Abstract
Wolbachia pipientis is the most common bacterial infection in the animal world and wields a vast influence on invertebrate reproduction, sex determination, speciation, and behavior worldwide. These avenues of research have made seminal gains, including the latest use of Wolbachia to alter mosquito populations and a strengthened focus on using anti-Wolbachia therapies against filarial nematode infections. This work is further bolstered by a more refined knowledge of Wolbachia biology spanning mechanisms to relevance. Here we tally the most up-to-date knowledge in the field and review the immense implications that this global infection has for the basic and applied life sciences.
Keywords: Wolbachia pipientis, vector control, filarial disease
The great Wolbachia pandemic
Wolbachia pipientis is an obligate, intracellular α-proteobacteria and a member of the Rickettsiales family. These gram-negative bacteria are not culturable outside of host cells and, as a result, knowledge on the symbiosis has only surged in the last two decades owing to readily available molecular techniques. Once considered an obscure bacterium in a few insect species, the most recent meta-analysis estimates that ~40% of all arthropod species are infected with Wolbachia [1] as well as 47% of the Onchocercidae family of filarial nematodes [2] (see Box 1 for description of strains). Arthropods are by far the most common group of animals with likely millions of species; thus, from a biodiversity perspective, Wolbachia infections are one of the great pandemics in the history of Life.
Box 1. Wolbachia strains.
The speciesWolbachia pipientisis divided into separate but related subclades or supergroups that are denoted by capital letters. For instance, supergroups A, B, E, and H infect arthropods while supergroups C and D reside in nematode species. The F group is unique in that it infects both arthropods and filarial nematodes. These supergroups can be further divided into specificWolbachiastrains that are named based on the host species they infect, such as thewMel strain found inDrosophila melanogasterand thewPip strain inCulex pipiens. Some hosts carry multiple supergroups, such asAedes albopictusthat harbors bothwAlbA andwAlbB. As with other species of bacteria, the genomes of differentWolbachiastrains can vary substantially while still maintaining a core set of genes.
While these infections rampantly transfer between different arthropod hosts on an evolutionary time scale, including a recent study that showed ingestion of infected arthropods can lead to infection [3], Wolbachia are predominantly transmitted vertically by maternal inheritance via infected stem cells of the ovaries to developing oocytes [4]. On top of the ability to both switch hosts and transmit vertically, Wolbachia use several parasitic and mutualistic mechanisms to increase the number of infected females in a new host population. These host manipulations are so successful for spreading the infection that they have led to proposals on how to commandeer Wolbachia for human benefit at global scales. For instance, the remarkable discoveries that infected mosquitos show resistance to dengue, Chikungunya virus, yellow fever, and even malaria [5,6] create a potentially cheap and sustainable system in which the great pandemic of Wolbachia can be used to control mosquito and other insect vectors. Equally exciting are areas that have focused on the mutualistic role that Wolbachia play with filarial nematodes, the causative agents of lymphatic filariasis and river blindness that afflict hundreds of millions of people [7,8]. This research aims to eliminate the Wolbachia infection and thereby reduce the fitness of the nematodes that depend on it. Here we will highlight recent advances in understanding the biology and spread of Wolbachia, its role in curbing human diseases, and the emerging fields of Wolbachia genomics and host-parasite interactions.
How did Wolbachia spread worldwide?
W. pipientis have an arsenal of host reproductive manipulations that propagated their worldwide prevalence, including feminization, parthenogenesis (see Glossary), male-killing, and cytoplasmic incompatibility (CI). These phenotypes serve to increase the frequency of infected females in a host population and therefore the scale of maternal transmission of Wolbachia to the next generation. While each of these modifications has been characterized at a foundational level, their underlying mechanisms are enigmatic [7].
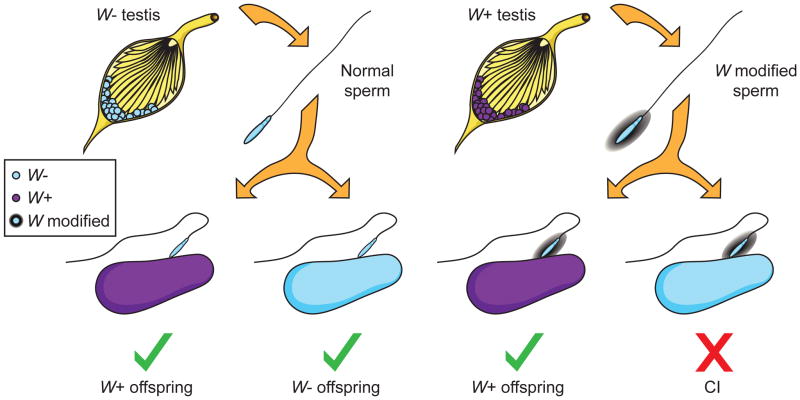
The most heavily studied adaptation of Wolbachia is CI, which occurs as a conditional reproductive failure that manifests as embryonic lethality between eggs from uninfected females and modified sperm from infected males (Figure 1). However, the reciprocal cross as well as crosses between males and females infected with the same Wolbachia strain are viable [10]. When compared to uninfected females, this unidirectional incompatibility underscores a dramatic, relative fitness increase for infected females - the transmitting gender of Wolbachia. Wolbachia have been repeatedly observed to spread via CI in natural and laboratory populations, with recent analyses in tsetse flies [8] and the grasshopper Chorthippus parallelus [9].
Figure 1. Wolbachia-induced cytoplasmic incompatibility.
Wolbachia (W, purple) infection causes a modification in the sperm that can be rescued by eggs of infected females but leads to embryonic death in uninfected embryos. Abbreviations: W−, Wolbachia-uninfected; W+, Wolbachia-infected; W modified, Wolbachia-modified sperm. Illustration by Robert M. Brucker.
How is cytoplasmic incompatibility induced?
One of the most pre-eminent questions in the Wolbachia field is how does the infection cause CI and other reproductive alterations? Extensive cytological studies of embryonic defects in model insects provide hints as to how CI causes lethality after fertilization. First, the majority of embryonic arrest is associated with shortcomings in the first mitotic division [13]. They include a failure of the paternal nuclear envelope to break down [14], delayed Cdk1 activation [14], an inability to correctly deposit maternal histones in the paternal genome, and slowed replication of the sperm DNA [15]. These delays in cell cycle progression are accompanied by severe chromosomal defects, specifically in the paternal DNA, which include incomplete condensation and failure to segregate correctly. In addition, CI embryos contain an excess of centrosomes that are unassociated with the pronuclei [16,17]. This latter defect can be explained by a combination of mitotic delays and incomplete chromosome condensation, which are known to dissociate centrosomes from nuclei [18].
