Abstract
TRAIL holds promise as an anti-cancer therapeutic but induces apoptosis in only a subset of tumor cell types. Moreover, many cancers that are responsive to TRAIL-mediated death exhibit partial sensitivity, with only a fraction of cells dying in response to treatment. Even within clonal populations of cultured cells, individual TRAIL-treated cells exhibit cell-to-cell variability both in timing of cell death and in whether a cell lives or dies. Fractional killing in these cell populations arises not from genetic differences among cells but rather from differences in gene expression states, fluctuations in protein levels, and the extent to which TRAIL-induced death or survival pathways become activated. In this study we ask how cell-to-cell variability manifests in cell types with different sensitivities to TRAIL, as well as how it changes in response to different combinations of drug treatments. We show that individual cells that survive treatment with TRAIL can regenerate the sensitivity of the starting population, demonstrating that transient heritability of resistance factors is a general property contributing to apoptotic sensitivity. Moreover, we show that the extent of cell-to-cell variability in timing and probability of apoptosis in response to treatment can be tuned using combinations of drugs that together increase apoptotic sensitivity compared to treatment with one drug alone. In the case of TRAIL, modulation of cell-to-cell variability using co-drugging sensitizes cells to apoptosis by altering the dynamics of initiator caspase activation and lowering the threshold for MOMP.
Keywords: apoptosis, death ligand, variability, co-drugging, TRAIL
INTRODUCTION
TRAIL (Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand) is a member of the TNF family of death ligands that induces apoptosis via an extrinsic receptor-mediated cell death pathway (Ashkenazi, 2008). TRAIL ligand and antibodies that function as receptor agonists are under investigation as anti-cancer drugs because of their observed ability to promote apoptosis in cancer cells while sparing normal tissue. However, many cancers are resistant to TRAIL-mediated apoptosis and others exhibit partial sensitivity, such that only a fraction of cells dies in response to treatment (Gonzalvez & Ashkenazi, 2010). These and related factors have complicated the clinical development of TRAIL and TRAIL receptor agonists.
TRAIL induces apoptosis via binding to DR4/5 receptors on the surface of target cells (Gonzalvez & Ashkenazi, 2010). Binding causes recruitment of Death Inducing Signaling Complex (DISC) proteins to the intracellular tails of DR4/5 receptors and activation of initiator caspases-8/10 (Kischkel et al, 1995; Martin et al, 1998). In some cell types (Type I cells), cleavage of effector caspases-3/7 by caspase-8/10 is sufficient to trigger cell death, but most cells (Type II cells) require mitochondrial outer membrane permeabilization (MOMP) to undergo apoptosis (Barnhart et al, 2003; Deng et al, 2002; Sun et al, 2002). MOMP is regulated by caspase-8/10 cleavage of Bid into tBid, followed by tBid translocation to the mitochondrial membrane where it activates pro-apoptotic Bcl-2 family proteins such as Bax/Bak (Eskes et al, 2000). When sufficient active Bax/Bak is present to overcome inhibition by resident anti-apoptotic Bcl-2 proteins, MOMP ensues, leading to release of Smac and cytochrome C into the cytosol (Li et al, 2002; Luo et al, 1998). Cytochrome C activates the caspase-9-containing apoptosome, while Smac displaces the inhibitor of apoptosis protein XIAP from caspase-3. These events result in a dramatic increase in effector caspase catalytic activity, ultimately leading to cleavage of the genome, proteome, and consequent cell death (Deveraux et al, 1997; Riedl & Salvesen, 2007).
Resistance to TRAIL is a natural feature of some cell types but may also be acquired following TRAIL treatment, and multiple mechanisms underlie resistance (Gonzalvez & Ashkenazi, 2010; Johnstone et al, 2008). Mutation or downregulation of DR4/5 receptors or upregulation of DcR1/2 decoy receptors, which bind TRAIL but lack signaling domains, account for TRAIL resistance in some cases but are not broadly prognostic (Ashkenazi & Dixit, 1999; Lee et al, 2001; MacFarlane et al, 2005). Changes in DISC signaling components, such as downregulation of caspase-8 or upregulation of the inhibitor protein c-FLIP, changes in the levels or activities of pro- or anti-apoptotic Bcl-2 family proteins, or changes in expression of IAP proteins such as XIAP can also cause resistance to TRAIL (Aldridge et al, 2011; Zhang & Fang, 2005). Survival signaling pathways, such as those mediated by the NF-κB transcription factor or pro-survival kinases, are also implicated in resistance (Falschlehner et al, 2007). Finally, it has been shown that post-translational modification of DR4/5 receptors affecting clustering and subsequent recruitment of DISC proteins can determine whether cells respond to treatment with TRAIL and whether they subsequently activate death or survival pathways (Mazurek et al, 2011; Song et al, 2007; Wagner et al, 2007).
Using combinations of drugs is widely considered to be a promising strategy for overcoming resistance to TRAIL and increasing tumor cell killing (Hellwig & Rehm, 2012; Johnstone et al, 2008). With the exception of cancers in which essential components of the extrinsic cell death pathway are mutated or silenced (resulting, for example, in inactivation of TRAIL receptors or caspase-8), co-drugging has been shown to increase the efficacy of TRAIL in cell lines, tumor models, and patients (Ashkenazi & Herbst, 2008). Sub-lethal doses of chemotherapy or ionizing radiation are known to sensitize resistant cancer cells to TRAIL-induced apoptosis via modulation of TRAIL receptors, DISC or mitochondrial components (Broaddus et al, 2005; Di Pietro et al, 2001; Ehrhardt et al, 2008; Galligan et al, 2005; Ganten et al, 2004; Keane et al, 1999; Morizot et al, 2010). Drugs that inhibit histone deacetylases or the proteasome (which modulate gene expression and protein stability, respectively) have also been shown to be effective in overcoming resistance to TRAIL in cell lines and tumor models (Bagci-Onder et al, 2012; Brooks et al, 2005; Butler et al, 2006; Frew et al, 2008; Inoue et al, 2004; Kabore et al, 2006; Vanoosten et al, 2005). Drugs that target pro-survival or mitogenic kinases such as the EGF receptor inhibitor gefitinib and the multikinase inhibitor sorafenib sensitize resistant cancer cells to TRAIL through a variety of mechanisms involving downregulation of the anti-apoptotic proteins Mcl-1, c-FLIP, and members of the IAP family (Kim et al, 2008; Ricci et al, 2007; Rosato et al, 2007; Shrader et al, 2007). Smac mimetics and XIAP inhibitors directly promote caspase-3 activity in resistant cell types (Bockbrader et al, 2005; Karikari et al, 2007). Finally, specific inhibition of Bcl-2 and Bcl-XL using the small molecule inhibitor ABT-737 (or its clinical version ABT-263) has shown promise in combination with TRAIL in certain contexts (Cristofanon & Fulda, 2012; Hetschko et al, 2007; Huang & Sinicrope, 2008; Lickliter et al, 2007; Song et al, 2008).
