Abstract
Helicobacter pylori induces less gastric inflammation in children than adults. Here we investigated whether this reduced inflammation involves dysregulated Th17 responses. H. pylori-infected children and adults in Santiago, Chile had similar levels of H. pylori colonization, proportions of bacteria containing cagA and s1/s2 vacA markers of virulence and strain genotypes (predominantly hpEurope), but the children had significantly reduced levels of gastric inflammation and neutrophil infiltration. The reduced neutrophil accumulation in infected children was accompanied by significantly fewer gastric Th17 cells and significantly lower levels of IL-17-specific mRNA and protein compared to infected adults. The gastric mucosa of H. pylori-infected children also contained higher numbers of IL-10+ cells and increased levels of both IL-10 and Foxp3 mRNA compared to that of infected adults. Thus, reduced gastric inflammation, including diminished neutrophil accumulation, in H. pylori-infected children compared with infected adults is likely due to down-regulated gastric Th17/IL-17 responses as a consequence of enhanced mucosal regulatory T cell activity in the children.
INTRODUCTION
The inflammation associated with H. pylori infection is mediated by gastric mucosal T helper 1 (Th1) cells through the release of IFN-γ, based on studies in animal models1–3 and adult humans4–8. Study of the infection in adult subjects addresses late H. pylori-induced mucosal responses, whereas the study of H. pylori infection in children, which has received little investigative attention, offers the opportunity to investigate early mucosal responses to the bacteria. To begin to elucidate the early, possibly age-related, mucosal events in H. pylori infection, we8, 9 and others10 have shown that childhood H. pylori infection is associated with significantly reduced levels of gastric inflammation and ulceration compared to adults. We also have shown that the gastric mucosa of H. pylori-infected children contains increased numbers of regulatory T (Treg) cells compared to the mucosa of infected adults8, suggesting Treg participation in the reduced Th1-mediated gastritis and ulceration in the children. In this connection, emerging evidence suggests that Treg-mediated immune regulation in humans may contribute to H. pylori persistence11, 12 and that inadequate or absent Treg responses in adult humans and mice are associated with increased mucosal inflammation during H. pylori infection12–15.
Recent studies also have shown that the levels of IL-17 are increased in the gastric mucosa of mice infected with H. pylori16–18, suggesting Th17 cells participate in H. pylori gastritis, at least in mice. Indeed, IL-17 is one of the earliest cytokines detected in the gastric mucosa of H. pylori-infected mice17. IL-17 mediates the recruitment and activation of polymorphonuclear neutrophils, a key cellular element in the inflammatory lesion associated with H. pylori infection19. Here we extend our study of the immunopathogenesis of H. pylori gastritis in children as a window into the early cellular responses to the infection by investigating gastric Th17 responses in H. pylori-infected children and adults living in Chile, where H. pylori is endemic.
RESULTS
Reduced levels of gastric inflammation in H. pylori-infected children compared with infected adults
To elucidate the immune mechanisms that initiate inflammation in early H. pylori infection, we first evaluated gastric tissue specimens from children and adults with or without H. pylori infection for inflammation. H. pylori infection was associated with gastric inflammation in both children and adults compared to their non-infected peers (each P=0.0002) (Figure 1a). However, the mean level of inflammation in the infected children was significantly less compared to that of the infected adults (P=0.002), based on histologic evaluation of coded biopsy specimens using the modified Sydney classification. The same biopsy specimens were evaluated for H. pylori colonization. The mean level of H. pylori colonization in the gastric mucosa of the children and adults was nearly identical (Figure 1b), despite the reduced level of gastritis in the children. Importantly, at each level of bacterial colonization, H. pylori-infected children displayed a reduced histology score compared to that of infected adults (P=0.018) (Figure 1c), indicating that the reduced mean histology score in the children (Figure 1a) was not the consequence of a disproportionally lower amount of inflammation in the children or an excessively higher amount of inflammation in the adults for any single level of colonization. Rather, H. pylori induced a proportionally lower inflammatory response in children compared to adults at each level of colonization. In addition, the amount of neutrophil infiltration into the gastric mucosa, based on the neutrophil score, was reduced at each level of colonization (P=0.01) (Figure 1d).
Figure 1.
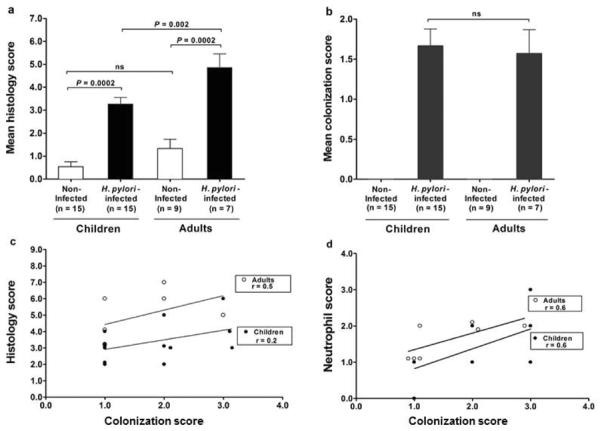
Levels of gastric inflammation and H. pylori colonization in children and adults. (a) H. pylori induced inflammation in the gastric mucosa of both children and adults, but inflammation was significantly less severe in the infected children. (b) Levels of H. pylori colonization in gastric mucosa were similar in infected children and adults. Levels of gastric inflammation and colonization are presented as the histology score and colonization score (mean +/− SEM), respectively (see Materials and Methods). H. pylori-infected children had reduced levels of (c) gastric inflammation based on the histology score and (d) neutrophil infiltration based on the neutrophil score compared to infected adults. (For comparison of lines in c P=0.018 and d P=0.01).
