Abstract
A typical white-matter integrity and elevated dopamine levels have been reported for individuals who stutter. We investigated how such abnormalities may lead to speech dysfluencies due to their effects on a syllable-sequencing circuit that consists of basal ganglia (BG), thalamus, and left ventral premotor cortex (vPMC). “Neurally impaired” versions of the neurocomputational speech production model GODIVA were utilized to test two hypotheses: (1) that white-matter abnormalities disturb the circuit via corticostriatal projections carrying copies of executed motor commands, and (2) that dopaminergic abnormalities disturb the circuit via the striatum. Simulation results support both hypotheses: in both scenarios, the neural abnormalities delay readout of the next syllable’s motor program, leading to dysfluency. The results also account for brain imaging findings during dysfluent speech. It is concluded that each of the two abnormality types can cause stuttering moments, probably by affecting the same BG-thalamus-vPMC circuit.
Keywords: stuttering, speech fluency, speech motor control, neural modeling, dopamine, white matter, basal ganglia, ventral premotor cortex, thalamus, brain imaging
1 Introduction
Developmental stuttering is a speech disorder characterized by frequent repetitions of syllables or parts of syllables (sound/syllable repetitions), and by audible and inaudible prolongations of articulatory positions (prolongations and blocks, respectively). Although approximately 1% of the population stutters (Van Riper, 1982), the etiology of the disorder is still unknown. The primary goal of this paper is to enhance our understanding of the underlying mechanisms by using a computational model to explore alternative neurological bases of stuttering.1 This approach provides detailed explanatory accounts for already published data, and frames testable hypotheses to guide future experiments. The model developed here extends recent instantiations of the DIVA (Directions into Velocities of Articulators) and GODIVA (Gradient Order DIVA) neurocomputational models of speech production (Bohland, Bullock, & Guenther, 2010; Golfinopoulos, Tourville, & Guenther, 2010; Guenther, 1995; Guenther, Ghosh, & Tourville, 2006; Guenther, Hampson, & Johnson, 1998; Nieto-Castanon, Guenther, Perkell, & Curtin, 2005; Tourville, Reilly, & Guenther, 2008).2
Brain imaging studies of developmental stuttering have disclosed various abnormalities in the brains of persons who stutter (PWS). In this paper we discuss two discoveries: a structural abnormality in white matter fibers (WMF) beneath the left precentral gyrus (Chang et al., 2008; Cykowski et al., 2010; Kell et al., 2009; Sommer et al., 2002; Watkins, Smith, Davis, & Howell, 2008),3 and evidence that dorsal striatum (Wu et al., 1997) has markedly elevated dopamine (DA), which is released by fibers from the SNc (substantia nigra pars compacta, see Watkins et al., 2008). Although both abnormalities have been suggested as possibly contributing to stuttering, it is unclear which functional neural circuits are implicated in the white matter impairment or the elevated dopamine levels. Also unknown are the functional consequences of these abnormalities, and how they lead to moments of stuttering (for selected proposals, see the aforementioned papers and S. Brown et al., 2005, p. 115; Giraud et al., 2008; Max et al., 2004). Lastly, an open question is whether the two abnormalities need to exist simultaneously for stuttering to emerge.
We believe that each of these abnormalities may separately lead to the dysfluencies that characterize stuttering. The location of the structural abnormality in the deep perisylvian operculum (Cykowski et al., 2010) that lies beneath the left precentral gyrus suggests that the abnormality is close to the ventral primary motor cortex, or vMC (Sommer et al., 2002). In accordance with this view, we follow the hypothesis that the impairment is in corticostriatal projections that originate in vMC and indicate the current motor state, and that the resulting transmission errors prevent the basal ganglia (BG) from detecting the proper motor context for shifting between syllables, i.e., terminating the previous syllable and initiating the motor program -- a stored sequence of motor commands -- for the next syllable (Alm, 2004, p. 358). Concerning the second abnormality, we build upon the claim that dopamine excess may adversely affect the striatum (Maguire, Yu, Franklin, & Riley, 2004), and we hypothesize that a specific part of the striatum, the putamen, would become dysfunctional. This dysfunction could hamper the BG’s ability to bias cortical competition (among motor programs for similar syllables) in favor of the motor program appropriate for the next syllable (cf. Alm, 2004; Lu et al., 2010; Watkins et al., 2008, p. 57).
These two dysfluency mechanisms -- a failure to cancel the activation of the previous syllable, and a failure to bias cortical competition in favor of the next syllable -- might be considered specific cases of the two common accounts of any perseveratory phenomenon: the failure to inhibit previous, and the failure to activate next (see, for example, Fischer-Baum & Rapp, 2012); both accounts were already proposed in the context of stuttering (Howell, 2007; MacKay & MacDonald, 1984, p. 274). However, in contrast with perseveration which is characterized by repetition of whole units, stuttering is mostly characterized by prolongations, blocks, and sound/syllable repetitions that do not respect segmental (phonemic) boundaries (Conture, 2001, p. 6). Therefore, we hypothesize that each of the two mentioned dysfluency mechanisms, rather than inducing repetition of the previous syllable, may lead to abnormally slow activation of all but the earliest part (see Howell, 2007) of the next syllable’s motor program (cf. S. Brown et al., 2005, p. 115; Packman, Code, & Onslow, 2007). All dysfluencies in stuttering may result from such delayed syllable activation, whereas the type of dysfluency (sound/syllable repetition, prolongation, block, etc.) depends on additional factors, including the reaction of the speaker to the delayed activation.
Both mechanisms described above involve the basal ganglia whose relation with stuttering is demonstrated by various lines of evidence (see Alm, 2004). This group of nuclei, which takes part in speech production (Crosson, 1992; Gracco & Abbs, 1987; Guenther, 2008), was first linked to stuttering indirectly: the association was made due to the BG’s interconnection with the supplementary motor area (SMA), which may also be involved in the disorder (Caruso, Abbs, & Gracco, 1988). Basal ganglia lesions are associated with the presence of acquired (neurogenic) stuttering following strokes (Theys et al., 2012) and traumatic brain injuries (Ludlow et al., 1987), and several case studies showed that such a scenario (acquired stuttering after a BG lesion) leads to speech disturbances similar to developmental stuttering in various behavioral and clinical dimensions (Heuer, Sataloff, Mandel, & Travers, 1996; Koller, 1983; Krishnan & Tiwari, 2011; Tani & Sakai, 2011). The BG also have critical role in sequence skill learning, which is deficient in PWS (e.g., Smits-Bandstra & De Nil, 2007).
Perhaps the strongest evidence for BG involvement in stuttering comes from pharmacological studies. The BG’s striatum receives the densest dopamine innervation in the brain, and in repeated findings, drugs that block type D2 dopamine receptors (D2Rs) have been shown to be effective in reducing stuttering (Brady, 1991; Maguire et al., 2004; Stager et al., 2005; Tran, Maguire, Franklin, & Riley, 2008). Although other parts of the brain also have D2Rs, it is likely that a major part of the ameliorative action of the D2R blockers was in the BG. Opponent processing in the BG, in which the D1R (D1 dopamine receptors) expressing striatal projection neurons promote action whereas the D2R-expressing striatal projection neurons oppose action (see Section 2.2), is fundamental to forebrain control of action in all jawed vertebrates (Reiner, 2009), and imbalances of D1R vs. D2R processing are associated with a wide range of disorders of movement and decision making. These include schizophrenia, bipolar disorder, and Parkinson’s disease. The use of D2R blockers to treat stuttering was motivated, for example, by similarities between stuttering and Tourette’s syndrome. Both begin in childhood, and both occur more frequently in males than in females. Unfortunately, treatment of stuttering with most D2 antagonists incurs problematic side effects (see Maguire et al., 2004).
A role for BG in stuttering is also supported by functional imaging studies (Braun et al., 1997; S. Brown et al., 2005; Chang, Kenney, Loucks, & Ludlow, 2009; Giraud et al., 2008; Ingham et al., 2004; Lu et al., 2010; Watkins et al., 2008; Wu et al., 1995). However, BG nuclei are small in size and/or complex in shape, so it is difficult to determine which BG nuclei are contributing to activation changes (e.g., Watkins et al., 2008). This may explain why, in a recent meta-analysis of functional imaging studies (S. Brown et al., 2005), PWS showed only one reliably detected abnormality in the BG: they lacked the weak but reliable above-baseline activation observed in the left globus pallidus of control subjects during speech.
Despite their limitations in highlighting problems in the BG, imaging studies are very useful in detecting cortical abnormalities. In the S. Brown et al. (2005) meta-analysis, the activation in the left precentral gyrus of fluent speakers extended from the vMC to the ventral premotor cortex (vPMC), whereas the activation in the left precentral gyrus of PWS was restricted to the vMC. That PWS fail to properly activate the left vPMC was confirmed by a Watkins et al. (2008) functional imaging experiment (see also Chang et al., 2011; Salmelin, Schnitzler, Schmitz, & Freund, 2000). The activation-failures reported by Watkins et al. were observed under conditions in which PWS were dysfluent, suggesting that the degree of deactivation of the vPMC may be causally related to actual moments of stuttering, and not solely to an underlying deficiency. The correlation of vPMC deactivation with actual stuttering is also consistent with observations that the left inferior lateral premotor cortex, which largely coincides with vPMC, is the only left hemisphere motor region in which the activation level for PWS of both sexes is inversely correlated with stutter rate (Ingham et al., 2004). Also relevant is Neumann et al.’s (2003) finding of abnormally low activation in the left precentral cortex (of which the vPMC is part). In that study, the only dysfluencies produced by the subjects were sentence-initial blocks.
There are additional clues supporting the involvement of vPMC in stuttering. The first is that the most common speech elements repeated by PWS are complete syllables or their initial parts. This problem may be the result of a vPMC malfunction because this brain region codes for syllables, as was recently demonstrated using functional imaging techniques (Bohland & Guenther, 2006; Ghosh, Tourville, & Guenther, 2008; Peeva et al., 2010). Another clue is the existence of vPMC-to-vMC projections (Barbas & Pandya, 1987; Passingham, 1993), which, according to the DIVA model, constitute feedforward or “well-learned” speech motor commands (Guenther et al., 2006). Simulations with the DIVA model showed that impaired readout of feedforward commands, which is one possible outcome of vPMC malfunction (see Section 2.2.3), may lead to sound/syllable repetitions (Civier, Tasko, & Guenther, 2010). Lastly, activation of the vPMC depends on reciprocal excitatory links with the thalamus (Hoover & Strick, 1993), and thalamic activation is gated by potent inhibitory outputs from BG. The vPMC is therefore likely to be affected by the BG problems discussed above. Because the vPMC both sends projections to (Takada, Tokuno, Nambu, & Inase, 1998), and (via the thalamus) receives projections from, the BG, we will refer to this closed circuit by the term BG-vPMC loop.
Although suggestive of a role for the BG in stuttering, systemic drug effects cannot reveal exact mechanisms of any disorder. Similarly, imaging studies that suggest a role for vPMC in stuttering do not explicate how that role may relate to a BG dysfunction. Imaging studies can be used, though, to evaluate hypotheses concerning BG dysfunction. Unfortunately, this method cannot be applied to most past hypotheses as they cannot predict whether particular brain regions would be over- or under-activated during stuttering (e.g., S. Brown et al., 2005, p. 114; but see Giraud et al., 2008; Wu et al., 1995); without a modeling strategy that progressively incorporates the BG’s complex internal circuits, including its many inhibitory connections, it will remain very difficult to predict neural activation patterns (cf. Alm, 2004, p. 335).
To begin to overcome such problems, our method is to formulate and test hypotheses using an extended version of the GODIVA model that includes both cortical and subcortical sites. We first describe the extended model, with an emphasis on the newly-developed BG-vPMC loop module. After introducing each abnormality (white matter impairment or elevated dopamine levels), we then confirm that the model is capable of generating speech behaviors that account for dysfluencies. As the GODIVA model is both neurocomputational and biologically plausible (compare with other models reviewed in Civier, 2010, p. 3), it can also predict entire patterns of blood-oxygenation-level-dependent (BOLD) responses observable across the brain regions simulated, much as cerebral and cerebellar activation patterns during normal and perturbed speech were predicted based on simulations of the GODIVA model’s counterpart, the DIVA model (Golfinopoulos et al., 2010; Guenther et al., 2006; Tourville et al., 2008). To evaluate our hypotheses, we compare these predicted BOLD responses to published functional imaging data.