The importance of understanding drug sensitivity at the level of individual cells has been demonstrated in several recent studies (Cohen et al, 2008; Sharma et al, 2010) and this is also true for TRAIL (Bagci-Onder et al, 2012; Spencer et al, 2009). The dynamics of TRAIL-mediated apoptosis in single cells have been most extensively studied in HeLa lines using live-cell microscopy of cells expressing fluorescent reporters for MOMP and caspase activity (Albeck et al, 2008a; Albeck et al, 2008b; Hellwig et al, 2008; Rehm et al, 2006) and recently these findings were extended to more clinically relevant settings such as glioblastoma (Bagci-Onder et al, 2012). Timing of cell death following TRAIL treatment was shown to vary dramatically from one cell to the next, even within a single cell clone (Albeck et al, 2008a; Spencer et al, 2009). Within an individual cell, death was shown to be controlled by a “snap-action, variable-delay switch” in which initiator caspases are slowly activated and effector caspases suddenly turned on only when MOMP is triggered and XIAP neutralized. The delay period is highly variable in length and its duration is determined both by the rate of initiator caspase activation and the threshold for MOMP, which is set by the levels of pro- and anti-apoptotic Bcl-2 proteins. However, once MOMP is triggered and effector caspases activated, cell death occurs at a rapid, relatively constant rate, on the order of minutes (Albeck et al, 2008a; Goldstein et al, 2000; Rehm et al, 2003). Variability in the timing of cell death is controlled in a non-genetic manner and appears to involve stochastic, naturally occurring differences in the concentrations of positive and negative regulators of apoptosis (Bhola & Simon, 2009; Gaudet et al, 2012; Spencer et al, 2009).
In this paper we investigate how cell-to-cell variability in both timing of cell death and cell fate (death vs. survival) manifests in cell types with different sensitivities to TRAIL and how the extent of cell-to-cell variability changes in response to therapeutic drugs individually and in combination. First, we illustrate how cell-to-cell variability affects the sensitivity read-out of a panel of cell types treated with TRAIL. Second, we show in multiple cell types that cells that survive treatment with TRAIL or other inducers of apoptosis regenerate the sensitivity of the original cell population within several days of outgrowth, demonstrating a non-genetic component to fractional killing. Third, we examine the effects of co-drugging on cell-to-cell variability and show that overall variability is reduced when cells are sensitized to TRAIL-mediated death using a variety of drugs. Finally, we demonstrate that modulating cell-to-cell variability contributes to sensitization to TRAIL-mediated apoptosis at least in part by lowering the threshold for MOMP.
RESULTS
Net sensitivity to death ligands reflects variability in timing of cell death combined with heterogeneity in fate
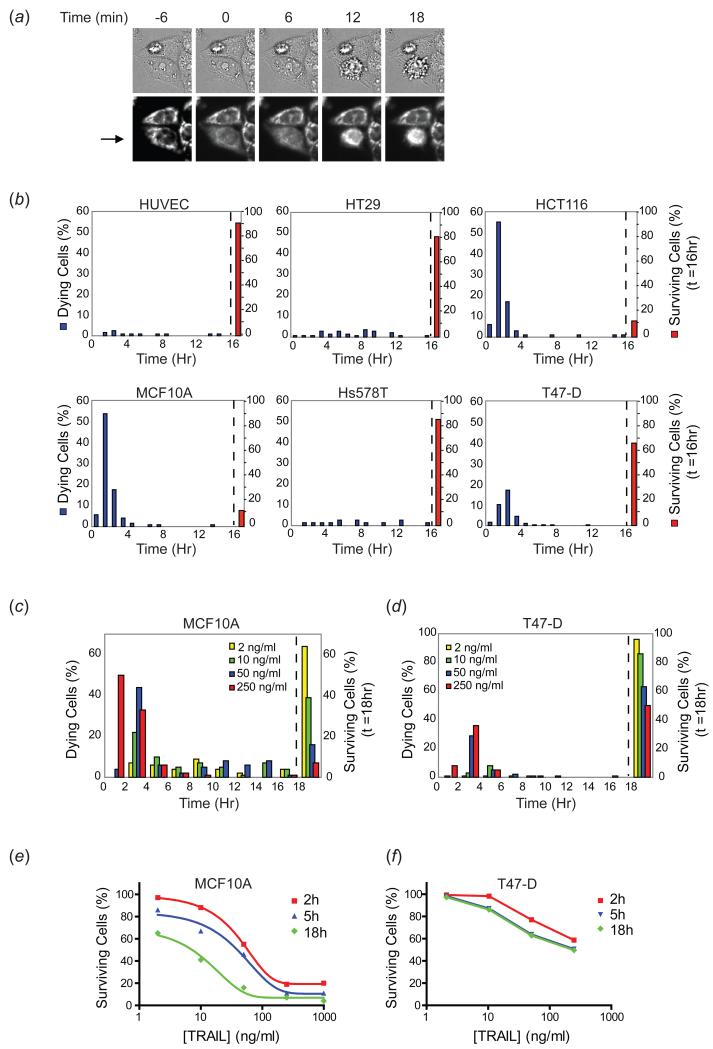
To explore the relationship between cell-to-cell variability and sensitivity to death ligands, we treated a panel of cell lines with recombinant TRAIL (50ng/ml) and monitored death times by live-cell microscopy using either the time of MOMP, as measured with an RFP-based reporter of mitochondrial membrane permeabilization (IMS-RP) or cellular morphology, which changes dramatically within minutes of MOMP (Albeck et al, 2008a) (Figure 1(a)). Cells that survived beyond a typical 16 hour movie were considered to be “survivors” since we observed that such cells were able to recover and successfully divide when TRAIL was removed. In agreement with previous reports, we found that primary endothelial cells (HUVEC) were almost completely resistant to TRAIL (90% surviving a 50ng/ml treatment) and breast cancer cell lines (Hs578T and T47-D) were only slightly more sensitive, with 85% and 65% survivors, respectively. Colon cancer lines exhibited greater variability in response: HCT116 cells were relatively sensitive (10-15% survivors) and HT29 cells relatively resistant (80% survivors). Non-transformed but immortalized MCF10A mammary epithelial cells were also sensitive at this dose of TRAIL (10-15% survivors) (Figure 1(b); (Rahman et al, 2009; Secchiero et al, 2003; van Geelen et al, 2003)). At a population level, sensitivity to TRAIL is a measure of heterogeneity in cell fate (death vs. survival), with some cell lines exhibiting a more even distribution between the two fates (and thus more heterogeneity in cell fate) than others.
Figure 1. Variability in timing of death and cell fate in different cell types treated with TRAIL.
(a) Representative microscopy images (20×, N.A.=0.75) depicting time of MOMP and the morphological changes used to score cell death in panel (b). Images are of HeLa cells expressing the MOMP reporter, and time (minutes) are indicated relative to time of MOMP (t=0) for the cell indicated by an arrow. For this cell, morphological changes associated with death began occurring within approximately 9-12 minutes of MOMP. (b) Death time distributions and percentage of surviving cells for primary human endothelial cells (HUVEC), MCF10A cells, and four cancer cell lines (HT29, HCT116, Hs578T, and T47-D) treated with TRAIL (50ng/ml) and imaged at 3 minute intervals for 16h. The red bar at 16 hours represents the percentage of cells surviving at the end of the 16h movie (right side of dotted line). At least 100 cells were analyzed for each cell type. (c-d) Death time distributions and percentage of surviving cells for MCF10A and T47-D cells treated with a range of doses of TRAIL and imaged by live-cell microscopy at 5 minute intervals for 18h. The percentage of surviving cells at the end of the 18h movie is plotted to the right of the dotted line. 100 cells were analyzed for each condition. (e-f) Dose response curves calculated from the time of death distributions. The percentage of surviving cells at three timepoints (2h, 5h, and 18h) across a range of doses was calculated from the time of death distributions and plotted as a dose response curve.
To explore the effects of variability in time of death on heterogeneity in fate, populations of MCF10A and T47-D cells were exposed to a range of TRAIL doses and imaged for 18h by live-cell microscopy. From the distributions of death times (Figure 1(c-d)) the percentage of cells still alive at 2h, 5h, or 18h was determined and plotted as a set of time-resolved dose-response curves (Figure 1(e-f)). Time was an important factor in determining sensitivity in the case of highly sensitive MCF10A cells (with the IC50 varying from 55ng/ml at 2h and 45ng/ml at 5h to 10ng/ml at 18h). In contrast, for T47-D cells, time was less important: T47-D cells either died within six hours of TRAIL exposure or survived indefinitely. Thus, variability in the timing of cell death affects the dose-dependent sensitivity of some cell types to TRAIL more than others.
