vaccination induces expansion of memory CD4 T cells with a mucosal homing phenotype that are readily susceptible to HIV-1. Proc Natl Acad Sci U S A. 2009;106(47):19940–19945. doi: 10.1073/pnas.0907898106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger G, Durand S, Fargier G, Nguyen XN, Cordeil S, Bouaziz S, Muriaux D, Darlix JL, Cimarelli A. APOBEC3A is a specific inhibitor of the early phases of HIV-1 infection in myeloid cells. PLoS Pathog. 2011;7(9):e1002221. doi: 10.1371/journal.ppat.1002221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boes M, Cerny J, Massol R, Op Den Brouw M, Kirchhausen T, Chen J, Ploegh HL. T-cell engagement of dendritic cells rapidly rearranges MHC class II transport. Nature. 2002;418(6901):983–988. doi: 10.1038/nature01004. [DOI] [PubMed] [Google Scholar]
- Bosinger SE, Li Q, Gordon SN, Klatt NR, Duan L, Xu L, Francella N, Sidahmed A, Smith AJ, Cramer EM, Zeng M, Masopust D, Carlis JV, Ran L, Vanderford TH, Paiardini M, Isett RB, Baldwin DA, Else JG, Staprans SI, Silvestri G, Haase AT, Kelvin DJ. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest. 2009;119(12):3556–3572. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosinger SE, Sodora DL, Silvestri G. Generalized immune activation and innate immune responses in simian immunodeficiency virus infection. Curr Opin HIV AIDS. 2011;6(5):411–418. doi: 10.1097/COH.0b013e3283499cf6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Silvestri G, Douek DC. Nonprogressive and progressive primate immunodeficiency lentivirus infections. Immunity. 2010;32(6):737–742. doi: 10.1016/j.immuni.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KN, Barratt-Boyes SM. Surface phenotype and rapid quantification of blood dendritic cell subsets in the rhesus macaque. J Med Primatol. 2009;38(4):272–278. doi: 10.1111/j.1600-0684.2009.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K, Gao W, Alber S, Trichel A, Murphey-Corb M, Watkins SC, Gambotto A, Barratt-Boyes SM. Adenovirus-transduced dendritic cells injected into skin or lymph node prime potent simian immunodeficiency virus-specific T cell immunity in monkeys. J Immunol. 2003;171(12):6875–6882. doi: 10.4049/jimmunol.171.12.6875. [DOI] [PubMed] [Google Scholar]
- Brown KN, Trichel A, Barratt-Boyes SM. Parallel loss of myeloid and plasmacytoid dendritic cells from blood and lymphoid tissue in simian AIDS. J Immunol. 2007;178(11):6958–6967. doi: 10.4049/jimmunol.178.11.6958. [DOI] [PubMed] [Google Scholar]
- Brown KN, Wijewardana V, Liu X, Barratt-Boyes SM. Rapid influx and death of plasmacytoid dendritic cells in lymph nodes mediate depletion in acute simian immunodeficiency virus infection. PLoS Pathog. 2009;5(5):e1000413. doi: 10.1371/journal.ppat.1000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, McElrath MJ, Casimiro DR, Gottesdiener KM, Chodakewitz JA, Corey L, Robertson MN. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372(9653):1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campillo-Gimenez L, Cumont MC, Fay M, Kared H, Monceaux V, Diop O, Muller-Trutwin M, Hurtrel B, Levy Y, Zaunders J, Dy M, Leite-de-Moraes MC, Elbim C, Estaquier J. AIDS progression is associated with the emergence of IL-17-producing cells early after simian immunodeficiency virus infection. J Immunol. 2010;184(2):984–992. doi: 10.4049/jimmunol.0902316. [DOI] [PubMed] [Google Scholar]
- Cao W, Bover L, Cho M, Wen X, Hanabuchi S, Bao M, Rosen DB, Wang YH, Shaw JL, Du Q, Li C, Arai N, Yao Z, Lanier LL, Liu YJ. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J Exp Med. 2009;206(7):1603–1614. doi: 10.1084/jem.20090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaleiro R, Baptista AP, Soares RS, Tendeiro R, Foxall RB, Gomes P, Victorino RM, Sousa AE. Major depletion of plasmacytoid dendritic cells in HIV-2 infection, an attenuated form of HIV disease. PLoS Pathog. 2009;5(11):e1000667. doi: 10.1371/journal.ppat.1000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A, Toomre D, Garrett W, Mellman I. Dendritic cell maturation triggers retrograde MHC class II transport from lysosomes to the plasma membrane. Nature. 2002;418(6901):988–994. doi: 10.1038/nature01006. [DOI] [PubMed] [Google Scholar]
- Chung E, Amrute SB, Abel K, Gupta G, Wang Y, Miller CJ, Fitzgerald-Bocarsly P. Characterization of virus-responsive plasmacytoid dendritic cells in the rhesus macaque. Clin Diagn Lab Immunol. 2005;12(3):426–435. doi: 10.1128/CDLI.12.3.426-435.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Co JG, Witwer KW, Gama L, Zink MC, Clements JE. Induction of innate immune responses by SIV in vivo and in vitro: differential expression and function of RIG-I and MDA5. J Infect Dis. 2011;204(7):1104–1114. doi: 10.1093/infdis/jir469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates PT, Barratt-Boyes SM, Zhang L, Donnenberg VS, O’Connell PJ, Logar AJ, Duncan FJ, Murphey-Corb M, Donnenberg AD, Morelli AE, Maliszewski CR, Thomson AW. Dendritic cell subsets in blood and lymphoid tissue of rhesus monkeys and their mobilization with Flt3 ligand. Blood. 2003;102(7):2513–2521. doi: 10.1182/blood-2002-09-2929. [DOI] [PubMed] [Google Scholar]
