Abstract
IgA nephropathy (IgAN) patients have elevated serum levels of immune complexes consisting of IgA1 with galactose-deficient hinge-region O-glycans (Gd-IgA1) and anti-glycan IgG. These immune complexes deposit in the kidney and activate mesangial cells. To confirm that the activity of these immune complexes depends on the interaction of Gd-IgA1 with anti-glycan IgG, we generated in vitro analogous immune complexes using Gd-IgA1 myeloma protein and anti-glycan IgG from cord blood of healthy women. The Gd-IgA1 and anti-glycan IgG from cord-blood serum formed IgA1–IgG immune complexes that resembled those in sera of patients with IgAN. Furthermore, the ability to activate cellular proliferation was dependent on a heat-sensitive serum factor. In summary, we developed a new protocol for in-vitro formation of IgA1–IgG immune complexes, thus providing a new tool for studies of the pathogenesis of IgAN.
Keywords: IgA nephropathy, Galactose-deficient IgA1, O-glycosylation, Anti-glycan IgG, Immune complexes, Mesangial cells
Abbreviations: Gal, galactose; IgAN, IgA nephropathy
1. Introduction
IgA nephropathy (IgAN) is characterized by IgA-containing immune complexes in the glomerular mesangium [1,2], with IgA exclusively of the IgA1 subclass [3]. IgA1 can be co-deposited with complement C3 and IgG or IgM or both [1,2]. Mesangial cellular proliferation and expansion of extracellular matrix are typical histological features. These glomerular changes lead to end-stage renal failure in 20–40% patients within 20 years of diagnosis [4–7]. The disease onset and/or recurrent episodes of macroscopic hematuria often coincide with mucosal infections, including those of upper respiratory tract and digestive system [4,8–11].
There is considerable evidence indicating that the mesangial deposits originate from circulating IgA1-containing immune complexes [12–24]. Analysis of the glycosylation of IgA1 in patients with IgAN has provided new insights into the mechanisms underlying immune-complex formation and activation of mesangial cells [21,24–27]. Specifically, circulating immune complexes in IgAN contain IgA1 with galactose (Gal)-deficient hinge-region O-linked glycans [21,24,25,28–33] and Gal-deficient IgA1 is the predominant glycosylation variant of IgA1 in the mesangium [34,35]. A relationship between Gal deficiency and nephritis also has been observed in other diseases: Gal-deficient IgA1 [36,37] and IgA–IgG circulating complexes [38] are found in sera of patients with Henoch–Schoenlein purpura who develop nephritis but not in sera of those patients who do not. Also, patients with IgA1 multiple myeloma have high levels of circulating IgA1, but only individuals with the aberrantly-glycosylated variant develop immune-complex glomerulonephritis [39,40].
In IgAN, the aberrant glycans or hinge-region glycopeptides of IgA1 are recognized by naturally occurring IgG and/or IgA1 anti-glycan antibodies and immune complexes are formed [21,24,41–44]. These IgA1-containing immune complexes are of a relatively large molecular mass [26,30,33] and, thus, are not efficiently cleared from the circulation by the liver and tend to deposit in the renal mesangium [45–50].
As only humans and hominoid primates have IgA1 [51], studies of IgAN have been hampered by the lack of appropriate animal models. Alternatively, cultured primary human mesangial cells present a convenient model to evaluate biologic activities of IgA1 complexes [25,26,30,52–64]. Using this model, we and others have demonstrated that immune complexes from sera of patients with IgAN containing galactose-deficient IgA1 activate mesangial cells and induce cellular proliferation [26,30,62,64,65].
Extensive studies of the detailed glycan structures of IgA1, fine specificities of anti-glycan antibodies, and biological activities are hampered by the low quantity of immune complexes isolated from sera of patients with IgAN. Therefore, to address this shortcoming, we developed a new protocol for in-vitro production of biologically active IgA1-containing immune complexes. We used cord-blood serum with high levels of anti-glycan IgG to bind to IgA1 myeloma proteins with Gal-deficient hinge-region O-glycans that mimic the aberrant IgA1 eluted from glomeruli of patients with IgAN [24,34,35,66–68] to form immune complexes. The advantage of cord-blood serum is that it contains almost exclusively IgG, with only trace amounts of IgM or IgA. Our results showed that formation of the biologically active immune complexes required Gal-deficient IgA1, anti-IgA1 IgG antibody, and a heat-sensitive serum factor. Future studies to identify this factor(s) and describe detailed mechanisms of immune-complex formation will help in designing a better therapeutic target in patients with IgAN.
2. Materials and methods
2.1. Serum samples
Cord-blood serum samples were collected from five pregnant women (one Caucasian and four African Americans) at the time of delivery. The donors did not have any evidence of renal disease as determined by normal values for serum creatinine concentration and urinary protein/creatinine ratio, and the absence of hematuria by urinalysis testing. Serum samples were obtained from three biopsy-proven IgAN patients and a healthy control. The UAB Institutional Review Board approved the study and informed written consent was obtained from each participant before collecting the blood samples.
2.2. IgA myeloma proteins
Two polymeric IgA1 (Mce and Gou) and one polymeric IgA2 (Fel) myeloma proteins were prepared from plasma of patients with IgA multiple myeloma [24,69]. Both IgA1 myeloma proteins had Gal-deficient hinge-region O-glycans [24,29].
2.3. Fractionation of immune complexes
Serum samples from patients with IgAN or healthy controls (0.5 ml) were filtered using 0.45 μm filter (Pall Corporation, Ann Arbor, MI, USA) and fractionated on a calibrated Superose 6 column (600 × 12 mm; Amersham Biosciences Corporation, Piscataway, NJ, USA) in phosphate-buffered saline (PBS). For cord-blood serum supplemented with IgA, a mixture of 160 μl cord-blood serum, 80 μg IgA myeloma protein and PBS to a final volume of 500 μl was incubated at 4 °C overnight, filtered and fractionated on a calibrated Superose 6 column. Fractions containing proteins of molecular mass apparently over 700 kDa were collected and every two fractions were pooled and added to the cultured mesangial cells [70]. IgA and IgG–IgA complexes were determined in the fractions by ELISA [25,71].
2.4. Cell cultures
Human mesangial cells were purchased from Cambrex Bio Science Walkersville, Inc. (Walkersville, MD, USA) and cultured, as described [25]. Cells from passages 3 or 4 were maintained in RPMI 1640 supplemented with 20% fetal calf serum (FCS), l-glutamine (2 mmol/l), penicillin G (100 U/ml) and streptomycin (0.1 mg/ml) in humidified 5% CO2 atmosphere at 37 °C. Twenty-four-well tissue-culture plates were seeded with the mesangial cells for proliferation experiments. At 85%–95% confluence, the mesangial cells were serum-starved in a medium containing 0.5% FCS for 24 h before experiments [70].
