Abstract
Background
In the treatment of burns, patients’ own skin is the preferred material to cover burn wounds, resulting in the need to create a donor site wound. Enhancement of healing of the donor site wound would be beneficial in burn patients. Insulin, an anabolic agent, is routinely used to treat hyperglycemia after injury. We investigated whether intensive insulin treatment (INS) increases fractional synthesis rate (FSR) of the donor site wound protein and decreases the length of hospitalization normalized for total body surface area burned (LOS/TBSA).
Methods
FSR of the donor site wound protein was measured in pediatric patients randomized to control (CNT) (n = 13) and INS (n = 10) treatments. Depending on the postoperative day when the tracer study was done studies were divided into “Early” (days < 5) and “Late” (days >=5) periods.
Results
FSR of the donor site wound protein was greater in the INS group at the “Early” period of wound healing (CNT vs. INS, 8.2±3.8 vs. 13.1±6.9 %/day, p: < 0.05); but not at the “Late” (CNT vs. INS, 19.7±4.6 vs. 16.6±4.0 %/day, p > 0.05). Despite these differences LOS/TBSA was not decreased in the INS group. Correlation analyses demonstrated that independently of the treatment regimen FSR positively correlated (p < 0.05) with time post creation of the donor site and negatively correlated (p < 0.05) with LOS/TBSA.
Conclusions
Insulin treatment increased FSR of the donor site wound protein in the early period of wound healing; FSR correlated with LOS/TBSA independently of the treatment regimen.
Keywords: Burn, insulin treatment, donor site wound, protein synthesis, stable isotopes
INTRODUCTION
In thermal injury, open wounds of the skin provide a port of entry for systemic and wound pathogens and promote a hypermetabolic state. Early closure of the burn wound is a unique problem in the treatment of burn patients. The patients’ own skin is preferred to cover burn wounds1,2, however, in severe burns, availability of donor site for skin harvesting is limited, and frequently the same donor site is used multiple times. This approach leads to numerous cycles of harvesting of autografts from the same donor site. Therefore, enhancement of donor site wound healing would be beneficial for burn patients. The effects of different pharmaceutic agents to enhance the healing of donor site wounds have been tested3-6. Among them, insulin is of particular interest due to its common use during the acute period after burn injury and its anabolic features6-10.
Observational and immunohistochemical analysis demonstrated that high doses of insulin improve healing of donor site wounds and increase levels of laminin and type IV collagen in the wound5. Sakurai et al.6, using a stable isotope tracer technique, demonstrated that the fractional synthesis rate (FSR) of the donor site wound protein appeared to be 41 % greater in the intensive insulin treatment group compared to the control group, but the results did not reach statistical significance.
In the past several years, our group has extensively studied and reported on amino acid kinetics of skin and donor site wounds in animal models and humans. Using stable isotope tracer techniques, we demonstrated that in rabbits, FSR of the donor site wound protein increases over time, ranging from 6.6 to 20.5 %/day from postoperative days 3 to 7, respectively11. Another report from our group demonstrated that there is almost a 50-fold difference in protein FSR in dermal and epidermal layers of skin12. Recently, we measured the FSR of the donor site wound protein in burn-injured patients receiving standard of care therapy 13. In this work, there were two important findings: (1) FSR of the donor site protein significantly and positively correlated with time post donor site creation; and (2) in the early period postoperatively (days 2-4) but not the late period (days 5-6) FSR of the donor site protein significantly correlated with a clinically important outcome – length of acute hospitalization normalized for total body surface area burned (LOS/TBSA)13. The first finding was consistent with our previous data from an animal model11. The second finding was a surprise but was logically acceptable because the faster wounds heal, the faster patients are discharged from a hospital. But a question why the correlation was significant in days 2-4 but not 5-6 was raised. We have speculated that this finding was probably related to distinct phases of skin wound healing11-15.