Survivor cells regenerate the original sensitivity profile
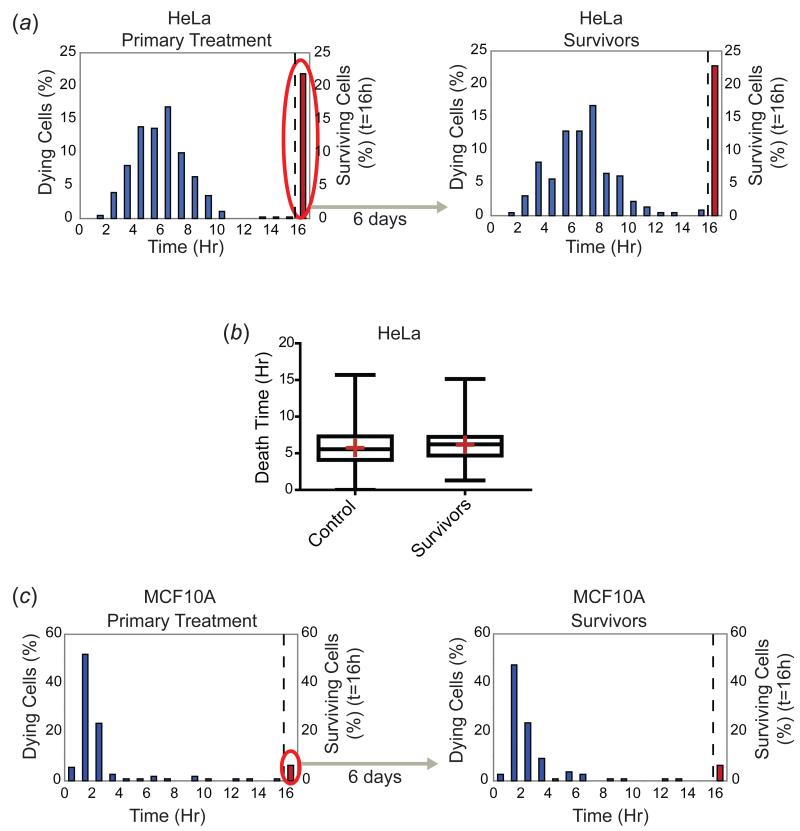
To investigate the origins of incomplete cell killing we treated cells with TRAIL, collected survivors, allowed them to grow for several days and then exposed them to a second TRAIL challenge. To minimize the impact of genetic heterogeneity on these measurements, we examined early-passage cell populations obtained by single-cell cloning. First, we treated HeLa cells with 50ng/ml TRAIL and followed them for 16h by live-cell microscopy, at which point about 20% of the cells were still alive. We then collected these survivors and expanded them for approximately one week in the absence of TRAIL. When the survivor population was re-challenged with 50ng/ml TRAIL and imaged for 16h, we observed that once again about 20% of the cells survived (Figure 2(a)). Moreover, not only the fraction of survivors, but also the shape of the time of death distribution was indistinguishable from that of the parental population: the mean, median, and variances of the time of death distributions did not vary significantly between control and survivor cells (p>0.05) (Figure 2(b)). Regeneration of the fraction of surviving cells as well as the shape of the death time distributions was observed for multiple cell types with varying degrees of sensitivity to TRAIL and with different shapes of death time distributions (Figure 2(c) and Figure S1(a)). HeLa cells surviving treatment with staurosporine also re-established the sensitivity profile of the starting population within two weeks of outgrowth, showing that the emergence of a sensitive cell population from an initially resistant population is true of inducers of both intrinsic and extrinsic apoptosis (Figure S1(b)). We have previously shown that with respect to the timing of TRAIL-mediated cell death, cell-to-cell variability is dominated by non-genetic factors including the unequal distribution of proteins between sister cells (Spencer et al, 2009). In the current study we show that cell populations exist in a dynamic state of “equilibrium” between and sensitive and resistant states that are unlikely to differ in genotype. Moreover, the shape of the death time distribution varies from one cell type to the next but appears to be a stable property of that line, since it can be regenerated from individual surviving cells.
Figure 2. Survivor cells re-acquire sensitivity to TRAIL and regenerate the time of death distribution of the original cell population.
(a) HeLa cell survivors of a 16 hour TRAIL treatment (50ng/ml) (red bar and oval, left panel) were grown for six days in the absence of TRAIL and then re-treated with the same dose of TRAIL (right panel). The cells were imaged by live-cell microscopy and time of death was scored based on morphological changes associated with cell death. Death time distributions (blue bars) and the percentage of surviving cells at the 16h timepoint (red bars, right side of dotted line) are plotted for both the primary treatment and the treatment of survivor cells. (b) Quantitation of the death time distributions of primary treatment control (naïve) and survivor cells from (a) monitored on the same day. Cells surviving at the 16h timepoint are excluded from the analysis. Neither the mean (red crosses), median (lines through boxes), nor variances of the death times were significantly different (p>0.05 for unpaired Welch’s t-test for distribution means, Mann-Whitney test for medians of non-Gaussian distributions, F test for variances). At least 200 cells were analyzed for each sample. (c) Time of death distributions and percentage of surviving cells plotted for control (naïve) and survivor MCF10A cells (six days after the initial treatment), treated with 50ng/ml TRAIL for 16h and imaged by live-cell microscopy.
Co-drugging decreases variability and increases sensitivity
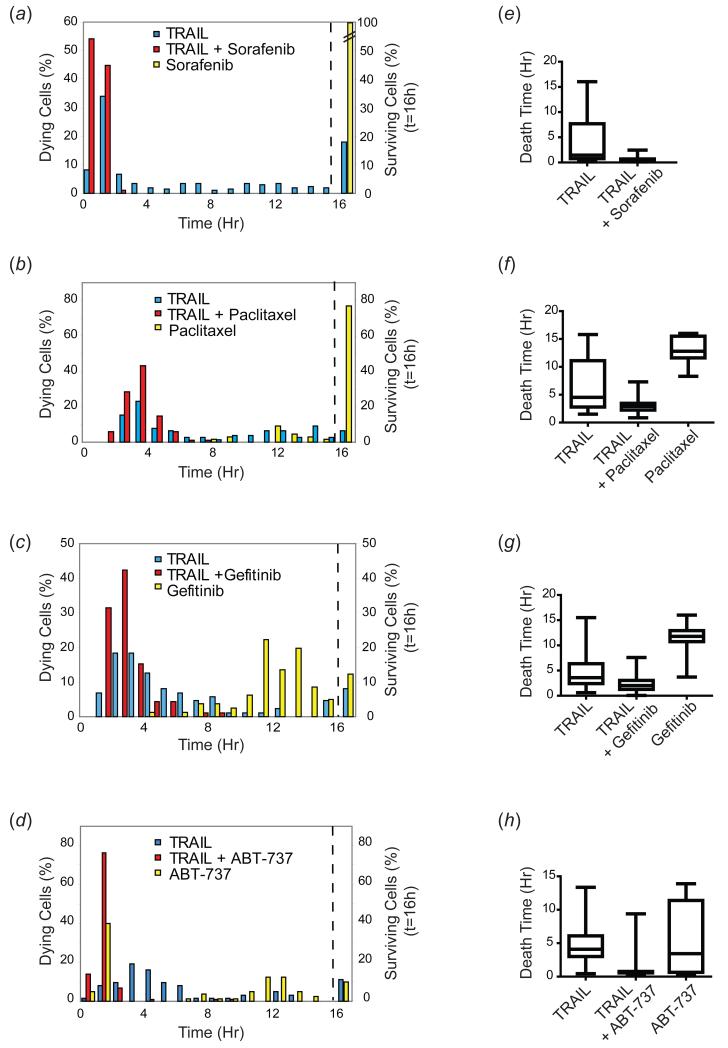