H. pylori from infected children and adults display similar genetic features
To determine whether the reduced inflammation in H. pylori-infected children could reflect infection by less virulent bacteria, we characterized the H. pylori isolated from 12 of the 15 infected children and all 7 of the infected adults for the presence of cagA and vacA alleles and for phylogeographic origin. Among these isolates, the proportion of bacteria that carried cagA and vacA (type s1/s2) markers of virulence was similar in infected children and adults (P=0.2 and 0.1, respectively) (Table 1). All of the strains containing s1 forms of vacA were classified as subtype s1b. H. pylori isolated from the children and adults also were subjected to nucleotide sequence analysis of the urel, muty, efp, ppa, atpA and trpC loci and then compared to 331 reference strains to assess phylogenetic similarity. As shown in the phylogenetic tree (Figure 2), all 12 of the isolates from children and 5 of the 7 isolates from adults were of European origin20,21, suggesting that hpEurope is the predominant H. pylori genotype present in this region of Chile. Thus, the Chilean children and adults in our study were colonized by H. pylori with similar cagA and vacA profiles and predominantly of the hpEurope genotype, indicating that host, not bacterial, factors contribute to the reduced gastritis in children.
Table 1.
H. pylori virulence factors
| Source of H. pylori |
|||
|---|---|---|---|
| Children | Adults | P value | |
| n=12 | n=7 | ||
| CagA, n (%) | 11 (92) | 5 (71) | 0.2 |
| VacA S alleles | 0.1 | ||
| s1 allele, n (%) | 4 (33) | 5 (71) | |
| s2 allele, n (%) | 8 (66) | 2 (29) | |
Figure 2.
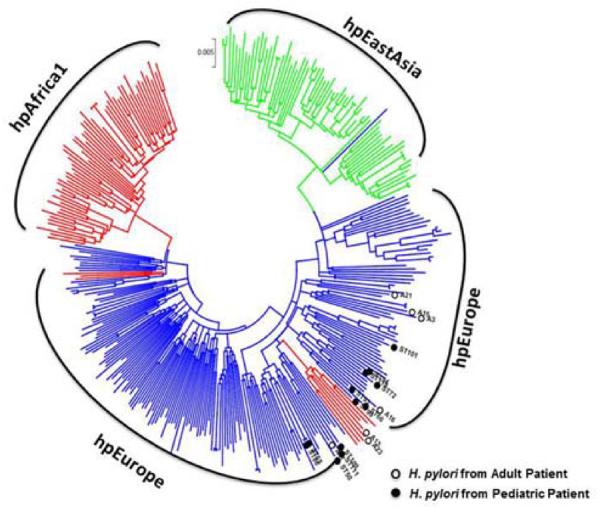
Phylogeographic origin of H. pylori strains. H. pylori isolated from the children (n=12, open circles) and adults (n=7, closed circles) were subjected to nucleotide sequence analysis of the urel, muty, efp, ppa, atpA and trpC loci. Concatenated nucleotide sequences of the loci from the 19 isolates were aligned to the corresponding sequences of 331 reference strains prior to phylogenetic analysis20, 50. The phylogenetic tree is drawn to scale with branch lengths in the same units as those of the evolutionary distances used to infer the tree. Major strain populations are designated as hpEurope (blue), hpAfrica1 (red) and hpEastAsia (green) (including subgroups hspEAsia (green) and hspAmerind (green). Seventeen of 19 H. pylori isolates were hpEurope and 2 were hpAfrica.
Reduced frequency of Th17 cells and IL-17 response in the gastric mucosa of H. pylori-infected children compared to adults
Since H. pylori gastritis is mediated by Th11–8 and possibly Th1716–18, 22 pathway responses, and since H. pylori-infected children display a reduced gastric Th1 response8, we next investigated whether infected children also have a reduced gastric Th17 response. To determine the prevalence of Th17 cells in gastric mucosa, we used immunofluorescence to analyze gastric biopsies from H. pylori-infected children and adults for CD3+ T cells that expressed IL-17 (Figure 3a). IL-17 expression was detected predominantly in CD3+ cells in the lamina propria and occasionally in CD3− cells in the epithelium and lamina propria (not shown). The proportion of CD3+ T cells that expressed IL-17 in the gastric lamina propria of H. pylori-infected adults was significantly increased compared to that of both non-infected adults (P=0.008) and infected children (P=0.024) (Figure 3b). In contrast, the proportion of gastric IL-17+/CD3+ T cells in infected children was not significantly increased compared that of non-infected children (Figure 3b). Thus, the number of Th17 cells in the gastric mucosa of H. pylori-infected children is significantly lower than that of infected adults.
Figure 3.
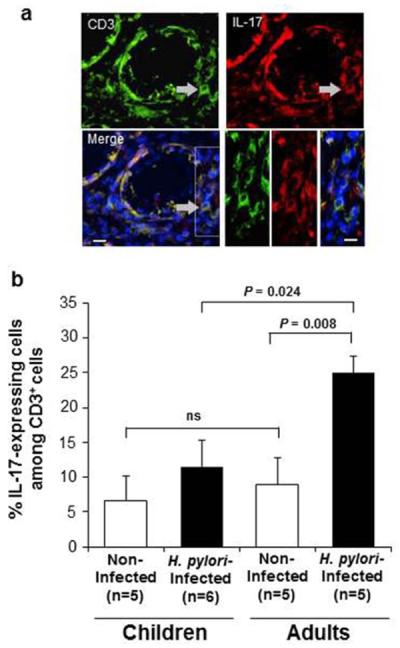
IL-17-expressing CD3+ T cells in the gastric mucosa of H. pylori-infected and uninfected children and adults. (a) Co-localization of CD3+ and IL-17+ mucosal cells in a gastric tissue section from a representative H. pylori-infected adult stained with goat anti-CD3 (Cy3) and goat anti-IL-17 (FITC) antibodies. Bar = 20 μm. (b) Percentage (mean +/−SEM) of CD3+ T cells that co-expressed IL-17 was significantly lower in H. pylori-infected children (n=6) compared to that of infected adults (n=5).
