Abstract
Objective
A recent meta-analysis has indicated that, in patients with dementia, the use of atypical antipsychotics is associated with an excess mortality. Later observational studies have suggested that conventional antipsychotics may pose an even greater risk of death. None of these studies could evaluate the risk associated with single antipsychotics nor could they provide any conclusive evidence concerning the risk among nursing home residents. We conducted a retrospective cohort study to compare the risk of death associated with atypical and conventional antipsychotics in a large population of nursing home residents with dementia.
Method
We identified 6,524 new users of atypical antipsychotics and 3,205 new users of conventional antipsychotics living in 1,581 Medicare- or Medicaid-certified nursing homes in 5 US states during the years 1998–2000. The outcome measure was all-cause mortality, which was determined during 6-months of follow-up.
Results
After adjusting for potential confounders relative to users of atypicals, the rate of death was increased for users of conventional antipsychotics (hazard ratio [HR], 1.26; 95% CI, 1.13–1.42). Relative to risperidone, a higher rate of death was documented for haloperidol (HR, 1.31; 95% CI, 1.13–1.53), phenothiazines (HR, 1.17; 95% CI, 1.00–1.38) and other conventional medications (HR, 1.32; 95% CI, 0.99–1.80). No atypical antipsychotic was associated with a differential risk relative to risperidone.
Conclusions
Conventional antipsychotics are associated with a higher risk of all-cause mortality than atypical agents. It seems advisable that they are not used in substitution for atypical antipsychotics among nursing home residents with dementia even when short-term therapy is being prescribed.
Although there are only a limited number of small clinical trials documenting the efficacy of atypical antipsychotics for the treatment of behavioral and psychological symptoms of dementia (BPSD), clinical guidelines have endorsed their utilization.1 Indeed, atypical antipsychotics have rapidly been adopted as a standard of care for BPSD,2 and they have largely replaced the older conventional antipsychotics.3
However, in April 2005, the US Food and Drug Administration (FDA) reviewed 17 randomized clinical trials (RCTs) involving risperidone, olanzapine, quetiapine, and aripiprazole and issued a public health advisory to warn about a 1.6–1.7 times higher risk of all-cause mortality relative to placebo.4 Yet, these RCTs were small, generally short in duration, and had a very low event rate, and reliable estimates of the mortality risk could be generated only when data were combined in a meta-analysis.5
More recently, some observational studies6–10 have suggested that conventional antipsychotics may pose an even greater risk of death compared to atypical agents. Following the evidence from such studies, the FDA has extended to conventional agents the warning of a possible increased risk of death associated with the use of these medications in patients with dementia.11 Nonetheless, all of these studies were done in outpatient users of antipsychotics based on prescription databases and regardless of the indication for use. Only 1 study8 included patients with dementia, and this study documented similar mortality risks for atypical and conventional antipsychotics. Information about the comparative risk of death associated with antipsychotics among nursing home residents with dementia is available only from an analysis of the Ontario database.12 In this study, there was a 26% increased risk of death associated with conventional relative to atypical antipsychotics but sensitivity analyses revealed that an unmeasured confounder could have eliminated the observed association. All of these studies considered the broad distinction in 2 separate classes of antipsychotics without analyzing the individual risk of the different medications.13
Thus, to date, there is really no conclusive evidence about the risk of death associated with atypical and conventional antipsychotics among patients with dementia residing in long-term care facilities, nor is there any information about different individual ingredient safety profiles. This is a very relevant issue since antipsychotic-use prevalence is over 30% in US nursing homes, with a clearly increasing trend.14 Also, there is evidence that the 2005 FDA report has produced a shift toward an increased use of conventional antipsychotics that are prescribed in substitution of atypical agents.15 Finally, especially among atypical antipsychotics, there are clear differences in the receptor-binding profiles16 and extrapyramidal safety characteristics such that some authors have indicated the need to overcome the misleading dichotomy between atypical and conventional antipsychotics and consider individual pharmacologic agents.17
We conducted a retrospective cohort study to compare the risk of death associated with atypical and conventional antipsychotics—as a class and also by ingredient—in a large population of elderly patients with dementia living in nursing homes in the United States.
METHOD
The present observational study was conducted based on the current standard methodological tools18 and is reported based on the guidelines of the strengthening the reporting of observational studies in epidemiology (STROBE) statement.19
Data Source
We used the Systematic Assessment of Geriatric drug use via Epidemiology (SAGE) database, which contains data from the Minimum Data Set (MDS).20,21 The MDS is a standardized, clinically-based instrument that collects information on each residents demographic, functional, medical, psychological, and cognitive status. Each Medicare- or Medicaid-certified nursing home conducts an MDS assessment of all residents upon admission and quarterly thereafter. Since 1998, the Center for Medicare and Medicaid Services (CMS) maintains a centralized repository of all MDS data, and these data are used for administrative and research purposes. The SAGE database links MDS data to Medicare enrollment files which contain information on vital status.
Study Population
Data were collected in the 1,581 nursing homes of 5 US States (Kansas, Maine, Mississippi, Ohio, and South Dakota) between January 1, 1998, and December 31, 2000. Eligible candidates were residents 65 years of age or above, with a diagnosis of dementia (Alzheimer’s disease or other types of dementia), who were new users of antipsychotics. Residents with a concomitant diagnosis of dementia and schizophrenia were excluded from the study population.