- Crostarosa F, Aravantinou M, Akpogheneta OJ, Jasny E, Shaw A, Kenney J, Piatak M, Lifson JD, Teitelbaum A, Hu L, Chudolij A, Zydowsky TM, Blanchard J, Gettie A, Robbiani M. A macaque model to study vaginal HSV-2/immunodeficiency virus co-infection and the impact of HSV-2 on microbicide efficacy. PLoS One. 2009;4(11):e8060. doi: 10.1371/journal.pone.0008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel MD, Kirchhoff F, Czajak SC, Sehgal PK, Desrosiers RC. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- de Jong MA, de Witte L, Taylor ME, Geijtenbeek TB. Herpes simplex virus type 2 enhances HIV-1 susceptibility by affecting Langerhans cell function. J Immunol. 2010;185(3):1633–1641. doi: 10.4049/jimmunol.0904137. [DOI] [PubMed] [Google Scholar]
- Desrosiers RC. The simian immunodeficiency viruses. Annu Rev Immunol. 1990;8:557–578. doi: 10.1146/annurev.iy.08.040190.003013. [DOI] [PubMed] [Google Scholar]
- Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Ait-Yahia S, Briere F, Zlotnik A, Lebecque S, Caux C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188(2):373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diop OM, Ploquin MJ, Mortara L, Faye A, Jacquelin B, Kunkel D, Lebon P, Butor C, Hosmalin A, Barre-Sinoussi F, Muller-Trutwin MC. Plasmacytoid dendritic cell dynamics and alpha interferon production during simian immunodeficiency virus infection with a nonpathogenic outcome. J Virol. 2008;82(11):5145–5152. doi: 10.1128/JVI.02433-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaghy H, Gazzard B, Gotch F, Patterson S. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood. 2003;101(11):4505–4511. doi: 10.1182/blood-2002-10-3189. [DOI] [PubMed] [Google Scholar]
- Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, Casazza JP, Kuruppu J, Kunstman K, Wolinsky S, Grossman Z, Dybul M, Oxenius A, Price DA, Connors M, Koup RA. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417(6884):95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- Durudas A, Milush JM, Chen HL, Engram JC, Silvestri G, Sodora DL. Elevated levels of innate immune modulators in lymph nodes and blood are associated with more-rapid disease progression in simian immunodeficiency virus-infected monkeys. J Virol. 2009;83(23):12229–12240. doi: 10.1128/JVI.01311-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes JD, Gordon SN, Zeng M, Chahroudi AM, Dunham RM, Staprans SI, Reilly CS, Silvestri G, Haase AT. Early resolution of acute immune activation and induction of PD-1 in SIV-infected sooty mangabeys distinguishes nonpathogenic from pathogenic infection in rhesus macaques. J Immunol. 2008;180(10):6798–6807. doi: 10.4049/jimmunol.180.10.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn BJ, Kastenmuller K, Wille-Reece U, Tomaras GD, Alam M, Lindsay RW, Salazar AM, Perdiguero B, Gomez CE, Wagner R, Esteban M, Park CG, Trumpfheller C, Keler T, Pantaleo G, Steinman RM, Seder R. Immunization with HIV Gag targeted to dendritic cells followed by recombinant New York vaccinia virus induces robust T-cell immunity in nonhuman primates. Proc Natl Acad Sci U S A. 2011;108(17):7131–7136. doi: 10.1073/pnas.1103869108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot D, He H, Hanabuchi S, Nehete PN, Zhang M, Chang M, Nehete B, Wang YH, Ma ZM, Lee HC, Ziegler SF, Courtney AN, Miller CJ, Sun SC, Liu YJ, Sastry KJ. TSLP production by epithelial cells exposed to immunodeficiency virus triggers DC-mediated mucosal infection of CD4+ T cells. Proc Natl Acad Sci U S A. 2009;106(39):16776–16781. doi: 10.1073/pnas.0907347106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank I, Pope M. The enigma of dendritic cell-immunodeficiency virus interplay. Curr Mol Med. 2002;2:229–248. doi: 10.2174/1566524024605716. [DOI] [PubMed] [Google Scholar]
- Frank I, Piatak M, Jr, Stoessel H, Romani N, Bonnyay D, Lifson JD, Pope M. Infectious and whole inactivated simian immunodeficiency viruses interact similarly with primate dendritic cells (DCs): differential intracellular fate of virions in mature and immature DCs. J Virol. 2002;76(6):2936–2951. doi: 10.1128/JVI.76.6.2936-2951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank I, Santos JJ, Mehlhop E, Villamide-Herrera L, Santisteban C, Gettie A, Ignatius R, Lifson JD, Pope M. Presentation of exogenous whole inactivated simian immunodeficiency virus by mature dendritic cells induces CD4+ and CD8+ T-cell responses. J Acquir Immune Defic Syndr. 2003;34(1):7–19. doi: 10.1097/00126334-200309010-00002. [DOI] [PubMed] [Google Scholar]
- Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20(1):73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- Gibbs JS, Lackner AA, Lang SM, Simon MA, Sehgal PK, Daniel MD, Desrosiers RC. Progression to AIDS in the absence of a gene for vpr or vpx. J Virol. 1995;69(4):2378–2383. doi: 10.1128/jvi.69.4.2378-2383.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujer C, Sundling C, Seder RA, Karlsson Hedestam GB, Lore K. Human and rhesus plas-macytoid dendritic cell and B-cell responses to Toll-like receptor stimulation. Immunology. 2011;134(3):257–269. doi: 10.1111/j.1365-2567.2011.03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Mlcochova P, Pelchen-Matthews A, Petit SJ, Mattiuzzo G, Pillay D, Takeuchi Y, Marsh M, Towers GJ. Simian immunodeficiency virus envelope glycoprotein counteracts tetherin/BST-2/CD317 by intracellular sequestration. Proc Natl Acad Sci U S A. 2009;106(49):20889–20894. doi: 10.1073/pnas.0907075106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney KB, Elliott J, Nassanian H, Song C, Soilleux E, McGowan I, Anton PA, Lee B. Binding and transfer of human immunodeficiency virus by DC-SIGN +cells in human rectal mucosa. J Virol. 2005;79(9):5762–5773. doi: 10.1128/JVI.79.9.5762-5773.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, Piatak M, Jr, Lifson JD, Nelson JA, Jarvis MA, Picker LJ. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15(3):293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeney JL, Dalgleish AG, Weiss RA. Origins of HIV and the evolution of resistance to AIDS. Science. 2006;313(5786):462–466. doi: 10.1126/science.1123016. [DOI] [PubMed] [Google Scholar]
- Hirsch VM, Olmsted RA, Murphey-Corb M, Purcell RH, Johnson PR. An African primate lentivirus (SIVsm) closely related to HIV-2. Nature. 1989;339(6223):389–392. doi: 10.1038/339389a0. [DOI] [PubMed] [Google Scholar]
- Hirsch VM, Sharkey ME, Brown CR, Brichacek B, Goldstein S, Wakefield J, Byrum R, Elkins WR, Hahn BH, Lifson JD, Stevenson M. Vpx is required for dissemination and patho-genesis of SIVsmPBj: evidence of macrophage-dependent viral amplification. Nat Med. 1998;4:1401–1408. doi: 10.1038/3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann-Lehmann R, Williams AL, Swenerton RK, Li PL, Rasmussen RA, Chenine AL, McClure HM, Ruprecht RM. Quantitation of simian cytokine and beta-chemokine mRNAs, using real-time reverse transcriptase-polymerase chain reaction: variations in expression during chronic primate lentivirus infection. AIDS Res Hum Retroviruses. 2002;18(9):627–639. doi: 10.1089/088922202760019329. [DOI] [PubMed] [Google Scholar]
- Hrecka K, Hao C, Gierszewska M, Swanson SK, Kesik-Brodacka M, Srivastava S, Florens L, Washburn MP, Skowronski J. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature. 2011;474(7353):658–661. doi: 10.1038/nature10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Pope M, O’Doherty U, Brown C, Miller CJ. Immunophenotypic characterization of SIV-infected cells in cervix, vagina and draining lymph nodes of chronically infected rhesus macaques. Lab Invest. 1998;78:435–451. [PubMed] [Google Scholar]
- Hu J, Miller CJ, O’Doherty U, Marx PA, Pope M. The dendritic cell-T cell milieu of the lymphoid tissue of the tonsil provides a locale in which SIV can reside and propagate at chronic stages of infection. AIDS Res Hum Retroviruses. 1999;15:1305–1314. doi: 10.1089/088922299310205. [DOI] [PubMed] [Google Scholar]
- Hu J, Gardner MB, Miller CJ. Simian immunodeficiency virus rapidly penetrates the cervi-covaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J Virol. 2000;74:6087–6095. doi: 10.1128/jvi.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatius R, Isdell F, O’Doherty U, Pope M. Dendritic cells from skin and blood of macaques both promote SIV replication with T cells from different anatomical sites. J Med Primatol. 1998;27:121–128. doi: 10.1111/j.1600-0684.1998.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Ignatius R, Steinman RM, Granelli-Piperno A, Messmer D, Stahl-Hennig C, Tenner-Racz K, Racz P, Frank I, Zhong L, Schlesinger-Frankel S, Pope M. Dendritic cells during infection with HIV-1 and SIV. In: Lotze MT, Thomson AW, editors. Dendritic cells. 2. Vol. 35. Academic; London, UK: 2001. p. 481. [Google Scholar]