Because Th17-derived IL-17 drives the recruitment and activation of neutrophils in the intestinal mucosa, we next measured the IL-17 response in gastric tissue specimens from the children and adults. H. pylori infection in the children did not induce increased gastric IL-17 mRNA expression, whereas infection in the adults was associated with a several-fold increase in IL-17 mRNA (P=0.03) (Figure 4a). Consistent with these results, the level of IL-17 mRNA in the children did not correlate with the magnitude of H. pylori colonization (r=−0.09) compared to the moderate positive correlation between the levels of IL-17 mRNA and colonization in the adults (r=+0.3). Consequently, the expression of IL-17 mRNA in the mucosa of H. pylori-infected children was significantly lower than that of infected adults (P=0.008) (Figure 4a). Among the subjects whose gastric tissue specimens provided sufficient total protein to analyze for IL-17 protein, gastric tissue from H. pylori-infected adults, but not infected children, had higher levels of IL-17 protein compared to that of uninfected subjects (P=0.04) (Figure 4b), and, consistent with these findings, the level of IL-17 protein in the infected children was significantly reduced compared to that of infected adults (P=0.02). The level of IFN-γ mRNA in the gastric tissue of infected children also was significantly lower than that of similarly infected adults (data not shown; P=0.02), corroborating our earlier finding of a reduced Th1 response in H. pylori-infected children8. Thus, the gastric IL-17 response, similar to the IFN-γ response, was significantly lower in H. pylori-infected children compared to the response of infected adults.
Figure 4.
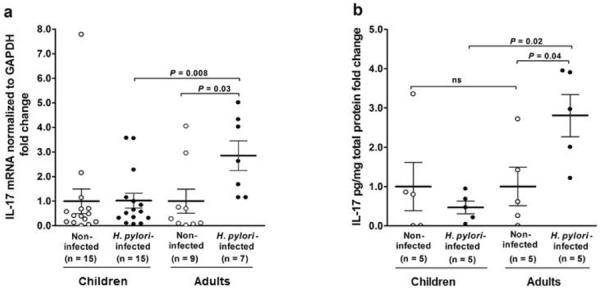
IL-17-specific mRNA and protein in gastric mucosa of non-infected and H. pylori-infected children and adults. IL-17 (a) mRNA and (b) protein in H. pylori-infected children were significantly lower compared to that of infected adults. mRNA expression was determined by real-time PCR and normalized to the housekeeping gene GAPDH. IL-17 pg protein was determined by ELISA and normalized to total mg protein. Data are presented as fold change (+/−SEM) in H. pylori-infected children and infected adults compared to non-infected cohort subjects.
Mucosal neutrophil infiltration correlates with IL-17 mRNA in H. pylori-infected adults but not children
Since polymorphonuclear neutrophil accumulation is a central feature of H. pylori gastritis and can be induced by IL-17, we investigated the relationship between tissue IL-17 responses and neutrophil accumulation in the gastric mucosa of H. pylori-infected children and adults. Among the 15 children with H. pylori infection, only 3 of 15 (20%) had high levels of neutrophil infiltration (neutrophil score 2–3) into the gastric mucosa, whereas, among the 7 adults with infection, 4 (57%) had similar amounts of high neutrophil infiltration (Figure 5). Moreover, among the infected children with high neutrophil infiltration, the level of IL-17 mRNA was significantly reduced compared to that of infected adults with the same amount of neutrophil infiltration (P=0.004). Thus, in the gastritis of H. pylori-infected children (Figure 1a), the decrease in neutrophil accumulation was associated with a significantly reduced mucosal IL-17 response.
Figure 5.
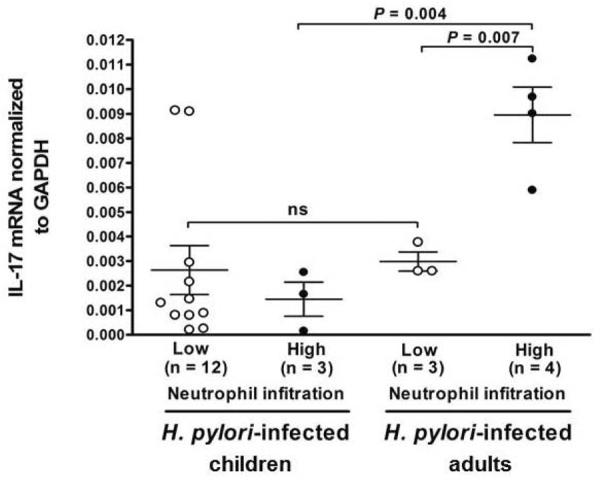
Levels of gastric IL17 mRNA and polymorphonuclear neutrophil infiltration in H. pylori-infected children and adults. Gastric mucosa from H. pylori-infected children (n=15) and adults (n=7) were analyzed for neutrophil infiltration and IL-17 mRNA expression as described in the Materials and Methods. Low neutrophil infiltration = neutrophil score 0–1 and high neutrophil infiltration = neutrophil score 2–3. Gastric IL-17 mRNA responses were lower in H. pylori-infected children than in infected adults. Values are presented as mean IL-17 mRNA normalized to GAPDH mRNA +/− SEM.
Increased levels of IL-10 and Foxp3 mRNA in the gastric mucosa of H. pylori-infected children compared to infected adults
To elucidate the mechanism by which the Th17 response might be down-regulated in children infected with H. pylori, we analyzed the same gastric tissue specimens for evidence of Treg cell responses. As shown in Figure 6a, gastric H. pylori infection in children was associated with a significant increase in the mucosal level of mRNA for the down-regulatory cytokine IL-10 compared to that of non-infected children (P=0.03). In contrast, H. pylori infection in adults was not associated with increased levels of IL-10 mRNA. Importantly, the gastric mucosa of H. pylori-infected children contained significantly higher levels of IL-10 mRNA compared to that of infected adults (P=0.03). Consistent with the increased gastric IL-10 response in H. pylori-infected children, IL-10+ cells were identified throughout the lamina propria, albeit at low frequency (Figure 6b). IL-10+ cells also were identified in the deep regions of the gastric glands, but more frequently than in the lamina propria, of infected children (Figure 6b). The cells that expressed IL-10 in the lamina propria were exclusively CD3+ T cells and not HLA-DR+ cells, whereas the IL-10-expressing cells in the glands were either CD3+ T cells or cytokeratin+ epithelial cells (Figure 6b).
Figure 6.