Exposure Assessment
To identify new users of antipsychotics, we first identified residents for whom antipsychotic use on a regular basis was documented in any MDS assessment during the study period (n = 61,781). We then selected the first assessment reporting any antipsychotic use (index assessment). Residents were considered “new” users if the MDS assessment prior to the index assessment documented no use of antipsychotics, not even on an as needed basis (n= 10,055). Such an operational definition for new users of antipsychotics has been adopted by the SAGE investigators in previous analyses and is like the one adopted in a published study22 investigating the association between antipsychotics and venous thromboembolism. Residents taking multiple antipsychotics were excluded (n = 326). The final sample (n = 9,729) included users of atypical antipsychotics (n = 6,524) and users of conventional antipsychotics (n = 3,205). Among users of atypical medications, we distinguished users of risperidone (n = 4,406), olanzapine (n= 1,563), quetiapine (n = 497), and clozapine (n = 58); among users of conventional agents, we distinguished users of haloperidol (n= 1,413), phenothiazines (including thioridazine [n = 546], perphenazine [n = 314], promazine [n = 305], chlorpromazine [n = 220], fluphenazine [n = 103], trifluoperazine [n = 32], and mesoridazine [n = 24]), and other conventional antipsychotics (including loxapine [n = 127], chlorprothixene [n = 59], thiothixene [n = 37], and molindone [n = 25]). These were the only antipsychotic medications used in the study period.
Outcome Assessment
Date of death was derived from Medicare enrollment files. We defined the length of follow-up to be 6 months. We chose a period of 6 months on the basis of the duration of trials in the FDA’s meta-analysis.4 The outcome measure of this study was defined as death occurring during this interval of time.
Potential Confounders
Residents’ sociodemographic characteristics (age, gender, and race/ethnicity) along with body mass index (BMI), indicators of functional and cognitive status, comorbid conditions, and concurrent drug use were considered as potential confounders. To evaluate functional status, we used the Activity of Daily Living scale (ADL),23 a 7-item, 5-level score based on the resident’s performance in 7 areas: dressing, eating, toileting, bathing, locomotion, transferring, and incontinence. We classified the degree of dependence as mild (ADL score, 0–1), moderate (ADL score, 2–3) or severe (ADL score, 4–5). The Cognitive Performance Scale (CPS)24 was used to measure cognitive status. The CPS is a validated scale embedded in the MDS that ranges from 0 (intact cognition) to 6 (severe dementia) and has a good correlation with the Mini-Mental State Exam.24 We categorized cognitive impairment as minimal (CPS score, 0–1), moderate (CPS score, 2–3), and severe (CPS score, 4–6).
Regarding BPSD, explicitly coded in the MDS are all of the disturbances that have been most commonly reported to occur, including aberrant motor behavior, aggression and agitation, hallucinations, delusion, depression, anxiety, irritability, or inappropriate behavior.25 The degree of severity of behavioral disturbances was evaluated based on the MDS behavioral index.26 Residents were considered to have severe symptoms if they were verbally or physically abusive and socially inappropriate every day. Residents with moderate behavioral symptoms were those showing aberrant motor or abusive behavior, but only on occasions.
The MDS clinical diagnoses section was used to assess residents’ comorbid conditions. The validity and accuracy of such diagnoses in the SAGE database have been previously demonstrated (positive predictive value [PPV] for diagnoses affecting functional status: 0.94; PPV for chronic clinical conditions: 0.70).20,21,26 Comorbidities considered were cardiovascular and cerebrovascular conditions, including hypertension, heart failure, ischemic heart disease, cardiac arrhythmias, diabetes, and history of stroke, and other chronic diseases, including chronic obstructive pulmonary disease and Parkinson’s disease.
Finally, we took into account the potential confounding effect of concomitant medication use, including cardiovascular drugs (diuretics, (β-blockers, angiotensin-converting enzyme inhibitors, calcium channel blockers, and digoxin), aspirin/antiplatelets and anticoagulants, benzodiazepines, and antidepressants.
Statistical Analysis
We used methods described by Kaplan and colleagues27 to estimate survival curves and compared these curves using Mantel-Haenszel tests.
We ran separate Cox multivariate regression models to estimate the effect of antipsychotics on the risk of death. In a first model, exposure to antipsychotics was categorized as use of atypical antipsychotics (reference category) and use of conventional antipsychotics. In a second model, individual ingredients were included and risperidone was used as the reference category. Additional models were used to estimate the risk of death associated with conventional relative to atypical antipsychotics, stratified by dementia type. We calculated person-time as number of days from the date of the index assessment to either death or end of follow-up. To rule out departures from the proportionality assumptions for each model, we examined the log-log survival function. From these models, we derived crude and adjusted estimates of effect and corresponding 95% confidence intervals (CIs). Models of mortality in the first 30 days, 31–90 days, and 91–180 days of drug use were also constructed. Since structural factors of facilities may affect antipsychotic use, we stratified the analysis by facility and calculated pooled estimates to minimize this potential confounding effect.28
Furthermore, we conducted sensitivity analyses to investigate the potential effect of an unmeasured confounder on the observed associations. As in a previous article12 on antipsychotics and death among patients with dementia, our sensitivity analyses investigated the effect of a hypothetical binary confounder on the observed hazard ratios (HRs) for the comparison between the risks of death associated with atypical and conventional antipsychotic use. We varied the prevalence of the hypothetical unmeasured confounder as well as the relative hazard of death associated with this unmeasured confounder.
All of the statistical analyses were performed using SAS V8 (SAS Institute Inc, Cary, North Carolina).
RESULTS
Among the 9,279 residents included in the study, 36% had a diagnosis of Alzheimer’s disease and the remaining 64% had a diagnosis of a different type of dementia. The prevalence of cardiovascular and cerebrovascular comorbidities was 66% for residents with Alzheimer’s disease and 80% for those with other dementia, thus indicating a presumable inclusion of vascular and mixed-dementia residents in the latter category.
