Abstract
Enoxacin inhibits binding between the B-subunit of vacuolar H+-ATPase (V-ATPase) and microfilaments, and also between osteoclast formation and bone resorption in vitro. We hypothesized that a bisphosphonate derivative of enoxacin, bis-enoxacin (BE), which was previously studied as a bone-directed antibiotic, might have similar activities. BE shared a number of characteristics with enoxacin: It blocked binding between the recombinant B-subunit and microfilaments and inhibited osteoclastogenesis in cell culture with IC50s of about 10 µM in each case. BE did not alter the relative expression levels of various osteoclast-specific proteins. Even though tartrate-resistant acid phosphatase 5b was expressed, proteolytic activation of the latent pro-enzyme was inhibited. However, unlike enoxacin, BE stimulated caspase-3 activity. BE bound to bone slices and inhibited bone resorption by osteoclasts on BE-coated bone slices in cell culture. BE reduced the amount of orthodontic tooth movement achieved in rats after 28 days. Analysis of these data suggests that BE is a novel anti-resorptive molecule that is active both in vitro and in vivo and may have clinical uses. Abbreviations: BE, bis-enoxacin; V-ATPase, vacuolar H+-ATPase; TRAP, tartrate-resistant acid phosphatase; αMEM D10, minimal essential media, alpha modification with 10% fetal bovine serum; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; RANKL, receptor activator of nuclear factor kappa B-ligand; NFATc1, nuclear factor of activated T-cells; ADAM, a disintegrin and metalloprotease domain; OTM, orthodontic tooth movement.
Keywords: osteoclastogenesis, anti-resorptive, osteoclast, V-ATPase, microfilaments, alendronate
Introduction
Various potential applications for anti-resorptive agents exist in dentistry. These include: blocking alveolar bone loss associated with periodontal infection (Graves et al., 2011), inhibiting replacement resorption linked to endodontic procedures (Rosenblatt, 2010), reducing root resorption associated with orthodontic treatment (Brezniak and Wasserstein, 2002), and manipulating patterns of orthodontic tooth movement and immobilizing teeth to provide orthodontic anchorage (Dunn et al., 2007; Holliday et al., 2009). Despite the opportunities for the exploitation of anti-resorptives, their use in the dental clinic has been limited, in part, because the commonly used anti-resorptives are linked to increased risk for the development of oral osteonecrosis (Hoff et al., 2011). However, it is possible that novel anti-resorptives that inhibit osteoclasts by alternative mechanisms can be identified that do not carry this risk and might prove appropriate for dental use.
Osteoclasts express high levels of a subpopulation of vacuolar H+-ATPases (V-ATPases) that are targeted to the plasma membrane (Blair et al., 1989; Toro et al., 2012a). V-ATPases that are destined to be delivered to the plasma membranes of osteoclasts bind microfilaments, and this interaction is mediated by the B2-subunit (Lee et al., 1999; Chen et al., 2004). We identified the actin-binding domain in the B2-subunit and showed that mutations which eliminated actin binding also prevented delivery of the B-subunit to the ruffled plasma membrane (Holliday et al., 2000; Chen et al., 2004; Zuo et al., 2006). Analysis of these data suggested that molecules that inhibit the interaction between microfilaments and V-ATPase might prove to be a new class of anti-resorptive agents.
By taking a reverse chemical genetic approach and using computational chemistry techniques, we identified the fluoroquinolone antibiotic enoxacin as an inhibitor of the interaction between the B2-subunit and microfilaments and of bone resorption in cell culture (Ostrov et al., 2009). Enoxacin functioned by altering vesicular trafficking patterns in osteoclasts (Toro et al., 2012b). In the present study, we show that a bisphosphonate derivative of enoxacin, bis-enoxacin (BE) (Herczegh et al., 2002), also has anti-resorptive activity in vitro and inhibits orthodontic tooth movement (OTM), an osteoclast-dependent process, in a rat model. We suggest that BE is a novel type of bone-targeted anti-osteoclastic agent that functions by a mechanism different from that of the anti-resorptives currently used in the clinic, which could prove to be suitable for dental applications.
Materials & Methods
Reagents and Antibodies
BE (Herczegh et al., 2002) was obtained from SynQuest Laboratories (Alachua, FL, USA; Cat. #8H77-B-06). The polyclonal anti-E, anti-B2, and anti-a3 subunit antibodies were described previously (Manolson et al., 2003; Chen et al., 2004). Anti-a disintegrin and metalloprotease domain (ADAM)8, ADAM12, and anti-nuclear factor of activated T-cells (NFATc1) were obtained from Santa Cruz, Biotechnology (Santa Cruz, CA, USA). Anti-tartrate-resistant acid phosphatase (TRAP) 5b was from BioLegend (San Diego, CA, USA). Bone slices were obtained from bovine cortical bone. The bone was dried, rough-cut, and sliced by a low-speed diamond saw (Buehler, Rockville, IN, USA). Unless otherwise noted, other antibodies and reagents were obtained from the Sigma Chemical Co. (St. Louis, MO, USA).
