Abstract
Properly conducted, an enrichment step can improve selectivity, sensitivity, yield, and most importantly, significantly reduce the time needed to isolate rare circulating tumor cells (CTCs). The enrichment process can be broadly categorized as positive selection versus negative depletion, or in some cases, a combination of both. We have developed a negative depletion CTC enrichment strategy that relies on the removal of normal cells using immunomagnetic separation in the blood of cancer patients. This method is based on the combination of magnetic and fluid forces in an axial, laminar flow in long cylinders placed in quadrupole magnets. Using this technology, we have successfully isolated CTCs from patients with breast carcinoma and squamous cell carcinoma of the head and neck. In contrast to a positive selection methodology, this approach provides an unbiased characterization of these cells, including markers associated with epithelial mesenchymal transition.
1 CTC Identification Relies on its Separation Strategy
With confirmation of cancer cells in the circulation over 50 years ago [1], one of the challenges has been developing technology with sufficient sensitivity and specificity to reliably examine the role of circulating tumor cells (CTCs) in cancer biology [2–5]. The prognostic and predictive relevance of CTCs as a validated biomarker has now been established by numerous studies in our institutions and by others [6–8]. Over the past several years cell separation technology has advanced substantially, and continues to evolve with research approaches which are of even greater sensitivity and suitable for rare CTC detection [9].
A milliliter of human blood contains an average of five billion RBCs, seven million WBCs, and 295 million platelets, and it is certainly a challenge to identify CTCs [10]. Given the generally accepted rarity of a CTC, (on the order of one cell per 1×106 nucleated cells) in the blood of patients with cancer, a general review of the literature will reveal a variety of techniques and procedures that either enrich and/or isolate, or merely detect and quantify CTCs. Older methods involving immunohistochemistry (IHC) with minimal pre-enrichment relied on direct human observation under the microscope of multiple slides [11]. This method was labor-intensive and time-consuming and had high false positivity. Newer approaches include the use of technologies, such as flow cytometry, in which CTCs are positively labeled with an antibody-fluorochrome conjugate, and molecular approaches, such as (reverse transcriptase-polymerase chain reaction) RT-PCR. However, for all three of these detection approaches, IHC, flow cytometry, and RT-PCR, it is highly advisable to use an enrichment step prior to the detection analysis [12].
2 Enrichment Methodologies
Properly conducted, the enrichment step can improve selectivity, sensitivity, yield, and most importantly, significantly reduce the time needed to perform the analysis. The enrichment process can be broadly categorized as positive selection versus negative depletion, or in some cases, a combination of both. An example of a positive selection system is the commercially available CellSearch™ System (Veridex LLC). The system is based on the enumeration of epithelial cells that are separated from blood by antibody-magnetic nanoparticle conjugates that target epithelial cell surface markers, EpCAM, and the subsequent identification of the CTCs with fluorescently labeled antibodies against cytokeratin (CK 8, 18, 19) and a fluorescent nuclear stain [8]. The CellSearch definition of a CTC is a nucleated cell lacking CD45 and expressing cytokeratins and EpCAM.
In negative depletion, what are believed to be normal hematopoeitic cells, such as CD45 positive cells, are targeted and subsequently removed, thereby enriching the blood cell suspension for the rare tumor cells. While less common than direct positive selection, such as with the use of the CellSearch system, a number of reports exist of the use of either red blood cell (RBC) lysis, or gradient separation to remove RBCs, followed by CD45 expressing cell removal, prior to analysis for potential CTCs. Both Iinuma et al. (2000) and Bilkenroth et al. (2001) used a Ficoll gradient to remove RBCs and targeted CD45 expressing cells with magnetic particles for further removal. They subsequently identified CTCs in these enriched peripheral blood samples from colorectal and renal carcinoma patients, respectively [13, 14]. Using a similar approach, Brakenhoff et al. (1999) and Partridge et al. (2003) identified disseminated tumor cells from the blood of head and neck of cancer patients [15, 16]. With respect to breast cancer, Tkaczuk et al., using an approach similar to the previously discussed approached, reported that negative depletion enrichment can isolate breast CTCs in all stages of breast cancer, including early stage breast cancer and that the number of CTCs correlated with disease outcomes and overall survival [17].
3 Advantages of CTC Pre-Enrichment by Depletion of Normal Cells (Negative Depletion)
Despite the success of the positive selection approach, there are significant limitations. Probably the greatest limitation is for a CTC to be detected, they must express the cell surface marker used to target the CTC. Commonly used positive selection technologies such as the CellSearch System and the CTC Chip [18] use antibodies targeting EpCAM (a commonly used epithelial cell surface marker). However, increasing evidence suggests that not all tumors and not all CTCs express EpCAM. One study indicated that only 70% of 134 tumors with different histological types expressed EpCAM [19]. In another study, the number of cytokeratin-negative cells with aneusomy outnumbered cytokeratin positive cells [20]. In addition, cell surface epithelial markers can be lost in cell lines derived from disseminated tumor cells. For example, all micrometastatic breast cancer cell lines derived from the bone marrow displayed loss of epithelial cytokeratins (CK8, CK18, and CK19) and ectopic expression of vimentin commonly present in mesenchymal cells compared to tumor-derived breast cell lines [21]. The cells not expressing epithelial markers would be missed by positive selection techniques even though these cells may be the most clinically relevant indicators of a tumor’s aggressive potential. This was experimentally shown by Sieuwerts et al. with breast cancer cell lines; the cancer cells with “normal like” phenotypes had the lowest recovery by CellSearch [22].
