Abstract
Background
African Americans have a higher incidence of prostate cancer and experience poorer outcomes compared to Caucasian Americans. Racial differences in care are well documented. However, few studies have characterized patients based on their prostate cancer risk category, which is required to differentiate appropriate from inappropriate guideline application.
Methods
The medical records of a population-based sample of 777 North Carolina men with newly diagnosed prostate cancer were studied to assess the association among patient race, clinical factors and National Comprehensive Cancer Network (NCCN) guideline-concordant prostate cancer care.
Results
African Americans presented with significantly higher Gleason scores (p=0.025) and prostate specific-antigen levels (p=0.008) than Caucasian Americans. However, when clinical T stage was considered as well, difference in overall risk category only approached statistical significance (p=0.055). Across risk categories, African Americans were less likely to have surgery (58.1% vs. 68.0%, p=0.004) and more likely to have radiation (39.0% vs. 27.4%, p=0.001) compared to Caucasian Americans. However, 83.5% of men received guideline-concordant care within one year of diagnosis, which did not differ by race in multivariable analysis (OR 0.83; 95% CI 0.54–1.25). Greater patient-perceived access to care was associated with greater odds of receiving guideline-concordant care (OR 1.06; 95% CI 1.01–1.12).
Conclusions
After controlling for NCCN risk category, there were no racial differences in receipt of guideline-concordant care. Efforts to improve prostate cancer treatment outcomes should focus on improving access to the health care system.
Keywords: Prostate Cancer, National Comprehensive Cancer Network, Quality of Care, North Carolina Health Care Access Project (HCaP-NC), Guideline-concordant care
Introduction
African-American (AA) men have a higher incidence of prostate cancer and experience poorer outcomes compared to Caucasian-American (CA) men.1–5 Racial differences in treatment have been suspected to account for the differential mortality. In particular, AAs with early stage prostate cancer receive less aggressive care, such as active surveillance or androgen deprivation therapy (ADT) alone, rather than surgery or radiation therapy;2, 3 AAs also are less likely than CAs to receive ADT for advanced disease.5–7 The National Comprehensive Cancer Network (NCCN) developed guidelines for the initial treatment of prostate cancer based on patient life expectancy and cancer aggressiveness (“risk categories”).8–10 Risk stratification is required to differentiate appropriate from inappropriate application of treatment options, but few studies have characterized patients based on risk categories. Treatment is influenced by the health care delivery environment and the patient’s willingness to receive, and ability to access and pay for, the care recommended.11 For AAs with prostate cancer in particular, insurance coverage, previous forgone care, trust in the healthcare system, marital status, and education level have been associated with care received for prostate cancer.12–14
No study of prostate cancer treatment has assessed directly whether there are racial differences in guideline concordance for initial treatment of prostate cancer care across all risk categories and across all patient age groups. This study fills that gap using a single-state, population-based cohort.
Materials and Methods
Data
Date were drawn from the North Carolina Health Care Access Project (HCaP-NC), a follow-up study of 811 men who were part of the North Carolina-Louisiana Prostate Cancer Project (PCaP) cohort, a population-based study of 2258 AA and CA men with newly diagnosed prostate cancer.15 The NC component originally enrolled 1031 men from July 2004 to October 2007 in 42 counties. AA men were oversampled and represented 49% of participants. Treatment and comorbidity data were abstracted from medical records. Demographic and patient-reported information was obtained from the baseline interview.
Measures
The outcome receipt of guideline-concordant care, was a binary measure derived from medical record abstraction. A determination was made based upon initial treatment received versus that recommended using the NCCN guidelines, which assign patients to one of six mutually exclusive prostate cancer risk categories based on clinical stage, Gleason grade, and prostate-specific antigen (PSA) level. The NCCN grades its guidelines based on the rigor of evidence on which they are based.10 The prostate guidelines represent uniform NCCN expert consensus based on lower level evidence, including clinical experience, although some recommended treatment options are based on higher-level evidence. The 2004 guideline was applicable at the time of first possible diagnosis date (July 1, 2004) and changes in the guideline through 2007 affected neither the risk categories nor the concordance algorithm (Figure 1). Only patients who had evidence of active surveillance or watchful waiting as a treatment plan were considered to have received “expectant management.” Patients who received no documented follow up were distinguished them from those who were followed for changes in PSA or symptoms according to the guideline.10 Treatment performed within six, 12 or 18 months of diagnosis was considered for calculating the outcome. Unadjusted results used either all treatment or initial treatment within the treatment window.
Figure 1.