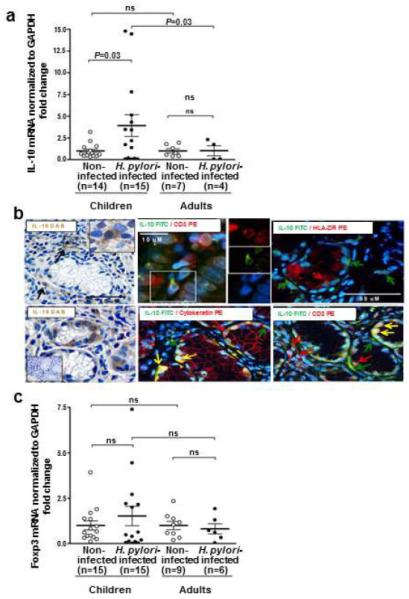
IL-10 and Foxp3 responses in the gastric mucosa of non-infected and H. pylori-infected children and adults. Gastric tissue specimens were analyzed for (a) IL-10 mRNA, (b) identity of the IL-10-expressing cells and (c) Foxp3 mRNA. Levels of IL-10 and Foxp3 mRNA, determined by real-time PCR and normalized to GAPDH mRNA, were higher in H. pylori-infected children than infected adults. Gastric IL-10 protein, detected by immunohistochemistry (left upper and lower panels) and immunofluorescence (right panels) in an H. pylori-infected child, co-localized in the lamina propria in CD3+ T cells and in the epithelium in cytokeratin+ epithelial cells and CD3+ T cells. Inset in the left lower panel shows control tissue without DAB staining. In the immunofluorescence panels, red and green arrows point to cells that stain with the indicated antibody of the same color; yellow arrows point to the merge, indicating co-localization.
The gastric mucosa of H. pylori-infected children also had higher levels of mRNA for the Treg transcription factor Foxp3 than that of both non-infected children and infected adults (Figure 6c), although the increase did not reach statistical significance. In addition, the Foxp3 and IL-17 mRNA levels in infected children exhibited a negative correlation (r=−0.08) compared to the positive, albeit not significant, correlation in adults (r=+0.25). Together, the increased IL-10 response and prominent Foxp3 response are consistent with an enhanced Treg response in the gastric mucosa of H. pylori-infected children.
DISCUSSION
To elucidate the mucosal regulation of H. pylori infection in children, which provides a window into the early host response to the bacteria, we characterized the bacteria, associated cellular infiltrate and mucosal cytokine response to the infection in children (and adults) residing in Santiago, Chile. Using pediatric and adult cohorts completely different from our previous study8, we first confirmed that H. pylori-infected children have reduced gastric inflammation compared to infected adults, despite similar mean levels of H. pylori colonization. Importantly, inflammation in the children was reduced at each level of bacterial colonization compared to that of adults, suggesting an overall down-regulation of the immune-mediated response to H. pylori in children. In addition, sequence analysis showed that the bacteria isolated from infected children and adults had similar cagA and vacA (s1b and s2) gene profiles and, in the first genotype analysis of H. pylori in Chile, that the bacteria were exclusively of European phylogeographic origin. Although genetic changes due to homologous sequence recombination commonly occur in H. pylori over time23, our gene expression studies indicate that differences in common virulence factors and bacterial strains are not the cause of the reduced inflammation in H. pylori-infected children compared to infected adults residing in this highly endemic region of the world.
The presence of only hpEurope strains in our subjects is consistent with the European ancestry in approximately two-thirds of the Chilean population, especially that of the cities24. Amerindians or persons of Amerindian admixture constitute most of the remaining third and persons of African descent less than 0.1% of the population25. In other regions of South America, persons of African ethnicity represent a substantial proportion of the population. In Colombia, for example, persons with at least some African ancestry represent 21% of the population and are concentrated along the Caribbean and Pacific coasts26. In the Pacific coast region, up to two-thirds of the inhabitants were recently shown to be colonized by hpAfrica1 strains27, likely introduced there in the 19th century through the importation of slaves infected with African strains20, 21. Importantly, strains of African origin dominated in the coastal residents and were less strongly associated with gastric cancer than European strains in the mountain residents27. In contrast, the high prevalence of only hpEurope strains in the Chilean children reported here assures prolonged exposure to hpEurope strains as the children develop into infected adults, which together with the high frequency of cancer-associated cagA+ and vacA-s1+ genotypes28, 29, likely contributes to the high rate of gastric cancer in the Chilean population.
IL-17 is the signature cytokine produced by Th17 cells and has a mediator role in the inflammation associated with certain autoimmune diseases and host defense against bacterial and fungal pathogens, particularly at mucosal surfaces30–32. Therefore, we characterized the mucosal Th17/IL-17 response in children and adults with H. pylori-associated gastritis. Whereas no differences in the frequency of Th17 cells were detected in the gastric mucosa of children and adults without H. pylori infection, children infected with the bacteria had significantly fewer gastric Th17 cells and significantly lower levels of gastric IL-17-specific mRNA and protein compared to that of similarly infected adults, indicating a potent reduction in the mucosal Th17 response in the children. The gastric mucosa of the infected children also contained less IFN-β mRNA, corroborating our earlier finding of a reduced Th1 response in H. pylori-infected children8. These findings indicate that adult H. pylori gastritis is the consequence of both Th17 and Th1 immune-mediated inflammatory pathways and that both pathways are down-regulated in the gastric mucosa of infected children.
Within the gastric inflammatory lesion of H. pylori-infected children, neutrophil infiltration was significantly lower and was associated with a reduced IL-17 response compared to that of infected adults. Thus, IL-17-producing cells in H. pylori-infected gastric mucosa likely contribute to local neutrophil accumulation, but this contribution is significantly less in children. H. pylori infection in the children also was accompanied by higher mucosal levels of mRNA for the transcription factor Foxp3 and the down-regulatory cytokine IL-10, consistent with an enhanced Treg response that may down-regulate the Th17-mediated gastric inflammation in the children. The increased IL-10 response is consistent with the finding of higher numbers of IL-10+ cells among CD3+ T cells in the lamina propria and epithelium of H. pylori-infected children. In addition to the presence of IL-10+CD3+ T cells in the epithelium, we also detected IL-10+cytokeratin+ cells in the epithelium, consistent with an epithelial source of IL-10 that has been reported by others33. In mice, the reciprocal polarization between Treg and Th17 cells is associated with inflammation of the central nervous system34, 35 and colon36 and commensal microbiota in the small intestine37. In the mouse stomach, Kao et al18 and Zhang et al38 showed that H. pylori-induced dendritic cells (DCs) skew the Th17/Treg balance toward a Treg-biased response that suppresses Th17 immunity through a CagA- and VacA-independent, TGF-β- and IL-10-dependent mechanism. In support of these findings, we recently showed that H. pylori was capable of stimulating human gastric DCs to produce IL-1039, 40, potentially supplementing Treg suppression of inflammation in the gastric mucosa. Although we have shown that macrophages in normal human intestinal mucosa are incapable of TGF-β and IL-10 production41, 42, the ability of human gastric macrophages to produce down-regulatory cytokines is not known.