Microfilament Binding Assay
BE was tested for its ability to inhibit interaction between rabbit muscle actin and bacterially expressed yeast B-subunit-maltose binding protein fusion protein (Vma2p-MBP) in a pelleting assay (Zuo et al., 2008; Ostrov et al., 2009). Vma2p-MBP (1 µM) or Vma2p-MBP plus microfilaments (1 µM) were mixed in the presence of various concentrations of BE or vehicle (ethanol) in an actin polymerizing buffer (F-buffer; 20 mM Tris, pH 7.4, 100 mM NaCl. 1 mM MgCl2, 0.1 mM CaCl2, 0.5 mM ATP, and 0.2 mM DTT) plus 10 µg/mL phalloidin to maximize filament polymerization. Ultracentrifugation in a Beckman Airfuge (Beckman Coulter, Fullerton, CA, USA) at 100,000 x g was carried out, and pellets and supernatants were collected, subjected to SDS-PAGE, and stained with Coomassie Brilliant Blue. Densitometry was performed from digital photographs with Image J software (NIH, Bethesda, MD, USA).
Generation of Osteoclasts
Mouse marrow osteoclasts were generated as described (Holliday et al., 1995). Swiss-Webster mice were killed by cervical dislocation, femora and tibia were dissected from adherent tissue, and marrow was removed by flushing with αMEM plus 10% fetal bovine serum (αMEM D10). The marrow was washed twice with αMEM D10, then seeded at a density of 1 x 106 cells/cm2 on tissue culture plates for 5 days in αMEM D10 plus 10−8 M 1,25-dihydroxyvitamin D3. We fed cultures on day 3 by replacing half the media per plate and adding fresh 1,25-dihydroxyvitamin D3. After 5 days in culture, osteoclasts appeared. These were detected as giant cells which stained positive for TRAP activity (a marker for mouse osteoclasts). The University of Florida Institutional Animal Care and Usage Committee approved all mouse and rat protocols.
Raw 264.7 cells were grown and differentiated by stimulation with recombinant receptor activator of nuclear factor kappa B-ligand (RANKL) into osteoclast-like cells as described previously (Krits et al., 2002) with 5 ng/mL RANKL. Mature osteoclast-like cells were scraped and replated on either glass coverslips or bone slices.
Caspase-3 Assay for Apoptosis
Cells were plated in 24-well plates at a density of 0.5 × 104 cells/cm2 and treated with 5 ng/mL RANKL plus or minus 50 µM enoxacin for 24, 48, and 72 hrs. Caspase-3 assays were performed as described previously (Toro et al., 2012b) with a caspase-3 assay kit (catalog No. APT131; Millipore, Temecula, CA, USA). The colorimetric reaction was quantified in a BioTek KC4 spectrophotometer (Winooski, VT, USA) at 405 nm.
Immunoblots
Immunoblots were performed by standard procedures in the SuperSignal WestDura chemiluminescence detection system (Pierce, Rockford, IL, USA). The samples were separated by SDS-PAGE, transferred to nitrocellulose, and probed with the antibodies described in the Fig. legends. Densitometry on blots was performed with ImageJ. Absorbance units were first adjusted to account for slight differences of actin in the samples, then the vehicle control samples of each comparison were converted to 100%, and the experimental results were adjusted accordingly. At least 3 blots were performed for each comparison.
BE/Enoxacin Absorption Assay
To measure binding of BE and enoxacin to bone, we placed 100-µm-thick, 1 cm2 bovine bone slices in 1 mL of PBS containing vehicle or enoxacin or BE (0.3-6.7 mg). After a six-hour incubation, the bones were removed. The control, enoxacin, and BE solutions were measured by spectrophotometry at 290 nm before and after incubation with the bone. The absorbance detected was compared with a concentration curve of BE or enoxacin to determine the concentration in solution. The amount bound to bone was calculated as the original amount in solution with the amount after bone incubation subtracted.
Resorption Assays
Resorption assays were performed by scanning electron microscopy (Holliday et al., 1995). We determined the area resorbed by taking random microscopic photos, then determined the area on Adobe Photoshop by overlaying a grid and counting grid intersections over pits vs. total grid intersections. Micrographs were taken at 200x. Pits were defined as continuous resorbed areas. Area per pit was calculated for each slice.