2.5. Proliferation assays
Proliferation of the cultured human mesangial cells was measured by 3H-thymidine uptake, as described [26,70]. Serum from healthy control or patient with IgAN or cord-blood serum was supplemented with IgA proteins and incubated at 4 °C overnight (unless otherwise stated). Then, the samples were filter-sterilized, mixed with culture medium containing 0.5% FCS, added in duplicates to mesangial cells, and the cells were incubated for 20 h at 37 °C in 5% CO2 atmosphere and then analyzed [26,70]. The culture medium supplemented with 10 ng/ml platelet-derived growth factor (PDGF; R&D Systems, Minneapolis, MN, USA) was used as a positive control. Medium alone served as a negative control. Average values were calculated from duplicates for each serum fraction and expressed relative to the negative control (cpm of sample/ cpm of the control) as relative proliferation. Alternatively, the data were expressed as Δcpm, calculated as cpm value of each sample from which the value measured for the control sample was subtracted.
2.6. ELISA
For IgA detection, polystyrene microtiter plates (Nalge Nunc International, Rochester, NY, USA) were coated overnight with 1 μg/ml goat anti-human IgA (Jackson ImmunoResearch Labs, West Grove, PA, USA) [25,71]. After washing and blocking with 1% bovine serum albumin (BSA; Sigma Chemical Company, St Louis, MO, USA) in PBS containing 0.05% Tween-20, serial 2-fold dilutions of duplicate samples and standard serum (The Binding Site, Birmingham, United Kingdom) in blocking solution were incubated overnight at room temperature. The bound IgA was detected by incubation with biotin-labeled goat anti-human IgA (BioSource International, Camarillo, TX, USA) for 3 h at 37 °C, followed by 1-h incubation with horseradish peroxidase-conjugated ExtrAvidin (Sigma). o-Phenylenediamine–H2O2 (Sigma) was used as substrate for peroxidase, and color development was stopped with 1 M sulphuric acid. The absorbance at 490 nm was measured using an automated ELISA reader (Bio-Tek Instruments Winooski, VT, USA). The concentrations were calculated based on calibration curves generated from standard serum. The results were expressed in μg/ml.
For measurement of IgG–IgA complexes, 50-fold-diluted fractions were applied on ELISA plates coated with goat anti-human IgG (Jackson ImmunoResearch Labs) and detected with biotin-labeled goat anti-human IgA (BioSource) and developed, as described above. Internal controls were included.
2.7. Depletion of IgA and IgG, and isolation of IgG
To remove IgA1 from serum of a patient with IgAN, serum was adsorbed on immobilized jacalin (1-ml bed volume; EY Laboratories, San Mateo, CA, USA), a lectin specific for O-glycans on IgA1. IgG was depleted from serum or cord-blood serum using GammaBind Plus Sepharose (Amersham Biosciences Corporation), using 1 ml of the sample mixed with the same amount of binding buffer (0.01 M sodium phosphate, 0.15 M NaCl, 0.01 M EDTA, pH 7.0). The flow-through was concentrated on Amicon Ultra-4 PL-50 Centrifugal Filter Devices (Millipore, Billerica, MA, USA) to a volume of 1 ml and used as IgG-depleted serum. To isolate IgG, the GammaBind Plus Sepharose column was washed with 5 ml binding buffer and the bound IgG was eluted with acidic buffer (0.5 M acetic acid adjusted to pH 3.0 with ammonium hydroxide) and the pH was adjusted to neutral with Tris–HCl buffer. Then, the IgG samples were desalted on a PD-10 column (GE HealthCare, Chalfont St. Giles, United Kingdom) equilibrated with PBS, and concentrated on Amicon Ultra-4 PL-50 Centrifugal Filter Devices (Millipore) to 1 ml volume. Purity of these samples was assessed after separation by SDS-PAGE under reducing conditions by silver staining, and by Western blotting with IgA- or IgG-specific antibodies.
2.8. Western blot
Proteins separated by SDS-PAGE were blotted on Immobilon P (Millipore) and detected with biotinylated goat IgG against human IgA or IgG (Vector Laboratories, Burlingame, CA, USA) followed by horseradish peroxidase-conjugated NeutrAvidin (Pierce Chemical Company, Rockford, IL, USA). The detection of IgA or IgG was accomplished by chemiluminescence using Supersignal substrate (Pierce) and visualization on Biomax film (Amersham, Biosciences Corp.) [70].
2.9. Statistical analyses
Data were expressed as mean ± SD or median values. Statistical analyses were performed using 2-tailed Student's t test with StatView 5.0 software (SAS). P value <0.05 was considered significant.
3. Results
3.1. In vitro-formed immune complexes containing Gal-deficient IgA1 stimulate proliferation of cultured primary human mesangial cells
Various concentrations of Gal-deficient IgA1 (Mce) myeloma protein with well-characterized structures of O-glycans [24,29,47,66–68,72] were added to serum from a healthy control or from an IgAN patient, or cord-blood serum. After 1-h incubation at 4 °C to allow formation of immune complexes, the mixtures were diluted with culture medium and added to serum-starved human mesangial cells. Cellular proliferation was measured after 20-h incubation. The sample with the most stimulatory activity was generated using cord-blood serum #1 (CBS1; 2% final concentration) supplemented with 20 μg/ml IgA1 (Fig. 1). In control experiments, immune complexes formed with serum from an IgAN patient stimulated cultured human mesangial cells to proliferate more than did the complexes formed with serum from a healthy control (Fig. 1). Supplementation with IgA1 increased formation of stimulatory complexes at lower serum concentrations and, conversely, led to inhibition of proliferation when more serum and IgA1 were used (Fig. 1). Uncomplexed IgA1 did not significantly affect cellular proliferation (Fig. 1), confirming our previous report [26].
Fig. 1.
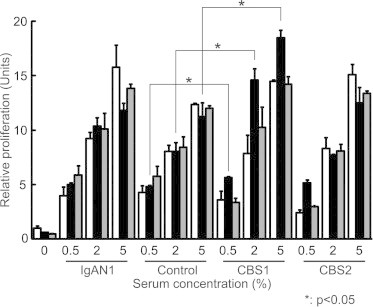
Proliferation of primary human mesangial cells stimulated with serum or cord-blood serum supplemented with Gal-deficient IgA1 myeloma protein. Gal-deficient IgA1 (Mce) myeloma protein was added to serum samples (final concentrations were 0.5%, 2%, and 5% serum; negative control was serum-free, denoted by 0%). Serum samples were from a healthy control (Control), an IgAN patient (IgAN1), and two samples of cord-blood serum (CBS1 and CBS2). IgA final concentrations were 0, 20, and 50 μg/ml of culture medium (marked by white, black, and gray columns, respectively). After 1-h incubation at 4 °C to allow formation of immune complexes, the samples were added with culture medium to serum-starved human mesangial cells, and cellular proliferation was measured after 20-h incubation. Data for cellular proliferation were expressed relative to the proliferation of cells with medium alone, considered 1. Mean and SD are shown (n = 8).
Next, we optimized conditions for the formation of immune complexes using cord-blood serum, based on variation of the factors known to affect antibody–antigen reactions, such as time, temperature, and antigen/antibody ratio. Thus, we varied incubation times (1 h at room temperature vs. overnight at 4 °C) and concentrations of Gal-deficient IgA1 (ranging from 1 to 50 μg/ml). We found that the immune complexes with most stimulatory activity were formed after overnight incubation at 4 °C using cord-blood serum supplemented with 10 μg/ml IgA1 and we used these conditions in further experiments.