One approach to sensitizing resistant tumors to TRAIL is to co-treat with a second drug, and many general and targeted chemotherapeutics have been shown to work at least in some cell lines (Ashkenazi & Herbst, 2008; Hellwig & Rehm, 2012). To examine the effects of co-drugging on cell-to-cell variability in response to TRAIL, we treated HeLa cells expressing a reporter for MOMP (IMS-RP) (Albeck et al, 2008a) with TRAIL alone or with TRAIL in combination with sorafenib, paclitaxel, gefitinib or ABT-737, chemotherapeutic drugs previously shown to sensitize cells to TRAIL-mediated apoptosis (Johnstone et al, 2008). All four drugs sensitized HeLa cells to TRAIL-induced death, increasing the fraction killed at 16h from ~80% to 100% at 50ng/ml TRAIL (as assayed by whether a cell had undergone MOMP by the 16h timepoint) (Figure 3(a-d)). In contrast, HeLa cells were relatively resistant to sorafenib and paclitaxel alone, and a significant fraction also survived with gefitinib or ABT-737 alone. In all cases, the timing of cell death was altered by co-drugging, with a significant decrease in the mean and median death times (p<0.001 for all combinations tested vs. TRAIL alone). Variability in death times was also reduced in co-drugged cells compared to cells treated with a single agent. To quantify this effect we estimated the interquartile range (IQR) of death times for the fraction of cells that underwent apoptosis (this is a more appropriate measure of variability than coefficient of variation because the death time histograms were not normally distributed). Cells treated with 50ng/ml TRAIL alone had an average IQR of 5.3 +/− 0.9h across all experiments, falling to 0.2 to 1.7h in co-drugged cells (Figure 3(e-h), and Table I). The effect was particularly striking for sorafenib which had little impact on its own but dramatically reduced variability in time of death when combined with TRAIL. We observed similar effects in other cell types such as MCF10A cells co-treated with TRAIL and gefitinib or sorafenib (Figure S2) and in U251 glioblastoma cells co-treated with TRAIL and the HDAC inhibitor MS-275 (Bagci-Onder et al, 2012). Thus, co-drugging increases sensitivity to TRAIL at least in part through a reduction in death time variability among individual cells.
Figure 3. Co-drugging sensitizes cells to TRAIL-mediated apoptosis and reduces variability in timing of cell death.
(a-d) Time of death distributions and percentage of surviving HeLa cells treated with TRAIL alone (50ng/ml in (a,c,d); 10ng/ml in (b)), with TRAIL (50ng/ml) + sorafenib (10μM; (a)); TRAIL (10ng/ml) + paclitaxel (300nM; (b)); TRAIL (50ng/ml) + gefitinib (10μM; (c)); TRAIL (50ng/ml) + ABT-737 (10μM; (d)), or with each drug alone, monitored for 16h by live-cell microscopy. For paclitaxel, cells were pre-treated with the drug for 24h to arrest them in mitosis prior to TRAIL addition at the start of the movie (in the continued presence of paclitaxel). Cells surviving at the end of the movie are plotted to the right of the dotted line. (e-h) Quantitation of death times for dying cells only. Box plots represent the spread of the data; the box spans the IQR, with the median indicated as a line through the box; bars span the range of the data, from the minimum to the maximum death time. 50-100 cells were analyzed for each condition.
Table I. Death time statistics for cells treated with TRAIL in combination with a second agent.
| TRAIL | TRAIL+Sorafenib | Sorafenib | |
|---|---|---|---|
| Median | 1.5h | 0.5h | -- |
| IQR | 7h | 0.4h | -- |
| Range | 16h | 2h | -- |
| CV | 111% | 63% | -- |
| TRAIL | TRAIL+Paclitaxel | Paclitaxel | |
| Median | 4.5h | 2.9h | 12.8h |
| IQR | 8.3h | 1.3h | 3.9h |
| Range | 14.3h | 6.4h | 7.7h |
| CV | 69% | 36% | 19% |
| TRAIL | TRAIL+Gefitinib | Gefitinib | |
| Median | 3.6h | 2h | 11.8h |
| IQR | 4h | 1.7h | 2.2h |
| Range | 14.9h | 7.6h | 12.3h |
| CV | 74% | 58% | 20% |
| TRAIL | TRAIL+ABT-737 | ABT-737 | |
| Median | 4.1h | 0.7h | 3.4h |
| IQR | 3.1h | 0.2h | 10.8h |
| Range | 12.9h | 9.1h | 13.7h |
| CV | 70% | 140% | 92% |
| TRAIL | TRAIL+CHX | TRAIL+CHX+MG-132 | |
| Median | 8.5h | 3.5h | 4.3h |
| IQR | 4.1h | 1.3h | 2.1h |
| Range | 13.5h | 5.2h | 6.3h |
| CV | 40% | 26% | 30% |
| TRAIL | TRAIL+CHX | TRAIL+CHXpre-treated | |
| Median | 8.5h | 3.5h | 0.9h |
| IQR | 4.1h | 1.3h | 0.2h |
| Range | 13.5h | 5.2h | 1.5h |
| CV | 40% | 26% | 25% |
Blocking protein synthesis decreases mean death time and variability, while stabilizing protein levels reverses this effect
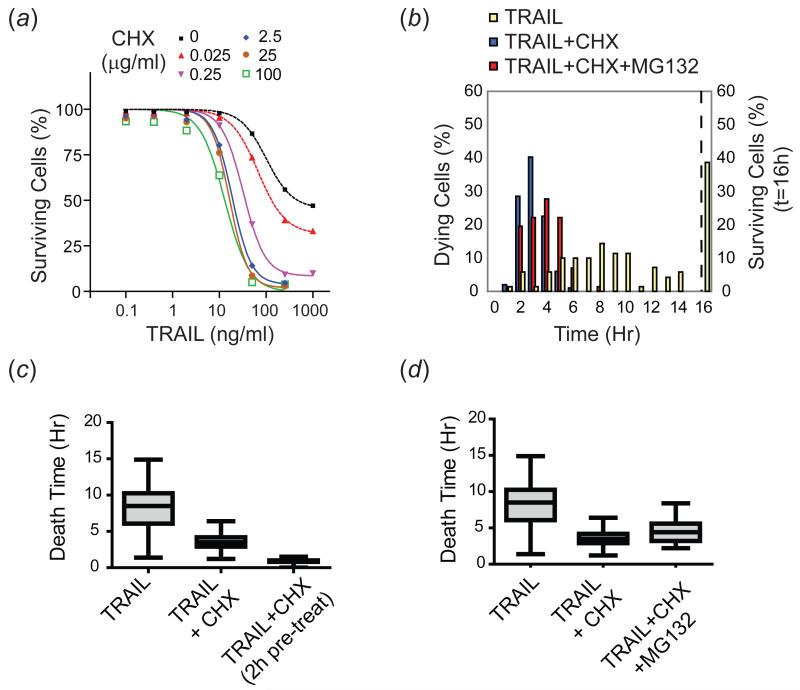
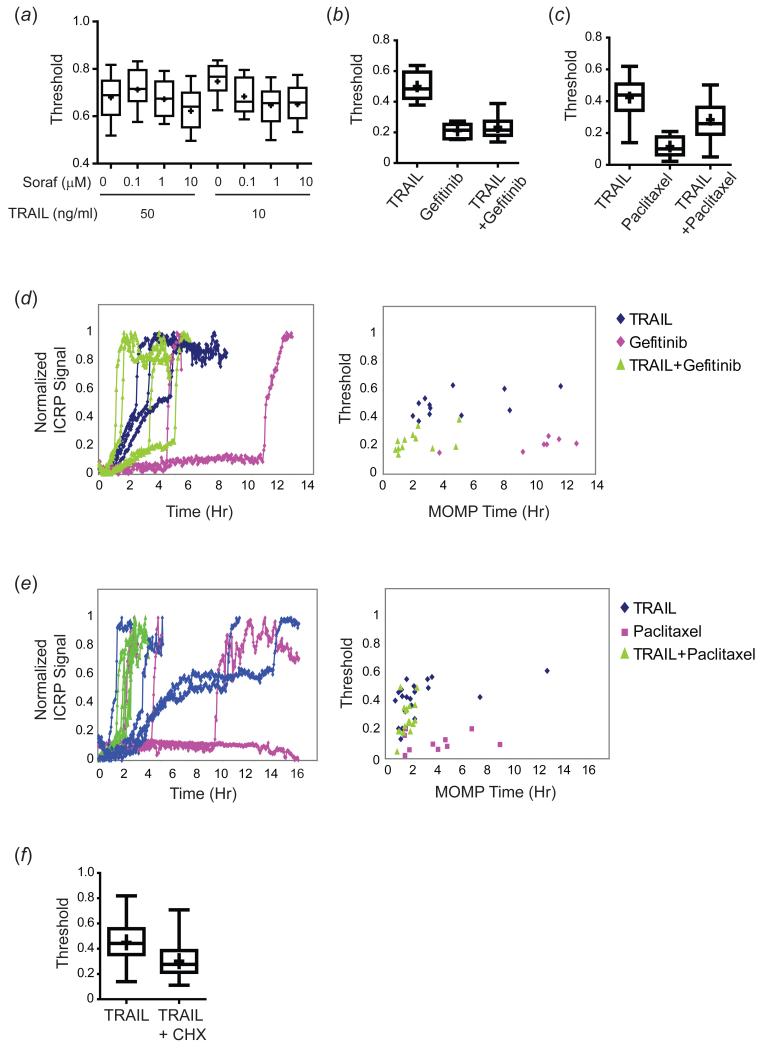
