Abstract
Sepsis is associated with an initial hyperinflammatory state; however, therapeutic trials targeting the inflammatory response have yielded disappointing results. It is now appreciated that septic patients often undergo a period of relative immunosuppression, rendering them susceptible to secondary infections. Interest in this phenomenon has led to the development of animal models to study the immune dysfunction of sepsis. In this review, we analyze the available models of sepsis-induced immunosuppression.
Introduction
Sepsis affects approximately 700,000 people annually, and was the 11th overall cause of death in 2009 [1]. Sepsis was initially regarded as an overly vigorous immune response to infection. Despite extensive research, few therapies have improved outcomes. Although activated protein C and the use of early goal-directed therapy can improve survival in selected patients [2, 3], immunosuppressive approaches targeting the inflammatory response have failed in clinical trials.
Subsequent studies have revealed that many patients with sepsis develop a state of immunologic quiescence, referred to as compensatory anti-inflammatory response syndrome (CARS). While this reflects an attempt to limit ongoing injury [4], patients display an attenuated immune response to infectious stimuli, thereby enhancing susceptibility to secondary infections. Many patients survive the initial bout of sepsis only to succumb to secondary infections. This may explain why trials of anti-inflammatory therapies such as high-dose corticosteroids and anti-cytokine agents failed to show benefit [5-10].
Models of Sepsis-induced immunosuppression
In vitro Models
The mammalian immune system recognizes pathogens through families of receptors known as pattern-recognition receptors. The Toll-like receptor (TLR) family recognizes molecular structures that are unique to pathogens and not present in mammalian cells, such as lipopolysaccharide (LPS), which is present on the outer membranes of gram-negative bacteria. LPS, also known as “endotoxin,” is recognized by Toll-like receptor 4, resulting in the production of proinflammatory cytokines. In vitro exposure of monocytes and macrophages to LPS results in the release of inflammatory cytokines, such as TNF-α, IL-6, and IL-8. [11, 12]. LPS was originally regarded as a major cause of the sepsis syndrome, given its proinflammatory effects and resultant shock when administered to animals experimentally.
However, in cells, repetitive exposure to LPS leads to decreased production of proinflammatory cytokines, particularly TNF-α [12]. This state of hyporesponsiveness following repeat challenge is termed endotoxin tolerance, and is reminiscent of the hyporesponsiveness observed in cells isolated from septic patients and experimental animals. Indeed, monocytes from patients with sepsis appear to display similar phenotypes as endotoxin-tolerant cells [13, 14]. Through in vitro studies, mechanisms that have been identified include downregulated expression of surface TLR4, alterations in the NF-kappa;B transcription factor complex for inflammatory gene expression, and upregulation of inhibitors of TLRs [15-17]. This phenomenon has also been observed with repeated stimulation by other TLR ligands, such as peptidoglycan (TLR2 agonist) [18]. Hence, the in vitro system of endotoxin tolerance is useful for elucidating specific molecular or cell-specific pathways that might also mediate sepsis-induced immunosuppression.
In vivo Models
Several in vivo models have been created to replicate sepsis in experimental animals, including mice, rats, dogs, guinea pigs, and rabbits. Most of the studies are performed in mice, given the availability of reagents to examine the immune response and the ease of generating genetically modified animals. Limitations with these models include the fact that they do not entirely reflect the clinical manifestations and temporal features of sepsis in human patients. Nonetheless, these models have been invaluable to unraveling the complexities of the immune response during sepsis. We will examine the most commonly used models, which include administration of bacterial components – particularly, endotoxin or LPS; systemic administration of live bacteria; and two similar models of septic peritonitis, the cecal ligation and puncture (CLP) model and the colon ascendens stent peritonitis (CASP) model.
