Abstract
Gleason grade is universally used for pathologic scoring the differentiation of prostate cancer. However, it is unknown whether prostate tumors arise well-differentiated and then progress to less differentiated forms or if Gleason grade is an early and largely unchanging feature. Prostate Specific Antigen (PSA) screening has reduced the proportion of tumors diagnosed at advanced stage, which allows assessment of this question on a population level. If Gleason grade progresses as stage does, one would expect a similar reduction in high grade tumors. We studied 1,207 Physicians’ Health Study and Health Professionals Follow-up Study participants diagnosed with prostate cancer 1982–2004 and treated with prostatectomy. We compared the distribution of grade and clinical stage across the pre-PSA and PSA screening eras. We re-reviewed grade using the ISUP 2005 revised criteria. The proportion of advanced stage tumors dropped more than six-fold, from the earliest period (12/1982–1/1993), 19.9% stage ≥T3, to the latest (5/2000–12/2004), 3% stage T3, none T4. The proportion of Gleason score ≥8 decreased substantially less, from 25.3% to 17.6%. A significant interaction between stage and diagnosis date predicting grade (p=0.04) suggests the relationship between grade and stage varies by time period. As the dramatic shift in stage since the introduction of PSA screening was accompanied by a more modest shift in Gleason grade, these findings suggest grade may be established early in tumor pathogenesis. This has implications for the understanding of tumor progression and prognosis, and may help patients diagnosed with lower grade disease feel more comfortable choosing active surveillance.
Keywords: prostate cancer, Gleason score, dedifferentiation
Introduction
For more than four decades, the Gleason score has been the most widely used grading system for prostate tumor differentiation and represents the best-established prognostic indicator for prostate cancer progression besides stage itself(1). Indeed, even within Gleason score 7, the predominance of pattern 4 (Gleason 4+3) carries more than a three-fold higher risk of prostate cancer mortality than Gleason pattern 3+4 (2). Pathologists and epidemiologists have long speculated on whether prostate tumors initially arise as well-differentiated tumors and then progress to higher grades over time, or whether grade is an early and largely unchanging feature of the tumor. This issue has important implications for understanding the basic biology of prostate cancer, and has clinical implications as well. If Gleason grade progresses little or not at all, patients who are diagnosed with lower grade disease may be more comfortable choosing active surveillance.
Several studies have attempted to answer this question by examining repeated biopsy specimens in individual patients(3–7). However, this question cannot readily be addressed through repeated biopsy because of the inherent heterogeneity of tumor nodules within the prostate. This heterogeneity is apparent given different Gleason grade findings from simultaneously collected needle cores within a single gland, and means that a later biopsy might well be targeting a different nodule or different part of the original nodule. Many of the biopsy studies are of low-grade patients (usually Gleason 6) undergoing active surveillance such that if cancer continues to be detected on subsequent biopsies it will continue to be either Gleason 6 or be upgraded, since cancers are rarely graded lower than Gleason 6. Once a higher grade tumor is detected, patients generally receive treatment and are therefore no longer followed up with additional biopsies. Thus, such studies are inherently more likely to show apparent Gleason progression due to these biases.
Although we cannot appropriately distinguish the two hypotheses regarding Gleason grade progression within individuals, the advent of PSA screening affords the opportunity to assess this issue on a population level. PSA screening provides a lead time of approximately 10 to 12 years (8–10). Thus, on the average, in repeatedly screened populations, tumors are diagnosed 10 to 12 years earlier in their natural history than in unscreened men; indeed, because of early detection, widespread screening has dramatically reduced the number and proportion of tumors with advanced stage at diagnosis and time of surgery ((11–14), reviewed in (15)). If Gleason grade progresses, one would expect a similar reduction in high grade tumors considering that tumors are now detected so much earlier in their natural history than before screening. One difficulty in such a comparison is that Gleason grading standards have shifted over time, so that scores below six are now rarely assigned, and assigning a higher grade has become more common, a trend described by Albertsen(16). Thus, standardized re-review of Gleason grade is essential for a valid assessment of possible change over calendar time. We therefore evaluated the change in distribution of re-reviewed Gleason scores in two prospective cohorts, with cases diagnosed 1982–2004, before and after introduction of widespread PSA screening, and compared this to the change in distribution of stage during that same interval.
