Abstract
Background
Conventional gene-therapy applications of hematopoietic stem cells (HSCs) involve purification of CD34+ progenitor cells from the mobilized peripheral blood, ex vivo transduction of the gene of interest into them, and reinfusion of the transduced CD34+ progenitor cells into patients. Eliminating the process of purification would save labor, time and money, while enhancing HSCs viability, transplantability and pluripotency. Lentiviral vectors have been widely used in gene therapy because they infect both dividing and nondividing cells and provide sustained transgene expression. One of the exceptions to this rule is quiescent primary lymphocytes, in which reverse transcription of viral DNA is not completed.
Methods
In the present study, we tested the possibility of targeting CD34+ progenitor cells within nonpurified human mobilized peripheral blood mononuclear cells (mPBMCs) utilizing vesicular stomatitis virus G (VSV-G) pseudotyped lentiviral vectors, based on the assumption that the CD34+ progenitor cells would be preferentially transduced. To further enhance the specificity of vector transduction, we also examined utilizing a modified Sindbis virus envelope (2.2) pseudotyped lentiviral vector, developed in our laboratory, that allows targeted transduction to specific cell receptors via antibody recognition.
Results
Both the VSV-G and 2.2 pseudotyped vectors achieved measurable results when they were used to target CD34+ progenitor cells in nonpurified mPBMCs.
Conclusions
Overall, the data obtained demonstrate the potential of ex vivo targeting of CD34+ progenitor cells without purification.
Keywords: gene therapy, targeting, lentiviral vector, hematopoietic stem cell, mobilized PBMCs
Introduction
The hematopoietic stem cell (HSC) is the progenitor of all types of blood cells. It is one of the best-characterized stem cells in the body and the only one that is currently being used in the clinic for the treatment of diseases such as congenital immunodeficiencies, leukemias and HIV [1-7]. The HSCs transplanted into patients are partially purified from mobilized peripheral blood using CD34+ antibody conjugated with magnetic beads, since CD34+ cells contain hematopoietic progenitor cells of which a small fraction is HSC [8-10]. For gene therapy application, these cells are transduced ex vivo, and reinfused into patients [11-14]. One of the adverse effects of these time-consuming, technically demanding, and costly procedures is the loss of HSC repopulating capacity [15-19]. Reducing the in vitro handling of HSCs would help preserve their inherent pluripotency, and the ideal way to achieve this reduction in handling would be to directly transduce CD34+ progenitor cells in the mobilized peripheral blood mononuclear cells (mPBMCs), bypassing the process of purification.
Lentiviral vectors have emerged as promising tools in gene therapy. They can infect a wide range of dividing and nondividing cells, including hematopoietic progenitor cells [20-24]. However, we have shown that HIV-1 is restricted for infection of quiescent primary T-lymohocytes [25], where HIV-1 DNA synthesis is initiated but reverse transcription is not completed [25]. Because human mPBMCs consist primarily of mobilized hematopoietic stem cells from the bone marrow and quiescent primary lymphocytes from the peripheral blood, we investigated the possibility that vesicular stomatitis virus G (VSV-G) pseudotyped lentiviral vectors derived from HIV-1 can be used to transfer genes into CD34+ progenitor cells, based on the assumption that the stem cells would be preferentially transduced in the nonpurified mPBMCs. However, because VSV-G is a broad-host virus envelope [26], nonspecific transduction could occur in other cell types resident in PBMCs such as activated T-cells and macrophages. To further limit gene transfer into CD34+ progenitor cells, we used a modified Sindbis virus envelope to pseudotype the lentiviral vectors and confer specificity at the level of cell entry.
We have previously developed and described a targeting lentiviral vector pseudotyped with a modified version of the Sindbis virus envelope proteins [27,28]. The ZZ domain of protein A has been incorporated into the Sindbis envelope, and vector transduction is achieved through antibody binding to specific antigens on the surface of the targeted cells. Several mutations, including deletion of amino acid 61–64 in the E3 protein and mutation of amino acids, K159A, E160A, and SLKQ68–71AAAA, in the E2 protein, have been generated in the Sindbis envelope glycoprotein to decrease the natural tropism of Sindbis and, consequently, its background infectivity, while maintaining high vector titers. Mutations of amino acids 226 and 227 in the E1 protein to S and G allow E1 to mediate fusion in the absence of cholesterol in the target membrane, thereby increasing both the tropism and infectivity of Sindbis pseudotyped vectors [28]. We have designated the resulting envelope 2.2. The 2.2 vector has successfully targeted human leukocyte antigen (HLA) class I, CD4, CD19, CD20, CD45, CD146, P-glycoprotein of melanoma cells, and prostate stem cell antigen [27-32]. We utilized this targeting vector to transduce CD34+ progenitors cells in nonpurified human mPBMCs as a way of eliminating the step of purification of hematopoietic progenitor cells, comprising a streamlined approach that could ultimately make transduction more efficient and less expensive.
One of the critical factors to achieve specific transduction in CD34+ progenitor cells utilizing the 2.2 targeting vector is to identify appropriate hematopoietic progenitor cell surface markers. Molecules that represent the stem cell pluripotency and, at the same time, can serve as a receptor for gene transfer into CD34+ progenitor cells are promising targets. CD34, CD133 and C-kit are three common markers for selection of human hematopoietic progenitor cells [12,33-35]. Their distribution in hematopoietic progenitor population has some overlap but is not completely identical. Besides antigen selection, antibody selection is important as well because not all the targeting reagents mediate the same transduction efficiency. Sinbis virus enters cells through the pH-dependent endocytic pathway and we hypothesize that antibodies that can induce endocytosis of an antigen may lead to better transduction of the 2.2 targeting vector. It is known that antibodies that bind to different epitopes of an antigen behave differently in inducing endocytosis. For example, anti-HPCA-1, but not anti-HPCA-2, induces internalization of CD34 [36]. We examined multiple antibodies to these three antigens, some of which induce endocytosis and some of which do not, for transduction efficiency. The antibodies providing the best transduction efficiency were subsequently used to target CD34+ progenitor cells in mPBMCs.
Overall, our studies are the first to establish that it is possible to target CD34+ progenitor cells within nonpurified mPBMCs using both VSV-G envelope and 2.2 envelope pseudotyped lentiviral vectors.