Endotoxin models
The earliest and simplest models of sepsis entail the systemic (intravenous or intraperitoneal) administration of bacterial products, particularly endotoxin or LPS, into animals [19]. Endotoxemia models employ either a single, large bolus injection of endotoxin, or a continuous infusion [20]. While high doses of LPS may cause the hemodynamic collapse and mortality seen in sepsis, the quality and kinetics of the immune response are markedly different from human sepsis. For example, intraperitoneal LPS (250 mcg) resulted in >85 % mortality, with peak levels of pro-inflammatory cytokines (e.g., TNF, IL-1, IL-6) occurring early (i.e., between 1.5-4 hours) after the insult, and decreasing levels starting at 8 hours [21]. Although this robust and transient burst of cytokine production is similar to what is observed in human subjects following experimental administration of intravenous endotoxin [22], patients with sepsis usually have a more gradual and prolonged increase in cytokine levels, limiting the utility of the endotoxemia model for understanding the pathogenesis of sepsis.
Nonetheless, the endotoxin model has been used to examine endotoxin-induced alterations in immune cell function. Intraperitoneal injections of 50 μg of LPS for two days followed by injection of 300 μg on day 4 resulted in an endotoxin tolerant state, which the authors found were characterized by decreased IFN-γ levels and decreased responsiveness to the inflammatory cytokine, IL-12 [23]. Loss of antigen-presenting cells such as dendritic cells (DCs) is believed to be a mechanism of sepsis-induced immunosuppression. Intravenous LPS in a murine model resulted in loss of splenic DCs and decreased ability of DCs to sensitize T lymphocytes at 48 hours [24]. Finally, in rat models of systemic LPS administration, alveolar macrophage function was found to be impaired, including phagocytosis of bacteria and production of reactive oxygen species [25, 26]. Hence, it appears that exposure to endotoxin in vivo can result in some features of sepsis-induced immunosuppression, despite not being completely representative of the immune response of clinical sepsis.
Cecal Ligation and Puncture
The cecal ligation and puncture (CLP) model is generally regarded as the “gold standard” for animal models of sepsis. In particular, it simulates the development of an abdominal abscess, resulting in the development of polymicrobial sepsis. In addition, this model has been demonstrated to be useful in examining the immunosuppression following sepsis by challenging the animal with a pathogen at various time points after CLP.
The procedure was originally developed by Wichterman and Chaudry [27] and was recently described in detail by Rittirsch et al [28]. A vertical incision is made along the midline of the murine abdominal wall, and the peritoneum entered. The cecum is then located and exteriorized, followed by ligation at the blind end, taking care to avoid bowel obstruction. The feces are pushed distally to the end of the cecum, followed by a single through-and-through puncture with a sterile needle. The cecum is then replaced into the abdominal cavity and the abdominal wall is closed. The severity of sepsis can be manipulated by varying the distance from the ileocecal junction that the cecum is ligated, increasing the size of the puncture, or increasing the number of punctures made. We and others have found in the murine model of CLP that a single puncture by a 26-gauge needle results in 100% survival [29]. Control animals undergo “sham” operation, which is performed by making an abdominal incision, exposing the cecum, and replacing it back into the abdominal cavity without ligation or puncture.
The immunosuppression following sepsis is examined in the CLP model by challenging septic animals with a secondary infection, most commonly with a respiratory pathogen such as Pseudomonas aeruginosa. CLP followed by intratracheal bacteria is considered a “two hit” model that aims to recapitulate the clinical scenario where the patient survives the initial insult of sepsis only to succumb to a secondary infection by a pathogen that normally would not cause disease in a healthy host. For example, Pseudomonas aeruginosa is a common cause of nosocomial pneumonia that almost never causes pneumonia in immunocompetent hosts. In our hands, animals rendered septic by CLP have 80% mortality when given intratracheal P. aeruginosa at a dose of 1×105 CFU, whereas animals undergoing either sham surgery or CLP alone, or sham-operated animals given the same dose of P. aeruginosa had 100% survival [29]. Even if the challenge dose of P. aeruginosa was decreased 10-fold (i.e., 1×104 CFU), septic animals still had significantly increased mortality compared to non-septic hosts, demonstrating that the CLP model indeed leads to marked impairment of pulmonary host defense. Other pathogens used in combination with CLP have included Candida albicans and Aspergillus fumigatus [30, 31], which are increasingly being appreciated as an important cause of secondary infections in patients with prolonged critical illness and reflective of an immunosuppressed state.