2 Neurocomputational modeling of serial speech production
2.1 The original GODIVA model
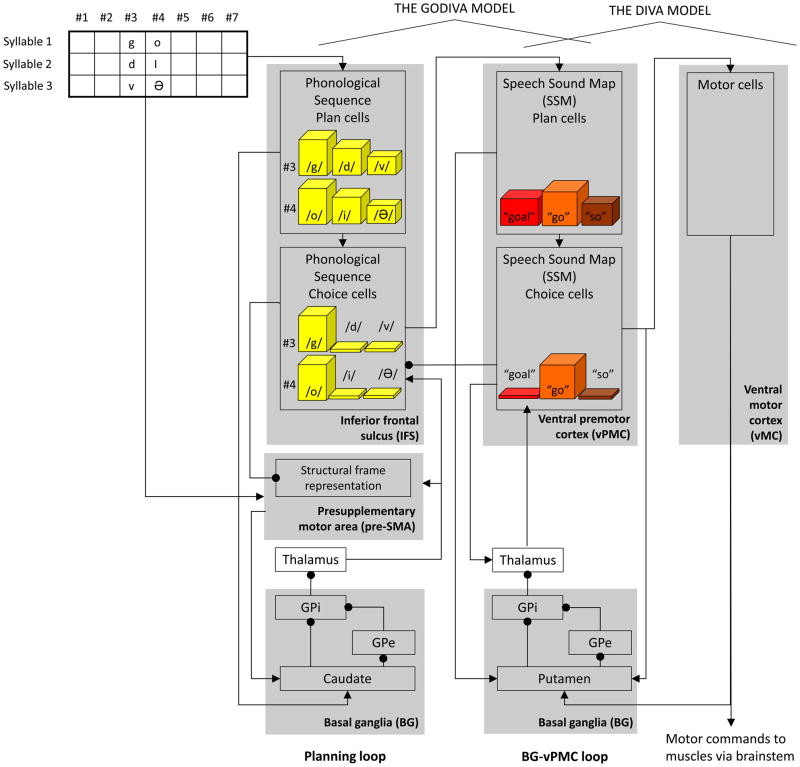
The GODIVA model (Bohland et al., 2010) explains how arbitrary utterances that fall within a speaker’s language rules can be represented in the brain, and how such utterances can be produced from a finite library of learned motor programs (well-learned syllables) when activated in the proper order.4 To this end, the GODIVA model simulates the activity in various cells, with each cell representing a population of adjacent neurons firing together. The activity of a cell in the model is a function of the activities of the cells projecting to it, the types (excitatory or inhibitory) and strengths of the projections, and the cell’s spontaneous decay (or recovery) rate. Based on functional imaging results, previous clinical studies, and previous theoretical models, the GODIVA model simulates activity in the following principal cortical cells (see boxes in the top of Fig. 1): phonological sequence plan and choice cells that code for sub-syllabic phonological content (phonemes) and reside in inferior frontal sulcus (IFS), and speech sound map (SSM) plan and choice cells that code for motor programs and sensory expectations for well-learned syllables and reside in the vPMC (Guenther et al., 2006). Each of the cortical regions mentioned is divided into two layers: a planning layer (where the plan cells reside), and a choice layer (where the choice cells reside). The planning and choice layers correspond to superficial and deep cortical layers, respectively (J. Brown, Bullock, & Grossberg, 2004). This paper focuses on the SSM cells, whose function is similar to the “mental syllabary” which was included in previous models of speech production (e.g., Levelt, Roelofs, & Meyer, 1999). For the sake of simplicity, we will not discuss here the roles of the structural frame representation in the presupplementary motor area (pre-SMA), and of the subcortical portion of the planning loop (bottom left of Fig. 1, for more details see Bohland et al., 2010).
Fig. 1.
“Box-and-arrow” schematic of the extended GODIVA model and its integration with the DIVA model. Although these models utilize feedback control, only the feedforward control portion of the model is shown because it is focal in this paper’s simulations of stuttering. The input to the model is the word “godiva”. GPi = internal globus pallidus. GPe = external globus pallidus. For simplicity, details of the model’s representation of the presupplementary motor area, and the projection from the vPMC planning layer to the thalamus, are omitted.
The input to the GODIVA model is a set of neural signals from higher-order linguistic areas. It specifies the phonemes of the target utterance, the order of their production, and their position inside the syllable (there are seven phoneme positions: three onset consonants, vowel nucleus, and three coda consonants). In the example given in Fig. 1, the input to the model (graphically presented at the top left corner) is the target utterance /godivə/ (“go” as in “go home”). The goal of the model is to choose the motor programs (“go”, “di”, and “və”) required for the production of the specified utterance, and to activate them in the appropriate order and timing.
Fig. 1 also demonstrates the activations of several cells during the planning and selection of the first syllable in /godivə/. The input to the model reaches the phonological sequence plan cells and creates activity gradients across the /g/, /d/, and /v/ phoneme cells in syllable position 3 (third onset consonant) and across the /o/, /i/, and /ə/ phoneme cells in syllable position 4 (vowel nucleus). In these gradients, the phonemes that come first in the utterance have higher activations. By choosing the most activated plan cells, the GODIVA model then generates a co-temporal activation of the phonological sequence choice cells coding /g/ and /o/. The choice cells, in turn, drive activity in the model’s vPMC planning layer, where multiple SSM plan cells representing possible motor programs become active. The cell for “go” fully matches the current phonological sequence representation in the inferior frontal sulcus, and the other cells match it partially (“goal”, whose third phoneme /l/ is not activated, and “so”, whose first phoneme /s/ is not activated). Due to its perfect match to the phonological representation, the “go” SSM plan cell becomes the most active cell in the vPMC planning layer. The next goal of the model is to produce the “go” syllable by choosing (increasing to supra-threshold activity) the corresponding SSM choice cell (“go”), while keeping the other SSM choice cells inactive. This will enable the “go” SSM choice cell reading out the appropriate stored motor programs and sensory expectations; instructions that will be passed to the DIVA portion of the model (Guenther et al., 2006) for motor execution (the right side of Fig. 1).
2.2 Extending the GODIVA model to account for stuttering
In this paper, the functionality of the GODIVA model is extended by connecting the vPMC with the BG; this forms a BG-vPMC loop (an illustration of the resulting extended GODIVA model is shown in Fig. 1). The BG-vPMC loop is separate from the planning loop that connects the BG to the inferior frontal sulcus and the pre-SMA. Although both loops pass through the BG, there is no interaction between the two. Just as the normal function of the BG-vPMC loop is to facilitate fluent production of rapid sequences of well-learned syllables, its dysfunction reduces fluency and permits the model to simulate stuttering.
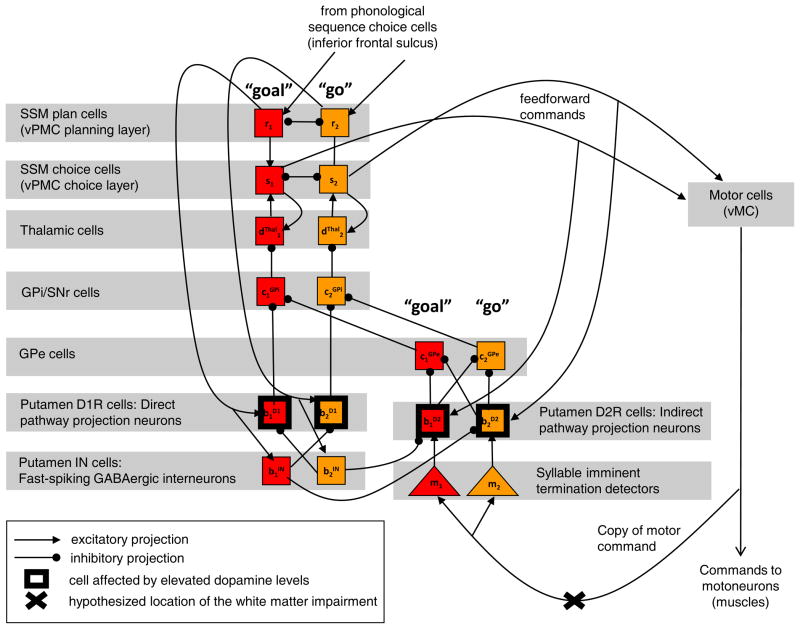
A close-up of the BG-vPMC loop is shown in Fig. 2. To implement the neural circuit, the GODIVA model hypothesizes projections from thalamic output cells to corresponding SSM choice cells. Following Mink (1996), the GODIVA model further hypothesizes that at each point, any of these projections may serve to either excite (via supra-baseline thalamic activation) or inhibit (via sub-baseline thalamic activation) the targeted SSM choice cell. The activation of each thalamic cell is determined by a chain of cells projecting from one to another (cortex-striatum-pallidum-thalamus). Although there is significant convergence between these chains in vivo, the model treats them as a set of competitive channels (only two of them are depicted), each modeled by two striatal projection neuron cells (putamen D1R cell and putamen D2R cell), one GABAergic striatal interneuron cell (putamen IN cell), one internal pallidum cell (GPi) and one external pallidum cell (GPe). All the cells represent spontaneously-decaying neurons except for the pallidal cells, which represent spontaneously-firing neurons. The channels interact in the striatum and continue to interact in the striatum’s projections to the GPe. In the rest of the BG’s nuclei, however, the channels remain independent of one another. Each one of the channels corresponds to a well-learned syllable (syllables “go” and “di” in the figure), and interacts with the idealized cortical column of the vPMC that codes the motor programs and sensory expectations for that syllable.
Fig. 2.
Idealized circuitry of the basal ganglia - ventral premotor cortex (BG-vPMC) loop. The cells of the circuit are represented by squares. Inside each square is the GODIVA model’s variable that codes for the cell’s activation level (see Section 1 of the Supplementary material for relevant equations). The model treats the cortico-striatal-pallidal-thalamic projections as a set of competitive channels. Two of these are included in the diagram and coded by an orange or a blue box for each cell type within the channel: one channel is for the well-learned syllable “go” (orange squares; variables with subscript 1), and the other channel is for the well-learned syllable “di” (blue squares; variables with subscript 2). Within each channel are two striatal projection neuron cells: a putamen D1R cell of the direct pathway, shown projecting to GPi/SNr, and a putamen D2R cell of the indirect pathway, shown projecting to GPe. Also shown for each channel are: one striatal GABAergic interneuron cell (putamen IN cell) shown as a mediator of feedforward inhibition, one internal pallidum cell (GPi/SNr), one external pallidum cell (GPe), and one thalamic cell. The cortical columns are shown as well, each represented by one SSM plan cell at the vPMC planning layer, and one SSM choice cell at the vPMC choice layer. The projections from the vPMC planning layer to the thalamus are omitted for simplicity. The deep-layer motor cells of the vMC, as well as their afferents (from the vPMC choice layer) and efferents (to the brainstem), are shown on the right. The corticostriatal white matter fibers that arise from the vMC’s efferents, feed into a stage (not modeled as a single cell, but algorithmically, so depicted as a triangle) that detects imminent syllable completion. This stage outputs a transient syllable-completion signal to the putamen D2R cells.
Each channel of the BG-vPMC loop is divided between two functionally-distinct direct and indirect pathways (Kravitz et al., 2010; Mink, 1996, p. 418). Fig. 2 illustrates that the pathways are segregated from one another in the striatum, and originate in the putamen’s predominant types of D1R and D2R expressing cells, respectively (Kravitz et al., 2010; but see Nadjar et al., 2006). Dopamine release facilitates signaling in the direct pathway, because DA binding with D1Rs is excitatory to the putamen D1R cells, whereas DA inhibits signaling in the indirect pathway, because DA binding with D2Rs is inhibitory to the putamen D2R cells (Gerfen, 1992).5 The direct pathway projects through inhibitory pathways from the putamen to the GPi (Albin, Young, & Penney, 1989), and the indirect pathway projects through inhibitory pathways from the putamen to the GPe (Percheron, Yelnik, & Francois, 1984), which in turn inhibits the GPi (Mink, 1996; A. Parent & Hazrati, 1995). The GPi, which is the convergence point of the two pathways, exerts inhibition on the thalamus. Rather than repeatedly mentioning the sign inversions implied by these chains of inhibition, the following discussion focuses on each pathway’s net effect on the cortex (via the excitatory thalamus-vPMC choice layer projection): excitatory for the direct pathway, and inhibitory for the indirect pathway.
