Abstract
The rapid progression of multiple myeloma is dependent upon cellular interactions within the bone marrow microenvironment. In vitro studies suggest that bone marrow stromal cells (BMSCs) can promote myeloma growth and survival and osteolytic bone disease. However, it is not possible to recreate all cellular aspects of the bone marrow microenvironment in an in vitro system, and the contributions of BMSCs to myeloma pathogenesis in an intact, immune competent, in vivo system are unknown. To investigate this, we utilized a murine myeloma model that replicates many features of the human disease. Co-inoculation of myeloma cells and a BMSC line isolated from myeloma-permissive mice in otherwise non-permissive mice resulted in myeloma development, associated with tumor growth within bone marrow and osteolytic bone disease. In contrast, inoculation of myeloma cells alone did not result in myeloma. BMSCs inoculated alone induced osteoblast suppression, associated with an increase in serum concentrations of the Wnt signaling inhibitor, Dkk1. Dkk1 was highly expressed in BMSCs and in myeloma-permissive bone marrow. Knockdown of Dkk1 expression in BMSCs decreased their ability to promote myeloma and the associated bone disease in mice. Collectively, our results demonstrate novel roles of BMSCs and BMSC-derived Dkk1 in the pathogenesis of multiple myeloma in vivo.
INTRODUCTION
The bone marrow is well known to support tumor growth and osteolytic bone disease in multiple myeloma. However, the contributions of this specialized microenvironment during early stages of myeloma development are poorly understood, largely because of the need for in vivo experiments that accurately replicate the complexity of tumor-host cell interactions. To address this, we have exploited the unique opportunities provided by the well-characterized 5T Radl myeloma model, where transplantation of 5T myeloma cells into recipient mice of the specific C57Bl/KaLwRijHsd (KaLwRij) substrain results in propagation of myeloma with many features of the human disease (1). One important feature of the Radl model is that 5T myeloma cells will only grow in syngeneic KaLwRij mice, and not in closely-related C57Bl6 mice. Since there is an emerging role for host fibroblasts in the establishment and progression of solid tumors (2–4), we hypothesized that fibroblasts, or bone marrow stromal cells (BMSCs), from myeloma-permissive KaLwRij mice may promote myeloma development in environments that are otherwise not permissive for myeloma. Furthermore, increasing evidence for elevated Dickkopf 1 (Dkk1) in patients with monoclonal gammopathy of undetermined significance (MGUS) (5–7) suggests a potential role for host-derived Dkk1 in the early stages of myeloma. We utilized a BMSC line derived from KaLwRij mice to determine the function of BMSCs and BMSC-derived Dkk1 in myeloma pathogenesis in vivo.
MATERIALS AND METHODS
Cell Culture
5TGM1-GFP myeloma cells were cultured as previously described (8). 14M1 BMSCs were originally isolated from KaLwRij mice bearing 5T myeloma. Bone marrow was flushed from long bones, and cells cultured in DMEM media with 10% fetal calf serum and L-glutamine. Media was changed every 3–4 days, and adherent cells were maintained in culture. The cell line was generated by continuous growth in vitro, with no requirement for cellular or viral immortalizing genes. Aliquots of early passage cells were frozen in 10% DMSO/90% FCS and stored in liquid nitrogen. ST2 BMSCs were purchased from Riken cell Bank, (Tsukuba, Japan) and maintained in DMEM media with 10% fetal calf serum and L-glutamine.
Stable Cell Lines
14M1 BMSCs were transfected with 1ug Dkk1shRNA or scrambled control (Santa Cruz Biotechnology, Santa Cruz, CA) using shRNA transfection reagent and standard protocols. Cells were cultured in DMEM with 10ug/ml puromycin. Knockdown of Dkk1 expression was confirmed by ELISA and cells were continuously cultured in puromycin to maintain knockdown. ST2 BMSCs were transfected with 250ng full-length Dkk1 cDNA (OriGene, Rockville, MD) or empty vector control according to the manufacturer's instructions. Cells were cultured in DMEM with 800ug/ml neomycin. Over-expression of Dkk1 was confirmed by ELISA and cells were continuously cultured in neomycin to maintain expression.
