Abstract
Adipose tissue is formed at stereotypic times and locations in a diverse array of organisms. Once formed, the tissue is dynamic, responding to homeostatic and external cues and capable of a 15-fold expansion. The formation and maintenance of adipose tissue is essential to many biological processes and when perturbed leads to significant diseases. Despite this basic and clinical significance, understanding of the developmental biology of adipose tissue has languished. In this Review, we highlight recent efforts to unveil adipose developmental cues, adipose stem cell biology and the regulators of adipose tissue homeostasis and dynamism.
Keywords: Adipose, Adipocyte, Development, Stem cells, Niche, Vascular niche
Introduction: fat facts
Adipose tissue plays a myriad of roles; it serves as a central nexus of metabolic communication and control, an arbiter of thermoregulation, a buffer against trauma and the cold, and a regulator of reproduction and satiety. Fat is also associated with emotionally charged issues, imparting various psychosocial imprints that have changed over the centuries: from a sign of wealth in the middle ages, to a Rubenesque celebration during the Renaissance, to fear and loathing in modern Hollywood (unless of course it is injected into the lips or other ‘cosmetically desirable’ locations). Despite the current social aversion to fat, the increase in the percentage of people who are overweight has been marked and is observed in nearly all regions of the world. The global prevalence of obesity is ∼15% and in the United States more than two thirds of the population is overweight (Hossain et al., 2007). The health care system is reeling not only from an obesity epidemic, but also from a host of obesity-related negative sequelae including diabetes, cardiovascular disease, cirrhosis and even cancer. The reasons for this increase are well known - increased food consumption and decreased exercise - but this is only because the body is predisposed to fat accumulation.
What is also becoming clear is that a variety of evolutionary and developmental forces have laid the foundation for the development of obesity. Both invertebrates and vertebrates have fat-storing tissues and many aspects of the mechanistic underpinnings are conserved. These include the developmental programs, transcriptional cascades and basic proteins that regulate fat synthesis, storage and lipolysis (McKay et al., 2003; Suh et al., 2006; Suh et al., 2008; Suh et al., 2007). It appears that fat tissues evolved primarily as a safe harbor to store energy in times of plenty and to provide fuel when food sources become insufficient. However, in addition to serving as purely a storage depot, adipose tissue is now recognized as the body’s largest endocrine organ, controlling many aspects of systemic physiology by secreting hormones (adipokines), lipids, cytokines and other factors (Gesta et al., 2007; Nawrocki and Scherer, 2004; Spiegelman and Flier, 2001). Although many of the regulatory molecules are not yet identified, they control a wide variety of biological actions, including appetite, glucose homeostasis, insulin sensitivity, aging, fertility and fecundity, and body temperature.
In mammals, adipose tissue forms in utero, in the peripartum period and throughout life. Notably, even in adult humans new adipocytes are generated continually and at substantial rates (Spalding et al., 2008). Adipose tissue is composed of adipose stem cells (the precursor cells that give rise to new adipocytes), adipocytes (the fat-storing cells) and various other cell types, which include mural, endothelial and neuronal cells. Adipose tissue comprises various discrete depots, such as inguinal, interscapular, perigonadal, retroperitoneal and mesenteric depots, which are placed in defined positions throughout the body. These various depots develop at specific and distinguishable pre- and postnatal times and they have discrete and distinct morphologies (Gregoire et al., 1998; Hossain et al., 2007; MacDougald and Mandrup, 2002). These observations support the notion that developmental cues are pivotal for the proper formation, migration and morphology of adipose tissue. Yet relatively little is known about adipocyte development because adipocytes are spread throughout the body, because of inherent difficulties in handling the enormous and fragile adipose cells, and because of historic reliance on cell culture models.
However, a new horizon is in sight; developmental tools are beginning to be utilized and interest in adipose tissue development is expanding. This recent tectonic shift towards a developmental field has begun to improve our understanding of adipose developmental biology and has positioned the field for extensive advancement. This is timely, as the obesity crisis has increased the demand for new therapeutic strategies for adipose-related illnesses. Thus, there is an urgent need to understand better the mechanisms and molecules that control the formation of adipocytes and the expansion of adipose tissue due to caloric excess. Here, we review the recent advancements in adipose tissue development, the discovery and regulation of adipose stem cells, adipocyte turnover, and potential therapeutic interventions for those suffering from obesity and diabetes.
Brown and white fat: flavors of the month
In mammals, there are two main classes of adipose tissue that are separated into major histological divisions, are molecularly distinct and are functionally distinguishable. White adipose tissue (WAT) is designed for energy storage whereas brown adipose tissue (BAT) dissipates energy and generates heat. WAT is far more prevalent and more biologically relevant, whereas BAT primarily functions in the neonatal period, although recent reports have shown BAT to be present and have functional roles in adults (Cypess et al., 2013; Lidell et al., 2013). Adipose tissue is also present in a variety of locations, such as palms, soles, scalp, periarticular regions and orbits, that are thought to have so called mechanical roles (Gesta et al., 2007). In addition, adipocytes are positioned throughout the skin and bone marrow, and recent evidence indicates that in those locations adipocytes regulate stem cell biology, for example by controlling epidermal and hematopoietic lineage decisions.
White adipose tissue: the great white whale
White adipose tissues coordinate systemic metabolism. This key role is highlighted by the metabolic dysfunctions (e.g. hyperglycemia, hyperlipidemia, hypertension, diabetes, liver disease, increased carcinogenesis, etc.) that are a consequence of too many (obesity) or too few (lipodystrophy) white adipocytes. There are two main divisions of white adipose tissue: subcutaneous and visceral. Subcutaneous and visceral depots are simply separated based upon gross anatomical location (Fig. 1A,B). Yet this gross placement appears to define a variety of essential features, including developmental timing, microscopic appearance, molecular signature and biological function. In rodents, subcutaneous depots, such as interscapular and inguinal, appear to form prior to visceral depots, which include retroperitoneal, perigonadal and mesenteric WAT (Fig. 1C,D). Each adipose depot has a specific, distinct and reproducible morphology and texture. Histologically, subcutaneous fat is heterogeneous and contains mature unilocular adipocytes intercalated with small multilocular adipocytes, whereas visceral fat is more uniform and appears to consist primarily of large unilocular adipocytes (Fig. 2A,B) (Tchernof et al., 2006; Tchkonia et al., 2007). Of note, retroperitoneal adipose depots, which are placed within the visceral class, have features that may be intermediate between subcutaneous and visceral depots. Current thinking indicates that subcutaneous and visceral depots are likely to have different contributions to metabolism, with inherent health ramifications. Increased subcutaneous fat deposition, sometimes called a pear-shaped or female pattern of distribution, might protect against certain aspects of metabolic dysfunction (Snijder et al., 2003a; Snijder et al., 2003b). However, visceral depots, also known as an apple or male pattern, are thought to be associated with metabolic complications and appear to increase the risk of diabetes, hyperlipidemia and cardiovascular disease (Grauer et al., 1984). Because of these still ill-defined associations, it has become popular to term subcutaneous adipose as ‘good fat’ and visceral as ‘bad fat’.
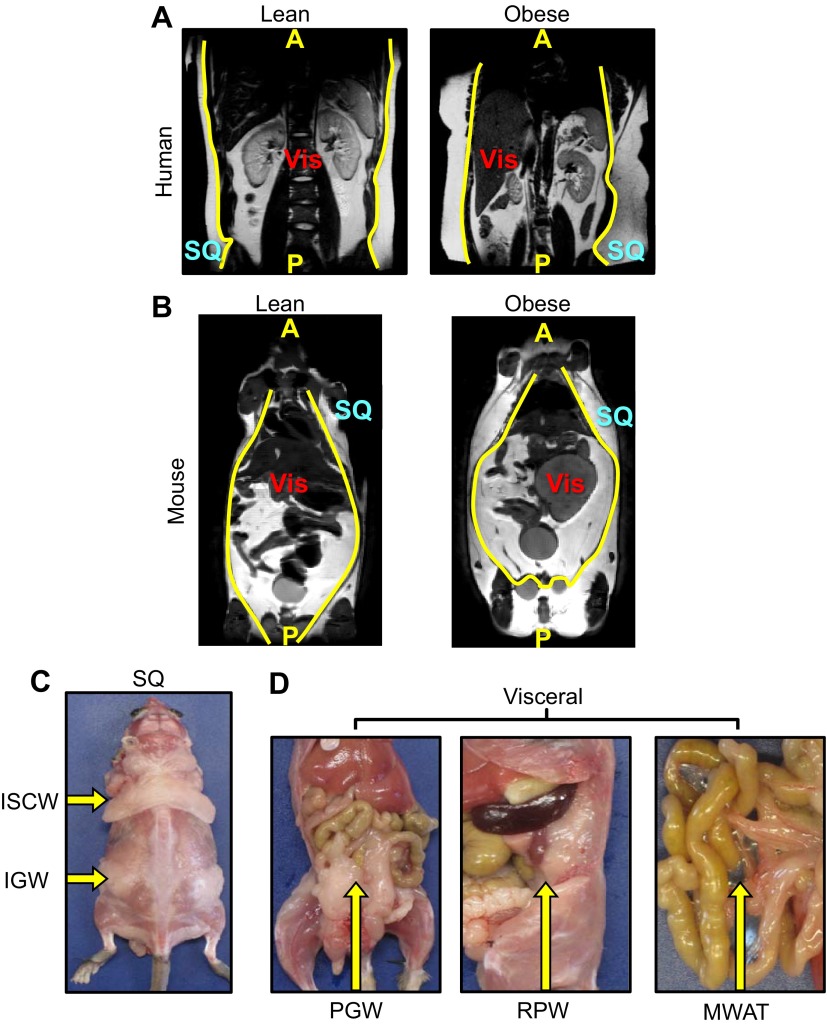
Fig. 1.