The CLP model has been useful in elucidating a myriad of mechanisms mediating the immunosuppressive effects of sepsis, including impairment of both innate and adaptive immunity. CLP-induced sepsis is evidenced by a relative shift from proinflammatory to anti-inflammatory cytokines in response to secondary bacterial challenge in the lung. For example, TNF-α and IL-6 levels were decreased while the anti-inflammatory cytokine IL-10 was elevated in the lungs of CLP mice that had undergone secondary infection with P. aeruginosa [32-34]. Type 2 cytokines (IL-4, IL-13, TGF-β, and CCL2) in the lung following CLP were noted to be elevated suggesting that immunosuppression occurs in the lung after CLP [30]. In addition, lung macrophages from septic mice had decreased inflammatory cytokine responses when stimulated ex vivo with LPS, compared to macrophages from non-septic animals [29, 32]. Similarly, dendritic cells isolated from the lungs following CLP produced increased levels of IL-10 and lower levels of IL-12 and TNF-α [30].
The immunosuppression of sepsis involves antigen-presenting cells such as dendritic cells (DC). Following CLP, mice succumb to intratracheal injection of Aspergillus fumigatus. This is partially due to DC dysfunction as intratracheal injection of bone-marrow derived DCs restores immune function to challenge with Aspergillus fumigatus [30]. Transfer of bone marrow-derived DCs from post-CLP operated mice led to higher bacterial load in the lung of mice infected with intranasal P. aeruginosa. This was associated with a decrease in IL-12 and IFN-γ, consistent with a transition from the inflammatory state of sepsis to the immunosuppressive state [35]. Hence, the CLP model has been useful for examining DC function during sepsis.
Apoptosis of leukocyte populations, including DCs, CD4 and CD8 T cells, occurs during sepsis and is a major contributor to the immunosuppression of sepsis [36]. DCs provide the costimulatory signal needed for activation of T lymphocytes. Following CLP, DC populations in the spleen of the mice showed increased caspase-3 activity and resultant apoptosis [37, 38]. By causing apoptosis of follicular DCs, the immunosuppression of sepsis may prevent the maturation of B cells and prevents class switching and proliferation. In addition, CLP induces upregulation of pro-apoptotic factors that reflects observations made in patients with sepsis. For example, programmed death-1 (PD-1) is an inhibitory molecule that is upregulated on CD4 and CD8 T cells, B cells, and monocytes in septic patients and has been linked to increased lymphocyte apoptosis and poor clinical outcomes.[39, 40] Similarly, the CLP model also leads to upregulation of PD1 and its ligand, PD-1L Interaction between PD-1 and PD-1L leads to increased apoptosis. Blockade of PD-L1 decreases lymphocyte apoptosis through caspase-mediated mechanisms in the spleen and thymus of septic animals following CLP, resulting in increased lymphocyte number and improved survival, thereby reversing the immunosuppression of sepsis. [41].
In a two hit model of sepsis using CLP followed by intravenous injection of Candida albicans, mechanisms of T cell suppression mice had decreased mortality after neutralization of CTLA-4, which has an inhibitory role on T cell function. Treatment with anti-CTLA-4 was able to decrease sepsis-induced apoptosis of lymphocytes [31]. T regulatory cells (Tregs) are regarded as a “suppressive” T cell population whose role in sepsis-induced immunosuppression has been investigated in the CLP model. Following CLP, Treg numbers increase in frequency, which is associated with decreased proliferation of CD4 T cells. Anti-GITR (glucocorticoid-induced tumor necrosis factor receptor family-related gene) prevents Tregs from functioning. A model using CLP followed by intranasal Legionella pneumophila showed that the immunosuppression of sepsis could be reversed with treatment with anti-GITR, suggesting that Tregs may be responsible for some of the immunosuppression of sepsis [42].
Inhibition of Toll-like receptors prevents early recognition of pathogens resulting in impaired clearance of secondary infection in sepsis. Short form MyD88, A20, IL-1 receptor-associated kinase (IRAK)-M [29, 43] and suppression of tumorigenicity 2 (ST2) are thought to be among the mechanisms of this inhibition. ST2L, a transmembrane product of the st2 gene, is a negative inhibitor of TLR signaling. IL-33 is the ligand for ST2. ST2 knockout mice reversed the decrease in production of TNF-alpha and IFN-gamma by CD4 + and CD8+ T cells, indicating its importance in the immunosupression of secondary infection following sepsis [33].