Based on these data, we speculated that the beneficial effect of intensive insulin treatment on the FSR of the donor site wound protein may be detectable in the early period of wound healing (days 2-4) but not the late period (days 5-6). Additionally, we aimed to determine if FSR of the donor site wound protein increases over time postoperatively and if there is a correlation between the FSR of the donor site wound protein and LOS/TBSA in patients receiving intensive insulin treatment similarly to the patients receiving standard of care therapy13.
METHODS
Patients
The study was conducted at the Shriners Hospital for Children in Galveston, TX, as a part of an ongoing research project between September 2006 and July 2008. The study protocol was approved by the Institutional Review Board of the University of Texas Medical Branch. Informed child assent and/or parental permission were obtained from each patient and parent or guardian before study enrollment. Patients under 18 years, either gender, and with greater than 30 % total body surface area (TBSA) burns requiring skin grafting were eligible for the study.
Immediately after admission, patients were randomized to receive standard of care or intensive insulin treatment. Because of the potential risk for hypoglycemia with insulin treatment, blinding between patient groups was not possible. Drug treatment was continued throughout the entire hospital stay until patients were discharged from the intensive care unit (ICU). A continuous infusion of recombinant human insulin (Nobolin, Novo Nordisk, Copenhagen, Denmark) titrated to glucose level was administered by the use of a constant infusion pump (IMED, San Diego, CA). In the control group, insulin infusion was started only if the blood glucose exceeded 220 mg/dL and the infusion was adjusted to maintain the serum blood glucose level at a value between 160–180 mg/dL. We chose blood glucose of 160-180 mg/dL in the control group because protein glycolysation occurs at 150-160 mg/dL, therefore we wanted to minimize patient risk with detectable differences in glucose between the groups. In the insulin treatment group, the insulin infusion started at 0.1 U/kg/hr; if the blood glucose level exceeded 110 mg/dL the infusion was adjusted to maintain euglycemia at 80–110 mg/dL. Adjustments of the insulin dose was based on measurements of whole blood glucose in undiluted arterial blood performed at 15 minute to 2 hour intervals with the use of a glucose analyzer (AccuCheck Advantage, Roche Diagnostics, Indianapolis, IN). The dose was adjusted according to a sliding scale by our team of intensive care nurses assisted by a study physician and the physicians involved in the clinical care of the patients.
By the time of the tracer study, the patients were on a steady state of enteral feeding of Vivonex R.T.F. or T.E.N. (Sandoz Nutritional Corp., Minneapolis, MN) at a rate of 1.2 times their resting energy expenditure (REE) as determined by weekly indirect calorimetry. 250 ml of Vivonex R.T.F. is composed of 12.5 g of proteins (as amino acids), 43.8 g of carbohydrates and 2.9 g of fat. 300 ml of Vivonex T.E.N. is composed of 11.5 g of proteins (as amino acids), 61.7 g of carbohydrates and 0.8 g of fat. One ml of nutrition contains one kcal of energy.
Medical care was determined by faculty surgeons, fellows and residents according to clinical protocols that have been previously described16. The patients received nutritional supplements including a multi-vitamin, folic acid, zinc and vitamin C.
During burn wound autografting surgery, skin was harvested using an electric dermatome set at 0.008-0.012 inches (Zimmer Inc, Warsaw, IN). The donor site was dressed with Scarlet Red (Sherwood Medical, St. Louis, MO) impregnated fine mesh gauze. On 2 to 6 days after their grafting surgery the subjects underwent a 5-hour stable isotope infusion study with ongoing feeding of Vivonex as described above. The amount of Vivonex given during the tracer study was recorded in order to calculate the amount of nutrients (e.g., amino acids, fat, carbohydrates) given for the duration of the study. Amount of nutrients was calculated based on the nutritional composition of Vivonex and expressed in grams of nutrients per weight per hour (g/kg/hr).