The two main functionalities that the BG-vPMC loop adds to the GODIVA model – biasing the competition in the cortex and initiating the next syllable based on contextual signals -- are described next. These functionalities are mediated by the direct and indirect pathways, respectively.
2.2.1 The direct pathway: biasing competition in the cortex to select a syllable
In the original GODIVA model, competition via recurrent lateral inhibition among SSM choice cells in the vPMC choice layer allows a single motor program to be chosen for output to the motor apparatus. Since the activities of the SSM choice cells reflect the activities of the SSM plan cells, the winner in the vPMC choice layer is the choice cell whose well-learned syllable matches the most the phonological sequence representation in the inferior frontal sulcus. However, the original GODIVA model ignores data and models showing that the information needed to resolve such a competition, and to gate onsets of voluntary actions, is not available in a single cortical patch but is available at the BG level due to convergent cortical afferents to zones of the striatum (J. Brown et al., 2004; Passingham, 1993). The direct pathway of the newly added BG-vPMC loop addresses these limitations of the original GODIVA model, and permits, for example, simulating delayed initiation of voluntary actions. Moreover, because the direct pathway is strongly modulated by dopamine (see Section 2.2), we would be able to simulate stuttering due to elevated dopamine levels.
Since the BG-vPMC loop ultimately projects (via the thalamus) to its cortical region of origin (see Fig. 2), the direct pathway is capable of biasing competition in the vPMC choice layer by exciting the SSM choice cells (hence, syllabic motor programs) that are most likely to win the competition, i.e., those SSM choice cells for programs that most closely match the phonological syllable (the better the match, the greater is the excitation). The direct pathway has this information as the BG-vPMC loop originates in the SSM plan cells, which code the degree of phonological match (see Section 2.1). The SSM choice cells for programs with poorer matches receive no boost via the direct pathway because their corresponding thalamic cells remain inhibited by GPi. This lack of thalamic excitation weakens such SSM cells in the competition. The dual role of the BG in strengthening desired programs and weakening competing programs (cf. Mink, 1996, p. 414) has been explored in numerous computational models, and is a kind of contrast enhancement.
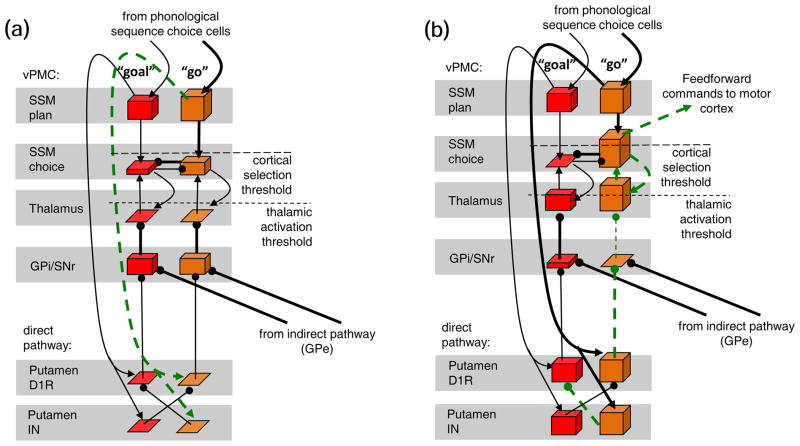
To demonstrate the selection of a winner SSM choice cell in the extended GODIVA model, Fig. 3 includes snapshots of the relevant subset of the BG-vPMC loop at the beginning (panel a) and end (panel b) of the selection process of “go”. The height of the bars represents cell activation level, and the thickness of the arrows represents strength of excitation or inhibition. In each panel, the green dashed arrows indicate the excitatory and inhibitory signals that drive the circuit into the next stage. Fig. 3(a) is a snapshot of the circuit after the phonological sequence choice cells drove activation in the SSM plan cells (see Section 2.1 and Fig. 1); indeed, the “go” SSM plan cell has higher activation than the “goal” SSM plan cell. As the SSM plan cell for “go” exerts more excitation (on its corresponding choice cell) than the SSM plan cell for “goal”, the pattern of activation in the vPMC planning layer is mirrored in the choice layer. Notice that although the SSM choice cell for “go” inhibits that for “goal”, this is not sufficient to decide the competition because the opposite inhibition, from “goal” to “go” operates as well. At that stage, the subcortical potion of the BG-vPMC loop is not affecting the cortex. The GPi cells are inhibited by the spontaneously-firing GPe, but since the inhibition is not complete, they still fire strong enough to keep the thalamic cells quiet.
Fig. 3.
The state of the basal ganglia - ventral premotor cortex (BG-vPMC) loop at two consecutive stages in the selection of the motor program adequate for the syllable “go”: (a) differential activation of the SSM plan and choice cells, (b) activation of the direct pathway biases cortical competition in favor of “go” SSM choice cell which reads out the motor program for “go”. The height of the bars represents cell activation level, and the thickness of the arrows represents strength of excitation/inhibition. Cortical and thalamic thresholds are marked by dashed lines. The rest of the conventions are as in Fig. 2. In each panel, the green dashed arrows indicate the excitatory or inhibitory signals that drive the circuit into the next stage. Only the relevant subset of the circuit is presented: the direct pathway, the GPi, the thalamus, and the vPMC.
To help the “go” SSM choice cell win the competition after all, the “go” SSM plan cell strongly activates its BG-vPMC loop channel (green dashed arrow in Fig. 3(a)). Fig. Fig. 3(b) shows the result of this subcortical activation (although subcortical participation is simultaneous, we treat it as a separate stage for clarity). First, the excitation from the SSM plan cell turns the putamen D1R cell for “go” into the most activated D1R cell. Through the direct pathway, the “go” putamen D1R cell is then strongly exciting the corresponding thalamic cell, pushing it well above the thalamic activation threshold. This is done via disinhibition: the “go” D1R cell inhibits the GPi cell, thus preventing the latter from inhibiting the thalamic cell. The cells in the channel for “goal” demonstrate exactly the opposite pattern, resulting in the “goal” thalamic cell being only weakly activated. As the thalamic cells excite the cortex in proportion to their activity, they ultimately bias cortical competition in favor of the SSM choice cell for “go”. The cell reaches cortical selection threshold, and reads out the feedforward commands.
The BG mechanisms described above provide the cortex with a supplemental form of lateral (or surround) inhibition (see Hikosaka, Takikawa, & Kawagoe, 2000, p. 963), thus allowing the cortex to make more rapid selection once the BG detect that the conditions for releasing a plan are satisfied. Increasing lateral inhibition hastens selection because it enables the most active SSM choice cell (the cell for the correct well-learned syllable) to suppress its competitors faster. Lateral inhibition provided by the BG, as opposed to that originating in the cortex, gives the central nervous system (CNS) extra degrees of freedom for controlling the extent of lateral inhibition (Gurney, Prescott, & Redgrave, 2001). Moreover, to increase the contrast enhancement provided to the cortex, the direct pathway of the extended GODIVA model is assisted by feedforward lateral inhibition from the GABAergic striatal interneurons (putamen IN cells). Because these interneurons, which are known to strongly inhibit other striatal neurons (J. Brown et al., 2004; Koos & Tepper, 1999), are driven by the SSM plan cells as well, each SSM plan cell will be effectively inhibiting the putamen D1R cells in the other channels (all channels other than the channel that corresponds to the specific SSM plan cell). This will increase the contrast between the putamen D1R cells, and ultimately, the contrast enhancement provided to cortex.6
2.2.2 The indirect pathway: using contextual signals to shift to the next syllable
To avoid undesired pauses when switching between syllables, the activity of the SSM choice cell for the current syllable must be quenched before the termination of syllable articulation, in order to allow enough time for the selection of the next syllable. The original GODIVA model employs a non-specific response signal that arrives from vMC and quenches the activity of the currently active SSM cell prior to the actual completion of articulation (Bohland et al., 2010). However, in order to accommodate data indicating that D2R antagonist drugs ameliorate stuttering, the extended GODIVA model utilizes the indirect pathway through the BG (Fig. 2). This indirect pathway provides an alternative method of shifting between syllables.
For a syllable motor program to be read out in full, the syllable’s SSM choice cell must be continuously excited by the thalamus, because without thalamic support, competition from the other SSM choice cells would suppress the activation of the choice cell for the current syllable. With the added indirect pathway, the model’s BG can use information received from the ventral motor cortex to determine when to quench the thalamic activation, which permits choice of, and shift to, the next syllable’s motor program (SSM choice). Because the projections from the motor cortex to the brainstem have collaterals that give rise to corticostriatal fibers (for rats, see Cowan & Wilson, 1994; Lei, Jiao, Del Mar, & Reiner, 2004; for monkeys, see M. Parent & Parent, 2006; but cf. Turner & Delong, 2000; for review, see Wilson, 2004), the putamen is expected to receive a copy of each motor command sent to the motoneurons (M. Parent & Parent, 2006; Wilson, 1995). In speech production, such an efference copy may be used to anticipate the imminent completion of the current syllable and to shift to the next syllable in a timely manner (Alm, 2004; cf. S. Brown et al., 2005; for non-speech tasks, see Deniau, Menetrey, & Charpier, 1996; Ueda & Kimura, 2003). In the model, the efference copy arrives at the D2R putamen cells (via the termination detectors, see Fig. 2), in accordance with findings that collaterals of deep-layer ventral motor cortex’s projections to the brainstem synapse preferentially on the striatal cells of the indirect pathway (Lei et al., 2004; Reiner, Hart, Lei, & Deng, 2010; but see Ballion et al., 2008).7
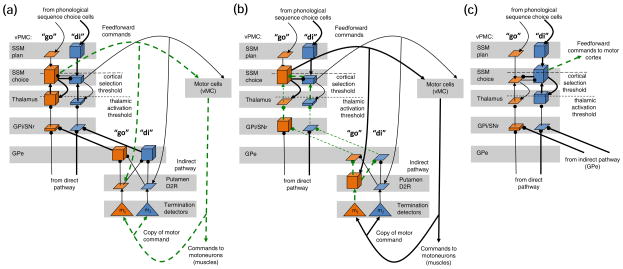
To demonstrate the shift from the “go” to the “di” syllables in the utterance /godivə/, Fig. 4 includes snapshots of the relevant subset of the BG-vPMC loop at the beginning, middle, and end of the transition between syllables (panels a, b, and c, respectively). For clarity, we removed the “goal” channel that appeared in Fig. 3, and instead included the channel for the upcoming syllable “di”. Fig. 4(a) is a snapshot of the circuit after the SSM choice cell for “go” was selected in the vPMC, and started reading out the feedforward motor commands. By this point, the high-order regions of the GODIVA model have already planned the next syllable, resulting in the phonological sequence choice cells strongly activating the “di” SSM plan cell. Since syllable selection was discussed in the previous section, this example omits the cells for “dig” and “do” which are activated as well, but do not affect the selection of “di”. The snapshot shows that the feedforward motor commands reach the vMC cells, and also prime the “go” putamen D2R cell.8 The vMC executes the motor commands, and the termination detectors constantly receive their copies.
Fig. 4.
The state of the basal ganglia - ventral premotor cortex (BG-vPMC) loop and its projections to the motor cortex at three consecutive stages in the shift from the “go” to the “di” syllables: (a) execution of the “go” syllable, (b) activation of the indirect pathway when the syllable is about to terminate, (c) quenching of the “go” choice cell paves the way to the execution of the “di” syllable. Conventions are as in Fig. 3. Only the relevant subset of the circuit is presented: the indirect pathway (not in panel c), the GPi, the thalamus, and the vPMC.