The duration of immunosuppression has also been examined in the CLP model. Four days after CLP, splenocytes taken from the mice show a reduced ability to produce IFN-γ, but had renewed ability to produce IFN-γ by day 7. This ability to produce IFN-γ correlated with the period of increased susceptibility to secondary infection with intranasal Pseudomonas aeruginosa. Mice given a secondary infection of P. aeruginosa had increased mortality at 4 days while those receiving a secondary infection at 7 days, had similar mortality to either CLP or pneumonia alone [44]. This time frame also correlated with a change in cytokine profile from an inflammatory state to an immunosuppressive state with higher levels of IL-10 and lower levels of IL-1α, IL-6, IFN-γ and G-CSF [44]. Other models of secondary infection (e.g., intranasal Legionella pneumophila) following CLP have demonstrated increased susceptibility lasting out to at least 30 days [42].
In summary, the CLP model has been invaluable for investigating the immune mechanisms and molecular pathways underlying sepsis-induced immunosuppression.
Colon Ascendens Stent Peritonitis
Another model of sepsis is the colon ascendens stent peritonitis (CASP) model where stents of varying size are placed in the cecum [45]. This allows a continuous stream of fecal material and bacteria to leak into the peritoneal space. In addition, adjusting the size of the stent (ranging from 14-22 gauge in diameter) can lead to a low versus a high mortality model [46]. Unlike the CLP model, the CASP model does not result in an abdominal abscess, rather it leads to formation of diffuse peritonitis [47]. Less is known about whether the CASP model accurately replicates the concept of immunosuppression following sepsis. However, both proinflammatory and anti-inflammatory cytokines rise concurrently rather than sequentially, and generally are higher than what is observed with CLP [47].
In contrast to the CLP model, proinflammatory cytokines (TNF-alpha and IL-1β) continue to rise in the CASP model [47], likely reflecting ongoing active inflammation as fecal matter and bacteria continue to leak from the stent. Interferon-gamma receptor deficient mice quickly die after the CASP surgery, indicating an ongoing uncontrolled proinflammatory state [46]. Consistent with CASP reflecting an ongoing state of inflammation that may lead to death is evidence that anti-inflammatory treatments improve survival. Treatment with the anti-inflammatory tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) decreases apoptosis in the thymus and decreases mortality in mice undergoing CASP [48]. Likewise, CCR4-/- mice undergoing CASP had improved survival, likely reflecting decreased inflammation with decreased levels of IL-6 and CCL2 [49]. Therefore, while CLP simulates a model of inflammation followed by immunosuppression, CASP simulates a model of ongoing severe inflammation. CASP is also more technically difficult to do than the CLP, in that care must be taken during placement to ensure stent patency.
Like sepsis in humans, use of activated protein C has been shown to improve survival in mice undergoing the CASP procedure [19]. In addition, the CASP model has similarities to sepsis in humans in that acute inflammatory lung injury can develop after development of sepsis [50].
Gram Positive Sepsis Models
Other less used models include peptidoglycan intraperitoneal injection [51] models of sepsis which mirror the intraperitoneal injection of LPS in gram negative sepsis animal models. This serves as a model for gram positive sepsis. In other more elaborate models, a fibrin clot full of Staphylococcus aureus is implanted in the peritoneum of a mouse [52].
In silico Models
More recently, there have been developments of mathematical models to reflect the acute inflammatory state [53]. While still in the early stages of development, a multidisciplinary group of researchers was able to come up with a model to closely mimic experimental data by using ordinary differential equations. In particular, the model was able to predict the inflammatory response in surgery/hemorrhage followed by LPS. With time, these models may be able to streamline animal studies and reduce animal use.