Orthodontic Tooth Movement
This study conforms to ARRIVE guidelines. For 2 wks prior to the application of orthodontic force, male rats (average starting weight, 340 g; Sprague Dawley, Charles River, MA, USA; 10 per group determined by power analysis) were injected daily S.C. with vehicle, 25 mg/kg BE, or 1 mg/kg alendronate. Experiments were performed in groups of 10, with animals assigned sequentially to treatment groups. After 2 wks, a 0.014” high tensile stainless steel Australian wire (A.J. Wilcock/Webster and Horsfall Ltd, West Midlands, UK) appliance was custom-fitted to a baseline epoxy model for each animal such that a 90° bend was placed at the mesiobuccal line angle of the right incisor (Appendix Fig. 1). The facial part of the wire wrapped around the mesiobuccal portion of the left incisor, and the distal arm of the wire extended back to the distal portion of the first molar. The wire was chosen to produce a light, continuous force (< 13 cN) when activated.
The maxillary teeth were isolated, and retention grooves were placed on the gingivodistal portion of both maxillary incisors and interproximally near the gingiva by means of a flame-tipped diamond bur. The incisors and maxillary first molar were etched with 35% phosphoric acid (Ultra Etch, Ultradent, South Jordan, UT, USA) and bond enhancer (Ortho Solo Bonding, Ormco, Orange, CA, USA), and then the wire was bonded with flowable composite (Henry Schein, Melville, NY, USA) at the gingival margins of the incisors such that the distal end lay passively against the palatal surface of the maxillary first molar. The appliance was activated by bonding to the buccal surface of the maxillary molar, producing a force that tipped the tooth in the palatal direction. To determine the amount of orthodontic tooth movement, we imaged epoxy models for each animal at every time-point (Zeiss AxioCam MR microscope, 10x magnification; Carl Zeiss, Göttingen, Germany). In total, 7 points were measured between each right and left molar tooth with Image J software (NIH) to determine the amount of palatal movement. The value for each respective set of points was subtracted from the pre-activation baseline value to determine the relative movement. Points 1 to 3 were averaged for assessing 1st molar movement.
Histology
Maxilla were excised, split, and fixed in 4% formaldehyde overnight. Maxillae were then decalcified in 40% EDTA, pH 7.4, for 2 wks, with daily changes of decalcification reagent. Samples were dehydrated through an ethanol series and embedded in paraffin, and 4-µm sections were cut, mounted on slides, and stained with H&E for visualization. An oral pathologist assessed the sections for osteoclasts (by numbers), inflammation, resorption, remodeling (on a 0-3 grading scale), and hypercementosis (plus/minus).
Statistics
Microscopists were pre-calibrated for their ability to identify TRAP+ osteoclast-like cells and then blinded to treatment groups. Results are expressed as mean ± SE. Samples were compared by one-way analysis of variance (ANOVA) and Student’s t test with the GraphPad Prism 5 program (GraphPad Software, La Jolla, CA, USA). All p values < .05 were considered significant. General Linear Model Repeated Measures analyses were completed for evaluation of the effects of time and drug treatment, and their interaction (time x treatment) on tooth movement. An ANOVA was used to compare the movement of the first molar within each treatment group over time following activation. Post hoc analyses were completed as needed for pairwise comparisons, with p < .05 being considered significant. Histology was analyzed by non-parametric ANOVA and the Kruskal-Wallis post-test.
Results
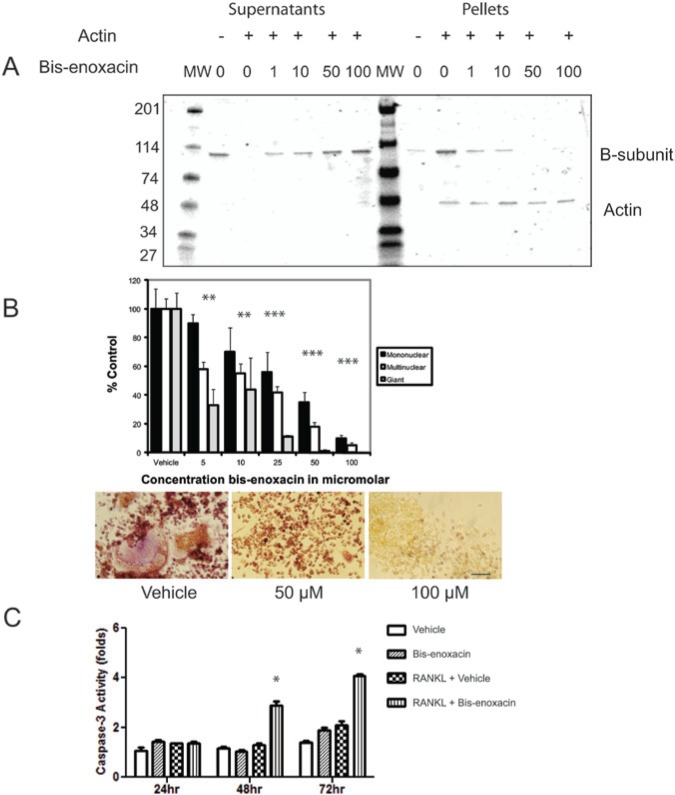
BE was tested for its ability to inhibit binding between rabbit muscle-actin microfilaments and the recombinant yeast B-subunit in an ultracentrifuge-based pelleting assay. BE inhibited the interaction dose-dependently based on densitometric monitoring of the increased B-subunit in supernatants (Fig. 1A).
