Abstract
Enhancement of sound-evoked responses in auditory cortex (ACx) following administration of systemic nicotine is known to depend on activation of extracellular-signaling regulated kinase (ERK), but the nature of this enhancement is not clear. Here, we show that systemic nicotine increases the density of cells immunolabeled for phosphorylated (activated) ERK (P-ERK) in mouse primary ACx (A1). Cortical injection of dihydro-β-erythroidine reduced nicotine-induced P-ERK immunolabel, suggesting a role for nicotinic acetylcholine receptors (nAChRs) located in A1 and containing α4 and β2 subunits. P-ERK expressing cells were distributed mainly in layers 2/3 and more sparsely in lower layers, with many cells exhibiting immunolabel within pyramidal-shaped somata and proximal apical dendrites. About one third of P-ERK positive cells also expressed calbindin. In the thalamus, P-ERK immunopositive cells were found in the nonlemniscal medial geniculate (MG) and adjacent nuclei, but were absent in the lemniscal MG. Pairing broad spectrum acoustic stimulation (white noise) with systemic nicotine increased P-ERK immunopositive cell density in ACx as well as the total amount of P-ERK protein, particularly the phosphorylated form of ERK2. However, narrow spectrum (tone) stimulation paired with nicotine increased P-ERK immunolabel preferentially at a site within A1 where the paired frequency was characteristic frequency (CF), relative to a second site with a spectrally-distant CF (two octaves above or below the paired frequency). Together, these results suggest that ERK is activated optimally where nicotinic signaling and sound-evoked neural activity converge.
Keywords: sensory, thalamocortical, nicotine, cholinergic, acetylcholine, MAPK
INTRODUCTION
Extracellular signal-regulated kinase (ERK), a subfamily of mitogen-activated protein kinases (MAPKs), has been implicated in sensory-associated learning by studies employing a variety of behavioral conditioning paradigms (e.g., Atkins et al., 1998; Berman et al., 1998; Schafe et al., 2000; Swank, 2000; Swank and Sweatt, 2001; Ye and Carew 2010). While the exact role of ERK in learning is unclear, it also is associated with conditions that can produce experience-dependent plasticity in sensory cortex. For example, in rat auditory cortex (ACx) deafness reduces the level of activated ERK, whereas stimulation of cochlear nerves in deafened rats elevates ERK activation (Tan et al., 2008). Similarly, visual stimulation activates ERK in visual cortex, and stimulation after dark rearing produces sustained ERK activation (Kaminska et al., 1999). These studies suggest that ERK activation may be common to sensory learning and plasticity in sensory cortex.
Sensory-associated learning involves filtering of behaviorally relevant sensory information, which may require activation of nicotinic acetylcholine receptors (nAChRs) by forebrain cholinergic systems. The drug nicotine enhances processing of attended sensory stimuli (Kassel, 1997; Levin et al., 2006; Hasselmo and Sarter, 2011), and can enhance the amplitude and lower the threshold of sensory-evoked cortical activity (Penschuck et al., 2002; Oldford and Castro-Alamancos, 2003; Liang et al., 2006; Disney et al., 2007; Kawai et al., 2011; Intskirveli and Metherate 2012). Importantly, a recent study has shown that nicotine-induced regulation of auditory processing depends on activation of ERK in primary ACx (A1) and thalamus (Intskirveli and Metherate, 2012). Thus, ERK emerges as a key molecule for regulating sensory processing, just as it is implicated in sensory learning and cortical plasticity.
If learning necessitates a convergence of sensory inputs with attention-related inputs in sensory cortex, an attractive notion is that ERK serves as a molecular node (Reissner et al. 2006) or coincidence detector (Sweatt 2001) to signal the requisite convergence. Since nicotinic mechanisms are important for attention (Hasselmo and Sarter 2011), convergence of sensory and nicotinic pathways in sensory cortex resulting in ERK activation may be critical for learning-related plasticity (London and Clayton 2008; Sweatt 2004; Welsby et al. 2009).
The present study aimed to explore this issue for ACx by investigating converging effects of systemically-administered nicotine and auditory-evoked neural activity on ERK activation in mouse ACx. We have investigated effects of nicotine on ERK activation and their dependence on nicotinic acetylcholine receptors. We examined the distribution of cells expressing activated (phosphorylated) ERK (P-ERK) in ACx and thalamus. And we sought to examine how nicotinic and auditory-evoked neuronal signaling interact to influence ERK activation in ACx.
MATERIALS AND METHODS
Animals
Adult mice (age 60–110 days; FVB strain) were used following NIH guidelines for animal use and as approved by the University of California, Irvine IACUC and by the Animal Use Committee at Soka University. Animals were housed in facilities with a 12/12 h light/dark cycle. On the day of experiments, mice were anesthetized using urethane (0.7 g/kg mouse, i.p., Sigma, Tokyo Kasei) and xylazine (13 mg/kg mouse, i.p., Phoenix Pharmaceuticals, Sigma). Supplemental doses were given as needed to maintain areflexia.
Anitibodies
For immunostaining of P-ERK, we used rabbit polyclonal and monoclonal antibodies (Cell Signaling Technology, Cat. # 9101 and 4370, respectively) raised against a synthetic peptide (KLH-coupled) corresponding to residues around Thr202/Tyr204 of human p44 MAPK. Each of these antibodies detects two bands for phosphorylated ERK1 and ERK2 according to manufacturer’s datasheet and has been used in many studies including Noma et al., 2008 (for #9101) and Zhang et al., 2010 (for #4370). Antibodies against calbindin D-28k (cl-300, Sigma) and calretinin (MAB1568, Chemicon) each recognize a single band in Western blot analysis using brain tissue samples (calbindin: Mastroberardino et al., 2002; calretinin: Fuentes-Santamaria et al., 2005). Both antibodies have been used in many previous immunohistochemical studies (e.g., calbindin: Cruikshank et al., 2001; calretinin; Nunzi et al., 2001; Mirich et al., 2002).
Bilateral cortical microinjection
Craniotomies (each ~1 mm by 1.5 mm) were performed to expose ACx bilaterally. Hamilton syringes fitted with glass pipettes (tips, 30–50 μm diameter) were filled with either artificial cerebrospinal fluid (ACSF; 145 mM NaCl, 2.5 mM, 1.8 mM CaCl2, 1 mM MgCl2, 25 mM glucose, 1.25mM NaH2PO4, 25 mM NaHCO3) or 1~10 μM dihydro-β-erythroidine (DHβE) in ACSF. To mark injection sites, solutions contained either Fluorescin or Lucifer yellow-dextran (Invitrogen). The injection site in A1 was determined physiologically, as described below (Electrophysiological localization of A1), and was 400–500 μm beneath the cortical surface. Solutions were simultaneously injected bilaterally at a rate of ~10 nl per minute over 10 min. After an additional 5 min, nicotine was injected (2 mg/kg tartrate salt, calculated to be 0.7 mg/kg free base, i.p., N5260, Sigma; pH adjusted to ~7.4 with NaOH).
Immunohistochemistry using 3,3′-diaminobenzidine (DAB)
Deeply anesthetized mice were perfused transcardially with phosphate buffered saline (PBS, 0.1 M sodium phosphate, 0.9% NaCl) followed with 4% paraformaldehyde (PFA) for ~10 min using a peristaltic pump (~10 ml/min). Brains were fixed overnight at 4°C and sectioned at 50 μm using a vibrating tissue slicer. For sections with drug/dye injections, the central four sections showing peak fluorescence for each dye were selected. After rinsing in 0.05 M Tris-buffered saline (TBS; pH 7.5), the sections were incubated in 3% H2O2 and 10% CH3OH for 5 min. Following wash in TBS, sections were incubated in 5% bovine serum albumin (BSA) and 0.3 % Triton X-100 (TX-100) for 90 min. After blocking, the sections were incubated in rabbit anti-phospho-ERK1/2 (Thr202/Tyr204) antibody (1:400 dilution, cat-9101; Cell Signaling Technology) in the blocking solution overnight at 4 °C. The sections were washed and incubated in biotinylated goat anti-rabbit IgG (1:250 dilution, Vector Laboratories) for 2 h at room temperature. The sections were then washed and incubated in 1:50 avidin-biotin-peroxidase complex reagent (ABC; Vector) for 90 min at room temperature. After wash in TBS and then 0.05 M Tris-buffer (TB; pH 7.4), sections were put into DAB (10 mg/50 ml TB) and reacted with 0.003% H2O2 for a few minutes. Stained sections were washed and mounted for analysis.
