Abstract
The kappa opioid receptors (KOR) are involved in mood disorders and addictive conditions. In vivo imaging studies of this receptor in humans have not been reported due to the lack of a selective ligand. We employed a recently developed selective KOR agonist tracer, [11C]GR103545, and performed a study in rhesus monkeys to estimate the in vivo receptor concentration (Bmax) and dissociation equilibrium constant (Kd).
Methods
Four rhesus monkeys underwent a total of 12 scans with [11C]GR103545 on the Focus 220 scanner under baseline and self-blocking conditions. The injected mass was 0.042±0.014 µg/kg for the baseline scans and ranged from 0.17 to 0.3 µg/kg for the self-blocking scans. The radiotracer was administered in a bolus plus infusion (B+I) protocol, and cerebellum used as reference region in kinetic analysis. Binding potential (BPND) values were computed as [(CROI/CREF)-1], where CROI and CREF are the mean of the radioactivity concentrations from 90 to 120 min post tracer administration in a given region of interest (ROI) and in the cerebellum. In six scans, arterial input functions and free fraction in plasma (fp) were measured, and a 2 -tissue compartment model was used to compute the volume of distribution in the cerebellum (VT_REF), which was then employed to estimate the free to non-displaceable concentration ratio (fND) as fp/VT_REF. A Scatchard plot was used to estimate Bmax, and KdND = Kd/fND, the Kd value with respect to the cerebellar concentration. Individual data were first analyzed separately, then pooled together. When KdND was allowed to vary among ROIs, results were very variable; therefore KdND was constrained to be constant across ROIs whereas Bmax was allowed to be ROI-dependent and animal-dependent.
Results
A global estimate of 1.72 nM was obtained for KdND. Estimated Bmax ranged from 0.3 to 6.1 nM across ROIs and animals. The Kd estimate of 0.048 nM, obtained by correcting KdND by the factor fND, was between the in vitro Kd values of 0.018 nM to 0.4 nM (obtained from functional assays in rabbit vas deferens and radioligand competition assays using cloned human receptors, respectively). Based on these data, a suitable tracer dose of 0.02 µg/kg was selected for use in humans.
Conclusions
The use of a B+I protocol with the KOR agonist tracer [11C]GR103545 permitted the successful estimation of Bmax and KdND in vivo. Based on the estimated Kd value, a tracer dose of 1.4 µg (3.38 nmol) for an average body weight of 70 k g was chosen as the mass dose limit in human studies using this novel agonist radiotracer.
Keywords: PET, Kappa opioid receptors, Agonist, In vivo affinity
INTRUDUCTION
Opioid receptors (ORs) belong to the superfamily of G-protein-coupled receptors and are subdivided into 4 classes: δ (DOR), κ (KOR), µ (MOR) and the recently discovered nociceptin receptors. The KOR, the most abundant brain OR in humans, is strongly related to substance abuse (cocaine, heroine, alcohol, opiate drugs) and KOR-targeted drugs have been therefore investigated for the treatment of addictions (1). Evidence suggests that dynorphin, the endogenous neurotransmitter which binds to the KOR, is involved in the body's natural addiction control mechanism (1). In addition, KORs are implicated in other brain disorders, including epilepsy (2, 3) and Alzheimer’s disease (4), and have recently been shown to play a role in the modulation of the cardiovascular system.
Several compounds labeled with positron emitters have been used in vivo to study the ORs (see (5) for a review). For example, the MOR-selective ligand [11C]carfentanil (6) and the less selective opioid ligand [18F]cyclofoxy (7) have been utilized in humans with Positron Emission Tomography (PET).
Only recently, the highly selective KOR ligand, ( ± )-4-methoxycarbonyl-2-[(1-pyrrolidinylmethyl]-1-[(3,4-dichlorophenyl)acetyl]-piperazine, known as GR89696, was labeled with C-11 and evaluated in mice (8). The (−)-isomer of the racemic GR89696 (GR103545) is more potent, and was also labeled and studied (9). GR103545 is a KOR agonist with in vitro Ki for the KOR of 0.02 ± 0.01 nM and excellent selectivity over MOR (6 × 102-fold) and DOR (2 × 104-fold) (10). An IC50 estimate of GR103545 for the KOR, measured in functional assays using rabbit vas deferens, was reported to be 0.018 nM (0.04 nM and 6.0 nM, respectively, for the racemic mixture GR89696 and the less potent (+)-enantiomer) (11). In mice, the uptake of the active isomer [11C]GR103545 correlated well with the known distribution of KOR, whereas the (+)-isomer, [11C](+)-GR89696, showed a homogeneous brain uptake (9). A PET study in baboons with [11C]GR89696, [11C]GR103545 and [11C](+)-GR89696 showed that [11C]GR103545 is a promising radioligand for imaging KOR, with a moderate rate of peripheral metabolism, a relatively high free fraction, and high specific binding signals (12).
This paper describes a study with [11C]GR103545 in rhesus monkeys to estimate in vivo the regional KOR concentration (Bmax) and the dissociation equilibrium constant (Kd). While these values are of general interest for all PET ligands, they are of higher importance for [11C]GR103545 because knowledge of Kd and receptor occupancy level associated with side effects are critical to minimize the manifestation of adverse events, especially in human studies, due to the agonist nature of this tracer (12).
Two aspects of this work differentiate it from many other studies to measure Bmax and Kd (13). First, this study was performed with bolus plus constant infusion of the radiotracer, an approach normally used to produce equilibrium in all brain regions. However, here, it was expected from simulations based on bolus data that equilibrium could not be reached in all regions within the time frame of the study limited by C-11 decay, and the goal was therefore to achieve equilibrium in the reference region (14). Second, it is conventional to occupy a large fraction of the receptors to estimate Bmax and Kd with low error. However, due to the agonist nature of GR103545, only a moderate level of occupancy was attempted, necessary to minimize side effects.