In the absence of data on the exact sperm modification used to cause embryonic lethality, investigators have turned to conceptual models that may explain CI-associated defects. They are based on the positions that (i) Wolbachia modify the sperm to cause severe defects in the timing and progression of mitosis, and (ii) this modification can be ‘rescued’ by a female infected with the same strain [19]. Males and females infected with genetically-distinct strains (Box 1) are bidirectionally or reciprocally incompatible. For many years, the two predominant models for CI have been (i) the lock and key model and (ii) the mistiming model. The lock and key model posits that Wolbachia place certain ‘locks’ on the paternal genome. A female infected with the same strain of Wolbachia has the appropriate ‘keys’ to remove these locks after fertilization and rescue the mitotic defects that may occur [20]. The mistiming model, however, suggests that CI results from mitotic mistiming between the maternal and paternal pronuclei [20]. An infected female is able to rescue this disparity by making compensatory changes in either pronucleus. The mistiming model can be expanded to suggest that the asynchrony actually occurs between the paternal pronuclei and maternal cytoplasm [7,18].
While each of the above models has its merits, neither fully explains current observations for CI. For instance, the lock and key model suggests that each strain has its own encrypted locks and keys. The known strain incompatibilities, however, quickly demand a questionably large number of different locks and keys, especially considering the speed with which new incompatibilities arise [22]. The mistiming model also fails to account for strain specificity. For example, data show that strain wSan of Drosophila santomea is capable of rescuing CI caused by strain wRi of Drosophila simulans and that wRi can rescue strain wMel of D. melanogaster. When tested, however, wSan is unable to rescue wMel [23,24], an incongruity not explained by the mistiming model. It is obvious that our understanding is incomplete and new simulations, such as the goalkeeper model proposed by Bossan et al. [25], are attempting to fill this gap. Briefly, this model posits that a rescuing strain of Wolbachia must act as a ‘goalkeeper’ to block CI. This action requires the bacteria to utilize two separate ‘factors’, which can range from mistiming of the parental genomes to various bacterial proteins or even phage components. These two factors are equivalent to a keeper jumping both far enough and high enough to block a soccer goal. They could also be altered by host conditions (equivalent to placing the goalkeeper on a stool or in a trench) and thereby explain the dependence on host genotype. This model is supported by growing evidence that multiple factors are involved in rescue [23].
Finally, it has been known for many years that titers of Wolbachia are, in general, positively associated with CI [26,27]. Each of the proposed models, whether it is through failure to place enough locks on the host genome, a struggle to induce mistiming, or a lack of sufficient modifying factors, can easily explain how low Wolbachia density fails to induce complete cytoplasmic incompatibility. Similar to mistiming, however, infection load cannot fully clarify the mechanism of CI.
Causal factors for CI
Current efforts to identify the causal factor for CI are varied and have been largely unsuccessful. Initial work looking at host gene expression in infected versus uninfected hosts implicated the host histone chaperone Hira [28]. Research has also focused on the unusually large number of Wolbachia proteins that contain ankyrin repeat domains. This domain is usually implicated in protein-protein interactions, and was thus a tempting candidate for host modifications. Multiple studies have looked at the link between these proteins and cytoplasmic incompatibility and, while some are regulated in a sex-specific manner, none were shown to be involved in CI [29–31]. Finally, there might be a link between reactive oxygen species (ROS) and the induction of CI. Specifically, Wolbachia infection leads to increased levels of ROS in testes and ovaries, and these reactive oxygen species lead to damaged spermatid DNA [32]. This discovery is interesting as DNA damage induced by ROS can account for several hallmarks of CI including defective paternal chromatin, delayed Cdk1 activation, and failed mitosis. Future research should determine how large a role DNA damage induced by reactive species actually plays in the induction of CI.
Future investigations into CI
While the exact mechanism of cytoplasmic incompatibility remains elusive, future studies will be strengthened by several developments within the field. One other bacterium, Cardinium hertigii, is known to induce CI, and in hosts infected by both bacteria, there is an additive effect to embryonic lethality [33]. The genome for Cardinium is now available, and comparative sequence analyses suggest an independent origin of CI [34]. A renewed focus on the other reproductive alterations induced by Wolbachia should also prove informative. Exciting, recent work demonstrates that male-killing, like CI, is associated with damaged paternal chromatin [35]. Further links between CI and male-killing are evident as CI-inducing Wolbachia from Drosophila recens elicit male-killing when transferred to Drosophila subquinaria, with similar effects observed in strains transferred between moths [36,37]. Other work shows that the male-killing strain wInn (from Drosophila innubila) fails to induce CI or male-killing in D. melanogaster and D. simulans. These results, however, can be explained by low titers, especially in sperm cysts, in the recipient hosts [38]. Finally, strains from Drosophila bifasciata that exhibit incomplete male-killing can also induce CI [39]. The growing links between the types of Wolbachia-induced sexual parasitism suggest a related underlying mechanism that warrants future exploration and modeling.
Commandeering the Wolbachia pandemic for vector control
The study of Wolbachia is a pre-eminent example of how basic science can translate to biomedical science. Once studied as an obscure reproductive modification, CI is now at the center stage of efforts to control the transmission of human pathogens through mosquito vectors. In particular, species infected with Wolbachia have increased resistance against dengue, Chikungunya, yellow fever, and West Nile viruses, as well as malaria and bacteria [6,40–42], though some hosts show increased susceptibility [43]. The twofold advantage of Wolbachia to both decrease pathogen replication and deterministically spread via CI in insect vectors has direct implications for quelling the transmission of infections to humans.
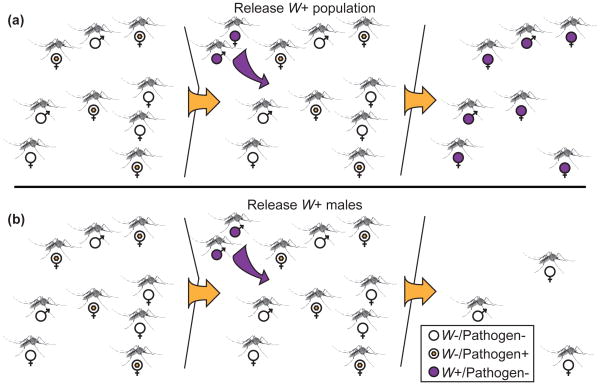
Two strategies take advantage of this system to reduce vector numbers and transmission competency. First, a large release of Wolbachia-infected, and therefore pathogen-depleted mosquitoes, could replace the local population of Wolbachia-uninfected animals through CI. As we discuss below, this Population Replacement Strategy (PRS) (Figure 2A) has made impressive progress in the past few years via the International Eliminate Dengue Project (EDP). A second strategy, known as the Incompatible Insect Technique (IIT) (Figure 2B), is to release only CI-inducing males into uninfected vector populations, which can then sterilize a large fraction of the females and drastically reduce overall vector numbers [44]. This population suppression has been successfully employed to control farm pests [45], and there is an ongoing field study on islands in the Indian Ocean, which aims to reduce Culex pipiens quinquefasciatus numbers to control filarial parasites and arboviruses [46]. A final technique, which utilizes the lifespan shortening ability of Wolbachia infection, has been proposed, but implementation is difficult, as models predict that a shortened lifespan substantially negates any fitness conferred by a genetic drive mechanism such as CI, making it exceedingly difficult to replace native populations [47].