In control experiments, cord-blood serum samples not supplemented with IgA1 showed low activity in the proliferation assay. Five different cord-blood serum samples were used; three (CBS1, CBS3, and CBS4) showed formation of stimulatory IgA immune complexes after supplementation with 10 μg/ml IgA1 and overnight incubation. Formation of stimulatory IgA1 immune complexes was verified with other cord-blood sera and the stimulatory effect was dependent on the presence of IgG antibodies binding to Gal-deficient IgA1.
To confirm and extend the experiments with cord-blood serum, we used two different Gal-deficient IgA1 myeloma proteins (Mce and Gou at 3 and 10 μg/ml final concentrations) and added an IgA1 myeloma protein to cord-blood serum (CBS3) or serum from an IgAN patient or a healthy control (2% serum final concentration in the culture medium) to form stimulatory immune complexes. Formation of immune complexes was required to stimulate mesangial cells to proliferate; only cord-blood serum or serum from an IgAN patient supported formation of stimulatory immune complexes (Fig. 2a and b).
Fig. 2.
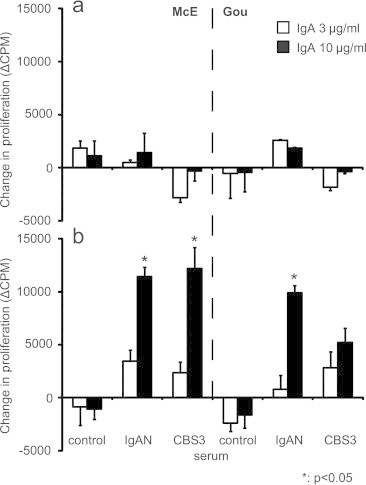
Proliferation of human mesangial cells stimulated with immune complexes formed in vitro using serum samples supplemented with different IgA1 myeloma proteins. Two different Gal-deficient IgA1 myeloma proteins, Mce and Gou (3 and 10 μg/ml final concentration), were added to cord-blood serum (CBS3), or serum from an IgAN patient or a healthy control (2% serum final concentration in the culture medium). After overnight incubation at 4 °C to allow formation of immune complexes, the samples were added to serum-starved human mesangial cells, and cellular proliferation was measured after 20-h incubation. Controls included stimulation with IgA1 proteins alone. Panel (a) shows proliferation with IgA1 proteins without allowing time for immune complex formation in serum samples (no complexes were formed) and panel (b) shows proliferation with IgA1-containing immune complexes. The changes in proliferation are shown as difference in thymidine uptake between a sample and a mock control (mock-treatment with no IgA1 added) (Δcpm). Mean and SD are shown (n = 6).
IgA1 (Gou) myeloma protein has some Gal-deficient O-glycans, as does IgA1 (Mce) myeloma protein. Therefore, cord-blood serum supplemented with either Gal-deficient IgA1 protein formed complexes that stimulated proliferation of mesangial cells (Fig. 2b). In contrast, IgA2 (Fel) myeloma protein without O-glycans did not form any complexes with cord-blood serum and did not influence proliferation of mesangial cells. Based on these results, we concluded that Gal-deficiency of the IgA1 O-glycans was critical for the formation of pathogenic IgA1–IgG complexes.
3.2. In vitro-formed immune complexes resemble those in sera of patients with IgAN
To further characterize the formed IgA1–IgG complexes, we used 80 μg Gal-deficient IgA1 (Mce) myeloma protein (final concentration 10 μg/ml in the culture medium) and 160 μl cord-blood serum (CBS3) and incubated the mixture overnight at 4 °C to allow formation of immune complexes. Cord-blood serum without added IgA1 served as a negative control. To determine the size of immune complexes that induced proliferation of mesangial cells, the samples were fractionated on a calibrated Superose 6 column and the resultant fractions were added to serum-starved mesangial cells. We determined that immune complexes >700 kDa were stimulatory and distributed in two peaks (fractions 32–36 and fractions 38–46) (Fig. 3a). The content of IgA1 and IgG–IgA1 immune complexes in these fractions was determined by ELISA (Fig. 3b and c). The stimulatory fractions contained IgA1–IgG immune complexes.
Fig. 3.
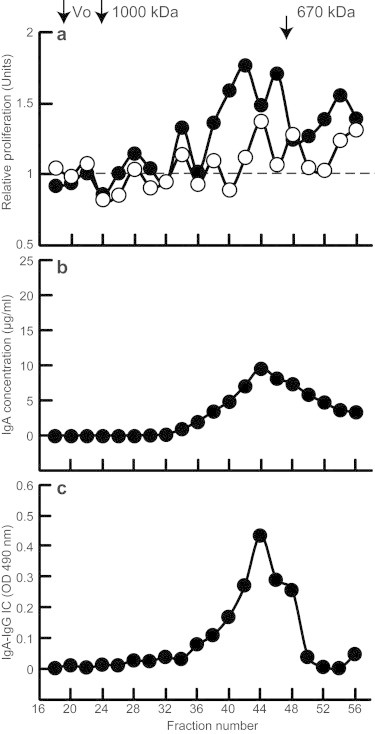
Proliferation of human mesangial cells stimulated with immune complexes formed in vitro using cord-blood serum supplemented with Gal-deficient IgA1 myeloma protein. Gal-deficient IgA1 (Mce) myeloma protein (80 μg, filled circles, corresponding to final concentration of 10 μg/ml culture medium) was added to 160 μl cord-blood serum. After overnight incubation at 4 °C to allow formation of immune complexes, the samples were fractionated on a calibrated Superose 6 column, and the resultant fractions were filter-sterilized and added to serum-starved human mesangial cells. Cellular proliferation was measured after overnight incubation. Panel (a) shows relative cellular proliferation (empty circles represent activity of cord-blood serum without any supplementation), panel (b) shows IgA concentration, and panel (c) shows the amount of IgG–IgA immune complexes in the collected fractions. V0, void volume.
For comparison, we also analyzed, in parallel, immune complexes isolated from serum samples of three patients with IgAN. Native serum samples were fractionated on the same Superose 6 column; resultant fractions were added to serum-starved mesangial cells and cellular proliferation was measured after 20-h incubation. As shown in Fig. 4, sera of each patient contained stimulatory IgA-containing immune complexes, predominantly in the large-molecular-mass fractions >700 kDa (fractions 22–36). In a control experiment, we removed IgA1 from the serum sample by jacalin-affinity chromatography before fractionation; most of the stimulatory activity was lost.
Fig. 4.