The stable isotope tracer study consisted of a primed (6.4 μmol/kg) continuous (0.16 μmol/kg/min) infusion of L-[ring-2H5]-phenylalanine (Cambridge Isotopes, Andover, MA), as previously described13. Punch biopsies of the donor site wound were obtained at 120 and 300 min after initiation of the tracer infusion. The timing for the infusion study was coordinated with clinically required graft staple removal procedure. Therefore, patients were given clinically necessary Midazolam (0.5 mg/kg, PO) and Ketamine (1 mg/kg, IV) and subcutaneous injection of 1% Lidocaine prior to obtaining tissue biopsy followed by staple removal procedure. Donor site wound biopsies were obtained using 3 mm biopsy punch needles (Sklar Tru-Punch, Sklar Inst., West Chester, PA). The samples were immediately frozen in liquid nitrogen for further analysis.
Serum insulin values were measured using ELISA kit (Diagnostic Systems Labs, Beckman Coulter, Webster, TX), according to manufacturer’s instructions.
The primary outcome of the study was to determine if intensive insulin treatment during acute hospitalization increases FSR of the donor site wound protein in burn injured patients. A secondary outcome was to determine if there was a correlation between FSR and LOS/TBSA or time postoperatively in burn injured patients undergoing intensive insulin treatment. Patients are normally discharged from the ICU when 95 % of initial total burn surface area has healed and there is no evidence of medical complications or general health conditions that require continued ICU care.
Sample processing
Twenty to twenty-five mg of wound was homogenized twice in 800 μL of 10% perchloric acid. The free intracellular enrichment of phenylalanine was measured from the supernatant after tissue homogenization and centrifugation. The bound phenylalanine incorporated into the protein of the wound was measured from the pellet obtained after centrifugation. The pellet was washed and dried, and the proteins were hydrolyzed in 6 N HCl at 110°C for 24 hours17. Amino acids were extracted with cation-exchange chromatography, and enrichment was determined after derivatization to tert-butyldimethylsilyl by gas chromatography-mass spectrometry (GC-MS HP 5989; Hewlett-Packard, Palo Alto, CA) with electron impact ionization. Ions 234 and 239 were monitored17.
Calculations
FSR calculations were based on the precursor-product method, using intracellular free and bound protein tracer enrichments in the wound biopsy as precursor and product, respectively. The following equation17 was used for the calculations:
where Ef and Es are the enrichments of free and bound amino acid respectively, and t is the time interval in min between two sequential biopsies. FSR was expressed in percent per day (%/day).
Statistics
Values are presented Mean±SE, unless otherwise stated. Student’s t test was used to analyze the difference in FSR of the donor site wound protein between the groups. Correlation analyses were performed by examining the Pearson Product moment. Multiple regression analysis was used to determine the relationship of the FSR of the donor site wound protein with other variables such as postoperative day when the infusion study was conducted and LOS/TBSA. Fisher exact test was used to evaluate the difference in gender distribution between the groups. A p value less than 0.05 was considered statistically significant.
RESULTS
During the study period there were 113 patients admitted to the hospital and eligible for the study. Of these patients, 93 patients were admitted within 7 days after the injury (acute admission). Of these 93 patients, 34 patients were randomized into the Control (CNT) (n = 21) and Insulin (INS) (n = 13) treatment groups and the others were randomized into other treatments. Of these 34 patients, 1 patient in each group died prior to tracer infusion study scheduling. Of the remaining 32 patients (CNT – 20, INS – 12), 5 patients in the CNT did not participate in the stable isotope tracer infusion study due to the lack of donor site wound. In total, 15 and 12 patients in the CNT and INS groups participated in the tracer infusion study. Out of these 27 patients, data from 2 patients in each group were excluded due to technical problems. Therefore, we are presenting data from 13 and 10 patients in the CNT and INS groups, respectively. The patients in the CNT group received the standard of care treatment only. The patients in the INS group received the standard of care plus intensive insulin treatment. None of these patients received any kind of anabolic investigational agents including oxandrolone, propranolol or recombinant human growth hormone.
There were no differences in demographics between the groups (Table 1). There were 5 girls and 8 boys in the CNT group and one girl and 9 boys in the INS group (p = 0.2). Inclusion of gender in correlation analysis as an independent variable demonstrated that gender did not have an effect on any of analyses (data are not presented). There was no difference in length of hospitalization normalized for total body surface area burned (LOS/TBSA) between the groups (Table 1).