Figure 2. Vector control strategies.
(A) Population Replacement Strategy switches a wild population of mosquitoes (pathogen carrying, Wolbachia uninfected) with a pathogen-free one through Wolbachia-induced CI. (B) In the Incompatible Insect Technique, a release of just Wolbachia infected males leads to high levels of CI and a reduction in the total vector population. Abbreviations: W−, Wolbachia-uninfected; W+, Wolbachia-infected; Pathogen+, pathogen-infected; Pathogen-, pathogen-uninfected. Illustration by Robert M. Brucker.
Eliminate Dengue Program
The Eliminate Dengue Program was originally established in Australia with the aim of using Wolbachia-based strategies to curb the spread of dengue, a mosquito-borne disease. Early efforts focused on using the wMelPop strain of Wolbachia [48], but in 2011, the EDP stably infected the mosquito vector of dengue, Aedes aegypti, with the wMel Wolbachia strain from D. melanogaster [49]. The feat was accomplished by passaging the bacteria for several years in an Aedes albopictus cell line before microinjection into the mosquitoes. The long term in vitro cultivation in mosquito cells led to attenuated virulence in the mosquito species in vivo and a normal host lifespan; yet, remarkably the wMel strain retained high rates of maternal transmission, the capacity to spread through experimental populations by CI, and the crucial refractoriness to dengue virus. Controlled release of these mosquitoes into a small number of Australian neighborhoods effectively replaced the native population with a dengue-free vector [50]. While data on whether the population replacement has reduced the incidence of human dengue cases will take many years to assess, the EDP is quickly scaling their approach throughout the world. Recent estimates suggest that dengue infects 390 million people per year with 96 million showing some level of disease severity [51]. The vast majority of these cases are in Southeast Asia and South America where the EDP has research centers in China, Indonesia, Vietnam, and Brazil. These locations will give the EDP a growing influence in the spread of dengue among the most heavily affected areas in the world.
The success of the EDP has inspired a broad push to identify applications for Wolbachia in other disease vectors (Box 2). Of particular interest are the anopheline mosquitoes, the main carriers of malaria. Every sampled species of Anopheles lacks Wolbachia, and while Anopheles gambiae can be somatically infected by Wolbachia strains from D. melanogaster and Aedes albopictus, stable germ line infection with high maternal transmission has historically been difficult [52,53]. Recently, however, that hurdle was overcome by stably infecting anopheline mosquitoes with microinjections of Wolbachia into eggs. The resultant mosquitoes show few defects, induce CI, and cause refractoriness to Plasmodium infection [54]. This exciting new work now places Wolbachia-based control of mosquitoes that transmit malaria within sight.
Box 2. Outstanding questions.
How is Wolbachia pipientis able to easily manipulate the host reproductive system and, specifically, how does it induce cytoplasmic incompatibility?
Can the success of the Eliminate Dengue Project be expanded into other mosquito-borne viruses such as Chikungunya and yellow fever?
Can Wolbachia-specific antibacterial drugs with no contraindications be developed to target filarial nematode infections?
Can bacteriophage WO be developed either into a transgenic vector for Wolbachia and/or an anti-Wolbachia therapeutic for treating filarial diseases?
There has also been work to identify the infection status of other mosquito species to test the applicability of population replacement by Wolbachia in the vectors of yellow fever and lymphatic filariasis [55]. Additionally, Wolbachia have been proposed as a possible means to control bed bugs [56] and tsetse flies [57]. In bed bugs, resistance to pyrethroid insecticides is common, and thus alternative methods using Wolbachia are welcome developments. Finally, the use of Wolbachia in tsetse flies is especially enticing, as they spread trypanosomes and sleeping sickness, and Wolbachia are already known to induce CI in some tsetse species [8]. Possible methods for vector control in tsetse flies, and their comparison to current techniques used in sub-Saharan Arica, are reviewed in Doudoumis et al. [57].
Caveats to Wolbachia-based vector control
While the discovery of Wolbachia-based anti-pathogen resistance [58,59] has attracted widespread attention for its role in vector control, the mechanism behind this super-charged host protection remains an area of active investigation. It was first hypothesized that Wolbachia, by virtue of its transgenerational persistence, simply primes the host immune system and thereby encourages the clearance of viral particles. Indeed, Wolbachia heat shock and surface proteins stimulate the expression of innate immunity genes including cytokines, defensins, proteases, and peptidoglycan-recognizing proteins [52,60,61]. These results, though, may arise from contamination originating in the E. coli expression systems utilized or non-physiologically relevant concentrations of protein. These alternatives are perhaps reinforced by data, which show that infected cell lines gain protection from viruses [62] even though they lack several components of the innate immune system, including fat bodies and phagocytosing blood cells. Interestingly, the Wolbachia strain wMelPop-CLA confers viral protection to both A. aegypti and D. melanogaster individuals, but it only upregulates immunity genes in the mosquitoes [63]. While ‘priming’ of the immune system might be insufficient to confer viral resistance, components of the innate immune system such as ROS and the Toll pathway are known to play large roles in Wolbachia-induced protection in A. aegypti [64]. Additionally, the innate antiviral siRNA pathway is not required for viral protection in infected D. melanogaster [65], and increased immunity is dependent on where Wolbachia localizes within D. simulans [66]. These conflicting results will hopefully be resolved in coming years by comparative studies of species that do not receive pathogen protection from Wolbachia infection [43,67] or are actually weakened by the bacteria [68]. Finally, recent evidence suggests that Wolbachia-associated expression of host miRNAs [69,70] assists regulation of dengue virus titers within mosquitoes [71].
Symbiotic transitions: from a pandemic to a mutualist
W. pipientis is well known for its parasitic phenotypes, yet it also has a mutualistic relationship with several invertebrate species. The archetypal example occurs in filarial nematodes, in which 47% of the Onchocercidae family are infected by Wolbachia [2], and both host and bacteria are completely dependent upon each other. Interestingly, almost every disease-causing species of filarial nematode are infected with Wolbachia. A watershed moment in the science of filarial diseases occurred when studies implicated Wolbachia as the chief cause of debilitating ailments such as river blindness and lymphatic filariasis [72], reviewed in [73]. The nature of this mutualism is slowly being elucidated, and there is strong evidence that the bacteria provide essential nutrients to the host, including riboflavin, heme, and flavin adenine dinucleotide (FAD) [74–76]. Recently, based on genome and transcriptome sequencing of Onchocerca worms, it was suggested that Wolbachia play a defensive, antibacterial role and have possible mitochondria-like actions such as providing energy and metabolites [8]. These observations are supported by work that shows dramatic increases in Wolbachia titers when the host is undergoing high levels of growth and division that demand increased metabolism [77–79].