The principal sociodemographic, functional, and clinical characteristics of residents in the study by type of antipsychotic are illustrated in Table 1. No major differences were evident between atypical and conventional antipsychotic users with respect to age, gender, race/ethnicity, and BMI. Functional and cognitive status tended to be severely impaired in greater proportion among conventional antipsychotic users. Behavioral and psychological symptoms of dementia were more prevalent among residents receiving atypical antipsychotics compared to those receiving conventional medications. Similarly, behavioral symptoms were more severe among users of atypical agents relative to users of conventional medications (18.4% vs 12.4%). All of the comorbid conditions were nearly equally represented in the 2 groups of antipsychotic users.
Table 1.
Sociodemographic, Functional, and Clinical Characteristics of Study Population by Antipsychotic Usea
| Characteristic | Users of Atypical Antipsychotics (n = 6,524) | Users of Conventional Antipsychotics (n = 3,205) |
|---|---|---|
| Age group, mean, y | 83.5 | 84.5 |
| Female gender | 71.8 | 72.0 |
| Race/ethnicity | ||
| White, not of Hispanic origin | 90.7 | 90.6 |
| Black, not of Hispanic origin | 8.4 | 8.3 |
| Other | 0.9 | 1.1 |
| Body mass index (kg/m2), mean | 23.9 | 23.3 |
| Functional impairment (ADL score) | ||
| Mild (0–1) | 12.0 | 8.9 |
| Moderate (2–3) | 54.2 | 49.8 |
| Severe (4–5) | 33.8 | 41.3 |
| Cognitive deficit (CPS score) | ||
| Mild (0–1) | 5.0 | 5.0 |
| Moderate (2–3) | 55.2 | 51.1 |
| Severe (4–6) | 39.8 | 43.9 |
| BPSD | ||
| Aberrant motor behavior | 31.8 | 24.0 |
| Verbal aggression | 34.3 | 28.1 |
| Physical aggression | 25.9 | 23.4 |
| Inappropriate behavior | 39.7 | 35.4 |
| Hallucinations | 7.2 | 5.3 |
| Delusions | 19.6 | 11.7 |
| Depression | 48.7 | 40.6 |
| Anxiety | 19.8 | 14.6 |
| Behavioral symptoms severity | ||
| Moderate | 62.3 | 43.4 |
| Severe | 18.4 | 12.4 |
| Cardiovascular and cerebrovascular comorbiditiesb | 75.5 | 77.1 |
| COPD | 15.2 | 16.3 |
| Parkinson’s disease | 7.7 | 7.6 |
| Alzheimer’s disease | 37.7 | 35.3 |
| Other dementia | 62.3 | 64.7 |
| Benzodiazepines | 21.6 | 18.5 |
| Antidepressants | 49.5 | 36.0 |
| Cardiovascular drugsc | 66.7 | 66.4 |
| Aspirin/antiplatelets/anticoagulants | 30.9 | 30.7 |
Values are shown as percents unless staled otherwise.
Residents were included in such category if they had at least 1 of the following conditions: hypertension, heart failure, ischemic heart disease, cardiac arrhythmias, diabetes, or history of stroke.
Residents were considered on cardiovascular drugs if they were taking at least 1 of the following medications: diuretics, β-blockers, angiotensin-converting enzyme inhibitors, calcium channel blockers, and digoxin.
Abbreviations: ADL=Activities of Daily Living, BPSD=Behavioral and Psychological Symptoms of Dementia, COPD=chronic obstructive pulmonary disease, CPS=Cognitive Performance Scale.
Consistently, there were no differences in concomitant medication use between the groups except that users of atypical antipsychotics were more likely to use benzodiazepines (21.6% vs 18.5%) and antidepressants (49.5% vs 36.0%) relative to users of conventional agents.
Among atypical antipsychotics, risperidone accounted for nearly 70% of prescriptions, followed by olanzapine (24% of prescriptions). Among conventional antipsychotics, haloperidol was the most commonly used medication (45% of prescriptions), followed by thioridazine (18% of prescriptions). Daily doses for antipsychotics were in accordance with recommendations for use in elderly patients. The mode of the daily dose was 1 mg for risperidone, 5 mg for olanzapine, 1 mg for haloperidol, and 20 mg for thioridazine.
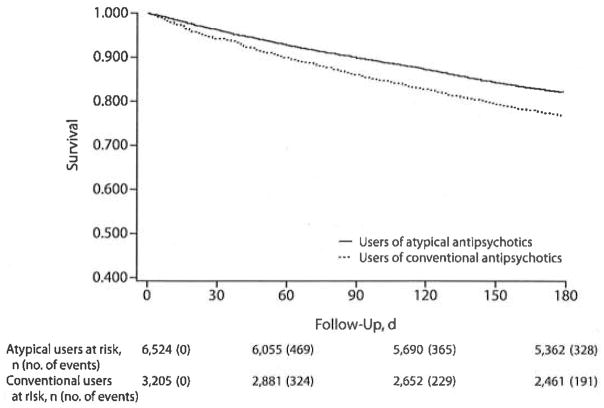
We identified 1,907 deaths during the 6-month follow-up time; the rate of death was 44.6 per 100 person-years. The median follow-up time was 180 days for users of both atypical and conventional antipsychotics. Figure 1 shows the survival curve by antipsychotic use. Survival curves for users of atypical antipsychotics and users of conventional agents differed at the Mantel-Haenszel test (P<.001). The occurrence of death started early (8–10 days) and was distributed throughout the entire follow-up time. The number of residents at risk decreased over time in an equal manner, and models of mortality in the first 30 days, 31–90 days, and 91–180 days of drug use gave similar results.