Figure 1.
BE inhibits binding between the V-ATPase B-subunit and microfilaments and osteoclast differentiation but stimulates caspase-3 activity in RANKL-treated RAW 264.7 cells. (A) The Recombinant Yeast B subunit (1 µM) was mixed with microfilaments (1 µM) together with vehicle or BE at the concentrations indicated. The B-subunit does not pellet during ultracentrifugation unless bound to microfilaments. The samples were subjected to ultracentrifugation, supernatants and pellets were collected, separated by SDS-PAGE, and stained with Coomassie Blue. BE dose-dependently shifted the B-subunit from pellet to supernatant, consistent with its blocking of the binding interaction. (B) RAW 264.7 cells were stimulated with RANKL and treated with vehicle or the concentrations of BE indicated. After 4 days in culture, cells were stained for TRAP activity, then counted by calibrated, blinded microscopists. The TRAP+ cells were grouped into mononuclear cells, multinuclear cells (2-10 nuclei), and giant cells (more than 10 nuclei). The control values were 114 giant cells, 299 multinuclear cells, and 980 mononuclear cells. BE reduced the number of multinuclear cells stained for TRAP activity (osteoclast-like cells) dose-dependently with an IC50 of approximately 10 µM. Asterisks indicate p < .05. The panels show representative photos from the experiment for vehicle or 50 and 100 µM BE. Scale bar equals 20 µm. (C) BE (50 µM) did not significantly alter caspase-3 activity levels in unstimulated RAW 264.7 cells but triggered a significant increase in caspase-3 activity in cells stimulated with RANKL. Asterisks indicate p < .05.
Raw 264.7 cells were stimulated to differentiate into osteoclast-like cells in the presence of various concentrations of BE which dose-dependently reduced the number of giant cells displaying TRAP activity with an IC50 of about 10 µM. To test whether BE reduced caspase-3 activity in the manner of enoxacin, we stimulated RAW 264.7 cells with either vehicle or RANKL and treated with vehicle or 50 µM BE (Fig. 1B). Caspase-3 assays were performed at 24, 48, and 72 hrs to test for apoptosis. BE stimulation increased caspase-3 activity in RANKL-stimulated cultures after 48 and 72 hrs (Fig. 1C).
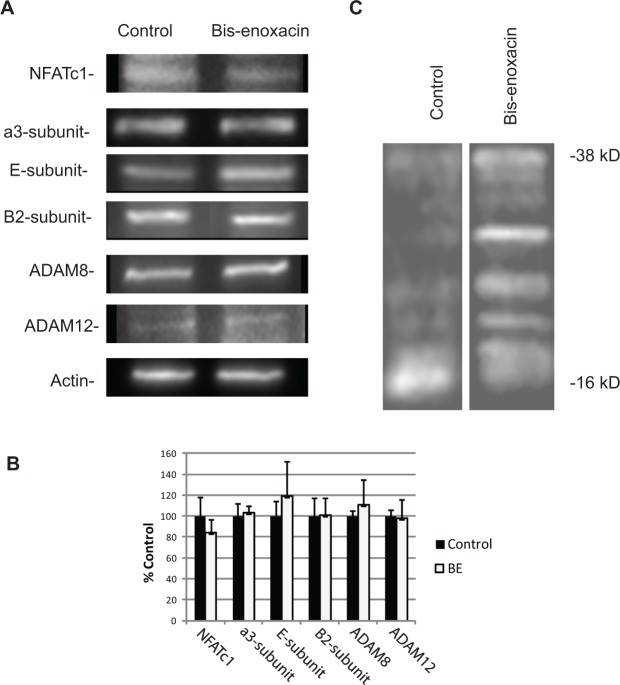
We performed quantitative Western blots to examine whether BE reduced the levels of osteoclast marker proteins. These included the a3-, B2-, and E-subunits of V-ATPase, ADAM8, ADAM12, and NFATc1 (Fig. 2A). No significant changes in the expression of these proteins were detected by densitometry of three or more comparison blots (Fig. 2B).
Figure 2.