Materials and methods
Cells
Human fetal livers in the age range 26–35 weeks were obtained from Advanced Bioscience Resources (ABR, Palo Alto, CA, USA) after approval from the Human Subjects Committee of the University of California, Los Angeles. To purify CD34+ progenitor cells, the fetal liver was washed three times with AIM-V media. Washed tissues were then cut to 3–4-mm pieces in AIM-V media with 10 mg/ml DNAse (Sigma, St. Louis, MO, USA), 1 mg/ml Collagenase (Sigma) and 1 mg/ml Hyaluronidase (Sigma). Cut tissues were further disrupted by passing the tissue fragments through a 60-ml syringe several times. Disrupted tissues were digested in more media at 37 °C for 1 h. To separate red and white blood cells, digested tissues were layered on Ficoll-Paque Plus (GE Healthcare, Piscataway, NJ, USA) and centrifuged for 20 min at 2400 r.p.m. 1201 g (Beckman Model TJ-6 centrifuge, Fullerton, CA, USA). The interphase cell layer was collected and washed with phosphate-buffered saline (PBS)/2% fetal calf serum (FCS) twice. Cells were resuspended in PBS/2% FCS and incubated with magnetic anti-CD34 antibody (Miltenyi Biotec, Bergisch Gladbach, Germany). Cells binding with the anti-CD34 antibody were separated by a MACS machine (Miltenyi Biotec). Human primary PBMCs were isolated from leukopacks, Ficoll purified and cultured in RPMI with 20% FCS (Omega Scientific, Tarzana, CA, USA). Frozen human mobilized PBMCs were obtained from AllCells (Emeryville, CA, USA). KG1a cells were obtained from Dr Kouki Morizono [30].
Virus production
All lentivirus vectors were produced by calcium-phosphate-mediated transient transfection of 293T cells, as previously described [27,28]. Briefly, 293T cells (1.8 × 107 cells) were transfected with 12.5 μg of pCMVR8.2ΔVPR, 12.5 μg of SIN18-RhMLV-E with central polypurine tract (termed cppt2e) [37] and optimized amounts (5–10 μg) of envelope-expressing plasmids. The viral vectors were harvested in OptiMEM (Invitrogen, Carlsbad, CA, USA) with 2% FCS and antibiotics. Lentiviral vectors were concentrated by ultracentrifugation at 18 000 5695 g RPI for 90 min at 4 °C by SW32 rotor (Beckman, Palo Alto, CA, USA). The pellets were resuspended in a 100-fold lower volume of HBSS. Virus were tittered in 293T cells and anti-HLA antibodies were used to titer the 2.2 vectors.
Cell transduction
5 × 104 CD34+ human fetal liver cells, human primary PBMCs, KG1a cells, and 2 × 105 mobilized human PBMCs were transduced with VSV-G (p24 = 20 ng/5 × 104 cell), 2.2 envelope (p24 = 100 ng/5 × 104 cell) conjugated with anti-HLA class I (1 μg/ml) (Sigma) or 2.2 envelope conjugated with anti-CD34 (1 μg/ml) clone My10 (Becton-Dickinson Biosciences, Franklin Lakes, NJ, USA), clone 8G12 (Becton-Dickinson Biosciences), clone TUKIII (Dako, Carpinteria, CA, USA), anti-C-kit (1 μg/ml) (Becton-Dickinson Biosciences), or anti-CD133 (1 μg/ml) (Miltenyi Biotech). Cells were incubated with virus in a retronectin-coated 48-well plate (Takara Bio Inc, Otsu, Japan) at 37 °C overnight. The next day, the transduced cells were treated with citric acid buffer (0.131 m citric acid, 0.066 m Na2HPO4, pH 5) for 10 s at room temperature. The buffer was then changed to IMEM media (SΛFC BioSciences, Lenexa, KS, USA) with 20% FCS, antibiotics, and 10 ng/ml interleukin (IL)-3, IL-6 and stem cell factor (SCF) (Amgen, Thousand Oaks, CA, USA). Three days post-infection, enhance green fluorescent protein (EGFP) expression was analysed by flow cytometry or plated in methylcellulose plates.
Colony forming assay
5 × 104 fetal liver CD34+ cells or 2 × 105 purified/nonpurified mobilized PBMCs were transduced with VSV-G envelope pseudotyped vectors or 2.2 targeting vectors and plated in 1 ml of methylcellulose medium supplemented with SCF, GM-CSF, IL-3 and Erythropoietin (Stem Cell Technology, Vancouver, Canada). Cells were cultured at 37 °C with 5% CO2 for 14 days then colonies (>30) were scored using fluorescence microscope (Olympus IX50, Centre Valley, PA, USA), colony pictures were taken using an Olympus camera DP10.
Results
Targeting human CD34+ progenitor cells using VSV-G pseudotyped lentiviral vectors
CD34+ cells were purified from fetal liver and cultured in IMDM with 20% FCS, IL-3, IL-6 and SCF [38]. As is typical, from day 0–3, we observed a decreased level (from 92% to 22%) of CD34 antigens presenting on the surface of the fetal liver CD34+ cells (Figure 1A), resulting from the differentiation of cells due to the incubation with cytokines [39,40].
Figure 1.
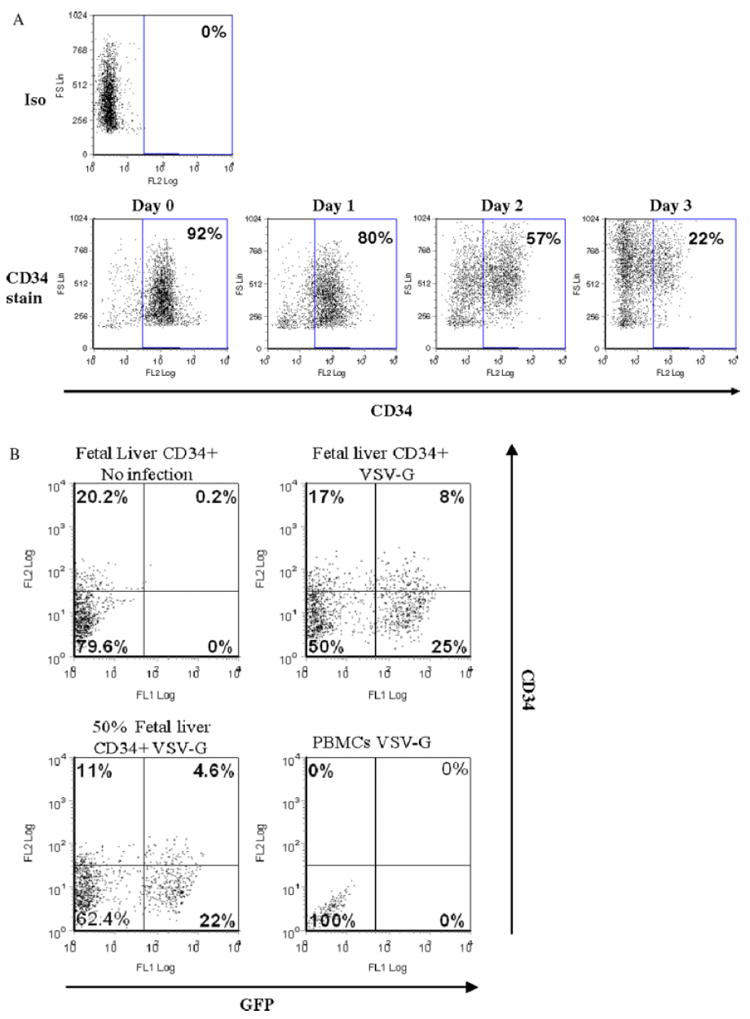
Human CD34+ progenitor cells can be targeted by VSV-G envelope pseudotyped lentiviral vectors. (A) 1 × 105 fetal liver CD34+ cells were stained with PE-conjugated CD34 antibodies (from day 0–3 after being cultured in IMDM with 20% FCS, IL-3, IL-6 and SCF. (B) 5 × 104 fetal liver CD34+ cells, fetal liver CD34+ cells mixed with nonstimulated PBMCs at a ratio of 1 : 1, and nonstimulated PBMCs alone were transduced by VSV-G envelope pseudotyped vectors. Negative controls were not transduced. Cells were transduced by VSV-G overnight. The next day, cells were washed and cultured in IMDM with 20% FCS, IL-3, IL-6 and SCF. Three days post-transduction, 1 × 105 cells were stained for CD34 and transductions were monitored by EGFP expression. Due to cytokine stimulation, the frequency of CD34+ cells decreased from approximately 90% on day 0 to that shown
To examine whether VSV-G envelope pseudotyped lentiviral vectors can specifically transduce CD34+ progenitor cells but not quiescent PBMCs, we transduced fetal liver CD34+ cells at day 0, nonstimulated PBMCs, and a mixture of fetal liver CD34+ cells with nonstimulated PBMCs. Nontransduced fetal liver CD34+ cells served as a negative control. With regard to the transduction efficiency by VSV-G pseudotyped vectors, the fetal liver CD34+ cells alone showed 33% transduction, whereas no transduction was observed in the nonstimulated PBMCs (Figure 1B). When we mixed fetal liver CD34+ cells with nonstimulated PBMCs at a 1 : 1 ratio, approximately 27% transduction was observed (Figure 1B), which was comparable to that of fetal liver CD34+ cells alone. These results suggest that VSV-G pseudotyped lentiviral vectors infect fetal liver CD34+ cells but not quiescent PBMCs, indicating its potential usefulness in targeting CD34+ progenitor cells in nonpurified mPBMCs.