The symbiosis between Wolbachia and filarial nematodes is tightly controlled. Studying specific cell lineages, it has been found that the parasite positions itself in the hypodermal chords of developing zygotes and later is able to specifically invade the gonads before sexual maturity is achieved [80,81]. The nematode maintains strict control over this interaction through host autophagy [82]. This inter-dependence is reinforced by horizontal gene transfer between the bacteria and host, such as in Brugia malayi [83]. There is also growing evidence that other filarial nematodes exist independent of Wolbachia and may have lost the bacteria after an ancient infection [2].
Although W. pipientis is required in many filarial nematodes, its mutualistic relationships with arthropods are more varied. In Aedes polynesiensis, infection is associated with decreased larval mortality and increased adult lifespan [84]. In other species, such as C. pipiens quinquefasciatus and brown planthoppers, Wolbachia increases the number of embryos surviving to adulthood but decreases adult lifespan [85,86]. In A. aegypti, however, Wolbachia decreases embryo survivability, and in the moth Ephestia kuehniella, infection reduces the number of viable sperm [87,88]. In other species, such as rice water weevils and the wasp Asorbara tabida, a Wolbachia infection is absolutely required for oogenesis [89,90]. Finally in Drosophila mauritiana, Wolbachia infections in the ovarian stem cells accelerate mitosis, leading to a fourfold increase in egg numbers compared to uninfected counterparts [91]. While these various phenotypes show little correlation with each other, one interesting hypothesis is that they might represent various stages of a parasitic-to-mutualistic continuum between Wolbachia and invertebrate hosts. A mutualistic or codependent relationship would be beneficial for both organisms and could be selected for over time. Interestingly, this exact transition has been observed in nature over the course of just a few decades with fruit flies [92].
Removing the mutualist to cure filarial diseases
In contrast to spreading the Wolbachia reproductive parasites in arthropods for vector control, the profound health repercussions for Wolbachia mutualisms are based on eliminating them. Specifically, in the filarial nematodes, curing Wolbachia can halt nematode growth, encourage apoptosis, and eventually lead to death of the worm [93]. These nematodes cause diseases such as lymphatic filariasis and onchocerciasis, which together account for 140 million infections a year. These afflictions threaten 1.4 billion people annually, yet alarmingly over 20 years have passed since the last anti-filarial drug was developed. More importantly, current treatment protocols are losing efficacy, and resistance is of growing concern [94].
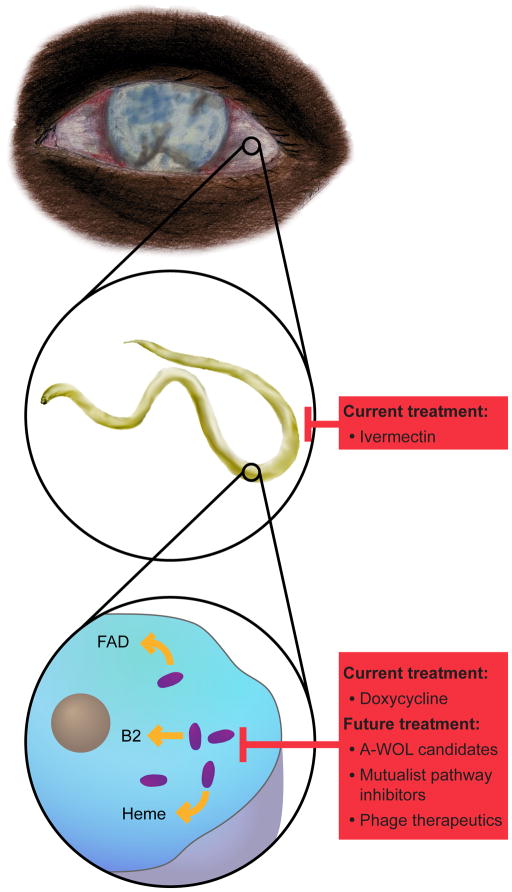
Research into Wolbachia-nematode interactions was boosted after the genomes for the main causative factor of lymphatic filariasis, B. malayi, and its Wolbachia symbiont, wBm, were published [74,75]. These studies enabled comparative genomic analyses of the pathways that complement missing functions in both the host and symbiont. As mentioned before, this work showed that B. malayi is reliant on factors such as riboflavin, heme, and FAD produced by the bacteria (Figure 3). Interestingly, it also revealed many of the specific metabolites that Wolbachia requires from its host. These include coenzyme A, biotin, and nicotinamide adenine dinucleotide (NAD), as well as ubiquinone, lipoic acid, folate, and pyridoxal phosphate. Whether any of these pathways can be successful drug targets is yet to be determined. Further candidates will also be elucidated as comparative genomics of nematodes and their Wolbachia continues with the more recent analyses of the uninfected nematode, Loa loa [95] and the F group Wolbachia [2].
Figure 3. Elimination of filarial nematodes.
Diseases such as river blindness and lymphatic filariasis, traditionally treated with anti-filarial medications, could benefit from anti-Wolbachia approaches that target the host-bacterial symbiosis. Illustration by Robert M. Brucker.
In addition to the factors mentioned above, other work has focused on identifying specific Wolbachia pathways and their role in the host-symbiont relationship. Initial results have recognized heme biosynthesis, DNA ligases, FtsZ, ClpP peptidase, lipoprotein biosynthesis, and pyruvate phosphate dikinase (PPDK) in the bacteria as promising targets for drug development [96–102]. While these pathways share little in common, the broad range of treatment candidates that they represent could enable Wolbachia-specific therapy. Finally, in a directed effort to discover drugs that treat river blindness and filariasis, the Anti-Wolbachia Consortium (A-WOL) has recently begun screening compounds that can target the infection in a mosquito cell line. These efforts have looked at over 2600 current drugs as well as 67 000+ other compounds with full results coming soon [73].
The race to find new anti-filarial and anti-Wolbachia treatments is urgent. Despite success in eliminating nematode infections with doxycycline in small groups of individuals [103], the lengthy treatment regimes, the potential for evolution of widespread antibiotic resistance in the endogenous microflora, and restrictions against use in children and pregnant women make massive administration of doxycycline problematic. Indeed, the gut flora of treated individuals could act as a reservoir for drug resistance genes [104,105], and intracellular bacteria, while more restricted in horizontal gene transfer than free living species, have still shown a capacity to gain resistance [106]. There is still hope for alternative drugs, such as an anti-filarial vaccine (Box 2), to supplement current treatments. In fact, recent work shows that mice immunized with a single Wolbachia protein show strong, although not complete, resistance to nematode infection [107]. More treatment avenues will also open as in-depth research is conducted on the recently sequenced genomes of several filarial nematode species and their accompanying Wolbachia infections [108].