The anatomical distributions of fat. (A,B) Coronal MRI of a lean (left) and an obese (right) human (A) and mouse (B); yellow lines delineate subcutaneous and visceral depots. A, anterior; P, posterior; SQ, subcutaneous; Vis, visceral. (C,D) Physical orientation, distribution and location of subcutaneous and visceral depots. Subcutaneous fat (C) is located in the inguinal (IGW) and interscapular (ISCW) regions. Visceral fat (D) is positioned at perigonadal (PGW), retroperitoneal (RPW) and mesenteric (MWAT) regions of the body. Yellow arrows indicate the denoted depot location within the body of the mouse.
Fig. 2.
Heterogeneity of fat. (A-C) Adipose depots are positioned at stereotypic times and locations with defined sizes; shown are subcutaneous (SQ) fat depots (A), perigonadal (visceral) fat depots (B) and intrascapular brown adipose tissue (C) from three unrelated mice. Histologically, white adipose depots differ in adipocyte size; subcutaneous depots are more heterogeneous, containing both uni- and multilocular adipocytes and interstitial tissue, whereas visceral depots are more uniform and contain mostly unilocular adipocytes. Morphologically, brown adipose tissue is very distinct from white adipose tissue with uniformity of multilocular lipid droplets.
What might account for these associations? It is plausible that the aforementioned histological differences may be at play. Subcutaneous depots contain an abundance of small multilocular adipocytes, which might be protective against metabolic derangement (Salans et al., 1973; Weyer et al., 2000). In addition, there is an increase in interstitial tissue in subcutaneous depots (Fig. 2A). In conjunction with histological differences, subcutaneous depots have increased rates of adipose turnover and new adipocyte formation, and it is postulated that ‘younger’ adipocytes do not confer metabolic dysfunction (Björntorp et al., 1971; Salans et al., 1973). Lipolytic rates also appear to be different between the two depots (Fisher et al., 2002). Other factors that might account for the differences include blood flow and proximity to metabolically important visceral organs, such as the liver, which produces glucose, lipids and cholesterols, and the intestine where food is absorbed. It is also known that aspects of regional fat deposition are inherited; for instance, it is widely appreciated that the propensity to accumulate subcutaneous versus visceral fat runs within families (Shi and Clegg, 2009). That is, one can only control whether one becomes fat, not where one puts it.
Subcutaneous and visceral adipose depots also respond to external stimuli in different ways. For example, in humans and mice, steroid hormones confer depot-specific consequences (Shi and Clegg, 2009); estrogen is thought to increase subcutaneous depots, whereas adipose tissues within the neck, or within the viscera, are more responsive to glucocorticoids. Furthermore, subcutaneous and visceral depots display unique and distinguishable responses to thiazolidinediones (TZDs), a class of drugs widely prescribed for diabetes (Tontonoz and Spiegelman, 2008). Additional support for complexity within the lineages derives from the clinical observation that lipodystrophy (pathological absence of fat) preferentially affects different adipose locations. For example, some patients with an inherited lipodystrophy lack all metabolic fat but retain normal amounts of mechanical fat (Herbst, 2012).
Transplantation methodologies have been employed in an attempt to probe whether the functional differences between visceral and subcutaneous depots might be intrinsic (autonomous) or extrinsic (non-autonomous). However, these studies have not yet produced a definitive answer, with some indicating inherent differences and others suggesting that these functional attributes alter when locations change. For example, Kahn and colleagues performed an elegant series of murine reciprocal transplants coupled with extensive metabolic analyses (Tran et al., 2008). The studies indicated that transplantation of subcutaneous depots into visceral locations improved metabolic parameters, whereas transplantation of visceral depots into subcutaneous locations did not (Tran et al., 2008). In addition to metabolic and morphological changes, a striking rearrangement in gene expression towards a subcutaneous signature occurred within three weeks of transplantation (Satoor et al., 2011). Thus, there appear to be both intrinsic and extrinsic forces that regulate adipose depot biology. The factors and relative contributions of each are likely to have fundamental and clinical significance and are areas of future exploration.
A variety of studies support the notion that distinct and definable molecular programs might underlie the functional differences between subcutaneous and visceral adipose tissues (Gesta et al., 2007). For example, gene expression profiling of adipose tissues in rodents and humans demonstrated divergent and discrete molecular signatures between the various depot locations. Notably, the pattern of gene expression, even within a depot, changes depending on adipocyte size (Jernås et al., 2006). Subcutaneous fat depots tend to express higher levels of leptin, angiotensinogen and glycogen synthase compared with visceral fat. Omental fat, part of the visceral compartment, expresses increased levels of the insulin receptor, 11β hydroxysteroid dehydrogenase (11β HSD) and interleukin 6 (IL6) (Gesta et al., 2007; Masuzaki et al., 2001). Large-scale gene expression analyses comparing rodent subcutaneous and visceral adipose progenitor signatures also identified a host of differentially expressed transcripts (Gesta et al., 2006). These include a group of developmental regulators such as homeobox (HOX) and forkhead box (FOX) family transcription factors (Gesta et al., 2006; Macotela et al., 2012). Moreover, the gene trends that were detected in mice were also observed in human adipose samples (Gesta et al., 2006). Additional data suggest that a subset of these genes correlate with body mass index (BMI) and by extension relate to metabolic dysfunction and cardiovascular disease (Gesta et al., 2006). The role of these genes in fat formation and regulation remains unclear. However, many of the identified genes are involved in developmental patterning, and it is possible that they might regulate aspects of adipose tissue formation. Studies on developmental signaling cascades have also highlighted the notion that developmental forces are important to adipose development and to regional distinctions. This can be illustrated by the WNT pathway, which regulates adipose tissue formation in a spatially significant manner (Longo et al., 2004; Zeve et al., 2012). In relation to gene changes, fat-storing cells derived from subcutaneous progenitors accumulate more lipid and express higher levels of PPARγ and C/EBPα upon differentiation compared with visceral progenitor cells (Baglioni et al., 2012; Tchkonia et al., 2002). Subcutaneous progenitor cells also have improved growth rate and different electrophysiological properties (Baglioni et al., 2012; Macotela et al., 2012). Collectively, there appear to be intrinsic genetic depot-specific differences in adipose stem cells that result in different adipogenic potential, genetic expression patterns, growth rates and biological properties. Thus, these studies support the notion that distinct developmental cues are likely to determine the complexity of the adipose lineage and that all fat stem cells and adipocytes are not created equally.
Brown adipose tissue: burning down the house
Brown adipocytes convert nutrients into chemical energy in the form of heat (Kajimura et al., 2010). The primary role of BAT appears to be in the neonatal period when temperature control is challenged (Gregoire et al., 1998; MacDougald and Mandrup, 2002; Spiegelman and Flier, 2001). Brown fat cells express a unique thermogenic and mitochondrial genetic program that promotes mitochondrial biogenesis, energy uncoupling and energy dissipation, which provides essential heat to the organism (Kajimura et al., 2010). Energy dissipation is accomplished by an abundance of mitochondria, hence the eponymous color of BAT, as well as by specialized proteins, including uncoupling protein 1 (UCP1). UCP1 collapses the electron gradient to generate heat rather than ATP (Cannon and Nedergaard, 2004). Similar to WAT, BAT is present at several stereotypic places and can be increased or decreased by environmental cues (Fig. 2C). However, the brown adipocytes that are present during neonatal life appear distinct from those that are present, or can be induced, in adults (Wu et al., 2012).
In the neonatal period, the most prominent BAT depot is located at the dorsal region between the scapulae (Cannon and Nedergaard, 2004). The cells in the interscapular region of the body are derived from the dermomytome, an unusual tripotent engrailed 1 (EN1)-positive cell lineage, which gives rise to brown fat bundles, the dermis and muscle cells (Atit et al., 2006). Studies also indicate that this specialized region of the body descends from a myogenic factor 5 (MYF5)-positive source, but it is clear that only a subset of brown adipocytes do so. Interestingly, recent data indicate that a subset of white adipocytes also arises from MYF5-positive cells (Sanchez-Gurmaches et al., 2012), and additional studies show that brown and white fat progenitor cells share commonality. Granneman and colleagues demonstrated that a platelet-derived growth factor receptor alpha (PDGFRα)-positive lineage traces to brown and white adipose tissue and that PDGFRα is present in proliferating brown and white adipose progenitors (Lee et al., 2012).
Brown adipocytes were once thought to be absent in adult humans; however, recent data indicate that some adults have energy-burning adipocytes with ‘brown-like’ characteristics, and promotion of their formation or maintenance has potential as an anti-obesity avenue (Cypess et al., 2009; Cypess et al., 2013; Lidell et al., 2013; Whittle et al., 2011). In small-scale studies, it appears that obesity reduces the appearance of brown-like cells and, because of the therapeutic possibilities, a variety of studies have been directed towards elucidating the molecular cascades and factors underlying brown fat formation, with the aim of triggering cells to have BAT-like energy-consuming actions (Cannon and Nedergaard, 2004; Kajimura et al., 2010; Seale et al., 2008; Seale et al., 2009). Many initial studies examined possible conservation of function between the white and brown lineages, and revealed that aspects of the overlapping core transcriptional machinery play related roles in the two lineages (Kajimura et al., 2010). In addition, various enzymes involved in lipogenesis and lipolysis are expressed in both types of adipocytes. However, it also appears that brown fat formation has unique aspects. For instance, bone morphogenetic protein 7 (BMP7) can induce cultured cells to undergo a brown adipocyte differentiation response (Tseng et al., 2008). In vivo studies also highlight the potential role of BMP7 in the formation or maintenance of BAT; Bmp7 null embryos have less brown fat compared with controls and virally overexpressing BMP7 increases brown but not white adipose tissue (Tseng et al., 2008). Thus, it appears that there are distinct signals that promote brown fat formation over white adipocyte formation and vice versa.