Quantification of labeled cells
DAB stained sections were imaged using an Aperio imaging system (Aperio Technologies, San Diego) or an Axioskop light microscope (Zeiss). Regions of interest (ROIs) for analysis in ACx were determined as described below (Anatomical localization of ACx). For analysis, pixel intensity of several small regions in ACx without apparent positive staining was averaged as background, and cells with at least 3-fold higher intensity were considered immunopositive for P-ERK. P-ERK positive cells within ROIs were examined and counted manually, blind to drug treatment, and the cell density (cells/mm2) computed using NIH ImageJ software. The minimum area for the identified cells was estimated to be ~28 μm2. We included labeled cells that were on and within the ROI borders. Our visual inspection of adjacent sections in three animals showed little evidence of stained cell bodies at the surface of the facing sections. Therefore, little overcount is expected (Guillery, 2002).
For sections injected with fluorescent dyes, injection sites were identified using a fluorescent microscope (Axioskop 20, Zeiss), and pictures were taken in both fluorescent and bright field for the ROI. The area was split into 5 bins (200 μm width, spanning layers 1–6; Fig. 2) with bin 3 containing the highest fluorescence intensity, so that results could be determined with respect to distance from the injection site center (bin 3). The sections were analyzed blind to injected drugs. The mean density of immunopositive cells for each hemisphere was determined from four adjacent brain sections and compared using ANOVA. Cell density for two different conditions was compared using a Student t-test.
Figure 2.
Nicotinic enhancement of P-ERK immunopositive cell density is mediated by α4β2*-nAChRs located in A1. (A) Example of a control microinjection site in physiologically defined A1 labeled with Fluorescin dye (a), and the same section stained for P-ERK immunolabel (b). Five 200 μm-wide bins (1–5) that span A1, with the middle bin (3) centered on the injection site, are indicated by solid and dashed lines for cell density analysis. Scale bar is 200 μm. (B) (left) Average P-ERK immunopositive cell density in A1 elicited in response to systemic nicotine following intracortical injection of ACSF or DHβE (1–10 μM). P-ERK labeled cells within solid lines in (A) were counted for cell density. (right) The percent decrease in P-ERK immunopositive cells due to DHβE injection. Difference in the cell density between the ACSF- and DHβE-injected was normalized to that for the ACSF-injected for each set of experiments and averaged (mean ± S.E.M, n = 4, *p < 0.01, unpaired t-test). (C) Average P-ERK immunopositive cell density in each of 5 bins in A1 for the ACSF- and DHβE-injected hemispheres. Error bars indicate S.E.M.
Immunofluorochemistry
Anesthetized animals were perfusion-fixed as above and post-fixed in the same fixative for 3 hours at room temperature or overnight at 4 °C. Coronal sections were prepared at 40 μm. Free-floating sections were blocked in a blocking solution (5% normal goat or donkey serum, 0.3% Triton X-100, 0.9% NaCl, 0.1 M sodium phosphate buffer, pH7.4) for at least 1 h, incubated in the blocking solution containing primary antibodies (rabbit anti-phospho-ERK1/2, 1/400 dilution, #4370, Cell Signaling; mouse anti-calbindin D-28k, 1/500 dilution; mouse anti-calretinin, 1/500 dilution) for 1.5 hours at room temperature or overnight at 4 °C with gentle agitation. After washing in PBS, sections were incubated for 1.5 hours at room temperature in the blocking solution containing secondary antibodies (1:250 dilution; Alexa Fluor-594-conjugated donkey anti-rabbit IgG, Alexa Fluor-488-conjugated donkey anti-mouse IgG, Molecular Probes/Invitrogen). Stained sections were mounted on glass slides using Vectashield (Vector) and imaged using an AxioCam digital camera mounted on an Axioskop 20 fluorescent microscope (Zeiss). Labeled cells usually had multiple processes extending from presumed cell bodies. Those at and within ROI borders have been included for cell counts.
Anatomical localization of ACx
To estimate the location of ACx in coronal sections for experiments without physiological verification, we used the method illustrated in Fig. 1A. We first constructed a horizontal line (perpendicular to the midline) through the center of the ventral division of the medial geniculate body (MGv), and constructed a second line through temporal cortex from the MGv at a 20° angle to the first line. This second line was assumed to run through ACx. The cortical ROI for counting cells was a ~1 mm2 region delimited by the cortical surface, the border between layer 6 and the subcortical white matter, and two lines parallel to, and ± 0.5 mm from, the 20° line. This method was found to identify ACx consistently in tissue that had been confirmed physiologically (see below) or histologically (cf., Cruikshank et al., 2002).
Figure 1.
Systemic nicotine increases density of P-ERK immunopositive cells in ACx. (A) Method for estimating ACx location is diagrammed on a control coronal section with P-ERK immunopositive cells visualized using DAB immunohistochemistry. The dotted box contains the ROI used for cell counting; the dotted circle outlines the MGv. Scale bar is 1 mm. (B) Auditory cortex and surrounding cortical areas indicated by a vertical line in (A) were magnified. More P-ERK labeled cells are found in the area ventral to ACx (e.g., perirhinal cortex, PRh). (C) DAB-stained sections showing P-ERK immunopositive cells in ACx of control and nicotine-exposed mice. Laminar boundaries are estimates based on published dimensions (Anderson et al., 2009). Bottom images are magnified from boxes in top images to show example cells including those with pyramidal shapes. Scale bars are 200 μm (top) and 50 μm (bottom). (D) (left) Average cell density of P-ERK immunopositive cells in ACx for control and nicotine-treated mice. (right) The percent increase due to systemic nicotine over control. For each set of experiments, the cell density for nicotine was normalized to that for control and averaged (n = 4, *p < 0.05, unpaired t-test). Error bars indicate S.E.M.
Acoustic stimulation
White noise and tone stimuli were digitally synthesized using MALab (Kaiser Instruments) on a Macintosh computer and were delivered through a speaker (ES-1 or FF-1 with ED-1 driver, Tucker-Davis Technologies) positioned ~3 cm in front of the left ear (open field). For calibration (SPL in decibels, dB, relative to 20 μPa) a microphone (model 4939 with Nexus amplifier, Brüel and Kjaer) was positioned in place of the animal at the tip of the left ear bar. Pure tones (100 ms duration, 5 ms rise/fall ramps) were presented to the contralateral ear with a frequency range of 3–40 kHz and an intensity range of 5 to 70 dB SPL. For Western blot experiments, white noise stimuli (60 dB SPL, 100 ms duration, 0.5 Hz rate) were digitally synthesized and controlled using custom-made software for Windows PC (developed by and freely available from the Computing and Engineering Core Facility, Center for Hearing Research, UC Irvine) and were delivered through a speaker (RX-DS620, Panasonic) positioned ~10 cm in front of the left ear.
Electrophysiological localization of A1
For functional localization of A1, we mapped tone-evoked responses across ACx using either local field potential (LFP) recordings from the cortical surface or multiunit recordings from the middle layers in order to determine the expected tonotopic arrangement (Kawai et al., 2007). In brief, responses to tones in 5 kHz steps at near-threshold intensities (−10 to 20 dB SPL) were filtered and amplified (1 Hz to 10 kHz, AI-401 or AI-405 CyberAmp380), and digitally recorded using AxoGraph X (AxoGraph Scientific) on a Mac computer. Reversal of tonotopy indicated the border with the anterior auditory field. We then located central A1 along the dorsoventral axis by determining the site with the shortest latency and largest amplitude surface LFP. After surface mapping, we confirmed tonotopy in A1 by recording multiunit activity in the middle layers using a tungsten electrode (1~2 MΩ) and constructing tuning curves. Characteristic frequency (CF; the frequency generating responses with the lowest threshold) determined this way matched within ~1 kHz those determined in surface LFP recordings.
