Abstract
Mutations in individuals with the lysosomal storage disorder Niemann-Pick disease, type C1 (NPC1) are heterogeneous, not localized to specific protein domains, and not correlated to time of onset or disease severity. We demonstrate direct correlation of the time of neurological symptom onset with the severity of lysosomal defects in NPC1 patient-derived fibroblasts. This is a novel assay for NPC1 individuals that may be predictive of NPC1 disease progression and broadly applicable to other lysosomal disorders.
1. Introduction
Advances in human genomic sequencing are revealing disease-causing mutations at younger ages, allowing improved patient management through earlier disease prediction. However in some diseases, such as the rare, recessive lysosomal storage disorder Niemann-Pick disease, Type C1 (NPC1; MIM #257220), accurate prognostic information is difficult because NPC1 disease is extremely heterogeneous in the timing of clinical presentation (early infancy to adulthood), is associated with a wide spectrum of causative NPC1 (Gene ID 4864) mutations, and shows little concordance between the predicted consequences of NPC1 gene mutation on protein function with time of onset or severity of the disease [1,2]. NPC1 encodes a transmembrane protein involved in intracellular cholesterol trafficking, and its mutation causes intracellular accumulation of unesterified cholesterol in late endosomal/lysosomal structures and marked accumulation of glycosphingolipids, especially in neuronal tissue. Consequently, NPC1 disease presents with hepatosplenomegaly and neurological degeneration that leads to premature death. Current diagnosis of NPC1 involves clinical assessments, gene sequencing and filipin staining of biopsied skin fibroblasts to confirm cholesterol accumulation in lysosomal compartments [3]. The inability of these existing clinical, genetic and biochemical parameters to predict time of onset of neurological symptoms highlights the need for new, more informative assays for NPC1 disease.
2. Methods
2.1 Cells and patient data
Dermal fibroblasts and clinical data from 27 NPC1 patients were obtained as part of a NICHD, National Institutes of Health, Institutional Review Board-approved NPC1 Natural History study (NCT00344331). Age of disease onset ranged from neonate to 39 years old, and clinical severity score was calculated as previously described [4] when patients entered into the study. Age of systemic onset was considered the age when patients first developed any symptoms, including those of non-neurological origin. Categorization of patients based on age of onset of their neurological symptoms was per current clinical guidelines [2,3]. Four control human fibroblast lines were purchased from Coriell Cell Repository. Culture conditions were as previously described [5] with additional supplementation of 2 mM L-glutamine.
2.2 FACS and LysoTracker Red dye staining
Cells were plated onto 60 mm tissue culture dishes and cultured to 70 – 80% confluency. Media was replaced 24 hours prior to analysis. On the day of analysis, cells were incubated at 37°C for 30 minutes in fresh media containing 1μM LysoTracker Red dye (Lysotracker) in 3 mL medium/60 mm dish. Next cells were given two PBS washes, then trypsinized, pelleted and resuspended in 500 μL medium. FACS data were collected on 10,000 cells using a FACSCalibur (Becton Dickinson Biosciences, Franklin Lakes, NJ) equipped with Cell Quest software and analyzed using FlowJo (Tree Star). Fold change in LysoTracker staining was calculated as the ratio of geometric means of stained/unstained samples. Duplicate biological replicates were analyzed on three different days, totaling 6 independent samples per cell line.
3. Results and discussion
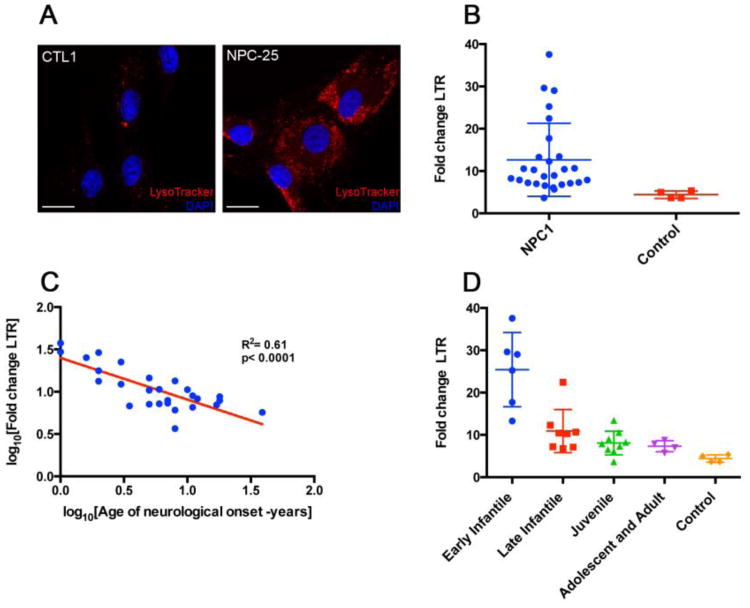
We tested if biochemical alterations in somatically-derived dermal fibroblasts from NPC1 patients correlated with age of disease onset. Cultured fibroblasts were analyzed for intensity of staining with LysoTracker, a modified fluorophore that is highly selective for acidic cellular compartments, including lysosomes. NPC1 patient fibroblasts exhibited microscopically visible increases in LysoTracker staining compared to control NPC1+/+ fibroblasts (Figure 1a). Quantification of this observation using FACS analysis to measure the fold-change in LysoTracker staining (over background fluorescence of unstained cells) for each fibroblast line showed significantly elevated LysoTracker staining in NPC1 patient fibroblasts (p<0.0001) with a large degree of variation; the staining intensity of these cells was 12.65 ± 8.6, while that of controls was 4.41 ± 0.77 (mean ± s.d.; Figure 1b). A statistical power post-test (http://statpages.org/postpowr.html) indicated the large difference between NPC1 patient and control cells gave a power of 84%, confirming the validity of these results from a relatively small sample size. This increased LysoTracker staining was associated with defective NPC1, as it was lessened by genetic rescue that was achieved via transfection of normal NPC1 cDNA as well as by administration of either methyl-β-cyclodextrin or δ-tocopherol, two compounds previously identified as alleviating NPC1-associated biochemical defects [5-7] (Supplemental Material Figures 1, 2).