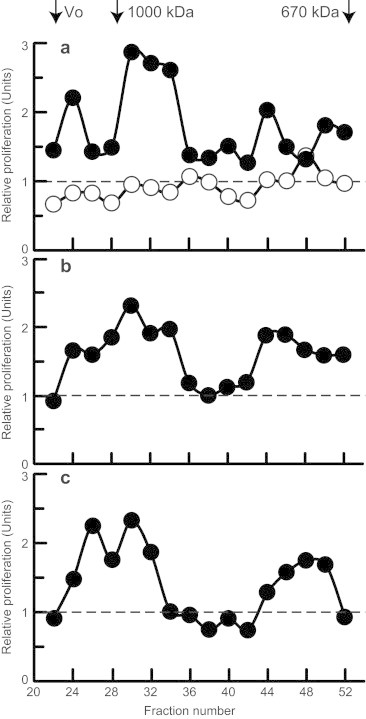
Proliferation of human mesangial cells stimulated with fractions of serum from three patients with IgAN. Native-serum samples from three IgAN patients (filled circles in panels a, b, and c) were fractionated on a calibrated Superose 6 column; the fractions were filter-sterilized and added to serum-starved human mesangial cells. Cellular proliferation was measured after 20-h incubation. IgA-depleted serum sample from one IgAN patient (empty circles in panel a) served as a negative control.
Together, these experiments showed that cord-blood serum contained IgG specific for Gal-deficient IgA1 and that addition of Gal-deficient IgA1 to cord-blood serum led to formation of biologically active immune complexes of large molecular mass. Functionally, these formed complexes were similar to the IgA1-containing complexes in the circulation of IgAN patients.
3.3. Formation of stimulatory IgA1-containing immune complexes requires a heat-sensitive serum factor
To extend our understanding of properties of IgA1-containing immune complexes, we formed IgA1 immune complexes using native or heat-inactivated cord-blood serum (CBS4). The samples were then fractionated by size-exclusion chromatography as before and their biological activities were measured using the proliferation assay. As shown in Fig. 5, the ability of cord-blood serum to form IgA1-containing stimulatory immune complexes decreased significantly after heat inactivation (Fig. 5a). Interestingly, immune complexes were formed with both the native and heat-inactivated serum samples (Fig. 5b and c), but the complexes formed in heat-inactivated serum were of larger molecular mass (Fig. 5c).
Fig. 5.
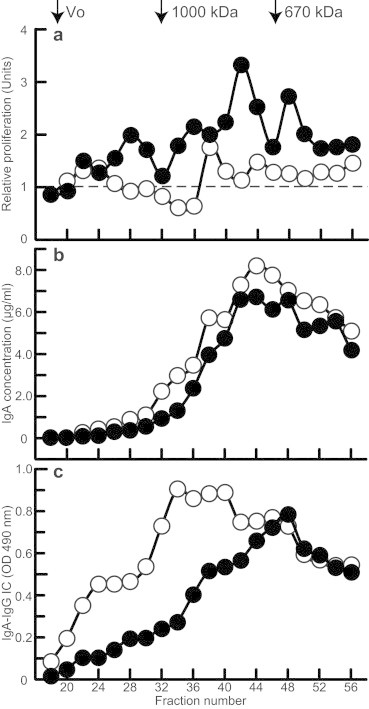
Proliferation of human mesangial cells stimulated with fractions of native or heat-inactivated cord-blood serum supplemented with Gal-deficient IgA1 myeloma protein. Gal-deficient IgA1 (Mce) myeloma protein (80 μg) was added to 160 μl of native (filled circles) or heat-inactivated (empty circles) cord-blood serum. After overnight incubation at 4 °C to allow formation of immune complexes, the samples were fractionated on a calibrated Superose 6 column, and the resultant fractions were filter-sterilized and added to serum-starved human mesangial cells, and cellular proliferation was measured. Panel (a) shows relative cellular proliferation. Panel (b) shows IgA concentration and panel (c) the amount of IgG–IgA complexes in the collected fractions. V0, void volume. Results of one of the two experiments are shown.
In summary, these studies showed that Gal-deficient IgA1, anti-IgA1 antibodies of IgG isotype, and a heat-sensitive serum factor are necessary for in-vitro formation of biologically active immune complexes.
4. Discussion
In this study, we demonstrated that biologically active immune complexes, functionally analogous to those present in sera of patients with IgAN, can be formed in vitro using components not derived from IgAN patients: Gal-deficient IgA1 myeloma proteins with precisely defined glycan structures [29,47,66–68,72] and cord-blood IgG from healthy women as a source of anti-glycan antibodies. Therefore, these results confirm and provide further structural and functional support for the role of immune complexes in sera of IgAN patients in the pathogenesis of their renal disease. As the amounts of immune complexes that can be obtained from sera of IgAN patients are modest, this protocol will facilitate quantitative analytical and structural studies of pathogenic IgA1-containing complexes. Generation of large amounts of such complexes in vitro will allow investigators to define in detail the precise localization of glycan-dependent epitopes in the Gal-deficient IgA1 hinge regions as well as specificities of the corresponding anti-glycan antibodies. The glycan structures of IgA1 myeloma proteins used in this study have been determined [29,44,66,67]. We propose that researchers exploit this information in the design of hinge-region glycopeptide structures to prevent formation of nephritogenic immune complexes [24].
We have shown that anti-glycan antibodies that can bind to Gal-deficient IgA1 are present in sera of healthy controls, but levels are higher in sera from patients with IgAN [44]. We screened cord-blood sera and found that three of the five samples also contained these anti-glycan IgG antibodies. The origin of the glycan-specific antibodies in sera of these apparently healthy mothers remains unclear. However, some viruses and bacteria express N-acetylgalactosamine-containing molecules on their surface in structures comparable to that of the hinge-region O-linked glycans of Gal-deficient IgA1 (for review see [27,44,49,65,73,74]). Accordingly, we speculate that an infection with one of these microorganisms induced production of glycan-specific antibodies that cross-react with Gal-deficient IgA1 [27,44,49,65]. Cord-blood serum contains maternal IgG, but other immunoglobulins are absent or present in only trace amounts. Furthermore, there were no intrinsic immune complexes that stimulated cellular proliferation of the cultured mesangial cells. Therefore, we tested the possibility of in-vitro formation of biologically active IgA1-containing immune complexes, with the overall goal to characterize the conditions necessary for production of stimulatory complexes that mimic the properties of complexes in the circulation of patients with IgAN.
We have defined the conditions that resulted in the formation of IgA1-containing immune complexes that stimulated proliferation of the mesangial cells. Importantly, in-vitro-generated immune complexes that displayed stimulatory activity for cultured human mesangial cells exhibited molecular properties of stimulatory immune complexes present in sera of IgAN patients. When used alone, Gal-deficient IgA1 or purified cord-blood IgG did not stimulate proliferation of mesangial cells. Additional control experiments revealed that purified cord-blood IgG and purified Gal-deficient IgA1 formed immune complexes in the absence of sera, but these complexes were not stimulatory. These experiments thus showed that cord-blood serum was necessary for generation of stimulatory IgA1–IgG immune complexes. However, when the cord-blood serum was heat-inactivated, immune complexes still formed, but did not activate mesangial cells. These results together showed that formation of the IgA1-containing biologically active immune complexes required Gal-deficient IgA1, anti-IgA1 IgG antibody, and a heat-sensitive serum factor. Although we tried to identify this heat-sensitive factor by routine proteomic approaches, the results were inconclusive. Therefore, future experiments will be needed to identify this factor(s). We speculate, based on the heat-sensitivity characteristic, that it may be a complement-regulating protein that affects the size of the formed complexes.