MATERIALS AND METHODS
Radiochemistry
Synthesis of high specific activity [11C]GR103545 was performed as described in Nabulsi et al. (15).
PET Imaging Experiments
Imaging Protocol
Twelve PET experiments were performed in four rhesus monkeys (2 males, 2 females, weight 7.9 ± 2.5 kg). Two monkeys (A and B) received two scanning sessions, each consisting of a high specific activity (SA) injection (baseline scan) in the morning followed by a low SA injection (self-blocking scan) in the afternoon, for a total of 4 scans each. Two monkeys (C and D) were scanned twice, once at baseline and once under self-blocking condition. The mass range of GR103545 was 0.02–0.05 µg/kg for the baseline scans, and 0.15–0.30 µg/kg for the self-blocking scans. Based on results in baboons (12), no more than 0.3 µg/kg of GR103545 was administered to minimize side effects. The injected activity dose was 113±78 MBq, and the specific activity at time of injection was 98 ± 45 MBq/nmol. A summary of the scan parameters is provided in Table 1.
Table 1.
Summary of scan parameters
| Animal | Scan Type | Activity Dose (MBq) |
Specific Activity at time of injection (MBq/nmol) |
Mass Dose (µg/kg) |
Tbol (min) | Kbol (min) |
|---|---|---|---|---|---|---|
| A | Baseline | 24.0 | 122.9 | 0.021 | 3 | 200 |
| A | Blocking | 183.5 | 136.2 | 0.16 | 3 | 150 |
| A | Baseline | 35.1 | 80.1 | 0.05 | 5 | 200 |
| A | Blocking | 144.7 | 65.6 | 0.30 | 5 | 200 |
| B | Baseline | 76.2 | 106.0 | 0.049 | 15 | 200 |
| B | Blocking | 220.5 | 62.9 | 0.30 | 15 | 100 |
| B | Baseline | 73.2 | 103.4 | 0.048 | 15 | 200 |
| B | Blocking | 190.9 | 79.1 | 0.20 | 15 | 100 |
| C | Baseline | 38.1 | 101.7 | 0.049 | 15 | 200 |
| C | Blocking | 34.8 | 23.8 | 0.171 | 2 | 150 |
| D | Baseline | 107.3 | 204.5 | 0.039 | 15 | 200 |
| D | Blocking | 232.4 | 95.1 | 0.22 | 15 | 100 |
PET experiments were carried out according to a protocol approved by the Yale University Institutional Animal Care and Use Committee. Animals were sedated with 10 mg/kg i.m. ketamine at least 2 h prior to the first radiotracer injection. Once sedated, endotracheal intubation was performed and the animals were placed on isoflurane (1.5%–3%). Once stable, the animals were moved into the scanner and a pressurized infusion set was connected to the animal’s indwelling arterial access port for blood pressure monitoring and arterial blood sampling. Vital signs were monitored throughout the PET scan.
PET scans were acquired on a FOCUS 220 scanner (Siemens Medical System, Knoxville, TN, USA). For each experiment, a transmission scan was performed before tracer injection, followed by a 120 min emission scan initiated at the start of tracer administration. After correcting for attenuation, deadtime, randoms, and scatters, list mode data were reformatted into 33 frames and reconstructed with the FORE algorithm, followed by 2D filtered-backprojection.
A bolus plus infusion (B+I) protocol (16) was employed for tracer administration with the goal of reaching equilibrium. The parameter Kbol, the ratio of bolus dose volume (mL) to infusion rate (mL/min), was set to 200 min for the baseline scans. In the self-blocking scans, Kbol was initially set to 150 min, and subsequently to 100 min, to decrease the relative contribution of the bolus component in order to reduce the peak concentration of GR103545. Value of Kbol was set higher for the baseline scans, where binding levels are higher, to approach equilibrium faster.
The bolus component was administered over a time equal to Tbol, set to 2 min for the first scans, and increased to 15 min in later scans to reduce the peak concentration of GR103545. Thus, the bolus fraction of the total dose of [11C]GR103545 was Kbol/(Kbol+T), with T = 120 min indicating the duration of the scan; the remaining portion of T/(Kbol+T) was infused at a constant rate. Values of Tbol and Kbol for each scan are reported in Table 1.
Plasma Input Function, Free Fraction and Metabolite Measurements
Arterial blood samples were collected to determine the metabolite-corrected plasma input function in 6 of 12 scans (in animals A and D). In these scans, 20 blood samples were drawn and plasma radioactivity concentration was measured with a gamma counter (WIZARD2, PerkinElmer, Waltham, MA, USA). The tail portion of the total plasma concentration curve was fitted to a 3-exponential function. Free fraction in plasma (fp) was measured by ultrafiltration of a reference blood sample taken before tracer injection to which was added a small amount of the radiotracer. In addition, selected samples were analyzed to assess the unchanged parent fraction with HPLC analysis. The parent fraction was calculated as the ratio of the sum of radioactivity in fractions containing the parent tracer to the total amount of radioactivity collected, and fitted to an integrated gamma function. This curve was also corrected for the timevarying extraction efficiency of radioactivity in acetonitrile and normalized to the extraction efficiency determined from the reference plasma sample. The final plasma input function was calculated as the product of the fitted total plasma curve, fitted HPLC fraction curve, and fitted acetonitrile extraction curve.
Data Analysis
To define regions of interest (ROIs) in each scan, two summed PET images (0–10 and 10–30 min) were registered to the MRI of each animal (6-parameter affine registration) by maximizing the normalized mutual information (NMI) of the images, and the registration that produced the higher NMI was chosen. Through a non-linear transformation, the individual MRI was registered to a rhesus monkey brain template MRI on which ROIs had already been defined. By combining the 2 registrations, a PET-to-template transformation was obtained; ROIs were then transferred to the original PET space and time-activity curves (TACs) were computed. The ROIs were cerebellum, amygdala, caudate, cingulate and frontal cortex, globus pallidus, hippocampus, insula, occipital cortex, putamen, temporal cortex and thalamus.