Depletion of normal cells prior to analysis (or negative depletion) has several other advantages including potential time/cost-efficiency and improved sample yield and purity allowing multiple biomarker analysis using immunocytochemistry [23] and RT-PCR [24]. Drawbacks to a negative selection methodology include the inability to obtain a high enough enrichment to be able to identify the “abnormal cells” or CTCs which exist in the specimen. Too many contaminating leukocytes may make it difficult to see the CTCs. Alternatively, a very high enrichment might result in the unintended loss of CTC, or other abnormal cells, with the removed normal cells. In addition, given that leukocytes are depleted using CD45 immunomagnetic separation, a CTC that expresses CD45 may be inadvertently removed from the sample, precluding its identification.
4 Depletion of Normal Cells Prior to Flow Cytometry or Other Optical Analyses
While a majority of the published studies on CTCs use a human observation of ICC to identify the cells, a number of studies use advanced electronic technology including flow cytometry and computer imaging. A purging of the sample of erythrocytes and PBMCs prior to FACS analysis is typically necessary in order to achieve the required high level of sensitivity as shown recently for a model of human breast carcinoma in athymic mice [25]. The mouse red blood cells were removed by lysis and the mouse PBMCs were removed by tagging with an anti pan-leukocyte antibody (anti CD45) attached to the magnetic bead followed by magnetic separation (EasySep kit from StemCell Technologies, Vancouver, BC, Canada). The limit of detection was one CTC in 105 mouse PBMCs based on a realistic metastatic tumor animal model and using a standard flow cytometer (four color XL-MCL from Beckman Coulter).
Rapid improvement in optical detection methods has opened the possibility of using laser scanning cytometry for CTC detection directly on blood smears on glass slides [26, 27]. A specialized system termed fiber-optic array scanning technology (FAST) has been tested on a model of metastatic colorectal tumor (HT29) spiked into whole blood from volunteer donors. The RBCs were removed by lysis and the remaining PBMC fraction was deposited on glass slides and stained for pan cytokeratin and cell nucleus markers. The combination of FAST screening followed by re-scanning of “hits” with a more conventional automated digital microscopy (ADM) resulted in average specificity of 1.5 × 1−5 and an average sensitivity of 98% at a scanning speed of 100 million PBMCs per hour (equivalent to approximately 5 mL whole blood per hour, excluding sample prep time).
Interestingly, the authors applied their technology to check for false negative results of CTC detection by a positive immunomagnetic CTC separation method based on expression of the epithelial cell adhesion molecular (EpCAM) marker. They reported two potential issues with the positive CTC enrichment when compared to FAST + ADM scan: (1) the positive immunomagnetic separation (using MACS microbeads and MiniMACS columns from Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) leads up to 50% CTC losses in the process and (2) furthermore, the positive immunomagnetic separation is highly sensitive to EpCAM marker down-regulation. The EpCAM and HER2/neu marker down-regulation has been observed in clinical studies of metastatic cancers [27–30]. These would not be an issue with the negative CTC enrichment, proposed in this study, because it does not rely on expression of any particular CTC marker.
5 Magnetic Depletion Technologies
The enrichment for targeted cells by depletion of the unwanted cells is a general strategy beyond the search for CTCs, and specialized magnetic separation instrumentation and reagents are available commercially [31, 32]. We have recently developed a negative CTC enrichment strategy that relies on a combination of viscous flow that facilitates recovery of the unlabeled cells (CTC) and the magnetic force that traps the labeled cells (leukocytes) from whole blood samples obtained from cancer patients [24, 33, 34]. The method is based on the combination of magnetic and fluid forces in an axial, laminar flow in long cylinders placed in quadrupole magnets [35–46]. The combination of magnetic and viscous shear forces, using specifically designed geometries and magnetic fields, lowers the likelihood of non-specific CTC losses, below those encountered during magnetic separation from static suspensions [17]. The method combines advantages of using flow and the magnetic field to achieve high throughput (mL/min) and high enrichment rates (by as much as 10,000-fold, i.e., increasing the CTC frequency in the sample, e.g., from 1:100,000 to 1:10) [24].
6 Clinical Results With CTCs or Cancer Associated Cells Identified With Negative Depletion
6.1 Breast Cancer
Using our enrichment system presented in Fig. 1, in an ongoing study we have identified CTCs in all breast cancer stages and elevated CTCs pretreatment or after one cycle of treatment. This negative depletion yielded an average log10 depletion of nucleated cells of 2.74 and an overall, average log10 depletion of 5.2 (> 100,000 enrichment). CTCs were detected in both localized, non-metastatic, and metastatic breast cancer patients and staining for mesenchymal and stem cell markers was successful [47, 48]. No CTCs have been identified in healthy volunteers and in buffy coats purchased from the Red Cross.