Identification of antibodies for targeting human CD34+ progenitor cells with 2.2 pseudotyped lentiviral vectors
Although capable of selective transduction, VSV-G envelope has a broad tropism that could allow transduction of other cell types within the nonpurified mPBMCs, such as activated T-cells and macrophages [41,42]. To further enhance transduction specificity, we employed a modified Sindbis virus envelope, 2.2 targeting envelope, to target CD34+ progenitor cells via antibodies directed to cell surface molecules expressed preferentially on hematopoietic progenitor cells [28]. We tested multiple antibodies directed to hematopoietic progenitor cell markers, including CD34, C-kit and CD133, for infectivity. Antibodies binding to distinct epitopes of the CD34 antigen (clone My10 (anti-HPCA1), 8G12 (anti-HPCA2) and TUKIII) were also examined. The 2.2 targeting envelope was utilized to pseudotype lentiviral vectors bearing EGFP reporters. VSV-G pseudotypes were also tested for transduction efficiency. Antibodies directed to HLA class I, which is ubiquitously expressed on the surface of all the leukocytes, served as positive controls, and transductions in the absence of antibodies served as negative controls.
We recently established that lowering the pH of the cell culture medium briefly after transduction can enhance gene transfer by 2.2 targeting vectors in primary hematopoietic cells [30], and we therefore treated the transduced cells with low pH buffer for 10 s after overnight transduction to obtain enhanced transduction efficiency. Approximately 25% of the fetal liver CD34+ cells transduced by VSV-G pseudotyped vectors expressed EGFP (Figure 2). For 2.2 targeting vectors, the highest level of EGFP transduction (68%) was achieved by antibodies directed to HLA class I (Figure 2), which is consistent with the highly expressed HLA markers present on the surface of the fetal liver CD34+ cells. Among the stem cell antibodies, antibodies directed to class I epitopes of CD34 (clone My10) led to an approximately twofold higher transduction compared to other antibodies (Figure 2). Transductions in the absence of antibodies showed nearly no transduction of the cells. Clone My10 was thus chosen as the best antibody in subsequent experiments for directing the 2.2 vector to CD34+ cells.
Figure 2.
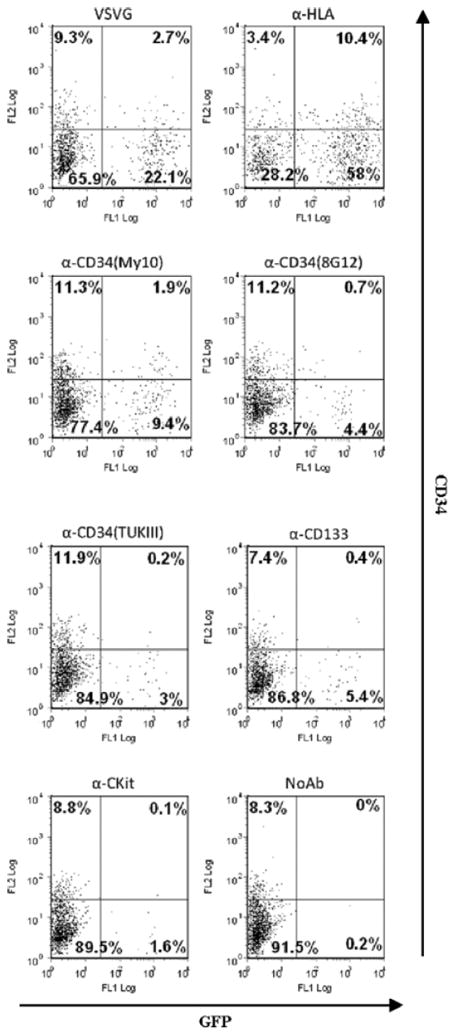
Targeting efficiency of antibodies directed against different epitopes of CD34+ progenitor cells. 5 × 104 fetal liver CD34+ cells were transduced by 2.2 targeting vectors. Antibodies to CD34 included clone My10, 8G12 and TUKIII. Other stem cell antibodies included anti-Ckit and anti-CD133. VSV-G envelope pseudotyped vectors were also tested for transduction efficiency. Anti-HLA directed transductions served as positive controls. Negative controls were transduction in the absence of antibody. Cells were treated with citric acid buffer (pH 5.5) for 10 s following overnight transduction. Cells were cultured in IMDM with 20% FCS, IL-3, IL-6 and SCF. Three days post-transduction, 1 × 105 cells were stained for CD34 and transductions were monitored by EGFP expression. Due to cytokine stimulation, the frequency of CD34+ cells decreased from approximately 90% on day 0 to that shown
Fetal liver CD34+ cells differentiate and loose the surface stem cell markers such as CD34 due to culture with cytokines [39,40]. Therefore, we confirmed the specificity of the CD34 (clone My10) antibody by testing the transduction efficiency of the 2.2 vector in the KG1a cell line, comprising an acute myelogenous leukemia cell line that expresses CD34 molecules, and stimulated PBMCs. CD34 antibody staining showed that 99.8% KG1a cells are positive for CD34. No CD34 is present on the stimulated PBMCs (Figure 3A). VSV-G pseudotyped vectors and 2.2 vectors conjugated with HLA antibodies were utilized as positive controls. For VSV-G pseudotyped vectors, the transduction efficiency was 4.6% and 16% in the KG1a cells and the stimulated PBMCs, respectively (Figure 3B). Transduction by 2.2 vectors via the HLA antibodies reached 14.1% in the KG1a cells and 2.7% in the stimulated PBMCs (Figure 3B). However, transduction by 2.2 vectors via the CD34 antibodies was only achieved in the KG1a cells with 17.1% efficiency, whereas no transduction was observed in the stimulated PBMCs (Figure 3B). These results indicated that transduction via the CD34 antibodies was specifically targeted to cells expressing CD34 antigens.