Western blot analysis
Following anesthesia, a needle with a catheter for saline or nicotine injection was placed intraperitoneally and mice were left untouched for ~1 h in front of a speaker. Saline or nicotine was then injected, and white noise stimulation was initiated (or no stimulation for control). After 6 min white noise exposure, animals were sacrificed by guillotine and brains quickly removed into ice-cold ACSF-HEPES (145 mM NaCl, 2.5 mM, 1.8 mM CaCl2, 1 mM MgCl2, 25 mM glucose, 1.25mM NaH2PO4, 5 mM HEPES, pH 7.4) and frozen at −80 °C. We cut coronal sections on a cryostat starting from the posterior end of the brain until the MG became visible, and then identified ACx using the method described above (see Anatomical localization of ACx) and removed ~1 mm3 blocks containing the identified region. Blocks were stored frozen until homogenization. Samples were prepared by homogenizing the frozen blocks in a homogenization-lysis buffer (10 mM Tris-HCl, pH 7.5, 1 mM EDTA, 2.5 mM Na-pyrophosphate, 1 mM Na-orthovanadate, 1 mM β-glycerophosphate, 1% Igepal CA-630, Complete EGTA-free protease inhibitor cocktail (Roche), 1 mM PMSF) using a 2 ml Dounce with a Teflon pestle. Sample aliquots were subjected to BCA assay (Pierce/Thermo Scientific) for protein concentration determination, and remaining samples were mixed in a 5× sample buffer (312.5 mM Tris, pH 6.8, 50% glycerol, 10% SDS, 5% β-mercaptoethanol, 0.00625% bromophenol blue). Following SDS-PAGE (10% T polyacrylamide gel, 2.6%C), samples were transferred to PVDF membrane (Millipore). The membranes were blocked and incubated with rabbit anti-GAPDH antibodies (#G9545, Sigma; sample loading internal control) and either mouse anti-Phospho-ERK1/2 (#9106, Cell Signaling) or rabbit anti-ERK1/2 antibodies (#4695, Cell Signaling) in a blocking solution (5% dry fat milk, 5% BSA, 0.1% Tween 20 in 50 mM Tris buffered saline, pH 7.5) for 1.5 h at room temperature or at 4 °C overnight. After washing, bound primary antibodies were recognized with horse anti-mouse (Cell Signaling) and donkey anti-rabbit (Jackson Immunoresearch) antibodies conjugated to horseradish peroxidase (HRP). Immunoblots were detected by HRP reaction with ECL reagents (ECL Plus or Prime, GE Healthcare) using LAS 3000 imaging system (Fujifilm), making sure that chemiluminescence detection was within a linear range, and were quantified by densitometric scans of autoradiographs using ImageJ software (NIH). To calculate the P-ERK/ERK ratio, pixel densities of ERKs and P-ERKs were normalized to those of GAPDH, the normalized P-ERK values were divided by the ERK values, and the PERK/ERK ratio for saline was used to normalize that for nicotine and white noise stimulation. Note that average percent difference in the GAPDH optical density (OD) between saline and paired nicotine and white noise stimulation, calculated by 100% × (ODpaired –ODsaline)/ODsaline, was 4.5 ± 15.3% (n = 7, unpaired t-test: p = 0.77), indicating little change in GAPDH level due to the paired stimulation.
Image preparation for figures
Captured TIFF and JPG images of DAB staining and fluorescence were processed using Photoshop CSS (Version 12.1 ×32). For Fig. 3, brightness and contrast were adjusted for better visualization of labeled cells, with adjustments applied to the entire image.
Figure 3.
Distribution of P-ERK and calbindin immunopositive cells in ACx. (A) Example immunofluorescence images showing P-ERK positive cells mainly in superficial layers, with fewer labeled in lower layers (left). Boxed area is magnified at right. White arrows indicate pyramidal shaped neurons with apical dendrites, and arrowheads indicate nonpyramdal shaped neurons. Scale bar is 200 μm (left) or 40 μm (right). (B) Double immunofluorescence images for calbindin (a), P-ERK (b), and overlay (c). Arrows point to neurons co-expressing both P-ERK and calbindin, which are shown at higher magnification in boxed areas (i) and (ii). Scale bar is 200 μm or 100 μm (boxed areas). (C) Double immunofluorescence images for calretinin (a), P-ERK (b), and overlay (c). Superficial layers were expanded on the right. Scale bar is 200 μm.
RESULTS
These studies were intended to characterize possible interactions of nicotine-induced and auditory-evoked ERK activation in A1. We first examined the effect of systemic nicotine on the distribution of P-ERK immunolabel in ACx (identified using anatomical landmarks) and its dependence on cortical nAChRs in A1 (identified physiologically). We also compared the distribution of P-ERK immunolabel to that of other cell markers with known distribution in the auditory forebrain. Then, we examined converging effects of nicotinic activation and two forms of acoustic stimulation on P-ERK immunolabel. Each of these results is presented in turn.
Systemic nicotine increases P-ERK immunopositive cells via nAChRs in A1
Adult mice were treated with nicotine (0.7 mg/kg free base, i.p.) for 10 min then sacrificed upon fixative perfusion, and coronal sections of fixed brains were processed for immunohistochemistry using antibodies against P-ERK and HRP-conjugated secondary antibodies for DAB reactions. In ACx as defined by a ROI in coronal sections using the MGv as a landmark (Fig. 1A), DAB stained cells were found in clusters in the superficial layers (layers 2/3) and sparsely in layers 5/6 (Fig. 1C). To quantify P-ERK positive cell density in ACx, we counted immunopositive cells in 4–6 sections per animal (details in Materials and Methods). In untreated control mice (brains processed simultaneously with treated brains), PERK positive cell density varied from 279 ± 22 cells/mm2 to 672 ± 25 cells/mm2 with an average of 501 ± 87 cells/mm2 (n = 4). Systemic nicotine increased the P-ERK positive cell density to 897 ± 205 cells/mm2 on average (Fig., 1D left, n = 4, paired t-test p = 0.067), an increase of 75 ± 27% (Fig. 1D right, nicotine data normalized to control for each set of experiments, unpaired t-test p < 0.05), demonstrating that systemic nicotine by itself (i.e., in the absence of acoustic stimulation) activates ERK in mouse ACx. Note, however, that the increase in P-ERK immunolabel was not restricted to the ROI, and appeared to occur similarly across many cortical areas.
To determine whether the effects of systemic nicotine involved nAChRs located within A1, in another set of mice we examined nicotine-induced P-ERK immunolabel following intracortical injection of DHβE (1 or 10 μM, ~0.1 μl), an antagonist at α4β2*-nAChRs (asterisk indicates presence of additional subunits). A1 was identified physiologically, and to control for inter-animal variability in P-ERK immunolabel, was exposed bilaterally so that vehicle (ACSF) could be injected into the contralateral A1 for a within-animal control. Control and drug solutions contained fluorescent dye for post-fixation localization of injection sites (Fig. 2Aa). Systemic nicotine was delivered after intracortical injections, followed by perfusion and tissue processing as before. In four mice (4–5 sections per animal), the density of P-ERK positive cells averaged 246 ± 101 cells/mm2 in control A1 and 147 ± 71 cells/mm2 in DHβE-injected A1 (Fig. 2B). The nicotinic antagonist reduced the density of nicotine-induced P-ERK immunolabeled cells by 44 ± 5% (Fig. 2B, unpaired t-test p < 0.01), suggesting that systemic nicotine activates ERK in A1 via α4β2*-nAChRs that also are located in A1.
