Dear Editors,
Kraepelin's differentiation of the major psychoses into Schizophrenia and “Manic-Depressive Insanity” (Bipolar Disorder) as distinct neuropsychiatric disorders has been a foundation for psychiatric classification for more than 100 years. Studies directly comparing Schizophrenia (SZ) and Bipolar Disorder with psychotic features (BDp) provide a valuable test of Kraepelin's model of specificity. While some results comparing the psychotic form of BD with schizophrenia support the Kraepelinian dichotomy (Arnone et al. 2009), others support the unitary or continuum concept of psychosis, which posits the two psychotic illnesses arise from common neurobiological processes (Van Snellenberg and de Candia 2009). One core phenotype the two psychotic disorders share is executive control and working memory deficits (WM) with these deficits tending to be more severe in schizophrenia (Altshuler et al. 2004; Seidman et al. 2002). While a growing number of functional neuroimaging studies (using diverse cognitive tasks) have directly compared SZ and BDp groups (Calhoun et al., 2008; Costefreda et al., 2011; McIntosh et al., 2008), only one examined executive functions (EFs), revealing no group differences in the dorsolateral prefrontal cortex (DLPFC; Hamilton et al 2009). Thus, the specificity of the neural substrates of EF deficits in schizophrenia and BDp remains an unresolved question.
We sought to directly compare DLPFC and medial prefrontal cortex (mPFC) activity in schizophrenia and BDp patients during WM. This was based on the importance of prefrontal dysregulation in psychotic disorders, and, in part, on our prior work (Whitfield-Gabrieli 2009), showing mPFC hyperactivation during 2-back working memory task performance in SZ and first-degree relatives of persons with SZ. Additionally, based on the observed behavioral differences we explored the possibility that brain activity and performance would differentially change over the course of two sequential WM task runs in SZ and BDp groups.
We studied 19 controls and 22 psychotic (BDp: n=12; SZ: n=10) patients. fMRI signal was acquired during performance of two experimental runs of 2-back and 0-back WM tasks at 1.5T. There were no significant differences on age (all subjects were in their 30's and 40's), parental socioeconomic status, handedness, or sex distribution between groups. Controls had significantly higher education than both groups and were significantly more likely to be Caucasian than the BDp group. Controls had significantly higher IQ estimates than individuals with schizophrenia. There were no significant differences on a single word oral reading score used to estimate premorbid IQ, 0-back task performance and reaction time, 2-back reaction time, or mood scores. On the 2-back task, BDp patients had significantly lower accuracy than controls, but were not different than SZ patients. Schizophrenia patients had lower 2-back task accuracy than controls, but this was not statistically significant. Of importance, there were no significant differences between the two patient groups.
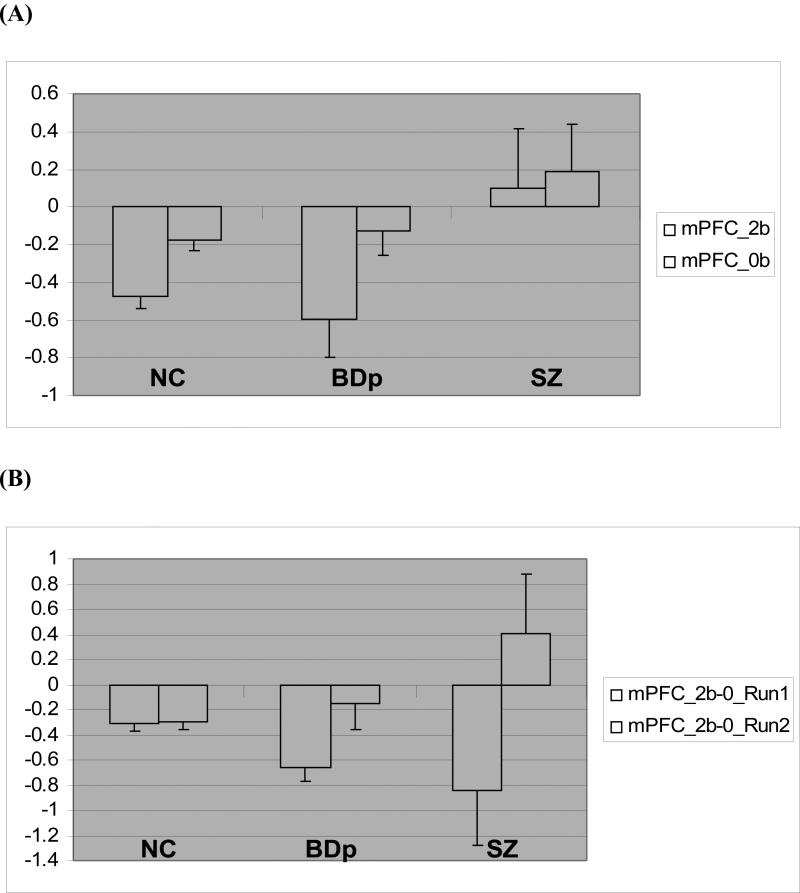
When activation during the 2-back > 0-back contrast was compared in the DLPFC region of interest (ROI), there were no significant differences in activity. However, schizophrenia patients exhibited greater activation than both control (t = 3.33, p<0.018) and BDp (t = 4.33, P<0.012) groups in the mPFC ROI (MNI coordinates −1 +47 −4), Fig 1A. Group differences between schizophrenia and BDp in mPFC remained significant after separately controlling for 2-back percent correct, Profile of Mood States Depression scores and IQ.