- Ignatius R, Tenner-Racz K, Messmer D, Gettie A, Blanchard J, Luckay A, Russo C, Smith S, Marx PA, Steinman RM, Racz P, Pope M. Increased macrophage infection upon subcutaneous inoculation of rhesus macaques with simian immunodeficiency virus-loaded dendritic cells or T cells but not with cell-free virus. J Virol. 2002;76(19):9787–9797. doi: 10.1128/JVI.76.19.9787-9797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquelin B, Mayau V, Targat B, Liovat AS, Kunkel D, Petitjean G, Dillies MA, Roques P, Butor C, Silvestri G, Giavedoni LD, Lebon P, Barre-Sinoussi F, Benecke A, Muller-Trutwin MC. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009;119(12):3544–3555. doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson B, Baribaud F, Pohlmann S, Ghavimi D, Mortari F, Doms RW, Iwasaki A. Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques. J Virol. 2002;76(4):1866–1875. doi: 10.1128/JVI.76.4.1866-1875.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia B, Serra-Moreno R, Neidermyer W, Rahmberg A, Mackey J, Fofana IB, Johnson WE, Westmoreland S, Evans DT. Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2. PLoS Pathog. 2009;5(5):e1000429. doi: 10.1371/journal.ppat.1000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur A, Di Mascio M, Barabasz A, Rosenzweig M, McClure HM, Perelson AS, Ribeiro RM, Johnson RP. Dynamics of T- and B-lymphocyte turnover in a natural host of simian immunodeficiency virus. J Virol. 2008;82(3):1084–1093. doi: 10.1128/JVI.02197-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestler HW, 3rd, Ringler DJ, Mori K, Panicali DL, Sehgal PK, Daniel MD, Desrosiers RC. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65(4):651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- Ketloy C, Engering A, Srichairatanakul U, Limsalakpetch A, Yongvanitchit K, Pichyangkul S, Ruxrungtham K. Expression and function of Toll-like receptors on dendritic cells and other antigen presenting cells from non-human primates. Vet Immunol Immunopathol. 2008;125(1–2):18–30. doi: 10.1016/j.vetimm.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Kornfeld C, Ploquin MJ, Pandrea I, Faye A, Onanga R, Apetrei C, Poaty-Mavoungou V, Rouquet P, Estaquier J, Mortara L, Desoutter JF, Butor C, Le Grand R, Roques P, Simon F, Barre-Sinoussi F, Diop OM, Muller-Trutwin MC. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J Clin Invest. 2005;115(4):1082–1091. doi: 10.1172/JCI23006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwa S, Kannanganat S, Nigam P, Siddiqui M, Shetty RD, Armstrong W, Ansari A, Bosinger SE, Silvestri G, Amara RR. Plasmacytoid dendritic cells are recruited to the colorectum and contribute to immune activation during pathogenic SIV infection in rhesus macaques. Blood. 2011;118(10):2763–2773. doi: 10.1182/blood-2011-02-339515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Segeral E, Yatim A, Emiliani S, Schwartz O, Benkirane M. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature. 2011;474(7353):654–657. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, Reilly CS, Peterson ML, Schultz-Darken N, Brunner KG, Nephew KR, Pambuccian S, Lifson JD, Carlis JV, Haase AT. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim ES, Emerman M. HIV: going for the watchman. Nature. 2011;474(7353):587–588. doi: 10.1038/474587a. [DOI] [PubMed] [Google Scholar]
- Lore K. Isolation and immunophenotyping of human and rhesus macaque dendritic cells. Methods Cell Biol. 2004;75:623–642. doi: 10.1016/s0091-679x(04)75026-8. [DOI] [PubMed] [Google Scholar]
- Lore K, Smed-Sorensen A, Vasudevan J, Mascola JR, Koup RA. Myeloid and plasmacytoid dendritic cells transfer HIV-1 preferentially to antigen-specific CD4+ T cells. J Exp Med. 2005;201(12):2023–2033. doi: 10.1084/jem.20042413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Wu X, Lu Y, Guo W, Andrieu JM. Therapeutic dendritic-cell vaccine for simian AIDS. Nat Med. 2003;9(1):27–32. doi: 10.1038/nm806. [DOI] [PubMed] [Google Scholar]
- Lu W, Arraes LC, Ferreira WT, Andrieu JM. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat Med. 2004;10(12):1359–1365. doi: 10.1038/nm1147. [DOI] [PubMed] [Google Scholar]
- Malleret B, Karlsson I, Maneglier B, Brochard P, Delache B, Andrieu T, Muller-Trutwin M, Beaumont T, McCune JM, Banchereau J, Le Grand R, Vaslin B. Effect of SIVmac infection on plasmacytoid and CDlc+myeloid dendritic cells in cynomolgus macaques. Immunology. 2008a;124(2):223–233. doi: 10.1111/j.1365-2567.2007.02758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malleret B, Maneglier B, Karlsson I, Lebon P, Nascimbeni M, Perie L, Brochard P, Delache B, Calvo J, Andrieu T, Spreux-Varoquaux O, Hosmalin A, Le Grand R, Vaslin B. Primary infection with simian immunodeficiency virus: plasmacytoid dendritic cell homing to lymph nodes, type I interferon, and immune suppression. Blood. 2008b;112(12):4598–4608. doi: 10.1182/blood-2008-06-162651. [DOI] [PubMed] [Google Scholar]