Figure 1. Fibroblasts from individuals with NPC1 show quantitative increases in lysosomal storage defects that correlate with age of onset of neurological symptoms.
(A) Confocal microscopic images of LysoTracker red staining in fibroblasts from an individual with NPC1 (NPC-25, right) compared to control fibroblasts (CTL1, left). Increased cellular staining in NPC1 is consistent with increased cholesterol storage in late endosomes/lysosomes. Nuclear DAPI staining in blue; scale bar = 20 μm. (B) Scatter dot plot comparing LysoTracker staining (LTR; y-axis) of individual, NPC1 patient-derived fibroblast lines (N=27) with staining of control fibroblast lines (N=4). NPC1 fibroblasts exhibit significantly greater LysoTracker staining than control cells (Student's two-tailed t-test, p<0.0001) with greater variance (NPC1 standard deviation = 8.6). (C) Linear regression of fold change increases in LysoTracker staining intensity of NPC1 cells (y-axis) versus the age of onset of neurological symptoms (x-axis) showed significant correlation. Each blue circle represents results from a single NPC1 patient cell line. Log10-transformed values are presented and were used for statistical analyses of these normally distributed data. (D) Scatter dot plot of NPC1 patients sorted into categories based upon neurological age of onset, as follows: early infantile (2 months – 2years) n=6; late infantile (2 – 6 years), n=8; juvenile (6 – 15 years), n=9; and adolescent/adulthood (≥15 years), n=4. LysoTracker staining intensity for early infantile was significantly different from that of all other categories (1-way ANOVA with Tukey's post-hoc test; p<0.0001). For (B-D), LysoTracker staining measurements are expressed as fold increase over unstained cells derived from the same individual, to remove any effects of autofluorescence). Box and whiskers plots in (B, D) show mean and standard deviation. Statistical analyses performed using Prism (GraphPad).
We next investigated if the levels of fibroblast LysoTracker staining correlated with any NPC1 disease clinical or genetic parameters. LysoTracker staining did not correlate with the clinical measurement of each patient's disease severity, nor was there correlation between disease severity and the predicted severities of the many various NPC1 mutations represented in this cohort, which were calculated using the MutPred algorithm (Supplemental Material Figure 3, Tables 1, 2). However, LysoTracker staining did show significant correlation with age of onset of systemic disease symptoms (p = 0.0002, R2 = 0.42; Supplemental Material Figure 3, Table 1), and even greater correlation with the age of neurological symptom onset (p < 0.0001, R2 = 0.61; Figure 1C, Table 1). When patients were grouped into four previously defined disease classes based upon neurological age of onset, LysoTracker staining in early infantile onset patients differed significantly from all other patients (p<0.0001, 1-way ANOVA-Tukey; Figure 4d). The mean fold-change in Lysotracker staining in early infantile onset patients was 25.4 ± 8.0 (s.d.), while the respective mean fold-changes of late infantile, juvenile, and adolescent/adult patients were 10.9 ± 4.7, 8.1 ± 2.6, and 7.32 ± 1.1.
4. Conclusions
We showed that lysosomal alterations in NPC1 patient-derived fibroblasts, when quantified by FACS analysis, directly correlate to the time of onset of neurological disease symptoms. Future work involving verification of these finding in additional groups of individuals will validate the use of this approach for clinical diagnostics. Adding this simple, inexpensive assay to the testing regimen given to individuals diagnosed with NPC1 could for the first time provide crucial information about disease progression. As the NPC1 field moves towards diagnosis prior to the onset of neurological symptoms [1,2,8], including NPC1 disease identification as a part of newborn genetic screening or in patients with splenomegaly, the addition of this somatic cell assay could provide great assistance for patients and their families in preparation for future life as well as improved disease management their physicians.