Concluding remarks and future perspectives
The field of Wolbachia research has matured considerably in recent years into large efforts with basic and applied approaches. From the humble beginnings of simple identification and classification, Wolbachia research has evolved into a globally important endeavor. Far from being an interesting side note in arthropod literature, this symbiont has shown great potential as a means for vector and filarial disease control. Success in controlling dengue fever in A. aegypti populations will hopefully translate over to other species, including the newly infected anopheline mosquitoes and tsetse flies. This research is paralleled by work in filarial nematodes that targets Wolbachia mutualists to eliminate diseases such as lymphatic filariasis and river blindness. Current drug screening efforts place these goals within reach. While there is continuing difficulty in the ability to genetically transform Wolbachia or to culture it outside host cells, making many modern techniques unavailable to researchers, much information has been gained thanks to new sequencing efforts. There are currently 19 sequenced strains of Wolbachia either completed or in progress [57,109,110] with more certainly to come. There has also been a growing appreciation for the role of bacteriophage WO in Wolbachia biology [111], with the lytic phage potentially offering a naturally-evolved anti-Wolbachia strategy for the treatment of filarial disease. The research community is compelled by surging amounts of evidence that Wolbachia have the potential to mediate diseases ranging from dengue fever and Chikungunya to malaria and lymphatic filariasis. These illnesses account for over 730 million infections every year and can be crushing social burdens for developing countries. The advancement of new techniques, and refinement of current ones, will hopefully allow the most abundant endosymbiont, W. pipientis, to make these diseases a thing of the past.
Highlights.
Wolbachia are one of the great pandemics of Life from a biodiversity perspective
The molecular basis of Wolbachia parasitism remains enigmatic
Wolbachia parasites can be deployed to curb natural mosquito vectors of dengue virus
Research on anti-Wolbachia drugs hold future promise to eradicate filarial disease
Acknowledgments
The preparation of this article was supported by awards NIH R01 GM085163 and NSF DEB 1046149 to S.R.B. and Cellular, Biochemical and Molecular Sciences Training grant support to D.L.P from NIH 5T32GM008554-17. We apologize in advance to our colleagues whose articles that we were unable to cite due to space limitations.
Glossary
- Bacteriophage WO
a temperate virus commonly found in arthropod Wolbachia
- Cytoplasmic incompatibility (CI)
a post-fertilization defect in chromatin where matings between infected males and Wolbachia-free females (or females harboring a different Wolbachia strain than that in the male) result in high levels of embryonic death
- Eliminate Dengue Project (EDP)
a worldwide effort to eliminate dengue virus through the spread of Wolbachia-infected mosquitoes
- Incompatible Insect Technique (IIT)
a method to reduce insect populations by sterilizing wild females with large releases of incompatible males
- Lock and key model
the concept that CI is caused by certain ‘locks’ placed on the paternal genome, which can arrest development and are only removed by specific ‘keys’ found in infected females
- Male-killing
the selective killing of male embryos
- Mistiming model
the concept that CI is induced by cell cycle timing defects during the first mitosis. Specifically, a delayed paternal pronuclei (when compared to the maternal pronuclei or cytoplasm), could account for cell cycle arrest and embryonic death
- Parthenogenesis
asexual reproduction whereby viable embryos develop from unfertilized eggs
- Population Replacement Strategy (PRS)
the use of a genetic drive mechanism, such as cytoplasmic incompatibility induced by Wolbachia infection, to replace a natural population with a laboratory-reared one
- Pronucleus
the nucleus of either the sperm or egg during fertilization
- Vertical transmission
the transmission of material from parent to offspring. Maternal transmission is a subtype, involving the specific passage from mother to child
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zug R, Hammerstein P. Still a host of hosts for Wolbachia: Analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS One. 2012;7:e38544. doi: 10.1371/journal.pone.0038544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferri E, et al. New insights into the evolution of Wolbachia infections in filarial nematodes inferred from a large range of screened species. PLoS One. 2011;6:e20843. doi: 10.1371/journal.pone.0020843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le Clec’h W, et al. Cannibalism and predation as paths for horizontal passage of Wolbachia between terrestrial isopods. PLoS One. 2013;8:e60232. doi: 10.1371/journal.pone.0060232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frydman HM, et al. Somatic stem cell niche tropism in Wolbachia. Nature. 2006;441:509–12. doi: 10.1038/nature04756. [DOI] [PubMed] [Google Scholar]
- 5.Iturbe-Ormaetxe I, et al. Wolbachia and the biological control of mosquito-borne disease. EMBO reports. 2011;12:508–18. doi: 10.1038/embor.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van den Hurk AF, et al. Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti. PLoS Neglected Tropical Diseases. 2012;6:e1892. doi: 10.1371/journal.pntd.0001892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melnikow E, et al. A Potential role for the interaction of Wolbachia surface proteins with the Brugia malayi glycolytic enzymes and cytoskeleton in maintenance of endosymbiosis. PLoS Neglected Tropical Diseases. 2013;7:e2151. doi: 10.1371/journal.pntd.0002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darby AC, et al. Analysis of gene expression from the Wolbachia genome of a filarial nematode supports both metabolic and defensive roles within the symbiosis. Genome Research. 2012;22:2467–2477. doi: 10.1101/gr.138420.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serbus LR, et al. The genetics and cell biology of Wolbachia-host interactions. Annual Review of Genetics. 2008;42:683–707. doi: 10.1146/annurev.genet.41.110306.130354. [DOI] [PubMed] [Google Scholar]
- 10.Ghelelovitch S. Genetic determinism of sterility in the cross-breeding of various strains of Culex autogenicus. C R Hebd Seances Acad Sci. 1952;234:2386–2388. [PubMed] [Google Scholar]
- 11.Alam U, et al. Wolbachia symbiont infections induce strong cytoplasmic incompatibility in the tsetse fly Glossina morsitans. PLoS Pathogens. 2011;7:e1002415. doi: 10.1371/journal.ppat.1002415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarasa J, et al. Wolbachia induced cytogenetical effects as evidenced in Chorthippus parallelus (Orthoptera) Cytogenetic and genome research. 2013;139:36–43. doi: 10.1159/000341572. [DOI] [PubMed] [Google Scholar]
- 13.Tram U, et al. Paternal chromosome segregation during the first mitotic division determines Wolbachia-induced cytoplasmic incompatibility phenotype. Journal of Cell Science. 2006;119:3655–63. doi: 10.1242/jcs.03095. [DOI] [PubMed] [Google Scholar]