The cerebellum was employed as the reference region (12) and values of binding potential (BPND) (17) were estimated using both Equilibrium Analysis (EA) and the Simplified Reference Tissue Model (SRTM). For EA, it was assumed that equilibrium was reached between 90 and 120 min and BPND_EA was computed using the standard expression
| Eq. 1 |
where CROI and CREF denote the mean concentrations in the ROI and cerebellum, respectively. For SRTM, the entire TAC was fitted to estimate BPND_SRTM. To reduce noise in the input function the tail of each cerebellum TAC was fitted to a 3-exponential function. Weights wi equal to were employed, with Δti,λ and CROI (i) denoting, respectively, the duration of the ith frame, the decay constant of C-11 and the measured concentration in the ith frame.
Where arterial input function data were available, the distribution volume (VT) was estimated using the 2-tissue (2T) compartment model, and BPND computed as [(VT_ROI-VT_REF)/VT_REF]. These values of BPND were compared with those derived from EA and SRTM.
For each pair of baseline—self-blocking scans performed on the same day, the percent occupancy of KOR due to GR103545 itself (RO%) was estimated with both EA and SRTM methods for each ROI as
| Eq. 2 |
and the average of RO% across ROIs, after the exclusion of outliers, was computed.
To estimate Bmax and KdND, instead of employing the linear Scatchard equation (18)
| Eq. 3 |
by fitting the concentration B' of the bound GR103545 at equilibrium, computed as (CROI-CREF)/SA, to BPND_EA or BPND_SRTM, the nonlinear version of Eq. 3 was used:
| Eq. 4 |
where F’ is CREF/SA. In Eq. 3 and 4 KdND is Kd/fND, where fND, the free fraction in the nondisplaceable pool, is assumed to be the same for all ROIs (17). In this case Eq. 4 is preferable to Eq. 3 as it is characterized by less noise-induced bias. Results of the fits, however, are displayed using Eq. 3, which is more intuitive from a graphical point of view. Bmax and KdND were estimated first from E q. 4, employing the mean concentrations between 90 and 120 min to compute CROI and CREF. Subtracting CREF from both sides of Eq. 4 and dividing by CREF yields:
| Eq. 5 |
Bmax and KdND were also estimated with Eq. 5, using the same CREF as in Eq. 4, but with BPND values derived from SRTM. Whereas Eq. 3 and 4 require equilibrium in both the reference region and target ROIs, Eq. 5 is based only on the assumption of the reference region at equilibrium if SRTM properly extrapolates the equilibrium BPND value.
Five data analysis approaches were employed, two applied to each individual animal, and 3 pooling across animals. Initially, individual estimates of Bmax and KdND for each animal and ROI were computed (individual analysis 1, IND1); KdND was then constrained to be constant across ROIs, obtaining, for each animal, one value of Bmax for every ROI and one global value of KdND (IND2). Subsequently all data were analyzed simultaneously and estimates of Bmax and KdND were obtained for each ROI (pooled analysis 1, POOL1 ). KdND was then constrained to be constant across ROIs, by computing one Bmax per ROI and one global value for KdND (POOL2). In the approach POOL3, Bmax was allowed to vary for each ROI and animal, whereas KdND was again constrained to be constant.
In addition, in the 6 sc ans where arterial measurements were available, a 2- tissue (2T) compartment model was employed with the metabolite-corrected input function to fit the TACs with a weighted-least-squares procedure to estimate the volume of distribution (VT) in all ROIs. The volume of distribution in the cerebellum (VT_REF) was employed as an estimate of the non-displaceable volume of distribution (VND), and fND was computed for each scan as fp/VND. The final estimate of Kd was obtained as KdND x fND.
RESULTS
Radiochemistry
The previous method for the preparation of [11C]GR103545 was a multiple step, low-yielding procedure that gives rise to a product with low and variable radiochemical yield, as well as low specific activity (8, 9, 12). Therefore, a new radiosynthetic method was developed in our laboratory to produce [11C]GR103545 in high radiochemical yield and specific activity (15). [11C]GR103545 used in this study was prepared with the new radiosynthetic method.
Administration of blocking doses of GR103545
No changes in the vital signs were observed in any of the baseline scans with [11C]GR103545. In the self-blocking scans, reductions in heart rate (range 10%–20%) and respiratory rate (range 10%–16%) were seen and recovery from anesthesia was slower than typical.
Calculation of BPND and receptor occupancy
Figure 1 displays axial, sagittal and coronal views of the uptake of [11C]GR103545 at 60–90 min post-injection for an image of a baseline scan. Images were post-smoothed with a 3D Gaussian filter with FWHM of 3 pixels. Figure 2 shows sample TACs for the cerebellum and ROIs with low, medium, and high uptake (occipital, temporal and cingulate cortex, respectively), from a typical baseline scan with the corresponding SRTM fits. The pattern in Figure 2 was typical, as equilibrium was generally reached in the cerebellum but not in the ROIs with higher uptake. Tables 2 and 3 list the mean ± SD of the percent increase in the TAC between 90 and 120 min for each ROI, along with the mean binding potentials from EA and SRTM analysis (Table 2, mean across baseline scans, n = 6; Table 3, mean across blocking scans, n = 6). In 13 of 132 fits, BPND_SRTM values were considered outliers (defined as BPND_SRTM > 3), so these values were set to 3.
Figure 1.
Example image showing uptake of [11C]GR103545 at 60–90 min postinjection during a baseline scan.
Figure 2.
Selected TACs from a typical [11C]GR103545 baseline scan with corresponding SRTM fits.
Table 2.