Similar quantitative somatic cell alterations measured by LysoTracker staining may also be informative regarding systemic phenotypes for the > 50 additional human lysosomal storage disorders exhibiting lipid accumulation in the lysosome [2,9]. Furthermore, as the defects in somatically-derived fibroblasts directly correlate with CNS-associated neurological symptoms, LysoTracker analysis of patient-derived somatic cells could be used to analyze genetic components that contribute to time of onset and severity of neurological disease, such as genomic analyses of variants that modify inherent lysosomal abnormalities, as well as a first screen for assessing efficacy of compounds in ameliorating the disease symptoms. For example, we recently demonstrated that treatment of fibroblasts with 40 μM δ-tocopherol reduced lysosomal storage defects (including LysoTracker staining) in NPC1, NPC2, Wolman, Batten, Fabry, Farber, Niemann-Pick A, Sanfilippo type B, and Tay-Sachs diseases [1,5].
Importantly, this novel ability to correlate a non-invasive somatic cell assay with cell autonomous neurodegenerative phenotypes may enable accurate prediction of the neurological disease progression of NPC1 disease, and this ability could be extended to other neurodegenerative disorders. This will require more extensive future studies, to verify the utility of this assay in neurological phenotype assessment and prediction in NPC1 disease as well as other neurological disorders. However, recent links between Parkinson disease and lysosomal function [3,10] open the possibility that the somatic cell analysis described herein may be broadly applicable to many neurodegenerative disorders.
Supplementary Material
Highlights.
Mutations in NPC1 disease are heterogeneous and not correlated to disease phenotypes.
Lysosomal staining of NPC1 patient fibroblasts was analyzed with LysoTracker Red.
NPC1 fibroblasts had increased staining correlating with neurological symptom onset.
This staining distinguished early infantile from later onset NPC1 patients.
This assay may improve diagnosis in NPC1 and other lysosomal storage disorders.
Acknowledgments
We thank Cynthia J. Tifft for helpful discussions, Laura Baxter for editing the manuscript, Stephen Wincovitch for support in imaging/microscopy, Larry N. Singh for assistance in mutation analysis software and the contributions of the caretakers, patients, and their families who participated in this study. This work was supported by the intramural research program of the National Human Genome Research Institute, National Institutes of Health, the intramural research program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the Ara Parseghian Medical Research Foundation, and a Bench to Bedside award from the Office of Rare Diseases and the National Institutes of Health Clinical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vanier MT, Millat G. Niemann-Pick disease type C. Clin Genet. 2003;64:269–281. doi: 10.1034/j.1399-0004.2003.00147.x. [DOI] [PubMed] [Google Scholar]
- 2.Vanier MT. Niemann-Pick disease type C. Orphanet J Rare Dis. 2010;5:16. doi: 10.1186/1750-1172-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patterson MC, Hendriksz CJ, Walterfang M, Sedel F, Vanier MT, Wijburg F. Recommendations for the diagnosis and management of Niemann-Pick disease type C: an update. Mol Genet Metab. 2012:330–344. doi: 10.1016/j.ymgme.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Yanjanin NM, Vélez JI, Gropman A, King K, Bianconi SE, Conley SK, et al. Linear clinical progression, independent of age of onset, in Niemann-Pick disease, type C. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:132–140. doi: 10.1002/ajmg.b.30969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu M, Liu K, Swaroop M, Porter FD, Sidhu R, Firnkes S, et al. δ- Tocopherol reduces lipid accumulation in Niemann-Pick type C1 and Wolman cholesterol storage disorders. J Biol Chem. 2012;287:39349–39360. doi: 10.1074/jbc.M112.357707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenbaum AI, Zhang G, Warren JD, Maxfield FR. Endocytosis of beta-cyclodextrins is responsible for cholesterol reduction in Niemann-Pick type C mutant cells. Proc Natl Acad Sci. 2010;107:5477–5482. doi: 10.1073/pnas.0914309107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swaroop M, Thorne N, Rao MS, Austin CP, McKew JC, Zheng W. Evaluation of cholesterol reduction activity of methyl-β-cyclodextrin using differentiated human neurons and astrocytes. J Biomol Screen. 2012;17:1243–1251. doi: 10.1177/1087057112456877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porter FD, Scherrer DE, Lanier MH, Langmade SJ, Molugu V, Gale SE, et al. Cholesterol oxidation products are sensitive and specific blood-based biomarkers for Niemann-Pick C1 disease. Sci Transl Med. 2010;2:56ra81. doi: 10.1126/scitranslmed.3001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schultz ML, Tecedor L, Chang M, Davidson BL. Clarifying lysosomal storage diseases. Trends in Neurosciences. 2011;34:401–410. doi: 10.1016/j.tins.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dehay B, Ramirez A, Martinez-Vicente M, Perier C, Canron MH, Doudnikoff E, et al. Loss of P-type ATPase ATP13A2/PARK9 function induces general lysosomal deficiency and leads to Parkinson disease neurodegeneration. Proc Natl Acad Sci. 2012;109:9611–9616. doi: 10.1073/pnas.1112368109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.