Model Comparison and Translation to Humans
While the in vitro models are useful for elucidating specific molecular pathways, they do not simulate the complexities of sepsis, which is a systemic illness. With LPS challenge and rechallenge, the in vitro model can be used to study how different cell types respond and contribute to the immunosuppression of sepsis. Monocytes in critically ill septic patients have lower HLA-DR and CD86 expression [54], making them less able to provide the costimulatory signal needed to activate T cells. T cells also had less CD28 expression in critically ill septic patients [54], again decreasing the costimulatory signal needed for activation. IRAK-M is a negative regulator of the TLR-4 pathway, partially responsible for the immunosuppression of sepsis, and IRAK-M mRNA levels are also increased in human monocytes after LPS injection [55] and during sepsis [56], similar to in vitro models.
The in silico models suffer from a different limitation in that they require knowledge of the underlying immune pathways and build upon that. They are in the early stages of development and, as of now, cannot predict new immunological pathways. What they are most useful for appears to be in streamlining animal studies.
The most representative models of human sepsis appear to be the two primary animal models of CLP and CASP. While the CLP model can be used to demonstrate the same immunosuppression of sepsis that is seen in human patients, it is not clear that the CASP model is able to recapitulate the immunosuppression of sepsis at the moment. Both models are able to simulate the initial hyperinflammatory state of sepsis. While the CLP model has an endpoint where an abdominal abscess forms, allowing for the immunosuppression of sepsis to emerge, the CASP model continues to allow bacteria and fecal content to leak into the peritoneal space causing an ongoing state of infection and inflammation. Closing the stent in the CASP model would be an easy adaptation to allow for the emergence of the immunosuppression of sepsis. This would allow for control of the size of the stent to simulate different degrees of sepsis and of the time the animal is exposed to the hyperinflammatory state of initial sepsis. Because the stent sizes are fixed, this would allow a more standardized approach to the initial state of sepsis whereas the CLP model has more variability as the degree of sepsis depends on the location, size and number of punctures of the cecum.
Conclusion
While treatments focused on the inflammatory phase of sepsis have largely been unsuccessful, treatments focused on reversing the immunosuppressive phase of sepsis may be beneficial. The CASP model simulates sepsis with ongoing inflammation, though it does not simulate sepsis-induced immunosuppression. The CLP model currently best represents an episode of sepsis followed by the immunosuppression of sepsis. During this ensuing phase, another infection can be introduced to test sepsis-induced immunoparalysis and the animal's response to this infection. It is hoped that treatments directed toward this second phase of sepsis can translate into human trials with improved outcomes in septic patients.
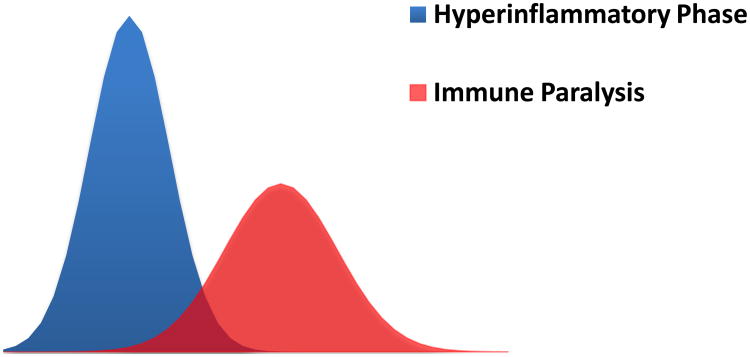
Figure 1.
Evolution of sepsis. A state of relative immune paralysis often develops concurrently or subsequent to the initial inflammatory state of sepsis, resulting in increased susceptibility to secondary infections. Although most patients survive the initial insult of sepsis, they can succumb to a secondary infection if the balance between pro- and anti-inflammatory immune responses is dysregulated.
Table. Comparison Summary Table.