Fig. 4(b) is a snapshot taken when the primed “go” D2R cell detects, via the termination detectors, a combination of motor commands indicating of upcoming syllable termination (e.g., motor commands to fully round the lips indicating the imminent completion of the well-learned syllable “go”). It immediately activates the indirect pathway which uses a chain of three inhibitory connections (via the GPe and GPi) to inhibit the thalamus and thereby terminate the current syllable. Notice that the “go” D2R cell has inhibitory connections to all GPe cells (only the connections to “go” and “di” are shown), which ultimately leads to inhibition of all thalamic cells. This diffuse inhibition by the indirect pathway (Mink, 1996) might also affect the thalamic cell for the upcoming syllable “di”, but owing to its excitation by the direct pathway (a chain of two inhibitory connections via the GPi), it survives the inhibition.
The new situation is that the “go” SSM choice cell, which keeps being inhibited by the “di” SSM choice cell, does not receive strong excitation from the “go” thalamic cell anymore. The result is a quenching of the “go” choice cell, with a concurrent increase in the activity of the “di” choice cell. Ultimately, the “di” choice cell reaches cortical activation threshold, and the production of “di” initiates (Fig. 4(c)).
2.2.3 Simulating distinct dysfluency types
The current extended GODIVA model is compatible with interpreting several types of dysfluencies as failures to activate the next syllable’s motor program in time. Blocks or prolongations occur when the model waits for the SSM choice cell for the next syllable to go above cortical selection threshold, and (to be better prepared for the production of the syllable) utilizes the waiting period to move the articulators to the configuration from which syllable execution starts. This scenario, which can account for the common observation that the articulators of PWS are in syllable-initial position during blocks and prolongations (e.g., Zimmermann, 1980), requires an auxiliary assumption: that despite being below cortical selection threshold, a SSM choice cell can still properly read out the motor commands that bring the articulators to their syllable-initial position (cf. Howell, 2007). Sound/syllable repetitions occur when instead of waiting for the SSM choice cell to go above threshold, the model forces the cell to read out the complete motor program right away. The result is impaired readout of feedforward motor commands that leads to production errors (e.g., incorrect tongue position). While the model can utilize sensory feedback in order to generate on-the-fly corrective motor commands, if at some point the error grows large enough, sound/syllable repetition will occur. This is because large errors force the model to “reset” (halt and repeat from the beginning) the current syllable. Simulations of sound/syllable repetitions are reported in a previous paper from our group (Civier et al., 2010).
It should be noted that although the GODIVA model is compatible with simulating various dysfluency types as a result of a central planning delay, its current instantiation does not try to predict which of them will be generated each time syllable activation is delayed. Selection between dysfluency types is made, instead, at random. Mechanism for selection between dysfluencies (cf. Howell, 2007) will be included in future version of the GODIVA model.
2.3 Implementation
The extended GODIVA model, like the original GODIVA model, is implemented computationally as a neural network governed by a set of ordinary differential equations that capture key features of membrane dynamics, including the bounded range of cell potentials (Grossberg, 1978). This approach permits computation of transient as well as steady-state network behavior, and allows model simulations to be constrained by neural activity data collected from monkeys performing non-speech motor sequencing tasks. The extended GODIVA model uses all the equations of the original GODIVA model (as specified in Bohland et al., 2010), except equations (13) and (14), which were modified, and equation (12), which was omitted for simplicity. The newly added equations of the extended GODIVA model, as well as the equations modified from the original GODIVA, are provided and described in detail in Section 1 of the Supplementary material.
2.4 Prediction of BOLD responses
One goal of the current modeling work is to generate predictions of entire patterns of BOLD responses associated with stuttering. To this end, we have identified likely neuroanatomical locations of the model’s components (Golfinopoulos et al., 2010; Guenther et al., 2006), which allow us to run simulated functional Magnetic Resonance Imaging (fMRI) experiments in which the model produces speech sounds. Based on the model’s cell activations, we then generate simulated hemodynamic response patterns.
The simulated BOLD responses were produced in a manner similar to that described in Guenther et al. (2006). The activity of each cell, or population of neurons, was convolved with an idealized hemodynamic response function that approximates the transformation from neuronal activity to hemodynamic response in the brain. The overall hemodynamic response for each brain region was calculated by summing the responses of the region’s cells. As a simplification, we used the same idealized response function for all regions in the model; unfortunately, we do not have enough data to determine if and how brain regions differ in their response functions. The BOLD response for each experimental condition was obtained by subtracting the hemodynamic response for the baseline condition from that of the experimental condition. To capture the effect on the BOLD response, of dysfluencies that occur either on or right after the first syllable, the calculation of the hemodynamic response for dysfluent utterances included neural activity from the beginning of the simulation up to the shift to the second syllable (the point where the “di” SSM choice cell overcomes the “go” cell). For fairness of comparison, the same duration of neural activity was also used to calculate the hemodynamic response for the baseline condition.
The prediction of BOLD responses (experimental condition minus baseline condition) adds up to the prediction of behavioral outcomes, and permits evaluating model output against previously reported fMRI results. As fMRI has low temporal resolution, reported BOLD responses are shaped by the relation between brain activities in the experimental and baseline conditions at each point of the speech task examined, and it is difficult to directly compare them with simulation results. The selected solution, of convolving model activations with an idealized hemodynamic response function, is only approximate, but provides a good estimation of the predicted BOLD response. Although a summation of model activations would have been simpler, it is inexact by nature, since it ignores the role of the hemodynamic response function in deciding the shape of the BOLD response.9
3 Results
Computer simulations of the extended GODIVA model producing its name (/godivə/) were performed to test our two hypotheses about the etiology of stuttering. One hypothesis involves elevated dopamine levels (affecting both the putamen D1R and D2R cells), and the other involves impaired white matter fibers (in corticostriatal projections originating in vMC). To assess each hypothesis, we used abnormal values for the critical parameters of the model that are listed either in the first or second row of Table 1, while keeping the parameters listed in the other row constant (more details on Table 1 are provided in the Discussion section). For each parameterization, we report the time course of activity in several key model components as well as the BOLD response predicted for that activity. To facilitate comparison between the different conditions, we report here on dysfluencies of only one type: blocks.
Table 1.
Critical parameter value ranges for normal and abnormal versions of the extended GODIVA model.
| Description | Functional significance | Notation | Normal values (persons who do not stutter) | Abnormal values (persons who stutter) | ||
|---|---|---|---|---|---|---|
| Simulated | Range | Simulated | Range | |||
| Dopamine levels | Binding of dopamine to D1 and D2 receptors in the putamen | βD1, βD2 | 1.0 | 0.7–1.45 | 1.6 | 0–0.7, 1.45< |
| Integrity of white matter fibers beneath the left precentral gyrus | Strength of signaling in the corticostriatal fibers that arise from collaterals of vMC-to- brainstem projections | λWMF | 1.0 | 0.35< | 0.1 | 0–0.35 |
To make predictions about differences in brain activations between PWS and fluent speakers, we also simulated the normal (unimpaired) version of the extended GODIVA model. All simulations assume that the motor programs for the “go”, “di”, and “və” syllables were among those acquired by the DIVA portion of the circuit (see Bohland et al., 2010).
3.1 Fluent speech
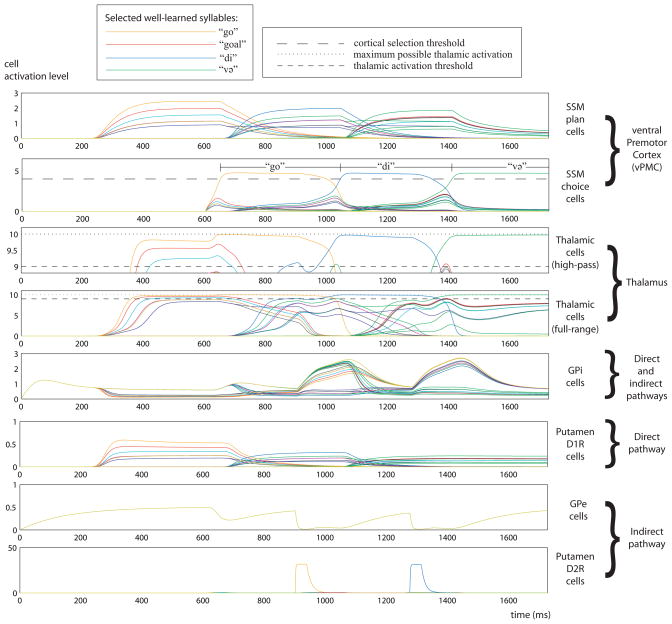
The activity in the BG-vPMC loop during fluent production of the sequence “go.di.və” by an intact version of the GODIVA model (normal GODIVA version) is shown in Fig. 5. The color of each cell activation in indicates the syllable to which the cell corresponds. After the phonological planning for the syllable “go” (depicted in Fig. 1, and described in 2.1), the most active SSM plan cell is the one that matches the phonological representation the best (in this case, the SSM plan cell for “go”, whose activation is plotted in orange). The tonic input signal, which initiates speaking by enabling selection of the next syllable in the vPMC choice layer, is applied starting from t=600 ms (see initiation signal I in equation 14, Supplementary material). Activity of the SSM choice cells is then observed, modulated by input from the thalamus, as described next.
Fig. 5.
Time course of simulated neural activity in the BG-vPMC loop. The activities of cells that correspond to different well-learned syllables (thus, to different channels and their corresponding cortical columns) are plotted in distinct colors. Shown from top to bottom are SSM plan cells, SSM choice cells, thalamic cells, GPi cells, putamen D1R cells, GPe cells, and putamen D2R cells. For clarity, the thalamic cell activations are shown both in high-pass and full-range views. Motor program execution is shown above the activations of the SSM choice cells. Note that the range of the y-axis varies from plot to plot.
The putamen D1R cells, driven by the input from the SSM plan cells, reflect the pattern of activity in the vPMC planning layer. The putamen D1R cells that are activated inhibit their corresponding GPi cells, driving the latter below their tonic level of activation. The weakening of some of the GPi cells (which is proportional to the strength of activation in the putamen D1R cells) removes the inhibition they usually exert on their corresponding thalamic cells. The thalamic cells -- like the putamen D1R cells -- now reflect the pattern of activity in the cortex (with the activation of the cell for “go”, in orange, being the highest).
As can be observed in Fig. 5, all well-learned syllables that bid for execution have their thalamic cells active to some degree. However, only those thalamic cells whose corresponding SSM plan cells have the strongest activations in the vPMC exceed the thalamic activation threshold. These thalamic cells (whose activation can be better observed in the high-pass plot of the thalamus) enable self-excitation in their target SSM choice cells, thus allowing the SSM choice cells to participate in the syllable-selection process that starts at t=600 ms. Because thalamic cells modulate cortical self-excitation in proportion to their level of activation, the SSM choice cell for “go”, whose thalamic cell has the highest activation in the thalamus, has the strongest self-excitation. This helps the SSM choice cell for “go” to quickly win the cortical competition. Once it exceeds the cortical selection threshold at around t=650 ms, the choice cell reads out the motor commands for the syllable “go”, and due to its high activation, also returns strong input to its corresponding thalamic cell. Consequently, the thalamic cell for “go” persists at strong activation above the thalamic activation threshold while the other thalamic cells decay. Note also that the choice cell’s threshold crossing at t=650 ms also initiates the process of replacing the current input to the planning layer with the inputs needed to prepare the next syllable (Bohland et al., 2010).
The shift between syllables begins at t=900 ms, when a phasic activation can be observed in the putamen D2R cell for “go”. This phasic activation is driven by the system’s detection of imminent syllable completion, and it results in a sharp decrease in the activity of the GPe cells (for simplicity, all GPe cells in the model have identical inputs, hence the same activity). Consequently, there are increases (of different extents) in the activities of all GPi cells. This ultimately leads to a phasic inhibition that quenches nearly all the thalamic cells, including the cell for the active SSM choice cell for “go”. The only thalamic cells spared are those that are excited via the direct pathway — in this case, the thalamic cells corresponding to the syllables which best match the next phonological syllable, /di/. At this point, another syllable-selection process takes place and the SSM choice cell for “di” is selected. The termination of “di” and the selection of “və” are performed in the same manner described above. The termination of “və” is not shown. In addition to detection of syllable completion, termination of utterance-final syllables in the current extended GODIVA model requires reset to zero of the input signal that initiates speaking.