Differentiation Studies
BMSCs were cultured in either control media, osteoblast differentiation media (10mM β-glycerophosphate, 50ug/ml ascorbic acid, 10ng/ml BMP-2) or adipocyte differentiation media (10nM dexamethasone, 5ug/ml insulin) for 7 days (osteoblast differentiation) or 10 days (adipocyte differentiation). Osteoblasts were identified by alkaline phosphatase staining, using a BCIP/NBT substrate kit and adipocytes by Oil Red-O staining.
ELISA, Western blotting and flow cytometry
The concentration of Dkk1 in conditioned media or sera was measured by ELISA, according to the manufacturers instructions (R & D Systems). Sera were assayed for monoclonal mouse IgG2bκ paraprotein as described (8). Western blotting and flow cytometry were performed as previously described, and outlined in Supplemental Data(9).
In vivo studies
Studies were conducted using weight-matched, 8–10 week old female C57BL/KaLwRijHsd (Harlan Netherlands B.V., The Netherlands) or C57Bl6 mice (Harlan U.S., Indianapolis, Indiana). Studies were approved by the Institution of Animal Care and Use Committee at Vanderbilt University. C57Bl6 or C57Bl/KaLwRij mice were intravenously inoculated with either 106 5TGM1-GFP cells alone, 5×105 5TGM1-GFP + 5×105 14M1 BMSCs or vehicle control (PBS). In separate studies, C57Bl6 mice were inoculated intravenously with (i) 106 5TGM1-GFP, 106 ST2 BMSCs or 5×105 5TGM1-GFP + 5×105 ST2 BMSCs (ii) 106 14M1 BMSCs, 106 14M1-Dkk1KD BMSCs, 5×105 5TGM1-GFP + 5×105 14M1 BMSCs, 5×105 5TGM1-GFP + 5×105 14M1-Dkk1KD BMSCs and (iii) 106 5TGM1-GFP, 106 ST2 BMSCs, 106 ST2-Dkk1 BMSCs, 5×105 5TGM1-GFP + 5×105 ST2 BMSCs, 5×105 5TGM1-GFP + 5×105 ST2-Dkk1 BMSCs. In another series of experiments, 5×105 5TGM1-GFP, 5×105 C4-2b-GFP or 5×105 MDA-231-GFP tumor cells, in the presence or absence of 5×105 14M1 BMSCs were injected into the tibias of C57Bl6 mice. GFP tagged tumor cell growth was measured and quantified using the CRi Maestro system. After the image was obtained, it was spectrally unmixed to remove the background fluorescence. Images were quantified using region of interest analysis software supplied with the Maestro system.
Histology, microCT and histomorphometric analyses
Histomorphometric analysis was performed to quantify bone volume and osteoclast and osteoblast surface area to bone surface area. Tibias and femurs were formalin-fixed, decalcified in 14% EDTA, paraffin–embedded, sectioned along the mid-sagittal plane in 4uM thick sections and stained with hematoxylin and eosin. To visualize osteoclasts, sections were stained for TRAP activity. For visualization of tetracycline labeling, spines were fixed in 70% ethanol and embedded in methylmethacrylate without prior decalcification. 6uM sections were cut and viewed unstained by epifluorescecen microscopy. Three non-consecutive sections were evaluated using Osteomeasure histomorphometry software, as described previously (9). Tibias were fixed in formalin and each bone was scanned at an isotropic voxel size of 12um using a microCT40 (SCANCO Medical, Bassersdorf, Switzerland). Images were analyzed using the SCANCO Medical Imaging Software and AMIRA 3-D graphics software (Mercury Computer Systems, Chelmsford, MA) to quantitate cortical bone lesions and trabecular bone volume, as described previously (9). Fluorescent in situ hybridization (FISH), immunohistochemistry and immunocytochemistry were performed as detailed in Supplemental Methods.
Statistical Analysis
Statistical significance was determined using a Mann-Whitney U test for nonparametric data, or one-way ANOVA followed by Tukey's post-hoc test. Data was considered significant given p≤0.05. Data are presented as means (± SEM) unless otherwise stated.