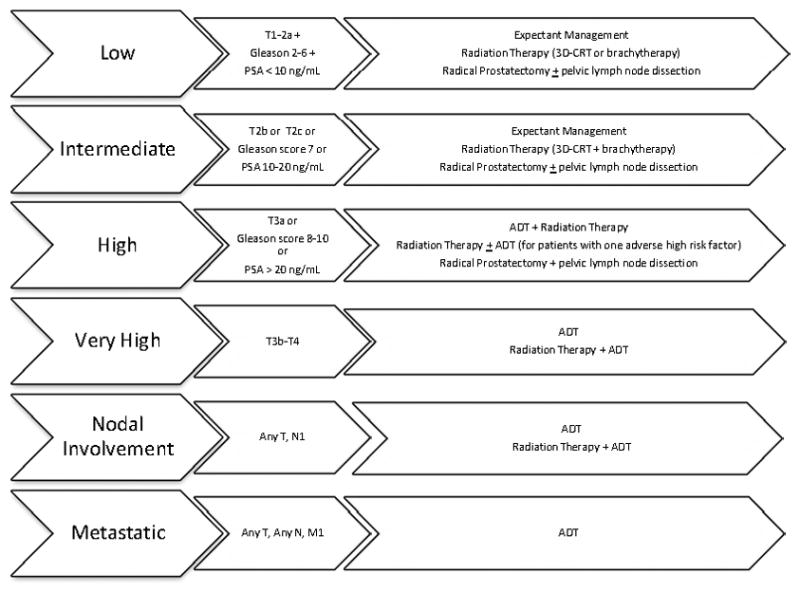
The 2004 to 2007 National Comprehensive Cancer Network guideline-concordant treatment options are shown for each prostate cancer risk category.
The primary independent variable, race, was assessed by participant self-report.
NCCN recommends care be tailored for each patient based on life expectancy.10 Comorbidity and patient age were controlled for using the Charlson Comorbidity Index (CCI) as a proxy for life expectancy.16 The CCI was scored from medical record abstraction. Weights were assigned to each condition and age category and summed into a single score. All liver disease was considered chronic.
The six risk and treatment combinations (Figure 1) were collapsed into five levels due to small sample sizes in the two highest risk categories (“metastatic” disease included both nodal involvement and metastases).
Five measures were included to assess how access moderated the effects of race on receipt of guideline-concordant care: Insurance status (yes vs. no), marital status (married vs. not), education level (more than high school vs. high school or less), medical mistrust (measured using a validated five-item scale),17, 18 and perceived access to care (measured using nine items resulting in a single summed score with possible values ranging from nine to 45).
Statistical Analysis
Racial differences were examined using Chi-squared tests and Fisher’s Exact tests for categorical variables, and t-tests for continuous variables. Maximum likelihood estimation (MLE) modeled the likelihood of receipt of NCCN guideline-concordant care. Likelihood ratio (LR) tests assessed inclusion of demographic characteristics and access variables. Model fit was assessed by comparing Akaike Information Criteria (AIC) and Hosmer and Lemeshow’s goodness-of-fit. Complete case analysis was used to address missing data.
Except for summarizing therapies received, all other descriptive and regression results adjusted for both the population sampling weights and PCaP response rate. All AAs were included in the cohort sampling frame, but only 44% of CAs were asked to participate.15 AA’s and CA’s observations were weighted by their respective response rates since response rates for inclusion in the original cohort differed by race. Descriptive statistics were expanded by these factors to represent the NC prostate cancer population. Sensitivity analyses assessed the effects of considering all comorbid liver disease as chronic; the appropriate treatment window length; and NCCN guideline considerations to move intermediate and high risk patients with multiple adverse factors to the next higher risk category. Standard errors were adjusted using robust variance estimators to account for the sampling and response weights. All analyses were conducted using Stata/IC 11.2.19
Results
AAs were significantly more likely than CAs to be uninsured (15.2% vs. 2.5%), to have completed no more than high school (54.0% vs. 25.7%), and to be younger (61.2 vs. 63.8 years) (all p<0.001) (Table 1). CCI was similar between AAs and CAs. AAs presented with significantly higher Gleason scores (p=0.025) and PSA levels (p=0.008), but their risk category only approached statistical difference from CA men when combined with the clinical T stage (p=0.055).
Table 1.