To elucidate the nature of interactions between IgA1–IgG immune complexes and mesangial cells, one would need to know the components of the immune complexes and the identities of receptors on mesangial cells. Mesangial cells do not express CD89 or asialoglycoprotein receptor but do express CD71, a transferrin receptor that binds polymeric IgA1 [25,26,61–63,75,76]. In another study, we used an IgA-specific protease to generate Fab and Fc fragments from polymeric Gal-deficient IgA1 and then, using the digested IgA1, formed immune complexes with native cord-blood serum (Yanagihara et al., unpublished data). Results of these experiments indicated that Fc fragments of IgA1 were present in the stimulatory complexes, thus supporting the role of an IgA1 receptor(s) in the cellular activation, likely in participation with other receptors, such as those for a heat-sensitive serum factor(s).
In summary, these findings provide new tools for studies of the pathogenesis of IgAN and will enable analysis of composition of the pathogenic immune complexes as well as of the signaling pathways induced in human mesangial cells. Such studies will thus have significant implications for the treatment of this common immune-complex renal disease [27,74].
Acknowledgments
This work was supported by the National Institutes of Health Grant nos. DK078244, DK082753, DK083663, and GM098539 and by the Center for Clinical and Translational Sciences of the University of Alabama at Birmingham (No. 1UL1RR025777), and grant no. NT11081 from the Ministry of Health of the Czech Republic.
References
- 1.Berger J., Hinglais N. Les dépôts intercapillaires d’IgA–IgG (Intercapillary deposits of IgA–IgG) Journal of Urology and Nephrology. 1968;74:694–695. [PubMed] [Google Scholar]
- 2.Jennette J.C. The immunohistology of IgA nephropathy. American Journal of Kidney Diseases. 1988;12:348–352. doi: 10.1016/s0272-6386(88)80022-2. [DOI] [PubMed] [Google Scholar]
- 3.Conley M.E., Cooper M.D., Michael A.F. Selective deposition of immunoglobulin A1 in immunoglobulin A nephropathy, anaphylactoid purpura nephritis, and systemic lupus erythematosus. Journal of Clinical Investigation. 1980;66:1432–1436. doi: 10.1172/JCI109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emancipator S.N. IgA nephropathy and Henoch-Schönlein syndrome. In: Jennette J.C., Olson J.L., Schwartz M.M., Silva F.G., editors. Heptinstall's pathology of the kidney. Lippincott-Raven Publishers; Philadelphia: 1998. pp. 479–539. [Google Scholar]
- 5.D’Amico G. Natural history of idiopathic IgA nephropathy: role of clinical and histological prognostic factors. American Journal of Kidney Diseases. 2000;36:227–237. doi: 10.1053/ajkd.2000.8966. [DOI] [PubMed] [Google Scholar]
- 6.Rychlik I., Jancova E., Tesar V., Kolsky A., Lacha J., Stejskal J., Stejskalova A., Dusek J., Herout V. The Czech registry of renal biopsies. Occurrence of renal diseases in the years 1994–2000. Nephrology Dialysis Transplantation. 2004;19:3040–3049. doi: 10.1093/ndt/gfh521. [DOI] [PubMed] [Google Scholar]
- 7.Yoshikawa N., Ito H., Nakamura H. Prognostic indicators in childhood IgA nephropathy. Nephron. 1992;60:60–67. doi: 10.1159/000186706. [DOI] [PubMed] [Google Scholar]
- 8.McCallum D., Smith L., Harley F., Yiu V. IgA nephropathy and thin basement membrane disease in association with Crohn disease. Pediatric Nephrology. 1997;11:637–640. doi: 10.1007/s004670050355. [DOI] [PubMed] [Google Scholar]
- 9.Bene M.C., Hurault De Ligny B., Kessler M., Faure G.C. Confirmation of tonsillar anomalies in IgA nephropathy: a multicenter study. Nephron. 1991;58:425–428. doi: 10.1159/000186474. [DOI] [PubMed] [Google Scholar]
- 10.Emancipator S.N., Lamm M.E. Biology of disease. IgA nephropathy: pathogenesis of the most common form of glomerulonephritis. Laboratory Investigation. 1989;60:168–183. [PubMed] [Google Scholar]
- 11.Emancipator S.N., Mestecky J., Lamm M.E. IgA nephropathy and related diseases. In: Mestecky J., Bienenstock J., Lamm M.E., Mayer L., McGhee J.R., Strober W., editors. Mucosal immunology. 3rd edition. Elsevier Academic Press; Amsterdam: 2005. pp. 1579–1600. [Google Scholar]
- 12.Coppo R., Amore A., Cirina P., Messina M., Basolo B., Segoloni G., Berthoux F., Boulahrouz R., Egido J., Alcazar R., Clarkson A.R., Woodroffe A.J. Characteristics of IgA and macromolecular IgA in sera from IgA nephropathy transplanted patients with and without IgAN recurrence. Contributions to Nephrology. 1995;111:85–92. doi: 10.1159/000423881. [DOI] [PubMed] [Google Scholar]
- 13.Coppo R., Amore A., Roccatello D., Amoroso A., Maffei S., Quattrocchio G., Molino A., Gianoglio B., Piccoli G. Complement receptor (CR1) and IgG or IgA on erythrocytes and in circulating immune complexes in patients with glomerulonephritis. Nephrology Dialysis Transplantation. 1989;4:932–938. doi: 10.1093/ndt/4.11.932. [DOI] [PubMed] [Google Scholar]
- 14.Odum J., Peh C.A., Clarkson A.R., Bannister K.M., Seymour A.E., Gillis D., Thomas A.C., Mathew T.H., Woodroffe A.J. Recurrent mesangial IgA nephritis following renal transplantation. Nephrology Dialysis Transplantation. 1994;9:309–312. [PubMed] [Google Scholar]
- 15.Julian B.A., Said M., Barker C.V. Allograft loss in IgA nephropathy. Journal of the American Society of Nephrology. 1998;9:91A. [Google Scholar]
- 16.Coppo R., Amore A., Cirina P., Messina M., Basolo B., Segoloni G., Berthoux F., Boulharouz R., Egido J., Alcazar R. IgA serology in recurrent and non-recurrent IgA nephropathy after renal transplantation. Nephrology Dialysis Transplantation. 1995;10:2310–2315. doi: 10.1093/ndt/10.12.2310. [DOI] [PubMed] [Google Scholar]
- 17.Berger J. Recurrence of IgA nephropathy in renal allografts. American Journal of Kidney Diseases. 1988;12:371–372. doi: 10.1016/s0272-6386(88)80027-1. [DOI] [PubMed] [Google Scholar]
- 18.Silva F.G., Chander P., Pirani C.L., Hardy M.A. Disappearance of glomerular mesangial IgA deposits after renal allograft transplantation. Transplantation. 1982;33:241–246. [PubMed] [Google Scholar]