Because nicotine consistently activated ERK in cortical regions ventral to ACx (e.g., perirhinal cortex, Fig. 1B) to a greater degree than in ACx or cortical regions located more dorsally (data not shown), we further analyzed the data to determine if a dorso-ventral gradient of P-ERK expression existed within A1. For each coronal section we defined five, 200 μm-wide bins that spanned layers 1–6, with the middle bin centered on the injection site, and compared P-ERK immunolabel across bins (averaging across animals for each bin) (Fig. 2C). For both ACSF- and DHβE-injected hemispheres, the density of P-ERK positive cells did not differ across the five bins (ANOVAs, p > 0.05), suggesting that P-ERK positive cells were distributed uniformly across the dorso-ventral extent of A1, and that DHβE diffused evenly from the center of the injection site.
A comparison of the percent increase in P-ERK positive cell density due to systemic nicotine (Fig. 1) and the percent decrease of the nicotine-induced effect due to DHβE (Fig. 2) implies a nicotine effect of similar magnitude in each case (ANOVA, p > 0.3). That is, systemic nicotine induced a 75 ± 27% increase in P-ERK cell density (Fig. 1D), which was similar to the reduction of P-ERK cell density (Fig. 2B, left), when the latter is expressed as a percentage of the P-ERK cell density in DHβE (i.e., 82 ± 15%). This result suggests there is a baseline level of endogenously activated ERK, which is elevated by systemic nicotine mostly via α4β2*-nAChRs in A1.
P-ERK immunofluorescence in the auditory thalamocortical system
Recent work indicates that nicotine enhancement of auditory-evoked responses in A1 requires activation of ERK both in A1 and in the thalamus and/or thalamocortical pathway (Intskirveli and Metherate, 2012). ERK activation in the thalamus appears to mediate nicotinic enhancement of inputs to A1, while ERK activation in A1 mediates enhancement of intracortical activity. In order to postulate potential mechanisms underlying ERK-mediated effects, we examined P-ERK in A1 and MG using immunofluorescent staining methods.
Immunofluoresent staining of nicotine-induced P-ERK (Fig. 3) was similar to immunohistological results (Fig. 1) in terms of laminar and areal distribution and inter-animal variability, although in general the absolute number of immunofluorescent-labeled cells appeared to be less. P-ERK positive immunofluorescence was more strongly biased towards layer 2/3 over the lower layers (Fig. 3A). Some stained cells had pyramidal–shaped somata with apical dendrites extending towards the cortical surface (arrows in Fig. 3A), but others appeared to be nonpyramidal (arrowheads in Fig. 3A). Since the calcium-binding protein calbindin is expressed highly in layer 2/3 of mouse A1, consistent with its general distribution within the nonlemniscal auditory forebrain (see thalamic staining below and Cruikshank et al., 2001), we examined possible co-distribution of calbindin and P-ERK. Calbindin immunolabel in A1 was distributed as expected, with dense staining especially in layers 2/3 (Fig 3Ba). In tissue that was double-labeled for calbindin and P-ERK, we found that 37 ± 3% of P-ERK immunolabled cells co-expressed calbindin (Fig. 3B; n = 3 mice).
Since another calcium binding protein, calretinin, also is preferentially expressed in superficial layers of rat A1 (Hof et al., 1999; Ouda et al., 2012), we also analyzed its distribution and co-expression with nicotine-induced P-ERK immunolabel. However, only a small number of neurons, distributed mostly in superficial layers, exhibited calretinin immunofluorescence, and none were co-labeled with P-ERK (0 out of 53 P-ERK positive cells, n = 3 mice; Fig. 3C).
Thus, nicotine-induced ERK activation in A1 appears in some calbindin expressing neurons, notably some layer 2/3 pyramidal and nonpyramidal neurons, but not in calretinin expressing neurons.
In the auditory thalamus, the distribution of calbindin is highly distinctive and has been shown to distinguish lemniscal and nonlemniscal subdivisions of the MG in several species including mouse (Cruikshank et al., 2001). This characteristic distribution was reproduced in our results, i.e., neurons in the lemniscal MGv exhibited weak or no calbindin staining, whereas the surrounding nonlemniscal subdivisions exhibited strong calbindin expression (Fig. 4, left column). A comparison with nicotine-induced P-ERK immunofluorescence in the same tissue revealed a striking result: P-ERK immunofluorescent somata occurred only in nonlemniscal regions of the auditory thalamus (Fig. 4, middle and right columns). Among the nonlemniscal subdivisions, P-ERK positive immunofluorescence was found mostly in the posterior intralaminar nucleus (PIL) and to a lesser extent in the dorsal and the medial divisions of the MG and the peripeduncular nucleus (PP). Although the regional distribution of P-ERK and calbindin immunofluorescence overlapped completely, this was not the case for individual cells; as in A1 (Fig. 3), only a minority of P-ERK positive cells co-expressed calbindin (arrowheads in Fig. 4).
Figure 4.
Distribution of calbindin and P-ERK immunopositive neurons in the nonlemniscal auditory thalamus. Examples of coronal sections from posterior (A), middle (B) and anterior (C) portions of the MG showing immunolabel for calbindin (1), P-ERK (2) and both (3). Arrowheads point to cells co-expressing calbindin and P-ERK. Bar is 200 μm. Abbreviations: MGv, the ventral division of medial geniculate body; PP, peripeduncular nucleus; PIL, Posterior intralaminar nucleus; MGd, the dorsal division of medial geniculate body; dLG, dorsal lateral geniculate neucleus; MGm, the medial division of medial geniculate body
Pairing systemic nicotine with broad spectrum (white noise) acoustic stimulation
Systemic nicotine enhances auditory-evoked cortical responses in humans, rats and mice (Harkrider and Chapman, 2001; Liang et al., 2006; Kawai et al., 2011), an effect that can be blocked by inhibiting ERK activation in the auditory forebrain (Intskirveli and Metherate, 2012). These data indicate that an interaction of nicotinic signaling with auditory-evoked responses leads to increased P-ERK in A1. We therefore determined whether pairing systemic nicotine with acoustic (white noise) stimulation could increase P-ERK immunolabel in cortex. For this experiment, we did not identify A1 physiologically, so as to avoid the possibility that cortical damage from the craniotomy or microelectrode insertion might activate ERK (Chotiner et al., 2010); instead we identified ACx from anatomical landmarks (e.g., Fig. 1). We injected nicotine systemically and presented repeated white noise bursts (60 dB SPL, 100 ms duration, 2 s interstimulus interval) for 6 min (by which time nicotine will have enhanced acoustic-evoked responses; Kawai et al., 2011), and then counted P-ERK immunopositive cells in the ACx contralateral to the speaker (cells were counted for six consecutive 40 μm-thick sections in each animal, n = 4). Control animals (n = 3) were placed in the acoustic chamber and injected with saline, but were not subject to acoustic stimulation. The results show that, as before, P-ERK immunolabeled cells were distributed mostly in the upper layers of ACx (Fig. 5A). Fig. 5B shows cell density for individual animals as well as the overall average to demonstrate variability. The pairing of nicotine with white noise stimulation produced 3.5 times more immunolabeled cells than did control stimulation (Fig. 5B).
Figure 5.
Pairing systemic nicotine with white noise stimulation increases the number of PERK immunopositive cells and ERK2 levels in ACx. (A) Example of P-ERK positive cells in ACx after exposure to nicotine and white noise stimulation. ROI, region of interest used to identify ACx for cell counting. Bar equals 100 μm. (B) Individual and average counts for PERK immunopositive cells in controls (n = 3) and mice exposed to nicotine and white noise (Nic+WN, n = 4). (C) (left) Western blots for ERK1/2 (left) and P-ERK1/2 (right) for protein samples prepared from blocks of ACx in mice treated with saline (Sal) or paired nicotine and white noise (Nic+WN). GAPDH was used as a loading control. (right) The ratio of the pixel densities for P-ERK over ERK for each ERK subtype. The pixel density of P-ERK1, P-ERK2, ERK1 and ERK2 were normalized to that of GAPDH in each lane before computing the ratio and averaging (n = 3 mice, * p < 0.05). All error bars indicate S.E.M.