Table 1.
Demographics and parameters. Data presented as Mean±SD. TBSA, total body surface area; LOS, length of stay.
| Parameter | Control | Insulin | p value |
|---|---|---|---|
| Number of patients | 13 | 10 | |
| Number of studies presented | 19 | 17 | |
| Age, years | 8±6 | 10±5 | 0.3 |
| Sex (F/M) | 5/8 | 1/9 | 0.2 |
| Weight during the study, kg | 39±26 | 36±18 | 0.9 |
| TBSA burned (%) | 56±12 | 57±13 | 0.9 |
| Third degree (%) | 43±19 | 45±13 | 0.8 |
| Delay in admission (days) | 2±1 | 2±2 | 0.7 |
| Insulin started day (days after admission) | 7±5 | 1±1 | 0.01 |
| Tracer study day (days after admission) | 14±8 | 12±5 | 0.4 |
| LOS (days) | 28±15 | 28±12 | 0.9 |
| LOS/TBSA | 0.50±0.19 | 0.47±0.12 | 0.7 |
Data from nineteen and seventeen tracer infusion studies from the CNT and INS groups, respectively, are presented. In the CNT group, 12 patients had measurements in the early period of wound healing and 5 had measurements in the late period of wound healing (some patients were studied at two different harvesting periods). Of the 10 patients in the INS group, 7 had measurements in the early period and 4 had measurements in the late period.
In INS group intensive insulin treatment was started 1±1 (Mean±SD) days after admission. In CNT group clinically required insulin treatment to maintain glucose levels of 160-180 mg/dl was started 7±5 (Mean±SD) days after admission (Table 1). Average amount of infused insulin was significantly greater in the INS group (CNT vs. INS, 22±10 vs. 117±12 IUnits/day, p < 0.05). Plasma daily (CNT vs. INS, 155±6 vs. 127±3 mg/dl, p < 0.05) or morning (CNT vs. INS, 150±6 vs. 120±3 mg/dl, p < 0.05) glucose values were significantly lower in the INS group.
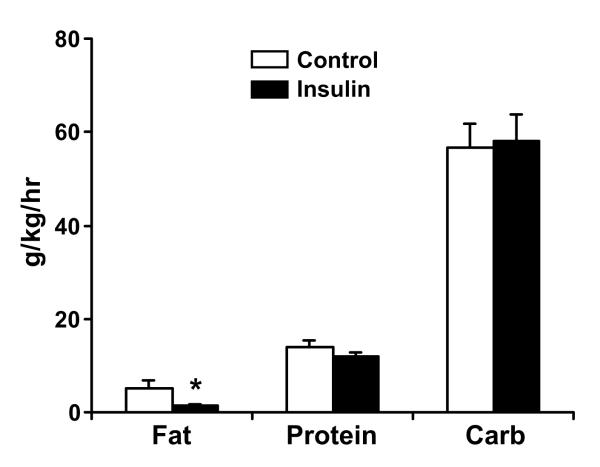
The average amount of feeding given during the tracer studies was not different between the groups (CNT vs. INS, 455±216 vs. 453±148 ml, Mean±SD, p > 0.05). Average amounts of carbohydrates and amino acids given during the tracer study were not different between the groups (Fig. 1). The average amount of fat was significantly higher (p < 0.05) in the CNT group (Fig. 1), however there was no correlation between the amount of fat given during the study and FSR (data are not presented).
Figure 1.
Average amounts of fat, carbohydrates (carb) and protein (as amino acids) given during stable isotope studies, expressed in g/kg weight/hr. Data are presented Mean±SE. *, p < 0.05, Control vs. Insulin group.