Figure 1.
Survival Curves of Users of Conventional Antipsychotics and Users of Atypical Antipsychotics
After adjusting for all potential confounders, relative to users of atypical antipsychotics, the rate of death was increased for users of conventional agents (adjusted HR, 1.26; 95% CI, 1.13–1.42) (Table 2).
Table 2.
Effect of Antipsychotics on the Risk of Death (atypical antipsychotics as reference category)
| Antipsychotic | No. of Events | Total Follow-Up (person-years) | Crude IR per 100 Person-Years | Crude HR | Adjusted HRa | 95% CI |
|---|---|---|---|---|---|---|
| Atypical | 1,162 | 2,904 | 40.0 | … | … | |
| Conventional | 745 | 1,372 | 54.3 | 1.41 | 1.26 | 1.13–1.42 |
Adjusted for age, race/ethnicity, gender, body mass index, Activities of Daily Living score, Cognitive Performance Scale score, severity of behavioral symptoms, cardiovascular and cerebrovascular comorbidities, and use of concomitant medications, including cardiovascular drugs, aspirin/antiplatelets/anticoagulants, benzodiazepines, and antidepressants.
Abbreviations: HR = hazard ratio, IR= incidence rate.
In Table 3, it is shown that, relative to risperidone, a higher rate of death was documented for haloperidol (adjusted HR, 1.31; 95% CI, 1.13–1.53), phenothiazines (adjusted HR, 1.17; 95% CI, 1.00–1.38), and other conventional medications (adjusted HR, 1.32; 95% CI, 0.99–1.80). No atypical antipsychotic was associated with a differential risk relative to risperidone. As shown in Table 4, no difference between the 2 types of antipsychotic medications was apparent in a subsample including residents with Alzheimer’s disease (adjusted HR, 1.02; 95% CI, 0.75–1.39). The excess mortality associated with conventional antipsychotics was found only among those residents with dementia other than Alzheimer’s (adjusted HR, 1.31; 95% CI, 1.14–1.50).
Table 3.
Effect of Antipsychotics on the Risk of Death (risperidone as reference category)
| Antipsychotic | No. of Events | Total Follow-Up (person-years) | Crude IR per 100 Person-Years | Crude HR | Adjusted HRa | 95% CI |
|---|---|---|---|---|---|---|
| Haloperidol | 357 | 594 | 60.0 | 1.46 | 1.31 | 1.13–1.53 |
| Phenothiazinesb | 320 | 778 | 49.9 | 1.25 | 1.17 | 1.00–1.38 |
| Other conventionalsc | 68 | 101 | 67.3 | 1.59 | 1.32 | 0.99–1.80 |
| Clozapine | 13 | 24 | 54.2 | 1.06 | 0.94 | 0.49–1.79 |
| Olanzapine | 258 | 702 | 36.7 | 0.91 | 0.95 | 0.80–1.12 |
| Quetiapine | 86 | 224 | 38.4 | 0.99 | 1.05 | 0.80–1.39 |
| Risperidone | 805 | 1,953 | 41.2 | … | … |
Adjusted for age, race/ethnicity, gender, body mass index, Activities of Daily Living score, Cognitive Performance Scale score, severity of behavioral symptoms, cardiovascular and cerebrovascular comorbidities, and use of concomitant medications, including cardiovascular drugs, aspirin/antiplatelets/anlicoagulants, benzodiazepines, and antidepressants.
Including thioridazine, perphenazine, promazine, chlorpromazine, fluphenazine, trifluoperazine, and mesoridazine.
Including chlorprothixene, thiothixene, loxapine, and molindone.
Abbreviations: HR=hazard ratio, IR= incidence rate.
Table 4.
Effect of Antipsychotic Use on the Risk of Death Stratified by Dementia Type
| Antipsychotic | Sample Restricted to Residents With Alzheimer’s Disease (n = 3,386)
|
Sample Restricted to Residents With Other Dementia (n = 5,893)
|
||
|---|---|---|---|---|
| Adjusted HRa | 95% CI | Adjusted HRa | 95% CI | |
| Atypical | … | … | ||
| Conventional | 1.02 | 0.75–1.39 | 1.31 | 1.14–1.50 |
Adjusted for age, race/ethnicity, gender, body mass index, Activities of Daily Living score, Cognitive Performance Scale score, severity of behavioral symptoms, cardiovascular and cerebrovascular comorbidities, and use of concomitant medications, including cardiovascular drugs, aspirin/antiplatelets/anticoagulants, benzodiazepines, and antidepressants.
Abbreviation: HR=hazard ratio.
Finally, sensitivity analyses conducted on the entire sample showed that an unmeasured confounder could not eliminate or diminish the observed associations, regardless of the variation of the associated risk (bivariate HR) and of possibly uneven distribution (Table 5).
Table 5.
| Bivariate HRc | Adjusted HR (95% CI) of Death
|
|||
|---|---|---|---|---|
| P1 = 0.2, P2= 0.1 | P1 = 0.4, P2 = 0.2 | P1 = 0.6, P2 = 0.3 | P1 = 0.6, P2 = 0.5 | |
| 2.00 | 1.27 (1.13–1.43) | 1.25 (1.11–1.40) | 1.28 (1.13–1.44) | 1.23 (1.09–1.39) |
| 1.50 | 1.27 (1.13–1.42) | 1.27 (1.13–1.43) | 1.28 (1.14–1.44) | 1.24 (1.10–1.39) |
| 0.75 | 1.27 (1.12–1.42) | 1.26 (1.12–1.42) | 1.26 (1.13–1.42) | 1.27 (1.13–1.42) |
| 1.50 | 1.26 (1.12–1.41) | 1.28 (1.14–1.44) | 1.25 (1.11–1.41) | 1.27 (1.13–1.43) |
P1 = prevalence of unmeasured confounder among conventional users.