Mean ± SD across baseline scans (n = 6) of the percent increase of the ROI TACs between 90 and 120 min1, and BPND values calculated from EA (BPND_EA) and SRTM (BPND_SRTM) analyses2.
| %INCREASE ± SD | BPND_EA ± SD | BPND_SRTM ± SD | |
|---|---|---|---|
| Cerebellum | −12 ± 11 | N/A | N/A |
| Amygdala | −2 ± 64 | 0.23 ± 0.35 | 0.74 ± 1.14 |
| Caudate | 24± 17 | 1.20 ± 0.36 | 1.78 ± 0.56 |
| Cingulate Cortex | 30± 17 | 1.69 ± 0.33 | 2.15 ± 0.67 |
| Frontal Cortex | 21 ± 10 | 1.03 ± 0.22 | 1.33 ± 0.55 |
| Globus Pallidus | 34 ± 37 | 1.33 ± 0.47 | 1.29 ± 0.37 |
| Hippocampus | 21 ± 28 | 0.59 ± 0.23 | 1.40 ± 1.26 |
| Insula | 25 ± 11 | 1.53 ± 0.21 | 2.03 ± 0.60 |
| Occipital Cortex | 7 ± 8 | 0.56 ± 0.16 | 0.60 ± 0.25 |
| Putamen | 4 ± 13 | 1.93 ± 0.93 | 1.87 ± 0.70 |
| Temporal Cortex | 15 ± 15 | 0.86 ± 0.13 | 1.07 ± 0.37 |
| Thalamus | −4 ± 23 | 0.45 ± 0.28 | 0.73 ± 1.12 |
BPND_SRTM higher than 3 were bounded to 3.
Table 3.
Mean ± SD across blocking scans (n = 6) of the percent increase of the ROI TACs between 90 and 120 min, and BPND values calculated from EA (BPND_EA) and SRTM (BPND_SRTM) analysis.
| %INCREASE ± SD | BPND_EA ± SD | BPND_SRTM ± SD | |
|---|---|---|---|
| Cerebellum | −0 ± 10 | N/A | N/A |
| Amygdala | 1 ± 53 | 0.24 ± 0.19 | 0.38 ± 0.29 |
| Caudate | 18 ± 10 | 1.03 ± 0.17 | 1.14 ± 0.34 |
| Cingulate Cortex | 31 ± 12 | 1.49 ± 0.31 | 2.40 ± 0.57 |
| Frontal Cortex | 28 ± 8 | 0.83 ± 0.27 | 1.57 ± 0.82 |
| Globus Pallidus | 33 ± 11 | 1.05 ± 0.29 | 1.46 ± 0.78 |
| Hippocampus | 6 ± 22 | 0.37 ± 0.14 | 0.86 ± 1.08 |
| Insula | 28 ± 8 | 1.29 ± 0.26 | 1.96 ± 0.34 |
| Occipital Cortex | 18 ± 6 | 0.52 ± 0.18 | 0.59 ± 0.26 |
| Putamen | 11 ± 15 | 1.79 ± 0.80 | 2.12 ± 0.76 |
| Temporal Cortex | 24 ± 9 | 0.76 ± 0.15 | 1.10 ± 0.22 |
| Thalamus | 9 ± 20 | 0.31 ± 0.15 | 0.70 ± 1.13 |
SRTM fits were in general of good quality. The mean ± SD across scans and ROIs of R1, the ratio of the influx rate K1 between the target region and the cerebellum, and , the efflux rate in the cerebellum, were 0.82 ± 0.26 mL·min−1·cm−3 and 0.05 ± 0.03 min−1, respectively. As expected, BPND_EA values were systematically lower than those of BPND_EA, because of the increasing trend of uptake in most ROIs (Fig. 1). SRTM estimates, on the other hand, were less stable than those of EA, with outliers (defined as BPND_SRTM estimates >3) in 13 of 132 fits. Despite the underestimation with EA, regional values of BPND_ED and BPND_SRTM were well correlated (r = 0.84), as shown in Figure 3. The slope m of the fitted line of equation y = mx was m = 0.77, indicating a 23% mean underestimation of BPND_EA compared to BPND_SRTM.
Figure 3.
Relationship between BPND_SRTM (x-axis) and BPND_EA (y-axis). The correlation was good (r = 0.84) with a line of regression of y = 0.77x (the intercept was set to 0). Thus, in this study, EA underestimated BPND.
For the studies for which V data were available BPND_EA and BPND_SRTM showed good correlation with BPND_2T (r = 0.81 and r = 0.70, respectively) but systematically underestimated the BPND_2T values; the equations of the fitted lines, with intercept set to 0, were y = 0.49x for BPND_EA and y = 0.79x for BPND_SRTM, with x denoting BPND_2T. The underestimation of BPND_SRTM is likely due to the fact that the 1T model does not adequately describe the cerebellum TACs (data not shown).
Percent receptor occupancy (RO%) values, as expected, were low due to the mass limit of 0.3 µg/kg for GR103545, with the highest RO% among all scans, averaged over ROIs after the exclusions of outliers, equal to 25% for EA and 29% for SRTM analysis.
Calculation of Bmax and KdND
Estimates of Bmax and KdND obtained using BPND_SRTM and Eq. 5 were often unreliable (i.e., either extremely high or zero) and/or characterized by large standard errors; therefore only estimates obtained fromEq.4 are presented in subsequent analyses.
When individual data were analyzed independently (IND1), the quality of fit in the Scatchard plots and of Bmax and KdND estimates was highly variable. Not surprisingly, results were dependent on baseline BPND: ROIs with high baseline BPND gave the best results in terms of physiological plausibility of the estimates and of percent standard error (%SE, range 10%–30%). Poorer estimates were obtained in low-binding ROIs because of the low level of receptor occupancy in this study. Some improvements were obtained when, for a given animal, KdND was constrained to be constant across ROIs (IND2). In this case, the estimates of KdND for the 2 animals with 4 scans each were similar (1.51 and 1.99 nM for animals A and B, respectively) and %SE values were low (34% and 23%, respectively). However, for the 2 animals with only 2 scans each, KdND estimates were poorer.