RESULTS AND DISCUSSION
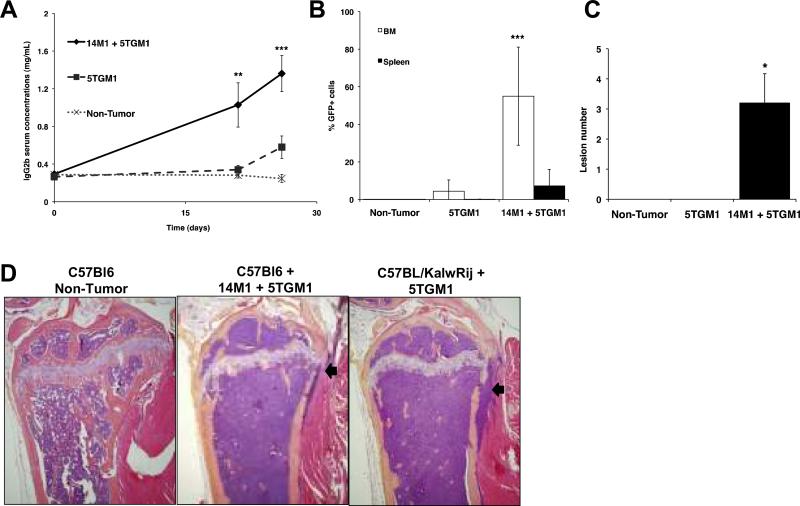
The importance of the host microenvironment in myeloma is exemplified by the 5T myeloma model, in which KaLwRij mice possess a unique host microenvironment that is permissive for 5T myeloma growth (10) We were able to induce myeloma development in non-permissive C57Bl6 mice by inoculating 5TGM1 myeloma cells in the presence of BMSCs isolated from KaLwRij mice (14M1 BMSCs). Importantly, myeloma induced by co-inoculation of myeloma cells and 14M1 BMSCs had many characteristics of both the original syngeneic KaLwRij model, and human myeloma. Mice demonstrated a time-dependent increase in serum paraprotein concentrations similar to the original KaLwRij model, and accumulation of myeloma cells in bone marrow and spleen (Figure 1A–B). Osteolytic bone disease is a major feature of multiple myeloma, and histomorphometric and microCT analysis confirmed a significant increase in osteolytic lesion number and osteoclast number, meanwhile demonstrating a significant decrease in trabecular bone volume and osteoblast number (Figure 1C, Supplementary Figure 1). Histological analysis revealed that C57Bl6 mice inoculated with 5TGM1 myeloma cells in the presence of 14M1 BMSCs displayed a striking similarity to the original KaLwRij myeloma model, with replacement of bone marrow by myeloma cells, loss of trabecular bone and erosion through cortical bone (Figure 1D). Inoculation of 5TGM1 myeloma cells alone into C57Bl6 mice did not result in the development of myeloma, with no significant accumulation of plasma cells within the bone marrow or evidence of osteolytic bone disease. There is increasing evidence from solid tumors to support a role for fibroblasts in tumor progression (11, 12). In contrast to previously published studies, where the fibroblasts were enhancing tumor growth, we have identified a role for fibroblasts or BMSCs whereby the tumor is unable to grow unless the fibroblasts are present, highlighting the critical role of these cells in myeloma pathogenesis.
Figure 1. 14M1 BMSCs promote myeloma development in non-permissive C57Bl6 mice.
C57Bl6 mice were inoculated with either 106 5TGM1-GFP cells alone, 5×105 5TGM1-GFP + 5×105 14M1 BMSCs or vehicle control (PBS). Co-inoculation of 5TGM1 myeloma cells plus 14M1 BMSCs significantly increased serum IgG2bκ concentrations (A) and tumor burden within bone marrow and spleen (B). Co-inoculation of 14M1 BMSCs and 5TGM1 myeloma cells resulted in the development of myeloma bone disease, associated with an increase in osteolytic lesions (C). Histological sections demonstrated the similarity between C57Bl6 mice co-inoculated with 14M1 BMSCs and 5TGM1 myeloma cells as compared with the original 5T model, where KalwRij mice are inoculated with 5TGM1 myeloma cells. Arrows indicate areas where tumor cells have eroded through cortical bone. (D). *p<0.05, **p<0.01, ***p<0.001 as compared to 5TGM1.