P2 = prevalence of unmeasured confounder among atypical users.
Hazard ratio of death associated with the unmeasured confounder.
Abbreviation: HR = hazard ratio.
DISCUSSION
The findings of this study document that, in a nontrial population of nursing home residents with dementia with long follow-up, those receiving conventional agents, especially haloperidol, are at a 26% greater risk of death than those on atypical antipsychotics. The excess mortality associated with the use of conventional antipsychotics is apparent within the initial 8–10 days of treatment and persists over 6 months. Also, the effect of conventional antipsychotics on increasing the risk of death relative to atypical medications seems to be clustered in residents with dementia other than Alzheimer’s disease.
The results of the present study are in agreement with other observational studies that were conducted in outpatient populations and included any users of antipsychotics for all of the different potential indications, especially psychiatric diseases. In the study by Wang et al,6 less than 40% of patients had a dementia diagnosis and only 18% had a prior history of nursing home stay. A stratified analysis on these subgroups gave an HR of 1.29 and 1.26, respectively. In the study by Schneeweiss et al,10 a mere 11% of patients had a dementia diagnosis and about a quarter had a prior history of nursing home stay. A stratified analysis on these subgroups gave an HR of 1.26 and 1.25, respectively. More recently, 1 large population-based study from Ontario, Canada has reported a 55% increased risk for death associated with conventional relative to atypical antipsychotics among community-dwelling elderly patients with dementia.12 The excess mortality associated with conventional antipsychotics relative to atypical agents was 26% in a cohort of nursing home residents.12
In RCTs, the risk of death associated with atypical antipsychotics has been shown to be similarly increased with risperidone, olanzapine, and quetiapine use.5 Likewise, a class effect has been invoked for the increased risk of death associated with conventional antipsychotic use, although with a different degree of severity.6,7,10,12 However, to date, nearly no data from placebo-controlled RCTs are available on mortality associated with conventional antipsychotic use in patients with dementia. Two of the trials included in the meta-analysis of atypical antipsychotics in dementia considered haloperidol as a comparator.5 The overall odds ratio of death for haloperidol was of similar magnitude to that for atypical antipsychotics but was not statistically significant.
In contrast with previous studies that presented aggregated data, the present one documented that, relative to risperidone and other atypical agents, all individual conventional antipsychotics may be associated with increased mortality, although haloperidol seems to be the single drug associated with the highest risk. Phenothiazines and also other conventional antipsychotics were associated with a similarly 20%-30% increased risk for death, although estimates of effect did not reach statistical significance. This finding is consistent with previous preliminary information generated by small observational studies. In a pilot study at a Veterans Affairs Medical Center including a total of 1,583 patients, Nasrallah et al29 documented a 4-fold increased mortality among haloperidol users relative to atypical users (cumulative of risperidone and olanzapine). Two subsequent studies in Australia7 and Canada10 based on prescription databases documented an over 2-fold increase in mortality associated with the use of haloperidol relative to either olanzapine or risperidone. However, 1 retrospective review30 found an opposite result: an increased risk of death with risperidone compared with haloperidol. No studies could determine the risk associated with conventional antipsychotics other than haloperidol. A single study7 of 551 chlorpromazine users documented a relative risk of 1.39 relative to olanzapine users but—as per authors’ concession—the study could not rule out confounding by indication since no clinical data were available.
Our study also concludes that, relative to risperidone, there seems to be no substantial difference among the other individual atypical antipsychotics with respect to the risk of death. At odds with the present findings, 1 previous study confronted older patients receiving only risperidone or olanzapine. Hollis et al7 documented a small but statistically significant increase in relative risk associated with risperidone use, but confounding by comorbidities and other clinical variables could not be excluded.
The mechanisms by which antipsychotic medications may contribute to death remain to be established. In the FDA meta-analysis,4,5 most deaths were due to cardiovascular events (mostly arrhythmias) and infections (pneumonia for the great majority). A review of the RCTs that evaluated olanzapine supports these proposed mechanisms of harm.31 Antipsychotic medication use is associated with a lengthening of QTc interval on electrocardiograms.32 Recently, atypical medications, such as ziprasidone, quetiapine, risperidone, and olanzapine, have been linked to QTc prolongation, although the highest estimate of risk has been documented for thioridazine.33 An increased risk of ischemic cerebrovascular events has been also linked to the use of atypical antipsychotics in patients with dementia.34 This evidence derives from secondary analyses of RCTs, and recent large observational studies35,36 have not confirmed it. Results from subgroup analyses in our study suggest that conventional antipsychotics would increase the risk of death relative to atypical medications only among residents with dementia other than Alzheimer’s disease. The MDS provides only information on whether or not the resident is affected by Alzheimer’s or by dementia other than Alzheimer’s. However, based on the documented higher prevalence of cardiovascular and cerebrovascular risk factors among residents with dementia other than Alzheimer’s, we believe that most patients in such a category are likely to have a vascular or mixed dementia. Findings from subgroup analyses would then support the hypothesis that cardiovascular and cerebrovascular risk factors may mediate the excess mortality associated with conventional antipsychotics. However, results should be interpreted with caution by taking into account the potential for misclassification of vascular/mixed dementia based on MDS data and a possible lack of statistical power to detect any effect of conventional antipsychotics in the Alzheimer’s disease group due to the reduced sample size. Alternative mechanisms such as sedation and extrapyramidal symptoms may contribute to swallowing problems and falls and, in turn, to an increased risk of pneumonia, other complications, and subsequent death.37,38
Whatever the underlying cause or causes are, we have found that the excess mortality associated with the use of conventional antipsychotics among nursing home residents with dementia becomes apparent within the initial 8–10 days of treatment and persists over 6 months. This pattern is remarkably similar to that documented by Gill et al,12 with a 180 days maximum follow-up, and, even more recently, in a study among older veterans over 5 years.7 That the most marked elevation of the risk of death with conventional antipsychotics is immediately after the initiation of treatment has also been confirmed in several other instances.6,10,38,39 In general, these studies have analyzed data within the initial 30–40 days, but there is also information that suggests that the risk of death increases as early as 8–10 days.38 In the latter studies, however, the risk tends to subside with time to a more or less pronounced extent.