- 19.Czerkinsky C., Koopman W.J., Jackson S., Collins J.E., Crago S.S., Schrohenloher R.E., Julian B.A., Galla J.H., Mestecky J. Circulating immune complexes and immunoglobulin A rheumatoid factor in patients with mesangial immunoglobulin A nephropathies. Journal of Clinical Investigation. 1986;77:1931–1938. doi: 10.1172/JCI112522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coppo R., Basolo B., Piccoli G., Mazzucco G., Bulzomi M.R., Roccatello D., De Marchi M., Carbonara A.O., Barbiano di Belgiojoso G. IgA1 and IgA2 immune complexes in primary IgA nephropathy and Henoch–Schönlein nephritis. Clinical and Experimental Immunology. 1984;57:583–590. [PMC free article] [PubMed] [Google Scholar]
- 21.Tomana M., Matousovic K., Julian B.A., Radl J., Konecny K., Mestecky J. Galactose-deficient IgA1 in sera of IgA nephropathy patients is present in complexes with IgG. Kidney International. 1997;52:509–516. doi: 10.1038/ki.1997.361. [DOI] [PubMed] [Google Scholar]
- 22.Schena F.P., Pastore A., Ludovico N., Sinico R.A., Benuzzi S., Montinaro V. Increased serum levels of IgA1-IgG immune complexes and anti-F(ab’)2 antibodies in patients with primary IgA nephropathy. Clinical and Experimental Immunology. 1989;77:15–20. [PMC free article] [PubMed] [Google Scholar]
- 23.Coppo R., Basolo B., Martina G., Rollino C., De Marchi M., Giacchino F., Mazzucco G., Messina M., Piccoli G. Circulating immune complexes containing IgA, IgG and IgM in patients with primary IgA nephropathy and with Henoch–Schönlein nephritis. Correlation with clinical and histologic signs of activity. Clinical Nephrology. 1982;18:230–239. [PubMed] [Google Scholar]
- 24.Tomana M., Novak J., Julian B.A., Matousovic K., Konecny K., Mestecky J. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. Journal of Clinical Investigation. 1999;104:73–81. doi: 10.1172/JCI5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novak J., Vu H.L., Novak L., Julian B.A., Mestecky J., Tomana M. Interactions of human mesangial cells with IgA and IgA-containing circulating immune complexes. Kidney International. 2002;62:465–475. doi: 10.1046/j.1523-1755.2002.00477.x. [DOI] [PubMed] [Google Scholar]
- 26.Novak J., Tomana M., Matousovic K., Brown R., Hall S., Novak L., Julian B.A., Wyatt R.J., Mestecky J. IgA1-containing immune complexes in IgA nephropathy differentially affect proliferation of mesangial cells. Kidney International. 2005;67:504–513. doi: 10.1111/j.1523-1755.2005.67107.x. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki H., Kiryluk K., Novak J., Moldoveanu Z., Herr A.B., Renfrow M.B., Wyatt R.J., Scolari F., Mestecky J., Gharavi A.G., Julian B.A. The pathophysiology of IgA nephropathy. Journal of the American Society of Nephrology. 2011;22:1795–1803. doi: 10.1681/ASN.2011050464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moldoveanu Z., Wyatt R.J., Lee J., Tomana M., Julian B.A., Mestecky J., Huang W.-Q., Anreddy S., Hall S., Hastings M.C., Lau K.K., Cook W.J., Novak J. Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney International. 2007;71:1148–1154. doi: 10.1038/sj.ki.5002185. [DOI] [PubMed] [Google Scholar]
- 29.Moore J.S., Kulhavy R., Tomana M., Moldoveanu Z., Suzuki H., Brown R., Hall S., Kilian M., Poulsen K., Mestecky J., Julian B.A., Novak J. Reactivities of N-acetylgalactosamine-specific lectins with human IgA1 proteins. Molecular Immunology. 2007;44:2598–2604. doi: 10.1016/j.molimm.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Novak J., Moldoveanu Z., Renfrow M.B., Yanagihara T., Suzuki H., Raska M., Hall S., Brown R., Huang W.Q., Goepfert A., Kilian M., Poulsen K., Tomana M., Wyatt R.J., Julian B.A., Mestecky J. IgA nephropathy and Henoch-Schoenlein purpura nephritis: aberrant glycosylation of IgA1, formation of IgA1-containing immune complexes, and activation of mesangial cells. Contributions to Nephrology. 2007;157:134–138. doi: 10.1159/000102455. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki H., Moldoveanu Z., Hall S., Brown R., Vu H.L., Novak L., Julian B.A., Tomana M., Wyatt R.J., Edberg J.E., Alarcón G.S., Kimberly R.P., Tomino Y., Mestecky J., Novak J. IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1. Journal of Clinical Investigation. 2008;118:629–639. doi: 10.1172/JCI33189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gharavi A.G., Moldoveanu Z., Wyatt R.J., Barker C.V., Woodford S.Y., Lifton R.P., Mestecky J., Novak J., Julian B.A. Aberrant IgA1 glycosylation is inherited in familial and sporadic IgA nephropathy. Journal of the American Society of Nephrology. 2008;19:1008–1014. doi: 10.1681/ASN.2007091052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mestecky J., Suzuki H., Yanagihara T., Moldoveanu Z., Tomana M., Matousovic K., Julian B.A., Novak J. IgA nephropathy: current views of immune complex formation. Contributions to Nephrology. 2007;157:56–63. doi: 10.1159/000102305. [DOI] [PubMed] [Google Scholar]
- 34.Allen A.C., Bailey E.M., Brenchley P.E.C., Buck K.S., Barratt J., Feehally J. Mesangial IgA1 in IgA nephropathy exhibits aberrant O-glycosylation: observations in three patients. Kidney International. 2001;60:969–973. doi: 10.1046/j.1523-1755.2001.060003969.x. [DOI] [PubMed] [Google Scholar]
- 35.Hiki Y., Odani H., Takahashi M., Yasuda Y., Nishimoto A., Iwase H., Shinzato T., Kobayashi Y., Maeda K. Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney International. 2001;59:1077–1085. doi: 10.1046/j.1523-1755.2001.0590031077.x. [DOI] [PubMed] [Google Scholar]
- 36.Allen A.C., Willis F.R., Beattie T.J., Feehally J. Abnormal IgA glycosylation in Henoch–Schönlein purpura restricted to patients with clinical nephritis. Nephrology Dialysis Transplantation. 1998;13:930–934. doi: 10.1093/ndt/13.4.930. [DOI] [PubMed] [Google Scholar]