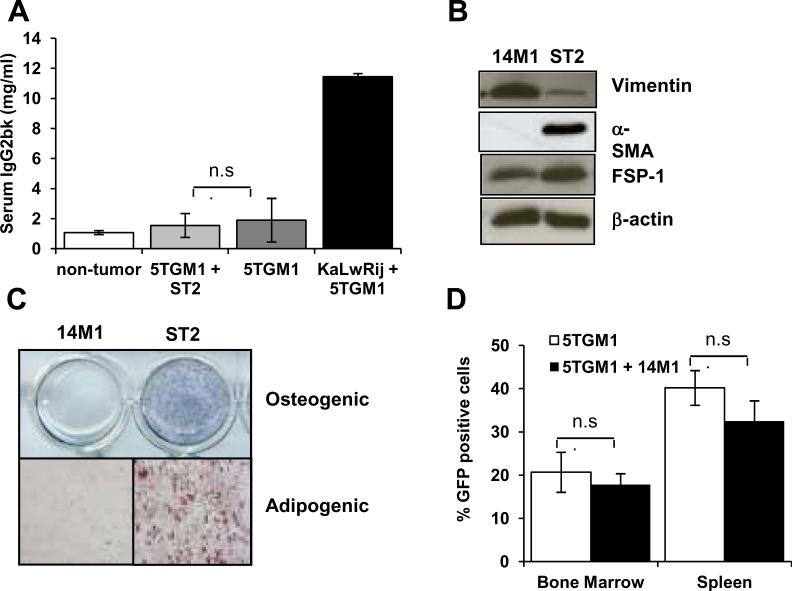
Co-inoculation of 14M1 BMSCs with 5TGM1 myeloma cells resulted in myeloma development in an otherwise non-permissive microenvironment. In contrast, coinoculation of 5TGM1 myeloma cells with normal ST2 BMSCs had no effect to promote myeloma development in C57Bl6 mice (Figure 2A), despite engraftment of ST2 cells in the bone marrow (5–15%). This suggests that the effect of 14M1 BMSCs is specific and not a general effect of all BMSCs. To determine whether the effect of 14M1 BMSCs was specific for myeloma cells, or whether it could promote the growth of other tumor cells, C57Bl6 mice were inoculated directly into the tibia with either 5TGM1 myeloma cells, C4-2b prostate cancer cells or MDA-231 breast cancer cells in the presence and absence of 14M1 BMSCs. All tumor cells were labeled with GFP. In vivo imaging detected tumor burden by GFP expression in only those mice co-inoculated with 5TGM1 myeloma cells in the presence of 14M1 BMSCs, demonstrating that 14M1 BMSCs could not promote breast or prostate cancer cell growth in a non-permissive microenvironment (data not shown). Together, these studies confirm that the effect of the 14M1 BMSCs is both cell type and disease specific.
Figure 2. Characterization of 14M1 BMSCs in vitro and in vivo.
C57Bl6 mice were inoculated with either 106 5TGM1-GFP cells alone, 5×105 5TGM1-GFP + 5×105 ST2 BMSCs or vehicle control (PBS). KaLwRij mice were inoculated with 5TGM1 myeloma cells as a positive control. Co-inoculation of ST2 BMSCs with 5TGM1 myeloma cells had no effect on tumor burden, as measured by serum IgG2bk (A). Western blot analysis demonstrated expression of vimentin and FSP-1 by 14M1 BMSCs (B). In contrast to ST2 BMSCs, 14M1 BMSCs did not differentiate into osteoblasts or adipocytes (C). KaLwRij mice were inoculated with either 106 5TGM1-GFP cells alone, 5×105 5TGM1-GFP + 5×105 14M1 BMSCs or vehicle control (PBS). Co-inoculation of 14M1 BMSCs with 5TGM1 myeloma cells had no effect on tumor burden, as measured by serum IgG2bk (D).