Population Estimated Summary Statistics by Race^
| Full Sample | African American | Caucasian American | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Sample Count | Population Estimate** (Linearized Standard Error) | Sample Count | Population Estimate (Linearized Standard Error) | Sample Count | Population Estimate (Linearized Standard Error) | P-value* | |
|
| |||||||
| n=777 | N=3736.7 | n=341 | N=897.4 | n=436 | N=2839.3 | ||
| Race | |||||||
| African American | 341 | 24.0% (0.01) | |||||
| Access | |||||||
| Uninsured | 63 | 5.6% (0.01) | 52 | 15.2% (0.02) | 11 | 2.5% (0.01) | <0.001 |
| Demographic | |||||||
| Married or Living as Married | 609 | 81.2% (0.01) | 240 | 70.4% (0.02) | 369 | 84.6% (0.02) | <0.001 |
| High School Education or Less | 296 | 32.5% (0.02) | 184 | 54.0% (0.03) | 112 | 25.7% (0.02) | <0.001 |
| Clinical T Stage | 0.028 | ||||||
| T1a-T2a | 507 | 64.3% (0.02) | 232 | 68.0% (0.03) | 275 | 63.1% (0.02) | |
| T2b-T2c | 262 | 35.0% (0.02) | 103 | 30.2% (0.02) | 159 | 36.5% (0.02) | |
| T3a | 2 | 0.1% (0.001) | 2 | 0.6% (0.004) | 0 | 0.0% | |
| T3b-T4 | 6 | 0.6% (0.003) | 4 | 1.2% (0.01) | 2 | 0.5% (0.003) | |
| Gleason Score | 0.025 | ||||||
| 2–6 | 464 | 61.7% (0.02) | 185 | 54.3% (0.03) | 279 | 64.0% (0.02) | |
| 7 | 237 | 29.0% (0.02) | 118 | 34.6% (0.03) | 119 | 27.3% (0.02) | |
| 8–10 | 76 | 9.3% (0.01) | 38 | 11.1% (0.02) | 38 | 8.7% (0.01) | |
| Mean (Linearized Standard Error) | |||||||
|
| |||||||
| Clinical Characteristics | |||||||
| PSA ng/mL | 9.48 (0.92) | 15.53 (2.87) | 7.58 (0.80) | 0.008 | |||
| Charlson Comorbidity Index | 2.58 (0.06) | 2.52 (0.09) | 2.59 (0.08) | 0.555 | |||
| Age in years | 63.15 (0.31) | 61.21 (0.42) | 63.76 (0.38) | <0.001 | |||
| Healthcare Perceptions | |||||||
| Mistrust Score | 19.84 (1.29) | 20.72 (1.90) | 19.56 (1.59) | 0.642 | |||
| Perceived Access to Care Score | 39.11 (0.15) | 37.72 (0.23) | 39.54 (0.19) | <0.001 | |||
Differences by race assessed with F-tests or Chi-squared tests
Estimates for the proportion of all men adjusted for sampling and response weights, which represent population proportions, not sample proportions
Clinical characteristics measured from diagnosis; demographic and patient-perceived data measured at baseline
Across all risk categories and combining all treatments received (Table 2), AAs received less surgery (58.1% vs. 68.0%, p=0.004), more radiation (39.0% vs. 27.4%, p<0.001), and more ADT (25.9% vs. 18.9%, p=0.022) than CAs, but similar rates of expectant management (5.9% vs. 9.0%, p=0.094) and brachytherapy (8.6% vs. 6.9%, p=0.403). When stratified by prostate cancer risk category, unadjusted treatment patterns were different by race only among men with intermediate risk (p=0.017). More AAs received ADT plus radiation than did CAs, which in this risk category is non-guideline-concordant. AAs also were more likely to receive radiation and less likely to receive both surgery and expectant management compared to CAs (Table 2).
Table 2.
Guideline-Concordant and Non-Guideline-Concordant Therapies Received by Race and Recurrence Risk/Severity Category
| Full Sample (n=777) | African Americans (n=341) | Caucasian Americans (n=436) | P-value^ | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Count | % | Count | % | Count | % | ||
| All Therapies Received* | |||||||
| Expectant Management | 59 | 8.2% | 20 | 5.9% (0.01) | 39 | 9.0% (0.01) | 0.094 |
| Radical Prostatectomy | 494 | 65.6% | 198 | 58.1% (0.03) | 296 | 68.0% (0.02) | 0.004 |
| Pelvic Lymph Node Dissection | 259 | 33.7% | 113 | 33.8% (0.02) | 146 | 33.2% (0.03) | 0.870 |
| Radiation Therapy | 252 | 30.2% | 133 | 39.0% (0.03) | 119 | 27.4% (0.02) | >0.001 |
| Brachytherapy | 59 | 7.3% | 29 | 8.6% (0.02) | 30 | 6.9% (0.01) | 0.403 |
| Androgen Deprivation Therapy | 170 | 20.6% | 88 | 25.9% (0.02) | 82 | 18.9% (0.02) | 0.022 |