- 37.Lau K.L., Wyatt R.J., Moldoveanu Z., Tomana M., Julian B.J., Hogg R.J., Lee J.Y., Huang W.-Q., Mestecky J., Novak J. Serum levels of galactose-deficient IgA in children with IgA nephropathy and Henoch–Schoenlein purpura. Pediatric Nephrology. 2007;22:2067–2072. doi: 10.1007/s00467-007-0623-y. [DOI] [PubMed] [Google Scholar]
- 38.Levinsky R.J., Barratt T.M. IgA immune complexes in Henoch–Schönlein purpura. Lancet. 1979;2:1100–1103. doi: 10.1016/s0140-6736(79)92505-4. [DOI] [PubMed] [Google Scholar]
- 39.van der Helm-van Mil A.H.M., Smith A.C., Pouria S., Tarelli E., Brunskill N.J., Eikenboom H.C. Immunoglobulin A multiple myeloma presenting with Henoch–Schönlein purpura associated with reduced sialylation of IgA1. British Journal of Haematology. 2003;122:915–917. doi: 10.1046/j.1365-2141.2003.04539.x. [DOI] [PubMed] [Google Scholar]
- 40.Zickerman A.M., Allen A.C., Talwar V., Olczak S.A., Brownlee A., Holland M., Furness P.N., Brunskill N.J., Feehally J. IgA myeloma presenting as Henoch–Schönlein purpura with nephritis. American Journal of Kidney Diseases. 2000;36:E19. doi: 10.1053/ajkd.2000.16221. [DOI] [PubMed] [Google Scholar]
- 41.Kokubo T., Hiki Y., Iwase H., Tanaka A., Nishikido J., Hotta K., Kobayashi Y. Exposed peptide core of IgA1 hinge region in IgA nephropathy. Nephrology Dialysis Transplantation. 1999;14:81–85. doi: 10.1093/ndt/14.1.81. [DOI] [PubMed] [Google Scholar]
- 42.Kokubo T., Hashizume K., Iwase H., Arai K., Tanaka A., Toma K., Hotta K., Kobayashi Y. Humoral immunity against the proline-rich peptide epitope of the IgA1 hinge region in IgA nephropathy. Nephrology Dialysis Transplantation. 2000;15:28–33. doi: 10.1093/ndt/15.1.28. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki H., Moldoveanu Z., Hall S., Brown R., Julian B.A., Wyatt R.J., Tomana M., Tomino Y., Novak J., Mestecky J. IgA nephropathy: characterization of IgG antibodies specific for galactose-deficient IgA1. Contributions to Nephrology. 2007;157:129–133. doi: 10.1159/000102454. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki H., Fun R., Zhang Z., Brown R., Hall S., Julian B.A., Chatham W.W., Suzuki Y., Wyatt R.J., Moldoveanu Z., Lee J.Y., Robinson J., Tomana M., Tomino Y., Mestecky J., Novak J. Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. Journal of Clinical Investigation. 2009;119:1668–1677. doi: 10.1172/JCI38468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haakenstad A.O., Mannik M. The biology of immune complexes. In: Talal N., editor. Autoimmunity. Genetic, immunologic, virologic, and clinical aspects. Academic Press; New York: 1977. pp. 277–360. [Google Scholar]
- 46.Mestecky J., Hashim O.H., Tomana M. Alterations in the IgA carbohydrate chains influence the cellular distribution of IgA1. Contributions to Nephrology. 1995;111:66–72. doi: 10.1159/000423879. [DOI] [PubMed] [Google Scholar]
- 47.Novak J., Tomana M., Kilian M., Coward L., Kulhavy R., Barnes S., Mestecky J. Heterogeneity of O-glycosylation in the hinge region of human IgA1. Molecular Immunology. 2000;37:1047–1056. doi: 10.1016/s0161-5890(01)00019-0. [DOI] [PubMed] [Google Scholar]
- 48.Julian B.A., Novak J. IgA nephropathy: an update. Current Opinion in Nephrology and Hypertension. 2004;13:171–179. doi: 10.1097/00041552-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Novak J., Julian B.A., Tomana M., Mestecky J. IgA glycosylation and IgA immune complexes in the pathogenesis of IgA nephropathy. Seminars in Nephrology. 2008;28:78–87. doi: 10.1016/j.semnephrol.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mestecky J., Tomana M., Moldoveanu Z., Julian B.A., Suzuki H., Matousovic K., Renfrow M.B., Novak L., Wyatt R.J., Novak J. The role of aberrant glycosylation of IgA1 molecules in the pathogenesis of IgA nephropathy. Kidney and Blood Pressure Research. 2008;31:29–37. doi: 10.1159/000112922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mestecky J., Moro I., Kerr M.A., Woof J.M. Mucosal immunoglobulins. In: Mestecky J., Bienenstock J., Lamm M.E., Mayer L., McGhee J.R., Strober W., editors. Mucosal immunology. 3rd edition. Elsevier Academic Press; Amsterdam: 2005. pp. 153–181. [Google Scholar]
- 52.Chen A., Chen W.P., Sheu L.F., Lin C.Y. Pathogenesis of IgA nephropathy: in vitro activation of human mesangial cells by IgA immune complex leads to cytokine secretion. Journal of Pathology. 1994;173:119–126. doi: 10.1002/path.1711730208. [DOI] [PubMed] [Google Scholar]
- 53.Chen A., Ding S.L., Sheu L.F., Song Y.B., Shieh S.D., Shaio M.F., Chou W.Y., Ho Y.S. Experimental IgA nephropathy. Enhanced deposition of glomerular IgA immune complex in proteinuric states. Laboratory Investigation. 1994;70:639–647. [PubMed] [Google Scholar]
- 54.Amore A., Cirina P., Conti G., Brusa P., Peruzzi L., Coppo R. Glycosylation of circulating IgA in patients with IgA nephropathy modulates proliferation and apoptosis of mesangial cells. Journal of the American Society of Nephrology. 2001;12:1862–1871. doi: 10.1681/ASN.V1291862. [DOI] [PubMed] [Google Scholar]
- 55.Gomez-Guerrero C., Gonzales E., Egido J. Evidence for a specific IgA receptor in rat and human mesangial cells. Journal of Immunology. 1993;151:7172–7181. [PubMed] [Google Scholar]
- 56.Gomez-Guerrero C., Gonzalez E., Hernando P., Ruiz-Ortega M., Egido J. Interaction of mesangial cells with IgA and IgG immune complexes: a possible mechanism of glomerular injury in IgA nephropathy. Contributions to Nephrology. 1993;104:127–137. doi: 10.1159/000422405. [DOI] [PubMed] [Google Scholar]
- 57.Gomez-Guerrero C., Lopez-Armada M.J., Gonzalez E., Egido J. Soluble IgA and IgG aggregates are catabolized by cultured rat mesangial cells and induce production of TNF-α and IL-6, and proliferation. Journal of Immunology. 1994;153:5247–5255. [PubMed] [Google Scholar]
- 58.Gomez-Guerrero C., Alonso J., Lopez-Armada M.J., Ruiz-Ortega M., Gomez-Garre D., Alcazar R., Gonzalez E., Egido J. Potential factors governing extracellular matrix production by mesangial cells: their relevance for the pathogenesis of IgA nephropathy. Contributions to Nephrology. 1995;111:45–54. doi: 10.1159/000423876. [DOI] [PubMed] [Google Scholar]