Limitations of the study need to be acknowledged. Specific causes of death were not available. There was no definitive information on whether the residents were indeed users of antipsychotics at the time of death. Also, the exact start and end dates of antipsychotic use were not available. This could have introduced a potential for misclassification of the exposure. However, such misclassification would likely have been nondifferential between study groups, thus introducing only a dilution of estimates. Also, it is noteworthy that models of mortality in the first 30 days, 31–90 days, and 91–180 days of drug use gave nearly identical results. With respect to dosages, we observed low variability among drug regimens, and we were not able to investigate dose-response relationships. In this respect, it is noteworthy that the literature has produced little convincing evidence of either a dose-relation6,10 or a dose-independency for haloperidol.7,38 Of note, no statistically significant relationship between antipsychotic dose and risk of death was found in a meta-analysis of olanzapine RCTs.31 Although we have taken due care of numerous potential confounders, residual confounding is always possible. In particular, a more severely impaired functional and cognitive status among users of conventional antipsychotics relative to residents on atypical medications could have produced an overestimate of the risk of death associated with conventional antipsychotics. Conversely, more severe BPSD and a higher prevalence of benzodiazepine and antidepressant use among residents on atypical agents could have biased results toward an underestimate of the effect. In spite of the convergence of results from sensitivity analyses, it is important to recognize that our study is based on nonexperimental data. There might be other factors with regard to patients who were newly prescribed either antipsychotic that we were unable to control for. Finally, patterns of antipsychotics use in 1998–2000 might have been slightly different from those in more recent years, thus affecting generalizability of the results. Yet, the breakdown of antipsychotic use in our study was similar to the one documented in the study by Gill et al12 including data from 1997 and 2003. Also, in a population-based study10 spanning between 1997 and 2004 when there were documented substantial changes in prescribing patterns, yearly adjusted mortality ratios varied little and in a non-systematic fashion.
The results of the present study have important clinical implications. First, conventional antipsychotics seem to be associated with a greater risk for death than atypical agents. Second, despite the fact that the estimated mortality rate was exceptionally high, an excess mortality linked to conventional antipsychotic use could still be detected. Third, the risk for death is possibly present even with short-term therapy. Finally, cardiovascular and cerebrovascular risk factors may mediate the effect of conventional antipsychotics on increasing the risk of death.
In conclusion, the findings of this study suggest that it may be inappropriate to switch to conventional agents for treating BPSD, as it has been documented to occur after the FDA alert.15 This is of particular importance since regulatory agencies worldwide have recommended that physicians not use atypical antipsychotics in dementia, leaving conventional agents as the only option for those patients requiring treatment.
The lifetime risk of developing BPSD among patients with dementia is close to 100%; each single patient will experience at least 1 neuropsychiatric symptom.40 Future research should be aimed at identifying those groups of patients who are the best candidates for pharmacologic treatment as well as those who are most susceptible to develop antipsychotic side effects. However, it should not be forgotten that data on the efficacy of antipsychotics are very limited41 and that, while withdrawal of these medications has generally no overall detrimental effect on functional and cognitive status, treatment with antipsychotics may be necessary and valuable to control BPSD, especially when severe.42,43 At this time, it seems advisable to encourage the adoption of nonpharmacologic interventions as the initial treatment of choice. Should a pharmacologic treatment be deemed necessary, it is recommended that physicians tailor their therapeutic choice on the individual patient, taking into account the available evidence on efficacy and safety, the individual clinical profile, and the response to treatment.44
For Clinical Use.
Conventional antipsychotics seem to be associated with a greater risk for death than atypical agents when used in elderly patients with behavioral and psychological symptoms of dementia (BPSD).
Cardiovascular risk factors may mediate the excess mortality associated with conventional antipsychotics among patients with BPSD.
Nonpharmacologic interventions are the first-choice treatment for BPSD. The choice of a specific pharmacologic treatment should take into account the current evidence on efficacy and safety and the individual cardiovascular risk profile.
Acknowledgments
Funding/support: National Institute on Aging, National Institutes of Health, #R37 AG11624-06s1.
Footnotes
Drug names: aripiprazole (Abilify), clozapine (FazaClo, Clozaril, and others), haloperidol (Haldol and others), molindone (Moban), olanzapine (Zyprexa), quetiapine (Seroquel), risperidone (Risperdal and others), thiothixene (Navane and others).
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, chlorpromazine, chlorprothixene, fluphenazine, loxapine, mesoridazine, perphenazine, promazine, thioridazine, trifluoperazine, aripiprazole, risperidone, clozapine, olanzapine, quetiapine, haloperidol, molindone, and thiothixene are not approved by the US Food and Drug Administration for the treatment of behavioral and psychological symptoms of dementia.