3.2 Dysfluent speech due to elevated dopamine levels
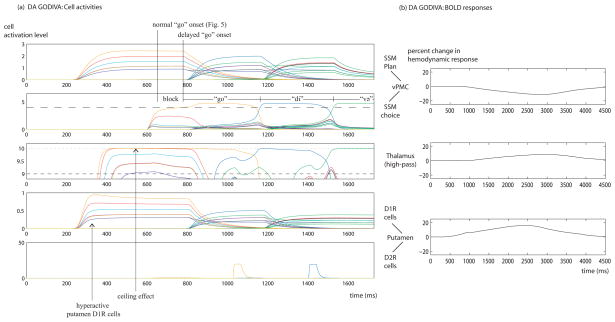
Fig. 6(a) shows the time course of activity in several key model components in a version of the extended GODIVA model with elevated dopamine levels (DA GODIVA version). The GPe and GPi are omitted for clarity, as their roles in the model are limited to relaying and integrating information between the putamen and the thalamus. In this version, both dopamine binding parameters βD1 and βD2 are set by default to 1.6, and the result is dysfluent speech. A comparison with the performance of the normal GODIVA version (Fig. 5) reveals that the DA GODIVA version reads out the motor program for the first syllable, “go”, too late. The orange line, indicating the activity of the SSM choice cell for “go”, exceeds threshold at t=777 ms, compared with t=650 ms for the normal GODIVA version. The behavioral correlate of the delayed SSM choice cell selection is a block (lasting for 127 ms) on the first syllable of the utterance /godivə/.
Fig. 6.
Time course of neural activities and predicted BOLD responses of the extended GODIVA model with elevated dopamine levels. (a) Neural activities. Conventions as in Fig. 5(b) The BOLD responses predicted by the model for elevated dopamine levels compared with the baseline of normal levels.
Fig. 6(b) shows the BOLD responses predicted by the model for elevated dopamine levels (DA GODIVA version) compared with the baseline of normal dopamine tone (normal GODIVA version). Since the BOLD responses of the different regions greatly differ in size -- a result of the regions having different number of cells, thus, baseline hemodynamic responses of different magnitudes -- the panels show the effect sizes relative to the peak of each region’s baseline hemodynamic response (compare with the normalization method used in Guenther et al., 2006, p. 293). Since the SSM plan and choice cells are intermixed and cannot be differentiated in imaging studies, the predicted decrease in vPMC BOLD response (DA GODIVA version < normal GODIVA version) takes both into account. Similarly, both the putamen D1R and D2R cells contribute to the predicted increase in putamen BOLD response (DA GODIVA version > normal GODIVA version), and both the GPe and GPi cells contribute to the predicted decrease in globus pallidus BOLD response (not plotted).
3.3 Dysfluent speech due to structural abnormality in white matter fibers
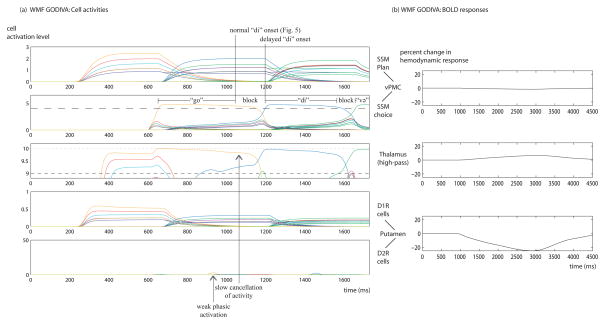
Fig. 7(a) and (b) similarly show neural activities and predicted BOLD responses (except for a predicted decrease in globus pallidus BOLD response, which is not plotted) for a version of the extended GODIVA model with white-matter fibers impairment (WMF GODIVA version). In this version of the model, the parameter λWMF, which represents the strength of the signal transmitted by the impaired corticostriatal fibers, is set to 0.1, and the result, again, is dysfluent speech. The modeling assumption is that the structural abnormality induces transmission errors, and those translate to generation of a weaker contextual signal in the putamen D2R cells. Although not reported here, similar results can be achieved by introducing noise into the information carried by these projections.
Fig. 7.
Time course of neural activities and predicted BOLD responses of the extended GODIVA model with structural abnormality in white matter fibers. (a) Neural activities. Conventions as in Fig. 5. (b) The BOLD responses predicted by the model for impaired white matter fibers compared with the baseline of intact fibers.
A comparison to the performance of the normal GODIVA version reveals that the WMF GODIVA version starts reading out the motor program of the “di” SSM too late. The winning SSM choice cell exceeds the cortical selection threshold at t=1194 ms, compared with t=1042 ms for the normal GODIVA version. The behavioral correlate is a block (152 ms long) on the second syllable of /godivə/. The reason for the delay is that the “go” SSM choice cell persists and prevents the selection of the “di” cell. The “go” syllable is produced normally, though. As motor programs have a fixed duration, they may terminate well before the suppression of the choice cell that launched them. There is another block between the second and third syllables, this time 93 ms long. The BOLD responses of the WMF GODIVA version were similar to the DA GODIVA version, other than the BOLD response of the putamen which decreased rather than increased relative to the normal GODIVA version.
4 Discussion
The simulations of the extended GODIVA model support two hypotheses of how neural abnormalities in stuttering prevent the SSM choice cell for the next syllable from being activated in a timely manner. In the case of elevated dopamine levels, the basal ganglia fail to bias cortical competition in favor of the SSM choice cell adequate for the next syllable, and in the case of white matter impairment, the BG fail to quickly cancel the activation of the SSM choice cell for the current syllable. It is this delayed selection of SSM choice cells that leads to dysfluencies in the speech produced by the model, supporting recent claims that the BG play a major role in stuttering (Alm, 2004; Giraud et al., 2008; Lu et al., 2010; Maguire et al., 2004). The delayed selection has several possible consequences; each leads to different dysfluencies: blocks, which are simulated in this paper, as well as prolongations, and sound/syllable repetitions (see Section 2.2.3). Although the reported blocks are atypically short (both are shorter than 200 ms; For comparison, blocks and prolongations with an average duration of 706 ms were reported by Zebrowski, 1994), this is because simulation parameters were set to reduce the durations of semi-static behavior in the middle of dysfluencies. This reduced the width of the plots while retaining detail at moments of dynamic model behavior, at starts and ends of dysfluencies. Longer dysfluencies can be produced with modest changes to model parameters.
Dopamine has an excitatory effect on the direct pathway, and in simulations, elevated dopamine levels ultimately lead to the creation of a “ceiling effect” in the thalamus (cf. Alm, 2004, p. 331). The strongly active direct pathway (note the increased activities of all putamen D1R cells in Fig. 6(a)) over-excites thalamus, pushing the activation of the thalamic cell for the correct well-learned syllable (“go”) to the highest possible level (marked by a dotted line); unfortunately, it does the same to one or more cells of incorrect well-learned syllables (“goal”). Although the “go” thalamic cell is still receiving stronger net-excitation than its competitors are, its activation cannot be increased above theirs because the cell has reached its ceiling. This interferes with the thalamus’s role in increasing signal-to-noise ratio, or contrast, in the cortex. The signal (the thalamic excitation of the SSM choice cell for “go”) is equal to the “noise” (the thalamic excitation of the SSM choice cells for other syllables), and does not provide the SSM choice cell for “go” with a competitive advantage. This results in slower-paced competition in the cortex, delaying the selection of the next syllable in line (the first syllable of the utterance, in this case).
With elevated dopamine levels in the model, dysfluencies do not happen on later syllables of the utterance /godivə/ because more SSM plan cells are active during the syllable selection process: the candidates for the next syllable, as well as the still-decaying candidates for the previous syllable. Due to lateral inhibition in the vPMC planning layer (see Bohland et al., 2010, as well as Section 1.1 of the Supplementary material), the larger number of active SSM plan cells results in lower activity for each of these cells, which translates to lower activity for each of the thalamic cells. No cell except the winning thalamic cell reaches the maximum possible activity level, and the “ceiling effect” is prevented. This modeling result predicts a bias toward dysfluencies on utterance-initial syllables, and thus, accounts for the fact that PWS stutter on the first word of a sentence more than any other word in it (see review in Bloodstein & Ratner, 2008). That said, depending on the number and fit of candidates for each syllable, elevated dopamine levels might also generate stuttering later in the utterance.10
Additional simulations demonstrated that the white matter impairment, which is assumed to affect the corticostriatal fibers that arise from vMC-to-brainstem projections, may lead to delayed selection of the SSM choice cell for the next syllable as well. In this case, the BG’s normal operation is disrupted, because the putamen D2R cells cannot reliably detect the context that indicates the imminent completion of syllable articulation (evident in the barely detectable transient activities in the putamen D2R cells for “go” and “di” in Fig. 7(a)). This prevents the indirect pathway from exerting strong inhibition on the cortex (via thalamus), and so the SSM choice cell for the currently executed syllable (and the thalamic cell that supports it) cannot be rapidly quenched. Staying active longer, the current SSM choice cell keeps inhibiting the choice cell that comes to replace it.
In contrast with the simulation for elevated dopamine levels, the white matter impairment simulation does not generate dysfluency on the first syllable of the utterance /godivə/. Impaired white-matter projections are hypothesized to disrupt shifts from one syllable to the next (including from the last syllable of a word to the first syllable of the following word), and they cannot affect utterance-initial syllables in the current form of the model. Thus this mechanism, by itself, cannot explain the frequent stuttering on first words of sentences (Bloodstein & Ratner, 2008).
Increasing our confidence in the validity of the two simulated hypotheses is the general agreement between previous imaging studies of dysfluent speech and the BOLD responses predicted by the simulations. It has been shown that activation levels in the left thalamus are correlated with moments of stuttering (Braun et al., 1997), and that the level of activation in the left globus pallidus is lower than normal in PWS (S. Brown et al., 2005). Furthermore, the simulations are consistent with repeated findings of abnormally low left vPMC activation in PWS, possibly associated with actual moments of stuttering (Ingham et al., 2004; Neumann et al., 2003; Watkins et al., 2008). There is a partial disagreement, though, regarding the BOLD response of the putamen. The highly significant correlation between the putamen’s activation levels and moments of stuttering (Braun et al., 1997) fits with the results of the DA GODIVA version, but not with those of the WMF GODIVA version.
The two hypotheses can be also evaluated in terms of their robustness, namely, whether the results of the simulations are independent of exact parameter values. According to our analysis, both hypotheses are robust because we could replicate their simulations over a wide range of parameter values. These ranges, of parameter values that produce simulations with dysfluencies, are listed in the rightmost column of Table 1. According to our prediction, they reflect the range of DA levels and severities of WMF impairments that would be observed in the brains of PWS. The table also lists the range of parameter values that produce simulations without dysfluencies. It is our prediction that these ranges would correspond to the brain of fluent speakers. Notice that not only too high dopamine levels, but also too low levels (βD1, βD2 < 0.7), produce simulations with dysfluencies. Although not explored in this paper, this might account for the dysfluencies observed in Parkinson’s disease patients (for review, see Goberman & Blomgren, 2003, p. 57).
The analysis of the simulations should not ignore the model’s principal limitation. Although the BG have complex architecture, for the sake of tractability, they were here modeled as a considerably simplified circuit, with certain elements omitted (subthalamic nucleus, presynaptic dopamine receptors, dopamine modulation of GABA-R mediated inhibition in the striatum, wide range of sensory inputs received by the striatum, etc.). However, since it was designed to capture the principal neural dynamics of the BG, the model is expected to survive the addition of any of the missing elements. For example, the addition of the subthalamic nucleus (STN) to the indirect pathway (as a GPe-to-GPi relay that replaces or augments the direct link between them) would not modify the net effect of the pathway on the cortex, predicting no change in the simulation results.11 Another simplification is that each cell in the model represents a neuron population rather than an individual neuron; it obviates the need for modeling the complex patterns of single-neuron firing, but by making the model more tractable, permits focusing on several fundamental network dynamics of the BG-vPMC loop (for similar approaches, see Bohland et al., 2010; J. Brown et al., 2004). To capture some of the complexities after all, the model includes nonlinearities as activation thresholds and saturation values, but those are still short of explaining certain firing patterns that might change network dynamics. For example, the pallidal neurons’ rebound burst, which might induce the late activation of the GPi relative to cortical input (Nambu et al., 2000), can be simulated by modeling individual neurons which exhibit T-type transient calcium currents (e.g., Rubchinsky et al., 2003).