In vitro adipocyte differentiation: artificial sweeteners
Pioneering studies on in vivo formation of adipose tissue were conducted in the first half of the 20th century but these were scattered and primarily observational (Clark and Clark, 1940; Napolitano, 1963; Napolitano and Gagne, 1963). Over the last few decades, the major focus of the field has been cell culture modeling primarily studying in vitro adipogenesis. The term adipogenesis first described the transition of cultured and confluent 3T3-L1 fibroblast cells to individual lipid-laden cells upon incubation in a powerful cocktail composed of artificial ‘inducers’: cAMP inducers, glucocorticoid agonists and insulin or insulin-like growth factor (IGF) (Green and Kehinde, 1975; Gregoire et al., 1998). These fat-storing cells appeared to have several characteristics of adipocytes and have served as the primary model. This well-studied system has led to the elucidation of the cellular and biological events that occur and the transcriptional hierarchy that exists during the conversion of these cells from fibroblasts to adipocyte-like cells (Rosen and Spiegelman, 2000; Tontonoz and Spiegelman, 2008). It appears that aspects of the 3T3-L1 adipogenic transition also occur in other in vitro systems, such as mouse embryonic fibroblasts (MEFs) and stromal vascular (SV) cells (see below), when induced with the adipogenic induction mix (Rosen and Spiegelman, 2000).
A significant advance derived from the 3T3-L1 cellular model was the identification of relevant molecules expressed during fat accumulation, most notably a transcriptional hierarchy. PPARγ, a nuclear hormone receptor, is the centerpiece of this transcriptional cascade and is necessary and sufficient for in vitro adipogenesis (Chawla et al., 1994; Tontonoz et al., 1994). PPARγ controls genes involved in lipid storage, lipid synthesis and glucose sensing (Barak et al., 1999; He et al., 2003; Miles et al., 2000). Multiple studies using conditional and hypomorphic alleles, all solidify the role of PPARγ in differentiation of mature adipocytes. However, a variety of data indicate that PPARγ might have significant roles in addition to those in adipocytes and indeed may play central roles in early adipose lineage development. For example, PPARγ is expressed by embryonic day (E)14.5, much earlier than adipocyte tissue development begins, and appears to mark the location of subcutaneous fat depots that are present perinatally (Barak et al., 1999; Brun et al., 1996). Furthermore, PPARγ is expressed in adipose stem cells and appears to have key functions in the adipose stem cell compartment, including roles in stem cell proliferation, self-renewal, and cell determination (Tang et al., 2011; Tang et al., 2008). Collectively, a variety of studies suggest that PPARγ is crucial both for adipocyte terminal differentiation and as a determinant that controls adipose tissue lineage development and location.
Elegant in vitro studies have also identified many additional genes that may be expressed in adipocytes or might regulate adipocyte differentiation and function. Multiple signaling molecules can initiate the cell model adipogenic cascade, including insulin, thyroid hormone, glucocorticoids and TGFβ superfamily members (BMP2/BMP4/GDF3) (Chapman et al., 1985; Flores-Delgado et al., 1987; Zamani and Brown, 2011). During the initiation of differentiation, a transcriptional cascade occurs with activation of multiple factors, such as KLF4, KLF5, SMAD1/5/8, CREB, KROX20 (EGR2), glucocorticoid receptor, STAT5A, STAT5B, C/EBPβ and C/EBPδ. In turn, these factors can alter PPARγ expression (Cristancho and Lazar, 2011; Siersbæk et al., 2012). Other transcription factors, such as thyroid receptor, C/EBPα, SREBP-1c (SREBF1), KLF15 and LXR (NR1H), are involved later in adipocyte maturation and regulate the expression of PPARγ as well as that of other genes involved in fat storage (Siersbæk et al., 2012). Terminal differentiation is characterized by the appearance of lipid droplets and the expression of lipid/carbohydrate-storage proteins [e.g. AP2 (FABP4), CD36, LPL, perilipin and GLUT4 (SLC2A4)] and adipokines (leptin) (Cawthorn et al., 2012). Multiple developmental signaling pathways, hormones and environmental cues also inhibit in vitro adipogenesis, such as canonical WNT signaling, retinoic acid, TNFα, Hedgehog (Hh) signaling, SMAD/TGFβ and HIF1α (Berry et al., 2012; Cawthorn et al., 2007; Ross et al., 2000; Suh et al., 2006; Sul, 2009; Wang et al., 2006). In summary, studies in cell culture models indicate that the formation of fat-storing cells is controlled by complex interactions of stimulatory and inhibitory signals. A key challenge for the field, however, is to determine whether these molecules have roles in adipose tissue formation in vivo.
Adipose stem cells: getting to the root of the stem
Although some aspects of in vitro models reflect in vivo events, it has become increasingly clear that in vivo adipocyte differentiation is distinct. Therefore, the identification and characterization of the adipose stem cell is essential to understanding adipose tissue development, formation and maintenance. However, our understanding of the developmental biology of adipose tissue has lagged and our understanding of adipose stem cells is primitive. Stem cells are crucial components for tissue development and maintenance, and the notion of adipose progenitors stems from the 1940s. In the 1940s, it was noted that fibroblast-like cells change morphology and acquire unilocular lipid droplets (Clark and Clark, 1940). The developmental or embryonic origin of adipose stem cells remains largely unexplored and a variety of basic insights, such as germ layer derivation, remain murky. Conventional wisdom holds that WAT originates from the mesoderm germ cell layer despite a lack of direct evidence supporting this hypothesis (Duan et al., 2007); however, not all adipose tissue originates from the mesoderm (Monteiro et al., 2009). For instance, a variety of studies, such as those using chick-quail chimeras and modern Cre-dependent cell marking studies in the mouse, indicate that craniofacial adipose depots originate from neural crest cells (Billon et al., 2007; Le Lièvre and Le Douarin, 1975).
Identifying and characterizing adipose stem cells
Recent efforts have begun to tease apart the endogenous adipose stem/progenitor compartment. Tissue dissociation studies have implicated the adipose stromal vascular fraction (SVF) as a possible site of origin for adipose stem cells. The SVF is a heterogeneous mixture of cells that can be isolated from adipose tissue after dissociation and centrifugation, in which adipocytes float and the remaining cells pellet (Hollenberg and Vost, 1968). The SVF is composed of a myriad of cells, which includes fibroblasts, endothelial cells, hematopoietic cells and neural cells. Yet within this multiplicity of cell types the SVF appears to contain bone fide adipose stem cells that proliferate, self-renew and differentiate (Rodeheffer et al., 2008; Tang et al., 2008; Yamamoto et al., 2007). The isolation, identification and characterization of such adipose stem cells has only recently been performed using two different approaches: tissue dissociation coupled with fluorescence-activated cell sorting (FACS) and lineage tracing. Utilizing cell surface markers, Friedman and colleagues identified a CD24+ cell population within the SVF that had high adipogenic potential in vitro (Rodeheffer et al., 2008). When this cell population was injected into wild-type mice it did not form a functional fat pad; however, when transplanted into A-Zip mice (an engineered lipodystrophic model that lacks fat) the cells were able to form adipose-like tissue. Thus, it appears that the A-Zip mice, and possibly lipodystrophic mice in general, harbor a pro-adipogenic environment presumably containing various signals that induce adipocyte differentiation (Rodeheffer et al., 2008).
Contemporaneously, Tang et al. also developed a flow-based method for isolating adipose stem cells that could form adipose tissue after transplantation, even in wild-type mice (Tang et al., 2008). More importantly, by exploiting the observation that PPARγ is expressed at low levels in adipocyte stem cells in order to label genetically and lineage trace adipocyte stem cells, they reported that PPARγ-expressing cells are present prior to birth and long before adipocytes are formed (Tang et al., 2008). Moreover, these cells proliferate, self-renew, differentiate and are capable of repopulating the stem cell pool and contributing to the formation of all adipocytes (Tang et al., 2011; Tang et al., 2008; Zeve et al., 2009).
Remarkably, a new notion arose from these studies, that these adipose stem cells are present in a vascular niche, residing as a subset of perivascular mural cells within adipose depots that express a variety of mural cell markers, such as PDGFRβ, α-smooth muscle actin and NG2 (CSPG4) (Fig. 3). Transplantation of PDGFRβ mural cells, isolated from adipose depots, reconstituted adipose depots in the recipients; yet equivalent PDGFRβ-positive mural cells from other organs did not give rise to adipocytes or a fat pad (Tang et al., 2008). These data support the notion that adipose stem cells reside at the vascular interface and resemble mural cells that can proliferate and differentiate into mature adipocytes.
Fig. 3.
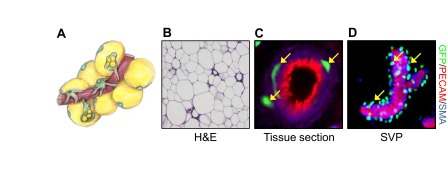
From the vascular niche to adipocyte. (A) Illustration conceptualizing the adipose vascular niche depicting blood vessels (red), adipose stem cells (green with green nuclei), mural cells (green with black nuclei) and mature adipocytes (yellow with green nuclei). (B) Mouse subcutaneous adipose tissue stained with Hematoxylin and Eosin (H&E) stain. (C) Immunofluorescence image of mouse subcutaneous adipose tissue section showing the expression of GFP (marking adipose stem cells, green), PECAM (an endothelial marker, red) and smooth muscle actin (SMA) (a mural cell marker, blue). (D) Stromal vascular particulates (SVPs) were isolated from mouse subcutaneous fat depots and immunostained for GFP (adipose stem cell, green), PECAM (endothelial marker, red) and SMA (mural cell marker, blue). Yellow arrows indicate adipose stem cells (green) residing at the vascular interface.