Materials and Methods
Study Population
Physicians’ Health Study (PHS)
The PHS began as a randomized, double-blind trial of aspirin and β-carotene in the prevention of cardiovascular disease and cancer among 22,071 healthy US physicians; written consent was obtained from each participant at the time of initial enrollment and the investigation was approved by the Human Subjects Committee at Brigham and Women’s Hospital. Men were excluded if they had any serious medical conditions at baseline including all cancers (except non-melanoma skin cancer)(17).
Participants are followed through annual questionnaires to collect data on diet, health and lifestyle behaviors, and medical history, and biannually through postcards to ascertain health endpoints, including prostate cancer. All self-reported prostate cancer cases are verified through medical record and pathology review. There is a high follow-up rate for cancer incidence (96%). Through this systematic medical record review we also abstract data on clinical information, including clinical stage.
Health Professionals Follow-up Study (HPFS)
The HPFS, an ongoing prospective cohort study on the causes of cancer and heart disease in men, consists of 51,529 U.S. health professionals who were aged 40–75 years in 1986(2). At baseline and biennially thereafter, participants responded to a mailed questionnaire that included questions on demographics, lifestyle, and medical history. When a participant reports a prostate cancer diagnosis medical and pathology records are obtained. Study investigators review these records to confirm the diagnosis and to abstract clinical information, including stage at diagnosis. Follow-up rates for cancer incidence exceed 94%. The Human Subjects Committee at the Harvard School of Public Health approved this study.
Tumor repository and Gleason re-review
We collected archival formalin-fixed paraffin embedded radical prostatectomy (RP) and trans-urethral resection of the prostate (TURP) specimens from men with incident prostate cancer in the PHS and HPFS (4.47% TURP, 95.53% RP). Pathology departments were instructed to send either the entire TURP or RP material collected or a representative sample. When only some blocks were provided, our pathologist (MF) confirmed that at least the main cancer nodule was present. Using the ISUP 2005 revised criteria(18), a single pathologist (MF), blinded to clinical data (including outcome and date of diagnosis), re-reviewed all H&E slides to assign major and minor Gleason grades, thereby avoiding the problem of the shift in Gleason scoring over the past several decades(16). Results describing the changes between original report and the re-review were previously reported, and we found the re-reviewed Gleason scores to significantly predict lethal outcome (2).
Statistical Analysis
Analyses were performed with SAS version 9.1 statistical software; all P values are two-sided. All individuals with a date of cancer diagnosis, clinical stage, and a re-reviewed Gleason score were included in this analysis. We combined data from PHS and HPFS and defined four time periods based on the quartiles of date at diagnosis (12/1982–1/1993, 1/1993–12/1996, 12/1996–5/2000, 5/2000–12/2004). These time periods span the pre-PSA and PSA eras. The proportions of four categories of Gleason score (≤6, 3+4, 4+3, ≥8) and three categories of clinical stage (T1/T2, T3, T4/N1/M1) were calculated for each of the four time periods. In a secondary analysis, we restricted to men diagnosed with clinical stage T1/T2 tumors and again examined the proportions of Gleason score across the same four time periods. A generalized linear model (GLM) was used to determine if the relationship between Gleason and stage depends on time period; re-reviewed Gleason grade was the outcome and clinical stage (3 categories), time periods, and an interaction variable between stage and time period were included in the model. The Type III F-test was used to determine significance of this interaction. A GLM was performed to determine the association of age at diagnosis with both Gleason score and time period separately; the Type III F-test for trend was used to determine significance of the associations.