This modeling study predicts that fluent speakers would exhibit stuttering-like behavior following an increase in brain dopamine levels, or an introduction of white matter impairment. The former option can be partially tested by giving subjects dopamine agonists. An experiment with apomorphine, for example, resulted in increased blinking and yawning (Blin et al., 1990). Such abnormal movements are not speech dysfluencies, but that the effect of apomorphine was limited to the oral and ocular systems, supports a role for dopamine in stuttering (the disorder affects most orofacial structures, including the eyes, see Bloodstein & Ratner, 2008). Unfortunately, the restricted range of dosages in experiments on healthy subjects precludes drawing firm conclusions. Another possibility is that the adverse effects of dopamine agonists on speech production depend on gene variations possessed by PWS only (for a demonstration that the effects of dopamine agonists might be gene-dependent, see Eisenegger et al., 2009). Much more data were collected on parkinsonians treated with the dopamine agonist L-dihydroxyphenylalanine (L-dopa). While the results are inconsistent, at least some of these patients become more dysfluent after drug administration (Goberman & Blomgren, 2003; Koller, 1983; Louis, Winfield, Fahn, & Ford, 2001). The elevated dopamine hypothesis predicts that this stuttering arises from an increase in dopamine to above-normal levels. The other model prediction -- stuttering due to white matter lesion in adulthood -- can be theoretically tested by analyzing cases of acquired stuttering. However, brain lesions are usually too diffuse to imitate the localized white matter impairment suggested by the model. Deep brain stimulation (DBS), which is a spatially localized intervention, might be more adequate for that purpose. Several recent case studies indeed report that DBS of subcortical structures induces stuttering in dystonian and parkinsonian patients (Allert et al., 2010; Nebel et al., 2009; Toft & Dietrichs, 2011). Although promising, DBS and L-dopa studies in patients should be interpreted with care since both Parkinson’s disease and dystonia are associated with abnormalities in the dopaminergic and/or basal ganglia systems (see Mink, 1996).
An unorthodox approach utilized in this paper is to compare between the BOLD responses predicted by the simulations, and the BOLD responses reported in the stuttering literature. The value of this approach is limited, however, by the ambiguous relationship between regional neural activities and activations of specific cell types in the GODIVA model. Unfortunately, there is not always a one-to-one relationship between brain regions whose BOLD responses are reported in the literature and specific cell types in the GODIVA model: the putamen contains putamen D1R and putamen D2R cells (among others), the vPMC contains both SSM plan and SSM choice cells, and the globus pallidus contains both GPi and GPe cells. (Although GPi and GPe cells reside in different parts of the globus pallidus, imaging studies of stuttering have not usually differentiated between them). The thalamus does correspond to single cell type in the GODIVA model. However, because abnormal thalamic cell activity may originate from either the direct or indirect pathways, it cannot indicate which of the pathways is dysfunctional. Given the above, we believe that studies measuring the BOLD response of the GPe (the part of the globus pallidus that belongs to the indirect pathway but not the direct pathway) in isolation, will prove most useful in testing current and future theories of BG involvement in stuttering.
The GODIVA model simulations presented here might also suggest on the etiology of other speech disorders that have manifestations similar to those produced by the model. Childhood apraxia of speech (CAS) is a good candidate, as it includes silent posturing (or prevocalic groping, see Hall, Jordan, & Robin, 2007), which is similar to the blocks simulated by GODIVA. However, to simulate CAS, the GODIVA model must also be able to generate the output abnormalities specific to that disorder. Terband and colleagues (Terband & Maassen, 2010; Terband, Maassen, Guenther, & Brumberg, 2009) used this approach with the GODIVA counterpart, the DIVA model. Though the DIVA model was initially used to simulate stuttering (Civier et al., 2010), they set the model’s feedback and reset parameters differently, and could generate searching articulatory behavior (or groping, see Hall et al., 2007), which characterizes CAS, but is absent from stuttering.
5 Conclusions
Based on GODIVA, a recently developed model of connected-speech planning and sequencing, we investigate two independent hypotheses of the etiology of stuttering: the “DA hypothesis” proposes that stuttering is caused by elevated dopamine levels affecting the putamen, and the “WMF hypothesis” proposes that stuttering is caused by structural abnormality to corticostriatal white matter fibers that arise from vMC-to-brainstem projections. According to the GODIVA model simulations, which support our predictions, either hypothesis can account for blocks (as well as other dysfluencies, see Section 2.2.3) in stuttering. The hypotheses predict the same dysfluency types, but partially different dysfluency locations: the DA hypothesis accounts for dysfluencies anywhere in the utterance (with a bias toward utterance-initial syllables), whereas the WMF hypothesis can only explain dysfluencies in the middle or end of the utterance.
Taking into account the simulation results and our literature review-based analysis of each hypothesis’s strengths and weaknesses (Section 2 of the Supplementary material), we reach the following conclusions. Despite the many studies reporting impaired white matter fibers in the vicinity of the vMC, the failure of the WMF hypothesis to perfectly match previous imaging studies, and its difficulty in accounting for frequent utterance-initial dysfluencies, as well as for non-oral motor control deficits (see Supplementary material), disqualify it from being, by itself, a unifying explanation for stuttering. It is possible that the white matter impairment is accompanied by another abnormality, or that the affected projections are different than hypothesized in this paper (e.g., superior longitudinal fasciculus III, see Cykowski et al., 2010). On the other hand, the close agreement between previous imaging studies and the BOLD responses predicted by the DA hypothesis, and the strengths of that hypothesis in explaining many features of stuttering (the disorder’s nonuniformity across motor systems, day-to-day variability in severity, the role of emotions, and the adaptation effect, see Supplementary material), suggest the DA hypothesis warrants further investigation. Unfortunately, at the moment there is only limited experimental evidence for elevated dopamine levels in PWS (Wu et al., 1997) or for dopamine agonist-induced stuttering in otherwise fluent speakers (see Discussion section). Integrating the above conclusions, we tentatively predict that in stuttering, elevated dopamine levels coexist with white matter impairment, and hypothesize that during brain development, one of these abnormalities might cause the other.
Supplementary Material
HIGHLIGHTS.
We examine the roles of dopamine excess and atypical white matter in stuttering
We simulate “neurally impaired” versions of the neurocomputational model GODIVA
Each abnormality causes stuttering by affecting the same BG-thalamus-vPMC circuit
The circuit selects/initiates the next syllable too late, resulting in dysfluency
The results account for brain imaging findings during dysfluent speech production
Acknowledgments
This study is part of the PhD dissertation of Oren Civier at Boston University and was supported by NIH/NIDCD grants R01 DC07683 and R01 DC02852 (P.I. Frank Guenther) and RO1 DC007603 (P.I. Ludo Max). We are grateful to Jason Bohland for developing the GODIVA model code and helping with the simulations, to Jason Tourville for the extensive knowledge and guidance with the prediction of BOLD responses, and to Gerald Maguire and Per Alm whose novel hypotheses and suggestions paved the way for this study. We thank Satrajit Ghosh, Alfonso Nieto-Castanon, and Jonathan Brumberg for the development of the DIVA model code, and Joseph Perkell, Amit Bajaj, and Michal Ben-Shachar for sharing their insights on stuttering and speech production. Lastly, thanks to Helen Barbas, Jonathan Mink, Hagai Bergman, Can Tan, and Alon Nevet for consulting on basal ganglia circuitry, to Vered Kronfeld, Paul Brocklehurst, and the three anonymous reviewers, for their comments on earlier versions of this manuscript, and to Maya Peeva, Shanqing Cai, Hayo Terband, and Elisa Golfinopoulos for their assistance.
Footnotes
In this paper, we always refer to developmental stuttering unless specified otherwise.
The DIVA and GODIVA models address different aspects of speech production. The DIVA model deals with motor control for articulation of single syllables, whereas the GODIVA model is involved in sequencing, selection, and initiation of syllable production in multi-syllabic speech.
Although abnormal fractional anisotropy (FA) values, which indicate structural abnormality, were detected in other brain regions as well, the finding of low FA values beneath the left precentral gyrus is the most consistent (see Cykowski et al., 2010). For the possibility that even beneath this limited region of cortex, PWS have several distinct abnormalities, see Section 2 of the Supplementary material.
Although a limited set of well-learned syllables is not sufficient for the production of all words in a given language, it usually serves for the production of most words (for example, the combinations of just 1000 syllables account for over 80% of words produced in English). See Bohland et al. (2010, pp. 1508–1509, 1516) for the mechanism that permits the model to produce those syllables whose motor programs are not included in the limited set stored in memory (for simplicity, this mechanism is not implemented here).
Although DA also inhibits cholinergic interneurons in the striatum via D2Rs, this action is synergistic because acetylcholine itself inhibits the direct path and facilitates the indirect path (see review in Tan & Bullock, 2008).
Whereas the GODIVA model gives the putamen IN cells an important role in the direct pathway, their role in the indirect pathway is less clear. However, since the anatomical data do not provide evidence that their efferents preferably synapse on putamen D1R cells, we implemented inhibition from the putamen IN cells to both putamen D1R and D2R cells (see Fig. 2). Yet, for this feedforward inhibition not to interfere with the normal operation of the indirect pathway, the inhibition of putamen D2R cells is modeled as weaker than the inhibition of the D1R cells. This is in line with the TELOS model of saccadic eye movements, which includes the same striatal feedforward-inhibition bias (see equations 33 and 34 in J. Brown et al., 2004). The fact that both the extended GODIVA and TELOS models benefit from a bias of feedforward inhibition toward the putamen D1R cells, predicts that such a bias may be important for the proper operation of the BG in the brain.
The GODIVA model represents an adult speaker, and as such, it is assumed that the weights between the vMC and the BG indirect pathway are already set such that the putamen D2R cell for the current syllable is excited when the syllable is about to complete. Modeling the process in which these weights are learned would be instructive because learning in the BG involves dopamine (see J. Brown et al., 2004; Frank & Claus, 2006) and is likely to be disrupted by hyper-activity in the dopaminergic system (Max, 2004; Max et al., 2004). This issue will be investigated in future papers.
The putamen D2 cells are informed of the current syllable through corticostriatal fibers that arise from collaterals of vPMC-to-vMC projections, and these fibers target the same putamen D2R cells that receive the aforementioned efference copy from vMC. Although not demonstrated for speech control areas, the convergence in the model’s striatum of fibers from two frontal areas characterized by strong cortico-cortical links (such as vPMC and vMC) is considered to be normative (reviewed in Wilson, 2004, p. 366). Finally, note that, of the two corticostriatal fiber bundles described, only the one that arises from collaterals of vMC efferents is hypothesized to be impaired in the brains of PWS.
For example, if the relation between brain activities in the experimental and control conditions varies throughout the speech task (experimental > baseline condition at some points, and experimental < baseline condition at others), the sign of the BOLD response peak might flip depending on the exact shape of the hemodynamic response: how fast it raises and drops, when it peaks, etc
Other factors that contribute to fluency by simultaneously activating many of the model’s SSM plan cells are: (a) a large number of syllables that are phonologically similar to the target syllable, and (b) many of these syllables occurring frequently in the language, thus, having their motor programs stored in memory. These model predictions are partially supported experimentally (Anderson, 2007).
The other roles of the STN should not be overlooked, though. Bullock and colleagues (J. Brown et al., 2004; Bullock, Tan, & John, 2009) suggest that the vPMC—STN—GPi—Thalamus pathway enhances lockout of competing plans during plan execution. In the context of the GODIVA model, this lockout mechanism may help to ensure that after a SSM choice cell is selected, it will be protected from disruption by other SSM choice cells that bid for execution. A primitive lockout mechanism is already included in the model (Section 2.2.2; also see Section 3.1), but since it relies on an activation function with a high exponent (see Section 1.1 of the Supplementary material), its biological plausibility is questionable. Yet another reason to include the STN in the model is the presumed contribution of this nucleus to contrast enhancement in the putamen (Bullock et al., 2009).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Oren Civier, Email: orenciv@gmail.com.