Bis-enoxacin (BE) did not change the relative levels of osteoclast marker proteins, but altered proteolytic processing of TRAP5b. (A) Raw 264.7 cells were stimulated with RANKL plus vehicle or 50 µM BE. After 5 days, the cellular protein was extracted in SDS-PAGE sample buffer and spun at 100,000 x g to remove nucleic acids. The proteins were separated by SDS-PAGE, blotted, and probed with the antibodies indicated. An initial blot was performed to determine the loading that matched the amount of actin in the samples being compared. The blots displayed are samples from at least 3 trials. Anti-E, anti-B2, and anti-a3 subunit antibodies were used at a 1:500 dilution; anti-a disintegrin and metalloprotease domain ADAM8 and ADAM12 were used at a 1:250 dilution; and anti-nuclear factor of activated T-cells (NFATc1) was diluted 1:600. (B) BE did not alter the relative protein levels. Differences were not statistically significant by ANOVA. (C) Raw 264.7 cells were treated with RANKL plus vehicle, BE (50 µM), or enoxacin (50 µM). After 5 days, cells were harvested, and the cellular total proteins were separated by SDS-PAGE, blotted, and probed with anti-TRAP5B (1:500 dilution). Like enoxacin, treatment with BE altered the proteolytic activation of TRAP5b, indicated by the low amount of 16 kDa-activated TRAP and multiple higher-molecular-weight bands in the enoxacin-treated cultures compared with the control. Full-length TRAP5b pro-enzyme is 38 kDa.
When treated with enoxacin, osteoclasts expressed little TRAP activity. The enzyme TRAP5b was expressed, but not proteolytically processed to the active form (Toro et al., 2012b). To determine whether lack of TRAP activity after BE treatment was due to the same mechanism, we immunoblotted RANKL-stimulated Raw 264.7 extracts from cells that had been treated with vehicle or 50 µM BE and probed them with an anti-TRAP5b antibody. BE, like enoxacin (Toro et al., 2012b), altered the proteolysis of TRAP 5b (Fig. 2C).
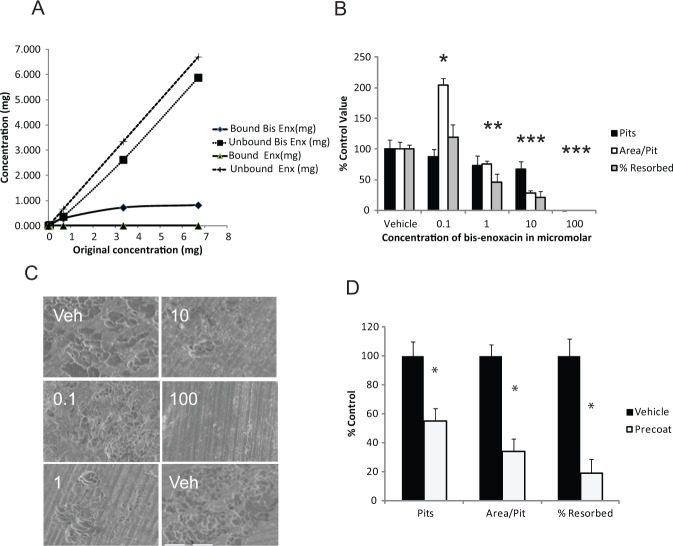
To confirm a previous study showing that BE binds bone (Herczegh et al., 2002), we incubated solutions containing enoxacin and BE with bone slices, then examined the amount removed from the solution by adsorption to the bone by spectrophotometry (Fig. 3A). We could not detect enoxacin adsorbed to the bone slices, but calculated that adsorbed BE saturated at 353 µg/cm2 of bone surface area.
Figure 3.
BE binds bone and blocks osteoclast bone resorption more effectively than enoxacin. (A) The indicated starting amounts of BE or enoxacin (in 1 mL total volume of water) were incubated with 1 cm2/100-µm-thick bone slices for 6 hrs. We determined the amount of BE or enoxacin bound to bone by subtracting the starting amount from the amount in soluble fraction after incubation. (B) BE reduced bone resorption after 3 days by 1,25-dihydroxyvitamin D3-stimulated mouse marrow cultures with an IC50 of 1 µM. The total area analyzed per bone image was 801,927 µm2. Mean control values analyzed were 27.5% resorption, 47.6 pits, and 3,961 µm2/pit. Asterisks indicate p < .05. (C) Representative scanning electron micrographs from experiments with data from (B). Electron micrographs are labeled with treatment group, vehicle (Veh), or the concentration in µM of BE. The scale bar in the lower left micrograph is 100 µm and is the same for all micrographs. (D) Bone slices were incubated with BE, then washed until BE was not detected in the wash. Slices were then incubated with 1,25-dihydroxyvitamin D3-stimulated mouse marrow cultures for 3 days. The total analyzed per bone image was 626,112 µm2. Mean control values were 15.5% resorption, 18 pits, and 5397 µm2/pit. Asterisks indicate p < .05.