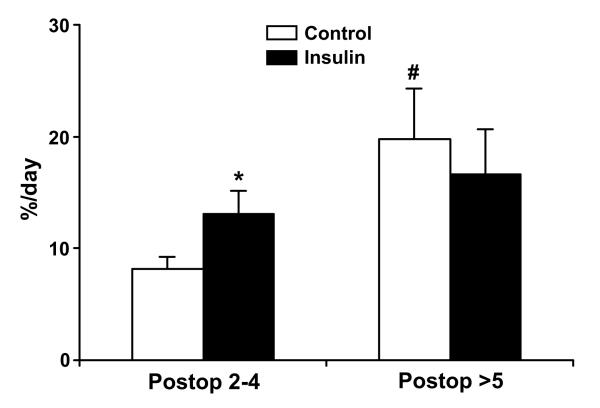
The stable isotope tracer infusion studies were conducted on average 4±1 (Mean±SD) days after grafting surgery in either group. The data for the FSR of the donor site wound are presented in Figure 2. FSR data analysis were performed separating results into “Early” and “Late” periods, based on the postoperative day when the metabolic study was performed. In the “Early” period the metabolic studies were performed at 2 to 4 days postoperatively, and in the “Late” period studies were done on postoperative days 5 and 6. In the “Early” period intensive insulin treatment significantly increased the FSR of the donor site wound protein (p < 0.05). This effect disappeared in the “Late” period and there was no significant difference in FSR between the two groups. Within the CNT group, the FSR of the wound protein was significantly higher (p < 0.05) in the “Late” period than in the “Early” period.
Figure 2.
Fractional synthesis rate (FSR) of donor site wound protein, %/day. Data are presented Mean±SD. *, p < 0.05, Control vs. Insulin group; #, p < 0.05, Late vs. Early period within the group.
The thigh was the location of donor site in 70 and 65 % of studies in the CNT and INS groups, respectively. Other locations were abdomen, shoulder and lower leg. There was no correlation between the location of the donor site and FSR.
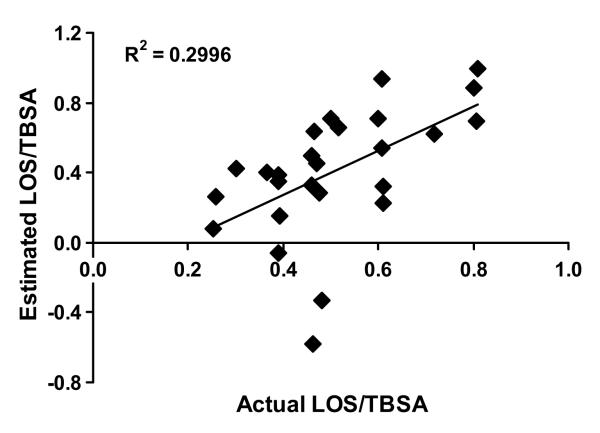
Independently of the treatment regimen, the FSR of the donor site wound protein significantly correlated (p < 0.05) with time post donor site creation and LOS/TBSA. Based on these data, we run multiple regression analysis to determine the relationship between the FSR of the donor site wound protein , time post surgery and LOS/TBSA. Based on data from the multiple regression analysis, we derived the following formula to calculate estimated
LOS/TBSA
Estimated LOS/TBSA = (FSR – 13.53 − 0.87 × Postoperative day) / (− 15.9). Using this equation, we calculated estimated values of LOS/TBSA for our study subjects and thereafter ran correlation analysis versus actual LOS/TBSA (Fig. 3). Correlation analysis demonstrated that the FSR of the donor site wound (corrected for the postoperative day when the study was conducted) accounted for 30 % of the variability (r2 = 0.299) in the actual LOS/TBSA. The linear regression analysis demonstrated a significant relationship (p < 0.05) between estimated and actual LOS/TBSA.
Figure 3.
Correlation analysis of estimated LOS/TBSA (length of acute hospitalization normalized for total burn surface area burned) versus actual LOS/TBSA for all studies in “Early” period. Estimated LOS/TBSA was calculated by the following formula derived from multiple linear regression analysis to determine the relationship between fractional synthesis rate (FSR) of donor site wound and postoperative day when the metabolic study was conducted and actual LOS/TBSA: Estimated LOS/TBSA = (FSR – 13.53 − 0.87 × Postoperative day) / (− 15.9). The linear regression analysis demonstrated a significant relationship (p < 0.05) between estimated and actual LOS/TBSA.