- Manches O, Bhardwaj N. Resolution of immune activation defines nonpathogenic SIV infection. J Clin Invest. 2009;119(12):3512–3515. doi: 10.1172/JCI41509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl JN, Barry AP, Vanderford TH, Kozyr N, Chavan R, Klucking S, Barrat FJ, Coffman RL, Staprans SI, Feinberg MB. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat Med. 2008;14(10):1077–1087. doi: 10.1038/nm.1871. [DOI] [PubMed] [Google Scholar]
- Manel N, Hogstad B, Wang Y, Levy DE, Unutmaz D, Littman DR. A cryptic sensor for HIV-1 activates antiviral innate immunity in dendritic cells. Nature. 2010;467(7312):214–217. doi: 10.1038/nature09337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangeat B, Turelli P, Caron G, Friedli M, Perrin L, Trono D. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature. 2003;424(6944):99–103. doi: 10.1038/nature01709. [DOI] [PubMed] [Google Scholar]
- Martinelli E, Tharinger H, Frank I, Arthos J, Piatak M, Jr, Lifson JD, Blanchard J, Gettie A, Robbiani M. HSV-2 infection of dendritic cells amplifies a highly susceptible HIV-1 cell target. PLoS Pathog. 2011;7(6) doi: 10.1371/journal.ppat.1002109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx PA, Li Y, Lerche NW, Sutjipto S, Gettie A, Yee JA, Brotman BH, Prince AM, Hanson A, Webster RG, et al. Isolation of a simian immunodeficiency virus related to human immunodeficiency virus type 2 from a west African pet sooty mangabey. J Virol. 1991;65(8):4480–4485. doi: 10.1128/jvi.65.8.4480-4485.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlhop E, Villamide LA, Frank I, Gettie A, Santisteban C, Messmer D, Ignatius R, Lifson JD, Pope M. Enhanced in vitro stimulation of rhesus macaque dendritic cells for activation of SIV-specific T cell responses. J Immunol Methods. 2002;260(1–2):219–234. doi: 10.1016/s0022-1759(01)00544-0. [DOI] [PubMed] [Google Scholar]
- Melhem NM, Liu XD, Boczkowski D, Gilboa E, Barratt-Boyes SM. Robust CD4+ and CD8+ T cell responses to SIV using mRNA-transfected DC expressing autologous viral Ag. Eur J Immunol. 2007;37(8):2164–2173. doi: 10.1002/eji.200636782. [DOI] [PubMed] [Google Scholar]
- Messmer D, Ignatius R, Santisteban C, Steinman RM, Pope M. The decreased replicative capacity of simian immunodeficiency virus SIVmac239Delta(nef) is manifest in cultures of immature dendritic cells and T cells. J Virol. 2000;74(5):2406–2413. doi: 10.1128/jvi.74.5.2406-2413.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messmer D, Jacque JM, Santisteban C, Bristow C, Han SY, Villamide-Herrera L, Mehlhop E, Marx PA, Steinman RM, Gettie A, Pope M. Endogenously expressed nef uncouples cytokine and chemokine production from membrane phenotypic maturation in dendritic cells. J Immunol. 2002;169(8):4172–4182. doi: 10.4049/jimmunol.169.8.4172. [DOI] [PubMed] [Google Scholar]
- Miller CJ, Li Q, Abel K, Kim EY, Ma ZM, Wietgrefe S, La Franco-Scheuch L, Compton L, Duan L, Shore MD, Zupancic M, Busch M, Carlis J, Wolinksy S, Haase AT. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol. 2005;79(14):9217–9227. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milush JM, Stefano-Cole K, Schmidt K, Durudas A, Pandrea I, Sodora DL. Mucosal innate immune response associated with a timely humoral immune response and slower disease progression after oral transmission of simian immunodeficiency virus to rhesus macaques. J Virol. 2007;81(12):6175–6186. doi: 10.1128/JVI.00042-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir KD, Gasper MA, Sundaravaradan V, Sodora DL. SIV infection in natural hosts: resolution of immune activation during the acute-to-chronic transition phase. Microbes Infect. 2011;13(1):14–24. doi: 10.1016/j.micinf.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcock DR, Thomas JA, Gagliardi TD, Gorelick RJ, Roser JD, Chertova EN, Bess JW, Jr, Ott DE, Sattentau QJ, Frank I, Pope M, Lifson JD, Henderson LE, Crise BJ. Elimination of retroviral infectivity by N-ethylmaleimide with preservation of functional envelope glycoproteins. J Virol. 2005;79(3):1533–1542. doi: 10.1128/JVI.79.3.1533-1542.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muszil B, Sauermann U, Motzkus D, Stahl-Hennig C, Sopper S. Increased APOBEC3G and APOBEC3F expression is associated with low viral load and prolonged survival in simian immunodeficiency virus infected rhesus monkeys. Retrovirology. 2011;8(1):77. doi: 10.1186/1742-4690-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehete PN, Nehete BP, Manuri P, Hill L, Palmer JL, Sastry KJ. Protection by dendritic cells-based HIV synthetic peptide cocktail vaccine: preclinical studies in the SHIV-rhesus model. Vaccine. 2005;23(17–18):2154–2159. doi: 10.1016/j.vaccine.2005.01.052. [DOI] [PubMed] [Google Scholar]
- Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451(7177):425–30. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- Nigam P, Velu V, Kannanganat S, Chennareddi L, Kwa S, Siddiqui M, Amara RR. Expansion of FOXP3+ CD8 T cells with suppressive potential in colorectal mucosa following a pathogenic simian immunodeficiency virus infection correlates with diminished antiviral T cell response and viral control. J Immunol. 2010;184(4):1690–1701. doi: 10.4049/jimmunol.0902955. [DOI] [PubMed] [Google Scholar]
- Nigam P, Kwa S, Velu V, Amara RR. Loss of IL-17-producing CD8 T cells during late chronic stage of pathogenic simian immunodeficiency virus infection. J Immunol. 2011;186(2):745–753. doi: 10.4049/jimmunol.1002807. [DOI] [PubMed] [Google Scholar]
- O’Doherty U, Ignatius R, Bhardwaj N, Pope M. Generation of monocyte-derived cells from the precursors in rhesus macaque blood. J Immunol Methods. 1997;207:185–194. doi: 10.1016/s0022-1759(97)00119-1. [DOI] [PubMed] [Google Scholar]
- Patterson LJ. The “STEP-wise” future of adenovirus-based HIV vaccines. Curr Med Chem. 2011;18(26):3981–3986. doi: 10.2174/092986711796957211. [DOI] [PubMed] [Google Scholar]
- Patterson S, Rae A, Hockey N, Gilmour J, Gotch F. Plasmacytoid dendritic cells are highly susceptible to human immunodeficiency virus type 1 infection and release infectious virus. J Virol. 2001;75(14):6710–6713. doi: 10.1128/JVI.75.14.6710-6713.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira LE, Ansari AA. A case for innate immune effector mechanisms as contributors to disease resistance in SIV-infected sooty mangabeys. Curr HIV Res. 2009;7(1):12–22. doi: 10.2174/157016209787048465. [DOI] [PubMed] [Google Scholar]
- Peretti S, Shaw A, Blanchard J, Bohm R, Morrow G, Lifson JD, Gettie A, Pope M. Immunomodulatory effects of HSV-2 infection on immature macaque dendritic cells modify innate and adaptive responses. Blood. 2005;106:1305–1313. doi: 10.1182/blood-2004-12-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker LJ, Hansen SG, Lifson JD. New paradigms for HIV/AIDS vaccine development. Annu Rev Med. 2012;63:95–111. doi: 10.1146/annurev-med-042010-085643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope M, Elmore D, Ho D, Marx P. Dendritic cell-T cell mixtures, isolated from the skin and mucosae of macaques, support the replication of SIV. AIDS Res Hum Retroviruses. 1997;13:819–827. doi: 10.1089/aid.1997.13.819. [DOI] [PubMed] [Google Scholar]
- Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, Gordon MA, Kolls JK, Dandekar S, Baumler AJ. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14(4):421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves RK, Fultz PN. Disparate effects of acute and chronic infection with SIVmac239 or SHIV-89.6P on macaque plasmacytoid dendritic cells. Virology. 2007;365(2):356–368. doi: 10.1016/j.virol.2007.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves RK, Wei Q, Stallworth J, Fultz PN. Systemic dendritic cell mobilization associated with administration of FLT3 ligand to SIV- and SHIV-infected macaques. AIDS Res Hum Retroviruses. 2009;25(12):1313–1328. doi: 10.1089/aid.2009.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro Dos Santos P, Rancez M, Pretet JL, Michel-Salzat A, Messent V, Bogdanova A, Couedel-Courteille A, Souil E, Cheynier R, Butor C. Rapid dissemination of SIV follows multi-site entry after rectal inoculation. PLoS One. 2011;6(5):e19493. doi: 10.1371/journal.pone.0019493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice GI, Bond J, Asipu A, Brunette RL, Manfield IW, Carr IM, Fuller JC, Jackson RM, Lamb T, Briggs TA, Ali M, Gornall H, Couthard LR, Aeby A, Attard-Montalto SP, Bertini E, Bodemer C, Brockmann K, Brueton LA, Corry PC, Desguerre I, Fazzi E, Cazorla AG, Gener B, Hamel BC, Heiberg A, Hunter M, van der Knaap MS, Kumar R, Lagae L, Landrieu PG, Lourenco CM, Marom D, McDermott MF, van der Merwe W, Orcesi S, Prendiville JS, Rasmussen M, Shalev SA, Soler DM, Shinawi M, Spiegel R, Tan TY, Vanderver A, Wakeling EL, Wassmer E, Whittaker E, Lebon P, Stetson DB, Bonthron DT, Crow YJ. Mutations involved in Aicardi-Goutieres syndrome implicate SAMHD1 as regulator of the innate immune response. Nat Genet. 2009;41(7):829–832. doi: 10.1038/ng.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanghavi SK, Reinhart TA. Increased expression of TLR3 in lymph nodes during simian immunodeficiency virus infection: implications for inflammation and immunodeficiency. J Immunol. 2005;175(8):5314–5323. doi: 10.4049/jimmunol.175.8.5314. [DOI] [PubMed] [Google Scholar]
- Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, Zhang ZQ, Simon AJ, Trigona WL, Dubey SA, Huang L, Harris VA, Long RS, Liang X, Handt L, Schleif WA, Zhu L, Freed DC, Persaud NV, Guan L, Punt KS, Tang A, Chen M, Wilson KA, Collins KB, Heidecker GJ, Fernandez VR, Perry HC, Joyce JG, Grimm KM, Cook JC, Keller PM, Kresock DS, Mach H, Troutman RD, Isopi LA, Williams DM, Xu Z, Bohannon KE, Volkin DB, Montefiori DC, Miura A, Krivulka GR, Lifton MA, Kuroda MJ, Schmitz JE, Letvin NL, Caulfield MJ, Bett AJ, Youil R, Kaslow DC, Emini EA. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415(6869):331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- Siddiqui RA, Krawczak M, Platzer M, Sauermann U. Association of TLR7 variants with AIDS-like disease and AIDS vaccine efficacy in rhesus macaques. PLoS One. 2011;6(10):e25474. doi: 10.1371/journal.pone.0025474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smed-Sorensen A, Lore K. Dendritic cells at the interface of innate and adaptive immunity to HIV-1. Curr Opin HIV AIDS. 2011;6(5):405–410. doi: 10.1097/COH.0b013e328349b06b. [DOI] [PubMed] [Google Scholar]
- Soderlund J, Nilsson C, Lore K, Castanos-Velez E, Ekman M, Heiden T, Biberfeld G, Andersson J, Biberfeld P. Dichotomy between CD1a+and CD83+ dendritic cells in lymph nodes during SIV infection of macaques. J Med Primatol. 2004;33(1):16–24. doi: 10.1111/j.1600-0684.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- Soumelis V, Scott I, Gheyas F, Bouhour D, Cozon G, Cotte L, Huang L, Levy JA, Liu YJ. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood. 2001;98(4):906–912. doi: 10.1182/blood.v98.4.906. [DOI] [PubMed] [Google Scholar]
- Spira AI, Marx PA, Patterson BK, Mahoney J, Koup RA, Wolinsky SM, Ho DD. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med. 1996;183(1):215–225. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl-Hennig C, Steinman RM, Tenner-Racz K, Pope M, Stolte N, Matz-Rensing K, Grobschupff G, Raschdorff B, Hunsmann G, Racz P. Rapid infection of oral mucosal-associated lymphoid tissue with simian immunodeficiency virus. Science. 1999;285:1261–1265. doi: 10.1126/science.285.5431.1261. [DOI] [PubMed] [Google Scholar]
- Stahl-Hennig C, Eisenblatter M, Franz M, Stoiber H, Tenner-Racz K, Suh YS, Jasny E, Falkensammer B, Ugucchioni M, Georgsson G, Baroni C, Dierich MP, Lifson JD, Steinman RM, Uberla K, Racz P, Ignatius R. A single vaccination with attenuated SIVmac 239 via the tonsillar route confers partial protection against challenge with SIVmac 251 at a distant mucosal site, the rectum. Front Biosci. 2007;12:2107–2123. doi: 10.2741/2215. [DOI] [PubMed] [Google Scholar]
- Sui Y, Zhu Q, Gagnon S, Dzutsev A, Terabe M, Vaccari M, Venzon D, Klinman D, Strober W, Kelsall B, Franchini G, Belyakov IM, Berzofsky JA. Innate and adaptive immune correlates of vaccine and adjuvant-induced control of mucosal transmission of SIV in macaques. Proc Natl Acad Sci U S A. 2010;107(21):9843–9848. doi: 10.1073/pnas.0911932107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunseri N, O’Brien M, Bhardwaj N, Landau NR. HIV-1 modified to package SIV Vpx efficiently infects macrophages and dendritic cells. J Virol. 2011;85(13):6263–6274. doi: 10.1128/JVI.00346-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teleshova N, Jones J, Kenney J, Purcell J, Bohm R, Gettie A, Pope M. Short term Flt3L treatment effectively mobilizes macaque myeloid dendritic cells. J Leukoc Biol. 2004a;75:1102–1110. doi: 10.1189/jlb.1103588. [DOI] [PubMed] [Google Scholar]
- Teleshova N, Kenney J, Jones J, Marshall J, Van Nest G, Dufour J, Bohm R, Lifson JD, Gettie A, Pope M. CpG-C immunostimulatory oligodeoxyribonucleotide activation of plasmacy-toid dendritic cells in rhesus macaques to augment the activation of IFN-gamma-secreting simian immunodeficiency virus-specific T cells. J Immunol. 2004b;173(3):1647–1657. doi: 10.4049/jimmunol.173.3.1647. [DOI] [PubMed] [Google Scholar]
- Tenner-Racz K, Hennig CS, Uberla K, Stoiber H, Ignatius R, Heeney J, Steinman RM, Racz P. Early protection against pathogenic virus infection at a mucosal challenge site after vaccination with attenuated simian immunodeficiency virus. Proc Natl Acad Sci U S A. 2004;101(9):3017–3022. doi: 10.1073/pnas.0308677101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp S, Derby NR, Singer R, Shaw A, Williams VG, Turville SG, Bess JW, Jr, Lifson JD, Robbiani M. Double-stranded RNA analog poly(I:C) inhibits human immunodeficiency virus amplification in dendritic cells via type I interferon-mediated activation of APOBEC3G. J Virol. 2009;83(2):884–895. doi: 10.1128/JVI.00023-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turville SG, Arthos J, Cameron PU, Mac Donald K, Clark G, Hart DN, Cunningham AL. Bitter sweet symphony: defining the role of dendritic cell gp120 receptors in HIV infection. J Clin Virol. 2001a;22(3):229–239. doi: 10.1016/s1386-6532(01)00194-9. [DOI] [PubMed] [Google Scholar]