Some recent observations raise the possibility that endothelial cells and hematopoietic stem cells might give rise to a subset of adipocytes by changing fate into mural cells and then converting to a progenitor population (Crossno et al., 2006; Gupta et al., 2012; Medici et al., 2010; Sera et al., 2009; Tran et al., 2012). Such lineage changes do not have significant precedence in other endothelial compartments but may turn out to be important in adipose biology. However, endothelial cell deletion of PPARγ did not alter fat formation, under normal chow conditions, as would be expected if endothelial cells are major components of the adipose fate (Kanda et al., 2009). Recently, data indicate that neither endothelial nor hematopoietic lineage markers label adipocytes even under high fat diet conditions (Berry and Rodeheffer, 2013).
Factors that regulate adipose stem cells
The observation that PPARγ marks adipocyte stem cells that reside in the perivascular niche raises several important questions about the functional role of PPARγ within these cells. TZDs, a group of glucose-lowering drugs prescribed for people with type 2 diabetes, are PPARγ agonists that promote insulin sensitivity and lower blood glucose. However, a common and distressing side effect for patients treated with TZDs is significant weight gain and increased fat mass (de Souza et al., 2001; Hiragun et al., 1988; Sandouk et al., 1993a; Sandouk et al., 1993b). This process is multifaceted in that TZDs stimulate new adipocyte formation and apoptosis of large adipocytes (Okuno et al., 1998), cause fat deposition remodeling from visceral to subcutaneous (Akazawa et al., 2000; Kelly et al., 1999), increase fluid retention (Muto et al., 2001) and augment appetite (Shimizu et al., 1998). This clinical observation coupled with the finding that PPARγ marks the adipose stem cell raised the possibility that TZDs might alter adipocyte stem cells, for example by inducing them to differentiate thereby increasing the number of adipocytes and leading to weight gain (Yki-Järvinen, 2004). Consistent with this hypothesis, in vivo administration of TZDs to mice enhanced differentiation of the adipose stem cell population and increased formation of additional mature adipocytes (Tang et al., 2011). Furthermore, rosiglitazone (a TZD and clinically prescribed medicine) enhanced adipose stem cell proliferation and reduced the fraction of adipose stem cells in the quiescent state. Intriguingly, chronic rosiglitazone administration reduced the number of adipose stem cells, reduced the adipogenic potential of the extant stem cell compartment and appeared to exhaust the adipocyte stem population (Tang et al., 2011). These studies indirectly implicated PPARγ in adipose stem cell proliferation, self-renewal, lineage decisions, stem and niche identity and adipocyte differentiation (Tang et al., 2011). Studies examining these notions are a major area of future experimentation.
The adipose stem cell niche: blood brothers
The niche, the microenvironment in which stem cells reside, is highly specialized and controls stem cell behaviors, such as proliferation, quiescence and differentiation. In the 1940s, Clark and Clark examined fat formation in rabbit ears using a chambered system to displace the tissue and watch capillary growth and fat formation into the chamber (Clark and Clark, 1940). The chamber methodology allowed ‘real-time’ visualization of fat formation in a quasi in vivo setting. These innovative experiments also indicated that numerous minute lipid droplets appeared first and coalesced to form a large unilocular droplet as the cell morphology changed (Clark and Clark, 1940). Electron micrographs from the 1960s provided further evidence that adipocyte progenitor cells reside at the blood vessel and ‘peel’ away as they begin the transition to mature adipocytes (Napolitano, 1963; Napolitano and Gagne, 1963). This notion was further supported by Hausman and colleagues who observed the appearance of vascular structures that slightly protrude before the formation of adipocytes (Crandall et al., 1997). Their microscopic studies also indicated that angiogenesis recruits adipose stem cells and stimulates these stem cells to differentiate (Crandall et al., 1997). These observations were supported by studies of Han et al., whose data suggested that angiogenesis precedes visceral epididymal adipose tissue depot development (Han et al., 2011). This relationship between vessels and adipose lineage cells appears to be reciprocal, as adipocyte stem cells stimulate blood vessel formation. For instance, in vivo mixing of adipose stem cells with endothelial cells showed that adipose stem cells stimulate vasculogenesis and indicate that adipocyte differentiation and angiogenesis occur in concert (Traktuev et al., 2009). Furthermore, adipose stem cells secrete multiple angiogenic regulators, some of which are transcriptionally regulated by PPARγ, including vascular endothelial growth factor (VEGF), angiopoietin-like 4, fibroblast growth factor 2 and matrix metalloproteinases (MMPs) (Cao, 2007; Fukumura et al., 2003; Gealekman et al., 2008; Kersten et al., 2000). Moreover, treating adipose tissue fragments with TZDs increased angiogenic sprout formation and similar results were obtained in vivo in mouse (Gealekman et al., 2008). Inhibiting or activating angiogenic factors controls adiposity; in the epididymal fat depot, blunting VEGF/VEGFR2 signaling inhibits fat pad formation (Han et al., 2011), and transgenically overexpressing VEGF in mature adipocytes enhances vasculogenesis and reduces adipocyte size (Nishimura et al., 2007; Sun et al., 2012), demonstrating the orchestration between blood vessels and adipocytes.
Although the niche is known to be a major element of stem cell control, examination of the niche has been hindered by difficulties in assessing it as an intact structural unit. The ability to study the adipose stem cell vascular niche has been aided by procedures that maintain the native structure of the microenvironment, thus allowing the isolation of stromal vascular particulates (SVPs) as an organotypic culture system (Tang et al., 2011; Tang et al., 2008). Isolation of SVPs showed that these vessels do not contain lipid-filled adipocytes but rather adipose stem cells that wrap around the blood vessel and express a bevy of mural cell markers (Fig. 3C,D). Upon adipogenic signals, such as TZDs, the stem cells on the SVP transition to lipid-filled adipocytes loosely associated with the particulate (Tang et al., 2008). Taken together, it seems likely that the vascular network is the niche for adipocyte stem cells, and that this microenvironment provides adipogenic cues to inhibit or initiate adipocyte differentiation. The identification of such cues is an area that may attract significant attention.
Adipocyte turnover: churning the fat
Stem cells play important roles in tissue development as well as in homeostatic maintenance and response to environmental stimuli. There are two distinguishable responses to adipose tissue expansion: hypertrophy and hyperplasia. However, the level of contributions of the two responses varies depending upon genetic background, modifier effects, diet, biological and hormonal milieu, and depot preference for fat storage. Adipose tissue can expand from 2-3% to 60-70% of body weight in response to positive energy balance (Fig. 1A,B; Fig. 4D) (Hossain et al., 2007). A hypertrophic response is characterized by pre-existing adipocytes enhancing triglyceride storage; estimates indicate that a single adipocyte can increase its volume two- to threefold (Hirsch and Batchelor, 1976; Salans et al., 1973). A prolonged hypertrophic response appears to be a causal link between adipose expansion and metabolic dysfunction such as reducing adipocyte insulin sensitivity (Hossain et al., 2007; MacDougald and Mandrup, 2002). During obesogenesis, there is significantly higher adipocyte turnover, shortened adipocyte lifespan and a markedly increased rate of apoptosis (Strissel et al., 2007). Hypertrophy also promotes local inflammation, for example by recruiting ‘unhealthy’ macrophages to adipose tissue thereby facilitating adipocyte cell death and other events that trigger maladaptive metabolic consequences (Osborn and Olefsky, 2012). Furthermore, hypertrophy provokes increases in several adipocyte extracellular signals, such as IGF1, IGFBP and TNFα, that are linked to negative outcomes. Of note, these factors appear able to alter various aspects of adipose stem cell biology, such as proliferation, quiescence and differentiation (Cao, 2007).
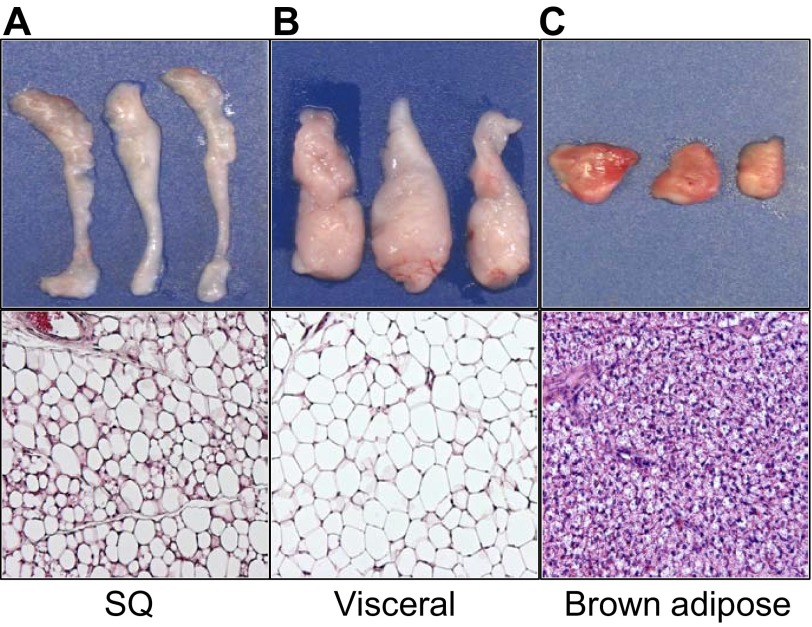
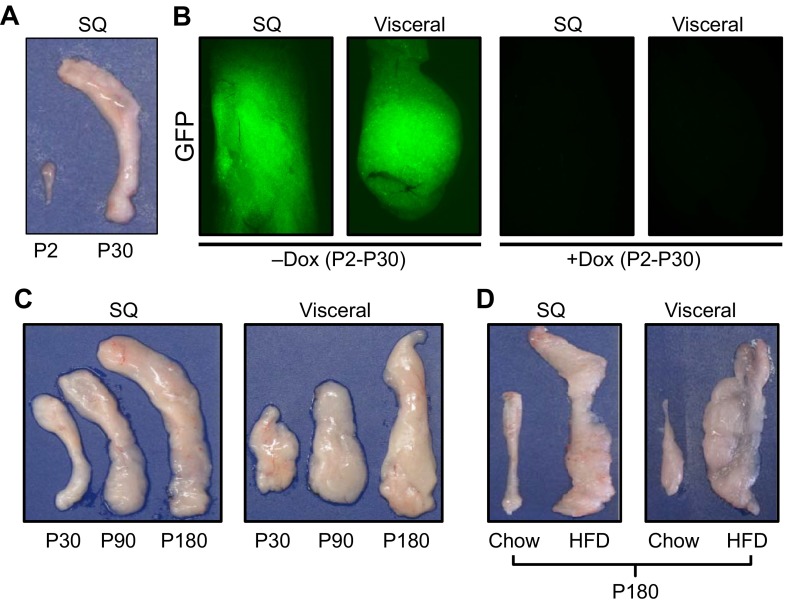
Fig. 4.