When data were pooled together, Bmax and KdND estimates substantially improved in terms of physiological plausibility, i.e., no negative values and no unreasonably high values, and %SE, when one scan (the blocking scan of animal D) was excluded. That scan was anomalous in that, although a high mass dose of 0.22 µg/kg was administered, a 0% mean RO was obtained with EA. Considered an outlier, data from that scan were excluded from the pooled analysis.
In the pooled analyses, when both Bmax and KdND were allowed to be ROI-dependent but constant across animals (POOL1), reasonable results were obtained in all ROIs but the thalamus. Excluding the thalamus, which has a low baseline BPND, KdND was in the range 0.7–8.2 nM (mean = 3.05 nM, median = 1.75 nM, SD = 75 %) and Bmax in the range 0.35–13.7 nM (mean = 3.76 nM, median 2.06 nM) with large %SE (range 50%-200% for both Bmax and KdND). When KdND was constrained to be constant across animals (POOL2), estimates were in good agreement with those from POOL1 (global KdND=2.54 nM, Bmax in the range 0.8–4.9 with mean and median of 2.64 and 2.54 nM, respectively) but with much lower %SE (41% for KdND, range 30%–40% for Bmax). Figure 4 shows examples of typical fits (caudate and putamen) for individual analysis (IND1) and for a pooled analysis (POOL1). Note that, for the putamen (Fig.4B), the slopes of the individual fitted lines are similar whereas the (extrapolated) intercepts on the x-axis (Bmax) are different, which suggests the need for a constant KdND and different Bmax values for the different animals. When KdND was constrained to a common value while allowing Bmax to be ROI- and animal-dependent (POOL3), the global estimate of KdND (1.72 nM) was in good agreement with the 2 individual KdND values reported above (1.51 and 1.99 nM) and with the median value from POOL1 (1.75 nM), and in reasonable agreement with the global value from POOL2 (2.54 nM). In addition, %SE of KdND in POOL3 was 17%, much lower than the 41% in POOL2.
Figure 4.
Examples of typical Scatchard plots for selected ROIs (caudate, top; putamen, bottom) based on individual and pooled analyses. Different symbols were used for each animal. The continuous and dashed lines represent the global fitted line and the fits of the individual data, obtained with POOL1 and IND1, respectively.
Furthermore, standard statistical tests (Akaike Information Criterion and Schwarz Criterion) selected method POOL3 as better than POOL2 and POOL1. The F-test performed between POOL2 and POOL3 also selected POOL3 as the better approach (p-value < 10−4).
Values of Bmax estimated from POOL3 are reported in Table 4. In addition, the table lists the corresponding predicted BPND for each ROI computed as the ratio of the ROI’s Bmax to the global KdND of 1.72 nM.
Table 4.
Mean ± SD of Bmax estimates across animals, obtained from pooled analysis (POOL3), and the corresponding BPND estimates, computed as ratio of Bmax to the global KdND of 1.72 nM.
| Bmax ± SD (nM) | BPND ± SD1 | |
|---|---|---|
| Amygdala | 0.55 ± 0.21 | 0.32 ± 0.13 |
| Caudate | 2.04 ± 0.68 | 1.19 ± 0.39 |
| Cingulate Cortex | 3.17 ± 0.53 | 1.84 ± 0.31 |
| Frontal Cortex | 1.81 ± 0.41 | 1.05 ± 0.24 |
| Globus Pallidus | 2.13 ± 0.75 | 1.24 ± 0.44 |
| Hippocampus | 0.90 ± 0.09 | 0.52 ± 0.05 |
| Insula | 2.65 ± 0.41 | 1.54 ± 0.24 |
| Occipital Cortex | 1.12 ± 0.30 | 0.65 ± 0.17 |
| Putamen | 3.75 ± 2.05 | 2.18 ±1.19 |
| Temporal Cortex | 1.62 ± 0.24 | 0.94 ± 0.14 |
| Thalamus | 0.71 ± 0.29 | 0.41 ± 0.17 |
SD values for BPND were extrapolated from the Bmax values by scaling with the global KdND.