An increase in P-ERK immunopositive cells implies that the amount of P-ERK protein is also increased. Since P-ERK immunohistochemical staining does not reveal differences in the extent of phosphorylation, we analyzed the expression level of P-ERK using Western blot analysis. Because the antibody against P-ERK detects phosphorylated forms of both ERK1 (44 kDa) and ERK2 (42 kDa), we examined changes in the amount of each P-ERK subtype; in each case we also determined the amount of (unphosphorylated) ERK and measures were normalized to GAPDH levels (Fig. 5C). Protein samples from cortical tissues containing ACx were prepared from mice exposed to saline alone or to paired nicotine and white noise stimulation. We found that paired stimulation elevated the P-ERK2/ERK2 ratio 38 ± 12% compared to control (unpaired t-test p < 0.05, n = 3), whereas no change was observed for the P-ERK1/ERK1 ratio (14 ± 14%, n = 3). There were no differences in the amount of ERK1 or ERK2 between groups (normalized to GAPDH level, unpaired t-tests, p > 0.4 for ERK1 and p > 0.5 for ERK2).
Together, these data show that pairing systemic nicotine with white noise acoustic stimulation increased the density of P-ERK immunolabeled cells and the amount of P-ERK2 in ACx.
Focal activation of ERK within A1 after pairing systemic nicotine with narrow-spectrum (tone) acoustic stimulation
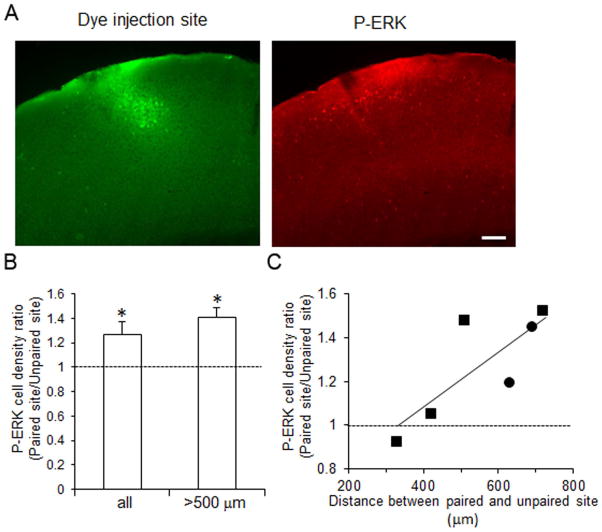
We next wished to determine whether the enhancement of ERK activation by nicotine paired with acoustic stimulation (Fig. 5) differed from that after nicotine alone (Fig. 1), and we wished to examine this issue using a within-animal design to control for inter-animal variability. We therefore mapped A1 in anesthetized mice and using fluorescent dye labeled two locations—one with a high frequency CF (28–32 kHz) and a second with a lower frequency CF (7–8.5 kHz). In each animal, CFs for the two labeled sites were separated by two octaves. We then paired systemic nicotine with repeated tone bursts of a single frequency, either the high or low CF, for 6 min and processed brains for P-ERK immunofluorescence (Fig. 6A) as before. The two dye injection sites were recovered, and at each site P-ERK immunolabled cells were counted across seven 40 μm-thick coronal sections centered on the dye injection. Comparison of P-ERK positive cell densities across the 7 sections for “paired” sites (CF tone paired with nicotine) or “unpaired” sites showed no difference (ANOVAs, p > 0.05, n = 6 each) and therefore cell densities were averaged across the 7 sections. The average cell density for the unpaired site was 119 ± 72 cells/mm2, while that for the paired site was 153 ± 46 cells/mm2. To control for inter-animal variability, we expressed P-ERK positive cell density as a ratio between the paired and unpaired sites for each animal (Fig. 6B). Although this ratio still varied considerably (see Fig. 6C for one explanation), P-ERK positive cell density at the paired site averaged 27 ± 10% higher than at the unpaired site (Fig. 6B, data for “all” sites; unpaired t-test, p < 0.05; similar results were observed for either high- or low-frequency paired tones).
Figure 6.
Pairing systemic nicotine with pure tone stimulation elevates P-ERK immunopositive cell density at a site in A1 where the paired tone is CF, relative to a second site with a spectrally-distant (two octaves away) CF. (A) Example showing fluorescent dye injection in A1 (left) and P-ERK immunolabeled cells in the same section (right) from a mouse stimulated with nicotine plus tone stimulation at the CF of the injection site. Bar is 200 μm. (B) Average ratio of P-ERK immunopositive cell density at paired/unpaired sites for all animals (“all”, n = 6, * p < 0.05), and for the subset of mice with >500 μm between injections sites (n = 4, * p < 0.02). Error bars indicate S.E.M. (C) P-ERK immunopositive cell density ratio increases with distance between injection sites. Symbols indicate whether paired tone was low frequency (squares) or high frequency (circles).
Although CFs at the paired and unpaired sites were always two octaves apart, the physical distance between the two sites varied considerably due to differences in the shape of the tonotopic map in A1. Since the distance between the two sites could be approximated by counting tissue sections between the two dye injections, we correlated anatomical distance with the ratio of P-ERK positive cell density at paired vs. unpaired sites (Fig. 6C). The results showed a striking positive correlation (regression coefficient, r2 = 0.81, p < 0.05, n = 6). Note that the P-ERK cell density ratio changed little for two animals with <500 μm separating the paired and unpaired sites. If we exclude these data, P-ERK positive cell density at the remaining paired sites averaged 41 ± 7% higher than at the unpaired sites (Fig. 6B, data for “>500 μm” sites, n = 4, unpaired t-test, p < 0.02). This analysis indicates that P-ERK positive cell density decreases with increasing anatomical distance from the paired site.
Overall, these results suggest that in A1 ERK is activated more effectively by systemic nicotine paired with acoustic stimulation (paired site) than by nicotine alone (unpaired site).
DISCUSSION
We have demonstrated for the mouse auditory forebrain that: (1) systemic nicotine increases the number of P-ERK immunopositive cells in A1 via α4β2*-nAChRs that also are located in A1; (2) some P-ERK expressing somata morphologically resemble pyramidal neurons while others appeared to be nonpyramidal; about one third were immunopositive for calbindin while none were immunopositive for calretinin; (3) P-ERK positive cells were found throughout the nonlemniscal auditory thalamus, in some cases co-labeled with calbindin immunopositivity, but were absent in the lemniscal MGv; (4) paired systemic nicotinic and broadband (white noise) acoustic stimulation increased the number of P-ERK immunopositive cells and the amount of P-ERK2, but not P-ERK1, in ACx; (5) paired nicotinic and narrow band (pure tone) acoustic stimulation increased the number of P-ERK immunopositive cells at cortical sites where the paired frequency was CF, relative to sites with a spectrally distant CF (not paired with nicotine). Together, these data suggest that both nicotine-induced and acoustic-evoked activity converge in A1 to activate ERK.
Distribution of nicotine-induced P-ERK immunopositive cells in ACx
Systemic nicotine consistently increased the density of P-ERK immunopositive cells in A1, most prominently in layer 2/3, and injection of DHβE in A1 showed that this effect depends on α4β2*-nAChRs. These findings are consistent with recent physiological studies showing that the effect of systemic nicotine to regulate tone-evoked responses in A1 is most prominent in the upper layers and depends on both α4β2*-nAChRs (Kawai et al., 2011) and subsequent activation of ERK (Intskirveli and Metherate, 2012). Together, the present and previous results indicate that sequential activation of α4β2*-nAChRs and ERK is required for nicotinic regulation of auditory information processing in A1.