- 59.Lopez-Armada M.J., Gomez-Guerrero C., Egido J. Receptors for immune complexes activate gene expression and synthesis of matrix proteins in cultured rat and human mesangial cells: role of TGF-β. Journal of Immunology. 1996;157:2136–2142. [PubMed] [Google Scholar]
- 60.Duque N., Gomez-Guerrero C., Egido J. Interaction of IgA with Fcα receptors of human mesangial cells activates transcription factor nuclear factor-κB and induces expression and synthesis of monocyte chemoattractant protein-1, IL-8, and IFN-inducible protein 10. Journal of Immunology. 1997;159:3474–3482. [PubMed] [Google Scholar]
- 61.Moura I.C., Arcos-Fajardo M., Gdoura A., Leroy V., Sadaka C., Mahlaoui N., Lepelletier Y., Vrtovsnik F., Haddad E., Benhamou M., Monteiro R.C. Engagement of transferrin receptor by polymeric IgA1: evidence for a positive feedback loop involving increased receptor expression and mesangial cell proliferation in IgA nephropathy. Journal of the American Society of Nephrology. 2005;16:2667–2676. doi: 10.1681/ASN.2004111006. [DOI] [PubMed] [Google Scholar]
- 62.Moura I.C., Arcos-Fajardo M., Sadaka C., Leroy V., Benhamou M., Novak J., Vrtovsnik F., Haddad E., Chintalacharuvu K.R., Monteiro R.C. Glycosylation and size of IgA1 are essential for interaction with mesangial transferrin receptor in IgA nephropathy. Journal of the American Society of Nephrology. 2004;15:622–634. doi: 10.1097/01.asn.0000115401.07980.0c. [DOI] [PubMed] [Google Scholar]
- 63.Moura I.C., Centelles M.N., Arcos-Fajardo M., Malheiros D.M., Collawn J.F., Cooper M.D., Monteiro R.C. Identification of the transferrin receptor as a novel immunoglobulin (Ig)A1 receptor and its enhanced expression on mesangial cells in IgA nephropathy. Journal of Experimental Medicine. 2001;194:417–425. doi: 10.1084/jem.194.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tam K.Y., Leung J.C., Chan L.Y., Lam M.F., Tang S.C., Lai K.N. Macromolecular IgA1 taken from patients with familial IgA nephropathy or their asymptomatic relatives have higher reactivity to mesangial cells in vitro. Kidney International. 2009;75:1330–1339. doi: 10.1038/ki.2009.71. [DOI] [PubMed] [Google Scholar]
- 65.Novak J., Mestecky J. IgA Immune-complex. In: Lai K.N., editor. Recent advances in IgA nephropathy. Imperial College Press and the World Scientific Publisher; Hong Kong: 2009. pp. 177–191. [Google Scholar]
- 66.Renfrow M.B., Cooper H.J., Tomana M., Kulhavy R., Hiki Y., Toma K., Emmett M.R., Mestecky J., Marshall A.G., Novak J. Determination of aberrant O-glycosylation in the IgA1 hinge region by electron capture dissociation Fourier transform-ion cyclotron resonance mass spectrometry. Journal of Biological Chemistry. 2005;280:19136–19145. doi: 10.1074/jbc.M411368200. [DOI] [PubMed] [Google Scholar]
- 67.Renfrow M.B., MacKay C.L., Chalmers M.J., Julian B.A., Mestecky J., Kilian M., Poulsen K., Emmett M.R., Marshall A.G., Novak J. Analysis of O-glycan heterogeneity in IgA1 myeloma proteins by Fourier transform ion cyclotron resonance mass spectrometry: implications for IgA nephropathy. Analytical and Bioanalytical Chemistry. 2007;389:1397–1407. doi: 10.1007/s00216-007-1500-z. [DOI] [PubMed] [Google Scholar]
- 68.Takahashi K., Wall S.B., Suzuki H., Smith A.D., Hall S., Poulsen K., Kilian M., Mobley J.A., Julian B.A., Mestecky J., Novak J., Renfrow M.B. Clustered O-glycans of IgA1: defining macro- and micro-heterogeneity by use of electron capture/transfer dissociation. Molecular and Cellular Proteomics. 2010;9:2545–2557. doi: 10.1074/mcp.M110.001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tomana M., Phillips J.O., Kulhavy R., Mestecky J. Carbohydrate-mediated clearance of secretory IgA from the circulation. Molecular Immunology. 1985;22:887–892. doi: 10.1016/0161-5890(85)90074-4. [DOI] [PubMed] [Google Scholar]
- 70.Novak J., Raskova Kafkova L., Suzuki H., Tomana M., Matousovic K., Brown R., Hall S., Sanders J.T., Eison T.M., Moldoveanu Z., Novak L., Novak Z., Mayne R., Julian B.A., Mestecky J., Wyatt R.J. IgA1 immune complexes from pediatric patients with IgA nephropathy activate cultured mesangial cells. Nephrology Dialysis Transplantation. 2011;26:3451–3457. doi: 10.1093/ndt/gfr448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matousovic K., Novak J., Tomana M., Kulhavy R., Julian B.A., Mestecky J. IgA1-containing immune complexes in the urine of IgA nephropathy patients. Nephrology Dialysis Transplantation. 2006;21:2478–2484. doi: 10.1093/ndt/gfl240. [DOI] [PubMed] [Google Scholar]
- 72.Gomes M.M., Suzuki H., Brooks M.T., Tomana M., Moldoveanu Z., Mestecky J., Julian B.A., Novak J., Herr A.B. Recognition of galactose-deficient O-glycans in the hinge region of IgA1 by N-acetylgalactosamine-specific snail lectins: a comparative binding study. Biochemistry. 2010;49:5671–5682. doi: 10.1021/bi9019498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Novak J., Moldoveanu Z., Julian B.A., Raska M., Wyatt R.J., Suzuki Y., Tomino Y., Gharavi A.G., Mestecky J., Suzuki H. Aberrant glycosylation of IgA1 and anti-glycan antibodies in IgA nephropathy: role of mucosal immune system. Advances in Otolaryngology. 2011;72:60–63. doi: 10.1159/000324607. [DOI] [PubMed] [Google Scholar]
- 74.Novak J., Julian B.A., Mestecky J., Renfrow M.B. Glycosylation of IgA1 and pathogenesis of IgA nephropathy. Seminars in Immunology. 2012;34:365–382. doi: 10.1007/s00281-012-0306-z. [DOI] [PubMed] [Google Scholar]
- 75.Leung J.C.K., Tsang A.W.L., Chan D.T.M., Lai K.N. Absence of CD89, polymeric immunoglobulin receptor, and asialoglycoprotein receptor on human mesangial cells. Journal of the American Society of Nephrology. 2000;11:241–249. doi: 10.1681/ASN.V112241. [DOI] [PubMed] [Google Scholar]
- 76.Leung J.C., Tsang A.W., Chan L.Y., Tang S.C., Lam M.F., Lai K.N. Size-dependent binding of IgA to HepG2, U937, and human mesangial cells. Journal of Laboratory and Clinical Medicine. 2002;140:398–406. doi: 10.1067/mlc.2002.129338. [DOI] [PubMed] [Google Scholar]