Comparison Summary Table of Models Used to Study Sepsis-Induced Immunosuppression
| In vivo model 1: Cecal Ligation and Puncture | In vivo model 2: Colon Ascendens Stent Peritonitis | In vitro model | In silico model | |
|---|---|---|---|---|
| Pros |
|
Mimics ongoing sepsis from polymicrobial diffuse peritonitis. | Allows study at cell-specific and molecular level | No laboratory equipment, animals or biological samples needed |
| Cons | Not able to replicate other causes of sepsis | May not simulate the immunosuppression of sepsis, given the ongoing inflammatory stimulus | Unable to integrate the immune system's response as a whole | Depends on previously discovered pathways and responses |
| Best use of model | Study of pathogenetic mechanisms and preclinical treatment trials | Study of the inflammatory phase of sepsis and possible treatment trials | Study of molecular and cell-specific pathways | Helps to streamline animal studies |
| References | [27] | [42] | [11] | [50] |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kochanek KD, et al. Deaths: Preliminary Data for 2009. National Vital Statistics Reports. 2011;59(4):1–51. [PubMed] [Google Scholar]
- 2.Rivers E, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. The New England journal of medicine. 2001;345(19):1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 3.Bernard GR, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. The New England journal of medicine. 2001;344(10):699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 4.Bone RC. Sir Isaac Newton, sepsis, SIRS, and CARS. Crit Care Med. 1996;24(7):1125–8. doi: 10.1097/00003246-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Cronin L, et al. Corticosteroid treatment for sepsis: a critical appraisal and meta-analysis of the literature. Crit Care Med. 1995;23(8):1430–9. doi: 10.1097/00003246-199508000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Abraham E, et al. Efficacy and safety of monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. TNF-alpha MAb Sepsis Study Group. JAMA. 1995;273(12):934–41. [PubMed] [Google Scholar]
- 7.Abraham E, et al. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. NORASEPT II Study Group. Lancet. 1998;351(9107):929–33. [PubMed] [Google Scholar]
- 8.Fisher CJ, jr, et al. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. The Soluble TNF Receptor Sepsis Study Group. N Engl J Med. 1996;334(26):1697–702. doi: 10.1056/NEJM199606273342603. [DOI] [PubMed] [Google Scholar]
- 9.Opal SM, et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit Care Med. 1997;25(7):1115–24. doi: 10.1097/00003246-199707000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Fisher CJ, jr, et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA. 1994;271(23):1836–43. [PubMed] [Google Scholar]
- 11.Pena OM, et al. Endotoxin tolerance represents a distinctive state of alternative polarization (m2) in human mononuclear cells. Journal of immunology. 2011;186(12):7243–54. doi: 10.4049/jimmunol.1001952. [DOI] [PubMed] [Google Scholar]
- 12.West MA, Koons A. Endotoxin tolerance in sepsis: concentration-dependent augmentation or inhibition of LPS-stimulated macrophage TNF secretion by LPS pretreatment. J Trauma. 2008;65(4):893–8. doi: 10.1097/TA.0b013e3181877fde. discussion 898-900. [DOI] [PubMed] [Google Scholar]
- 13.Adib-Conquy M, et al. NF-kappaB expression in mononuclear cells of patients with sepsis resembles that observed in lipopolysaccharide tolerance. Am J Respir Crit Care Med. 2000;162(5):1877–83. doi: 10.1164/ajrccm.162.5.2003058. [DOI] [PubMed] [Google Scholar]
- 14.Munoz C, et al. Dysregulation of in vitro cytokine production by monocytes during sepsis. J Clin Invest. 1991;88(5):1747–54. doi: 10.1172/JCI115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziegler-Heitbrock HW, et al. Tolerance to lipopolysaccharide involves mobilization of nuclear factor kappa B with predominance of p50 homodimers. J Biol Chem. 1994;269(25):17001–4. [PubMed] [Google Scholar]