Since nicotinic regulation in A1 has multiple interrelated features, the underlying mechanisms are likely also complex. Acoustic (tone) stimulation appears to activate first, via thalamocortical inputs, the region of A1 where the tone frequency is CF, followed by subsequent activation of other regions in A1 via horizontal intracortical pathways (Kaur et al., 2004, Kawai et al., 2011). Systemic nicotine both enhances the CF-evoked response and suppresses responses to spectrally-distant tones, effects that may involve enhancement of thalamocortical inputs, facilitation and/or disinhibition of local intracortical activity, and suppression of activity along (or activated by) horizontal intracortical pathways (Liang et al., 2006; Kawai et al., 2007; Kawai et al., 2011). Pharmacological inhibition of ERK activation either in the thalamus or A1 shows that P-ERK is required for nicotinic regulation of thalamocortical inputs, or local and long-distance intracortical activity (Intskirveli and Metherate, 2012). The present results reflect initial steps towards identifying the underlying neuronal elements, and indicate the potential involvement within A1 of ERK signaling in both excitatory and inhibitory neurons (see next paragraph, and final Discussion section), whereas the unexpected finding of P-ERK immunopositivity in the nonlemniscal, but not lemniscal, auditory thalamus restricts the possible mechanisms for enhanced thalamocortical input (see next Discussion section).
Nicotinic enhancement of local cortical excitability by activated ERK could involve a variety of mechanisms, including phosphorylation of voltage-gated potassium channels (Yuan et al., 2002; Hu et al., 2003; Schrader et al., 2009), facilitation of postsynaptic AMPA receptors (Zhu et al, 2002) or presynaptic enhancement of glutamate release (Jovanovic et al., 2000; Pereira et al., 2002). Increased excitability due to disinhibition also is a possibility; however the specific involvement of calretinin-containing neurons, although they have been implicated in cortical disinhibition (Gonchar and Burkhalter, 2003; Wang et al., 2004), may not be a factor given the lack of cells co-labeled with P-ERK and calretinin immunopositivity.
Nicotinic suppression of long-distance intracortical activity may involve P-ERK mediated facilitation of lateral inhibition. Calbindin-expressing interneurons suppress pyramidal neurons by contacting their distal dendrites (DeFelipe et al., 1990; Kawaguchi, 1993; Conde et al., 1994; Kawaguchi and Kondo, 2002, Markram et al., 2004), and may be involved in inter-column inhibitory modulation (Rao et al., 1999, 2000; Lund et al., 2003). Since α4β2*-nAChRs are expressed in many cortical interneurons (Xiang et al., 1998; Porter et al., 1999) and nicotine activates ERK in some calbindin-immunopositive nonpyramidal neurons, we speculate that some of these interneurons may contribute to nicotine-enhanced lateral inhibition.
Throughout this study we have noted variability in P-ERK positive cell density among different sets of experiments. One potential source of variability is the use of polyclonal vs. monoclonal primary antibodies (raised against same antigen, per manufacturer datasheet). However, since pilot experiments (not shown) comparing their immunofluorescence in the same animals produced similar results, a more likely possibility is the difference in visualization method (i.e., DAB vs. immunofluorescence). Signal amplification of DAB may have raised the baseline level of staining, resulting in higher cell counts. In addition to variability of immunostaining between sets of experiments, we also noted variability among animals within an experiment (leading to the use of within-animal controls, where possible). Sources of variability might be methodological (e.g., staining conditions, rearing conditions, time or day of experiments), or variability may be functionally important, just as inter-animal variability in nAChR density and function in the auditory forebrain correlates with behavioral performance in an auditory-cued conditioning task (Liang et al., 2008, Bieszczad et al., 2012). In any case, variability among animals treated similarly appears to be due to factors that are not controlled in our study. Nonetheless, in each experiment we observed consistent nicotinic effects on P-ERK positive cell density using percent-change or ratio measures, or within-animal controls.
Distribution of nicotine-induced P-ERK immunopositive cells in auditory thalamus
An unexpected result was that systemic nicotine produced P-ERK immunopositive cells in the nonlemniscal auditory thalamus—notably the medial and dorsal MG and PIL/PP—but not in the lemniscal MGv. This result was unexpected because MGv neurons provide the major thalamocortical input to the middle layers of A1 (typically, layers 3 and 4; Smith and Populin, 2001), and it is this input that is enhanced by systemic nicotine. Moreover, the nicotinic enhancement is blocked by injecting an inhibitor of ERK activation into the initial portion of the thalamocortical pathway (within the thalamus proper) (Intskirveli and Metherate 2012). It is conceivable that ERK does exist within lemniscal thalamocortical cells but is rapidly transported towards the terminals and therefore undetected in the soma. A future study of lemniscal axon terminals could resolve this issue. Nonetheless, the present data suggest an alternative explanation, i.e., that ERK-dependent thalamocortical regulation is mediated by nonlemniscal thalamic nuclei. Possible mechanisms may involve nicotinic regulation of nonlemniscal auditory thalamocortical projections to the upper layers of ACx (Frost and Caviness, 1980; LeDoux et al., 1985; Linke, 1999), interactions among lemniscal and nonlemniscal nuclei within the MG complex, or interactions within the thalamocortical pathway itself (Kawai et al., 2007), where lemniscal and nonlemniscal axons may course together. Interestingly, since the nonlemniscal MG and PIL have been implicated in auditory fear conditioning (Apergis-Schoute et al., 2005; Ping and Schafe, 2010), and ERK is implicated in learning-related plasticity (Atkins et al., 1998; Ye and Carew, 2010), it is possible that nicotinic activation of ERK in the auditory thalamus contributes to auditory learning.
Nicotine and sound pairing enhances ERK activation
A major finding of the present study is that pairing systemic nicotine with tones elevates P-ERK immunolabeling at a cortical site where the paired frequency is CF, relative to P-ERK immunopositivity at sites with spectrally-distant CFs (two octaves from the paired frequency). Since neural circuits throughout A1 are exposed to nicotine, preferential ERK activation at the “paired-tone” site suggests that nicotinic signaling and tone-evoked activity converge to synergistically elevate P-ERK. Lesser activation of ERK at the distant site may result solely from nicotinic signaling (in the absence of tone-evoked activity) or possibly to active regulation of activity via horizontal intracortical pathways (e.g., mediating lateral inhibition). While these results are consistent with those from experiments showing that pairing systemic nicotine with white noise increased P-ERK immunolabeling to a greater degree (~3.5-fold, Fig. 5) than nicotine stimulation alone (~1.8-fold, Fig. 1), the possible role of intracortical activity in regulating ERK will be the topic of future investigation.
The signaling mechanisms upstream to ERK activation that depend on α4β2*-nAChRs are not clear. For example, nicotine may activate presynaptic α4β2*-nAChRs to enhance glutamate release (Dickinson et al. 2008), which then could activate postsynaptic NMDA receptors to produce calcium influx and ERK phosphorylation (Kurino et al., 1995; Zhu et al., 2002). Nicotine also could act postsynaptically to depolarize cells and activate voltage-gated calcium channels, as proposed for postsynaptic α7-nAChRs (Chang and Berg, 2001; Dickinson et al., 2008) or DHβE-insensitive nAChRs (Steiner et al., 2007). Future studies need to identify both upstream signaling mechanisms as well as downstream targets of P-ERK in A1.
Acknowledgments
We thank Drs. Duo Zhang and Fan-Gang Zeng of the Computing and Engineering Core, Center for Hearing Research for the Windows-based white noise stimulator. The authors’ research was supported by the National Institutes of Health (R01 DA12929, P30 DC08369 (to R.M.) and RO3 DC08204 (to H.D.K.)) and the UC Irvine Center for Hearing Research.