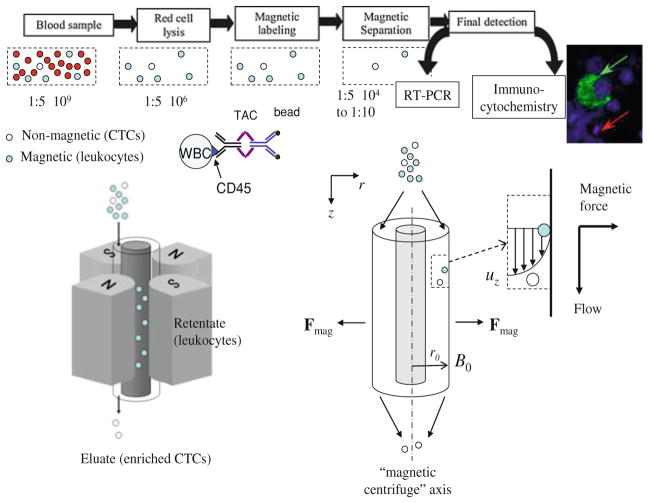
Fig. 1.
CTC enrichment by magnetic depletion of normal cells. Top: Flow diagram of the experimental protocol indicating approximate number ratios of CTCs to normal cells. Bottom: Quadrupole magnet and annular shell separation channel for separation of non-magnetic CTCs from normal cells (tagged with tetrameric antibody complex, TAC) using magnetic and viscous shear flow forces. The radial magnetic forces act as a type of “magnetic centrifuge” on the magnetically tagged normal white blood cells (WBCs) retaining them inside the channel while the CTCs are washed out by the flowing fluid
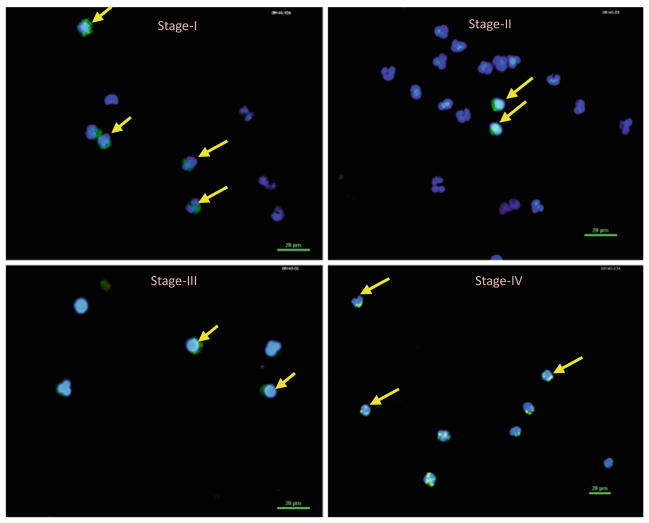
Figure 2 is a set of photographs of microscopic images of an immunocyto-chemically stained, peripheral blood from stage I through IV breast cancer patients. Nuclear staining with DAPI (blue color) and cytokeratin staining with pan cytokeratin 8/18/19 (green color) were used. Cells staining for both were counted as CTCs. The apparently normal volunteer donor blood was used as a control and showed no visible staining (results not shown).
Fig. 2.
CTCs in stages I–IV breast cancer obtained by magnetic CD45 + cell depletion as shown in Fig. 1. Nuclear staining with DAPI and cykeratin staining with pan cytokeratin 8/18/19 was used. Cells staining for both were counted as CTCs. The apparently normal volunteer donor blood was used as a control and showed no visible staining (results not shown)
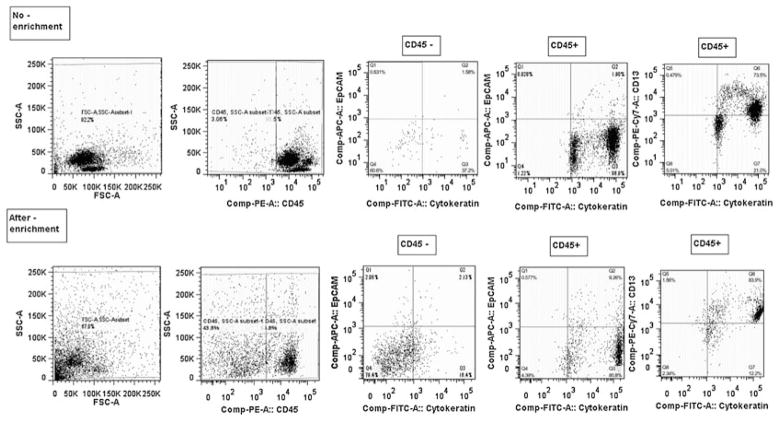
An argument can be made that this approach does not conclusively prove that the cells that are positive for nuclei and cytokeratins are also negative for CD45, despite magnetic depletion for CD45. To address this concern Fig. 3 is presented, which is a set of representative, multiparameter flow analysis of a blood sample from a woman with metastatic triple negative breast cancer prior to therapy. The top row is pre-enrichment and the bottom row is post-enrichment by our magnetic CD45 + cell depletion approach. For this specific enrichment, a 3 log10 of the nucleated cells, based on hemocytometer counting, was obtained (i.e. a 1000-fold enrichment). The first column on the left-hand side of this figure is the ungated dot plot of the side scatter versus forward scatter is presented. A clear decrease in the location of a typical lymphocyte population in the dot plots is observed. The next column on the left is the CD45 stained population. Even after CD45 depletion, a population of CD45 is still present, even after the significant lymphocyte depletion. The next column is further analysis of the CD45− population, gated in the second column. The CD45− population, after the magnetic enrichment, clearly has events that are positive for cytokeratin and mostly negative for EpCAM, although a number of EpCAM and cytokeratin positive events are present. Interesting, the CD45+ population, the fourth column from the left, has highly cytokeratin positive cells, mostly negative for EpCAM, but a noticeable number positive for both EpCAM and cytokeratin. Finally, all of these cytokerain positive cells are positive for CD13. CD13 can be expressed on granulocytes, monocytes, endothelial, and epithelial cells. These results, using this negative enrichment process, present a number of potentially interesting subpopulations for further analysis, and also suggest that the absolute number of CTCs may not be the most relevant biomarkers as the majority of these cells are probably neither capable of establishing metastatic niches nor becoming dormant [49]. Further characterization of this subpopulations and molecular marker analysis may elucidate pathways for the development and progression of metastatic disease which can be used to develop novel targeted therapies.
Fig. 3.