Figure 3.

Test of transduction specificity via antibodies to CD34. (A) 1 × 105 KG1a cells and stimulated PBMCs (ST PBMCs) were stained with PE-conjugated isotype and antibodies to CD34. (B) 5 × 104 KG1a cells and stimulated PBMCs were transduced by VSV-G envelope pseudotyped vectors and 2.2 targeting vectors for 2 h followed by treatment with citric acid buffer (pH 5.5) for 10 s. HLA antibodies were used as a positive control. Antibodies to CD34 (clone My10) were utilized for targeting. Cells were cultured in RPMI medium with 10% FCS and 1% L-glutamine/penicillin-G/Streptomycin. Three days post-transduction, 1 × 105 cells were monitored by EGFP expression
Targeting CD34+ progenitor cells in mixed cell populations
Because our ultimate goal is to target mobilized CD34+ cells in nonfractionated peripheral blood, we used PBMCs as the irrelevant cell type for assessing targeting specificity. Because the percentage of CD34+ cells within mPBMCs is approximately 1–2%, to mimic the status of mPBMCs, we added 1% of fetal liver CD34+ cells to nonstimulated PBMCs. A ratio of 50% was also examined as a positive control for successful transduction in the mixed cell populations. VSV-G pseudotypes and 2.2 vectors with antibodies directed to CD34 (clone My10) were tested for transduction efficiency within the mixed cell populations. Transduction efficiency was monitored by EGFP expression 3 days after treatment with the vectors. Because nonstimulated PBMCs are refractory to transduction, the decrease of the fetal liver CD34+ cells within the mixed cell populations resulted in a reduced number of both VSV-G and 2.2 transduced cells (Figure 4). In the ‘50% mix’ cell populations, VSV-G and anti-CD34 showed 25% and 5.5% EGFP+ cells, respectively (Figure 4). In the ‘1% mix’ cell populations, EGFP+ cells were all under 3% in the transduced cells by different vectors (Figure 4). Transduction was not detected in the PBMCs monoculture. Although transduction was obvious in the ‘50% mix’ populations, EGFP+ cells were scarcely evident in the ‘1% mix’ populations.
Figure 4.
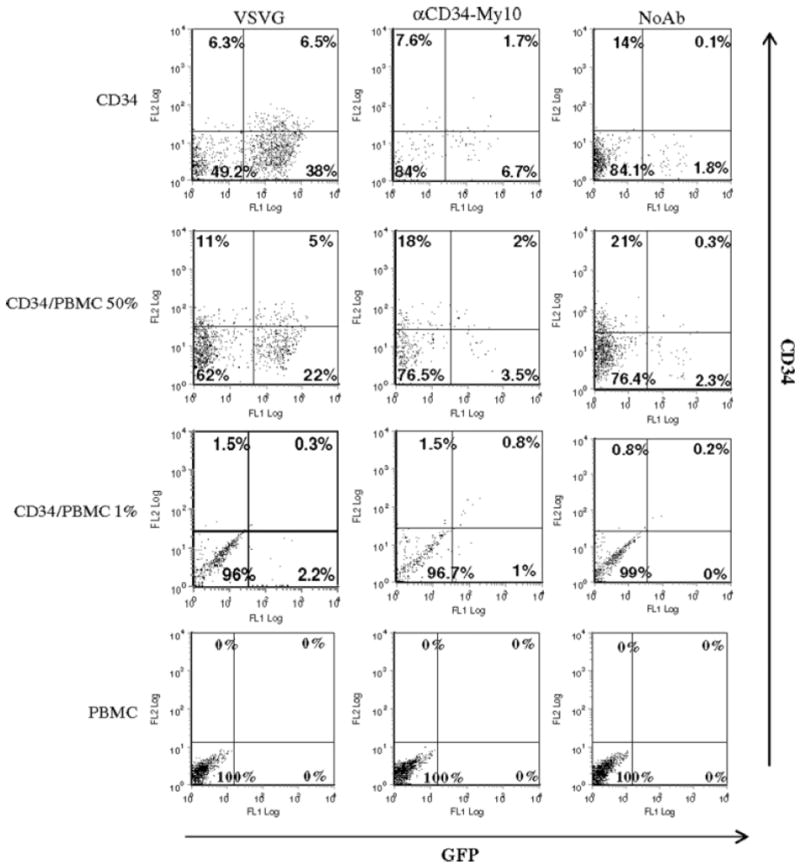
Targeting of CD34+ progenitor cells within mixed populations. Fetal liver CD34+ cells were mixed with nonstimulated PBMCs at different ratios (50% and 1%), resulting in a total number of 5 × 104 cells. 2.2 targeting vectors conjugated with CD34 antibody (clone My10) were used for transduction. VSV-G envelope pseudotyped vectors were also tested for transduction efficiency. Cells were treated with citric acid buffer (pH 5.5) for 10 s following overnight transduction. Three days post-transduction, 1 × 105 cells were stained for CD34 and transductions were monitored by EGFP expression. Due to cytokine stimulation, the frequency of CD34+ cells decreased from approximately 90% on day 0 to that shown
To overcome the limitations in sensitivity seen with flow-cytometry and to directly assay transduction of multipotent progenitor CD34+ cells, we performed colony-forming assays to determine the level of EGFP transduced progenitors. We plated the ‘1% mix’ fetal liver CD34+ cells transduced by VSV-G pseudotyped vectors and 2.2 targeting vectors in a methylcellulose medium supplemented with cytokines. Fetal liver CD34+ cells alone serve as a positive control. The resultant colonies, including colony forming unit of granulocyte (CFU-G), macrophage (CFU-M), granulocyte and macrophage (CFU-GM), and granulocyte, erythrocyte, macrophage and megakaryocyte (CFU-GEMM), were scored by fluorescence microscopy at day 14 (Figure 5). Within the 5 × 104 cells that were plated, the number of total colonies in the ‘1% mix’ cells was in the range 35–50 in all the transductions, which was approximately 1% of the total colonies observed in the fetal liver CD34+ cells alone (Table 1), and therefore consistent with the fraction of CD34+ cells in the ‘1% mix’. Importantly, in the ‘1% mix’ cells, the highest percentage of EGFP+ colonies (15%) was observed in the cells transduced with 2.2 targeting vectors using the clone My10 antibody (Table 1). Even though very few transduced cells were evident by flow cytometry in the 1% mix populations, almost 15% of the colonies were transduced, indicating targeting with a high degree of specificity. Transductions by VSV-G envelope showed 12% of EGFP+ colonies. Transductions in the absence of antibody showed no EGFP+ colonies (Table 1). Transductions directed by anti-HLA antibody served as a positive control.