BE inhibited bone resorption by 1,25-dihydroxyvitamin D3-stimulated mouse marrow osteoclasts with an IC50 of about 1 µM (Figs. 3B, 3C; Appendix Fig. 2). We hypothesized that this difference in inhibitory activity compared with enoxacin was the result of BE being concentrated to the bone surface and being mobilized as the osteoclasts began resorbing. To test this idea, we pre-coated bone slices with excess (10 mM) BE, then washed the slices extensively, until they no longer leached off detectable amounts of BE, as measured by spectrophotometry. We then loaded osteoclasts atop the BE-coated slices and allowed the osteoclasts 3 days for resorption. We found that bone-bound BE reduced the amount of bone resorbed (Fig. 3D).
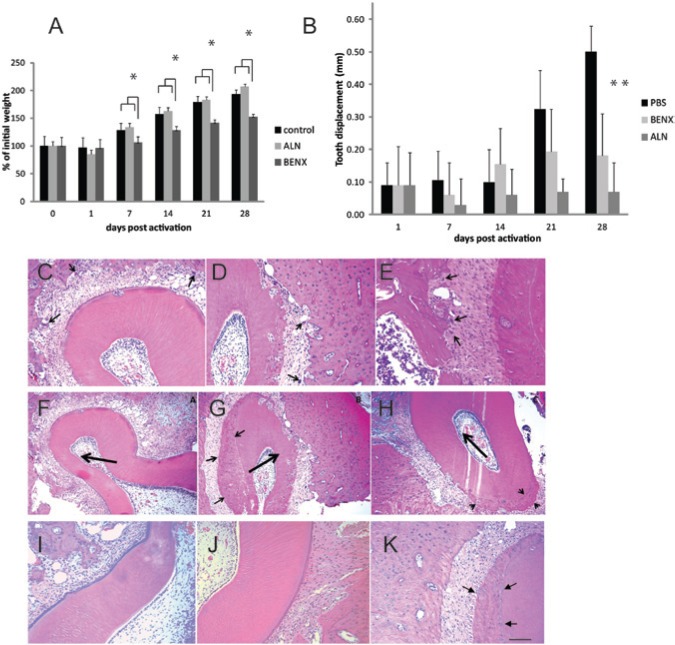
We then tested the effect of BE on OTM, an osteoclast-dependent process. Although rats injected with BE did not display signs of illness, they gained weight more slowly than either control or alendronate-treated animals (Fig. 4A). We found that 25 mg/kg/day BE significantly inhibited orthodontic tooth movement after 28 days (Fig. 4B). Histological examination of the alveolar bone around teeth subjected to orthodontic force in BE-treated rats did not reveal a reduction in osteoclast numbers or other histological parameters in the bone around teeth of either BE or alendronate-treated rats, compared with controls (Figs. 4C-4H, Appendix Fig. 3). Both BE and alendronate exhibited hypercementosis, which was not prominent in controls (Figs. 4F-4H).
Figure 4.
BE blocks orthodontic tooth movement in a rat model. Rats were subcutaneously injected with vehicle, alendronate 1 mg/kg/day, or BE 25 mg/kg/day for 2 wks prior to the activation of appliances and for 4 wks during orthodontic force application. (A) Rats in all 3 treatment groups gained weight after activation of the orthodontic apparatus. Ten animals per group were weighed; the groups were averaged and normalized to the pre-activation weight of each group. Note that although all 3 groups showed a slight decline in weight after activation, all groups subsequently gained weight consistently. (B) Both BE (BENX) and alendronate (ALN) reduced orthodontic tooth movement compared with vehicle, a result consistent with the inhibition of osteoclast bone resorption. The difference between alendronate and BE was not significant. Maxillae were collected after 28 days of orthodontic force application, fixed, and prepared for histology. Orthodontically treated sides of control rats (C), alendronate-treated rats (D), and BE-treated rats (E) displayed abundant osteoclasts, indicated by arrows in the H&E-stained sections. Hypercementosis (indicated by arrows) was evident in all rats (4 per group) treated with alendronate (G) and BE (H). Hypercementosis was less conspicuous in controls and detected in only 2 of the 4 control rats (F). The direction of orthodontic force application is indicated by the large arrow. Generally, as expected, osteoclasts were more abundant on the pressure side of teeth. Hypercementosis tended to occur on the tension side. Panels I-K are examples from the non-orthodontically treated sides of rats. Vehicle-treated rats (I) showed few osteoclasts or hypercementosis. BE- (J) and alendronate-treated rats also displayed few osteoclasts. However, all alendronate-treated rats exhibited hypercementosis even without orthodontic force application. The scale bar in K is equal to 80 µm in C-E and I-K and 130 µm in F-H.
Discussion
We show for the first time that BE inhibits osteoclasts in vitro and blocks OTM, an osteoclast-dependent process in vivo. Enoxacin was previously identified as a novel anti-resorptive agent (Ostrov et al., 2009). Fluoroquinolones can be conjugated to a bisphosphonate backbone so that they bind bone. Such conjugates were originally reported as potential bone-targeted antibiotics for the treatment of osteomyelitis; the bisphosphonate derivative was shown to bind bone while retaining its antibiotic activity (Herczegh et al., 2002). In this study, we show that BE, like enoxacin, blocks binding between the pure recombinant B-subunit and microfilaments. In cell culture, BE displayed anti-osteoclastic properties comparable with those of enoxacin. Thus, the bisphosphonate backbone did not compromise the anti-resorptive activity derived from the enoxacin moiety.