Sub-lethal concentrations of the protein synthesis inhibitor cycloheximide (CHX) are well known to sensitize many types of cells to TRAIL-induced apoptosis (Albeck et al, 2008a; Kang et al, 2003). This effect is dramatic in HeLa cells which exhibit both dose-responsive cell killing by TRAIL and dose-responsive sensitization when cells are treated with TRAIL + CHX (Figure 4(a)). Several explanations have been suggested for this sensitizing effect, including a reduction in the production of pro-survival factors, particularly those that are short-lived or induced by TRAIL treatment, or activation of a JNK-mediated stress response (Clohessy et al, 2006; Kang et al, 2003; Kreuz et al, 2001; Sah et al, 2003; Wajant et al, 2000). To study the effects of CHX on variability in death time, HeLa cells were exposed to TRAIL (50ng/ml) with or without the addition of CHX at the lowest saturating concentration for TRAIL sensitization (2.5μg/ml). Under these circumstances, mean death time fell significantly (p<0.0001; (Albeck et al, 2008a)) and IQR decreased from ~4.1h for TRAIL alone to 1.3h for TRAIL + CHX (these curves passed a normality test, allowing us to estimate a CV of 40% for TRAIL vs. 26% for TRAIL + CHX; Figure 4(b-c); Table I). The effect of CHX was enhanced when cells were pre-treated with CHX for 2h prior to addition of TRAIL (IQR for TRAIL + pre-treatment with CHX was ~0.2h; Figure 4(c) and Table I). Since CHX acts rapidly to block potential TRAIL-induced protein synthesis, this implies that CHX is altering the basal state of the apoptotic machinery.
Figure 4. Cycloheximide reduces mean death time and variability in death times, and proteasome inhibition partially reverses this process.
(a) Dose-response curves of HeLa cells treated with increasing doses of TRAIL +/− increasing doses of CHX for 4h, monitored for cell death by flow cytometry using an antibody that recognizes cleaved PARP. (b) Time of death distributions and percentage of surviving cells for HeLa cells treated with TRAIL (50ng/ml; yellow), TRAIL + CHX (2.5μg/ml; blue), or TRAIL + CHX + MG-132 (10μM; red) for 16h and imaged by live-cell microscopy. Percent cells surviving at the end of the movie is plotted to the right of the dotted line. Shown is a representative of three independent experiments that yielded similar results. (c) Quantitation of the spread of the death times for cells pre-treated with CHX for 2h prior to treatment with TRAIL + CHX, compared to co-treatment with TRAIL + CHX or treatment with TRAIL alone. (d) Quantitation of the spread of the death times in (b). Median, IQR, and range of death times for each treatment appear in the box and whisker plots as described for Figure 3. 50-100 cells were analyzed per condition.
We reasoned that if degradation of short-lived anti-apoptotic proteins were important for the observed sensitization by CHX, then stabilization of these proteins using a proteasome inhibitor might counteract this effect. We found that this was the case: when HeLa cells were treated with TRAIL in combination with both 2.5μg/ml CHX and 10μM of the proteasome inhibitor MG-132, median death time increased (p=0.0002) and variability was also increased (IQR=1.3h for TRAIL + CHX vs. 2.1h for TRAIL + CHX + MG-132; Figure 4(b), (d) and Table I). Notably, the shift was only a fraction of that needed to recover the time of death profile observed for treatment with TRAIL alone; this is consistent with the fact that MG-132 by itself sensitizes cells to TRAIL-induced apoptosis, due to the multiple targets of this inhibitor (Laussmann et al, 2011; Sayers & Murphy, 2006). A similar proteasome inhibitor-mediated delay in cell death has been observed by others (Laussmann et al, 2012) and attributed to stabilization of the anti-apoptotic DISC protein FLIP. Thus, degradation of short-lived anti-apoptotic proteins is likely to have a significant effect on the timing and probability of TRAIL-mediated cell death. Moreover, variability in death times can be modulated by affecting the balance of overall protein synthesis and degradation. Since CHX and MG-132 alter the synthesis and degradation of many apoptotic regulators, including Mcl-1, FLIP, and XIAP (Clohessy et al, 2006; Kreuz et al, 2001; Lane et al, 2006), further work is required to determine precisely which proteins play the most significant roles in different cell types.