Our finding that H. pylori-infected children have down-regulated Th17 responses in the gastric mucosa has important implications for the development of an effective H. pylori vaccine. First, in animal model studies, IL-17 has been implicated as a key mediator of vaccine-induced reduction in H. pylori infection43–45 through the stimulation of local chemokines, including MIP-2, KC and LIX, which recruit neutrophils to the gastric mucosa43. Second, since children will be an especially important target population for H. pylori-specific vaccination in resource-poor nations, reduction in the gastric IL-17 response in children infected with H. pylori is a potentially confounding issue that will need to be addressed in the development of such a vaccine. Thus, the Th17/IL-17 results presented here implicate discordant regulation of the gastric mucosal response of children and adults to H. pylori. Further elucidation of the immune-mediated mechanism(s) of H. pylori-induced mucosal inflammation during early host development will provide important information for the design of more effective intervention strategies against this challenging pathogen, especially for children in countries with high rates of H. pylori infection and gastric cancer.
METHODS
Patients
Consecutive subjects with abdominal symptoms residing in Santiago, Chile, including 30 children less than 13 years of age and 16 adults greater than 18 years of age, were evaluated in this IRB-approved study. The mean age of the children was 10.4 +/−2.9 years old and the adults 48 +/−13.1 years old. Female gender dominated in both the children (62%) and the adults (69%). All subjects were of European ethnicity, and the mean socioeconomic levels based on the Department of Economy criteria were similar for the groups. Acceptance into the study was based on the presence of symptoms of recurrent abdominal pain, burning abdominal discomfort, hematemesis or chronic vomiting. Exclusion criteria included (a) use of antibiotics, antacid, H2-blocker, proton-pump inhibitor, bismuth compound, non-steroidal anti-inflammatory drug or immunosuppressive agent during the two weeks prior to endoscopy; (b) presence of an autoimmune disease; and (c) stool exam positive for ova or parasites.
Assessment of H. pylori infection
Subjects provided six antral biopsies by esophagogastroduodenoscopy for the following studies: (1) Rapid urease test (Rapid Urea Test; Bios Chile, Santiago, Chile) to detect H. pylori; (2) Histologic analysis and microscopic detection of H. pylori; (3) Genotype analysis; (4) Immunofluorescence studies; (5) Cytokine protein determination; and (6) Analysis of cytokine gene expression. A study subject was judged colonized with H. pylori if either the rapid urease test or the microscopic evaluation was positive for the bacteria.
Evaluation of gastric H. pylori histopathology and colonization
Serial sections of formalin-fixed, paraffin-embedded, H&E-stained gastric tissue specimens were evaluated for inflammation and bacterial colonization by two pathologists without knowledge of other results. Briefly, 5 random fields in 3 tissue sections per biopsy were scored (0–3) according to the modified Sydney classification system46 for the level of neutrophils and mononuclear cells, number of lymphoid follicles, mucosal atrophy and intestinal metaplasia. Thereafter, a histology score reflecting the level of inflammation8 was determined by adding the score of each parameter. Similarly, a neutrophil score reflected the level of neutrophils in 5 random fields in 3 sections per coded biopsy with 0=absent, 1=mild, 2=moderate and 3=high. A colonization score was calculated based on H. pylori density (0=absent, 1=mild, 2=moderate, 3=intense).
H. pylori virulence factor and genotype analysis
Gastric biopsies were placed in Brucella broth, frozen immediately in liquid nitrogen and subsequently cultured on plates with Brucella agar, 5% horse blood and H. pylori-selective supplement (Dent; Oxoid, Basingstoke, UK). The plates were cultured for 7 days in 10% CO2 and then passaged three times. A single colony was identified as H. pylori using colony morphology and the rapid urease test (Rapid Urea Test, Bios Chile, Santiago, Chile). DNA from the remaining colonies was extracted using the Qiagen DNeasy Minikit (Qiagen, Valencia, CA) and quantified by spectrophotometry. Virulence factor cagA was amplified by PCR, as previously described47. Strains without cagA amplification by PCR were confirmed as cagA negative by amplifying the cag pathogenicity island empty site as previously described48. To analyze the s-region of vacA, the nucleotide sequences of PCR products were compared to variants of four known vacA s-region subtypes (s1a, s1b, s1c and s2)49.
Nucleotide sequences for H. pylori housekeeping genes (ureI, mutY, efp, ppa, atpA and trpC) also were amplified by PCR20, 50. PCR products were subsequently purified using the Wizard SV gel and PCR Clean-up kit (Promega, Fitchburg, WI) and stored at −20°C. PCR products were sequenced by the University of Alabama at Birmingham DNA Sequencing and Analysis Core. To determine the relationship between strains of H. pylori infecting patients in this study and globally distributed H. pylori isolates, we retrieved data from a multi-locus sequence typing (MLST) database (http://pubmlst.org/helicobacter) for 331 reference isolates with appropriate geographic distribution. Concatenated nucleotide sequences for H. pylori MLST loci (ureI, mutY, efp, ppa, atpA and trpC) from seven isolates obtained from children and three isolates obtained from adults as well as the 331 isolates retrieved from the MLST database were analyzed using Mega4 software51. Sequences were aligned using the ClustalW algorithm and phylogenetic relationships were produced using the Kimura two-parameter model for nucleotide substitution and neighbor-joining clustering. A phylogenetic structure was constructed as a bootstrap consensus tree inferred from 1000 replicates.