Fat expansion. (A) Isolated postnatal day (P)2 and P30 subcutaneous (inguinal) fat depots, demonstrating the extensive expansion that occurs within this short window of time. (B) Adipo-trak mice (Tang et al., 2008) were treated with placebo (-Dox) or doxycycline (+Dox) at denoted times. In the absence of doxycycline the GFP marker is expressed and the adipose depots are green. In the presence of doxycycline, all GFP signal is lost, indicating that there was a massive recruitment of new stem cells and adipocyte replacement. (C) White adipose depots enlarge with age; subcutaneous and visceral fat depots were isolated from 30-, 90- and 180-day-old mice. Depot weight nearly quintupled between 30 and 180 days. (D) Subcutaneous inguinal (SQ) and visceral perigonadal depots obtained from mice on a standard diet (chow) or a high fat diet (HFD), demonstrating the dramatic expansion in response to HFD.
Adipose tissue can also expand by forming new adipocytes, and newly formed, small adipocytes might be protective against metabolic dysregulation (de Souza et al., 2001; Okuno et al., 1998; Strissel et al., 2007). In the 1960s, it was shown in rodents that cells within adipose depots proliferate, that adipocytes are renewed and that adipocytes have a finite lifespan (Hellman and Hellerstrom, 1961; Hollenberg and Vost, 1968). Indeed, caloric excess stimulates the adipose stem cell compartment to proliferate and differentiate. For example, Joe et al. reported a significant increase in bromodeoxyuridine (BrdU) incorporation in the SVF of subcutaneous adipose depots in response to high-fat diet, whereas visceral fat increased as a result of hypertrophy (Joe et al., 2009). Other studies have begun to examine the source(s) of high-fat diet-induced increases in proliferation observed in the adipose lineage. Several studies have since aimed to gain a better understanding of adipocyte turnover but have yielded mixed findings. In humans, it was found that 8.4% of adipocytes turnover every year (Spalding et al., 2008). However, estimates indicate that young adult mice generate ∼15% of adipocytes each month. The stem cell compartment also appears to be active with some estimates suggesting that 4.8% of mouse adipose stem cells are replicating at a given time (Rigamonti et al., 2011). These findings suggest that adipose stem cell proliferation not only contributes to adipocyte formation but also replenishes the stem cell pool. Support for this notion comes from the findings that during postnatal development [specifically, the first 30 days of life (P0-P30 in mice)], there is rapid turnover and replenishing events in the adipose depot (Fig. 4A,B) (Tang et al., 2008). Furthermore, adipose tissue maintains its ability to expand as the organism ages, and aging is associated with increased percentage of body fat, which cannot only be attributed to hypertrophy (Fig. 4C). Further studies to examine adipose stem cell proliferation are needed to define the mechanisms that control these events, how the stem cell divisions regulate adipose biology and whether interventions directed towards cycle cell manipulation are therapeutically tenable.
Adipose stem cells presumably confer the ability of adipose depots to turnover, and they may also underlie the ability of adipose tissue to regenerate. In humans, lipectomy and liposuction are commonly performed medical procedures that remove excess adipose tissue. However, removal of adipose tissue causes other fat depots to remodel with both hypertrophic and hyperplastic responses (Mauer et al., 2001; Reyne et al., 1983), and full regeneration of the removed fat pad also occurs leading to repeat operations. Experiments using rodent model systems have aimed to characterize this regenerative phenomenon. When inguinal fat (subcutaneous) depots were removed from rats the fat pad was regenerated within ∼13 weeks (Larson and Anderson, 1978). Lipectomies in obese mice stimulated the rate of local and global lipogenesis and stimulated adipocyte differentiation (Bueno et al., 2011). This indicates that stem cells are potential sources of the renewal, respond to environmental cues and provide experimental paradigms for further exploration.
The therapeutic adipose stem cell
The ability to isolate and track the responses of adipocyte stem cells has provided a platform for the characterization and manipulation of these cells, with the aim of providing some beneficial therapeutic outcomes. Recent studies show that TZDs improve symptoms of the metabolic syndrome in part by mobilizing adipocyte progenitors to differentiate into new insulin-sensitive adipocytes (Tang et al., 2011). This indicates that adipose stem cells are pharmacologically accessible, that increased formation of new adipocytes may be beneficial and that adipose stem cells may be subject to additional types of manipulation. Efforts to control differentiation or proliferation might thus serve as weight loss intervention for overweight or pre-obese individuals. Alternatively, other methods directed at inducing adipocyte differentiation in order to maintain new small insulin-sensitive adipocytes (healthy adipocytes) might provide metabolic relief to those suffering with obesity and metabolic dysfunction. In addition to pharmacological intervention, adipose stem cells are uniquely poised for regenerative and genetic therapy. WAT provides minimally invasive access to large numbers of progenitor cells necessary for cell-based therapeutics. Furthermore, rejection is not a concern because these cells can be harvested from and administered to the same person. In principle, applications could include reprogramming cells into other lineages or inducing cells into the adipose lineage, for example to improve wound healing or to use as a ‘filler’ to repair defects or other cosmetic concerns. Plastic and reconstructive surgeons have long been plagued with the inconsistencies of adipose grafting in wound healing and soft tissue augmentation (Sterodimas et al., 2012). However, new technologies enriching graft material for adipose progenitor cells are being developed and show initial promise (Gir et al., 2012). An issue in surgical applications is that transplants of adipose depots tend to fail and necrose because of the lack of vascular supply. However, transplanted adipose stem cells recruit vessel formation and the formed adipose depots are well vascularized overcoming the cause of traditional transplant failure (Han et al., 2011; Satoor et al., 2011; Tran et al., 2008).
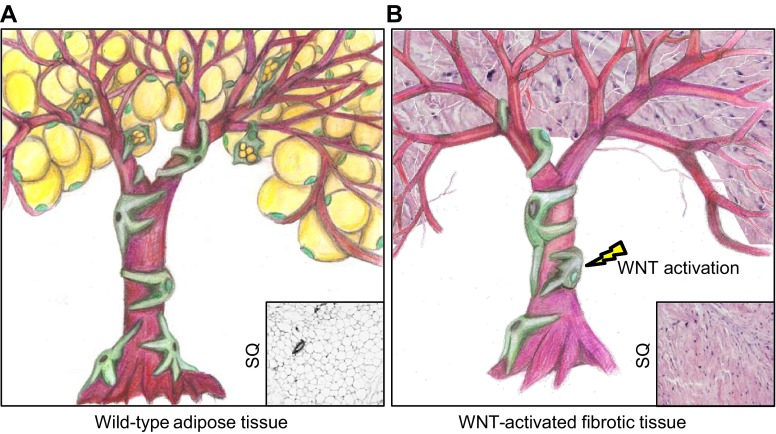
The ability to induce adipose stem cells into a variety of lineages, such as skeletal myocytes for muscle degeneration, cardiac myocytes for a failing heart, or neural fates for Alzheimer’s disease, also has significant potential. Confirmation and identification of the conditions necessary to promote lineage variation might lead to production of cells or tissues amenable for autologous transplant of modified tissue to treat a host of degenerative diseases. Yet the full range of lineage capacity/plasticity of isolated adipose progenitor cells remains unknown. For instance, altering canonical WNT signaling (via a conditional allele of β-catenin) in adipose stem cells disrupted adipose development in mice (Fig. 5) (Zeve et al., 2009). These mutant mice had a marked paucity of adipocytes, and a lipodystrophic phenotype, with ensuing hypertriglyceridemia. Notably, these mutants displayed essentially a complete loss of subcutaneous adipose tissue accompanied by a lineage change in the subcutaneous adipose stem cells. Of note, the secretome of WNT mutant stem cells was altered and the cells produced high levels of a glucose-lowering hormone, glucodyne, which had many actions similar to insulin but functioned through distinct mechanisms (Zeve et al., 2012).
Fig. 5.