References
- Anderson LA, Christianson GB, Linden JF. Mouse auditory cortex differs from visual and somatosensory cortices in the laminar distribution of cytochrome oxidase and acetylcholinesterase. Brain Res. 2009;1252:130–142. doi: 10.1016/j.brainres.2008.11.037. [DOI] [PubMed] [Google Scholar]
- Apergis-Schoute AM, Debiec J, Doyere V, LeDoux JE, Schafe GE. Auditory fear conditioning and long-term potentiation in the lateral amygdala require ERK/MAP kinase signaling in the auditory thalamus: a role for presynaptic plasticity in the fear system. J Neurosci. 2005;25(24):5730–5739. doi: 10.1523/JNEUROSCI.0096-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1(7):602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- Berman DE, Hazvi S, Rosenblum K, Seger R, Dudai Y. Specific and differential activation of mitogen-activated protein kinase cascades by unfamiliar taste in the insular cortex of the behaving rat. J Neurosci. 1998;18(23):10037–10044. doi: 10.1523/JNEUROSCI.18-23-10037.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieszczad KM, Kant R, Constantinescu CC, Pandey SK, Kawai HD, Metherate R, Weinberger NM, Mukherjee J. Nicotinic acetylcholine receptors in rat forebrain that bind (1)(8)F-nifene: relating PET imaging, autoradiography, and behavior. Synapse. 2012;66(5):418–434. doi: 10.1002/syn.21530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KT, Berg DK. Voltage-gated channels block nicotinic regulation of CREB phosphorylation and gene expression in neurons. Neuron. 2001;32(5):855–865. doi: 10.1016/s0896-6273(01)00516-5. [DOI] [PubMed] [Google Scholar]
- Chotiner JK, Nielson J, Farris S, Lewandowski G, Huang F, Banos K, de Leon R, Steward O. Assessment of the role of MAP kinase in mediating activity-dependent transcriptional activation of the immediate early gene Arc/Arg3.1 in the dentate gyrus in vivo. Learn Mem. 2010;17(2):117–129. doi: 10.1101/lm.1585910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde F, Lund JS, Jacobowitz DM, Baimbridge KG, Lewis DA. Local circuit neurons immunoreactive for calretinin, calbindin D-28k or parvalbumin in monkey prefrontal cortex: distribution and morphology. J Comp Neurol. 1994;341(1):95–116. doi: 10.1002/cne.903410109. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Killackey HP, Metherate R. Parvalbumin and calbindin are differentially distributed within primary and secondary subregions of the mouse auditory forebrain. Neuroscience. 2001;105(3):553–569. doi: 10.1016/s0306-4522(01)00226-3. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Rose HJ, Metherate R. Auditory thalamocortical synaptic transmission in vitro. J Neurophysiol. 2002;87(1):361–384. doi: 10.1152/jn.00549.2001. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Hendry SH, Hashikawa T, Molinari M, Jones EG. A microcolumnar structure of monkey cerebral cortex revealed by immunocytochemical studies of double bouquet cell axons. Neuroscience. 1990;37(3):655–673. doi: 10.1016/0306-4522(90)90097-n. [DOI] [PubMed] [Google Scholar]
- Dickinson JA, Kew JN, Wonnacott S. Presynaptic alpha 7- and beta 2-containing nicotinic acetylcholine receptors modulate excitatory amino acid release from rat prefrontal cortex nerve terminals via distinct cellular mechanisms. Mol Pharmacol. 2008;74(2):348–359. doi: 10.1124/mol.108.046623. [DOI] [PubMed] [Google Scholar]
- Disney AA, Aoki C, Hawken MJ. Gain modulation by nicotine in macaque v1. Neuron. 2007;56(4):701–713. doi: 10.1016/j.neuron.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost DO, Caviness VS., Jr Radial organization of thalamic projections to the neocortex in the mouse. J Comp Neurol. 1980;194(2):369–393. doi: 10.1002/cne.901940206. [DOI] [PubMed] [Google Scholar]
- Fuentes-Santamaria V, Alvarado JC, Taylor AR, Brunso-Bechtold JK, Henkel CK. Quantitative changes in calretinin immunostaining in the cochlear nuclei after unilateral cochlear removal in young ferrets. J Comp Neurol. 2005;483(4):458–475. doi: 10.1002/cne.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonchar Y, Burkhalter A. Distinct GABAergic targets of feedforward and feedback connections between lower and higher areas of rat visual cortex. J Neurosci. 2003;23(34):10904–10912. doi: 10.1523/JNEUROSCI.23-34-10904.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery RW. On counting and counting errors. J Comp Neurol. 2002;447(1):1–7. doi: 10.1002/cne.10221. [DOI] [PubMed] [Google Scholar]
- Gulyas AI, Hajos N, Freund TF. Interneurons containing calretinin are specialized to control other interneurons in the rat hippocampus. J Neurosci. 1996;16(10):3397–3411. doi: 10.1523/JNEUROSCI.16-10-03397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkrider AW, Champlin CA. Acute effect of nicotine on non-smokers: II. MLRs and 40-Hz responses. Hear Res. 2001;160(1–2):89–98. doi: 10.1016/s0378-5955(01)00346-x. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology. 2011;36(1):52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof PR, Glezer, Conde F, Flagg RA, Rubin MB, Nimchinsky EA, Vogt Weisenhorn DM. Cellular distribution of the calcium-binding proteins parvalbumin, calbindin, and calretinin in the neocortex of mammals: phylogenetic and developmental patterns. J Chem Neuroanat. 1999;16(2):77–116. doi: 10.1016/s0891-0618(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Hu K, Huang CS, Jan YN, Jan LY. ATP-sensitive potassium channel traffic regulation by adenosine and protein kinase C. Neuron. 2003;38(3):417–432. doi: 10.1016/s0896-6273(03)00256-3. [DOI] [PubMed] [Google Scholar]
- Intskirveli I, Metherate R. Nicotinic neuromodulation in auditory cortex requires MAPK activation in thalamocortical and intracortical circuits. J Neurophysiol. 2012;107(10):2782–2793. doi: 10.1152/jn.01129.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra TS. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci. 2000;3(4):323–329. doi: 10.1038/73888. [DOI] [PubMed] [Google Scholar]
- Kaminska B, Kaczmarek L, Zangenehpour S, Chaudhuri A. Rapid phosphorylation of Elk-1 transcription factor and activation of MAP kinase signal transduction pathways in response to visual stimulation. Mol Cell Neurosci. 1999;13(6):405–414. doi: 10.1006/mcne.1999.0757. [DOI] [PubMed] [Google Scholar]
- Kassel JD. Smoking and attention: a review and reformulation of the stimulus-filter hypothesis. Clin Psychol Rev. 1997;17(5):451–478. doi: 10.1016/s0272-7358(97)00032-9. [DOI] [PubMed] [Google Scholar]
- Kaur S, Lazar R, Metherate R. Intracortical pathways determine breadth of subthreshold frequency receptive fields in primary auditory cortex. J Neurophysiol. 2004;91(6):2551–2567. doi: 10.1152/jn.01121.2003. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci. 1993;13(11):4908–4923. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kondo S. Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex. J Neurocytol. 2002;31(3–5):277–287. doi: 10.1023/a:1024126110356. [DOI] [PubMed] [Google Scholar]
- Kawai H, Lazar R, Metherate R. Nicotinic control of axon excitability regulates thalamocortical transmission. Nat Neurosci. 2007;10(9):1168–1175. doi: 10.1038/nn1956. [DOI] [PubMed] [Google Scholar]
- Kawai HD, Kang HA, Metherate R. Heightened nicotinic regulation of auditory cortex during adolescence. J Neurosci. 2011;31(40):14367–14377. doi: 10.1523/JNEUROSCI.1705-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurino M, Fukunaga K, Ushio Y, Miyamoto E. Activation of mitogen-activated protein kinase in cultured rat hippocampal neurons by stimulation of glutamate receptors. J Neurochem. 1995;65(3):1282–1289. doi: 10.1046/j.1471-4159.1995.65031282.x. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Ruggiero DA, Reis DJ. Projections to the subcortical forebrain from anatomically defined regions of the medial geniculate body in the rat. J Comp Neurol. 1985;242(2):182–213. doi: 10.1002/cne.902420204. [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 2006;184(3–4):523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Liang K, Poytress BS, Chen Y, Leslie FM, Weinberger NM, Metherate R. Neonatal nicotine exposure impairs nicotinic enhancement of central auditory processing and auditory learning in adult rats. Eur J Neurosci. 2006;24(3):857–866. doi: 10.1111/j.1460-9568.2006.04945.x. [DOI] [PubMed] [Google Scholar]