DISCUSSION
Early closure of the burn wound is a unique problem in the treatment of burn patients. Presently, the patients’ own skin is preferred to cover burn wounds. However, as a result a donor site wound is created. A donor site wound by itself is another breach in the integrity of skin in burn patients. Furthermore, multiple grafting surgeries may be required in major burns, which may lead to multiple harvesting from the same donor site. Therefore, enhancement of donor site wound healing is beneficial for burn patients. The effect of insulin (INS) treatment on donor site wound healing is of particular interest due to its common use during the acute period after burn injury and its anabolic features6-9.
Observational and immunohistochemical analysis demonstrated that high doses of INS improve donor site wound healing5. Stable isotope tracer techniques enable the quantitative measurement of protein turnover rates, i.e., synthesis and breakdown. Using the tracer incorporation method, Sakurai et al.6 demonstrated that intensive INS treatment increased the FSR of the wound protein by 41 % compared to the control group, but the results did not reach statistical significance.
Recently, we measured the FSR of the donor site wound protein in burn-injured patients receiving standard of care therapy13. We drew two basic conclusions from this study: (1) the FSR of the donor site protein significantly and positively correlated with time post donor site creation; (2) in the early period postoperatively (days 2-4) but not in the late period (days 5-6) the FSR of the donor site wound protein significantly correlated with a clinically important outcome - length of hospitalization normalized for total body surface area burned (LOS/TBSA)13. The first finding was consistent with the data of Zhang et al.11 obtained from an animal model. The second finding was a surprise, but we speculated that the difference between the early and late periods was probably related to distinct phases of skin wound healing and existence of dermal and epidermal layers in skin11-14. Nevertheless, our conclusion from that work was that the synthesis of dermal and not epidermal protein was the clinically important parameter in burns with regard to length of stay13. Based on these data, we have studied the effect of intensive INS treatment on synthesis of donor site wound protein at the early (days 2-4) and the late (days 5-6) period of wound healing.
The main result of this study was that intensive INS treatment significantly increased donor site wound protein synthesis in the early but not the late period of wound healing following burn injury. This result was in accordance with our hypothesis that the effect of intervention on donor site wound protein metabolism was distinguishable at the early period of wound healing. The absence of the effect of INS on the FSR of the donor site wound protein in the later period suggests that the wound protein turnover was already at its maximal level without intensive INS treatment. In a previous experiment we classified the seventh day postoperatively as the flow phase of wound metabolism11, which was characterized by a 4-fold increase in the synthesis of wound proteins. The intensive INS treatment was not able to further stimulate the already increased protein synthesis in the flow phase. However, the anabolic effect of INS might be manifested by an improved net balance in this later period of wound healing. We have reported that acute INS infusion with amino acids increased the net balance of skin wound proteins at the seventh day postoperatively without detectable changes in synthesis or breakdown rate9. Usually this type of partial thickness donor wound heals by the seventh day postoperatively5, meaning that the epidermal layer is developed. Although there is rapid turnover of proteins for matrix formation and remodeling of the wound area, a further increase in protein synthetic rate would not be a biological priority. On the other hand, INS stimulation of wound protein deposition would favor the deposition and maturation of dermal collagen. Therefore, it is logically meaningful that INS stimulates the FSR of the donor site wound protein only during the earlier period of wound healing.
There were two different types of Vivonex used in these studies, namely R.T.F. and T.E.N. This was due to the fact that when the study was conducted, R.T.F. was being replaced by T.E.N. in our clinical settings. The average amount of fat (but not carbohydrates and amino acids) given during the tracer infusion study was significantly higher in the CNT group. This is explained by usage of Vivonex R.T.F. in 60 % of studies in CNT group compared to 30 % in INS group. However, inclusion of the type of Vivonex or the amount of infused fat as independent variables in the correlation analysis demonstrated no change in the conclusions of the study (data are not presented).