| Initial Therapy** | |||||||
| Low risk Disease | 284 | 118 | 166 | .262 | |||
| Expectant ManagementGC | 32 | 11.3% | 13 | 11.0% | 19 | 11.5% | |
| Radical ProstatectomyGC | 186 | 65.5% | 72 | 61.0% | 114 | 68.7% | |
| Radiation Therapy or BrachytherapyGC | 33 | 11.6% | 21 | 17.8% | 12 | 7.2% | |
| Primary Androgen Deprivation Therapy | 9 | 3.2% | 3 | 2.5% | 6 | 3.6% | |
| ADT + Radiation Therapy | 8 | 2.8% | 3 | 2.5% | 5 | 3.0% | |
| No Therapy Documented | 16 | 5.6% | 6 | 5.1% | 10 | 6.0% | |
| Intermediate risk Disease | 371 | 158 | 213 | 0.017 | |||
| Expectant ManagementGC | 25 | 6.7% | 7 | 4.4% | 18 | 8.5% | |
| Radical Prostatectomy ± Lymphnode DissectionGC | 243 | 65.5% | 95 | 60.1% | 148 | 69.5% | |
| Radiation Therapy ± BrachytherapyGC | 37 | 10.0% | 19 | 12.0% | 18 | 8.5% | |
| Primary Androgen Deprivation Therapy | 12 | 3.2% | 6 | 3.8% | 6 | 2.8% | |
| Brachytherapy Alone | 15 | 4.0% | 6 | 3.8% | 9 | 4.2% | |
| ADT+ Radiation Therapy | 31 | 8.4% | 22 | 14.0% | 9 | 4.2% | |
| No Therapy Documented | 8 | 2.2% | 3 | 1.9% | 5 | 2.4% | |
| High risk Disease | 97 | 51 | 46 | 0.262 | |||
| ADT + Radiation TherapyGC | 46 | 47.4% | 25 | 49.0% | 21 | 45.7% | |
| Radical Prostatectomy +Lymph Node DissectionGC | 39 | 40.2% | 18 | 35.3% | 21 | 45.7% | |
| Radiation Therapy ± ADTGC | 4 | 4.1% | 3 | 5.9% | 1 | 2.2% | |
| Primary ADT | 2 | 2.1% | 1 | 2.0% | 1 | 2.2% | |
| Brachytherapy Alone | 1 | 1.0% | 0 | 0.0% | 1 | 2.2% | |
| Radiation + Brachytherapy | 1 | 1.0% | 0 | 0.0% | 2.2% | ||
| No Therapy Documented | 4 | 4.1% | 4 | 7.8% | 0 | 0.0% | |
| Very High | 6 | 4 | 2 | 0.600 | |||
| Androgen Deprivation TherapyGC | 1 | 16.7% | 1 | 25.0% | 0 | 0.0% | |
| Radiation Therapy + ADTGC | 4 | 66.7% | 3 | 75.0% | 1 | 50.0% | |
| Radical Prostatectomy | 1 | 16.7% | 0 | 0.0% | 1 | 50.0% | |
| Nodal Involvement | 8 | 3 | 5 | 0.571 | |||
| Androgen Deprivation TherapyGC | 3 | 37.5% | 1 | 33.3% | 2 | 40.0% | |
| Radiation Therapy + ADTGC | 1 | 12.5% | 1 | 33.3% | 0 | 0.0% | |
| Radical Prostatectomy | 2 | 25.0% | 0 | 0.0% | 2 | 40.0% | |
| Expectant Management | 1 | 12.5% | 0 | 0.0% | 1 | 20.0% | |
| Radiation Alone | 1 | 12.5% | 1 | 33.3% | 0 | 0.0% | |
| Metastatic | 11 | 7 | 4 | 0.818 | |||
| Androgen Deprivation TherapyGC | 3 | 27.3% | 2 | 28.6% | 1 | 25.0% | |
| Radiation Therapy + ADT | 6 | 54.6% | 4 | 57.1% | 2 | 50.0% | |
| Radical Prostatectomy | 1 | 9.1% | 0 | 0.0% | 1 | 25.0% | |
| No Therapy Documented | 1 | 9.1% | 1 | 14.3% | 0 | 0.0% | |
Guideline-concordant for Recurrence Risk Category based on 2004 NCCN Prostate Cancer Treatment Guidelines
Therapies delivered regardless of treatment window, i.e., the first treatment the patient received whether or not therapy delivered in the treatment window
Only initial therapy delivered regardless of treatment window
p-value based on Fisher’s Exact test for difference in proportions for AA and CA among all therapies delivered in the recurrence risk category
The use of non-guideline concordant primary ADT was low (≤ 3.2%) across all risk categories of clinically localized disease. The proportion of men who received no therapy was low across all disease classifications. Expectant management was the least used guideline concordant therapy for men with low and intermediate risk disease, the only categories for which it is recommended, with 11.3% and 6.7% receiving it, respectively.
Population-adjusted rates showed that 83.5% of men received guideline-concordant care within one year of diagnosis, which did not differ by patient race (Table 3). Guideline concordance was relatively high in all categories, except for metastatic disease. Receipt of guideline-concordant initial treatment differed by race only among men with intermediate risk disease (concordance 75.3% AA vs. 85.9% CA, p=0.009).
Table 3.