Multiparameter flow analysis of a blood sample from a woman with metastatic triple negative breast cancer prior to therap. Top row is pre-enrichment and the bottom row is post-enrichment by magnetic CD45 + cell depletion. Note the presence of cytokeratin + and CD45 ± cells after enrichment. Also note the large number of CD45 + cells that are cytokeratin positive and all CD13 positive. Considering efficient depletion of lymphocytes the data show that the CD45 + cells in the depleted fraction are all CD13 positive and cytokeratin positive
7 Squamous Cell Carcinoma of the Head and Neck
In squamous cell carcinoma of the head and neck (SCCHN), another epithelial malignancy, there are limited studies on CTCs in the literature to date. Partridge et al. (2003) used a negative depletion methodology to identify disseminated tumor cells in SCCHN patients. The detection of disseminated tumor cells pre-operatively or intra-operatively indicated an increased risk of local/distant recurrence and decreased survival [16]. Using flow cytometry, Hristozova et al. (2011), reported that detection of CTCs (CD45-CK + EpCAM +) in inoperable SCCHN, correlated with a higher incidence of regional metastasis and that concurrent chemoradiotherapy reduced their frequency [50].
Our published early prospective clinical results of 48 patients with SCCHN with a mean follow-up of 19 months, showed a statistically significant worse disease-free survival in patients with CTC present in the blood taken at the time of surgical resection (p = 0.01), [51]. There was no correlation between the presence of CTC and tumor site, overall stage, nodal status, smoking or alcohol use, or the use of adjuvant therapy. On a number of samples, using Confocal microscopy with multimarker staining, we have found expression of other markers on the CTC from SCCHN patients, including EGFR, CD44, and vimentin. We have optimized our detection methodology to be able to obtain an overall average enrichment of 5.66 log10, and as high as >7 log10 total enrichment of CTCs in the blood of patients with SCCHN. Using our technique, we have identified 0 to over 3000 CTCs per mL of blood collected from SCCHN patients.
8 Epiethelial Mesenchymal Transition
The ectopic expression of vimentin, with the corresponding loss of cytokeratins is a proposed mechanism for the epithelial to mesenchymal transition (EMT). This process is hypothesized to be a marker for aggressiveness of tumor cells to establish metastatic sites [21]. Recent reports indicate that EMT potentially takes place during tumor cell invasion. Such a transition is characterized by the decrease in epithelial markers and the increase in mesenchymal markers [52–55]. Previously published studies show that more aggressive breast cell lines and tumors, such as basaloid subtype, [56, 57] have mesenchymal markers and increased stem cell markers [58–61]. It is possible that these cells have undergone EMT, a highly regulated process during which tumor cells lose epithelial characteristics and gain invasive mesenchymal and stem cell-like features [54, 62–64]. In contrast, less aggressive breast cancer lines, such as hormone receptor positive cells, have less mesenchymal features [65, 66]. Although conclusive evidence for EMT in vivo has not been established, there is emerging evidence for the role of EMT in generating mammary stem cells as a model for breast cancer invasiveness and metastases [58]. Positive selection technology, such as CellSearch has been shown to miss up to 98% of cells with high CD44 expression, and low EpCAM expression. Recently, CTCs with mesenchymal and stem cell markers, based on RT-PCR analysis of blood samples, was reported in the metastatic setting [67, 68]. However, the analysis was limited to a small set of markers by RT-PCR and not combined with the benefits of direct visualization with ICC.