Because the proportion of Gleason score ≥8 tumors could be altered both by changes in the occurrence of those tumors or by changes in occurrence of lower grade tumors, as our main analysis, we estimated the incidence rate of re-reviewed tumors across the four time periods among the entire PHS and HPFS cohorts combined. This was performed by counting the number of re-reviewed Gleason ≥8, Gleason 4+3, Gleason 3+4 and Gleason ≤6 tumors diagnosed, and calculating the total person-years (p-y) contributed by all participants in each time period. Person-time was counted from the start date of the time period to diagnosis of prostate cancer, death from any cause, or the end of the time period.
Results
Clinical characteristics of the PHS (N=420) and HPFS (N=787) participants are described in Table 1. While screening information is not available from the PHS trial, participants in the overall HPFS were asked about their PSA testing during the previous 2 years beginning in 1994. In 1994, only 42% of participants had had a PSA test in the previous 2 years, but this percentage steadily increased over time to 81% in 2000. From 2002–2008, this percentage decreased slightly to 74–76% in each 2 year period.
Table 1.
Clinical characteristics of PHS and HPFS prostate cancer cases with re-reviewed Gleason score and clinical stage
| PHS (N=420) | HPFS (N=787) | |
|---|---|---|
| Years of diagnosis, range | 1982–2004 | 1986–2004 |
| Age at diagnosis, mean (s.d.) | 66.1 (6.2) | 65.6 (6.2) |
| Re-reviewed Gleason score (%) | ||
| 2–6 | 125 (29.8) | 142 (18.0) |
| 7 (3+4) | 151 (36.0) | 295 (37.5) |
| 7 (4+3) | 77 (18.3) | 189 (24.0) |
| 8–10 | 67 (16.0) | 161 (20.4) |
| Clinical stage (%) | ||
| T1/T2 | 392 (93.3) | 713 (90.6) |
| T3 | 25 (6.0) | 48 (6.1) |
| T4/N1/M1 | 3 (0.1) | 26 (3.3) |
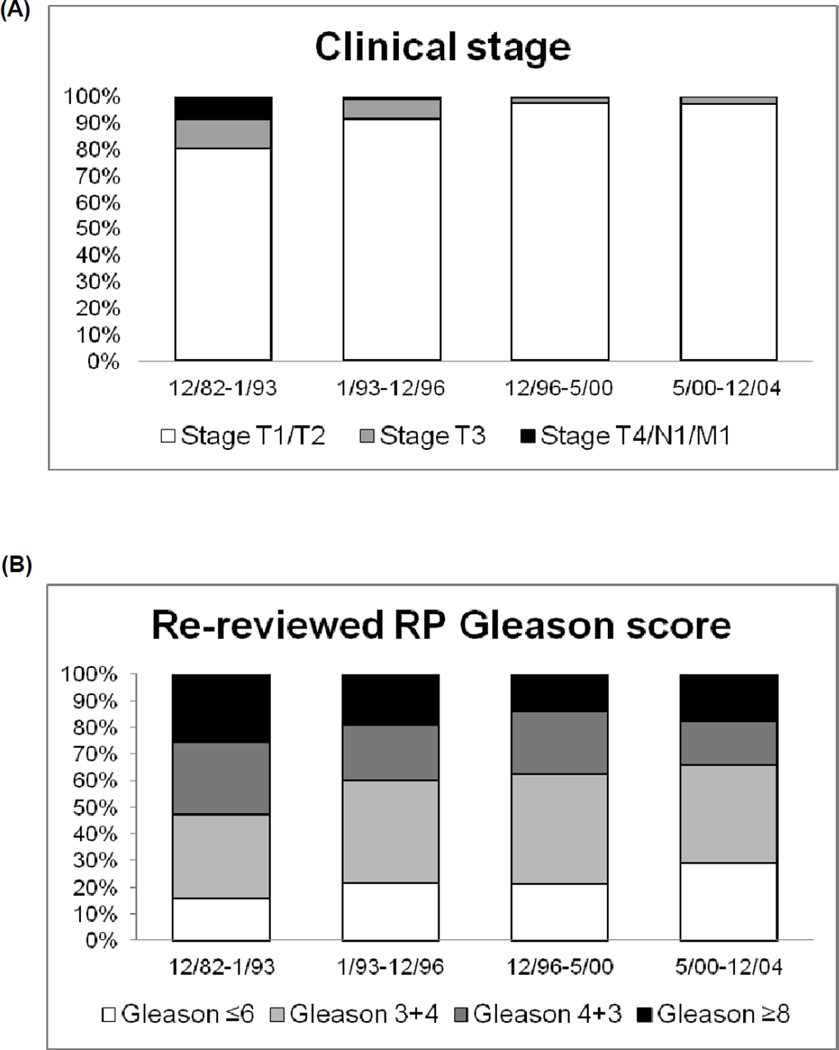
The distribution of clinical stage and Gleason score across time periods are presented in Figure 1. In the earliest period (12/1982–1/1993, pre-PSA), 19.9% were stage T3 or higher. This proportion decreased steadily until the last period (5/2000–12/2004), with only 3% of tumors stage T3, and none stage T4 or M1. In contrast to the dramatic 6-fold shift in stage at diagnosis, the proportion of Gleason score ≥8 decreased only moderately, from 25.3% in the first period to 17.6% to the most recent period, a shift of only 30%. When restricting to men with stage T1/T2, the proportion of Gleason score 8–10 decreased even less across time periods, from 19.5% to 16%.