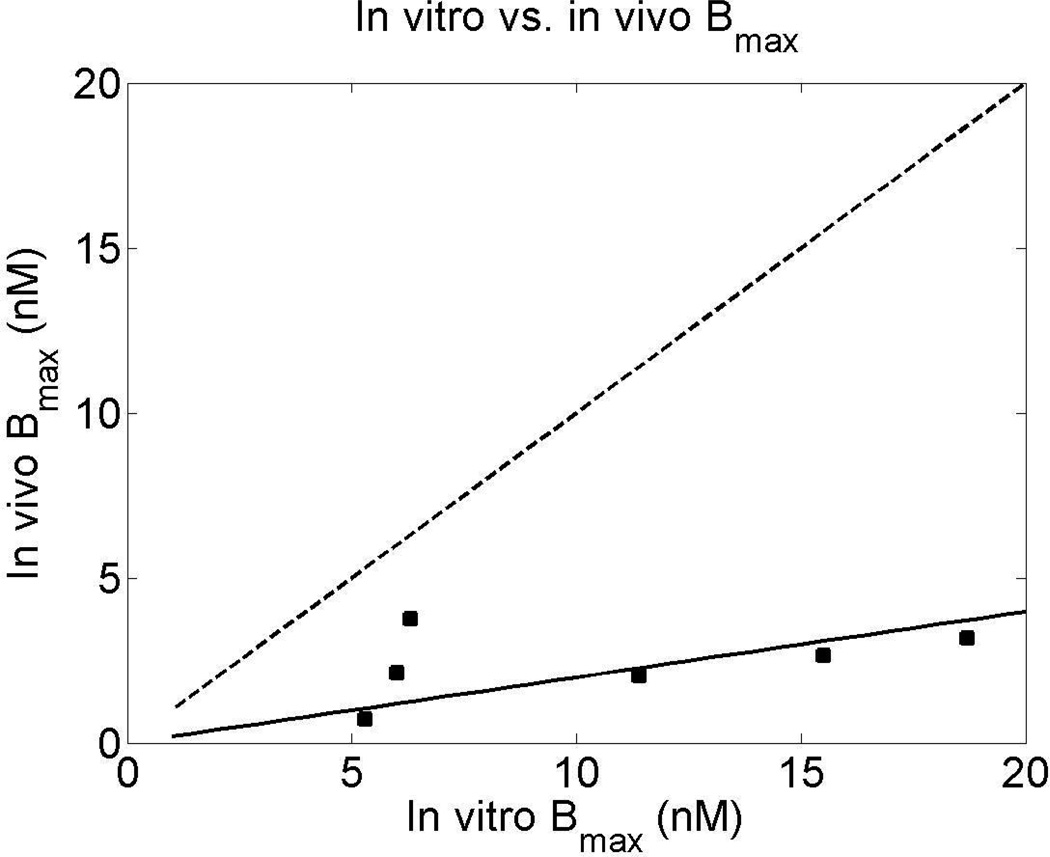
Figure 5 illustrates the relationship between in vitro and in vivo Bmax estimates, with the dashed and continuous lines representing the identity and fitted line y = mx, respectively. The in vitro estimates, reported in (19), were computed from sections of monkey brain (macaque fascicularis) with the autoradiographic method and the radioligand [3H]dynorphin. The correlation between these two sets of estimates was reasonable (r = 0.39), although the in vivo estimates were substantially lower than the in vitro values (slope = 0.20).
Figure 5.
Relationship between in vitro and in vivo Bmax estimates: the continuous and dashed lines represent, respectively, the fitted line (y=0.20x) and the line of identity (y=x). The ROIs for which both estimates were available are caudate, cingulate cortex, globus pallidus, insula, putamen and thalamus.
Using the 2T compartmental model, estimates for the volume of distribution in the cerebellum (VT_REF) were 8.6± 0.87 mL·cm−3 for animal B (n = 4 scans) and 14.7 ± 0.91 mL·cm−3 for animal D (n = 2 scans); fP values were 0.26 ± 0.01 and 0.39 ± 0.02 for animals B and D, respectively, and consistent with the expected increase in VT with increasing fP. Estimates of fND were quite similar across the 6 scans (0.028± 0.004). The mean value of fND was employed to estimate Kd from Kd = KdND x fND = 1.72 × 0.028 = 0.048 nM. This value was in good agreement with the IC50 value of 0.018 nM measured with functional assays using rabbit vas deferens (11) and the in vitro Ki value of 0.02 nM determined by radioligand competition assays using cloned human kappa opioid receptor (10).
DISCUSSION
GR103545 is a ligand with a high selectivity for KOR and reports in the literature demonstrated the potential of [11C]GR103545 for PET imaging in the non-human primate brain (10, 12). In the present study [11C]GR103545 was employed to estimate the in vivo regional KOR concentration (Bmax) and the dissociation equilibrium constant (Kd) using a B+I tracer administration protocol. Regional BPND values were derived using both Equilibrium Analysis (EA) and SRTM methods. Estimates of BPND_EA and BPND_SRTM were well correlated, with BPND_EA consistently lower than BPND_SRTM because of the increasing trend of tracer uptake in most ROIs in the 90–120 min time window. The lack of simultaneous equilibrium in all ROIs was expected, as preliminary simulations showed that the range of [11C]GR103545 kinetics in rhesus monkeys was too wide for equilibrium to be achieved in all ROIs at the same time window. For values of Kbol such that equilibrium was reached in the reference region, equilibrium was not reached in the high-binding regions, which had an increasing trend in the last frames of the PET scan. For higher values of Kbol such that equilibrium was achieved in the high-binding regions, equilibrium was not reached in the reference region, which showed a decreasing trend.
The EA method provided estimates of KdND and Bmax more reliable than those derived from SRTM. The quality of fit in the Scatchard plots was very variable, with the ROIs with high BPND yielding the best results. The reason for this sometimes unsatisfactory fit quality was the limit on the injected mass (0.3 µg/kg): higher masses were not administered, as they were not expected to be well tolerated by the animals. As a consequence, percent receptor occupancy values were low, with the highest mean RO% among all scans equal to 25% for EA and 29% for SRTM. Our data were therefore all located in the first portion of the Scatchard plots, which produced larger errors than would have been found if a wider range of occupancies could have been achieved.