Identification and enumeration of gastric Th17 cells
Snap frozen biopsies were cut into 5 μm sections, fixed in acetone (10 min) and rinsed in PBS with 0.05% Tween. After blocking in Dako serum-free protein block for 30 min at room temperature, the sections were incubated with goat anti-human IL-17 (10 μg/mL; R&D Systems, Minneapolis, MN) and rabbit anti-human CD3 (1:300; Sigma-Aldrich, St. Louis, MO) for 4 h at room temperature, followed by incubation with Cy3 donkey anti-goat IgG (1:200; Jackson Immunity, West Grove, PA) and fluorescein isothiocyanate (FITC) donkey anti-rabbit IgG (1:50; Jackson Immunity, West Grove, PA) for 30 min at room temperature. Isotype-matched, irrelevant antibodies were included as controls with each staining experiment. Cell nuclei were labeled with DAPI. Sections were mounted, sealed and stored at 4°C until analysis by fluorescence microscopy (Nikon Eclipse T2000-U, equipped with a CoolSnap ES digital camera and NIS Elements BR2.30 software). The proportion of IL-17+ cells in the CD3+ T cell population was determined on digital images by counting CD3+ cells with and without co-expression of_IL-17 using ImageJ1.46R software (Wayne Rasband, National Institutes of Health, USA, hppt://imagej.nih.gov/ij). Regions of interest were set to exclude surface and glandular epithelial cells. Three or more randomly selected areas from two slides per specimen were analyzed by two independent investigators.
Identification of gastric IL-10 cells
Biopsies fixed in 4% formalin, embedded in paraffin and cut in 7um sections were rehydrated and rinsed in water. Sections were subjected to antigen retrieval (citrate buffer, DAKO Target Retrieval Solution; 20 min >92°C by microwave), endogenous peroxidase inhibition and casein protein blocking (each 20 min, room temperature, CSA System DAKO), and incubated overnight at 4°C with primary antibody (DAKO Antibody Diluent with Background Reducing Components). Primary antibodies included a) mouse anti-human IL-10 IgG2b (1:200, Santa Cruz); b) rabbit anti-human CD3 IgG (1:400, Sigma-Aldrich); c) mouse anti-human HLA-DR IgG1 (1:20, BD Biosciences); and d) mouse anti-human cytokeratin IgG1 (1:50, Cell Signaling). IL-10 positive cells were identified by tyramide signal amplification (1:100, goat anti-mouse IgG2b-HRP; 1:50, biotinylated tyramide; 1:1000, streptavidin-HRP, PerkinElmer) followed by diaminobenzidine staining and counterstained with hematoxylin, or by tyramide signal amplification as above using tyramide-FITC (1:50, Molecular Probes 488). Nuclei were counterstained with DAPI (1:500). CD3+ cells were detected with donkey anti-rabbit IgG (1:400, Jackson Immunity); HLA-DR+ and cytokeratin+ cells were detected with goat anti-mouse IgG1 (1:100, Molecular Probes 594) and nuclei were counterstained with DAPI. Control slides were included with every experiment (n=12). Sections were stored and analyzed as above.
Cytokine protein determination
Biopsy specimens were homogenized in 750 μL PBS, centrifuged (12,000g, 5 min, 4°C) and the supernatant collected and frozen (−70°C) until assayed. The concentration of IL-17 was measured by ELISA (R&D Systems, Minneapolis, MN) and expressed as pg/mg protein. Total protein was measured using the bicinchoninic acid method (Pierce, Rockford, IL) and expressed as mg/mL.
Real-Time RT-PCR analysis for cytokine gene expression
Gastric specimens were snap frozen in liquid nitrogen and kept at −80°C until processed. Total RNA was extracted using the Qiagen RNeasy Minikit (Qiagen, Valencia, CA). RNA concentration and purity were determined by spectrophotometry, and RNA integrity was assesed by agarose gel electrophoresis. RNA then was treated with DNAse I (Invitrogen, Carlsbad, CA) for 15 min, heat-inactivated at 65°C to avoid genomic DNA amplification during real-time PCR and reverse transcribed into first-strand cDNA using the affinityScript qPCR cDNA synthesis kit (Agilent Technologies Inc, Santa Clara, CA) with oligo dT as primers. Real-time PCR was performed on duplicate cDNA samples with custom-made primer sets using Brilliant II SYBR green QPCR master mix in an Mx3000p Real-Time PCR System machine (both from Agilent Technologies). The following primer pairs for IL-17 (FW: 5'ACCAATCCCAAAAGGTCCTC3'; RV: 5'GGGGACAGAGTTCATGTGGT3'); IL-10 (FW: 5'GTGATGCCCCAAGCTGAGA3'; RV: 5'CACGGCCTTGCTCTTGTTTT3'); Foxp3 (FW: 5'AGAAGCAGCGGACACTCAAT3'; RV: 5'GAAAGGAGGATGGACGAACA3'); IFN-γ (FW: 5'GAATTGGAAAGAGGAGAGTGAC3'; RV: 5'TGTATTGCTTTGCGTTGGAC3') and GAPDH (reference gene) (FW: 5'AACCTGCCAAATATGATGAC3'; RV: 5'GTTGTCATACCAGGAAATGAG3') were used for amplification.
PCR fragments for each cytokine and the reference gene obtained by RT-PCR were cloned into the pGEM-T Easy Vector System (Promega, Litchburg, WI). Plasmids extracted from selected clones using the Wizard SV miniprep DNA purification systems (Promega, Fitchburg, WI) containing expected inserts were quantified by spectrophotometry. Serial dilutions of the vector-insert were used as standard curves to quantify the levels of gene expression. mRNA levels were analyzed by comparing the differences in fold change in cytokine mRNA normalized to GAPDH mRNA.
Statistical analysis
Comparisons between groups were performed using Student's t-test or the Mann-Whitney U test when appropriate for continuous data. Categorical data were analyzed using the Chi-square test and the Fisher exact test. Spearman's rank correlation and the Goodness-of-Fit test were used to analyze relationships, although only the Spearman correlation coefficient is presented as analyses were uniformly consistent with each other. Comparison of curve fit values was performed using an F-test. Statistical significance was defined as a P value of less than .05.