Figure 1.
Proportion of prostate cancer cases with (a) clinical stage T1/T2, T3, and T4/N1/M1 across four different time periods and (b) re-reviewed radical prostatectomy Gleason score ≤6, 7, and ≥8.
Using a generalized linear model, we observed a significant interaction between stage and ordinal categories of date at diagnosis to predict Gleason score (p=0.04), demonstrating that the relationship between Gleason score and stage is not uniform across time periods. Age at diagnosis is associated with Gleason score, with older men tending to have higher Gleason score (p<0.0001); however, when examined by category of date of diagnosis, the association is only significant in the three PSA era time periods, becoming more significant over time (Table 2). Age at diagnosis is additionally not associated with categories of date of diagnosis (p=0.10). This suggests that the change over time we observe for Gleason score is not due to a change in age at diagnosis and may represent an increase in screening of younger men detecting more indolent, lower grade tumors.
Table 2.
Mean age at diagnosis by Gleason score across time periods
| Mean age at diagnosis | |||||
|---|---|---|---|---|---|
| Time period | Gleason 2–6 | Gleason 3+4 | Gleason 4+3 | Gleason 8–10 | ptrend |
| 12/82–1/93 | 66.8 | 66.5 | 66.8 | 67 | 0.70 |
| 1/93–12/96 | 64.6 | 64.4 | 66.7 | 66.3 | 0.02 |
| 12/96–5/00 | 63.4 | 65.2 | 65.5 | 67.3 | 0.003 |
| 5/00–12/04 | 64.7 | 65.4 | 66.7 | 68.1 | 0.0003 |
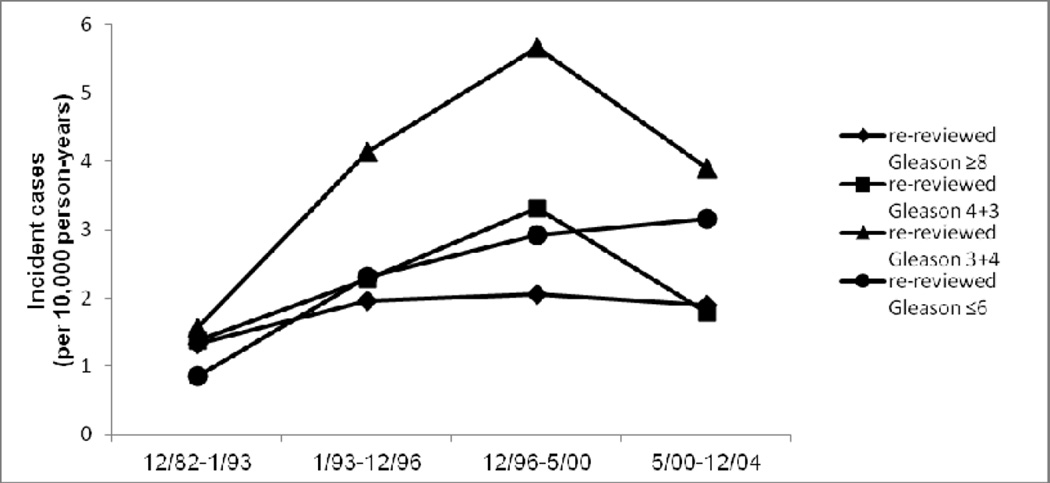
Widespread PSA screening not only advanced the time of diagnosis of prostate cancer, but also has increased the prevalence of detected indolent tumors that would otherwise never have been diagnosed. Thus, prostate cancers diagnosed in the PSA era are enriched with early stage and low grade. For this reason, the change in incidence of Gleason ≥8 tumors provides a stronger test of the hypothesis for Gleason progression over time than the proportion of ≥8 tumors among all tumors, which is also affected by the incidence of low grade cases