14M1 BMSCs were originally isolated from myeloma-bearing KaLwRIj mice, and so represent host stromal cells from the permissive KaLwRij bone marrow microenvironment. 14M1 BMSCs expressed both vimentin and fibroblast-specific protein-1, indicative of fibroblasts (Figure 2B). Immunocytochemistry for FSP-1 demonstrated expression by all cells, confirming that 14M1 BMSCs are comprised entirely of fibroblasts. (Supplementary Figure 2). Further characterization demonstrated no osteogenic or adipogenic differentiation potential, commonly seen in BMSCs (Figure 2C). In vivo analysis confirmed engraftment of 14M1 BMSCs, with flow cytometric analysis detecting approximately 13% BMSCs in the bone marrow following co-inoculation of 14M1 cells labeled with DsRedII with myeloma cells (Supplemental Data Figure 3). Inoculation of 14M1 BMSCs was unable to support the growth of human myeloma cells and had no effect on the proportion of B, T or NK cells suggesting that 14M1 cells were not immunosuppressive (Supplementary Figure 4). To further characterize the in vivo effects of 14M1 BMSCs, 5TGM1 myeloma cells and 14M1 BMSCs were co-inoculated into KaLwRij mice, which are already permissive for 5T myeloma development. Although 5TGM1 myeloma cell inoculation resulted in tumor growth and osteolytic bone disease, the co-inoculation of 14M1 BMSCs did not further increase tumor burden in bone marrow or spleen (Figure 2D) or osteolysis (data not shown), suggesting that the 14M1 BMSCs were not acting directly on the myeloma cells to increase myeloma growth.
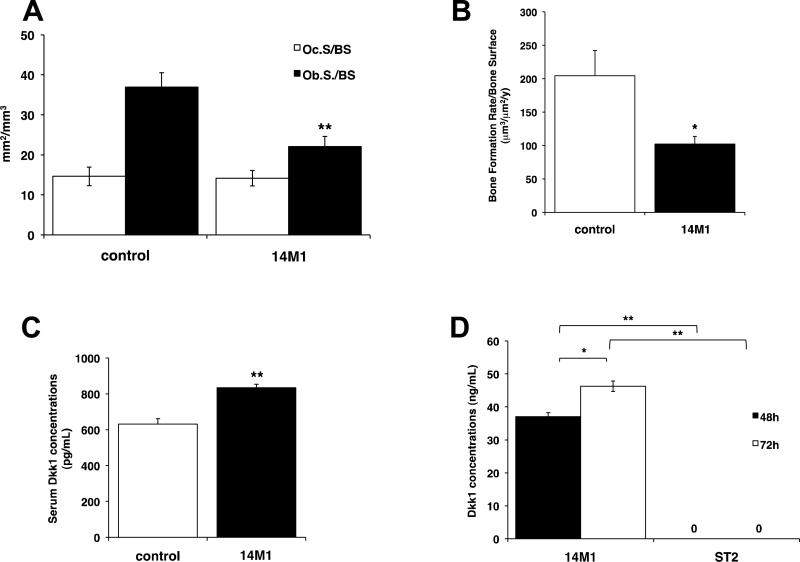
Figure 3. 14M1 BMSCs have direct effects to decrease bone formation.
C57Bl6 mice were inoculated with 106 14M1 BMSCs and sacrificed after a period of 4 weeks. Inoculation of 14M1 BMSCs had no effect on osteoclast numbers, but significantly decreased osteoblast numbers (A), rates of bone formation (B) and increased serum concentrations of Dkk1 (C). 14M1 BMSCs secrete high concentrations of Dkk1 (D). *p<0.05, **p<0.01, as compared to control.