Number and Population-Adjusted° Proportion Receiving NCCN Guideline-Concordant Prostate Cancer Care by Race and Prostate Cancer Risk Category
| Full Sample | African Americans | Caucasian Americans | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Sample Count | Population Estimate | Sample Count | Population Estimate | Sample Count | Population Estimate | P-value* | |
|
| |||||||
| n=777 | N=3736.7 | n=341 | N=897.4 | n=436 | N=2839.3 | ||
|
| |||||||
| Count | % | Count | % | Count | % | ||
| Guideline- Concordant Care^ | 641 | 83.5% | 272 | 79.8% | 369 | 84.6% | 0.076 |
| Low Risk (n=284) | 245 | 85.9% | 103 | 87.3% | 142 | 85.5% | 0.674 |
| Intermediate Risk (n=371) | 302 | 83.4% | 119 | 75.3% | 183 | 85.9% | 0.009 |
| High Risk (n=97) | 83 | 86.1% | 43 | 84.3% | 40 | 87.0% | 0.712 |
| Very High (n=6) | 5 | 72.3% | 4 | 100.0% | 1 | 50.0% | 0.333** |
| Metastatic (n=19) | 6 | 32.3% | 3 | 30.0% | 3 | 33.30% | 1.000** |
p-value based on Chi squared test for difference in proportions unless noted
p-value based on Fisher’s Exact test for difference in proportions
Guideline concordance based on 2004 NCCN Prostate Cancer Treatment Guidelines
Population adjusted proportions represent the proportion of patients in the category expected in the North Carolina population from which the sample was drawn, while the count (n) indicates the number of men represented in the study sample.
Model 2, which excluded trust, marital status and education level, demonstrated the best model fit among three models tested, though findings were generally consistent among all models (Table 4). In multivariable analysis, receipt of guideline-concordant care did not differ by race (OR 0.83; 95% CI 0.54–1.25), but patients with metastatic cancer had lower odds of receiving guideline-concordant care (OR 0.09; 95% CI 0.03–0.31). Metastatic patients had 91% lower odds of receiving guideline-concordant care than low-risk, clinically localized patients. In addition, patient-perceived access to care was associated with greater odds of receiving guideline-concordant care (OR 1.06; 95% CI 1.01–1.12); however, insurance status was not (OR 0.82; 95% CI 0.36, 1.62). Sensitivity analyses varying the classification of liver disease and varying the treatment window from six to 18 months did not affect results.
Table 4.
Odds Ratios on Receipt of Guideline-Concordant Care^ for Prostate Cancer
| Model 1# | Model 2# | Model 3# | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Odds Ratio | (95% Confidence Interval¥) | Odds Ratio | (95% Confidence Interval¥) | Odds Ratio | (95% Confidence Interval¥) | |
|
| ||||||
| African-American Race | 0.71 | (0.49, 1.05) | 0.83 | (0.54, 1.25) | 0.87 | (0.56, 1.37) |
| Charlson Comorbidity Index | 0.82*** | (0.74, 0.91) | 0.83*** | (0.75, 0.92) | 0.83*** | (0.75, 0.93) |
| Intermediate Risk | 0.81 | (0.51, 1.29) | 0.84 | (0.53, 1.34) | 0.84 | (0.52, 1.34) |
| High Risk | 1.10 | (0.52, 2.35) | 1.17 | (0.55, 2.48) | 1.20 | (0.57, 2.55) |
| Very High Risk | 0.46 | (0.05, 4.51) | 0.45 | (0.04, 5.02) | 0.47 | (0.04, 5.75) |
| Metastatic | 0.08*** | (0.02, 0.25) | 0.09*** | (0.03, 0.31) | 0.09*** | (0.03, 0.32) |
| Not Insured | 0.82 | (0.36, 1.62) | 0.76 | (0.36, 1.59) | ||
| Perceived Access to Care | 1.06* | (1.01, 1.12) | 1.06* | (1.00, 1.12) | ||
| Married or Living as Married | 1.15 | (0.67, 1.96) | ||||
| High School Education or Less | 0.82 | (0.52, 1.31) | ||||
| Mistrust | 1.01 | (1.00, 1.01) | ||||
| Constant | 11.41*** | (6.85, 19.00) | 1.00 | (0.13, 7.86) | 1.06 | (0.11, 10.02) |
|
| ||||||
| n | 777 | 777 | 777 | |||
| McFadden’s Pseudo R2 | 0.0617 | 0.0730 | 0.0762 | |||
| Akaike Information Criterion | 690.22 | 686.09 | 689.76 | |||
statistically significant at <0.05
statistically significant at <0.01
statistically significant at <0.001
Guideline concordance based on 2004 NCCN Prostate Cancer Treatment Guidelines
Models estimated using population and response weights
95% CI calculated with linearized standard errors
Discussion
In a North Carolina population-based cohort of men with incident prostate cancer diagnosed between 2004 and 2007, AA race was not associated with receipt, or lack of receipt, of guideline-concordant care. These findings appear to contradict several studies demonstrating that AAs experience different patterns of prostate cancer care than CAs;3, 5, 20 however, these studies looked at broad patterns of care without consideration of risk status or guideline recommendations. When patients were stratified by risk categories, racial differences in care were found to be differences among appropriate treatment options for most risk categories.