diagnosed. While the incidence of re-reviewed Gleason ≤6 prostate cancer increased from 0.9 per 10,000 p-y in the first time period to 3.2 per 10,000 p-y in the last time period, and for Gleason 3+4 from 1.6 per 10,000 p-y to 3.9 per 10,000 p-y, the incidence rates of re-reviewed Gleason 4+3 and ≥8 cases showed no parallel change. Gleason 4+3 increased in the middle time periods, the rate came back down to baseline in the last time period (rates were 1.4, 2.3, 3.3, 1.8 per 10,000 p-y across the four time periods). For Gleason ≥8, rates were 1.3, 2.0, 2.0, and 1.9 per 10,000 p-y across the four time periods, respectively (Figure 2). In contrast, the incidence rate of late stage disease decreased markedly over time, with no similar decrease in early stage disease; this result was similar for all prostate cancer cases in PHS/HPFS and in the subset with re-reviewed Gleason score (Supplementary Figure 1).
Figure 2.
Incidence rate (per 10,000 person-years) of re-reviewed Gleason ≤6, 3+4, 4+3 and ≥8 disease across four time periods.
Discussion
Our data confirm the dramatic shift toward lower stage disease in prostate cancer cases diagnosed in the pre-PSA era to more recent years. In contrast, the decrease in the proportion of high grade (Gleason 8–10) cancers across this time period is considerably less dramatic than the shift in clinical stage, and we observed no significant reduction in the incidence of high grade cancers over time. The moderate decrease in the proportion of high grade cases may be due to the increased diagnosis of low-volume, non-progressing disease – cases that would otherwise never have been detected if not for PSA screening. These additional cases are more likely to be indolent and therefore low grade disease. Thus, the analysis of absolute incidence of Gleason 8–10 over time provides a better assessment of change, and demonstrated little evidence for a decrease. These results are supported by an analysis utilizing Surveillance, Epidemiology and End Results (SEER) Program data. These data show that with the introduction of PSA screening and throughout the 1990s, the incidence rate of well and poorly differentiated cancers increased slightly, but most of the increase in the rate was due to moderately differentiated tumors(19).