Figure 5.
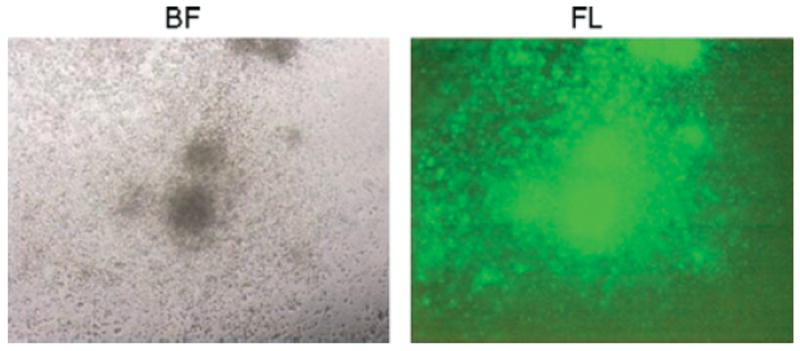
Colony-forming assay of 1% mix CD34+ cells. 5 × 104 cells of a mixture of 1% fetal liver CD34+ cells with nonstimulated PBMCs were transduced overnight by VSV-G envelope pseudotyped vectors or 2.2 targeting vectors overnight followed by a low-pH treatment. Antibodies to HLA class I and CD34 (clone My10) were used in the 2.2 transductions. The number of CFU-GM and CFU-GEMM colonies were scored under brightfield (BF), and EGFP+ colonies were scored under fluorescence (FL) after 14 days of culture in a methycellulose medium. The left panels show representative colonies under transmitted light. The right panels show the corresponding colonies under fluorescent light. EGFP+ colonies are green in color. Original magnification is × 20 for all panels
Table 1.
Colony-forming assay of fetal liver CD34+ cells and ‘1% mix’ CD34+ cells
| Total colonies | EGFP+ colonies | % EGFP+ colonies | |
|---|---|---|---|
| CD34 cells | |||
| VSVG | 4500 | 1525 | 34 |
| α-HLA | 4750 | 1200 | 25 |
| α-CD34 (My10) | 4375 | 400 | 9 |
| NoAb | 4625 | 0 | 0 |
| 1% CD34+ cells | |||
| VSVG | 50 | 6 | 12 |
| α-HLA | 48 | 5 | 10 |
| α-CD34 (My10) | 40 | 6 | 15 |
| NoAb | 35 | 0 | 0 |
5 × 104 of nonmix fetal liver CD34+ cells and a mixture of 1% fetal liver CD34+ cells with nonstimulated PBMCs were transduced overnight using VSV-G pseudotyped vectors or 2.2 targeting vectors followed by a low-pH treatment. Antibodies to HLA class I and CD34 (clone My10) were used in the 2.2 transductions. The number of CFU-G, CFU-M, CFU-GM, and CFU-GEMM colonies were scored under brightfield, and EGFP+ colonies were scored under fluorescence after 14 days of culture in a methycellulose medium supplemented with stem cell factor, GM-CSF, IL-3, and erythropoietin (Stem Cell Technologies). Compared to nonmix fetal liver CD34+ cells, the number of EGFP+ cells in the ‘1% mix’ fetal liver CD34+ cells were not significantly different from the expected efficiency (1%) in the α-CD34 (My10) group (p > 0.05). For the ‘1% mix’ cells, the group transduced by 2.2 with anti-CD34 (clone My10) achieved the highest number of EGFP+ colonies.
Targeting CD34+ progenitor cells in human mobilized PBMCs
The successful targeting of CD34+ progenitor cells within mixed cell populations provides evidence for the feasibility of directly targeting CD34+ progenitor cells in nonpurified human mPBMCs. Therefore, we confirmed our results in human mPBMCs, which were transduced by VSV-G pseudotyped vectors and 2.2 targeting vectors via anti-CD34 (clone My10). Transductions directed by anti-HLA antibody served as a positive control. Purified mPBMCs were also included for comparison of transduction efficiency with the nonpurified mPBMCs. Cells were stained for PE-conjugated CD34 antibodies before transduction to assess the expression of CD34 on the cell surface. The percentage of CD34+ cells was 1.6% and 71% in nonpurified mPBMCs and CD34 enriched mPBMCs, respectively (Figure 6). Transduced cells were plated in the methylcellulose medium and scored for EGFP + colonies 14 days after they were cultured. In nonpurified mPBMCs, cells transduced by VSV-G pseudotypes and 2.2 targeting vectors via CD34 antibodies (clone My10) yielded 7% and 4% EGFP+ colonies, respectively (Table 2). Importantly, the frequency of GFP+ colonies obtained with 2.2/αCD34 was similar regardless of whether the CD34+ cells were purified or not. Cells transduced in the absence of antibodies showed no EGFP+ colonies (Table 2). Therefore, we successfully demonstrated that transduction of CD34+ progenitor cells via lentiviral vectors in nonpurified mPBMCs was achievable.
Figure 6.

CD34 staining of fetal liver CD34+ cells and mPBMCs. 1 × 105 fetal liver CD34+ cells, nonpurified mPBMCs and purified mPBMCs were stained with PE-conjugated CD34 antibodies for 30 min then PE+ cells were scored by flow cytometry
Table 2.
Colony-forming assay of purified and nonpurified human mPBMCs
| Total colonies | EGFP+ colonies | % EGFP+ colonies | |
|---|---|---|---|
| Purified hPBMCs | |||
| VSVG | 3470 | 520 | 15 |
| α-HLA | 3300 | 396 | 12 |
| α-CD34 (My10) | 3180 | 191 | 6 |
| NoAb | 3050 | 0 | 0 |
| Nonpurified hPBMCs | |||
| VSVG | 112 | 8 | 7 |
| α-HLA | 108 | 12 | 8 |
| α-CD34 (My10) | 113 | 5 | 4 |
| NoAb | 120 | 0 | 0 |
2 × 105 purified and nonpurified human mPBMCs were transduced by VSV-G envelope pseudotyped vectors or 2.2 targeting vectors overnight followed by a low-pH treatment. Antibodies of HLA class I and CD34 (clone My10) were used in the 2.2 transductions. The number of CFU-G, CFU-M, CFU-GM, and CFU-GEMM colonies were scored under brightfield, and EGFP+ colonies were scored under fluorescence after 14 days of culture in a methycellulose medium supplemented with stem cell factor, GM-CSF, IL-3, and erythropoietin (Stem Cell Technologies). Transduction efficiency by α-CD34 (My10) in nonpurified mPBMCs is not significantly different from those in purified mPBMCs (p > 0.05).