Patients received relatively high levels of guideline concordant care, which should be reassuring for prostate cancer patients in NC. Another study that examined treatment assigned based on D’Amico prostate cancer risk categories found similar proportions of concordance for low- and intermediate risk patients (>80%), but lower proportions for high-risk patients (60%) than were found in this study.21 No national benchmarks have been set for prostate cancer care, but 80% adherence is a frequently used benchmark for care. Future efforts to improve prostate cancer quality in NC should consider 80% adherence to be achievable and consider establishing higher goals.
In intermediate risk disease, although overall quality of care was relatively high, we found racial differences in guideline concordance. Compared to CA men, more AA men in this risk category received radiation therapy combined with ADT. Although evidence supporting addition of ADT to radiation in intermediate risk cancer was emerging,22 it was not yet recommended in 2004. Physicians treating these patients may have been innovators or early adopters of this treatment advance, which was eventually incorporated into the 2010 NCCN Guideline. However, the 2004 guidelines also allowed for moving patients with multiple risks to the next higher risk category. In this case, higher risk would have made ADT guideline-concordant. Reclassifying the 106 intermediate risk disease (and the 11 eligible high risk disease patients) into the next higher category did not change overall results with regard to race, CCI and perceived access to care. More intermediate-risk AA men received therapy consistent with the higher risk category. We do not know whether physicians treating these patients were innovators or reclassifying these patients based on multiple risk factors; however, this raises the possibility that intermediate risk AAs may have actually received better care than similar-risk CAs.
In men with nodal and metastatic disease, overall adherence was low. Only 32.3% of these men appeared to receive appropriate care. Although few men were receiving ADT as their initial treatment, it does not appear that physicians were delaying ADT until symptoms arose, as has been suggested may be appropriate.23 Rather, overuse of aggressive care—either surgery for those with nodal involvement only, or radiation for those with metastases—led to most of the guideline discordance observed. However, the 2004 NCCN guidelines suggest that surgery may be appropriate for those with T3 stage and nodal involvement,10 thus some of this care may be clinically appropriate. In addition, unlike clinical populations of prevalent disease, we observed very few men with metastatic disease in this population-based cohort of incident prostate cancer, thus estimates for this group may be unreliable.
While focusing on quality improvement in certain risk categories may be premature in light of evolving evidence and guideline nuances, patients’ perceived access to care was associated with receipt of guideline-concordant care. Overall, perceived access to care was moderately high, but those who had the lowest levels of perceived access at baseline were less likely to receive guideline-concordant care. Financial limitations (co-payments) and physical access to care may both inhibit care. Lack of continuity in care or decreased levels of comfort communicating with the healthcare provider also can represent diminished access. Thus, improving these aspects of care delivery may be the most important quality improvement targets in NC.
No association was found between having insurance and receiving guideline-concordant care; however, the numbers of uninsured in this study were small and therefore, the analysis of the contribution of insurance status lacked precision.
Guideline-concordant care in the high-risk NC cohort was estimated at 86.1% overall compared to 60% in a study of SEER-Medicare21 and 50% among the CaPSURE cohort.24 CaPSURE is a continuously collected prostate cancer registry, initiated in 1996. Thus, treatment patterns described from the registry represent the collective treatment provided over more than a decade. During this time changes in reimbursement have occurred;25 new technologies have emerged;26 and standards of care have changed.10, 27 Thus, comparison to the 2004 guideline may not be appropriate, and comparisons of the CaPSURE cohort to our 2004–2007 cohort may not be valid. The differing age of the cohorts also may partially explain the different findings among studies. The SEER-Medicare study is censored below age 65, whereas HCaP-NC had no lower age limit (60% are less than 65 years old). In CaPSURE, 46% of men are less than 65 years old.24 Nonetheless, when we restrict our cohort to those over 65 years of age, guideline-concordance for the high risk group falls only slightly to 86% overall.