Although we cannot rule out the possibility that Gleason grade progresses within an individual, we conclude that it is not a major feature of prostate cancer. Our results are additionally supported by Falzarano et al.(15) who found that upon Gleason score re-review, there was no difference in Gleason distribution between the early PSA and late PSA eras(15). This topic was explored in a slightly different way by Pashayan et al.(20), comparing prostate cancer detected in a PSA screened population to cancers diagnosed clinically during the same time period (2002–2005). The authors observed the proportion of advanced stage decreased with PSA screening, but this seemed to be mainly in cases with Gleason score <7, again confirming the strong association between grade and stage as well as the potential for over-diagnosis. Studies have also attempted to estimate the proportion of tumors where Gleason might progress using statistical modeling(21, 22), finding that dedifferentiation is a likely and common event. However, numerous assumptions are required in these statistical models, and the observed data are also compatible with little or no progression in Gleason grade. Sowalsky et al.(23) and Kovtun et al. (24) report molecular data suggesting the potential for Gleason progression, but because of the cross-sectional nature of the studies, such conclusions are not definitive.
If the Gleason grade of a tumor typically does not progress, this information can affect clinical management of prostate cancer diagnosed with a low Gleason score. These findings do not answer the question of whether other tumor foci with higher Gleason scores were missed by the random biopsy sampling. Nonetheless, the knowledge that Gleason score largely does not progress may make the choice of active surveillance more appealing for patients with low grade disease. If we suppose that a Gleason score 3+3 will remain 3+3 for the entire course of the disease, active surveillance could be considered a definitive treatment for selected patients with ≤10 years life expectancy and could significantly delay (potentially forever) the treatment of selected patients with >10 years life expectancy. This option would prevent side effects from radiation or surgery for patients who do not need these more aggressive treatments. This will become more important with the development of improved imaging and molecular tools to assess the entire prostate at diagnosis.
Our findings have implications for understanding the biology of prostate cancer as well. Gleason score is a strong predictor of prostate cancer-specific death, and seems set early in the disease process; this suggests that later influences, such as diet, lifestyle, or environmental factors, might be important to trigger disease progression among men with low grade disease. Alternatively, earlier influences, such as genetics, may drive the development of a subtype of cancer that is more aggressive in a way that is not related to differentiation status. Lavery and Droller(25) posit that Gleason patterns 3 and 4 represent separate cancer diatheses, and the faster proliferation of pattern 4 compared to 3 may explain the apparent evolution of pattern 3 to 4. Different risk factors (genetics or environmental) may lead separately to the development of Gleason grade 3 or grade 4 disease.
There are limitations of this study to consider. We restricted the analysis to patients with radical prostatectomy so that we were able to acquire the tissue to re-review the Gleason score. This is a crucial aspect of the study since we avoid the issue with changes in grading criteria over time; however, this does assume that the indications for prostatectomy are constant over time, which may be questioned. Clinicians and surgeons may have changed their practices regarding which patients based on Gleason score are eligible for radical prostatectomy. Additionally, because we do not deal with biopsy tissue sampling variability, the clinical implications are currently restricted. However, if a similar trend is observed with biopsy specimens this should confirm our results.
A major strength of this study is its large size and our ability to compare time periods spanning the pre-PSA and PSA era. This allows for the observation at the population level that the shift in Gleason scores over time is much less dramatic than the shift in stage. Although we cannot exclude the potential for progression of Gleason score in some individual cases, we therefore conclude that such progression is not a major feature of prostate cancer.
Supplementary Material
Acknowledgements
We are grateful to the participants and staff of the Health Professionals Follow-up Study for their valuable contributions. In addition we would like to thank the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY.
Grant Support: This work was supported by the Dana-Farber/Harvard Cancer Center Prostate SPORE (5P50CA090381-08) and the National Cancer Institute (5R01CA141298).The PHS was supported by grants CA34944, CA40360, and CA097193 from the National Cancer Institute and HL26490 and HL34595 from the National Heart, Lung, and Blood Institute. The HPFS was supported by CA55075. KLP was supported by a National Research Service Award (T32 CA009001-32); JAS is supported by T32 CA009001-32. KLP and LAM are supported by the Prostate Cancer Foundation.
Footnotes
Conflict of Interest: The authors disclose no potential conflicts of interest.