Figure 1.
(A) Graph depicts parameter estimates (mean BOLD fMRI signal change, with standard error bars) extracted from the mPFC cluster for the 0-back (0B) and 2-back (2B) conditions relative to fixation (represented by the zero baseline) for each group. Statistical analysis was performed for 2-back minus 0-back condition. One-way ANOVA for between group differences: F=5.692, p<0.007. Post-hoc Bonferroni correction: SZ>BDp, p< 0.005 (B) mPFC parameter estimates (mean BOLD fMRI signal change, with standard error bars) extracted separately from each task run. Parameter estimates were extracted from the significant mPFC cluster for the 0-back (0B) and 2-back (2B) conditions relative to fixation (represented by the zero baseline) for each group and shown as 2-back minus 0-back condition. GLM analysis for group x task interaction: F=4.62, p<0.017. Post hoc analysis, Run 1: NC>BDp (p<0.011) and Run 2: SZ>NC (p<0.05), SZ>BDp (p<0.05)
When 2-back data were analyzed for the task runs separately, there was a significant group-by-2-back performance accuracy interaction (p<0.05). Post-hoc tests revealed that controls performed better than BDp only in run 1 and better than SZ only in run 2 (p <0.05, Bonferonni corrected). Parameter estimates were extracted from the DLPFC and mPFC ROIs for each run separately. There was a significant group-by-task run interaction on DLPFC activity (F = 4.099, P<0.025), with post-hoc tests showing significantly less DLPFC activity in SZ in run 1 and greater activity in SZ in run 2 compared to controls (DLPFC activity did not differentiate BDp and controls). In the mPFC, there was a significant group-by-task interaction on mPFC activity (Fig 1B). Post hoc tests showed significant differences between controls and BDp in run 1 and between SZ and both controls and BDp in run 2 (p <0.05, Bonferonni corrected).
Patients with schizophrenia vs. BDp showed a different pattern of mPFC activity even in the face of comparable WM performance and demographic similarity. This suggests that despite many similarities between schizophrenia and BDp, brain functioning may be different in the two illnesses. Parameter estimates extracted from the mPFC revealed that patients with schizophrenia exhibit reduced mPFC suppression during the 2-back task. This finding is consistent with our previous work in younger patients with schizophrenia and in first-degree relatives (Whitfield-Gabrieli et al 2009). Of preliminary interest are the exploratory findings of different patterns of performance and brain activity over time within the experimental session (van Raalten et al., 2008). This intriguing finding needs to be studied with a larger sample and preferably, in unmedicated patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Altshuler LL, Ventura J, van Gorp WG, Green MF, Theberge DC, Mintz J. Neurocognitive function in clinically stable men with bipolar I disorder or schizophrenia and normal control subjects. Biological Psychiatry. 2004;56:560–569. doi: 10.1016/j.biopsych.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. British Journal of Psychiatry. 2009;195:194–201. doi: 10.1192/bjp.bp.108.059717. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Maciejewski PK, Pearlson GD, Kiehl KA. Temporal lobe and “default” hemodynamic brain modes discriminate between schizophrenia and bipolar disorder. Human Brain Mapping. 2008;29:1265–1275. doi: 10.1002/hbm.20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costafreda SG, Fu CH, Picchioni M, Touloupoulou T, McDonald C, Walshe M, Prata D, Murray RM, McGuire PK. Pattern of neural responses to verbal fluency shows diagnostic specificity for schizophrenia and bipolar disorder. BMC Psychiatry. 2011;11:18. doi: 10.1186/1471-244X-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton LS, Altshuler LL, Townsend J, Bookheimer SY, Phillips OR, Fischer J, Woods RP, Mazziotta JC, Toga AW, Nuechterlein KH, Narr KL. Alterations in functional activation in euthymic bipolar disorder and schizophrenia during a working memory task. Human Brain Mapping. 2009;30:3958–3969. doi: 10.1002/hbm.20820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AM, Whalley HC, McKirdy J, Hall J, Sussmann JE, Shankar P, Johnstone EC, Lawrie SM. Prefrontal function and activation in bipolar disorder and schizophrenia. American Journal of Psychiatry. 2008;165:378–84. doi: 10.1176/appi.ajp.2007.07020365. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Kremen WS, Koren D, Faraone SV, Goldstein JM, Tsuang MT. A comparative profile analysis of neuropsychological functioning in patients with schizophrenia and bipolar psychoses. Schizophrenia Research. 2002;53:31–44. doi: 10.1016/s0920-9964(01)00162-1. [DOI] [PubMed] [Google Scholar]
- van Raalten TR, Ramsey NF, Jansma JM, Jager G, Kahn RS. Automatization and working memory capacity in schizophrenia. Schizophrenia Research. 2008;100:161–171. doi: 10.1016/j.schres.2007.10.035. [DOI] [PubMed] [Google Scholar]
- Van Snellenberg JX, de Candia T. Meta-analytic evidence for familial coaggregation of schizophrenia and bipolar disorder. Archives of General Psychiatry. 2009;66:748–755. doi: 10.1001/archgenpsychiatry.2009.64. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, Wojcik J, Gabrieli JD, Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proceedings of the National Academy of Sciences U S A. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]