- Liang K, Poytress BS, Weinberger NM, Metherate R. Nicotinic modulation of tone-evoked responses in auditory cortex reflects the strength of prior auditory learning. Neurobiol Learn Mem. 2008;90(1):138–146. doi: 10.1016/j.nlm.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke R. Organization of projections to temporal cortex originating in the thalamic posterior intralaminar nucleus of the rat. Exp Brain Res. 1999;127(3):314–320. doi: 10.1007/s002210050801. [DOI] [PubMed] [Google Scholar]
- London SE, Clayton DF. Functional identification of sensory mechanisms required for developmental song learning. Nat Neurosci. 2008;11(5):579–586. doi: 10.1038/nn.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund JS, Angelucci A, Bressloff PC. Anatomical substrates for functional columns in macaque monkey primary visual cortex. Cereb Cortex. 2003;13(1):15–24. doi: 10.1093/cercor/13.1.15. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5(10):793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Mastroberardino PG, Iannicola C, Nardacci R, Bernassola F, De Laurenzi V, Melino G, Moreno S, Pavone F, Oliverio S, Fesus L, Piacentini M. ‘Tissue’ transglutaminase ablation reduces neuronal death and prolongs survival in a mouse model of Huntington’s disease. Cell Death Differ. 2002;9(9):873–880. doi: 10.1038/sj.cdd.4401093. [DOI] [PubMed] [Google Scholar]
- Mirich JM, Williams NC, Berlau DJ, Brunjes PC. Comparative study of aging in the mouse olfactory bulb. J Comp Neurol. 2002;454(4):361–372. doi: 10.1002/cne.10426. [DOI] [PubMed] [Google Scholar]
- Noma N, Tsuboi Y, Kondo M, Matsumoto M, Sessle BJ, Kitagawa J, Saito K, Iwata K. Organization of pERK-immunoreactive cells in trigeminal spinal nucleus caudalis and upper cervical cord following capsaicin injection into oral and craniofacial regions in rats. J Comp Neurol. 2008;507(3):1428–1440. doi: 10.1002/cne.21620. [DOI] [PubMed] [Google Scholar]
- Nunzi MG, Birnstiel S, Bhattacharyya BJ, Slater NT, Mugnaini E. Unipolar brush cells form a glutamatergic projection system within the mouse cerebellar cortex. J Comp Neurol. 2001;434(3):329–341. doi: 10.1002/cne.1180. [DOI] [PubMed] [Google Scholar]
- Oldford E, Castro-Alamancos MA. Input-specific effects of acetylcholine on sensory and intracortical evoked responses in the “barrel cortex” in vivo. Neuroscience. 2003;117(3):769–778. doi: 10.1016/s0306-4522(02)00663-2. [DOI] [PubMed] [Google Scholar]
- Ouda L, Burianova J, Syka J. Age-related changes in calbindin and calretinin immunoreactivity in the central auditory system of the rat. Exp Gerontol. 2012;47(7):497–506. doi: 10.1016/j.exger.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Penschuck S, Chen-Bee CH, Prakash N, Frostig RD. In vivo modulation of a cortical functional sensory representation shortly after topical cholinergic agent application. J Comp Neurol. 2002;452(1):38–50. doi: 10.1002/cne.10361. [DOI] [PubMed] [Google Scholar]
- Pereira DB, Carvalho AP, Duarte CB. Non-specific effects of the MEK inhibitors PD098,059 and U0126 on glutamate release from hippocampal synaptosomes. Neuropharmacology. 2002;42(1):9–19. doi: 10.1016/s0028-3908(01)00162-9. [DOI] [PubMed] [Google Scholar]
- Ping J, Schafe GE. The NO-cGMP-PKG signaling pathway coordinately regulates ERK and ERK-driven gene expression at pre- and postsynaptic sites following LTP-inducing stimulation of thalamo-amygdala synapses. Neural Plast. 2010;2010:540940. doi: 10.1155/2010/540940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JT, Cauli B, Tsuzuki K, Lambolez B, Rossier J, Audinat E. Selective excitation of subtypes of neocortical interneurons by nicotinic receptors. J Neurosci. 1999;19(13):5228–5235. doi: 10.1523/JNEUROSCI.19-13-05228.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. Isodirectional tuning of adjacent interneurons and pyramidal cells during working memory: evidence for microcolumnar organization in PFC. J Neurophysiol. 1999;81(4):1903–1916. doi: 10.1152/jn.1999.81.4.1903. [DOI] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory. J Neurosci. 2000;20(1):485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissner KJ, Shobe JL, Carew TJ. Molecular nodes in memory processing: insights from Aplysia. Cell Mol Life Sci. 2006;63(9):963–974. doi: 10.1007/s00018-006-6022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning. J Neurosci. 2000;20(21):8177–8187. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader LA, Ren Y, Cheng F, Bui D, Sweatt JD, Anderson AE. Kv4.2 is a locus for PKC and ERK/MAPK cross-talk. Biochem J. 2009;417(3):705–715. doi: 10.1042/BJ20081213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PH, Populin LC. Fundamental differences between the thalamocortical recipient layers of the cat auditory and visual cortices. J Comp Neurol. 2001;436(4):508–519. doi: 10.1002/cne.1084. [DOI] [PubMed] [Google Scholar]
- Steiner RC, Heath CJ, Picciotto MR. Nicotine-induced phosphorylation of ERK in mouse primary cortical neurons: evidence for involvement of glutamatergic signaling and CaMKII. J Neurochem. 2007;103(2):666–678. doi: 10.1111/j.1471-4159.2007.04799.x. [DOI] [PubMed] [Google Scholar]
- Swank MW. Phosphorylation of MAP kinase and CREB in mouse cortex and amygdala during taste aversion learning. Neuroreport. 2000;11(8):1625–1630. doi: 10.1097/00001756-200006050-00006. [DOI] [PubMed] [Google Scholar]
- Swank MW, Sweatt JD. Increased histone acetyltransferase and lysine acetyltransferase activity and biphasic activation of the ERK/RSK cascade in insular cortex during novel taste learning. J Neurosci. 2001;21(10):3383–3391. doi: 10.1523/JNEUROSCI.21-10-03383.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76(1):1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14(3):311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Tan J, Widjaja S, Xu J, Shepherd RK. Cochlear implants stimulate activity-dependent CREB pathway in the deaf auditory cortex: implications for molecular plasticity induced by neural prosthetic devices. Cereb Cortex. 2008;18(8):1799–1813. doi: 10.1093/cercor/bhm206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Tegner J, Constantinidis C, Goldman-Rakic PS. Division of labor among distinct subtypes of inhibitory neurons in a cortical microcircuit of working memory. Proc Natl Acad Sci U S A. 2004;101(5):1368–1373. doi: 10.1073/pnas.0305337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsby PJ, Rowan MJ, Anwyl R. Intracellular mechanisms underlying the nicotinic enhancement of LTP in the rat dentate gyrus. Eur J Neurosci. 2009;29(1):65–75. doi: 10.1111/j.1460-9568.2008.06562.x. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Huguenard JR, Prince DA. Cholinergic switching within neocortical inhibitory networks. Science. 1998;281(5379):985–988. doi: 10.1126/science.281.5379.985. [DOI] [PubMed] [Google Scholar]
- Ye X, Carew TJ. Small G protein signaling in neuronal plasticity and memory formation: the specific role of ras family proteins. Neuron. 68(3):340–361. doi: 10.1016/j.neuron.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan LL, Adams JP, Swank M, Sweatt JD, Johnston D. Protein kinase modulation of dendritic K+ channels in hippocampus involves a mitogen-activated protein kinase pathway. J Neurosci. 2002;22(12):4860–4868. doi: 10.1523/JNEUROSCI.22-12-04860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Blackman BE, Schonemann MD, Zogovic-Kapsalis T, Pan X, Tagliaferri M, Harris HA, Cohen I, Pera RA, Mellon SH, Weiner RI, Leitman DC. Estrogen receptor beta-selective agonists stimulate calcium oscillations in human and mouse embryonic stem cell-derived neurons. PLoS One. 2010;5(7):e11791. doi: 10.1371/journal.pone.0011791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, Qin Y, Zhao M, Van Aelst L, Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110(4):443–455. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]