BE bound bone and reduced bone resorption with an IC50 of about 1 µM in vitro. This is consistent with the concept that BE has 2 activities: bone-binding derived from the bisphosphonate and anti-resorptive derived from enoxacin. Moreover, our results are also consistent with the accepted mechanism of nitrogen-containing bisphosphonates, where moieties that inhibit farnesyl diphosphate synthase are positioned in the same location as enoxacin (Russell, 2011). However, the fact that BE, in contrast to enoxacin, increases caspase-3 activity in osteoclast-like cells shows that the bisphosphonate backbone does alter the activity of enoxacin. Although caspase-3 activity is widely used as a surrogate for apoptosis, it has also been shown to be required for osteoclast formation (Szymczyk et al., 2006).
As an initial test of the ability of BE to inhibit osteoclast-dependent processes in vivo, we showed that BE inhibited orthodontic tooth movement in rats. Numerous osteoclasts were observed in alveolar bone associated with orthodontically manipulated teeth in both alendronate- and BE-treated rats. It was somewhat surprising to find large numbers of osteoclasts in the alendronate- and BE-treated rats. However, this finding is consistent with other reports (Srisubut et al., 2007; Yamamota-Silva et al., 2013). In addition, alendronate was associated with conspicuous hypercementosis, both on teeth subjected to orthodontic force and on the control sides. This finding hints that anti-resorptives might prove useful to reduce root resorption and improve cementum repair after tooth replantation.
Like enoxacin, BE blocks osteoclastogenesis and bone resorption without altering the expression levels of numerous osteoclast-specific proteins. These results are consistent with our proposal (Toro et al., 2012b) that, by blocking V-ATPase-microfilament-binding, enoxacin perturbs the specialized vesicular trafficking that is required for osteoclast differentiation and bone-resorptive activity. Enoxacin has also been reported to have anti-cancer activity, due its ability to enhance microRNA activity (Melo et al., 2011; Sousa et al., 2013). Further testing is required to determine whether BE stimulates microRNAs and/or has anti-cancer activity. If so, it may prove useful to treat bone cancer.
In summary, BE, a bisphosphonate derivative of enoxacin, inhibits osteoclast formation and bone resorption in a manner that resembles the novel mechanism by which enoxacin functions. Importantly, we show that BE inhibits OTM, a process that requires osteoclast activity in vivo. BE represents a “designer” bisphosphonate which was rationally developed with the goal of producing a bone-targeted version of enoxacin that selectively inhibits osteoclasts (Ostrov et al., 2009; Toro et al., 2012). The novel mechanistic characteristics of BE may allow it to have novel uses in the treatment of osteoclast-dependent pathologies.
Supplementary Material
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This study was supported by the NIDCR, National Institutes of Health (Grants R21 DE-19862 01A1 to LSH and T32 DE07200 to EJT).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Blair HC, Teitelbaum SL, Ghiselli R, Gluck S. (1989). Osteoclastic bone resorption by a polarized vacuolar proton pump. Science 245:855-857. [DOI] [PubMed] [Google Scholar]
- Brezniak N, Wasserstein A. (2002). Orthodontically induced inflammatory root resorption. Part I: The basic science aspects. Angle Orthod 72:175-179. [DOI] [PubMed] [Google Scholar]
- Chen SH, Bubb MR, Yarmola EG, Zuo J, Jiang J, Lee BS, et al. (2004). Vacuolar H+-ATPase binding to microfilaments: regulation in response to phosphatidylinositol 3-kinase activity and detailed characterization of the actin-binding site in subunit B. J Biol Chem 279:7988-7998. [DOI] [PubMed] [Google Scholar]
- Dunn MD, Park CH, Kostenuik PJ, Kapila S, Giannobile WV. (2007). Local delivery of osteoprotegerin inhibits mechanically mediated bone modeling in orthodontic tooth movement. Bone 41:446- 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves DT, Li J, Cochran DL. (2011). Inflammation and uncoupling as mechanisms of periodontal bone loss. J Dent Res 90:143-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herczegh P, Buxton TB, McPherson JC, III, Kovacs-Kulyassa A, Brewer PD, Sztaricskai F, et al. (2002). Osteoadsorptive bisphosphonate derivatives of fluoroquinolone antibacterials. J Med Chem 45:2338-2341. [DOI] [PubMed] [Google Scholar]