The inability of 14M1 BMSCs to promote myeloma in an already permissive environment suggested that the cells might instead act directly on the host microenvironment to render it permissive for myeloma. To investigate this, we inoculated C57Bl6 mice with 14M1 BMSCs alone. After 4 weeks, we detected a 23.4% decrease in trabecular bone (control, 7.38 ± 1.50 vs. 14M1 BMSCs, 5.65 ± 2.13), associated with a significant decrease in osteoblast number and bone formation rates, but no change in osteoclast number (Figure 3A–3B). In control experiments, inoculation of ST2 BMSCs was found to have no effect on trabecular bone volume, bone cell number or bone formation rates (data not shown). In support of a decrease in trabecular bone volume contributing to myeloma development, KaLwRij mice were also found to have a significant reduction in trabecular bone volume (Supplementary Figure 5) when compared to age- and sex-matched C57Bl6 mice.
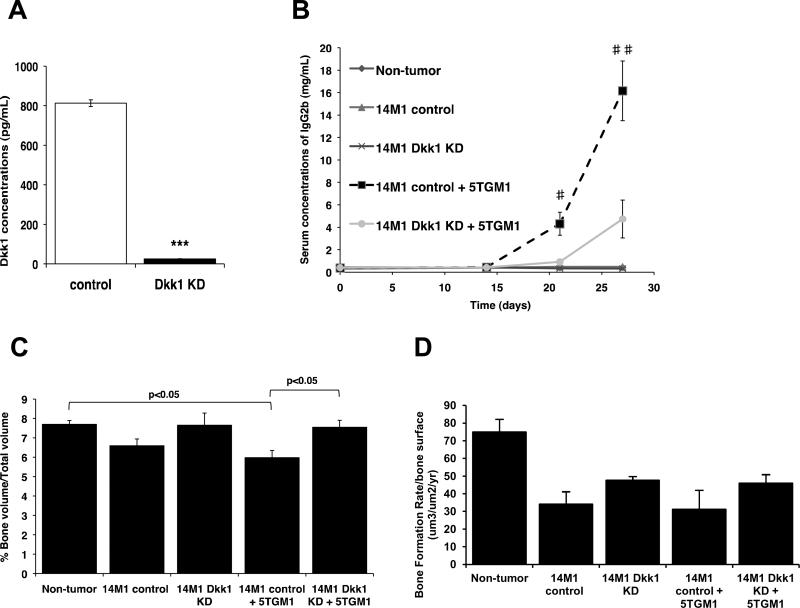
Since a major mediator of osteoblast suppression in myeloma bone disease is Dkk1 (13–15), we measured Dkk1 serum concentrations, and observed a significant increase in C57Bl6 mice inoculated with 14M1 BMSCs, as compared with either C57Bl6 mice or C57Bl6 mice inoculated with ST2 BMSCs (Figure 3C). 14M1 BMSCs were found to express high concentrations of Dkk1 in vitro (Figure 3D), and investigation of Dkk1 expression in myeloma-permissive KaLwRij mice demonstrated a significant 160.8% increase in Dkk1 serum concentrations (p<0.05) and increased expression of Dkk1 in KaLwij BMSCs (Supplementary Figure 6), suggesting a possible role for Dkk1 in myeloma-permissive microenvironments. In support of this, immunohistochemistry revealed an increase in Dkk1 expression within the bone marrow of mice inoculated with 14M1 BMSCs alone, as compared with non-tumor bearing mice (Supplementary Figure 7). Therefore, we determined the contribution of BMSC-derived Dkk1 in myeloma pathogenesis in vivo. 14M1 BMSCs were stably transfected with Dkk1 shRNA or scrambled control, and knockdown of Dkk1 expression was confirmed by ELISA (Figure 4A). Knockdown of Dkk1 expression had no effect on the growth rate of 14M1 BMSCs in vitro (data not shown). C57Bl6 mice were inoculated with 5TGM1 myeloma cells in the presence of either 14M1 scrambled control BMSCs or 14M1 DKK1 knockdown BMSCs. Those mice inoculated with 5TGM1 cells in the presence of 14M1 DKK1 knockdown BMSCs had a significant reduction in tumor development, as measured by serum IgG2bk titers, when compared to mice co-inoculated with 5TGM1 myeloma cells and 14M1 scrambled control BMSCs (Figure 4B). Knockdown of Dkk1 expression in the 14M1 BMSCs prevented the reduction in trabecular bone volume observed in those mice co-inoculated with 5TGM1 myeloma cells and 14M1 BMSCs (Figure 4C), and this was associated with an increase in rates of bone formation (Figure 4D). ST2 BMSCs were stably transfected to over-express Dkk1, however co-inoculation of these BMSCs with 5TGM1 myeloma cells in C57Bl6 mice did not result in myeloma (Supplementary Figure 8).