Discussion
The potential applications of stem cell-based gene therapy are tremendous. Current protocols for transducing hematopoietic stem cells involve ex vivo purification of CD34+ progenitor cells, which is a process that can alter many of the functional properties of these cells [19,43,44]. In the present study, we describe a technology that represents a potential method for preserving the salient properties of hematopoietic progenitor cells, while allowing cell transduction with reasonable efficiency and at lower cost.
Lentiviral vectors have been reported to transduce nondividing cells such as neurons and other terminally differentiated cells efficiently [23,45], which makes lentiviral vectors appealing potential vectors in gene therapy. Transduction of relatively nondividing hematopoietic progenitor cells by lentiviral vectors has also been successful [46]. The exception is quiescent primary T-lymphocytes, in which gene transfer does not occur due to incomplete reverse transcription of viral DNA [25]. We used this exception to our advantage in the present studies, by successfully targeting CD34+ progenitor cells within non-purified mPBMCs utilizing VSV-G pseudotyped lentiviral vectors, knowing that the quiescent lymphocytes would not be transduced. Although targeting of CD34+ progenitor cells was achieved using VSV-G envelope pseudotyped lentiviral vectors, the broad-host range properties of VSV-G remain a potential safety concern in gene therapy. The small but significant fraction of activated T-cells, B-cells, macrophages and dendritic cells within the mPBMCs could still be transduced. In an attempt to overcome this limitation, we utilized a modified Sindbis virus envelope to further restrict transduction to CD34+ progenitor cells.
The 2.2 modified Sindbis virus envelope contains the antibody binding peptide ZZ and was developed in our laboratory to pseudotype lentiviral vectors [28]. The antibody directed transduction provides enhanced specificity of targeting by limiting transduction to the cells of interest. After binding to the cell membrane, Sindbis virus enters cells via the clathrin endocytosis pathway [47]. This suggests that endocytosis may play an important role in the transduction with 2.2 targeting vectors, and thus we tested antibodies to different hematopoietic progenitor cell markers that had previously been examined for endocytosis, as well as some that had not. We were able to identify an antibody to CD34 (clone My10, anti-HPCA1), as the best antibody for directing the 2.2 targeting vector during transduction. Among the anti-CD34 antibodies that we tested, anti-HPCA1 was previously reported to induce endocytosis most efficiently. Therefore, this finding is consistent with our hypothesis that antibodies that can induce endocytosis mediate more efficient transduction.
Using the 2.2 targeting vector and CD34 antibody (clone My10), we were able to achieve an approximate 8–11% transduction in fetal liver CD34+ cells. No transduction was observed in PBMC monoculture. In the colony-forming assay, for which 1% of fetal liver CD34+ cells were mixed with nonstimulated PBMCs prior to transduction, 2.2 vectors with CD34 antibodies resulted in 15% EGFP+ colonies, whereas VSV-G pseudotypes achieved 12% EGFP+ colonies, suggesting a better targeting to the CD34+ progenitor cells by 2.2 vectors. One explanation for the better targeting is that the binding of 2.2 targeting vector to cells via CD34 antibodies was concentrated in the hematopoietic progenitor cell populations, whereas binding of VSV-G envelope pseudotyped vectors was dispersed into progenitor and nonprogenitor cells. In the colony-forming assay of the transduced nonpurified mPBMCs, VSV-G pseudotypes and 2.2 targeting vector with CD34 antibodies achieved 7% and 4%, respectively, indicating a feasibility of targeting CD34+ progenitor cells without purification of mPBMCs. The transduction efficiency achieved by both VSV-G pseudotypes and 2.2 targeting vectors were lower in mPBMCs compared to fetal liver CD34+ cells. For the 2.2 targeting vectors, the decreased levels of targeting CD34+ cells may be due to the lower density of CD34 antigens on the surface of mPBMCs compared to fetal liver CD34+ cells. Although VSV-G pseudotypes were equally effective at transducing the CD34+ cells in nonpurified mPBMCs, the broad host range of VSV-G envelope means that these pseudotypes can also infect cells in PBMCs, such as activated T and B cells, macrophages and dendritic cells. This lack of specificity for CD34+ cells precludes application for selective gene transduction in a clinical setting.
The results obtained in the present study suggest that the purification of CD34+ cells from mPBMCs could potentially be eliminated for lentiviral vector mediated gene transduction of CD34+ progenitor cells. Preserving function of HSCs and improving their repopulating capacity are critical issues in stem cell gene therapy. Researchers have reported the impairment of the pluripotency of HSCs via immunomagnetic column selections [44,48]. The best and most efficient way to overcome this limitation is eliminating the purification step and transducing hematopoietic progenitor cells directly within the nonfractionated cell populations, as proposed by the present study. In addition, it is noteworthy that many studies have shown that the presence of cells other than the hematopoietic stem cells, such as lymphoid and myeloid cells, can enhance allogeneic stem cell transplantation [49-53]. Therefore, our approach also provides an opportunity to enable transduction of progenitor cells in the nonfractionated cell populations and improve hematopoietic stem cell transplantation.
In conclusion, our ex vivo targeting of CD34+ progenitor cells by 2.2 envelope pseudotyped lentiviral vectors opens a new avenue of research, which may eventually allow the efficient transduction of hematopoietic progenitor cells directly in patients while conserving their functional properties and eliminating the labor-intensive and costly steps involved in ex vivo transduction. Future understanding and modification of the Sindbis targeting vector may allow for direct in vivo targeting of CD34+ progenitor cells. We recognize that there are developmental barriers to be overcome before clinical application. Issues such as the frequency of transduced cells in a nonpurified population necessary for efficient blood system reconstitution and the relative efficiency of reconstitution of nonpurified progenitor cells in comparison to standard approaches with purified cells can best be addressed in future animal model systems.
Acknowledgments
We thank E. Bayrd for editorial assistance with the manuscript. We thank D. S. An for help with fetal liver CD34 experiment. We thank M. Kamata for help with the manuscript submission. Flow cytometry was performed using the flow cytometry facility at the UCLA AIDS Institute. This work was supported by US National Institutes of Health grants AI039975, AI069350 and CA-120327. There are no ethical issues regarding this study.