- 14.Tram U. Role of delayed nuclear envelope breakdown and mitosis in Wolbachia-induced cytoplasmic incompatibility. Science. 2002;296:1124–1126. doi: 10.1126/science.1070536. [DOI] [PubMed] [Google Scholar]
- 15.Landmann F, et al. Wolbachia-mediated cytoplasmic incompatibility is associated with impaired histone deposition in the male pronucleus. PLoS Pathogens. 2009;5:e1000343. doi: 10.1371/journal.ppat.1000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Callaini G, et al. Wolbachia-induced delay of paternal chromatin condensation does not prevent maternal chromosomes from entering anaphase in incompatible crosses of Drosophila simulans. Journal of Cell Science. 1997;110:271–80. doi: 10.1242/jcs.110.2.271. [DOI] [PubMed] [Google Scholar]
- 17.Lassya CW, Karrb TL. Cytological analysis of fertilization and early embryonic development incompatible crosses of Drosophila simulans. Mechanisms of Development. 1996;57:47–58. doi: 10.1016/0925-4773(96)00527-8. [DOI] [PubMed] [Google Scholar]
- 18.Takada S, et al. Drosophila checkpoint kinase 2 couples centrosome function and spindle assembly to genomic integrity. Cell. 2003;113:87–99. doi: 10.1016/s0092-8674(03)00202-2. [DOI] [PubMed] [Google Scholar]
- 19.Werren JH. Biology of Wolbachia. Annual Review of Entomology. 1997;42:587–609. doi: 10.1146/annurev.ento.42.1.587. [DOI] [PubMed] [Google Scholar]
- 20.Poinsot D, et al. On the mechanism of Wolbachia-induced cytoplasmic incompatibility: confronting the models with the facts. BioEssays. 2003;25:259–65. doi: 10.1002/bies.10234. [DOI] [PubMed] [Google Scholar]
- 21.Ferree PM, Sullivan W. A genetic test of the role of the maternal pronucleus in Wolbachia-induced cytoplasmic incompatibility in Drosophila melanogaster. Genetics. 2006;173:839–47. doi: 10.1534/genetics.105.053272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duron O, et al. Rapid evolution of Wolbachia incompatibility types. Proceedings of the Royal Society B: Biological Sciences. 2012;279:4473–80. doi: 10.1098/rspb.2012.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zabalou S, et al. Multiple rescue factors within a Wolbachia strain. Genetics. 2008;178:2145–60. doi: 10.1534/genetics.107.086488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poinsot D, et al. Wolbachia transfer from Drosophila melanogaster into D. simulans: Host effect and cytoplasmic incompatibility relationships. Genetics. 1998;150:227–237. doi: 10.1093/genetics/150.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bossan B, et al. A new model and method for understanding Wolbachia-induced cytoplasmic incompatibility. PLoS One. 2011;6:e19757. doi: 10.1371/journal.pone.0019757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bourtzis K, et al. Wolbachia Infection and cytoplasmic incompatibility in Drosophila species. Genetics. 1996;144:1063–1073. doi: 10.1093/genetics/144.3.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinkins SP, et al. Wolbachia pipientis: bacterial density and unidirectional cytoplasmic incompatibilty between infected populations of Aedes albopictus. Experimental Parasitology. 1995;81:284–291. doi: 10.1006/expr.1995.1119. [DOI] [PubMed] [Google Scholar]
- 28.Zheng Y, et al. Wolbachia-induced cytoplasmic incompatibility is associated with decreased Hira expression in male Drosophila. PLoS One. 2011;6:e19512. doi: 10.1371/journal.pone.0019512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamada R, et al. Functional test of the influence of Wolbachia genes on cytoplasmic incompatibility expression in Drosophila melanogaster. Insect molecular biology. 2011;20:75–85. doi: 10.1111/j.1365-2583.2010.01042.x. [DOI] [PubMed] [Google Scholar]
- 30.Papafotiou G, et al. Regulation of Wolbachia ankyrin domain encoding genes in Drosophila gonads. Research in microbiology. 2011;162:764–72. doi: 10.1016/j.resmic.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 31.Duron O, et al. Variability and expression of ankyrin domain genes in Wolbachia variants infecting the mosquito Culex pipiens. Journal of bacteriology. 2007;189:4442–8. doi: 10.1128/JB.00142-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brennan LJ, et al. Disruption of redox homeostasis leads to oxidative DNA damage in spermatocytes of Wolbachia-infected Drosophila simulans. Insect Molecular Biology. 2012 doi: 10.1111/j.1365-2583.2012.01155.x. [DOI] [PubMed] [Google Scholar]
- 33.Zhu LY, et al. Wolbachia strengthens cardinium-induced cytoplasmic incompatibility in the spider mite Tetranychus piercei McGregor. Current Microbiology. 2012;65:516–23. doi: 10.1007/s00284-012-0190-8. [DOI] [PubMed] [Google Scholar]
- 34.Penz T, et al. Comparative genomics suggests an independent origin of cytoplasmic incompatibility in Cardinium hertigii. PLoS Genetics. 2012;8:e1003012. doi: 10.1371/journal.pgen.1003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riparbelli MG, et al. Wolbachia-mediated male killing is associated with defective chromatin remodeling. PLoS One. 2012;7:e30045. doi: 10.1371/journal.pone.0030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sasaki T, et al. Wolbachia variant that induces two distinct reproductive phenotypes in different hosts. Heredity. 2005;95:389–93. doi: 10.1038/sj.hdy.6800737. [DOI] [PubMed] [Google Scholar]
- 37.Jaenike J. Spontaneous emergence of a new Wolbachia phenotype. Evolution. 2007;61:2244–52. doi: 10.1111/j.1558-5646.2007.00180.x. [DOI] [PubMed] [Google Scholar]
- 38.Veneti Z, et al. Loss of reproductive parasitism following transfer of male-killing Wolbachia to Drosophila melanogaster and Drosophila simulans. Heredity. 2012;109:306–12. doi: 10.1038/hdy.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hurst GD, et al. Male-killing Wolbachia in Drosophila: a temperature-sensitive trait with a threshold bacterial density. Genetics. 2000;156:699–709. doi: 10.1093/genetics/156.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moreira La, et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell. 2009;139:1268–78. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 41.Glaser RL, Meola Ma. The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS One. 2010;5:e11977. doi: 10.1371/journal.pone.0011977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong ZS, et al. Wolbachia-mediated antibacterial protection and immune gene regulation in Drosophila. PLoS One. 2011;6:e25430. doi: 10.1371/journal.pone.0025430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hughes GL, et al. Wolbachia strain wAlbB enhances infection by the rodent malaria parasite Plasmodium berghei in Anopheles gambiae mosquitoes. Applied and Environmental Microbiology. 2012;78:1491–5. doi: 10.1128/AEM.06751-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Connor L, et al. Open release of male mosquitoes infected with a Wolbachia biopesticide: field performance and infection containment. PLoS Neglected Tropical Diseases. 2012;6:e1797. doi: 10.1371/journal.pntd.0001797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Apostolaki A, et al. Transinfection of the olive fruit fly Bactrocera oleae with Wolbachia: towards a symbiont-based population control strategy. Journal of Applied Entomology. 2011;135:546–553. [Google Scholar]