Manipulating the adipose stem cell: kill the stem, kill the tree. (A) Illustration conceptualizing the importance of adipose stem cells to the formation of adipose tissue. This illustration demonstrates the extensive branching of the vascular network and shows that adipose stem cells (green nuclei) and mural cells (black nuclei) reside on the vasculature. Adipose stem cells then transition to a multilocular progenitor giving rise to the unilocular adipocyte. Inset: Histological image of subcutaneous fat from a wild-type mouse. (B) Disrupting adipose stem cells by activating WNT signaling results in a fate change that promotes the formation of fibrotic tissue (purple). Inset: Histological image of subcutaneous fat from a mouse with activated WNT signaling.
Conclusions
In recent decades, attention to adipose tissue has increased in parallel with a rising epidemic of obesity and its negative effects on whole body metabolism and increased incidence of various illnesses and conditions. Only recently have research efforts shifted to understanding the developmental biology of this tissue (Han et al., 2011; Rodeheffer et al., 2008; Tang et al., 2011; Tang et al., 2008). The complexity of the various adipose lineages (white, brown, induced brown, subcutaneous, visceral, etc.), the difficulty in working with such a fragile cell type, and the non-contiguous nature of this tissue has made it difficult to understand its developmental origin, and multiple origins might exist. However, with the generation of developmental tools, such as lineage tracing, researchers are now poised to understand the developmental cues and origin of adipose tissue and to answer a slew of interesting and undefined questions. For instance, what are the cell types of origin of the adipose lineage? What is the timing of adipose lineage determination and specification? Do adipose stem cells arise in situ on the blood vessel or do they migrate and arrive from elsewhere? What are the signals derived from the blood vessel niche that stimulate these stem cells to proliferate and differentiate or that hold them in the quiescent state? What is the importance of adipose stem cells to the homeostasis and maintenance of the fat pad under both normal energy intake and excess nutrient load? Do other anti-diabetes drugs alter adipose stem cell biology, similar to TZD treatment? Do growth factors and development signaling pathways alter stem cell behavior and adipocyte formation? In the midst of the obesity epidemic, the recent discoveries and the answers to these open-ended questions would provide hope that there is light at the end of the tunnel.
Acknowledgments
We thank all current and former members of the Graff lab for their helpful comments and discussion points leading to refinement of our concepts. We especially thank Dr Ayoung Jo and Kia Huang for providing data images for specific figures, and Dr Todd Soesbe for MRI imaging.We apologize for the editorial constraints that limit the ability to cite a vast number of papers that pioneered the way for the above studies and notions for future advances.
Footnotes
Funding
J.M.G. is supported by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Institutes of Health [R01-DK066556, R01-DK064261 and R01-DK088220]. D.C.B. is supported by the National Heart, Lung and Blood Institute [5T32HL007360-34]. Deposited in PMC for release after 12 months.
Competing interests statement
J.M.G. is a co-founder and shareholder of Reata Pharmaceuticals.
References
- Akazawa S., Sun F., Ito M., Kawasaki E., Eguchi K. (2000). Efficacy of troglitazone on body fat distribution in type 2 diabetes. Diabetes Care 23, 1067–1071 [DOI] [PubMed] [Google Scholar]
- Atit R., Sgaier S. K., Mohamed O. A., Taketo M. M., Dufort D., Joyner A. L., Niswander L., Conlon R. A. (2006). Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev. Biol. 296, 164–176 [DOI] [PubMed] [Google Scholar]
- Baglioni S., Cantini G., Poli G., Francalanci M., Squecco R., Di Franco A., Borgogni E., Frontera S., Nesi G., Liotta F., et al. (2012). Functional differences in visceral and subcutaneous fat pads originate from differences in the adipose stem cell. PLoS ONE 7, e36569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak Y., Nelson M. C., Ong E. S., Jones Y. Z., Ruiz-Lozano P., Chien K. R., Koder A., Evans R. M. (1999). PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol. Cell 4, 585–595 [DOI] [PubMed] [Google Scholar]
- Berry R., Rodeheffer M. S. (2013). Characterization of the adipocyte cellular lineage in vivo. Nat. Cell Biol. 15, 302–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry D. C., DeSantis D., Soltanian H., Croniger C. M., Noy N. (2012). Retinoic acid upregulates preadipocyte genes to block adipogenesis and suppress diet-induced obesity. Diabetes 61, 1112–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billon N., Iannarelli P., Monteiro M. C., Glavieux-Pardanaud C., Richardson W. D., Kessaris N., Dani C., Dupin E. (2007). The generation of adipocytes by the neural crest. Development 134, 2283–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björntorp P., Bengtsson C., Blohmé G., Jonsson A., Sjöström L., Tibblin E., Tibblin G., Wilhelmsen L. (1971). Adipose tissue fat cell size and number in relation to metabolism in randomly selected middle-aged men and women. Metabolism 20, 927–935 [DOI] [PubMed] [Google Scholar]
- Brun R. P., Tontonoz P., Forman B. M., Ellis R., Chen J., Evans R. M., Spiegelman B. M. (1996). Differential activation of adipogenesis by multiple PPAR isoforms. Genes Dev. 10, 974–984 [DOI] [PubMed] [Google Scholar]
- Bueno A. A., Habitante C. A., Oyama L. M., Estadella D., Ribeiro E. B., Oller do Nascimento C. M. (2011). White adipose tissue re-growth after partial lipectomy in high fat diet induced obese wistar rats. J. Physiol. Sci. 61, 55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B., Nedergaard J. (2004). Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 [DOI] [PubMed] [Google Scholar]
- Cao Y. (2007). Angiogenesis modulates adipogenesis and obesity. J. Clin. Invest. 117, 2362–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthorn W. P., Heyd F., Hegyi K., Sethi J. K. (2007). Tumour necrosis factor-alpha inhibits adipogenesis via a beta-catenin/TCF4(TCF7L2)-dependent pathway. Cell Death Differ. 14, 1361–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthorn W. P., Scheller E. L., MacDougald O. A. (2012). Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J. Lipid Res. 53, 227–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman A. B., Knight D. M., Ringold G. M. (1985). Glucocorticoid regulation of adipocyte differentiation: hormonal triggering of the developmental program and induction of a differentiation-dependent gene. J. Cell Biol. 101, 1227–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla A., Schwarz E. J., Dimaculangan D. D., Lazar M. A. (1994). Peroxisome proliferator-activated receptor (PPAR) gamma: adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology 135, 798–800 [DOI] [PubMed] [Google Scholar]
- Clark E. R., Clark E. L. (1940). Microscopic studies of the new formation of fat in living adult rabbits. Am. J. Anat. 67, 255–285 [Google Scholar]
- Crandall D. L., Hausman G. J., Kral J. G. (1997). A review of the microcirculation of adipose tissue: anatomic, metabolic, and angiogenic perspectives. Microcirculation 4, 211–232 [DOI] [PubMed] [Google Scholar]
- Cristancho A. G., Lazar M. A. (2011). Forming functional fat: a growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 12, 722–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossno J. T., Jr, Majka S. M., Grazia T., Gill R. G., Klemm D. J. (2006). Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. J. Clin. Invest. 116, 3220–3228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess A. M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A. B., Kuo F. C., Palmer E. L., Tseng Y. H., Doria A., et al. (2009). Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360, 1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess A. M., White A. P., Vernochet C., Schulz T. J., Xue R., Sass C. A., Huang T. L., Roberts-Toler C., Weiner L. S., Sze C., et al. (2013). Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat. Med. 19, 635–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza C. J., Eckhardt M., Gagen K., Dong M., Chen W., Laurent D., Burkey B. F. (2001). Effects of pioglitazone on adipose tissue remodeling within the setting of obesity and insulin resistance. Diabetes 50, 1863–1871 [DOI] [PubMed] [Google Scholar]
- Duan S. Z., Ivashchenko C. Y., Whitesall S. E., D’Alecy L. G., Duquaine D. C., Brosius F. C., 3rd, Gonzalez F. J., Vinson C., Pierre M. A., Milstone D. S., et al. (2007). Hypotension, lipodystrophy, and insulin resistance in generalized PPARgamma-deficient mice rescued from embryonic lethality. J. Clin. Invest. 117, 812–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. M., Thörne A., Hamsten A., Arner P. (2002). Fatty acid binding protein expression in different human adipose tissue depots in relation to rates of lipolysis and insulin concentration in obese individuals. Mol. Cell. Biochem. 239, 95–100 [PubMed] [Google Scholar]
- Flores-Delgado G., Marsch-Moreno M., Kuri-Harcuch W. (1987). Thyroid hormone stimulates adipocyte differentiation of 3T3 cells. Mol. Cell. Biochem. 76, 35–43 [DOI] [PubMed] [Google Scholar]