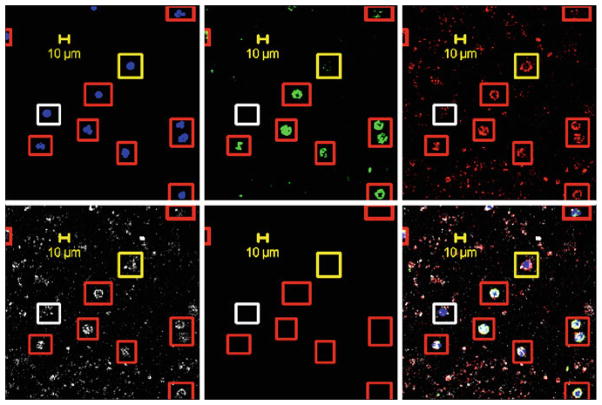
Our work has shown that both CTCs from SCCHN and breast cancer express markers associated with EMT. Figure 4 is a representative set of confocal images from a negatively depleted blood sample from a patient with stage IV triple negative breast cancer. Markers shown are with DAPI/nuclei, FITC/cytokeratins, AF594/vimentin, and APC/CD44. In the composite, the cells in red boxes are positive for all four markers, while cells encircled with yellow boxes are negative for cytokeratins; yet positive for the other three markers. White boxes are cells negative for cytokeratins, vimentin, CD44, and positive for DAPI/nuclei.
Fig. 4.
Representative, confocal images from a negatively depleted blood sample from a patient with stage IV triple negative breast cancer. Markers shown are with DAPI/nuclei, FITC/cytokeratins, AF594/vimentin, and APC/CD44. In the composite, the cells in red boxes are positive for all four markers, while cells encircled with yellow boxes are negative for cytokeratins, yet positive for the other three markers. White boxes are cells negative for cytokeratins and vimentin, and CD44, positive for DAPI/nuclei
Additional studies are needed to better understand the role of this subpopulation of CTCs in both women with localized, early stage, and metastatic breast cancer and to assess how these expression markers change with treatment. Investigating the up-regulation of mesenchymal markers in CTCs provides a valuable opportunity to understand mechanisms underlying metastasis which may lead to future novel therapies. The concept that CTC directly contributes to the metastatic process and undergoes EMT is an intriguing hypothesis. However, limitations in the current methods of CTC collection and phenotypic characterization with positive enrichment (EpCAM-based) technology have impaired efforts to test this clinically relevant hypothesis. A negative depletion method can help to eliminate the selection bias of CTCs, to provide an objective assessment of any atypical cells found in the blood.
9 Current Assumptions about “Normal Cell”
In addition to the currently accepted definition of a CTC (Cytokeratin and/or EpCAM positive nucleated cell that is CD45 negative), multiple groups are beginning to note other atypical cells in the blood of patients with cancer. These include CD45 positive cells that also have cytokeratin or EGFR. These cells have been called “double positives” by some groups including Toner et al. because they have both hematopoietic and epithelial markers. These findings raise the question: can a CD45 positive cell be a cancer cell? The exact origins of these cells are still under debate. The possibilities include the fusion of hematopoietic cells to circulating cancer cells, non-specific binding of CD45 antibodies to isolated cells, or most intriguingly cancer cells originating in the bone marrow with stem cell-like features. Additional studies are underway to help answer these questions.
Contributor Information
Maryam Lustberg, Internal Medicine, Division of Medical Oncology, The Ohio State University, Columbus, OH, USA.
Kris R. Jatana, Department of Otolaryngology—Head and Neck Surgery, The Ohio State University and Nationwide Children’s Hospital, Columbus, OH, USA
Maciej Zborowski, Department of Biomedical Engineering, Cleveland Clinic, Cleveland, OH, USA.