Daniel Bullock, Email: danb@bu.edu.
Ludo Max, Email: LudoMax@uw.edu.
Frank H. Guenther, Email: guenther@bu.edu.
References
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends in Neurosciences. 1989;12:366–374. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Allert N, Kelm D, Blahak C, Capelle HH, Krauss JK. Stuttering induced by thalamic deep brain stimulation for dystonia. Journal of Neural Transmission. 2010;117(5):617–620. doi: 10.1007/s00702-010-0380-0. [DOI] [PubMed] [Google Scholar]
- Alm PA. Stuttering and the basal ganglia circuits: A critical review of possible relations. Journal of Communication Disorders. 2004;37(4):325–369. doi: 10.1016/j.jcomdis.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Anderson JD. Phonological neighborhood and word frequency effects in the stuttered disfluencies of children who stutter. Journal of Speech, Language, and Hearing Research. 2007;50(1):229–247. doi: 10.1044/1092-4388(2007/018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballion B, Mallet N, Bezard E, Lanciego JL, Gonon F. Intratelencephalic corticostriatal neurons equally excite striatonigral and striatopallidal neurons and their discharge activity is selectively reduced in experimental parkinsonism. European Journal of Neuroscience. 2008;27(9):2313–2321. doi: 10.1111/j.1460-9568.2008.06192.x. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and frontal cortical connections of the premotor cortex (area 6) in the rhesus monkey. Journal of Comparative Neurology. 1987;256(2):211–228. doi: 10.1002/cne.902560203. [DOI] [PubMed] [Google Scholar]
- Blin O, Masson G, Azulay JP, Fondarai J, Serratrice G. Apomorphine-induced blinking and yawning in healthy volunteers. British Journal of Clinical Pharmacology. 1990;30(5):769–773. doi: 10.1111/j.1365-2125.1990.tb03848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodstein O, Ratner NB. A handbook on stuttering. 6. Clifton Park, NY: Thomson/Delmar Learning; 2008. [Google Scholar]
- Bohland JW, Bullock D, Guenther FH. Neural representations and mechanisms for the performance of simple speech sequences. Journal of Cognitive Neuroscience. 2010;22:1504–1529. doi: 10.1162/jocn.2009.21306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohland JW, Guenther FH. An fMRI investigation of syllable sequence production. Neuroimage. 2006;32(2):821–841. doi: 10.1016/j.neuroimage.2006.04.173. [DOI] [PubMed] [Google Scholar]
- Brady JP. The pharmacology of stuttering: A critical review. American Journal of Psychiatry. 1991;148:1309–1316. doi: 10.1176/ajp.148.10.1309. [DOI] [PubMed] [Google Scholar]
- Braun AR, Varga M, Stager S, Schulz G, Selbie S, Maisog JM, et al. Altered patterns of cerebral activity during speech and language production in developmental stuttering. An H2(15)O positron emission tomography study. Brain. 1997;120:761–784. doi: 10.1093/brain/120.5.761. [DOI] [PubMed] [Google Scholar]
- Brown JW, Bullock D, Grossberg S. How laminar frontal cortex and basal ganglia circuits interact to control planned and reactive saccades. Neural Networks. 2004;17(4):471–510. doi: 10.1016/j.neunet.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Brown S, Ingham RJ, Ingham JC, Laird AR, Fox PT. Stuttered and fluent speech production: An ALE meta-analysis of functional neuroimaging studies. Human Brain Mapping. 2005;25(1):105–117. doi: 10.1002/hbm.20140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock D, Tan CO, John YJ. Computational perspectives on forebrain microcircuits implicated in reinforcement learning, action selection, and cognitive control. Neural Networks. 2009;22(5–6):757–765. doi: 10.1016/j.neunet.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso AJ, Abbs JH, Gracco VL. Kinematic analysis of multiple movement coordination during speech in stutterers. Brain. 1988;111:439–456. doi: 10.1093/brain/111.2.439. [DOI] [PubMed] [Google Scholar]
- Chang SE, Erickson KI, Ambrose NG, Hasegawa-Johnson MA, Ludlow CL. Brain anatomy differences in childhood stuttering. Neuroimage. 2008;39(3):1333–1344. doi: 10.1016/j.neuroimage.2007.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Horwitz B, Ostuni J, Reynolds R, Ludlow CL. Evidence of left inferior frontal-premotor structural and functional connectivity deficits in adults who stutter. Cerebral Cortex. 2011;21(11):2507–2518. doi: 10.1093/cercor/bhr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Kenney MK, Loucks TM, Ludlow CL. Brain activation abnormalities during speech and non-speech in stuttering speakers. Neuroimage. 2009;46(1):201–212. doi: 10.1016/j.neuroimage.2009.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civier O. Doctoral dissertation. Vol. 70. Boston University; 2010. Computational modeling of the neural substrates of stuttering and induced fluency; p. 10. Dissertation Abstracts International. [Google Scholar]
- Civier O, Tasko SM, Guenther FH. Overreliance on auditory feedback may lead to sound/syllable repetitions: Simulations of stuttering and fluency-inducing conditions with a neural model of speech production. Journal of Fluency Disorders. 2010;35:246–279. doi: 10.1016/j.jfludis.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conture EG. Stuttering: Its nature, diagnosis, and treatment. Boston: Allyn and Bacon; 2001. [Google Scholar]
- Cowan RL, Wilson CJ. Spontaneous firing patterns and axonal projections of single corticostriatal neurons in the rat medial agranular cortex. Journal of Neurophysiology. 1994;71(1):17–32. doi: 10.1152/jn.1994.71.1.17. [DOI] [PubMed] [Google Scholar]
- Crosson B. Subcortical functions in language and memory. New York: Guilford; 1992. [Google Scholar]
- Cykowski MD, Fox PT, Ingham RJ, Ingham JC, Robin DA. A study of the reproducibility and etiology of diffusion anisotropy differences in developmental stuttering: A potential role for impaired myelination. Neuroimage. 2010;52(4):1495–1504. doi: 10.1016/j.neuroimage.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deniau JM, Menetrey A, Charpier S. The lamellar organization of the rat substantia nigra pars reticulata: Segregated patterns of striatal afferents and relationship to the topography of corticostriatal projections. Neuroscience. 1996;73(3):761–781. doi: 10.1016/0306-4522(96)00088-7. [DOI] [PubMed] [Google Scholar]
- Eisenegger C, Knoch D, Ebstein RP, Gianotti LR, Sandor PS, Fehr E. Dopamine receptor D4 polymorphism predicts the effect of L-dopa on gambling behavior. Biological Psychiatry. 2009;67(8):702–706. doi: 10.1016/j.biopsych.2009.09.021. [DOI] [PubMed] [Google Scholar]
- Fischer-Baum S, Rapp B. Underlying cause(s) of letter perseveration errors. Neuropsychologia. 2012;50(2):305–318. doi: 10.1016/j.neuropsychologia.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Claus ED. Anatomy of a decision: Striato-orbitofrontal interactions in reinforcement learning, decision making, and reversal. Psychological Review. 2006;113(2):300–326. doi: 10.1037/0033-295X.113.2.300. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: Multiple levels of compartmental organization in the basal ganglia. Annual Review of Neuroscience. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- Ghosh SS, Tourville JA, Guenther FH. A neuroimaging study of premotor lateralization and cerebellar involvement in the production of phonemes and syllables. Journal of Speech, Language, and Hearing Research. 2008;51(5):1183–1202. doi: 10.1044/1092-4388(2008/07-0119). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud AL, Neumann K, Bachoud-Levi AC, von Gudenberg AW, Euler HA, Lanfermann H, et al. Severity of dysfluency correlates with basal ganglia activity in persistent developmental stuttering. Brain and Language. 2008;104:190–199. doi: 10.1016/j.bandl.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Goberman AM, Blomgren M. Parkinsonian speech disfluencies: Effects of L-dopa-related fluctuations. Journal of Fluency Disorders. 2003;28:55–70. doi: 10.1016/s0094-730x(03)00005-6. [DOI] [PubMed] [Google Scholar]
- Golfinopoulos E, Tourville JA, Guenther FH. The integration of large-scale neural network modeling and functional brain imaging in speech motor control. Neuroimage. 2010;52:862–874. doi: 10.1016/j.neuroimage.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracco VL, Abbs JH. Programming and execution processes of speech movement control: Potential neural correlates. In: Keller E, Gopnik M, editors. Motor and sensory processes of language. Hillsdale, NJ: L. Erlbaum Associates; 1987. pp. 163–201. [Google Scholar]
- Grossberg S. A theory of human memory: Self-organization and performance of sensory-motor codes, maps, and plans. In: Rosen R, Snell F, editors. Progress in theoretical biology. Vol. 5. New York: Academic Press; 1978. pp. 233–374. [Google Scholar]
- Guenther FH. Speech sound acquisition, coarticulation, and rate effects in a neural network model of speech production. Psychological Review. 1995;102(3):594–621. doi: 10.1037/0033-295x.102.3.594. [DOI] [PubMed] [Google Scholar]
- Guenther FH. Neuroimaging of normal speech production. In: Ingham RJ, editor. Neuroimaging in communication sciences and disorders. San Diego, CA: Plural Pub; 2008. [Google Scholar]
- Guenther FH, Ghosh SS, Tourville JA. Neural modeling and imaging of the cortical interactions underlying syllable production. Brain and Language. 2006;96(3):280–301. doi: 10.1016/j.bandl.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther FH, Hampson M, Johnson D. A theoretical investigation of reference frames for the planning of speech movements. Psychological Review. 1998;105(4):611–633. doi: 10.1037/0033-295x.105.4.611-633. [DOI] [PubMed] [Google Scholar]
- Gurney K, Prescott TJ, Redgrave P. A computational model of action selection in the basal ganglia. II. Analysis and simulation of behaviour. Biological Cybernetics. 2001;84(6):411–423. doi: 10.1007/PL00007985. [DOI] [PubMed] [Google Scholar]
- Hall P, Jordan L, Robin D. Developmental apraxia of speech: Theory and clinical practice. 2. Austin, TX: Pro-Ed; 2007. [Google Scholar]
- Heuer RJ, Sataloff RT, Mandel S, Travers N. Neurogenic stuttering: Further corroboration of site of lesion. Ear, Nose, and Throat Journal. 1996;75(3):161–168. [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiological Reviews. 2000;80(3):953–978. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- Hoover JE, Strick PL. Multiple output channels in the basal ganglia. Science. 1993;259(5096):819–821. doi: 10.1126/science.7679223. [DOI] [PubMed] [Google Scholar]
- Howell P. A model of serial order problems in fluent, stuttered and agrammatic speech. Human Movement Science. 2007;26(5):728–741. doi: 10.1016/j.humov.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham RJ, Fox PT, Ingham JC, Xiong J, Zamarripa F, Hardies LJ, et al. Brain correlates of stuttering and syllable production: Gender comparison and replication. Journal of Speech, Language, and Hearing Research. 2004;47(2):321–341. doi: 10.1044/1092-4388(2004/026). [DOI] [PubMed] [Google Scholar]
- Kell CA, Neumann K, von Kriegstein K, Posenenske C, von Gudenberg AW, Euler H, et al. How the brain repairs stuttering. Brain. 2009;132:2747–2760. doi: 10.1093/brain/awp185. [DOI] [PubMed] [Google Scholar]
- Koller WC. Dysfluency (stuttering) in extrapyramidal disease. Archives of Neurology. 1983;40(3):175–177. doi: 10.1001/archneur.1983.04050030069014. [DOI] [PubMed] [Google Scholar]
- Koos T, Tepper JM. Inhibitory control of neostriatal projection neurons by gabaergic interneurons. Nature Neuroscience. 1999;2(5):467–472. doi: 10.1038/8138. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466(7306):622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan G, Tiwari S. Revisiting the acquired neurogenic stuttering in the light of developmental stuttering. Journal of Neurolinguistics. 2011;24:383–396. [Google Scholar]