- Fukumura D., Ushiyama A., Duda D. G., Xu L., Tam J., Krishna V., Chatterjee K., Garkavtsev I., Jain R. K. (2003). Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ. Res. 93, e88–e97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gealekman O., Burkart A., Chouinard M., Nicoloro S. M., Straubhaar J., Corvera S. (2008). Enhanced angiogenesis in obesity and in response to PPARgamma activators through adipocyte VEGF and ANGPTL4 production. Am. J. Physiol. 295, E1056–E1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesta S., Blüher M., Yamamoto Y., Norris A. W., Berndt J., Kralisch S., Boucher J., Lewis C., Kahn C. R. (2006). Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc. Natl. Acad. Sci. USA 103, 6676–6681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesta S., Tseng Y. H., Kahn C. R. (2007). Developmental origin of fat: tracking obesity to its source. Cell 131, 242–256 [DOI] [PubMed] [Google Scholar]
- Gir P., Oni G., Brown S. A., Mojallal A., Rohrich R. J. (2012). Human adipose stem cells: current clinical applications. Plast. Reconstr. Surg. 129, 1277–1290 [DOI] [PubMed] [Google Scholar]
- Grauer W. O., Moss A. A., Cann C. E., Goldberg H. I. (1984). Quantification of body fat distribution in the abdomen using computed tomography. Am. J. Clin. Nutr. 39, 631–637 [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. (1975). An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell 5, 19–27 [DOI] [PubMed] [Google Scholar]
- Gregoire F. M., Smas C. M., Sul H. S. (1998). Understanding adipocyte differentiation. Physiol. Rev. 78, 783–809 [DOI] [PubMed] [Google Scholar]
- Gupta R. K., Mepani R. J., Kleiner S., Lo J. C., Khandekar M. J., Cohen P., Frontini A., Bhowmick D. C., Ye L., Cinti S., et al. (2012). Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell Metab. 15, 230–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Lee J. E., Jin J., Lim J. S., Oh N., Kim K., Chang S. I., Shibuya M., Kim H., Koh G. Y. (2011). The spatiotemporal development of adipose tissue. Development 138, 5027–5037 [DOI] [PubMed] [Google Scholar]
- He W., Barak Y., Hevener A., Olson P., Liao D., Le J., Nelson M., Ong E., Olefsky J. M., Evans R. M. (2003). Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc. Natl. Acad. Sci. USA 100, 15712–15717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman B., Hellerstrom C. (1961). Cell renewal in the white and brown fat tissue of the rat. Acta Pathol. Microbiol. Scand. 51, 347–353 [DOI] [PubMed] [Google Scholar]
- Herbst K. L. (2012). Rare adipose disorders (RADs) masquerading as obesity. Acta Pharmacol. Sin. 33, 155–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiragun A., Sato M., Mitsui H. (1988). Preadipocyte differentiation in vitro: identification of a highly active adipogenic agent. J. Cell. Physiol. 134, 124–130 [DOI] [PubMed] [Google Scholar]
- Hirsch J., Batchelor B. (1976). Adipose tissue cellularity in human obesity. Clin. Endocrinol. Metab. 5, 299–311 [DOI] [PubMed] [Google Scholar]
- Hollenberg C. H., Vost A. (1968). Regulation of DNA synthesis in fat cells and stromal elements from rat adipose tissue. J. Clin. Invest. 47, 2485–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain P., Kawar B., El Nahas M. (2007). Obesity and diabetes in the developing world—a growing challenge. N. Engl. J. Med. 356, 213–215 [DOI] [PubMed] [Google Scholar]
- Jernås M., Palming J., Sjöholm K., Jennische E., Svensson P. A., Gabrielsson B. G., Levin M., Sjögren A., Rudemo M., Lystig T. C., et al. (2006). Separation of human adipocytes by size: hypertrophic fat cells display distinct gene expression. FASEB J. 20, 1540–1542 [DOI] [PubMed] [Google Scholar]
- Joe A. W., Yi L., Even Y., Vogl A. W., Rossi F. M. (2009). Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells 27, 2563–2570 [DOI] [PubMed] [Google Scholar]
- Kajimura S., Seale P., Spiegelman B. M. (2010). Transcriptional control of brown fat development. Cell Metab. 11, 257–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda T., Brown J. D., Orasanu G., Vogel S., Gonzalez F. J., Sartoretto J., Michel T., Plutzky J. (2009). PPARgamma in the endothelium regulates metabolic responses to high-fat diet in mice. J. Clin. Invest. 119, 110–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly I. E., Han T. S., Walsh K., Lean M. E. (1999). Effects of a thiazolidinedione compound on body fat and fat distribution of patients with type 2 diabetes. Diabetes Care 22, 288–293 [DOI] [PubMed] [Google Scholar]
- Kersten S., Mandard S., Tan N. S., Escher P., Metzger D., Chambon P., Gonzalez F. J., Desvergne B., Wahli W. (2000). Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J. Biol. Chem. 275, 28488–28493 [DOI] [PubMed] [Google Scholar]
- Larson K. A., Anderson D. B. (1978). The effects of lipectomy on remaining adipose tissue depots in the Sprague Dawley rat. Growth 42, 469–477 [PubMed] [Google Scholar]
- Le Lièvre C. S., Le Douarin N. M. (1975). Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J. Embryol. Exp. Morphol. 34, 125–154 [PubMed] [Google Scholar]
- Lee Y. H., Petkova A. P., Mottillo E. P., Granneman J. G. (2012). In vivo identification of bipotential adipocyte progenitors recruited by β3-adrenoceptor activation and high-fat feeding. Cell Metab. 15, 480–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidell M. E., Betz M. J., Dahlqvist Leinhard O., Heglind M., Elander L., Slawik M., Mussack T., Nilsson D., Romu T., Nuutila P., et al. (2013). Evidence for two types of brown adipose tissue in humans. Nat. Med. 19, 631–634 [DOI] [PubMed] [Google Scholar]
- Longo K. A., Wright W. S., Kang S., Gerin I., Chiang S. H., Lucas P. C., Opp M. R., MacDougald O. A. (2004). Wnt10b inhibits development of white and brown adipose tissues. J. Biol. Chem. 279, 35503–35509 [DOI] [PubMed] [Google Scholar]
- MacDougald O. A., Mandrup S. (2002). Adipogenesis: forces that tip the scales. Trends Endocrinol. Metab. 13, 5–11 [DOI] [PubMed] [Google Scholar]
- Macotela Y., Emanuelli B., Mori M. A., Gesta S., Schulz T. J., Tseng Y. H., Kahn C. R. (2012). Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes 61, 1691–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuzaki H., Paterson J., Shinyama H., Morton N. M., Mullins J. J., Seckl J. R., Flier J. S. (2001). A transgenic model of visceral obesity and the metabolic syndrome. Science 294, 2166–2170 [DOI] [PubMed] [Google Scholar]
- Mauer M. M., Harris R. B., Bartness T. J. (2001). The regulation of total body fat: lessons learned from lipectomy studies. Neurosci. Biobehav. Rev. 25, 15–28 [DOI] [PubMed] [Google Scholar]
- McKay R. M., McKay J. P., Avery L., Graff J. M. (2003). C elegans: a model for exploring the genetics of fat storage. Dev. Cell 4, 131–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici D., Shore E. M., Lounev V. Y., Kaplan F. S., Kalluri R., Olsen B. R. (2010). Conversion of vascular endothelial cells into multipotent stem-like cells. Nat. Med. 16, 1400–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles P. D., Barak Y., He W., Evans R. M., Olefsky J. M. (2000). Improved insulin-sensitivity in mice heterozygous for PPAR-gamma deficiency. J. Clin. Invest. 105, 287–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro M. C., Wdziekonski B., Villageois P., Vernochet C., Iehle C., Billon N., Dani C. (2009). Commitment of mouse embryonic stem cells to the adipocyte lineage requires retinoic acid receptor beta and active GSK3. Stem Cells Dev. 18, 457–463 [DOI] [PubMed] [Google Scholar]
- Muto S., Miyata Y., Imai M., Asano Y. (2001). Troglitazone stimulates basolateral rheogenic Na+/HCO3-cotransport activity in rabbit proximal straight tubules. Exp. Nephrol. 9, 191–197 [DOI] [PubMed] [Google Scholar]
- Napolitano L. (1963). The Differentiation of White Adipose Cells. An Electron Microscope Study. J. Cell Biol. 18, 663–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitano L., Gagne H. T. (1963). Lipid-depleted white adipose cells: an electronmicroscope study. Anat. Rec. 147, 273–293 [DOI] [PubMed] [Google Scholar]
- Nawrocki A. R., Scherer P. E. (2004). The delicate balance between fat and muscle: adipokines in metabolic disease and musculoskeletal inflammation. Curr. Opin. Pharmacol. 4, 281–289 [DOI] [PubMed] [Google Scholar]
- Nishimura S., Manabe I., Nagasaki M., Hosoya Y., Yamashita H., Fujita H., Ohsugi M., Tobe K., Kadowaki T., Nagai R., et al. (2007). Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes 56, 1517–1526 [DOI] [PubMed] [Google Scholar]
- Okuno A., Tamemoto H., Tobe K., Ueki K., Mori Y., Iwamoto K., Umesono K., Akanuma Y., Fujiwara T., Horikoshi H., et al. (1998). Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J. Clin. Invest. 101, 1354–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn O., Olefsky J. M. (2012). The cellular and signaling networks linking the immune system and metabolism in disease. Nat. Med. 18, 363–374 [DOI] [PubMed] [Google Scholar]
- Reyne Y., Nougues J., Vezinhet A. (1983). Adipose tissue regeneration in 6-month-old and adult rabbits following lipectomy. Proc. Soc. Exp. Biol. Med. 174, 258–264 [DOI] [PubMed] [Google Scholar]
- Rigamonti A., Brennand K., Lau F., Cowan C. A. (2011). Rapid cellular turnover in adipose tissue. PLoS ONE 6, e17637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeheffer M. S., Birsoy K., Friedman J. M. (2008). Identification of white adipocyte progenitor cells in vivo. Cell 135, 240–249 [DOI] [PubMed] [Google Scholar]
- Rosen E. D., Spiegelman B. M. (2000). Molecular regulation of adipogenesis. Annu. Rev. Cell Dev. Biol. 16, 145–171 [DOI] [PubMed] [Google Scholar]