Sensitizing agents decrease the threshold for cell death
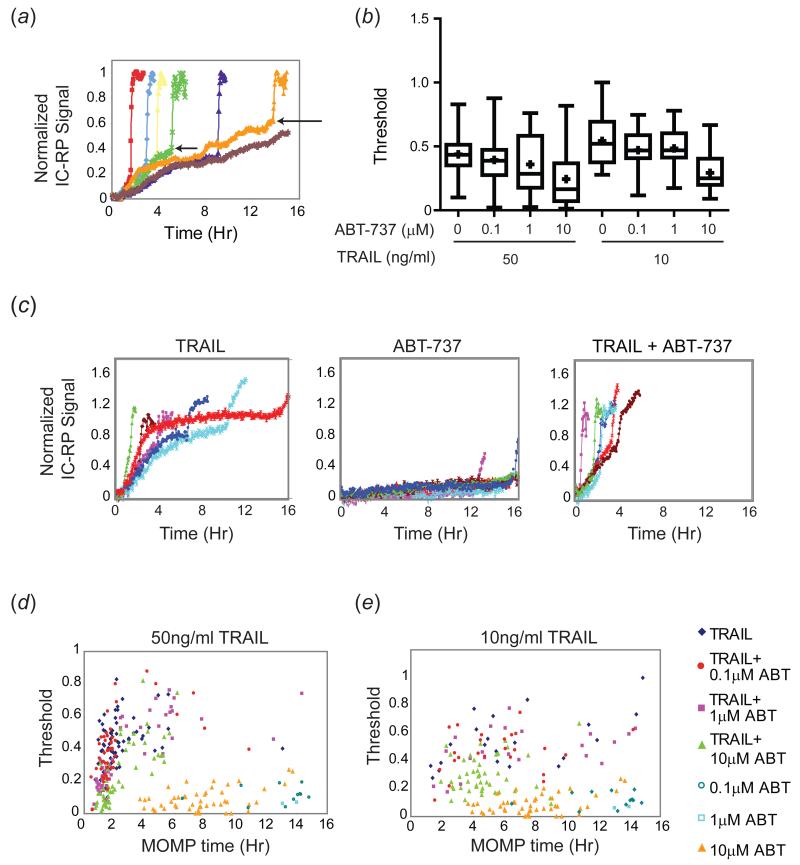
Previous work in our lab demonstrated that variability in factors upstream of MOMP has the greatest contribution to variability in timing of cell death; these factors can be divided into those that alter the rate of caspase-8 cleavage of its substrates (particularly Bid) and those that alter the threshold of cleaved Bid (tBid) necessary to trigger MOMP. Across a population of cells, the trajectory of pre-MOMP initiator caspase activity (as measured using a substrate caspase-8/10 FRET reporter (IC-RP)) varies in steepness and maximum level. Experimentally, the maximum level is defined as the normalized IC-RP signal at the time of MOMP for a particular TRAIL-treated cell (this is not necessarily equal to the amount of tBid because cleaved reporter and Bid have different half-lives; Figure 5(a), arrows) and it represents a threshold in the integrated activity of caspase-8/10 needed to trigger MOMP. Single-cell variation in the rate of approach to this threshold and in its height appears to generate the variable delay period between the time that TRAIL binds to DR4/5 receptors and the onset of cell death (Albeck et al, 2008a; Spencer et al, 2009).
Figure 5. Bcl-2 inhibition lowers the threshold for cell death.
(a) Schematic illustrating sample IC-RP trajectories for individual HeLa cells (each indicated by a different color) treated with TRAIL (50ng/ml). Arrows indicate levels of IC-RP activity (threshold, y-axis) at the time of MOMP (x-axis) for two individual cells. (b) Box plots indicating the MOMP threshold (height of the IC-RP FRET trajectory at the time of MOMP) for a population of 50-100 HeLa cells treated with the indicated doses of TRAIL and ABT-737. Initiator caspase FRET trajectories for individual cells were normalized to the minimum and maximum intensity for each trajectory, and the threshold was calculated as the normalized intensity at the time of MOMP. Box plots span the entire range of the data. (c) IC-RP trajectories of representative individual HeLa cells treated with TRAIL (50ng/ml), ABT-737 (1μM), or TRAIL + ABT-737 for 16h and imaged for 16h by live-cell microscopy. (d-e) Dot plots showing the MOMP threshold plotted relative to time of MOMP for individual cells treated with the indicated doses of TRAIL and ABT-737.
In principle, the sensitization of cells to TRAIL-mediated death and the reduction in variability in death times caused by co-drugging could be mediated by either an increase in the rate of initiator caspase substrate cleavage, or by a decrease in the MOMP threshold (or by a combination of the two) (Figure 5(a)). Chemotherapeutic drugs have been shown to affect the balance of mitochondrial pro- and anti-apoptotic proteins (Callus et al, 2008; Davids et al, 2012; Letai, 2008) and thus might be expected to alter the threshold in particular. As a positive control for this hypothesis, we first measured the MOMP threshold in cells treated with TRAIL and ABT-737, a Bcl-2 /Bcl-XL inhibitor (thought to work by blocking the binding of Bcl-2/Bcl-XL to pro-apoptotic Bcl-2 proteins) as compared to treatment with TRAIL alone. When HeLa cells expressing IC-RP and the MOMP reporter were treated with TRAIL plus ABT-737 and analyzed by live-cell microscopy, we observed a decrease in MOMP threshold that was dose-dependent for ABT-737 (Figure 5(b-c)). Treatment with ABT-737 alone did not lead to a significant increase in pre-MOMP initiator caspase activity over time, even in cells that died (as would be expected for a drug that does not activate extrinsic cell death); this results in the relatively flat pre-MOMP IC-RP trajectories in ABT-737-treated cells (Fig 5(c), middle panel). The threshold tended to increase with later MOMP times both with and without ABT-737 in TRAIL-treated cells, and was higher, on average, at 10ng/ml TRAIL than at 50ng/ml TRAIL, but an overall decrease in the threshold was observed with ABT-737 even at matched MOMP times for either dose of TRAIL alone (Figure 5(d-e)). Thresholds for ABT-737 treatment alone are plotted to indicate the baseline activity of the reporter (Figure 5(d-e)).
Next, we monitored initiator caspase activity and MOMP in HeLa cells treated with TRAIL plus a variety of other anti-cancer drugs. Sorafenib in combination with TRAIL was particularly interesting since it did not kill HeLa cells on its own even at the highest dose tested (10μM for 16h). Co-treatment with sorafenib led to a dose-responsive decrease in the MOMP threshold compared to TRAIL alone (Figure 6(a)). Combinations of TRAIL (50ng/ml) plus gefitinib (10μM) and TRAIL (50ng/ml) plus paclitaxel (300nM, with a 24h paclitaxel pre-treatment) also reduced the threshold for MOMP compared with TRAIL treatment alone (Figure 6(b-c)); thresholds for gefitinib and paclitaxel alone are plotted to indicate baseline activity of the reporter (which exhibited little or no pre-MOMP IC-RP activity in response to the drugs alone; Figure 6(d-e)). Thus, these agents reduced the threshold for TRAIL-induced MOMP, presumably by affecting the levels or states of pro- and anti-apoptotic mitochondrial proteins. Treatment of cells with TRAIL + CHX also reduced the threshold compared with TRAIL treatment alone, demonstrating that inhibition of protein synthesis-- and a consequent reduction in the levels of short-lived anti-apoptotic mitochondrial proteins-- can contribute to a reduction in the MOMP threshold (CHX alone did not kill any cells and is excluded from the plot; Figure 6(f)). Thus, co-drugging with the agents tested led to more rapid and less variable death times than death by TRAIL alone at least in part by lowering the threshold for MOMP.
Figure 6. Co-drugging lowers the threshold for cell death.
(a) Box plots indicating the MOMP threshold for a population of 50-100 HeLa cells treated with the indicated doses of TRAIL and sorafenib and analyzed as in Figure 5. Boxes span the 10th-90th percentile of the data (excluding outliers in the top and bottom 10%); means are significantly different (p<0.05). (b) Box plots indicating the MOMP threshold for HeLa cells treated with TRAIL (50ng/ml), gefitinib (10μM), or TRAIL + gefitinib for 16h. Box plots span the entire range of the data. (c) Box plots indicating the MOMP threshold for HeLa cells treated with TRAIL (50ng/ml) alone, or pre-treated with paclitaxel (300nM) for 24h and then treated with TRAIL + paclitaxel or paclitaxel alone for an additional 16h. Box plots span the entire range of the data. (d) Representative FRET trajectories (colored lines, left panel) and MOMP thresholds (colored dots, right panel) for individual HeLa cells treated with TRAIL (50ng/ml; blue lines and dots), gefitinib (10μM; pink lines and dots); or TRAIL + gefitinib (green lines and dots) for 16h. (e) Representative FRET trajectories (colored lines, left panel) and MOMP thresholds (colored dots, right panel) for individual HeLa cells treated with TRAIL (50ng/ml; blue lines and dots), paclitaxel (300nM; pink lines and dots); or TRAIL + paclitaxel (green lines and dots) for 16h. Paclitaxel-treated cells were pre-treated with paclitaxel for 24h. (f) Box plots indicating the MOMP threshold for HeLa cells treated with TRAIL (50ng/ml) with or without CHX (2.5μg/ml) for 16h; CHX alone did not kill any cells and is excluded from the plot. Box plots span the entire range of the data.