- 46.Atyame CM, et al. Cytoplasmic incompatibility as a means of controlling Culex pipiens quinquefasciatus mosquito in the islands of the south-western Indian Ocean. PLoS Neglected Tropical Diseases. 2011;5:e1440. doi: 10.1371/journal.pntd.0001440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schraiber JG, et al. Constraints on the use of lifespan-shortening Wolbachia to control dengue fever. Journal of Theoretical Biology. 2012;297:26–32. doi: 10.1016/j.jtbi.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 48.McMeniman CJ, et al. Stable introduction of a life-shortening Wolbachia infections into the mosquito Aedes aegypti. Science. 2009;323:141–144. doi: 10.1126/science.1165326. [DOI] [PubMed] [Google Scholar]
- 49.Walker T, et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011;476:450–3. doi: 10.1038/nature10355. [DOI] [PubMed] [Google Scholar]
- 50.Hoffmann AA, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476:454–7. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- 51.Bhatt S, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kambris Z, et al. Wolbachia stimulates immune gene expression and inhibits plasmodium development in Anopheles gambiae. PLoS Pathogens. 2010;6:e1001143. doi: 10.1371/journal.ppat.1001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jin C, et al. The virulent Wolbachia strain wMelPop efficiently establishes somatic infections in the malaria vector Anopheles gambiae. Applied and Environmental Microbiology. 2009;75:3373–6. doi: 10.1128/AEM.00207-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bian G, et al. Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science. 2013;340:748–751. doi: 10.1126/science.1236192. [DOI] [PubMed] [Google Scholar]
- 55.Osei-Poku J, et al. Identification of Wolbachia strains in mosquito disease vectors. PLoS One. 2012;7:e49922. doi: 10.1371/journal.pone.0049922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meriweather M, et al. A 454 survey reveals the community composition and core microbiome of the common bed bug (Cimex lectularius) across an urban landscape. PLoS One. 2013;8:e61465. doi: 10.1371/journal.pone.0061465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doudoumis V, et al. Tsetse-Wolbachia symbiosis: Comes of age and has great potential for pest and disease control. Journal of Invertebrate Pathology. 2012;112:S94–S103. doi: 10.1016/j.jip.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teixeira L, et al. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biology. 2008;6:e2. doi: 10.1371/journal.pbio.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hedges LM, et al. Wolbachia and virus protection in insects. Science. 2008;322:702. doi: 10.1126/science.1162418. [DOI] [PubMed] [Google Scholar]
- 60.Kamalakannan V, et al. Wolbachia heat shock protein 60 induces pro-inflammatory cytokines and apoptosis in monocytes in vitro. Microbes and Infection. 2012;14:610–8. doi: 10.1016/j.micinf.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pinto SB, et al. Wolbachia surface protein induces innate immune responses in mosquito cells. BMC Microbiology. 2012;12(Suppl 1):S11. doi: 10.1186/1471-2180-12-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frentiu FD, et al. Wolbachia-mediated resistance to dengue virus infection and death at the cellular level. PLoS One. 2010;5:e13398. doi: 10.1371/journal.pone.0013398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rancès E, et al. The relative importance of innate immune priming in Wolbachia-mediated dengue interference. PLoS Pathogens. 2012;8:e1002548. doi: 10.1371/journal.ppat.1002548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pan X, et al. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. PNAS. 2012;109:E23–E31. doi: 10.1073/pnas.1116932108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hedges LM, et al. The small interfering RNA pathway is not essential for Wolbachia-mediated antiviral protection in Drosophila melanogaster. Applied and Environmental Microbiology. 2012;78:6773–6. doi: 10.1128/AEM.01650-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Osborne SE, et al. Antiviral protection and the importance of Wolbachia density and tissue tropism in Drosophila simulans. Applied and Environmental Microbiology. 2012;78:6922–9. doi: 10.1128/AEM.01727-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Longdon B, et al. Male-killing Wolbachia do not protect Drosophila bifasciata against viral infection. BMC Microbiology. 2012;12(Suppl 1):S8. doi: 10.1186/1471-2180-12-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Graham RI, et al. Wolbachia in a major African crop pest increases susceptibility to viral disease rather than protects. Ecology letters. 2012;15:993–1000. doi: 10.1111/j.1461-0248.2012.01820.x. [DOI] [PubMed] [Google Scholar]
- 69.Hussain M, et al. Wolbachia uses host microRNAs to manipulate host gene expression and facilitate colonization of the dengue vector Aedes aegypti. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9250–5. doi: 10.1073/pnas.1105469108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Osei-Amo S, et al. Wolbachia-Induced aae-miR-12 miRNA negatively regulates the expression of MCT1 and MCM6 genes in Wolbachia-infected mosquito cell line. PLoS One. 2012;7:e50049. doi: 10.1371/journal.pone.0050049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang G, et al. Wolbachia uses a host microRNA to regulate transcripts of a methyltransferase, contributing to dengue virus inhibition in Aedes aegypti. Proceedings of the National Academy of Sciences of the United States of America. 2013;108:9250–9255. doi: 10.1073/pnas.1303603110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saint André AV, et al. The role of endosymbiotic Wolbachia bacteria in the pathogenesis of river blindness. Science. 2002;295:1892–5. doi: 10.1126/science.1068732. [DOI] [PubMed] [Google Scholar]
- 73.Taylor MJ, et al. Wolbachia filarial interactions. Cellular Microbiology. 2012 doi: 10.1111/cmi.12084. [DOI] [PubMed] [Google Scholar]
- 74.Foster J, et al. The Wolbachia genome of Brugia malayi: endosymbiont evolution within a human pathogenic nematode. PLoS Biology. 2005;3:e121. doi: 10.1371/journal.pbio.0030121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ghedin E, et al. Draft genome of the filarial nematode parasite Brugia malayi. Science. 2007;317:1756–1760. doi: 10.1126/science.1145406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hosokawa T, et al. Wolbachia as a bacteriocyte-associated nutritional mutualist. PNAS. 2010;107:769–74. doi: 10.1073/pnas.0911476107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fenn K, Blaxter M. Quantification of Wolbachia bacteria in Brugia malayi through the nematode lifecycle. Molecular and Biochemical Parasitology. 2004;137:361–4. doi: 10.1016/j.molbiopara.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 78.Fischer K, et al. Tissue and stage-specific distribution of Wolbachia in Brugia malayi. PLoS Neglected Tropical Diseases. 2011;5:e1174. doi: 10.1371/journal.pntd.0001174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McGarry HF, et al. Population dynamics of Wolbachia bacterial endosymbionts in Brugia malayi. Molecular and Biochemical Parasitology. 2004;135:57–67. doi: 10.1016/j.molbiopara.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 80.Landmann F, et al. Asymmetric Wolbachia segregation during early Brugia malayi embryogenesis determines its distribution in adult host tissues. PLoS Neglected Tropical Diseases. 2010;4:e758. doi: 10.1371/journal.pntd.0000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Landmann F, et al. Both asymmetric mitotic segregation and cell-to-cell invasion are required for stable germline transmission of Wolbachia in filarial nematodes. Biology Open. 2012;1:536–47. doi: 10.1242/bio.2012737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Voronin D, et al. Autophagy regulates Wolbachia populations across diverse symbiotic associations. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E1638–1646. doi: 10.1073/pnas.1203519109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dunning Hotopp JC, et al. Widespread lateral gene transfer from intracellular bacteria to multicellular eukaryotes. Science. 2007;317:1753–6. doi: 10.1126/science.1142490. [DOI] [PubMed] [Google Scholar]