Unmeasured factors may be contributing to the differences found. Prostate cancer treatment is highly provider-dependent.24, 28, 29 Our cohort may be seen by a limited number of providers in a single state and may not reflect patterns of care present in other regions of the country. Differences may reflect true differences in care in the populations studied. NC is not represented among the SEER-Medicare registries and care delivery in NC may differ markedly from those represented. For example, the distribution of AAs among healthcare delivery systems may differ. The majority of the AA populations in the SEER sample are concentrated in urban areas: Los Angeles, Detroit, Atlanta, San Francisco;30 whereas NC AAs are more widely distributed outside of urban areas.31 Emerging evidence suggests racial differences in healthcare may result from geographic differences.32
This study has a number of strengths. The study was designed to detect racial differences in prostate cancer care. The quality of care was assessed using the well accepted NCCN guidelines, which assign treatment by prostate cancer risk categories. Still, the study has limitations. Whether the lack of implementation of guideline-concordant care results from patient or provider preference was not addressed.9, 33, 34 The standard errors could be too small because clustering by provider was not controlled for. However, since the main hypothesis was not significant, the inability to control for clustering should not change the results. Patients may have preferences for the aggressiveness of their care, independent of clinical practice guidelines. However, patient level factors, which were included in the analysis, did not explain the greater likelihood of discordant care provided in metastatic disease. Finally, while NCCN guidelines are commonly used and represent the best level of evidence available, the optimal treatment of clinically localized prostate cancer lacks a strong evidence base. While we found care to be concordant with guidelines and distributed equitably between racial groups, we do not know whether the guidelines themselves represent the best care available. Moreover, the guidelines provide multiple treatment options for localized disease. Some guideline-concordant treatments were not used as frequently as others. Rates of expectant management were lower in this NC cohort than in national cohorts,26 but this may be due to the much younger age of the NC prostate cancer population.
Conclusion
NCCN guideline adherence was high in NC from 2004 to 2007 and did not differ by race. Health policy makers and population scientists should not attribute racial differences in prostate cancer outcomes to racial differences in treatment choices unless these differences persist after adjustment for prostate cancer risk category.
Acknowledgments
Funding:
North Carolina-Louisiana Prostate Cancer Project (PCaP) and North Carolina Health Access Project (HCaP-NC) are carried out as collaborative studies supported by the Department of Defense contract DAMD 17-03-2-0052 and the American Cancer Society award RSGT-08-008-01-CPHPS, respectively. Ms. Ellis was supported by a National Cancer Institute training grant (R25CA116339).
The University of North Carolina, Chapel Hill Institutional Review Board provided approval for and oversight. Participants provided informed consent. The authors thank the staff, advisory committees and research subjects for their important contributions.
References
- 1.Barocas DA, Penson DF. Racial variation in the pattern and quality of care for prostate cancer in the USA: mind the gap. BJU Int. 2010;106:322–328. doi: 10.1111/j.1464-410X.2010.09467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morris CR, Snipes KP, Schlag R, Wright WE. Sociodemographic factors associated with prostatectomy utilization and concordance with the physician data query for prostate cancer (United States) Cancer Causes Control. 1999;10:503–511. doi: 10.1023/a:1008951009959. [DOI] [PubMed] [Google Scholar]
- 3.Schymura MJ, Kahn AR, German RR, et al. Factors associated with initial treatment and survival for clinically localized prostate cancer: results from the CDC-NPCR Patterns of Care Study (PoC1) BMC Cancer. 2010;10:152. doi: 10.1186/1471-2407-10-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spencer BA, Miller DC, Litwin MS, et al. Variations in quality of care for men with early-stage prostate cancer. J Clin Oncol. 2008;26:3735–3742. doi: 10.1200/JCO.2007.13.2555. [DOI] [PubMed] [Google Scholar]
- 5.Zeliadt SB, Potosky AL, Etzioni R, Ramsey SD, Penson DF. Racial disparity in primary and adjuvant treatment for nonmetastatic prostate cancer: SEER-Medicare trends 1991 to 1999. Urology. 2004;64:1171–1176. doi: 10.1016/j.urology.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 6.Carson AP, Howard DL, Carpenter WR, et al. Trends and racial differences in the use of androgen deprivation therapy for metastatic prostate cancer. J Pain Symptom Manage. 2010;39:872–881. doi: 10.1016/j.jpainsymman.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shahinian VB, Kuo YF, Gilbert SM. Reimbursement policy and androgen-deprivation therapy for prostate cancer. N Engl J Med. 2010;363:1822–1832. doi: 10.1056/NEJMsa0910784. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network; National Comprehensive Cancer Network, editor. Practice Guidelines in Oncology. 2010. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines™) [Google Scholar]