ACKNOWLEDGMENTS
The authors thank Robin G. Lorenz, M.D., Ph.D. for assistance with the tyramide signal amplification. This study was supported by Fondecyt (#1100654, #1085232) and Conicyt RUE29 (Chile); the National Institutes of Health (DK-54495, DK-84063, AI-83539, AI-83027, RR-20136, AI068009, CA116087, and the Cell and Molecular Pathology Core of the UAB Mucosal HIV and Immunobiology Center, DK-64400); UAB Autoimmunity, Immunology and Transplantation Pilot Program; UAB Center for Clinical and Translational Science Pilot Program (UL1 TR000168); the Department of Veterans Affairs, and the Fulbright Fellowship Program, Department of State (USA).
Footnotes
DISCLOSURE The authors declare no conflict of interest.
REFERENCES
- 1.Mohammadi M, Czinn S, Redline R, Nedrud J. Helicobacter-specific cell-mediated immune responses display a predominant Th1 phenotye and promote a delayed-type hypersensitivity response in the stomachs of mice. J Immunol. 1996;156(12):4729–4738. [PubMed] [Google Scholar]
- 2.Smythies LE, Waites KB, Lindsey RJ, Harris PR, Ghiara P, Smith PD. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-γ, gene-deficient mice. J Immunol. 2000;165(2):1022–1029. doi: 10.4049/jimmunol.165.2.1022. [DOI] [PubMed] [Google Scholar]
- 3.Mattapallil JJ, Dandekar S, Canfield DR, Solnick JV. A predominant Th1 type of immune response is induced early during acute Helicobacter pylori infection in rhesus macaques. Gastroenterology. 2000;118(2):307–315. doi: 10.1016/s0016-5085(00)70213-7. [DOI] [PubMed] [Google Scholar]
- 4.Karttunen R, Karttunen T, Ekre H-P, T., MacDonald TT. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut. 1995;36:341–345. doi: 10.1136/gut.36.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haeberle HA, Kubin M, Bamford KB, Garofalo R, Graham DY, El-Zaatari F, et al. Differential stimulation of interleukin-12 (IL-12) and IL-10 by live and killed Helicobacter pylori in vitro and association of IL-12 production with γ interferon-producing T cells in the human gastric mucosa. Infect Immun. 1997;65(10):4229–4235. doi: 10.1128/iai.65.10.4229-4235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Elios MM, Manghetti M, De Carli M, Costa F, Baldari CT, Burroni D, et al. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J Immunol. 1997;158(2):962–967. [PubMed] [Google Scholar]
- 7.Bamford KB, Fan X, Crowe SE, Leary JF, Gourley WK, Luthra GK, et al. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114(3):482–492. doi: 10.1016/s0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- 8.Harris PR, Wright SW, Serrano C, Riera F, Duarte I, Torres J, et al. Helicobacter pylori gastritis in children is associated with a regulatory T-cell response. Gastroenterology. 2008;134(2):491–499. doi: 10.1053/j.gastro.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Guiraldes E, Duarte I, Peña A, Godoy A, Espinosa M, Bravo R, et al. Proinflammatory cytokine expression in gastric tissue from children with Helicobacter pylori-associated gastritis. Journal of pediatric gastroenterology and nutrition. 2001;33(2):127–132. doi: 10.1097/00005176-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Whitney AE, Guarner J, Hutwagner L, Gold BD. Helicobacter pylori gastritis in children and adults: comparative histopathologic study. Annals of Diagnostic Pathology. 2000;4(5):279–285. doi: 10.1053/adpa.2000.17871. [DOI] [PubMed] [Google Scholar]
- 11.Lundgren A, Stromberg E, Sjoling A, Lindholm C, Enarsson K, Edebo A, et al. Mucosal FOXP3-expressing CD4+ CD25high regulatory T cells in Helicobacter pylori-infected patients. Infect Immun. 2005;73(1):523–531. doi: 10.1128/IAI.73.1.523-531.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson K, Kenefeck R, Pidgeon EL, Shakib S, Patel S, Polson RJ, et al. Helicobacter pylori-induced peptic ulcer disease is associated with inadequate regulatory T cell responses. Gut. 2008;57(10):1375–1385. doi: 10.1136/gut.2007.137539. [DOI] [PubMed] [Google Scholar]
- 13.Raghavan S, Fredriksson M, Svennerholm AM, Holmgren J, Suri-Payer E. Absence of CD4+CD25+ regulatory T cells is associated with a loss of regulation leading to increased pathology in Helicobacter pylori-infected mice. Clin Exp Immunol. 2003;132(3):393–400. doi: 10.1046/j.1365-2249.2003.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rad R, Brenner L, Bauer S, Schwendy S, Layland L, da Costa CP, et al. CD25+/Foxp3+ T cells regulate gastric inflammation and Helicobacter pylori colonization in vivo. Gastroenterology. 2006;131(2):525–537. doi: 10.1053/j.gastro.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Alam MS, Kurtz CC, Rowlett RM, Reuter BK, Wiznerowicz E, Das S, et al. CD73 is expressed by human regulatory T helper cells and suppresses proinflammatory cytokine production and Helicobacter felis-induced gastritis in mice. J Infect Dis. 2009;199(4):494–504. doi: 10.1086/596205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiomi S, Toriie A, Imamura S, Konishi H, Mitsufuji S, Iwakura Y, et al. IL-17 is involved in Helicobacter pylori-induced gastric inflammatory responses in a mouse model. Helicobacter. 2008;13(6):518–524. doi: 10.1111/j.1523-5378.2008.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Algood HM, Gallo-Romero J, Wilson KT, Peek RM, Jr., Cover TL. Host response to Helicobacter pylori infection before initiation of the adaptive immune response. FEMS Immunol Med Microbiol. 2007;51(3):577–586. doi: 10.1111/j.1574-695X.2007.00338.x. [DOI] [PubMed] [Google Scholar]
- 18.Kao JY, Zhang M, Miller MJ, Mills JC, Wang B, Liu M, et al. Helicobacter pylori immune escape is mediated by dendritic cell-induced Treg skewing and Th17 suppression in mice. Gastroenterology. 2010;138(3):1046–1054. doi: 10.1053/j.gastro.2009.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117(1):60–69. doi: 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falush D, Wirth T, Linz B, Pritchard JK, Stephens M, Kidd M, et al. Traces of human migrations in Helicobacter pylori populations. Science. 2003;299(5612):1582–1585. doi: 10.1126/science.1080857. [DOI] [PubMed] [Google Scholar]