Previously, we have shown that intensive insulin treatment significantly decreases LOS/TBSA at acute admission, from 1.03±0.1 in the CNT group to 0.7±0.9 in the INS group7. In the current work, we hypothesized that LOS/TBSA would be decreased in the INS group. However, we found that LOS/TBSA was comparable between the groups (Table 1). The LOS/TBSA in the CNT group in the current study was almost half of the LOS/TBSA from the previous study7, which is consistent with a recent American Burn Association report that there is a downward trend in US hospital stay18. The difference in the LOS/TBSA of the CNT groups of the previous and current studies might explain the difference in the effects of INS on LOS/TBSA. Unfortunately, we do not have a solid explanation for why we did not detect decreased LOS with increased FSR in the INS group. A power analysis based on the obtained results of this study demonstrated that 613 patients would be needed to detect a statistically significant difference in LOS/TBSA between CNT and INS with power = 0.8 and alpha = 0.05. We note, though, that a correlation between two variables does not mean that there is a clear-cut cause and effect relationship between the variables. It would seem that insulin treatment increases FSR in these patients via a mechanism that unfortunately does not also decrease LOS. We can only speculate why this would be the case, but perhaps the rate of wound healing is no longer the determinant of LOS once LOS is lowered to a certain point, when other factors such as presence of infection come into play.
We derived a formula to calculate estimated LOS/TBSA for our study subjects based on a multiple linear regression analysis that evaluated the relationship between the FSR of the donor site wound protein, the postoperative day when the metabolic study was conducted, and the LOS/TBSA. The linear regression analysis demonstrated a significant relationship (p < 0.05) between estimated and actual LOS/TBSA, with r2 = 0.299, demonstrating that the FSR of the donor site wound protein corrected for the postoperative day accounted for 30 % of the variability of the actual LOS/TBSA. However, this correlation coefficient was much lower than the number we derived for the CNT group in our previous work13. Analysis of the current results demonstrated that the estimated LOS/TBSA was a negative number for three studies in the INS group (i.e. −0.06, −0.33 and –0.58 days/TBSA). These negative numbers were due to the high FSR values in those three studies (18.01, 21.4 and 26.4 %/day, respectively). LOS/TBSA cannot be a negative number. When we excluded these results from correlation analysis, r2 equaled 0.51. Sensitivity analysis for the model suggested that if the FSR of the wound protein was higher than 16 %/day, then the model calculated a negative estimated LOS/TBSA. The regression model was designed for the studies conducted at the early period of wound healing (days 2-4), but values of FSR higher than 16 %/day seem to belong to the later period results (days 5-6)13, or when the wound is mostly regenerated. These two factors may explain why the model fails when FSR values are high.
In conclusion, intensive insulin treatment increases the synthesis rate of donor site wound proteins in the early period postoperatively of wound healing. In addition, the synthesis rate of donor site wound protein increases over time after the creation of the donor site of the wound and correlates with LOS/TBSA, independently of therapeutic intervention.
Acknowledgements
The authors wish to thank the study volunteers and their families for their patience and dedication, and the clinical and research staff of Shriners Hospital for Children, Galveston, TX for their help with conducting the clinical portion of the study; Tara Cocke, Christopher Danesi, Ann Hightower and Cindy Locklin for excellent technical performance, and Guy Jones and Jariwala Guarang for performing GC-MS analyses. Authors also thank Steve Schuenke for an excellent technical assistance in the preparation of this manuscript.