DISCUSSION
An extensive literature exists on the sensitivity of cells to death ligands such as TRAIL and FasL individually and in combination with various anti-cancer drugs. The vast majority of these papers involve endpoint, population-average measures of apoptosis in combination with detailed biochemical or genetic analysis of a few apoptotic regulators. In this paper we ask whether dynamic, single-cell assays such as those used to develop mathematical models of receptor-mediated apoptosis in HeLa cells (Albeck et al, 2008a; Albeck et al, 2008b; Fricker et al, 2010; Hellwig et al, 2008; Neumann et al, 2010; Rehm et al, 2003; Spencer & Sorger, 2011) might usefully be applied to understanding TRAIL-sensitivity and resistance in genetically diverse tumor lines with and without co-drugging. Our focus on assaying key reactions that control extrinsic apoptosis in individual cells provides a window into the physiology of cell death and sets the stage for physicochemical modeling of combination therapy. By examining diverse cell lines that would traditionally be considered to cover the range from TRAIL-sensitive to relatively resistant we observe a diversity of death time distributions. In all lines examined we find that cells can switch between states of sensitivity and resistance over a time period of several days and that fractional killing is a stable property of specific cell types. Cells from a sensitive line that survive an initial TRAIL challenge give rise, within several days of outgrowth, to a population of cells with the same degree of sensitivity and distribution of death times; the same is true of relatively resistant lines. Variability in the time at which single cells in a population die is likely to be determined in a non-genetic fashion (Spencer et al, 2009), whereas the shape of the death time distribution appears to be a stable property of a particular cell line undergoing treatment with a particular dose of TRAIL. Anti-cancer drugs that increase sensitivity to TRAIL modulate the timing of cell death, at least in part by lowering the threshold for MOMP.
We and others have previously demonstrated that individual HeLa cells in a clonal population exposed to death ligands such as TRAIL and FasL differ markedly in the time at which they die and that even at high ligand doses, a fraction of cells survives (Albeck et al, 2008a; Neumann et al, 2010; Spencer et al, 2009). Fractional killing has also been observed following treatment of cells with agents that activate intrinsic cell death pathways (Cohen et al, 2008; Gascoigne & Taylor, 2008) and we find that it is associated with variability in time of death that is qualitatively similar to that observed with TRAIL (Figure 3 and Figure S1(b)). However, the connection between variability in time of death and fractional kill has not previously been examined in detail for either extrinsic or intrinsic apoptosis. In population-based studies it is conventional to measure the sensitivity of cells to a pro-death ligand based on fixed-time, end-point measurement of the ligand concentration necessary to kill 50% of the cells in the initial population (the IC50). When only a subset of cells dies at the highest ligand doses, it is more informative to report the maximum fractional killing (Emax) and the ligand concentration at which half of this level of killing is observed (EC50; (Barretina et al, 2012)). Particularly in the case of fractionally sensitive cells, it is thus important to distinguish between IC50 and EC50 at multiple timepoints when evaluating sensitivity to apoptosis.
We observe that even among sensitive cell lines the shape of the death time distribution (sharp and narrow vs. wide and evenly spread) is remarkably variable; the same is true for resistant lines. For example, both T47-D and HCT116 cells exhibited narrowly distributed and early death time distributions but T47-D cells were significantly more resistant than HCT116 cells (with a total of 35% of T47-D cells vs. 85% of HCT116 dying at a 50ng/ml TRAIL treatment over 16h). In contrast, TRAIL-resistant Hs578T and HT29 cells exhibited broad death time distributions in which a few cells died sporadically throughout a 16h time-lapse experiment. The role of dose in shaping the death time distribution was dependent on cell type: dose had a large effect on the shape of the death time distribution in MCF10A cells but had a much smaller effect on the shape of the death time distribution in T47-D cells.
The clear separation in time between the T47-D, HCT116 and MCF10A cells that died soon after exposure to TRAIL (within ~4-6h at 50ng/ml TRAIL) and those that were resistant to prolonged ligand exposure (>12-16h) is suggestive of two distinct subpopulations. However, in multiple cell lines tested, and in multiple clones derived from the same parental culture, cells that survived treatment with TRAIL were not permanently resistant. Within several days of outgrowth surviving cells had regenerated the same fractional sensitivity and death time distribution as the starting population. Conventional acquired resistance to pro-apoptotic ligands can be observed following long-term or repeated exposure (Lane et al, 2006; Li et al, 2011) and this resistance is stable and genetically determined. In contrast, the resistance observed in our experiments is transient and is likely to involve non-genetic factors such as epigenetic switching, differences in cell cycle state, stochastic fluctuations in the levels of pro- and anti-apoptotic proteins and differential induction of survival signaling (Brock et al, 2009; Cohen et al, 2008; Neumann et al, 2010; Sharma et al, 2010; Spencer et al, 2009). In principle, these processes could interact on different timescales to influence the initial efficacy of a pro-apoptotic drug and the outgrowth of resistant populations. Our results raise the important question of how death time distributions and Emax are independently and stably encoded in different cell types.
We observed that treating cells with both TRAIL and a second pro-apoptotic drug reduces the mean and variability in death times and increases the maximum fraction of cells killed. To begin to dissect these effects we treated cells with the protein synthesis inhibitor CHX and the proteasome inhibitor MG-132, whose effects on protein levels are understood in broad terms. Treating cells with sub-lethal concentrations of CHX is known to reduce the levels of potent but short-lived anti-apoptotic proteins such as Mcl-1, c-FLIP and XIAP and thus to sensitize cells to death ligands (Clohessy et al, 2006; Kreuz et al, 2001; Lane et al, 2006). Under some conditions, proteasome inhibitors can also sensitize cells to TRAIL-induced apoptosis via inhibition of NF-κB-mediated pro-survival signaling or caspase-3 degradation (Albeck et al, 2008a; Lane et al, 2006), stabilization of TRAIL receptors or tBid (Breitschopf et al, 2000; Johnson et al, 2003; Kabore et al, 2006), or alteration of the DISC (Laussmann et al, 2011; Sayers et al, 2003). We observed that co-treating cells with TRAIL and sub-lethal doses of CHX reduced variability in death times and that further addition of MG-132 reversed this, at least partially (under these conditions, virtually all cells were ultimately sensitive to 16h of TRAIL exposure). Antagonism between CHX and proteasome inhibition has also been observed using the clinical-grade proteasome inhibitor bortezomib (Laussmann et al, 2012). These data strongly suggest that short-lived anti-apoptotic proteins play a significant role in the variability of death times. Cell-to-cell variability in the levels of proteins is often ascribed to stochastic fluctuation rates of transcription and protein synthesis; inhibition of these processes is thus expected to decrease variability while blocking protein degradation would in principle reverse this effect and maintain variability as we observe.