Several analysis methods were applied to these data. In the best approach (POOL3) to derive global estimates of KdND and Bmax, KdND was constrained to a common global value whereas Bmax was allowed to be ROI and animal-dependent. A global estimate of 1.72 nM was obtained for KdND, with Bmax ranging from 0.3 to 6.1 nM depending on the ROI and animal. The subsequent Kd estimate of 0.048 nM, obtained by correcting KdND by fND, was in good agreement with the IC50 or Ki value measured in vitro. As for Bmax, there was a good correlation between the in vivo and in vitro estimates, although the in vivo values were substantially lower (roughly 20% of the in vitro estimates). This underestimation is partly a consequence of the underestimation of BPND and B' by the EA method. It can be shown that under the hypothesis of BPND underestimation by a factor independent of its value (BPND_TRUE = k x BPND_EA, k > 1), Bmax values from the Scatchard plot is underestimated by the same factor k, whereas KdNDEA = KdNDTRUE. In addition, the in vitro values were estimated with audioradiography using [3H]dynorphin (19): although all dynorphins primarily exert their effects on the KOR, these peptides have some affinity for DOR, MOR, and NMDA-type glutamate receptor as well (20), which could have caused the estimated Bmax values to be positively biased. Another factor that might contribute to the underestimation of in vivo Bmax is the effects of the anaesthetic agents ketamine and isoflurane. Ketamine appears to bind to opioid receptors (21), even though its kappa-modulated (agonist) effect on behaviour in rats was detectable only at a high dose (20 mg/kg, i.p.) and shortly (10 min) after dosing (21, 22). In our study, ketamine was used only at the beginning of the experiment to immobilize the animals, and isoflurane was used thereafter to maintain anaesthesia. By the time of the blocking scan it was generally 6–7 h after ketamine administration, or ∼3 plasma half-lives (t1/2 ∼ 2 h for i. m. dosing). Therefore, it seems unlikely that ketamine would occupy the KOR and cause an underestimation of Bmax, although this possibility can not be completely ruled out due to the lack of in vivo studies to assess KOR occupancy by ketamine at the dose used in our study. As for the effect of isoflurane, there have been conflicting reports on its interaction with the OR in general and KOR in particular. However, the overwhelming evidence appears to indicate it as a non-specific anaesthetic agent, as opposed to a specific effect on KOR (or other ORs). Hence, given the factors cited here, our hypothesis is that the true values of regional Bmax are higher than those reported in this study and lower than those obtained from autoradiography studies (19).
GR103545 is a potent KOR agonist and a low injected mass is critical to limit the pharmacological effect of the ligand, especially in applications in humans. Even with the limit of 0.3 µg/kg, there were evident side effects including reduction in heart and respiratory rates, and increased post-scan recovery time. We have developed a new radiosynthetic method (15), which provides [11C]GR103545 with specific activity 7 times higher on average than the previous radiosynthetic method (12). Thus, for human scans, this will permit a substantial reduction in the injected mass of GR103545 while maintaining an acceptable radioactivity level of [ 11C]GR103545, essential for the quality of PET images.
Data from the present study permit the estimation of an appropriate mass dose for human studies to maintain trace dose levels. For this calculation, the cerebellum B/I TACs were converted from Bq/mL to nM using the SA of the radiotracer for each scan. These B/I TACs were then mathematically converted to what would have been obtained from a bolus injection, given knowledge of the infusion protocol used, specifically Kbol. The TACs were then normalized by dividing by the injected dose of GR103545 per body weight (µg/kg) and averaged across animals, and a predicted mass concentration (CCER) could then be obtained by multiplying the resulting curve by a selected injected mass (µg/kg).
Receptor occupancy (RO) was then calculated as:
| Eq. 6 |
Based on the concentration in the non-displaceable pool (FND) and the dissociation constant (KdND), assuming instantaneous equilibrium. The concentration in the cerebellum (CCER) was used as FND, assuming no specific binding in that region. Maximum occupancy was determined based on the maximum value in the averaged cerebellum curve, and final occupancy was determined from the value at 120 min postinjection. KdND was assumed to be 2.71 nM, the value estimated in this study. Maximum occupancy values for doses of 0.01, 0.02, and 0.04 µg/kg were 1.3%, 2.6%, and 5.1%. Final occupancy values were 0.6%, 1.1%, and 2.2%. Similar results were found when using the mean normalized metabolite-corrected plasma TACs multiplied by the free fraction (F), in place of the cerebellum TAC, and using Kd in place of KdND in Eq. 6 and the equation RO% =100 x F/(F + Kd) for occupancy estimation.
Based on these computations, a dose of 0.02 µg/kg was selected as appropriate in human studies. With the current specific activity of [11C]GR103545 obtained from our new radiosynthetic method, this mass dose should allow the injection of [11C]GR103545 in radioactivity doses sufficient to provide PET images of acceptable statistical quality. For example, assuming an average body weight of 70 kg for a human subject, this mass dose limit will allow the injection of 1.4 µg, or 3.38 nmol of mass, which translates into 406 MBq (11 mCi) of radioactivity dose based on the average specific activity (120 MBq/nmol) at time of injection in the baseline scans.
CONCLUSIONS
We have successfully used the KOR agonist [11C] GR103545 to estimate its in vivo Kd and regional KOR Bmax estimates in rhesus monkeys. The in vivo Kd estimate allows the selection of an appropriate tracer mass dose limit, important to optimize the imaging quality while minimizing the potential side effect of this agonist radiotracer in human studies. In our ongoing PET studies in humans the limit of 0.02 µg/kg derived from this study is being used to obtain acceptable images without causing any observable physiological effects, thus validating the result that this mass dose fulfill the tracer dose requirement.