- 84.Brelsfoard C, Dobson S. Wolbachia effects on hostfitness and the influence of male aging on cytoplasmic incompatibility in Aedes polynesiensis. Journal of Medical Entomology. 2011;48:1008–1015. doi: 10.1603/me10202. [DOI] [PubMed] [Google Scholar]
- 85.De Almeida F, et al. Effects of Wolbachia on fitness of Culex quinquefasciatus (Diptera; Culicidae) Infection, Genetics and Evolution. 2011;11:2138–43. doi: 10.1016/j.meegid.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 86.Zhang H, et al. Population dynamics of noncytoplasmic incompatibility-inducing Wolbachia in Nilaparvata lugens and its effects on host adult life span and female fitness. Environmental Entomology. 2010;39:1801–9. doi: 10.1603/EN10051. [DOI] [PubMed] [Google Scholar]
- 87.Turley AP, et al. Transinfected Wolbachia have minimal effects on male reproductive success in Aedes aegypti. Parasites & Vectors. 2013;6:36. doi: 10.1186/1756-3305-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lewis Z, et al. Wolbachia infection lowers fertile sperm transfer in a moth. Biology letters. 2011;7:187–9. doi: 10.1098/rsbl.2010.0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen SJ, et al. Identification and Biological Role of the Endosymbionts Wolbachia in Rice Water Weevil (Coleoptera: Curculionidae) Environmental Entomology. 2012;41:469–477. doi: 10.1603/EN11195. [DOI] [PubMed] [Google Scholar]
- 90.Dedeine F, et al. Removing symbiotic Wolbachia bacteria specifically inhibits oogenesis in a parasitic wasp. PNAS. 2001;98:6247–52. doi: 10.1073/pnas.101304298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fast EM, et al. Wolbachia enhance Drosophila stem cell proliferation and target the germline stem cell niche. Science. 2011;334:990–2. doi: 10.1126/science.1209609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weeks AR, et al. From parasite to mutualist: rapid evolution of Wolbachia in natural populations of Drosophila. PLoS Biology. 2007;5:e114. doi: 10.1371/journal.pbio.0050114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Landmann F, et al. Anti-filarial activity of antibiotic therapy is due to extensive apoptosis after Wolbachia depletion from filarial nematodes. PLoS Pathogens. 2011;7:e1002351. doi: 10.1371/journal.ppat.1002351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Slatko BE, et al. The Wolbachia endosymbiont as an anti-filarial nematode target. Symbiosis. 2010;51:55–65. doi: 10.1007/s13199-010-0067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Desjardins Ca, et al. Genomics of Loa loa, a Wolbachia-free filarial parasite of humans. Nature Genetics. 2013;45:495–500. doi: 10.1038/ng.2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu B, et al. The heme biosynthetic pathway of the obligate Wolbachia endosymbiont of Brugia malayi as a potential anti-filarial drug target. PLoS Neglected Tropical Diseases. 2009;3:e475. doi: 10.1371/journal.pntd.0000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shrivastava N, et al. Molecular characterization of NAD+-dependent DNA ligase from Wolbachia endosymbiont of lymphatic filarial parasite Brugia malayi. PLoS One. 2012;7:e41113. doi: 10.1371/journal.pone.0041113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Palayam M, et al. Preliminary analysis to target pyruvate phosphate dikinase from Wolbachia endosymbiont of Brugia malayi for desiging anti-filarial agents. Interdisciplinary Sciences: Computational Life Sciences. 2012;4:74–82. doi: 10.1007/s12539-011-0109-2. [DOI] [PubMed] [Google Scholar]
- 99.Li Z, et al. Targeting the Wolbachia cell division protein FtsZ as a new approach for antifilarial therapy. PLoS Neglected Tropical Diseases. 2011;5:e1411. doi: 10.1371/journal.pntd.0001411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schiefer A, et al. The ClpP peptidase of Wolbachia endobacteria is a novel target for drug development against filarial infections. The Journal of Antimicrobial Chemotherapy. 2013 doi: 10.1093/jac/dkt105. [DOI] [PubMed] [Google Scholar]
- 101.Sharma R, et al. Filamentation temperature-sensitive protein Z (FtsZ) of Wolbachia, endosymbiont of Wuchereria bancrofti: A potential target for anti-filarial chemotherapy. Acta Tropica. 2013;125:330–8. doi: 10.1016/j.actatropica.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 102.Johnston KL, et al. Lipoprotein biosynthesis as a target for anti-Wolbachia treatment of filarial nematodes. Parasites & Vectors. 2010;3:99. doi: 10.1186/1756-3305-3-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gayen P, et al. A double-blind controlled field trial of doxycycline and albendazole in combination for the treatment of bancroftian filariasis in India. Acta Tropica. 2013;125:150–6. doi: 10.1016/j.actatropica.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 104.Sjölund M, et al. Persistence of resistant Staphylococcus epidermidis after single course of clarithromycin. Emerging Infectious Diseases. 2005;11:1389–93. doi: 10.3201/eid1109.050124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jernberg C, et al. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. The ISME journal. 2007;1:56–66. doi: 10.1038/ismej.2007.3. [DOI] [PubMed] [Google Scholar]
- 106.Dugan J, et al. Tetracycline Resistance in Chlamydia suis Mediated by Genomic Islands Inserted into the Chlamydial inv-Like Gene. Antimicrobial Agents and Chemotherapy. 2004;48:3989–3995. doi: 10.1128/AAC.48.10.3989-3995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nag JK, et al. Recombinant translation initiation factor-1 of Wolbachia is an immunogenic excretory secretory protein that elicits Th2 mediated immune protection against Brugia malayi. Comparative immunology, microbiology and infectious diseases. 2013;36:25–38. doi: 10.1016/j.cimid.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 108.Scott AL, et al. Filarial and Wolbachia genomics. Parasite Immunology. 2013;34:121–129. doi: 10.1111/j.1365-3024.2011.01344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mavingui P, et al. Whole-genome sequence of Wolbachia strain wAlbB, an endosymbiont of tiger mosquito vector Aedes albopictus. Journal of Bacteriology. 2012;194:1840. doi: 10.1128/JB.00036-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maren Ellegaard K, et al. Comparative Genomics of Wolbachia and the Bacterial Species Concept. PLoS Genetics. 2013;9:e1003381. doi: 10.1371/journal.pgen.1003381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Metcalf Ja, Bordenstein SR. The complexity of virus systems: the case of endosymbionts. Current Opinion in Microbiology. 2012;15:546–52. doi: 10.1016/j.mib.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]