- Lei W, Jiao Y, Del Mar N, Reiner A. Evidence for differential cortical input to direct pathway versus indirect pathway striatal projection neurons in rats. The Journal of Neuroscience. 2004;24(38):8289–8299. doi: 10.1523/JNEUROSCI.1990-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levelt WJM, Roelofs A, Meyer AS. A theory of lexical access in speech production. Behavioral and Brain Sciences. 1999;22(1):1–38. doi: 10.1017/s0140525x99001776. [DOI] [PubMed] [Google Scholar]
- Louis ED, Winfield L, Fahn S, Ford B. Speech dysfluency exacerbated by levodopa in Parkinson’s disease. Movement Disorders. 2001;16(3):562–565. doi: 10.1002/mds.1081. [DOI] [PubMed] [Google Scholar]
- Lu C, Peng D, Chen C, Ning N, Ding G, Li K, et al. Altered effective connectivity and anomalous anatomy in the basal ganglia-thalamocortical circuit of stuttering speakers. Cortex. 2010;46(1):49–67. doi: 10.1016/j.cortex.2009.02.017. [DOI] [PubMed] [Google Scholar]
- Ludlow CL, Rosenberg J, Salazar A, Grafman J, Smutok M. Site of penetrating brain lesions causing chronic acquired stuttering. Annals of Neurology. 1987;22(1):60–66. doi: 10.1002/ana.410220114. [DOI] [PubMed] [Google Scholar]
- MacKay DG, MacDonald MC. Stuttering as a sequencing and timing disorder. In: Perkins WH, Curlee R, editors. Nature and treatment of stuttering: New directions. San Diego: College Hill; 1984. pp. 261–282. [Google Scholar]
- Maguire GA, Yu BP, Franklin DL, Riley GD. Alleviating stuttering with pharmacological interventions. Expert Opinion on Pharmacotherapy. 2004;5(7):1565–1571. doi: 10.1517/14656566.5.7.1565. [DOI] [PubMed] [Google Scholar]
- Max L. Stuttering and internal models for sensorimotor control: A theoretical perspective to generate testable hypotheses. In: Maassen B, Kent RD, Peters HFM, van Lieshout PHHM, Hulstijn W, editors. Speech motor control in normal and disordered speech. Oxford, UK: Oxford University Press; 2004. pp. 357–387. [Google Scholar]
- Max L, Guenther FH, Gracco VL, Ghosh SS, Wallace ME. Unstable or insufficiently activated internal models and feedback-biased motor control as sources of dysfluency: A theoretical model of stuttering. Contemporary Issues in Communication Science and Disorders. 2004;31:105–122. [Google Scholar]
- Mink JW. The basal ganglia: Focused selection and inhibition of competing motor programs. Progress in Neurobiology. 1996;50(4):381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Nadjar A, Brotchie JM, Guigoni C, Li Q, Zhou SB, Wang GJ, et al. Phenotype of striatofugal medium spiny neurons in parkinsonian and dyskinetic nonhuman primates: A call for a reappraisal of the functional organization of the basal ganglia. The Journal of Neuroscience. 2006;26(34):8653–8661. doi: 10.1523/JNEUROSCI.2582-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Hamada I, Kita H, Imanishi M, Akazawa T, et al. Excitatory cortical inputs to pallidal neurons via the subthalamic nucleus in the monkey. Journal of Neurophysiology. 2000;84:289–300. doi: 10.1152/jn.2000.84.1.289. [DOI] [PubMed] [Google Scholar]
- Nebel A, Reese R, Deuschl G, Mehdorn HM, Volkmann J. Acquired stuttering after pallidal deep brain stimulation for dystonia. Journal of Neural Transmission. 2009;116(2):167–169. doi: 10.1007/s00702-008-0173-x. [DOI] [PubMed] [Google Scholar]
- Neumann K, Euler HA, von Gudenberg AW, Giraud AL, Lanfermann H, Gall V, et al. The nature and treatment of stuttering as revealed by fMRI: A within- and between-group comparison. Journal of Fluency Disorders. 2003;28:381–409. doi: 10.1016/j.jfludis.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Nieto-Castanon A, Guenther FH, Perkell JS, Curtin HD. A modeling investigation of articulatory variability and acoustic stability during American English /r/ production. Journal of the Acoustical Society of America. 2005;117(5):3196–3212. doi: 10.1121/1.1893271. [DOI] [PubMed] [Google Scholar]
- Packman A, Code C, Onslow M. On the cause of stuttering: Integrating theory with brain and behavioral research. Journal of Neurolinguistics. 2007;20:353–362. [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Research Brain Research Reviews. 1995;20(1):128–154. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- Parent M, Parent A. Single-axon tracing study of corticostriatal projections arising from primary motor cortex in primates. Journal of Comparative Neurology. 2006;496(2):202–213. doi: 10.1002/cne.20925. [DOI] [PubMed] [Google Scholar]
- Passingham R. The frontal lobes and voluntary activity. Oxford, UK: Oxford University Press; 1993. [Google Scholar]
- Peeva MG, Guenther FH, Tourville JA, Nieto-Castanon A, Anton JL, Nazarian B, et al. Distinct representations of phonemes, syllables, and supra-syllabic sequences in the speech production network. Neuroimage. 2010;50(2):626–638. doi: 10.1016/j.neuroimage.2009.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percheron G, Yelnik J, Francois C. A golgi analysis of the primate globus pallidus. III. Spatial organization of the striato-pallidal complex. Journal of Comparative Neurology. 1984;227(2):214–227. doi: 10.1002/cne.902270207. [DOI] [PubMed] [Google Scholar]
- Reiner A. You cannot have a vertebrate brain without a basal ganglia. In: Groenewegen HJ, Voorn P, Berendse HW, Mulder AB, Cools AR, editors. The basal ganglia IX, advances in behavioral biology. Vol. 58. New York: Springer; 2009. pp. 3–24. [Google Scholar]
- Reiner A, Hart NM, Lei W, Deng Y. Corticostriatal projection neurons - dichotomous types and dichotomous functions. Frontiers in Neuroanatomy. 2010;4:142. doi: 10.3389/fnana.2010.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubchinsky LL, Kopell N, Sigvardt KA. Modeling facilitation and inhibition of competing motor programs in basal ganglia subthalamic nucleus-pallidal circuits. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(24):14427–14432. doi: 10.1073/pnas.2036283100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmelin R, Schnitzler A, Schmitz F, Freund HJ. Single word reading in developmental stutterers and fluent speakers. Brain. 2000;123:1184–1202. doi: 10.1093/brain/123.6.1184. [DOI] [PubMed] [Google Scholar]
- Smits-Bandstra S, De Nil LF. Sequence skill learning in persons who stutter: Implications for cortico-striato-thalamo-cortical dysfunction. Journal of Fluency Disorders. 2007;32:251–278. doi: 10.1016/j.jfludis.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Sommer M, Koch MA, Paulus W, Weiller C, Buchel C. Disconnection of speech-relevant brain areas in persistent developmental stuttering. Lancet. 2002;360(9330):380–383. doi: 10.1016/S0140-6736(02)09610-1. [DOI] [PubMed] [Google Scholar]
- Stager SV, Calis K, Grothe D, Bloch M, Berensen NM, Smith PJ, et al. Treatment with medications affecting dopaminergic and serotonergic mechanisms: Effects on fluency and anxiety in persons who stutter. Journal of Fluency Disorders. 2005;30:319–335. doi: 10.1016/j.jfludis.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Takada M, Tokuno H, Nambu A, Inase M. Corticostriatal projections from the somatic motor areas of the frontal cortex in the macaque monkey: Segregation versus overlap of input zones from the primary motor cortex, the supplementary motor area, and the premotor cortex. Experimental Brain Research. 1998;120(1):114–128. doi: 10.1007/s002210050384. [DOI] [PubMed] [Google Scholar]
- Tan CO, Bullock D. A dopamine-acetylcholine cascade: Simulating learned and lesion-induced behavior of striatal cholinergic interneurons. Journal of Neurophysiology. 2008;100(4):2409–2421. doi: 10.1152/jn.90486.2008. [DOI] [PubMed] [Google Scholar]
- Tani T, Sakai Y. Analysis of five cases with neurogenic stuttering following brain injury in the basal ganglia. Journal of Fluency Disorders. 2011;36:1–16. doi: 10.1016/j.jfludis.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Terband H, Maassen B. Speech motor development in childhood apraxia of speech (CAS): Generating testable hypotheses by neurocomputational modeling. Folia Phoniatrica et Logopaedica. 2010;62:134–142. doi: 10.1159/000287212. [DOI] [PubMed] [Google Scholar]
- Terband H, Maassen B, Guenther FH, Brumberg J. Computational neural modeling of speech motor control in childhood apraxia of speech (CAS) Journal of Speech, Language, and Hearing Research. 2009;52(6):1595–1609. doi: 10.1044/1092-4388(2009/07-0283). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theys C, De Nil L, Thijs V, van Wieringen A, Sunaert S. A crucial role for the cortico-striato-cortical loop in the pathogenesis of stroke-related neurogenic stuttering. Human Brain Mapping. 2012 doi: 10.1002/hbm.22052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft M, Dietrichs E. Aggravated stuttering following subthalamic deep brain stimulation in Parkinson’s disease - two cases. BMC Neurology. 2011;11:44. doi: 10.1186/1471-2377-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourville JA, Reilly KJ, Guenther FH. Neural mechanisms underlying auditory feedback control of speech. Neuroimage. 2008;39(3):1429–1443. doi: 10.1016/j.neuroimage.2007.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran NL, Maguire GA, Franklin DL, Riley GD. Case report of aripiprazole for persistent developmental stuttering. Journal of Clinical Psychopharmacology. 2008;28(4):470–472. doi: 10.1097/JCP.0b013e31817ea9ad. [DOI] [PubMed] [Google Scholar]
- Turner RS, Delong MR. Corticostriatal activity in primary motor cortex of the macaque. The Journal of Neuroscience. 2000;20(18):7096–7108. doi: 10.1523/JNEUROSCI.20-18-07096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Kimura M. Encoding of direction and combination of movements by primate putamen neurons. European Journal of Neuroscience. 2003;18(4):980–994. doi: 10.1046/j.1460-9568.2003.02814.x. [DOI] [PubMed] [Google Scholar]
- Van Riper C. The nature of stuttering. Englewood Cliffs, NJ: Prentice-Hall; 1982. [Google Scholar]
- Watkins KE, Smith SM, Davis S, Howell P. Structural and functional abnormalities of the motor system in developmental stuttering. Brain. 2008;131:50–59. doi: 10.1093/brain/awm241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ. The contribution of cortical neurons to the firing pattern of striatal spiny neurons. In: Houk JC, Davis JL, Beiser DG, editors. Models of information processing in the basal ganglia. Cambridge, MA: The MIT Press; 1995. pp. 29–50. [Google Scholar]
- Wilson CJ. Basal ganglia. In: Shepherd GM, editor. The synaptic organization of the brain. 5. Oxford, UK: Oxford University Press; 2004. [Google Scholar]
- Wu JC, Maguire G, Riley G, Fallon J, Lacasse L, Chin S, et al. A positron emission tomography [18F]deoxyglucose study of developmental stuttering. Neuroreport. 1995;6(3):501–505. doi: 10.1097/00001756-199502000-00024. [DOI] [PubMed] [Google Scholar]
- Wu JC, Maguire G, Riley G, Lee A, Keator D, Tang C, et al. Increased dopamine activity associated with stuttering. Neuroreport. 1997;8(3):767–770. doi: 10.1097/00001756-199702100-00037. [DOI] [PubMed] [Google Scholar]
- Zebrowski PM. Duration of sound prolongation and sound/syllable repetition in children who stutter: Preliminary observations. Journal of Speech and Hearing Research. 1994;37(2):254–263. doi: 10.1044/jshr.3702.254. [DOI] [PubMed] [Google Scholar]
- Zimmermann G. Articulatory behaviors associated with stuttering: A cinefluorographic analysis. Journal of Speech and Hearing Research. 1980;23(1):108–121. doi: 10.1044/jshr.2301.108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.