Jeffrey J. Chalmers, Email: chalmers@chbmeng.ohio-state.edu, Professor William G. Lowrie Department of Chemical and Biomolecular Engineering, The Ohio State University, 140 W. 19th Avenue, Columbus, OH 43210, USA
References
- 1.Engell HC. Cancer cells in the circulating blood; a clinical study on the occurrence of cancer cells in the peripheral blood and in venous blood draining the tumour area at operation. Acta Chir Scand Suppl. 1955;201:1–70. [PubMed] [Google Scholar]
- 2.Goldblatt SA, Nadel EM. Cancer cells in the circulating blood: a critical review ii. 1965;9:6–20. [PubMed] [Google Scholar]
- 3.Herbeuval R, Duheille J, Goedert-Herbeuval C. Diagnosis of unusual blood cells by immunofluorescence. Acta Cytol. 1965;9:73–82. [PubMed] [Google Scholar]
- 4.Kiseleva NS, Magamadov YC. Hematogenous dissemination of tumour cells and metastases formation in Ehrlich ascites tumour. Neoplasma. 1972;19:257–275. [PubMed] [Google Scholar]
- 5.Stevenson JL, Von Haam E. The application of immunofluorescence techniques to the cytodiagnosis of cancer. Acta Cytol. 1966;10:15–20. [PubMed] [Google Scholar]
- 6.Budd GT, Cristofanilli M, Ellis MJ, et al. Circulating tumor cells versus imaging–predicting overall survival in metastatic breast cancer. Clin Cancer Res. 2006;12:6403–6409. doi: 10.1158/1078-0432.CCR-05-1769. [DOI] [PubMed] [Google Scholar]
- 7.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 8.Riethdorf S, Fritsche H, Muller V, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the Cell Search system. Clin Cancer Res. 2007;13:920–928. doi: 10.1158/1078-0432.CCR-06-1695. [DOI] [PubMed] [Google Scholar]
- 9.Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer. 2008;8:329–340. doi: 10.1038/nrc2375. [DOI] [PubMed] [Google Scholar]
- 10.McKenzie S. Textbook of Hematology. Williams and Wilkens, Inc; Maryland: 1996. [Google Scholar]
- 11.Braun S, Pantel K, Muller P, et al. Cytokeratin-positive cells in the bone marrow and survival of patients with stage I, II, or III breast cancer. N Engl J Med. 2000;342:525–533. doi: 10.1056/NEJM200002243420801. [DOI] [PubMed] [Google Scholar]
- 12.Gross HJ, Verwer B, Houck D, et al. Model study detecting breast cancer cells in peripheral blood mononuclear cells at frequencies as low as 10(-7) Proc Natl Acad Sci U S A. 1995;92:537–541. doi: 10.1073/pnas.92.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iinuma H, Okinaga K, Adachi M, et al. Detection of tumor cells in blood using CD45 magnetic cell separation followed by nested mutant allele-specific amplification of p53 and K-ras genes in patients with colorectal cancer. Int J Cancer. 2000;89:337–344. doi: 10.1002/1097-0215(20000720)89:4<337::aid-ijc4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 14.Bilkenroth U, Taubert H, Riemann D, et al. Detection and enrichment of disseminated renal carcinoma cells from peripheral blood by immunomagnetic cell separation. Int J Cancer. 2001;92:577–582. doi: 10.1002/ijc.1217. [DOI] [PubMed] [Google Scholar]
- 15.Brakenhoff RH, Stroomer JG, ten Brink C, et al. Sensitive detection of squamous cells in bone marrow and blood of head and neck cancer patients by E48 reverse transcriptase-polymerase chain reaction. Clin Cancer Res. 1999;5:725–732. [PubMed] [Google Scholar]
- 16.Partridge M, Brakenhoff R, Phillips E, et al. Detection of rare disseminated tumor cells identifies head and neck cancer patients at risk of treatment failure. Clin Cancer Res. 2003;9:5287–5294. [PubMed] [Google Scholar]
- 17.Tkaczuk KH, Goloubeva O, Tait NS, et al. The significance of circulating epithelial cells in Breast Cancer patients by a novel negative selection method. Breast Cancer Res Treat. 2008;111:355–364. doi: 10.1007/s10549-007-9771-9. [DOI] [PubMed] [Google Scholar]
- 18.Nagrath S, Sequist LV, Maheswaran S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–1239. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Went PT, Lugli A, Meier S, et al. Frequent EpCam protein expression in human carcinomas. Hum Pathol. 2004;35:122–128. doi: 10.1016/j.humpath.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 20.Fehm T, Sagalowsky A, Clifford E, et al. Cytogenetic evidence that circulating epithelial cells in patients with carcinoma are malignant. Clin Cancer Res. 2002;8:2073–2084. [PubMed] [Google Scholar]
- 21.Willipinski-Stapelfeldt B, Riethdorf S, Assmann V, et al. Changes in cytoskeletal protein composition indicative of an epithelial-mesenchymal transition in human micrometastatic and primary breast carcinoma cells. Clin Cancer Res. 2005;11:8006–8014. doi: 10.1158/1078-0432.CCR-05-0632. [DOI] [PubMed] [Google Scholar]
- 22.Sieuwerts AM, Kraan J, Bolt J, et al. Anti-epithelial cell adhesion molecule antibodies and the detection of circulating normal-like breast tumor cells. J Natl Cancer Inst. 2009;101:61–66. doi: 10.1093/jnci/djn419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 24.Tong X, Yang L, Lang JC, et al. Application of immunomagnetic cell enrichment in combination with RT-PCR for the detection of rare circulating head and neck tumor cells in human peripheral blood. Cytometry B Clin Cytom. 2007;72:310–323. doi: 10.1002/cyto.b.20177. [DOI] [PubMed] [Google Scholar]