Statement of Author Contributions: KLP, MJS, MF, and JAS conceived the project. KLP performed the analysis. RF, SF, ML, and MF provided pathological data and expertise. HDS, MJS, LAM, JLJ and EG collected study data and materials, and provided study support. All authors were provided comments and were involved in writing the paper, and had final approval of the submitted version.
References
- 1.Andren O, Fall K, Franzen L, Andersson SO, Johansson JE, Rubin MA. How well does the Gleason score predict prostate cancer death? A 20-year followup of a population based cohort in Sweden. J Urol. 2006;175(4):1337–1340. doi: 10.1016/S0022-5347(05)00734-2. [DOI] [PubMed] [Google Scholar]
- 2.Stark JR, Perner S, Stampfer MJ, Sinnott JA, Finn S, Eisenstein AS, et al. Gleason score and lethal prostate cancer: does 3 + 4 = 4 + 3? J Clin Oncol. 2009;27(21):3459–3464. doi: 10.1200/JCO.2008.20.4669. PMCID: 2717753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choo R, Danjoux C, Morton G, Szumacher E, Sugar L, Gardner S, et al. How much does Gleason grade of follow-up biopsy differ from that of initial biopsy in untreated, Gleason score 4–7, clinically localized prostate cancer? Prostate. 2007;67(15):1614–1620. doi: 10.1002/pros.20648. [DOI] [PubMed] [Google Scholar]
- 4.Sheridan TB, Carter HB, Wang W, Landis PB, Epstein JI. Change in prostate cancer grade over time in men followed expectantly for stage T1c disease. J Urol. 2008;179(3):901–904. doi: 10.1016/j.juro.2007.10.062. discussion 4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porten SP, Whitson JM, Cowan JE, Cooperberg MR, Shinohara K, Perez N, et al. Changes in prostate cancer grade on serial biopsy in men undergoing active surveillance. J Clin Oncol. 2011;29(20):2795–2800. doi: 10.1200/JCO.2010.33.0134. [DOI] [PubMed] [Google Scholar]
- 6.Johansson JE, Andren O, Andersson SO, Dickman PW, Holmberg L, Magnuson A, et al. Natural history of early, localized prostate cancer. JAMA. 2004;291(22):2713–2719. doi: 10.1001/jama.291.22.2713. [DOI] [PubMed] [Google Scholar]
- 7.Epstein JI, Walsh PC, Carter HB. Dedifferentiation of prostate cancer grade with time in men followed expectantly for stage T1c disease. J Urol. 2001;166(5):1688–1691. [PubMed] [Google Scholar]
- 8.Draisma G, Etzioni R, Tsodikov A, Mariotto A, Wever E, Gulati R, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101(6):374–383. doi: 10.1093/jnci/djp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finne P, Fallah M, Hakama M, Ciatto S, Hugosson J, de Koning H, et al. Lead-time in the European Randomised Study of Screening for Prostate Cancer. Eur J Cancer. 2010;46(17):3102–3108. doi: 10.1016/j.ejca.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 10.Savage CJ, Lilja H, Cronin AM, Ulmert D, Vickers AJ. Empirical estimates of the lead time distribution for prostate cancer based on two independent representative cohorts of men not subject to prostate-specific antigen screening. Cancer Epidemiol Biomarkers Prev. 2010;19(5):1201–1207. doi: 10.1158/1055-9965.EPI-09-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galper SL, Chen MH, Catalona WJ, Roehl KA, Richie JP, D'Amico AV. Evidence to support a continued stage migration and decrease in prostate cancer specific mortality. J Urol. 2006;175(3 Pt 1):907–912. doi: 10.1016/S0022-5347(05)00419-2. [DOI] [PubMed] [Google Scholar]