- 16.Nomura F, et al. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface toll-like receptor 4 expression. J Immunol. 2000;164(7):3476–9. doi: 10.4049/jimmunol.164.7.3476. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi K, et al. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110(2):191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 18.Nakayama K, et al. Involvement of IRAK-M in peptidoglycan-induced tolerance in macrophages. J Biol Chem. 2004;279(8):6629–34. doi: 10.1074/jbc.M308620200. [DOI] [PubMed] [Google Scholar]
- 19.Kerschen EJ, et al. Endotoxemia and sepsis mortality reduction by non-anticoagulant activated protein C. J Exp Med. 2007;204(10):2439–48. doi: 10.1084/jem.20070404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fink MP, Heard SO. Laboratory models of sepsis and septic shock. J Surg Res. 1990;49(2):186–96. doi: 10.1016/0022-4804(90)90260-9. [DOI] [PubMed] [Google Scholar]
- 21.Remick DG, et al. Comparison of the mortality and inflammatory response of two models of sepsis: lipopolysaccharide vs. cecal ligation and puncture. Shock. 2000;13(2):110–6. doi: 10.1097/00024382-200013020-00004. [DOI] [PubMed] [Google Scholar]
- 22.Copeland S, et al. Acute inflammatory response to endotoxin in mice and humans. Clin Diagn Lab Immunol. 2005;12(1):60–7. doi: 10.1128/CDLI.12.1.60-67.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balkhy HH, Heinzel FP. Endotoxin fails to induce IFN-gamma in endotoxin-tolerant mice: deficiencies in both IL-12 heterodimer production and IL-12 responsiveness. Journal of immunology. 1999;162(6):3633–8. [PubMed] [Google Scholar]
- 24.De Smedt T, et al. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J Exp Med. 1996;184(4):1413–24. doi: 10.1084/jem.184.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christman JW, et al. Alveolar macrophage function is selectively altered after endotoxemia in rats. Infect Immun. 1988;56(5):1254–9. doi: 10.1128/iai.56.5.1254-1259.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mason CM, et al. Intraportal lipopolysaccharide suppresses pulmonary antibacterial defense mechanisms. J Infect Dis. 1997;176(5):1293–302. doi: 10.1086/514125. [DOI] [PubMed] [Google Scholar]
- 27.Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock--a review of laboratory models and a proposal. J Surg Res. 1980;29(2):189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 28.Rittirsch D, et al. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4(1):31–6. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deng JC, et al. Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M. J Clin Invest. 2006;116(9):2532–42. doi: 10.1172/JCI28054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benjamim CF, et al. Reversal of long-term sepsis-induced immunosuppression by dendritic cells. Blood. 2005;105(9):3588–95. doi: 10.1182/blood-2004-08-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inoue S, et al. Dose-Dependent Effect of Anti-CTLA-4 on Survival in Sepsis. Shock. 2011;36(1):38–44. doi: 10.1097/SHK.0b013e3182168cce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen GH, et al. Intrapulmonary TNF gene therapy reverses sepsis-induced suppression of lung antibacterial host defense. J Immunol. 2000;165(11):6496–503. doi: 10.4049/jimmunol.165.11.6496. [DOI] [PubMed] [Google Scholar]
- 33.Hoogerwerf JJ, et al. Loss of suppression of tumorigenicity 2 (ST2) gene reverses sepsis-induced inhibition of lung host defense in mice. Am J Respir Crit Care Med. 2011;183(7):932–40. doi: 10.1164/rccm.201006-0934OC. [DOI] [PubMed] [Google Scholar]
- 34.Steinhauser ML, et al. IL-10 is a major mediator of sepsis-induced impairment in lung antibacterial host defense. J Immunol. 1999;162(1):392–9. [PubMed] [Google Scholar]
- 35.Pastille E, et al. Modulation of dendritic cell differentiation in the bone marrow mediates sustained immunosuppression after polymicrobial sepsis. J Immunol. 2011;186(2):977–86. doi: 10.4049/jimmunol.1001147. [DOI] [PubMed] [Google Scholar]
- 36.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6(11):813–22. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 37.Tinsley KW, et al. Sepsis induces apoptosis and profound depletion of splenic interdigitating and follicular dendritic cells. J Immunol. 2003;171(2):909–14. doi: 10.4049/jimmunol.171.2.909. [DOI] [PubMed] [Google Scholar]
- 38.Hotchkiss RS, et al. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat Immunol. 2000;1(6):496–501. doi: 10.1038/82741. [DOI] [PubMed] [Google Scholar]