- 25.Allan AL, Vantyghem SA, Tuck AB, et al. Detection and quantification of circulating tumor cells in mouse models of human breast cancer using immunomagnetic enrichment and multiparameter flow cytometry. Cytometry A. 2005;65:4–14. doi: 10.1002/cyto.a.20132. [DOI] [PubMed] [Google Scholar]
- 26.Hsieh HB, Marrinucci D, Bethel K, et al. High speed detection of circulating tumor cells. Biosens Bioelectron. 2006;21:1893–1899. doi: 10.1016/j.bios.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 27.Krivacic RT, Ladanyi A, Curry DN, et al. A rare-cell detector for cancer. Proc Natl Acad Sci U S A. 2004;101:10501–10504. doi: 10.1073/pnas.0404036101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braun S, Hepp F, Sommer HL, et al. Tumor-antigen heterogeneity of disseminated breast cancer cells: implications for immunotherapy of minimal residual disease. Int J Cancer. 1999;84:1–5. doi: 10.1002/(sici)1097-0215(19990219)84:1<1::aid-ijc1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 29.Kasimir-Bauer S, Otterbach F, Oberhoff C, et al. Rare expression of target antigens for immunotherapy on disseminated tumor cells in breast cancer patients without overt metastases. Int J Mol Med. 2003;12:969–975. [PubMed] [Google Scholar]
- 30.Thurm H, Ebel S, Kentenich C, et al. Rare expression of epithelial cell adhesion molecule on residual micrometastatic breast cancer cells after adjuvant chemotherapy. Clin Cancer Res. 2003;9:2598–2604. [PubMed] [Google Scholar]
- 31.EasySep. StemCell Technologies; [last accessed October 2009]. www.stemcell.com/product_catalog/easysep.aspx. [Google Scholar]
- 32.LD Columns. Miltenyi Biotec GmbH; [last accessed October 2009]. www.miltenyibiotec.com/en/PG_115_167_LD_Columns.aspx. [Google Scholar]
- 33.Lara O, Tong X, Zborowski M, et al. Enrichment of rare cancer cells through depletion of normal cells using density and flow-through, immunomagnetic cell separation. Exp Hematol. 2004;32:891–904. doi: 10.1016/j.exphem.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Yang L, Lang JC, Balasubramanian P, et al. Optimization of an enrichment process for circulating tumor cells from the blood of head and neck cancer patients through depletion of normal cells. Biotechnol Bioeng. 2009;102:521–534. doi: 10.1002/bit.22066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chalmers JJ, Zborowski M, Sun L, et al. Flow through, immunomagnetic cell separation. Biotechnol Prog. 1998;14:141–148. doi: 10.1021/bp970140l. [DOI] [PubMed] [Google Scholar]
- 36.Hoyos M, McCloskey K, Moore L, et al. Pulse-injection studies of blood progenitor cells in a quadrupole magnetic flow sorter. Sep Sci Technol. 2002;37:1–23. [Google Scholar]
- 37.Jin X, Zhao Y, Richardson A, et al. Differences in magnetically induced motion of diamagnetic, paramagnetic, and superparamagnetic microparticles detected by cell tracking velocimetry. Analyst. 2008;133:1767–1775. doi: 10.1039/b802113a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jing Y, Moore LR, Schneider T, et al. Negative selection of hematopoietic progenitor cells by continuous magnetophoresis. Exp Hematol. 2007;35:662–672. doi: 10.1016/j.exphem.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 39.McCloskey KE, Moore LR, Hoyos M, et al. Magnetophoretic cell sorting is a function of antibody binding capacity. Biotechnol Prog. 2003;19:899–907. doi: 10.1021/bp020285e. [DOI] [PubMed] [Google Scholar]
- 40.Moore LR, Rodriguez AR, Williams PS, et al. Progenitor cell isolation with a high-capacity quadrupole magnetic flow sorter. J Magn Magn Mater. 2001;225:277–284. [Google Scholar]
- 41.Nakamura M, Decker K, Chosy J, et al. Separation of a breast cancer cell line from human blood using a quadrupole magnetic flow sorter. Biotechnol Prog. 2001;17:1145–1155. doi: 10.1021/bp010109q. [DOI] [PubMed] [Google Scholar]
- 42.Tong X, Xiong Y, Zborowski M, et al. A novel high throughput immunomagnetic cell sorting system for potential clinical scale depletion of T cells for allogeneic stem cell transplantation. Exp Hematol. 2007;35:1613–1622. doi: 10.1016/j.exphem.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams PS, Zborowski M, Chalmers JJ. Flow rate optimization for the quadrupole magnetic cell sorter. Anal Chem. 1999;71:3799–3807. doi: 10.1021/ac990284+. [DOI] [PubMed] [Google Scholar]
- 44.Zborowski M, Chalmers JJ. Magnetic cell separation. Elsevier Science; Amsterdam: 2008. p. 464. [Google Scholar]
- 45.Zborowski M, Moore LR, Williams PS, et al. Separations based on magnetophoretic mobility. Sep Sci Technol. 2002;37:3611–3633. [Google Scholar]
- 46.Zborowski M, Williams PS, Sun L, et al. Cylindrical SPLITT and quadrupole magnetic field in application to continuous-flow magnetic cell sorting. J Liq Chromatogr Relat Tech. 1997;20:2887–2905. [Google Scholar]