ACKNOWLEDGMENTS
The authors acknowledge the staff at the Yale PET Center, especially Krista Fowles and the NHP team. This study was funded by the Yale-Pfizer Bioimaging Alliance. This publication was also made possible by CTSA Grant UL1 RR024139 from the National Center for Research Resources (NCRR) and the National Center for Advancing Translational Science (NCATS), components of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
REFERENCES
- 1.Shippenberg TS. The dynorphin/kappa opioid receptor system: a new target for the treatment of addiction and affective disorders? Neuropsychopharmacology. 2009 Jan;34(1):247. doi: 10.1038/npp.2008.165. [DOI] [PubMed] [Google Scholar]
- 2.de Lanerolle NC, Williamson A, Meredith C, et al. Dynorphin and the kappa 1 ligand [3H]U69,593 binding in the human epileptogenic hippocampus. Epilepsy Res. 1997 Oct;28(3):189–205. doi: 10.1016/s0920-1211(97)00044-2. [DOI] [PubMed] [Google Scholar]
- 3.Tortella FC, DeCoster MA. Kappa opioids: therapeutic considerations in epilepsy and CNS injury. Clin Neuropharmacol. 1994 Oct;17(5):403–416. [PubMed] [Google Scholar]
- 4.Mathieu-Kia AM, Fan LQ, Kreek MJ, Simon EJ, Hiller JM. Mu-, delta- and kappa-opioid receptor populations are differentially altered in distinct areas of postmortem brains of Alzheimer's disease patients. Brain Res. 2001 Mar 2;893(1–2):121–134. doi: 10.1016/s0006-8993(00)03302-3. [DOI] [PubMed] [Google Scholar]
- 5.Henriksen G, Willoch F. Imaging of opioid receptors in the central nervous system. Brain. 2008 May;131(Pt 5):1171–1196. doi: 10.1093/brain/awm255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dannals RF, Ravert HT, Frost JJ, Wilson AA, Burns HD, Wagner HN., Jr Radiosynthesis of an opiate receptor binding radiotracer: [11C]carfentanil. Int J Appl Radiat Isot. 1985 Apr;36(4):303–306. doi: 10.1016/0020-708x(85)90089-4. [DOI] [PubMed] [Google Scholar]
- 7.Cohen RM, Andreason PJ, Doudet DJ, Carson RE, Sunderland T. Opiate receptor avidity and cerebral blood flow in Alzheimer's disease. J Neurol Sci. 1997 May 29;148(2):171–180. doi: 10.1016/s0022-510x(96)05315-4. [DOI] [PubMed] [Google Scholar]
- 8.Ravert HT, Mathews WB, Musachio JL, Scheffel U, Finley P, Dannals RF. [11C]-methyl 4-[(3,4-dichlorophenyl)acetyl]-3-[(1-pyrrolidinyl)-methyl]-1- piperazinecarboxylate ([11C]GR89696): synthesis and in vivo binding to kappa opiate receptors. Nucl Med Biol. 1999 Oct;26(7):737–741. doi: 10.1016/s0969-8051(99)00043-8. [DOI] [PubMed] [Google Scholar]
- 9.Ravert HT, Scheffel U, Mathews WB, Musachio JL, Dannals RF. [(11)C]-GR89696, a potent kappa opiate receptor radioligand; in vivo binding of the R and S enantiomers. Nucl Med Biol. 2002 Jan;29(1):47–53. doi: 10.1016/s0969-8051(01)00285-2. [DOI] [PubMed] [Google Scholar]
- 10.Schoultz BW, Hjornevik T, Willoch F, et al. Evaluation of the kappa-opioid receptor-selective tracer [(11)C]GR103545 in awake rhesus macaques. European journal of nuclear medicine and molecular imaging. 2010 Jun;37(6):1174–1180. doi: 10.1007/s00259-010-1384-6. [DOI] [PubMed] [Google Scholar]
- 11.Naylor A, Judd DB, Lloyd JE, Scopes DI, Hayes AG, Birch PJ. A potent new class of kappa-receptor agonist: 4-substituted 1-(arylacetyl)-2-[(dialkylamino)methyl]piperazines. J Med Chem. 1993 Jul 23;36(15):2075–2083. doi: 10.1021/jm00067a004. [DOI] [PubMed] [Google Scholar]
- 12.Talbot PS, Narendran R, Butelman ER, et al. 11C-GR103545, a radiotracer for imaging kappa-opioid receptors in vivo with PET: synthesis and evaluation in baboons. J Nucl Med. 2005 Mar;46(3):484–494. [PubMed] [Google Scholar]
- 13.Farde L, Eriksson L, Blomquist G, Halldin C. Kinetic analysis of central [11C]raclopride binding to D2-dopamine receptors studied by PET--a comparison to the equilibrium analysis. J Cereb Blood Flow Metab. 1989 Oct;9(5):696–708. doi: 10.1038/jcbfm.1989.98. [DOI] [PubMed] [Google Scholar]
- 14.Holden JE, Jivan S, Ruth TJ, Doudet DJ. In vivo receptor assay with multiple ligand concentrations: an equilibrium approach. J Cereb Blood Flow Metab. 2002 Sep;22(9):1132–1141. doi: 10.1097/00004647-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Nabulsi NB, Zheng MQ, Ropchan J, et al. [11C]GR103545: novel one-pot radiosynthesis with high specific activity. Nucl Med Biol. Feb;38(2):215–221. doi: 10.1016/j.nucmedbio.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 16.Carson RE, Channing MA, Blasberg RG, et al. Comparison of bolus and infusion methods for receptor quantitation: application to [18F]cyclofoxy and positron emission tomography. J Cereb Blood Flow Metab. 1993 Jan;13(1):24–42. doi: 10.1038/jcbfm.1993.6. [DOI] [PubMed] [Google Scholar]
- 17.Innis RB, Cunningham VJ, Delforge J, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007 Sep;27(9):1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 18.Scatchard G. Ann NY Acad Sci. 1949;61:660–672. [Google Scholar]
- 19.Slater P, Cross A. Audioradiography of monkey brain with [3H]dynorphin. Neuropeptides. 1986;8(1):71–76. doi: 10.1016/0143-4179(86)90067-3. [DOI] [PubMed] [Google Scholar]
- 20.Drake CT, Chavkin C, Milner TA. Opioid systems in the dentate gyrus. Prog Brain Res. 2007;163:245–263. doi: 10.1016/S0079-6123(07)63015-5. [DOI] [PubMed] [Google Scholar]
- 21.Nemeth CL, Paine TA, Rittiner JE, et al. Role of kappa-opioid receptors in the effects of salvinorin A and ketamine on attention in rats. Psychopharmacology (Berl) 2010 Jun;210(2):263–274. doi: 10.1007/s00213-010-1834-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirota K, Okawa H, Appadu BL, Grandy DK, Devi LA, Lambert DG. Stereoselective interaction of ketamine with recombinant mu, kappa, and delta opioid receptors expressed in Chinese hamster ovary cells. Anesthesiology. 1999 Jan;90(1):174–182. doi: 10.1097/00000542-199901000-00023. [DOI] [PubMed] [Google Scholar]