Figure 4. BMSC-derived Dkk1 creates a microenvironment that is permissive for myeloma development.
14M1 BMSCs were stably transfected with Dkk1 shRNA (Dkk1 KD) or scrambled control (control), resulting in a significant reduction in secretion of Dkk1 (A). Co-inoculation of C57Bl6 mice with 5TGM1 myeloma cells plus 14M1 Dkk1 KD cells resulted in a significant decrease in tumor burden, as measured by serum IgG2bκ (B), and increase in trabecular bone volume (C) and bone formation rates (D) as compared to 5TGM1 myeloma cells plus 14M1 control cells. *p<0.05, **p<0.01, ***p<0.001 as compared to control. #p<0.05, ##p<0.01 as compared to 14M1 control + 5TGM1.
Dkk1 plays a key role in the osteoblast suppression of myeloma bone disease, but the majority of studies to date have focused upon the role of myeloma-derived Dkk1 (13, 14). Stromal cells and osteoblasts are known sources of Dkk1 (16, 17), and the present study provides compelling evidence for a key role of stromal-derived Dkk1 to promote myeloma development. There is increasing evidence to suggest that cells of the bone marrow microenvironment are altered in MGUS, and that Dkk1 is increased in patients with MGUS, associated with a loss of trabecular bone (5–7). In support of this, our studies demonstrate increased host-derived Dkk1 in myeloma-permissive KaLwRij mice that is associated with a significant reduction in trabecular bone volume. Furthermore, we have discovered a novel role of BMSCs to promote myeloma establishment and progression in an otherwise non-permissive environment. This is mediated, in part, through stromal-derived Dkk1, however the inability of over-expression of Dkk1 in normal BMSCs to support myeloma development reveals that Dkk1 alone is not permissive for myeloma development and suggests a requirement for additional factors. Furthermore, although Dkk1 expression is undetectable in vitro, 5TGM1 myeloma cells do express Dkk1 in vivo, raising the possibility that tumor-derived Dkk1 may compensate for a loss in host-derived Dkk1 (Supplementary Figure 7).
It is intriguing to speculate whether the effect of 14M1 BMSCs is associated with a generalized osteoblast suppression that renders the bone marrow microenvironment favorable for myeloma. In support of this, recent studies have implicated changes in the bone marrow, associated with bone loss, that contribute to the initiation of hematopoietic cell diseases(18–20). An anti-human Dkk1 neutralizing antibody (BHQ880) is currently in phase I/II clinical trials in multiple myeloma, for the treatment of the osteolytic bone disease. The present study identifies a novel role for stromal-derived Dkk1 in myeloma pathogenesis, by rendering the host microenvironment favorable for myeloma growth and development. Therefore, targeting Dkk1, such as with a neutralizing antibody, may have additional applications, including preventing myeloma progression during early stages of disease development. Collectively, our studies reveal the importance of host-derived Dkk1 and BMSCs in the pathogenesis of myeloma in vivo, highlighting the importance of changes in the local bone marrow microenvironment in the early stages of myeloma development.
Supplementary Material
Acknowledgments
GRANT SUPPORT This work was supported by NCI through R01 CA 137116 (C.M.E) and P01 CA-40035 (G.R.M) and by the Leukemia Research Foundation (C.M.E) and the Elsa U. Pardee Foundation (C.M.E). We gratefully acknowledge support from the Vanderbilt University Institute of Imaging Science and the Vanderbilt Medical Center (VMC) Flow Cytometry Shared Resource. The VMC Flow Cytometry Shared Resource is supported by the Vanderbilt Ingram Cancer Center (P30 CA68485) and the Vanderbilt Digestive Disease Research Center (DK058404).
Financial Support; This work was supported by NCI through R01 CA 137116 (C.M.E) and P01 CA-40035 (G.R.M) and by the Leukemia Research Foundation (C.M.E) and the Elsa U. Pardee Foundation (C.M.E).