References
- 1.van Griensven J, De Clercq E, Debyser Z. Hematopoietic stem cell-based gene therapy against HIV infection: promises and caveats. AIDS Rev. 2005;7:44–55. [PubMed] [Google Scholar]
- 2.Verfaillie CM, McIvor RS, Zhao RC. Gene therapy for chronic myelogenous leukemia. Mol Med Today. 1999;5:359–366. doi: 10.1016/s1357-4310(99)01507-5. [DOI] [PubMed] [Google Scholar]
- 3.Aiuti A, Slavin S, Aker M, et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296:2410–2413. doi: 10.1126/science.1070104. [DOI] [PubMed] [Google Scholar]
- 4.Richter J, Karlsson S. Clinical gene therapy in hematology: past and future. Int J Hematol. 2001;73:162–169. doi: 10.1007/BF02981933. [DOI] [PubMed] [Google Scholar]
- 5.Su L, Lee R, Bonyhadi M, et al. Hematopoietic stem cell-based gene therapy for acquired immunodeficiency syndrome: efficient transduction and expression of RevM10 in myeloid cells in vivo and in vitro. Blood. 1997;89:2283–2290. [PubMed] [Google Scholar]
- 6.Banerjea A, Li MJ, Remling L, et al. Lentiviral transduction of Tar Decoy and CCR5 ribozyme into CD34+ progenitor cells and derivation of HIV-1 resistant T cells and macrophages. AIDS Res Ther. 2004;1:2. doi: 10.1186/1742-6405-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li MJ, Kim J, Li S, et al. Long-term inhibition of HIV-1 infection in primary hematopoietic cells by lentiviral vector delivery of a triple combination of anti-HIV shRNA, anti-CCR5 ribozyme, and a nucleolar-localizing TAR decoy. Mol Ther. 2005;12:900–909. doi: 10.1016/j.ymthe.2005.07.524. [DOI] [PubMed] [Google Scholar]
- 8.Satterthwaite AB, Burn TC, Le Beau MM, et al. Structure of the gene encoding CD34, a human hematopoietic stem cell antigen. Genomics. 1992;12:788–794. doi: 10.1016/0888-7543(92)90310-o. [DOI] [PubMed] [Google Scholar]
- 9.Fina L, Molgaard HV, Robertson D, et al. Expression of the CD34 gene in vascular endothelial cells. Blood. 1990;75:2417–2426. [PubMed] [Google Scholar]
- 10.Huyhn A, Dommergues M, Izac B, et al. Characterization of hematopoietic progenitors from human yolk sacs and embryos. Blood. 1995;86:4474–4485. [PubMed] [Google Scholar]
- 11.Vose JM, Bierman PJ, Lynch JC, et al. Transplantation of highly purified CD34 + Thy-1+ hematopoietic stem cells in patients with recurrent indolent non-Hodgkin’s lymphoma. Biol Blood Marrow Transplant. 2001;7:680–687. doi: 10.1053/bbmt.2001.v7.pm11787531. [DOI] [PubMed] [Google Scholar]
- 12.Isidori A, Motta MR, Tani M, et al. Positive selection and transplantation of autologous highly purified CD133(+) stem cells in resistant/relapsed chronic lymphocytic leukemia patients results in rapid hematopoietic reconstitution without an adequate leukemic cell purging. Biol Blood Marrow Transplant. 2007;13:1224–1232. doi: 10.1016/j.bbmt.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Consolini R, Legitimo A, Calleri A. The hematopoietic stem cell: biology and clinical applications. Pathologica. 2001;93:2–14. [PubMed] [Google Scholar]
- 14.Gunsilius E, Gastl G, Petzer AL. Hematopoietic stem cells. Biomed Pharmacother. 2001;55:186–194. doi: 10.1016/s0753-3322(01)00051-8. [DOI] [PubMed] [Google Scholar]
- 15.Charbord P, Newton I, Voillat L, et al. The purification of CD34 cells from human cord blood: comparison of separation techniques and cytokine requirements for optimal growth of clonogenic progenitors. Br J Haematol. 1996;94:449–454. doi: 10.1046/j.1365-2141.1996.d01-1822.x. [DOI] [PubMed] [Google Scholar]
- 16.Kimura T, Minamiguchi H, Wang J, et al. Impaired stem cell function of CD34+ cells selected by two different immunomagnetic beads systems. Leukemia. 2004;18:566–574. doi: 10.1038/sj.leu.2403211. [DOI] [PubMed] [Google Scholar]
- 17.Watts MJ, Sullivan AM, Ings SJ, et al. Evaluation of clinical scale CD34+ cell purification: experience of 71 immunoaffinity column procedures. Bone Marrow Transplant. 1997;20:157–162. doi: 10.1038/sj.bmt.1700879. [DOI] [PubMed] [Google Scholar]
- 18.Bruno A, Caravita T, Adorno G, et al. Positive selection of CD34+ cells by immunoadsorption: factors affecting the final yield and hematopoietic recovery in patients with hematological malignancies and solid tumors. Transfus Apher Sci. 2002;26:103–110. doi: 10.1016/s1473-0502(01)00157-4. [DOI] [PubMed] [Google Scholar]
- 19.Watts MJ, Somervaille TC, Ings SJ, et al. Variable product purity and functional capacity after CD34 selection: a direct comparison of the CliniMACS (v2. 1) and Isolex 300i (v2. 5) clinical scale devices. Br J Haematol. 2002;118:117–123. doi: 10.1046/j.1365-2141.2002.03561.x. [DOI] [PubMed] [Google Scholar]
- 20.Scherr M, Eder M. Gene transfer into hematopoietic stem cells using lentiviral vectors. Curr Gene Ther. 2002;2:45–55. doi: 10.2174/1566523023348237. [DOI] [PubMed] [Google Scholar]
- 21.Chinnasamy N, Chinnasamy D, Toso JF, et al. Efficient gene transfer to human peripheral blood monocyte-derived dendritic cells using human immunodeficiency virus type 1-based lentiviral vectors. Hum Gene Ther. 2000;11:1901–09. doi: 10.1089/10430340050129512. [DOI] [PubMed] [Google Scholar]
- 22.Naldini L. Lentiviruses as gene transfer agents for delivery to non-dividing cells. Curr Opin Biotechnol. 1998;9:457–463. doi: 10.1016/s0958-1669(98)80029-3. [DOI] [PubMed] [Google Scholar]
- 23.Naldini L, Blomer U, Gallay P, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 24.Kafri T, Blomer U, Peterson DA, et al. Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nat Genet. 1997;17:314–317. doi: 10.1038/ng1197-314. [DOI] [PubMed] [Google Scholar]
- 25.Zack JA, Haislip AM, Krogstad P, et al. Incompletely reverse-transcribed human immunodeficiency virus type 1 genomes in quiescent cells can function as intermediates in the retroviral life cycle. J Virol. 1992;66:1717–1725. doi: 10.1128/jvi.66.3.1717-1725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitt MA, Buonocore L, Prehaud C, et al. Membrane fusion activity, oligomerization, and assembly of the rabies virus glycoprotein. Virology. 1991;185:681–688. doi: 10.1016/0042-6822(91)90539-n. [DOI] [PubMed] [Google Scholar]