- Hoff AO, Toth B, Hu M, Hortobagyi GN, Gagel RF. (2011). Epidemiology and risk factors for osteonecrosis of the jaw in cancer patients. Ann NY Acad Sci 1218:47-54. [DOI] [PubMed] [Google Scholar]
- Holliday LS, Dean AD, Greenwald JE, Glucks SL. (1995). C-type natriuretic peptide increases bone resorption in 1,25-dihydroxyvitamin D3-stimulated mouse bone marrow cultures. J Biol Chem 270:18983-18989. [DOI] [PubMed] [Google Scholar]
- Holliday LS, Lu M, Lee BS, Nelson RD, Solivan S, Zhang L, et al. (2000). The amino-terminal domain of the B subunit of vacuolar H+-ATPase contains a filamentous actin binding site. J Biol Chem 275:32331-32337. [DOI] [PubMed] [Google Scholar]
- Holliday LS, Ostrov DA, Wronski TJ, Dolce C. (2009). Osteoclast polarization and orthodontic tooth movement. Orthod Craniofac Res 12:105-112. [DOI] [PubMed] [Google Scholar]
- Krits I, Wysolmerski RB, Holliday LS, Lee BS. (2002). Differential localization of myosin II isoforms in resting and activated osteoclasts. Calcif Tissue Int 71:530-538. [DOI] [PubMed] [Google Scholar]
- Lee BS, Gluck SL, Holliday LS. (1999). Interaction between vacuolar H(+)-ATPase and microfilaments during osteoclast activation. J Biol Chem 274:29164-29171. [DOI] [PubMed] [Google Scholar]
- Manolson MF, Yu H, Chen W, Yao Y, Li K, Lees RL, et al. (2003). The a3 isoform of the 100-kDa V- ATPase subunit is highly but differentially expressed in large (>or=10 nuclei) and small (<or= nuclei) osteoclasts. J Biol Chem 278:49271-49278. [DOI] [PubMed] [Google Scholar]
- Melo S, Villanueva A, Moutinho C, Davalos V, Spizzo R, Ivan C, et al. (2011). Small molecule enoxacin is a cancer-specific growth inhibitor that acts by enhancing TAR RNA-binding protein 2-mediated microRNA processing. Proc Natl Acad Sci USA 108:4394-4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrov DA, Magis AT, Wronski TJ, Chan EK, Toro EJ, Donatelli RE, et al. (2009). Identification of enoxacin as an inhibitor of osteoclast formation and bone resorption by structure-based virtual screening. J Med Chem 52:5144-5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt A. (2010). The best treatment for avulsed permanent teeth. Evid Based Dent 11:42-43. [DOI] [PubMed] [Google Scholar]
- Russell RG. (2011). Bisphosphonates: the first 40 years. Bone 49:2-19. [DOI] [PubMed] [Google Scholar]
- Sousa E, Graça I, Baptista T, Vieira FQ, Palmeira C, Henrique R, et al. (2013). Enoxacin inhibits growth of prostate cancer cells and effectively restores microRNAs processing. Epigenetics 8:548-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srisubut S, Teerakapong A, Vattraphodes T, Taweechaisupapong S. (2007). Effect of local delivery of alendronate on bone formation in bioactive glass grafting in rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 104:e11-e16. [DOI] [PubMed] [Google Scholar]
- Szymczyk KH, Freeman TA, Adams CS, Srinivas V, Steinbeck MJ. (2006). Active caspase-3 is required for osteoclast differentiation. J Cell Physiol 209:836-844. [DOI] [PubMed] [Google Scholar]
- Toro EJ, Ostrov DA, Wronski TJ, Holliday LS. (2012a). Rational identification of enoxacin as a novel V-ATPase-directed osteoclast inhibitor. Curr Protein Pept Sci 13:180-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro EJ, Zuo J, Ostrov DA, Catalfamo D, Bradaschia-Correa V, Arana-Chavez V, et al. (2012b). Enoxacin directly inhibits osteoclastogenesis without inducing apoptosis. J Biol Chem 287:17894-17904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto-Silva FP, Bradaschia-Correa V, Lima LA, Arana-Chavez VE. (2013). Ultrastructural and immunohistochemical study of early repair of alveolar sockets after the extraction of molars from alendronate-treated rats. Microsc Res Tech 76:633-640. [DOI] [PubMed] [Google Scholar]
- Zuo J, Jiang J, Chen SH, Vergara S, Gong Y, Xue J, et al. (2006). Actin binding activity of subunit B of vacuolar H(+)-ATPase is involved in its targeting to ruffled membranes of osteoclasts. J Bone Miner Res 21:714-721. [DOI] [PubMed] [Google Scholar]
- Zuo J, Vergara S, Kohno S, Holliday LS. (2008). Biochemical and functional characterization of the actin-binding activity of the B subunit of yeast vacuolar H+-ATPase. J Exp Biol 211(Pt 7):1102-1108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.