- 12.Moore AL, Dimitropoulou P, Lane A, Powell PH, Greenberg DC, Brown CH, et al. Population-based prostate-specific antigen testing in the UK leads to a stage migration of prostate cancer. BJU Int. 2009;104(11):1592–1598. doi: 10.1111/j.1464-410X.2009.08652.x. [DOI] [PubMed] [Google Scholar]
- 13.Derweesh IH, Kupelian PA, Zippe C, Levin HS, Brainard J, Magi-Galluzzi C, et al. Continuing trends in pathological stage migration in radical prostatectomy specimens. Urol Oncol. 2004;22(4):300–306. doi: 10.1016/j.urolonc.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Jhaveri FM, Klein EA, Kupelian PA, Zippe C, Levin HS. Declining rates of extracapsular extension after radical prostatectomy: evidence for continued stage migration. J Clin Oncol. 1999;17(10):3167–3172. doi: 10.1200/JCO.1999.17.10.3167. [DOI] [PubMed] [Google Scholar]
- 15.Falzarano SM, Magi-Galluzzi C. Prostate cancer staging and grading at radical prostatectomy over time. Adv Anat Pathol. 2011;18(2):159–164. doi: 10.1097/PAP.0b013e31820cb506. [DOI] [PubMed] [Google Scholar]
- 16.Albertsen PC, Hanley JA, Barrows GH, Penson DF, Kowalczyk PD, Sanders MM, et al. Prostate cancer and the Will Rogers phenomenon. J Natl Cancer Inst. 2005;97(17):1248–1253. doi: 10.1093/jnci/dji248. [DOI] [PubMed] [Google Scholar]
- 17.Final report on the aspirin component of the ongoing Physicians' Health Study. Steering Committee of the Physicians' Health Study Research Group. N Engl J Med. 1989;321(3):129–135. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 18.Montironi R, Cheng L, Lopez-Beltran A, Scarpelli M, Mazzucchelli R, Mikuz G, et al. Original Gleason system versus 2005 ISUP modified Gleason system: the importance of indicating which system is used in the patient's pathology and clinical reports. Eur Urol. 2010;58(3):369–373. doi: 10.1016/j.eururo.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 19.Crawford ED. Epidemiology of prostate cancer. Urology. 2003;62(6 Suppl 1):3–12. doi: 10.1016/j.urology.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Pashayan N, Pharoah P, Neal DE, Hamdy F, Donovan J, Martin RM, et al. Stage shift in PSA-detected prostate cancers - effect modification by Gleason score. J Med Screen. 2009;16(2):98–101. doi: 10.1258/jms.2009.009037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Draisma G, Postma R, Schroder FH, van der Kwast TH, de Koning HJ. Gleason score, age and screening: modeling dedifferentiation in prostate cancer. Int J Cancer. 2006;119(10):2366–2371. doi: 10.1002/ijc.22158. [DOI] [PubMed] [Google Scholar]
- 22.Pashayan N, Pharoah P, Neal DE, Hamdy F, Donovan J, Martin RM, et al. PSA-detected prostate cancer and the potential for dedifferentiation--estimating the proportion capable of progression. Int J Cancer. 2011;128(6):1462–1470. doi: 10.1002/ijc.25471. [DOI] [PubMed] [Google Scholar]
- 23.Sowalsky AG, Ye H, Bubley GJ, Balk SP. Clonal progression of prostate cancers from Gleason grade 3 to grade 4. Cancer Res. 2013;73(3):1050–1055. doi: 10.1158/0008-5472.CAN-12-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovtun IV, Cheville JC, Murphy SJ, Johnson SH, Zarei S, Kosari F, et al. Lineage relationship of Gleason patterns in Gleason score 7 prostate cancer. Cancer Res. 2013;73(11):3275–3284. doi: 10.1158/0008-5472.CAN-12-2803. [DOI] [PubMed] [Google Scholar]
- 25.Lavery HJ, Droller MJ. Do Gleason patterns 3 and 4 prostate cancer represent separate disease states? J Urol. 2012;188(5):1667–1675. doi: 10.1016/j.juro.2012.07.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.