- 27.Morizono K, Bristol G, Xie YM, et al. Antibody-directed targeting of retroviral vectors via cell surface antigens. J Virol. 2001;75:8016–8020. doi: 10.1128/JVI.75.17.8016-8020.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morizono K, Xie Y, Ringpis GE, et al. Lentiviral vector retargeting to P-glycoprotein on metastatic melanoma through intravenous injection. Nat Med. 2005;11:346–352. doi: 10.1038/nm1192. [DOI] [PubMed] [Google Scholar]
- 29.Morizono K, Chen IS. Targeted gene delivery by intravenous injection of retroviral vectors. Cell Cycle. 2005;4:854–856. doi: 10.4161/cc.4.7.1789. [DOI] [PubMed] [Google Scholar]
- 30.Morizono K, Ringpis GE, Pariente N, et al. Transient low pH treatment enhances infection of lentiviral vector pseudotypes with a targeting Sindbis envelope. Virology. 2006;355:71–81. doi: 10.1016/j.virol.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 31.Pariente N, Mao SH, Morizono K, et al. Efficient targeted transduction of primary human endothelial cells with dual-targeted lentiviral vectors. J Gene Med. 2007;10:242–248. doi: 10.1002/jgm.1151. [DOI] [PubMed] [Google Scholar]
- 32.Pariente N, Morizono K, Virk MS, et al. A novel dual-targeted lentiviral vector leads to specific transduction of prostate cancer bone metastases in vivo after systemic administration. Mol Ther. 2007;15:1973–1981. doi: 10.1038/sj.mt.6300271. [DOI] [PubMed] [Google Scholar]
- 33.Wognum AW, Eaves AC, Thomas TE. Identification and isolation of hematopoietic stem cells. Arch Med Res. 2003;34:461–475. doi: 10.1016/j.arcmed.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Egeland T, Tjonnfjord G, Steen R, et al. Positive selection of bone marrow-derived CD34 positive cells for possible stem cell transplantation. Transplant Proc. 1993;25:1261–1263. [PubMed] [Google Scholar]
- 35.Kobari L, Giarratana MC, Pflumio F, et al. CD133+ cell selection is an alternative to CD34+ cell selection for ex vivo expansion of hematopoietic stem cells. J Hematother Stem Cell Res. 2001;10:273–281. doi: 10.1089/15258160151134980. [DOI] [PubMed] [Google Scholar]
- 36.Krauter J, Hartl M, Hambach L, et al. Receptor-mediated endocytosis of CD34 on hematopoietic cells after stimulation with the monoclonal antibody anti-HPCA-1. J Hematother Stem Cell Res. 2001;10:863–871. doi: 10.1089/152581601317210953. [DOI] [PubMed] [Google Scholar]
- 37.Kung SK, Bonifacino A, Metzger ME, et al. Lentiviral vector-transduced dendritic cells induce specific T cell response in a nonhuman primate model. Hum Gene Ther. 2005;16:527–532. doi: 10.1089/hum.2005.16.527. [DOI] [PubMed] [Google Scholar]
- 38.An DS, Wersto RP, Agricola BA, et al. Marking and gene expression by a lentivirus vector in transplanted human and nonhuman primate CD34(+) cells. J Virol. 2000;74:1286–1295. doi: 10.1128/jvi.74.3.1286-1295.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nolta JA, Kohn DB. Comparison of the effects of growth factors on retroviral vector-mediated gene transfer and the proliferative status of human hematopoietic progenitor cells. Hum Gene Ther. 1990;1:257–268. doi: 10.1089/hum.1990.1.3-257. [DOI] [PubMed] [Google Scholar]
- 40.Yoshikawa Y, Hirayama F, Kanai M, et al. Stromal cell-independent differentiation of human cord blood CD34 + CD38− lymphohematopoietic progenitors toward B cell lineage. Leukemia. 2000;14:727–734. doi: 10.1038/sj.leu.2401713. [DOI] [PubMed] [Google Scholar]
- 41.Gasmi M, Glynn J, Jin MJ, et al. Requirements for efficient production and transduction of human immunodeficiency virus type 1-based vectors. J Virol. 1999;73:1828–1834. doi: 10.1128/jvi.73.3.1828-1834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Costello E, Munoz M, Buetti E, et al. Gene transfer into stimulated and unstimulated T lymphocytes by HIV-1-derived lentiviral vectors. Gene Ther. 2000;7:596–604. doi: 10.1038/sj.gt.3301135. [DOI] [PubMed] [Google Scholar]
- 43.Cardoso AA, Watt SM, Batard P, et al. An improved panning technique for the selection of CD34+ human bone marrow hematopoietic cells with high recovery of early progenitors. Exp Hematol. 1995;23:407–412. [PubMed] [Google Scholar]
- 44.de Wynter EA, Coutinho LH, Pei X, et al. Comparison of purity and enrichment of CD34+ cells from bone marrow, umbilical cord and peripheral blood (primed for apheresis) using five separation systems. Stem Cells. 1995;13:524–532. doi: 10.1002/stem.5530130510. [DOI] [PubMed] [Google Scholar]
- 45.Mitrophanous K, Yoon S, Rohll J, et al. Stable gene transfer to the nervous system using a non-primate lentiviral vector. Gene Ther. 1999;6:1808–1818. doi: 10.1038/sj.gt.3301023. [DOI] [PubMed] [Google Scholar]
- 46.Montini E, Cesana D, Schmidt M, et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- 47.DeTulleo L, Kirchhausen T. The clathrin endocytic pathway in viral infection. EMBO J. 1998;17:4585–4593. doi: 10.1093/emboj/17.16.4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perseghin P, Gaipa G, Dassi M, et al. CD34+ stem cell recovery after positive selection of ‘overloaded’ immunomagnetic columns. Stem Cells Dev. 2005;14:740–743. doi: 10.1089/scd.2005.14.740. [DOI] [PubMed] [Google Scholar]
- 49.Hanash AM, Levy RB. Donor CD4 + CD25+ T cells promote engraftment and tolerance following MHC-mismatched hematopoietic cell transplantation. Blood. 2005;105:1828–1836. doi: 10.1182/blood-2004-08-3213. [DOI] [PubMed] [Google Scholar]
- 50.Gangopadhyay NN, Shen H, Landreneau R, et al. Isolation and tracking of a rare lymphoid progenitor cell which facilitates bone marrow transplantation in mice. J Immunol Methods. 2004;292:73–81. doi: 10.1016/j.jim.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 51.Gangopadhyay NN, Hoffman RA, Shen H, et al. Bone marrow-derived CD8alpha + TCR− cells that facilitate allogeneic bone marrow transplantation are a mixed population of lymphoid and myeloid progenitors. Exp Hematol. 2007;35:1847–1857. doi: 10.1016/j.exphem.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 52.Kaufman CL, Colson YL, Wren SM, et al. Phenotypic characterization of a novel bone marrow-derived cell that facilitates engraftment of allogeneic bone marrow stem cells. Blood. 1994;84:2436–2446. [PubMed] [Google Scholar]
- 53.Shizuru JA, Jerabek L, Edwards CT, et al. Transplantation of purified hematopoietic stem cells: requirements for overcoming the barriers of allogeneic engraftment. Biol Blood Marrow Transplant. 1996;2:3–14. [PubMed] [Google Scholar]