- 9.Wilt TJ, Shamliyan T, Taylor B, et al. Agency for Healthcare Research and Quality, editor. Comparative Effectiveness Review. Rockville, MD: 2008. Comparative Effectiveness of Therapies for Clinically Localized Prostate Cancer. [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network; National Comprehensive Cancer Network, editor. NCCN Practice Guidelines in Oncology. 2004. Prostate Cancer. [DOI] [PubMed] [Google Scholar]
- 11.Andersen RM, Davidson PL, Ganz PA. Symbiotic relationships of quality of life, health services research and other health research. Qual Life Res. 1994;3:365–371. doi: 10.1007/BF00451728. [DOI] [PubMed] [Google Scholar]
- 12.Sadetsky N, Lubeck DP, Latini DM, et al. Demographics, insurance coverage, and utilization of medical services in newly diagnosed prostate cancer: data from caPSURE. Manag Care Interface. 2005;18:25–30. [PubMed] [Google Scholar]
- 13.Talcott JA, Spain P, Clark JA, et al. Hidden barriers between knowledge and behavior: the North Carolina prostate cancer screening and treatment experience. Cancer. 2007;109:1599–1606. doi: 10.1002/cncr.22583. [DOI] [PubMed] [Google Scholar]
- 14.Ward E, Halpern M, Schrag N, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58:9–31. doi: 10.3322/CA.2007.0011. [DOI] [PubMed] [Google Scholar]
- 15.Schroeder JC, Bensen JT, Su LJ, et al. The North Carolina-Louisiana Prostate Cancer Project (PCaP): methods and design of a multidisciplinary population-based cohort study of racial differences in prostate cancer outcomes. Prostate. 2006;66:1162–1176. doi: 10.1002/pros.20449. [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.LaVeist TA, Nickerson KJ, Bowie JV. Attitudes about racism, medical mistrust, and satisfaction with care among African American and white cardiac patients. Med Care Res Rev. 2000;57 (Suppl 1):146–161. doi: 10.1177/1077558700057001S07. [DOI] [PubMed] [Google Scholar]
- 18.LaVeist TA, Isaac LA, Williams KP. Mistrust of health care organizations is associated with underutilization of health services. Health Serv Res. 2009;44:2093–2105. doi: 10.1111/j.1475-6773.2009.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.StataCorp LP. Stata/IC 11.2 for Windows. College Station, TX: StataCorp LP; 2011. [Google Scholar]
- 20.Gross CP, Smith BD, Wolf E, Andersen M. Racial disparities in cancer therapy: did the gap narrow between 1992 and 2002? Cancer. 2008;112:900–908. doi: 10.1002/cncr.23228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen RC, Carpenter WR, Hendrix LH, et al. African American and Caucasian Prostate Cancer Patients. Receipt of Guideline-Concordant Treatment in older. Under Review. [Google Scholar]
- 22.D’Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA. 2004;292:821–827. doi: 10.1001/jama.292.7.821. [DOI] [PubMed] [Google Scholar]
- 23.Conti PD, Atallah AN, Arruda H, Soares BG, El Dib RP, Wilt TJ. Intermittent versus continuous androgen suppression for prostatic cancer. Cochrane Database Syst Rev. 2007:CD005009. doi: 10.1002/14651858.CD005009.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28:1117–1123. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medicare Prescription Drug, Improvement, and Modernization Act. United States of America: 2003. [Google Scholar]
- 26.Dinan MA, Robinson TJ, Zagar TM, et al. Changes in Initial Treatment for Prostate Cancer Among Medicare Beneficiaries, 1999–2007. Int J Radiat Oncol Biol Phys. 2012 doi: 10.1016/j.ijrobp.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bahnson RR, Hanks GE, Huben RP, et al. NCCN Practice Guidelines for Prostate Cancer. Oncology (Williston Park) 2000;14:111–119. [PubMed] [Google Scholar]
- 28.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Determinants of androgen deprivation therapy use for prostate cancer: role of the urologist. J Natl Cancer Inst. 2006;98:839–845. doi: 10.1093/jnci/djj230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shahinian VB, Kuo YF, Freeman JL, Orihuela E, Goodwin JS. Characteristics of urologists predict the use of androgen deprivation therapy for prostate cancer. J Clin Oncol. 2007;25:5359–5365. doi: 10.1200/JCO.2006.09.9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Committee on Cancer Research Among Minorities and the Medically Underserved, Institute of Medicine. The Unequal Burden of Cancer:An Assessment of NIH Research and Programs for Ethnic Minorities and the Medically Underserved. The National Academies Press; 1999. [PubMed] [Google Scholar]
- 31.State Center for Health Statistics and Office of Minority Health and Health Disparities. North Carolina Minority Health Facts. Raleigh, NC: Department of Health and Human Services; 2005. African Americans. [Google Scholar]
- 32.Sarrazin MS, Campbell ME, Richardson KK, Rosenthal GE. Racial segregation and disparities in health care delivery: conceptual model and empirical assessment. Health Serv Res. 2009;44:1424–1444. doi: 10.1111/j.1475-6773.2009.00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jayadevappa R, Malkowicz SB, Chhatre S, Gallo J, Schwartz JS. Racial and ethnic variation in health resource use and cost for prostate cancer. BJU Int. 2010;106:801–808. doi: 10.1111/j.1464-410X.2010.09227.x. [DOI] [PubMed] [Google Scholar]
- 34.Parsons JK, Kwan L, Connor SE, Miller DC, Litwin MS. Prostate cancer treatment for economically disadvantaged men: a comparison of county hospitals and private providers. Cancer. 2010;116:1378–1384. doi: 10.1002/cncr.24856. [DOI] [PubMed] [Google Scholar]