Financial disclosure: Drs Liperoti, Onder, Landi, Lapane, Mor, Bernabei, and Gambassi have no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
References
- 1.American Geriatrics Society, American Association for Geriatric Psychiatry. Consensus statement on improving the quality of mental health care in US nursing homes: management of depression and behavioral symptoms associated with dementia. J Am Geriatr Soc. 2003;51(9):1287–1298. doi: 10.1046/j.1532-5415.2003.51415.x. [DOI] [PubMed] [Google Scholar]
- 2.Mossman D, Lehrer DS. Conventional and atypical antipsychotics and the evolving standard of care. Psychiatr Serv. 2000;51(12):1528–1535. doi: 10.1176/appi.ps.51.12.1528. [DOI] [PubMed] [Google Scholar]
- 3.Rapoport M. Antipsychotic use in the elderly: shifting trends and increasing costs. Int J Geriatr Psychiatry. 2005;20(8):749–753. doi: 10.1002/gps.1358. [DOI] [PubMed] [Google Scholar]
- 4.FDA Public Health Advisory. [Accessed August 24, 2009];Deaths with antipsychotics in elderly patients with behavioral disturbances. http://www.fda.gov/Drugs/DrugSafety/PublicHealthAdvisories/ucm053171.htm.
- 5.Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294(15):1934–1943. doi: 10.1001/jama.294.15.1934. [DOI] [PubMed] [Google Scholar]
- 6.Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs atypical antipsychotic medications. N Engl J Med. 2005;353(22):2335–2341. doi: 10.1056/NEJMoa052827. [DOI] [PubMed] [Google Scholar]
- 7.Hollis J, Grayson Forrester L, Brodaty H, et al. Antipsychotics medication dispensing and risk of death in veterans and war widows 65 years and older. Am J Geriatr Psychiatry. 2007;15(11):932–941. doi: 10.1097/JGP.0b013e31813547ca. [DOI] [PubMed] [Google Scholar]
- 8.Trifirò G, Verhamme KM, Ziere G, et al. All-cause mortality associated with atypical and typical antipsychotics in demented outpatients. Pharmacoepidemiol Drug Saf. 2007;16(5):538–544. doi: 10.1002/pds.1334. [DOI] [PubMed] [Google Scholar]
- 9.Kales HC, Valenstein M, Myra Kim H, et al. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry. 2007;164(10):1568–1576. doi: 10.1176/appi.ajp.2007.06101710. [DOI] [PubMed] [Google Scholar]
- 10.Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ. 2007;176(5):627–632. doi: 10.1503/cmaj.061250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.FDA alert. Information for health care professionals. [Accessed August 24, 2009];Antipsychotics. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetylnformationforPatientsandProviders/DrugSafelyInformationforHeathcareProfessionals/ucm084149.htm.
- 12.Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146(11):775–786. doi: 10.7326/0003-4819-146-11-200706050-00006. [DOI] [PubMed] [Google Scholar]
- 13.Trifirò G, Spina E, Gambassi G. Use of antipsychotics in elderly patients with dementia: do atypical and conventional agents have a similar safety profile? Pharmacol Res. 2009;59(1):1–12. doi: 10.1016/j.phrs.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Center for Medicare-Medicaid Services. [Accessed April 27, 2008];OSCAR data 2002–2008. http://www.ascp.com.
- 15.Fukuda K, Tanaka K. Influence of the FDA talk paper upon the use of atypical antipsychotics for psychotic symptoms in older patients with dementia in Japan. Psychogeriatrics. 2006;6(2):81–86. [Google Scholar]
- 16.Snyder EM, Murphy MR. Schizophrenia therapy: beyond atypical antipsychotics. Nat Rev Drug Discov. 2008;7(6):471–472. doi: 10.1038/nrd2571. [DOI] [PubMed] [Google Scholar]
- 17.Fischer-Barnicol D, Lanquillon S, Haen E, et al. Typical and atypical antipsychotics: the misleading dichotomy: results from the Working Group “Drugs in Psychiatry” (AGATE) Neuropsychobiology. 2008;57(1–2):80–87. doi: 10.1159/000135641. [DOI] [PubMed] [Google Scholar]
- 18.Sanderson S, Tatt ID, Higgins JP. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol. 2007;36(3):666–676. doi: 10.1093/ije/dym018. [DOI] [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, et al. on behalf of the Iniciativa STROBE. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 20.Bernabei R, Gambassi G, Lapane KL, et al. Characteristics of the SAGE database: a new resource for research on outcomes in long-term care. J Gerontol A Biol Sci Med Sci. 1999;54(1):M25–M33. doi: 10.1093/gerona/54.1.m25. [DOI] [PubMed] [Google Scholar]
- 21.Gambassi G, Landi F, Peng L, et al. Validity of diagnostic and drug data in standardized nursing home assessments: potential for geriatric pharmacoepidemiology. Med Care. 1998;36(2):167–179. doi: 10.1097/00005650-199802000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Liperoti R, Pedone C, Lapane KL, et al. Venous thromboembolism among elderly patients treated with atypical and conventional antipsychotic agents. Arch Intern Med. 2005;165(22):2677–2682. doi: 10.1001/archinte.165.22.2677. [DOI] [PubMed] [Google Scholar]