- 21.Linz B, Balloux F, Moodley Y, Manica A, Liu H, Roumagnac P, et al. An African origin for the intimate association between humans and Helicobacter pylori. Nature. 2007;445(7130):915–918. doi: 10.1038/nature05562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caruso R, Fina D, Paoluzi OA, Del Vecchio Blanco G, Stolfi C, Rizzo A, et al. IL-23-mediated regulation of IL-17 production in Helicobacter pylori-infected gastric mucosa. European Journal of Immunology. 2008;38(2):470–478. doi: 10.1002/eji.200737635. [DOI] [PubMed] [Google Scholar]
- 23.Kraft C, Stack A, Josenhans C, Niehus E, Dietrich G, Correa P, et al. Genomic changes during chronic Helicobacter pylori infection. J Bacteriol. 2006;188(1):249–254. doi: 10.1128/JB.188.1.249-254.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cruz-Coke R, Moreno RS. Genetic epidemiology of single gene defects in Chile. J Med Genet. 1994;31(9):702–706. doi: 10.1136/jmg.31.9.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medina EL, Kaempffer AMR. Elementos de Salud Publica. Biblioteca Digital. Universidad de Chile. 2011;Section 5.2.6 [Google Scholar]
- 26.National Administration Department of Statistics (DANE) Government of Columbia. 2007
- 27.de Sablet T, Piazuelo MB, Shaffer CL, Schneider BG, Asim M, Chaturvedi R, et al. Phylogeographic origin of Helicobacter pylori is a determinant of gastric cancer risk. Gut. 2011;60(9):1189–1195. doi: 10.1136/gut.2010.234468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basso D, Zambon CF, Letley DP, Stranges A, Marchet A, Rhead JL, et al. Clinical relevance of Helicobacter pylori cagA and vacA gene polymorphisms. Gastroenterology. 2008;135(1):91–99. doi: 10.1053/j.gastro.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 29.Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, et al. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer research. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 30.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-β induces development of the T(H)17 lineage. Nature. 2006;441(7090):231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 31.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annual Review of Immunology. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 32.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28(4):454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bodger K, Bromelow K, Wyatt JI, Heatley RV. Interleukin 10 in Helicobacter pylori associated gastritis: immunohistochemical localisation and in vitro effects on cytokine secretion. J Clin Pathol. 2001;54(4):285–292. doi: 10.1136/jcp.54.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 35.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, et al. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453(7191):65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 36.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317(5835):256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 37.Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host & Microbe. 2008;4(4):337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang M, Liu M, Luther J, Kao JY. Helicobacter pylori directs tolerogenic programming of dendritic cells. Gut Microbes. 2010;1(5):325–329. doi: 10.4161/gmic.1.5.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bimczok D, Clements RH, Waites KB, Novak L, Eckhoff DE, Mannon PJ, et al. Human primary gastric dendritic cells induce a Th1 response to H. pylori. Mucosal Immunology. 2010;3(3):260–269. doi: 10.1038/mi.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bimczok D, Grams JM, Stahl RD, Waites KB, Smythies LE, Smith PD. Stromal regulation of human gastric dendritic cells restricts the Th1 response to Helicobacter pylori. Gastroenterology. 2011;141(3):929–938. doi: 10.1053/j.gastro.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH, et al. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115(1):66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smythies LE, Shen R, Bimczok D, Novak L, Clements RH, Eckhoff DE, et al. Inflammation anergy in human intestinal macrophages is due to Smad-induced IκBα expression and NF-κB inactivation. J Biol Chem. 2010;285(25):19593–19604. doi: 10.1074/jbc.M109.069955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeLyria ES, Redline RW, Blanchard TG. Vaccination of mice against H pylori induces a strong Th-17 response and immunity that is neutrophil dependent. Gastroenterology. 2009;136(1):247–256. doi: 10.1053/j.gastro.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Velin D, Favre L, Bernasconi E, Bachmann D, Pythoud C, Saiji E, et al. Interleukin-17 is a critical mediator of vaccine-induced reduction of Helicobacter infection in the mouse model. Gastroenterology. 2009;136(7):2237–2246. doi: 10.1053/j.gastro.2009.02.077. [DOI] [PubMed] [Google Scholar]
- 45.Hitzler I, Oertli M, Becher B, Agger EM, Muller A. Dendritic cells prevent rather than promote immunity conferred by a helicobacter vaccine using a mycobacterial adjuvant. Gastroenterology. 2011;141(1):186–196. doi: 10.1053/j.gastro.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20(10):1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 47.van Doorn LJ, Figueiredo C, Sanna R, Pena S, Midolo P, Ng EK, et al. Expanding allelic diversity of Helicobacter pylori vacA. J Clin Microbiol. 1998;36(9):2597–2603. doi: 10.1128/jcm.36.9.2597-2603.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sicinschi LA, Correa P, Bravo LE, Schneider BG. A positive assay for identification of cagA negative strains of Helicobacter pylori. J Microbiol Methods. 2003;55(3):625–633. doi: 10.1016/s0167-7012(03)00206-9. [DOI] [PubMed] [Google Scholar]
- 49.Rudi J, Kolb C, Maiwald M, Kuck D, Sieg A, Galle PR, et al. Diversity of Helicobacter pylori vacA and cagA genes and relationship to VacA and CagA protein expression, cytotoxin production, and associated diseases. J Clin Microbiol. 1998;36(4):944–948. doi: 10.1128/jcm.36.4.944-948.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Achtman M, Azuma T, Berg DE, Ito Y, Morelli G, Pan ZJ, et al. Recombination and clonal groupings within Helicobacter pylori from different geographical regions. Mol Microbiol. 1999;32(3):459–470. doi: 10.1046/j.1365-2958.1999.01382.x. [DOI] [PubMed] [Google Scholar]
- 51.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24(8):1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]