- Turville SG, Arthos J, Mac Donald K, Lynch G, Naif H, Clark G, Hart DN, Cunningham AL. HIV gp120 receptors on human dendritic cells. Blood. 2001b;98(8):2482–2488. doi: 10.1182/blood.v98.8.2482. [DOI] [PubMed] [Google Scholar]
- Turville SG, Santos JJ, Frank I, Cameron PU, Wilkinson J, Miranda-Saksena M, Dable J, Stossel H, Romani N, Piatak M, Jr, Lifson JD, Pope M, Cunningham AL. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood. 2004;103(6):2170–2179. doi: 10.1182/blood-2003-09-3129. [DOI] [PubMed] [Google Scholar]
- Turville SG, Peretti S, Pope M. Lymphocyte-dendritic cell interactions and mucosal acquisition of SIV/HIV infection. Curr Opin HIV AIDS. 2006;1(1):3–9. doi: 10.1097/01.COH.0000194109.14601.20. [DOI] [PubMed] [Google Scholar]
- Vagenas P, Aravantinou M, Williams VG, Jasny E, Piatak M, Jr, Lifson JD, Salazar AM, Blanchard JL, Gettie A, Robbiani M. A tonsillar PolyICLC/AT-2 SIV therapeutic vaccine maintains low viremia following antiretroviral therapy cessation. PLoS One. 2010;5(9):e12891. doi: 10.1371/journal.pone.0012891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, Stephens EB, Guatelli J. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe. 2008;3(4):245–252. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- Velu V, Kannanganat S, Ibegbu C, Chennareddi L, Villinger F, Freeman GJ, Ahmed R, Amara RR. Elevated expression levels of inhibitory receptor programmed death 1 on simian immunodeficiency virus-specific CD8 T cells during chronic infection but not after vaccination. J Virol. 2007;81(11):5819–5828. doi: 10.1128/JVI.00024-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villamide-Herrera L, Ignatius R, Eller M, Wilkinson K, Griffin C, Mehlhop E, Jones J, Han S-H, Lewis MG, Parrish S, VanCott TC, Lifson JD, Schlesinger S, Mascola JR, Pope M. Macaque dendritic cells infected with SIV-recombinant canarypox ex vivo induce SIV-specific immune responses in vivo. AIDS Res Hum Retroviruses. 2004;20:871–884. doi: 10.1089/0889222041725136. [DOI] [PubMed] [Google Scholar]
- Wagner GS, Miller CJ, McChesney MB. CD4+ T cells and monocytes elicited by immunization of rhesus monkeys with antigen-pulsed dendritic cells control SIV replication. AIDS Res Hum Retroviruses. 2002;18(2):143–148. doi: 10.1089/08892220252779683. [DOI] [PubMed] [Google Scholar]
- Wijewardana V, Soloff AC, Liu X, Brown KN, Barratt-Boyes SM. Early myeloid dendritic cell dysregulation is predictive of disease progression in simian immunodeficiency virus infection. PLoS Pathog. 2010;6(12):e1001235. doi: 10.1371/journal.ppat.1001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Bashirova AA, Martin TD, Villamide L, Mehlhop E, Chertov AO, Unutmaz D, Pope M, Carrington M, KewalRamani VN. Rhesus macaque dendritic cells efficiently transmit primate lentiviruses independently of DC-SIGN. Proc Natl Acad Sci U S A. 2002;99(3):1568–1573. doi: 10.1073/pnas.032654399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Wang X, Pahar B, Moroney-Rasmussen T, Alvarez X, Lackner AA, Veazey RS. Increased B7-H1 expression on dendritic cells correlates with programmed death 1 expression on T cells in simian immunodeficiency virus-infected macaques and may contribute to T cell dysfunction and disease progression. J Immunol. 2010;185(12):7340–7348. doi: 10.4049/jimmunol.1001642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Chen D, Konig R, Mariani R, Unutmaz D, Landau NR. APOBEC3B and APOBEC3C are potent inhibitors of simian immunodeficiency virus replication. J Biol Chem. 2004;279(51):53379–53386. doi: 10.1074/jbc.M408802200. [DOI] [PubMed] [Google Scholar]
- Zhang ZQ, Wietgrefe SW, Li Q, Shore MD, Duan L, Reilly C, Lifson JD, Haase AT. Roles of substrate availability and infection of resting and activated CD4+ T cells in transmission and acute simian immunodeficiency virus infection. Proc Natl Acad Sci U S A. 2004;101(15):5640–5645. doi: 10.1073/pnas.0308425101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wilson SJ, Landford WC, Virgen B, Gregory D, Johnson MC, Munch J, Kirchhoff F, Bieniasz PD, Hatziioannou T. Nef proteins from simian immunodeficiency viruses are tetherin antagonists. Cell Host Microbe. 2009;6(1):54–67. doi: 10.1016/j.chom.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer ML, Larregina AT, Castillo CM, Capuano S, Jr, Falo LD, Murphy-Corb M, Reinhart TA, Barratt-Boyes SM. Disrupted homeostasis of Langerhans cells and interdigitating dendritic cells in monkeys with AIDS. Blood. 2002;99(8):2859–2868. doi: 10.1182/blood.v99.8.2859. [DOI] [PubMed] [Google Scholar]