- 39.Guignant C, et al. Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit Care. 2011;15(2):99. doi: 10.1186/cc10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, et al. Upregulation of programmed death-1 on T cells and programmed death ligand-1 on monocytes in septic shock patients. Crit Care. 2011;15(1):70. doi: 10.1186/cc10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, et al. PD-L1 blockade improves survival in experimental sepsis by inhibiting lymphocyte apoptosis and reversing monocyte dysfunction. Crit Care. 2010;14(6):220. doi: 10.1186/cc9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nascimento DC, et al. Role of regulatory T cells in long-term immune dysfunction associated with severe sepsis. Crit Care Med. 2010;38(8):18–25. doi: 10.1097/CCM.0b013e3181e78ad0. [DOI] [PubMed] [Google Scholar]
- 43.Lyn-Kew K, et al. IRAK-M regulates chromatin remodeling in lung macrophages during experimental sepsis. PLoS One. 2010;5(6):e11145. doi: 10.1371/journal.pone.0011145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muenzer JT, et al. Characterization and modulation of the immunosuppressive phase of sepsis. Infect Immun. 2010;78(4):1582–92. doi: 10.1128/IAI.01213-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Traeger T, et al. Colon ascendens stent peritonitis (CASP)--a standardized model for polymicrobial abdominal sepsis. J Vis Exp. 2010;(46) doi: 10.3791/2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zantl N, et al. Essential role of gamma interferon in survival of colon ascendens stent peritonitis, a novel murine model of abdominal sepsis. Infect Immun. 1998;66(5):2300–9. doi: 10.1128/iai.66.5.2300-2309.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maier S, et al. Cecal ligation and puncture versus colon ascendens stent peritonitis: two distinct animal models for polymicrobial sepsis. Shock. 2004;21(6):505–11. doi: 10.1097/01.shk.0000126906.52367.dd. [DOI] [PubMed] [Google Scholar]
- 48.Cziupka K, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) improves the innate immune response and enhances survival in murine polymicrobial sepsis. Crit Care Med. 2010;38(11):2169–74. doi: 10.1097/CCM.0b013e3181eedaa8. [DOI] [PubMed] [Google Scholar]
- 49.Traeger T, et al. Detrimental role of CC chemokine receptor 4 in murine polymicrobial sepsis. Infect Immun. 2008;76(11):5285–93. doi: 10.1128/IAI.00310-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neumann B, et al. Mechanisms of acute inflammatory lung injury induced by abdominal sepsis. Int Immunol. 1999;11(2):217–27. doi: 10.1093/intimm/11.2.217. [DOI] [PubMed] [Google Scholar]
- 51.Sweeney TE, et al. Differential regulation of the PGC family of genes in a mouse model of Staphylococcus aureus sepsis. PLoS One. 2010;5(7):e11606. doi: 10.1371/journal.pone.0011606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murphey ED, Fang G, Sherwood ER. Pretreatment with the Gram-positive bacterial cell wall molecule peptidoglycan improves bacterial clearance and decreases inflammation and mortality in mice challenged with Staphylococcus aureus. Crit Care Med. 2008;36(11):3067–73. doi: 10.1097/CCM.0b013e31818c6fb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vodovotz Y, et al. In silico models of acute inflammation in animals. Shock. 2006;26(3):235–44. doi: 10.1097/01.shk.0000225413.13866.fo. [DOI] [PubMed] [Google Scholar]
- 54.Manjuck J, et al. Decreased response to recall antigens is associated with depressed costimulatory receptor expression in septic critically ill patients. The Journal of laboratory and clinical medicine. 2000;135(2):153–60. doi: 10.1067/mlc.2000.104306. [DOI] [PubMed] [Google Scholar]
- 55.van't Veer C, et al. Induction of IRAK-M is associated with lipopolysaccharide tolerance in a human endotoxemia model. Journal of immunology. 2007;179(10):7110–20. doi: 10.4049/jimmunol.179.10.7110. [DOI] [PubMed] [Google Scholar]
- 56.Escoll P, et al. Rapid up-regulation of IRAK-M expression following a second endotoxin challenge in human monocytes and in monocytes isolated from septic patients. Biochemical and biophysical research communications. 2003;311(2):465–72. doi: 10.1016/j.bbrc.2003.10.019. [DOI] [PubMed] [Google Scholar]