Footnotes
Conflict-of-interest Disclosure: The authors declare no competing financial interest.
REFERENCES
- 1.Radl J, Croese JW, Zurcher C, Van Den Enden-Vieveen MHM, Margreet de Leeuw A. Animal model of human disease; multiple myeloma. Am J Pathol. 1988;132:593–7. [PMC free article] [PubMed] [Google Scholar]
- 2.Hayward SW, Wang Y, Cao M, et al. Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Res. 2001;61:8135–42. [PubMed] [Google Scholar]
- 3.Bhowmick NA, Chytil A, Plieth D, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–51. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 4.Parrinello S, Coppe JP, Krtolica A, Campisi J. Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J Cell Sci. 2005;118:485–96. doi: 10.1242/jcs.01635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drake M, Ng A, Kumar S, et al. Increases in Seum Levels of Dickkopf 1 are associated with Alterations in Skeletal Microstructure in Monoclonal Gammopathy of Undetermined Significance. Journal of Bone and Mineral Research. 2009;24(Suppl 1) abstract. [Google Scholar]
- 6.Corre J, Mahtouk K, Attal M, et al. Bone marrow mesenchymal stem cells are abnormal in multiple myeloma. Leukemia. 2007;21:1079–88. doi: 10.1038/sj.leu.2404621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Todoerti K, Lisignoli G, Storti P, et al. Distinct transcriptional profiles characterize bone microenvironment mesenchymal cells rather than osteoblasts in relationship with multiple myeloma bone disease. Exp Hematol. 38:141–53. doi: 10.1016/j.exphem.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Dallas SL, Garrett IR, Oyayobi BO, et al. Ibandronate reduces osteolytic lesions but not tumour burden in a murine model of myeloma bone disease. Blood. 1999;93:1697–706. [PubMed] [Google Scholar]
- 9.Edwards CM, Edwards JR, Lwin ST, et al. Increasing Wnt signaling in the bone marrow microenvironment inhibits the development of myeloma bone disease and reduces tumor burden in bone in vivo. Blood. 2008;111:2833–42. doi: 10.1182/blood-2007-03-077685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radl J, de Glopper E, Schuit HER, Zurcher C. Idiopathic paraprotienemia:II. Transplantation of the paraprotein-producing clone from old to young C587Bl/KaLwRij mice. J Immunol. 1979;122:609–13. [PubMed] [Google Scholar]
- 11.Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell. 17:135–47. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 12.Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 13.Tian E, Zhan F, Walker R, et al. The role of the Wnt-signaling antagonist in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349:2483–94. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 14.Yaccoby S, Ling W, Zhan F, Walker R, Barlogie B, Shaughnessy JD., Jr. Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood. 2007;109:2106–11. doi: 10.1182/blood-2006-09-047712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heath DJ, Chantry AD, Buckle CH, et al. Inhibiting Dickkopf-1 (Dkk1) removes suppression of bone formation and prevents the development of osteolytic bone disease in multiple myeloma. J Bone Miner Res. 2009;24:425–36. doi: 10.1359/jbmr.081104. [DOI] [PubMed] [Google Scholar]
- 16.Gregory CA, Gunn WG, Reyes E, et al. How Wnt signaling affects bone repair by mesenchymal stem cells from the bone marrow. Ann N Y Acad Sci. 2005;1049:97–106. doi: 10.1196/annals.1334.010. [DOI] [PubMed] [Google Scholar]
- 17.Morvan F, Boulukos K, Clement-Lacroix P, et al. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res. 2006;21:934–45. doi: 10.1359/jbmr.060311. [DOI] [PubMed] [Google Scholar]
- 18.Walkley CR, Olsen GH, Dworkin S, et al. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell. 2007;129:1097–110. doi: 10.1016/j.cell.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walkley CR, Shea JM, Sims NA, Purton LE, Orkin SH. Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell. 2007;129:1081–95. doi: 10.1016/j.cell.2007.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raaijmakers MH, Mukherjee S, Guo S, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 464:852–7. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.