- 47.Lustberg MB, Balasubramanian P, Lang JC, Ruppertt AS, Carothers S, Berger MJ, Mrozek E, Ramaswamy B, Layman RC, Chalmers J, Shapiro CLS. Mesenchymal markers are present on circulating tumor cells in breast cancer AACR special conference on EMT and cancer progression and treatment. Poster presentation taking place; Arlington. 28 Feb–2 Mar 2010.2010. [Google Scholar]
- 48.Lustberg MB, Balasubramanian P, Lang JC, Ruppertt AS, Carothers S, Berger MJ, Mrozek E, Ramaswamy B, Layman RC, Chalmers J, Shapiro CLS. Isolation of circulating tumor cells (CTCs) with mesenchymal and stem cell markers in localized and metastatic breast cancer using a novel negative selection enrichment. AACR National Meeting; 2010. p. Abstract # 5105. [Google Scholar]
- 49.Pantel K, Alix-Panabieres C, Riethdorf S. Cancer micrometastases. Nat Rev Clin Oncol. 2009;6:339–351. doi: 10.1038/nrclinonc.2009.44. [DOI] [PubMed] [Google Scholar]
- 50.Hristozova T, Konschak R, Stromberger C, et al. The presence of circulating tumor cells (CTCs) correlates with lymph node metastasis in nonresectable squamous cell carcinoma of the head and neck region (SCCHN) Ann Oncol. 2011;22(8):1878–1885. doi: 10.1093/annonc/mdr130. [DOI] [PubMed] [Google Scholar]
- 51.Jatana KR, Balasubramanian P, Lang JC, et al. Significance of circulating tumor cells in patients with squamous cell carcinoma of the head and neck: initial results. Arch Otolaryngol Head Neck Surg. 2010;136:1274–1279. doi: 10.1001/archoto.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paterlini-Brechot P, Benali NL. Circulating tumor cells (CTC) detection: clinical impact and future directions. Cancer Lett. 2007;253:180–204. doi: 10.1016/j.canlet.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 53.Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–8326. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- 54.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Natl Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 55.Yang J, Mani SA, Weinberg RA. Exploring a new twist on tumor metastasis. Cancer Res. 2006;66:4549–4552. doi: 10.1158/0008-5472.CAN-05-3850. [DOI] [PubMed] [Google Scholar]
- 56.Blick T, Widodo E, Hugo H, et al. Epithelial mesenchymal transition traits in human breast cancer cell lines. Clin Exp Metastasis. 2008;25:629–642. doi: 10.1007/s10585-008-9170-6. [DOI] [PubMed] [Google Scholar]
- 57.Sarrio D, Rodriguez-Pinilla SM, Hardisson D, et al. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 2008;68:989–997. doi: 10.1158/0008-5472.CAN-07-2017. [DOI] [PubMed] [Google Scholar]
- 58.Mani S, Guo W, Liao MJ, et al. The epithelial mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morel A, Lievre M, Thomas C, et al. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS ONE. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Galie M, Konstantinidou G, Peroni D, et al. Mesenchymal stem cells share molecular signature with mesenchymal tumor cells and favor early tumor growth in syngeneic mice. Oncogene. 2008;27:2542–2551. doi: 10.1038/sj.onc.1210920. [DOI] [PubMed] [Google Scholar]
- 61.Santisteban M, Reiman JM, Asiedu MK, et al. Immune-Induced epithelial to mesenchymal transition in vivo generates breast cancer stem cells. Cancer Res. 2009;69:2887–2895. doi: 10.1158/0008-5472.CAN-08-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kokkinos MI, Wafai R, Wong MK, et al. Vimentin and epithelial-mesenchymal transition in human breast cancer–observations in vitro and in vivo. Cells Tissues Organs. 2007;185:191–203. doi: 10.1159/000101320. [DOI] [PubMed] [Google Scholar]
- 63.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4:448–456. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- 64.Pantel K, Alix-Panabieres C. The clinical significance of circulating tumor cells. Nat Clin Pract Oncol. 2007;4:62–63. doi: 10.1038/ncponc0737. [DOI] [PubMed] [Google Scholar]
- 65.Sommers CL, Heckford SE, Skerker JM, et al. Loss of epithelial markers and acquisition of vimentin expression in adriamycin- and vinblastine-resistant human breast cancer cell lines. Cancer Res. 1992;52:5190–5197. [PubMed] [Google Scholar]
- 66.Thompson EW, Paik S, Brunner N, et al. Association of increased basement membrane invasiveness with absence of estrogen receptor and expression of vimentin in human breast cancer cell lines. J Cell Physiol. 1992;150:534–544. doi: 10.1002/jcp.1041500314. [DOI] [PubMed] [Google Scholar]
- 67.Theodoropoulos PA, Polioudaki H, Agelaki S, et al. Circulating tumor cells with a putative stem cell phenotype in peripheral blood of patients with breast cancer. Cancer Lett. 2009;288(1):99–106. doi: 10.1016/j.canlet.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 68.Aktas B, Tewes M, Fehm T, et al. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 2009;11:R46. doi: 10.1186/bcr2333. [DOI] [PMC free article] [PubMed] [Google Scholar]