- Ross S. E., Hemati N., Longo K. A., Bennett C. N., Lucas P. C., Erickson R. L., MacDougald O. A. (2000). Inhibition of adipogenesis by Wnt signaling. Science 289, 950–953 [DOI] [PubMed] [Google Scholar]
- Salans L. B., Cushman S. W., Weismann R. E. (1973). Studies of human adipose tissue. Adipose cell size and number in nonobese and obese patients. J. Clin. Invest. 52, 929–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gurmaches J., Hung C. M., Sparks C. A., Tang Y., Li H., Guertin D. A. (2012). PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from Myf5 precursors. Cell Metab. 16, 348–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandouk T., Reda D., Hofmann C. (1993a). Antidiabetic agent pioglitazone enhances adipocyte differentiation of 3T3-F442A cells. Am. J. Physiol. 264, C1600–C1608 [DOI] [PubMed] [Google Scholar]
- Sandouk T., Reda D., Hofmann C. (1993b). The antidiabetic agent pioglitazone increases expression of glucose transporters in 3T3-F442A cells by increasing messenger ribonucleic acid transcript stability. Endocrinology 133, 352–359 [DOI] [PubMed] [Google Scholar]
- Satoor S. N., Puranik A. S., Kumar S., Williams M. D., Ghale M., Rahalkar A., Karandikar M. S., Shouche Y., Patole M., Bhonde R., et al. (2011). Location, location, location: Beneficial effects of autologous fat transplantation. Sci. Rep. 1, 81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P., Bjork B., Yang W., Kajimura S., Chin S., Kuang S., Scimè A., Devarakonda S., Conroe H. M., Erdjument-Bromage H., et al. (2008). PRDM16 controls a brown fat/skeletal muscle switch. Nature 454, 961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P., Kajimura S., Spiegelman B. M. (2009). Transcriptional control of brown adipocyte development and physiological function—of mice and men. Genes Dev. 23, 788–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sera Y., LaRue A. C., Moussa O., Mehrotra M., Duncan J. D., Williams C. R., Nishimoto E., Schulte B. A., Watson P. M., Watson D. K., et al. (2009). Hematopoietic stem cell origin of adipocytes. Exp Hematol 37, 1108–1120, e1-e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Clegg D. J. (2009). Sex differences in the regulation of body weight. Physiol. Behav. 97, 199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H., Tsuchiya T., Sato N., Shimomura Y., Kobayashi I., Mori M. (1998). Troglitazone reduces plasma leptin concentration but increases hunger in NIDDM patients. Diabetes Care 21, 1470–1474 [DOI] [PubMed] [Google Scholar]
- Siersbæk R., Nielsen R., Mandrup S. (2012). Transcriptional networks and chromatin remodeling controlling adipogenesis. Trends Endocrinol. Metab. 23, 56–64 [DOI] [PubMed] [Google Scholar]
- Snijder M. B., Dekker J. M., Visser M., Bouter L. M., Stehouwer C. D., Kostense P. J., Yudkin J. S., Heine R. J., Nijpels G., Seidell J. C. (2003a). Associations of hip and thigh circumferences independent of waist circumference with the incidence of type 2 diabetes: the Hoorn Study. Am. J. Clin. Nutr. 77, 1192–1197 [DOI] [PubMed] [Google Scholar]
- Snijder M. B., Dekker J. M., Visser M., Yudkin J. S., Stehouwer C. D., Bouter L. M., Heine R. J., Nijpels G., Seidell J. C. (2003b). Larger thigh and hip circumferences are associated with better glucose tolerance: the Hoorn study. Obes. Res. 11, 104–111 [DOI] [PubMed] [Google Scholar]
- Spalding K. L., Arner E., Westermark P. O., Bernard S., Buchholz B. A., Bergmann O., Blomqvist L., Hoffstedt J., Näslund E., Britton T., et al. (2008). Dynamics of fat cell turnover in humans. Nature 453, 783–787 [DOI] [PubMed] [Google Scholar]
- Spiegelman B. M., Flier J. S. (2001). Obesity and the regulation of energy balance. Cell 104, 531–543 [DOI] [PubMed] [Google Scholar]
- Sterodimas A., Boriani F., Magarakis E., Nicaretta B., Pereira L. H., Illouz Y. G. (2012). Thirtyfour years of liposuction: past, present and future. Eur. Rev. Med. Pharmacol. Sci. 16, 393–406 [PubMed] [Google Scholar]
- Strissel K. J., Stancheva Z., Miyoshi H., Perfield J. W., 2nd, DeFuria J., Jick Z., Greenberg A. S., Obin M. S. (2007). Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 56, 2910–2918 [DOI] [PubMed] [Google Scholar]
- Suh J. M., Gao X., McKay J., McKay R., Salo Z., Graff J. M. (2006). Hedgehog signaling plays a conserved role in inhibiting fat formation. Cell Metab. 3, 25–34 [DOI] [PubMed] [Google Scholar]
- Suh J. M., Zeve D., McKay R., Seo J., Salo Z., Li R., Wang M., Graff J. M. (2007). Adipose is a conserved dosage-sensitive antiobesity gene. Cell Metab. 6, 195–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh J. M., Stenesen D., Peters J. M., Inoue A., Cade A., Graff J. M. (2008). An RGS-containing sorting nexin controls Drosophila lifespan. PLoS ONE 3, e2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sul H. S. (2009). Minireview: Pref-1: role in adipogenesis and mesenchymal cell fate. Mol. Endocrinol. 23, 1717–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K., Wernstedt Asterholm I., Kusminski C. M., Bueno A. C., Wang Z. V., Pollard J. W., Brekken R. A., Scherer P. E. (2012). Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc. Natl. Acad. Sci. USA 109, 5874–5879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W., Zeve D., Suh J. M., Bosnakovski D., Kyba M., Hammer R. E., Tallquist M. D., Graff J. M. (2008). White fat progenitor cells reside in the adipose vasculature. Science 322, 583–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W., Zeve D., Seo J., Jo A. Y., Graff J. M. (2011). Thiazolidinediones regulate adipose lineage dynamics. Cell Metab. 14, 116–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernof A., Bélanger C., Morisset A. S., Richard C., Mailloux J., Laberge P., Dupont P. (2006). Regional differences in adipose tissue metabolism in women: minor effect of obesity and body fat distribution. Diabetes 55, 1353–1360 [DOI] [PubMed] [Google Scholar]
- Tchkonia T., Giorgadze N., Pirtskhalava T., Tchoukalova Y., Karagiannides I., Forse R. A., DePonte M., Stevenson M., Guo W., Han J., et al. (2002). Fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. Am. J. Physiol. 282, R1286–R1296 [DOI] [PubMed] [Google Scholar]
- Tchkonia T., Lenburg M., Thomou T., Giorgadze N., Frampton G., Pirtskhalava T., Cartwright A., Cartwright M., Flanagan J., Karagiannides I., et al. (2007). Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am. J. Physiol. 292, E298–E307 [DOI] [PubMed] [Google Scholar]
- Tontonoz P., Spiegelman B. M. (2008). Fat and beyond: the diverse biology of PPARgamma. Annu. Rev. Biochem. 77, 289–312 [DOI] [PubMed] [Google Scholar]
- Tontonoz P., Hu E., Graves R. A., Budavari A. I., Spiegelman B. M. (1994). mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 8, 1224–1234 [DOI] [PubMed] [Google Scholar]
- Traktuev D. O., Prater D. N., Merfeld-Clauss S., Sanjeevaiah A. R., Saadatzadeh M. R., Murphy M., Johnstone B. H., Ingram D. A., March K. L. (2009). Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circ. Res. 104, 1410–1420 [DOI] [PubMed] [Google Scholar]
- Tran T. T., Yamamoto Y., Gesta S., Kahn C. R. (2008). Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 7, 410–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran K. V., Gealekman O., Frontini A., Zingaretti M. C., Morroni M., Giordano A., Smorlesi A., Perugini J., De Matteis R., Sbarbati A., et al. (2012). The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab. 15, 222–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng Y. H., Kokkotou E., Schulz T. J., Huang T. L., Winnay J. N., Taniguchi C. M., Tran T. T., Suzuki R., Espinoza D. O., Yamamoto Y., et al. (2008). New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454, 1000–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Kim K. A., Kim J. H., Sul H. S. (2006). Pref-1, a preadipocyte secreted factor that inhibits adipogenesis. J. Nutr. 136, 2953–2956 [DOI] [PubMed] [Google Scholar]
- Weyer C., Foley J. E., Bogardus C., Tataranni P. A., Pratley R. E. (2000). Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia 43, 1498–1506 [DOI] [PubMed] [Google Scholar]
- Whittle A. J., López M., Vidal-Puig A. (2011). Using brown adipose tissue to treat obesity - the central issue. Trends Mol. Med. 17, 405–411 [DOI] [PubMed] [Google Scholar]
- Wu J., Boström P., Sparks L. M., Ye L., Choi J. H., Giang A. H., Khandekar M., Virtanen K. A., Nuutila P., Schaart G., et al. (2012). Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N., Akamatsu H., Hasegawa S., Yamada T., Nakata S., Ohkuma M., Miyachi E., Marunouchi T., Matsunaga K. (2007). Isolation of multipotent stem cells from mouse adipose tissue. J. Dermatol. Sci. 48, 43–52 [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H. (2004). Thiazolidinediones. N. Engl. J. Med. 351, 1106–1118 [DOI] [PubMed] [Google Scholar]
- Zamani N., Brown C. W. (2011). Emerging roles for the transforming growth factor-beta superfamily in regulating adiposity and energy expenditure. Endocr. Rev. 32, 387–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeve D., Tang W., Graff J. (2009). Fighting fat with fat: the expanding field of adipose stem cells. Cell Stem Cell 5, 472–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeve D., Seo J., Suh J. M., Stenesen D., Tang W., Berglund E. D., Wan Y., Williams L. J., Lim A., Martinez M. J., et al. (2012). Wnt signaling activation in adipose progenitors promotes insulin-independent muscle glucose uptake. Cell Metab. 15, 492–504 [DOI] [PMC free article] [PubMed] [Google Scholar]