- 23.Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. J Gerontol A Biol Sci Med Sci. 1999;54(11):M546–M553. doi: 10.1093/gerona/54.11.m546. [DOI] [PubMed] [Google Scholar]
- 24.Hartmaier SL, Sloane PD, Guess HA, et al. The MDS cognition scale: a valid instrument for identifying and staging nursing home residents using the Minimum Data Set. J Am Geriatr Soc. 1994;42(11):1173–1180. doi: 10.1111/j.1532-5415.1994.tb06984.x. [DOI] [PubMed] [Google Scholar]
- 25.Lyketsos CG, Sheppard JE, Steinberg M, et al. Neuropsychiatric symptoms in Alzheimer’s disease clusters into three groups: the Cache County Study. Int J Geriatr Psychiatry. 2001;16(11):1043–1053. doi: 10.1002/gps.448. [DOI] [PubMed] [Google Scholar]
- 26.Gambassi G, Lapane KL, Sgadari A, et al. Measuring health outcomes for older people using the SAGE database. Can J Aging. 2000;19(suppl 2):67–86. [Google Scholar]
- 27.Kaplan EL, Meier P. Non parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 28.Rochon PA, Stukel TA, Bronskill SE, et al. Variation in nursing home antipsychotic prescribing rates. Arch Intern Med. 2007;167(7):676–683. doi: 10.1001/archinte.167.7.676. [DOI] [PubMed] [Google Scholar]
- 29.Nasrallah HA, White T, Nasrallah AT. Lower mortality in geriatric patients receiving risperidone and olanzapine versus haloperidol: preliminary analysis of retrospective data. Am J Geriatr Psychiatry. 2004;12(4):437–439. doi: 10.1176/appi.ajgp.12.4.437. [DOI] [PubMed] [Google Scholar]
- 30.Hennessy S, Bilker WB, Knauss JS, et al. Cardiac arrest and ventricular arrhythmia in patients taking antipsychotic drugs: cohort study using administrative data. BMJ. 2002;325(7372):1070–1075. doi: 10.1136/bmj.325.7372.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kryzhanovskaya LA, Jeste DV, Joung CA, et al. A review of treatment-emergent adverse events during olanzapine clinical trials in elderly patients with dementia. J Clin Psychiatry. 2006;67(6):933–945. doi: 10.4088/jcp.v67n0610. [DOI] [PubMed] [Google Scholar]
- 32.Reilly JG, Ayis SA, Ferrier IN, et al. QTc-interval abnormalities and psychotropic drug therapy in psychiatric patients. Lancet. 2000;355(9209):1048–1052. doi: 10.1016/s0140-6736(00)02035-3. [DOI] [PubMed] [Google Scholar]
- 33.Stöllberger C, Huber JO, Finsterer J. Antipsychotic drugs and QT prolongation. Int Clin Psychopharmacol. 2005;20(5):243–251. doi: 10.1097/01.yic.0000166405.49473.70. [DOI] [PubMed] [Google Scholar]
- 34.Herrmann N, Lanctot KL. Do atypical antipsychotics cause stroke? CNS Drugs. 2005;19(2):91–103. doi: 10.2165/00023210-200519020-00001. [DOI] [PubMed] [Google Scholar]
- 35.Liperoti R, Gambassi G, Lapane KL, et al. Cerebrovascular events among elderly nursing home patients treated with conventional or atypical antipsychotics. J Clin Psychiatry. 2005;66(9):1090–1096. doi: 10.4088/jcp.v66n0901. [DOI] [PubMed] [Google Scholar]
- 36.Herrmann N, Mamdani M, Lanctot KL. Atypical antipsychotics and risk of cerebrovascular accidents. Am J Psychiatry. 2004;161(6):1113–1115. doi: 10.1176/appi.ajp.161.6.1113. [DOI] [PubMed] [Google Scholar]
- 37.Liperoti R, Onder G, Lapane KL, et al. Conventional or atypical antipsychotics and the risk of femur fracture among elderly patients: results of a case-control study. J Clin Psychiatry. 2007;68(6):929–934. doi: 10.4088/jcp.v68n0616. [DOI] [PubMed] [Google Scholar]
- 38.Knol W, van Marum RJ, Jansen PA, et al. Antipsychotic drug use and risk of pneumonia in elderly people. J Am Geriatr Soc. 2008;56(4):661–666. doi: 10.1111/j.1532-5415.2007.01625.x. [DOI] [PubMed] [Google Scholar]
- 39.Rochon PA, Normand S-L, Gomes T, et al. Antipsychotic therapy and short-term serious events in older adults with dementia. Arch Intern Med. 2008;168(10):1090–1096. doi: 10.1001/archinte.168.10.1090. [DOI] [PubMed] [Google Scholar]
- 40.Steinberg M, Shao H, Zandi P, et al. cache County Investigators. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2008;23(2):170–177. doi: 10.1002/gps.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneider LS, Tariot PN, Dagerman KS, et al. CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355(15):1525–1538. doi: 10.1056/NEJMoa061240. [DOI] [PubMed] [Google Scholar]
- 42.Ballard C, Lana MM, Theodoulou M, et al. DART AD Investigators. A randomized, blinded, placebo-controlled trial in dementia patients continuing or stopping neuroleptics (the DART-AD trial) PLoS Med. 2008;5(4):E76. doi: 10.1371/journal.pmed.0050076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ballard CG, Thomas A, Fossey J, et al. A 3-month randomized, placebo-controlled, neuroleptic discontinuation study in 100 people with dementia: the neuropsychiatric inventory median cutoff is a predictor of clinical outcome. J Clin Psychol. 2004;65:114–119. doi: 10.4088/jcp.v65n0120. [DOI] [PubMed] [Google Scholar]
- 44.Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33(5):957–970. doi: 10.1038/sj.npp.1301492. [DOI] [PMC free article] [PubMed] [Google Scholar]