Grant support: NIGMS T32-GM08256, NIGMS P50-GM60338, NIGMS R01-GM56687, NIDRR H133A020102 and Shrine grants 84090, 86060, 87060, 85070, 86000.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kogan L, Govrin-Yehudain J. Vertical (two-layer) skin grafting: new reserves for autologic skin. Ann Plast Surg. 2003;50:514–6. doi: 10.1097/01.SAP.0000044150.03940.4E. [DOI] [PubMed] [Google Scholar]
- 2.Archer SB, Henke A, Greenhalgh DG, Warden GD. The use of sheet autografts to cover extensive burns in patients. J Burn Care Rehabil. 1998;19:33–8. doi: 10.1097/00004630-199801000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Herndon DN, Barrow RE, Kunkel KR, Broemeling L, Rutan RL. Effects of recombinant human growth hormone on donor-site healing in severely burned children. Ann Surg. 1990;212:424–9. doi: 10.1097/00000658-199010000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilpin DA, Barrow RE, Rutan RL, Broemeling L, Herndon DN. Recombinant human growth hormone accelerates wound healing in children with large cutaneous burns. Ann Surg. 1994;220:19–24. doi: 10.1097/00000658-199407000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pierre EJ, Barrow RE, Hawkins HL, Nguyen TT, Sakurai M, Desai A, et al. Effects of insulin on wound healing. J Trauma. 1998;44:342–5. doi: 10.1097/00005373-199802000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Sakurai Y, Aarsland A, Herndon DN, Chinkes DL, Pierre E, Nguen TT, et al. Stimulation of muscle protein synthesis by long-term insulin infusion in severely burned patients. Ann Surg. 1995;222:283–97. doi: 10.1097/00000658-199509000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas SJ, Morimoto K, Herndon DN, Ferrando AA, Wolfe RR, Klein GL, et al. The effect of prolonged euglycemic hyperinsulinemia on lean body mass after severe burn. Surgery. 2002;132:341–7. doi: 10.1067/msy.2002.126871. [DOI] [PubMed] [Google Scholar]
- 8.Zhang XJ, Chinkes DL, Wolf SE, Wolfe RR. Insulin but not growth hormone stimulates protein anabolism in skin wound and muscle. Am J Physiol Endocrinol Metab. 1999;276:E712–20. doi: 10.1152/ajpendo.1999.276.4.E712. [DOI] [PubMed] [Google Scholar]
- 9.Zhang XJ, Chinkes DL, Irtun Ø , Wolfe RR. Anabolic action of insulin on skin wound protein is augmented by exogenous amino acids. Am J Physiol Endocrinol Metab. 2002;282:E1308–15. doi: 10.1152/ajpendo.00361.2001. [DOI] [PubMed] [Google Scholar]
- 10.Hemmila MR, Taddonio MA, Arbabi S, Maggio PM, Wahl WL. Intensive insulin therapy is associated with reduced infectious complications in burn patients. Surgery. 2008;144:629–37. doi: 10.1016/j.surg.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang XJ, Chinkes DL, Cox RA, Wolfe RR. The flow phase of wound metabolism is characterized by stimulated protein synthesis rather than cell proliferation. J Surg Res. 2006;135:61–7. doi: 10.1016/j.jss.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Zhang XJ, Chinkes DL, Wolfe RR. Measurement of protein metabolism in epidermis and dermis. Am J Physiol Endocrinol Metab. 2003;284:E1191–01. doi: 10.1152/ajpendo.00460.2002. [DOI] [PubMed] [Google Scholar]
- 13.Tuvdendorj D, Chinkes DL, Zhang XJ, Aarsland A, Herndon DN. Donor site wound protein synthesis correlates with length of acute hospitalization in severely burned children. Wound Repair Regener. 2010;18:277–83. doi: 10.1111/j.1524-475X.2010.00584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viziam CB, Matoltsy AG, Mescon H. Epithelialization of small wounds. J Invest Dermat. 1964;43:499–507. [PubMed] [Google Scholar]
- 15.Odland G, Ross R. Human wound repair. J Cell Biol. 1968;39:135–151. doi: 10.1083/jcb.39.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pereira C, Murphy K, Jeschke M, Herndon DN. Post burn muscle wasting and the effects of treatments. Int. J. Biochem. Cell Biol. 2005;37:1948–61. doi: 10.1016/j.biocel.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Wolfe RR, Chinkes DL. Isotope tracers in metabolic research. John Wiley & Sons, Inc.; New Jersey: 2005. publication. [Google Scholar]
- 18.Miller S, Jeng J, Bessey P, Caruso D, Gomez M, Kagan R, et al. National Burn Repository. (version 2) 2005 Report. www.ameriburn.org/NBR2005.pdj.