In TRAIL-treated cells the timing of cell death appears to be determined by the rate of accumulation of tBid (a pro-apoptotic Bcl-2 family member generated by caspase 8/10-mediated cleavage of Bid) and the threshold at which tBid can trigger MOMP. In the case of TRAIL, this threshold is set primarily by the levels of anti-apoptotic Bcl-2 family proteins (or by the ratio of these proteins with pro-apoptotic Bcl-2 family members). It is logical to assume that when the threshold is lower, less time is needed for cleaved and active tBid molecules to accumulate to a level that exceeds the threshold and triggers cell death. To demonstrate that this is indeed the case we treated cells with TRAIL and ABT-737, a small molecule that inhibits Bcl-2 and Bcl-XL (Huang & Sinicrope, 2008; Oltersdorf et al, 2005). As expected, we observed a dose-dependent reduction in the MOMP threshold, causing cells to die earlier. A variety of other anti-cancer drugs have been shown to synergize with TRAIL in cell killing and the proposed mechanisms are diverse: p53-dependent or -independent transcriptional upregulation of TRAIL receptors or caspase-8 (Di Pietro et al, 2001; Ehrhardt et al, 2008; Sheikh et al, 1998; Wu et al, 1997), downregulation of anti-apoptotic pathway components such as c-FLIP and Mcl-1 (Galligan et al, 2005; Kim et al, 2008; Morizot et al, 2010; Stagni et al, 2010) or transcriptional upregulation of pro-apoptotic BH3-only proteins such as Bim or Puma (Letai, 2008). It is not yet possible to measure the MOMP threshold for inducers of intrinsic apoptosis such as gefitinib, sorafenib, or paclitaxel in single cells (although BH3-profiling makes it possible in cell populations (Deng et al, 2007)), in large part because we do not know which pro-apoptotic regulators play the role that tBid does in extrinsic apoptosis. However, by measuring the caspase-8/10 threshold needed to trigger MOMP in cells treated with both TRAIL and an intrinsic apoptosis inducer it is possible to infer the latter as the reduction in threshold relative to TRAIL alone. By analogy with mitochondrial priming, this reduction in threshold could involve either the inhibition of anti-apoptotic proteins such as Mcl-1 or the production of pro-apoptotic proteins such as Bim (Certo et al, 2006). We observed that when TRAIL was combined with sorafenib, gefitinib or paclitaxel the threshold for MOMP was significantly lower than for TRAIL alone. In the large body of literature on sorafenib, gefitinib, and paclitaxel a common element is their effect on Mcl-1, a protein expected to regulate the MOMP threshold directly (Chen et al, 2010; Henson et al, 2003; Meng et al, 2007; Ricci et al, 2007; Rosato et al, 2007; Sanchez-Perez et al, 2009; Wertz et al, 2011).
The second factor expected to change the timing of TRAIL-induced cell death is the rate of IC-RP cleavage. When we used a phenomenological rate equation to fit for caspase-8/10 activity in pre-MOMP cells (Spencer et al, 2009), we observed that earlier death times were associated with faster rates of IC-RP cleavage in both TRAIL-treated and co-drugged HeLa cells expressing the IC-RP reporter. Previous studies showed that in cells treated with TRAIL plus CHX, the rate of IC-RP cleavage for an individual cell was more predictive of time of death than the threshold (Spencer et al, 2009). In our current analysis, we observed that the shapes of the IC-RP trajectories were altered in co-drugged cells compared to cells treated with TRAIL alone (complicating efforts to apply a uniform function to compare rates among the different treatments), but we did not find consistent evidence demonstrating that increases in rate were a major determinant for the sensitizing effects we observed with co-drugging. Nevertheless, given that gefitinib, sorafenib, and paclitaxel are implicated in affecting not only the levels of mitochondrial proteins but also the levels of DISC proteins and DR4/5 receptors (Nimmanapalli et al, 2001; Rosato et al, 2007; Singh et al, 2003), this question warrants further analysis.
In conclusion, we have shown that variability in the time and probability of TRAIL-mediated cell death varies from one cell type to the next and is a stable property of a particular cell type. Co-drugging cells with TRAIL and a second anti-cancer drug has a significant impact on cell death time, at least partly by lowering the threshold for MOMP. Non-genetic variation among cells may impact the extent of synergism observed when drugs are combined (Fitzgerald et al, 2006), and the degree of synergy may in turn influence cell-to-cell variability in death times. Future studies in cell types exhibiting a range of fractional sensitivities could in principle shed light on these effects and aid in the design of more potent combination therapies.
MATERIALS & METHODS
Cell culture and generation of stable cell lines
HeLa cells expressing IC-RP and the mitochondrial reporter IMS-RP were generated as described (Albeck et al, 2008a) and cultured in DMEM containing 10% FBS, 5mM L-glutamine, and 1% penicillin/streptomycin (Gibco). MCF10A and HCT116 cells expressing IMS-RP were generated using a retrovirus produced by co-transfection of 293T cells (ATCC) with pCL-ampho and pBabe-IMS-RP and were cultured as described (Aldridge et al, 2011; Debnath et al, 2003). HT29 (ATCC) were cultured in McCoy’s 5a Medium; T47-D were grown in RPMI, and Hs578T cells were grown in DMEM, supplemented with 10% FBS, 2mM L-glutamine, and 1% penicillin/streptomycin (Neve et al, 2006). HUVEC (primary human endothelial cells) were maintained in M200 medium containing Low-Serum Growth Supplement (Cascade Biologics). Single-cell clones were generated by serial dilution followed by expansion and testing for TRAIL-sensitivity that was either similar to or enhanced compared to that of the parental cell line.
Cell treatments
SuperKiller TRAIL was purchased from Alexis Biochemicals; other reagents include ABT-737 (Selleck Chemicals), gefitinib and sorafenib (LC Laboratories), paclitaxel (Sigma Aldrich), cycloheximide (Sigma Aldrich), MG-132 (Calbiochem), and staurosporine (EMD Biosciences).
Measurement of cell death by flow cytometry
Following treatment, floating dead cells were combined with trypsinized adherent cells, fixed with 4% paraformaldehyde, permeablized in methanol and labeled with Alexa Fluor 488-conjugated anti-cleaved PARP (cPARP) antibodies (Becton Dickinson) as described (Albeck et al, 2008a). Flow cytometry was performed on a FACsCalibur (BD Biosciences), and data were analyzed using FlowJo software (TreeStar). Fitting of IC50 curves for the percentage of cPARP-negative (live) cells was performed using Graphpad Prism.
Live-cell microscopy
Cells were seeded in glass-bottom chamber slides (Nunc) and imaged at 3-5 minute intervals for 16-18h as described (Albeck et al, 2008a). For HUVEC, Hs578T, and T47-D cells, chamber slides were pre-coated with 50μg/ml fibronectin (Invitrogen) to facilitate cell adhesion. Cells were imaged in a 37°C humidified chamber either in CO2-independent media (Invitrogen) supplemented with 1% FBS, 2mM L-glutamine, and 1% penicillin-streptomycin, or in ~5% CO2 in phenol-free medium containing full growth supplements. Unless otherwise indicated, treatments were performed 15-30 minutes prior to the start of the movie. Time of death was monitored as the time of MOMP in cells expressing the IMS-RP reporter (Albeck et al, 2008a) or by morphological changes associated with apoptosis. Cells were imaged either on a Deltavision microscope (Applied Precision) at 10-20× magnification or on a Nikon TE2000E (Nikon Imaging Center, Harvard Medical School).
Survivor re-challenge experiment
Cells seeded in glass-bottom chamber slides (Nunc) were treated with 50ng/ml TRAIL and imaged at 3 minute intervals for 16 hours; survivors were collected by trypsinization, replated and recovered for 6 days in the absence of TRAIL. Survivor cells were then replated into chamber slides, re-treated with 50ng/ml TRAIL, and re-imaged for 16h under identical conditions, alongside control cells that had not been previously exposed to TRAIL. Alternatively, cells were treated for 7-24h with 50ng/ml TRAIL in 10cm dishes; surviving cells were collected by trypsinization, replated into 10cm dishes, and allowed to recover for 6-7 days in the absence of TRAIL. Survivor cells were then seeded alongside control (naïve) cells into glass-bottom chamber slides, treated with 50ng/ml TRAIL, and imaged for 16h by live cell microscopy. For the staurosporine survivor re-challenge experiment, HeLa cells growing in 6-well dishes were treated for 16h with 2μM staurosporine; surviving cells were collected by trypsinization, replated, and allowed to recover for two weeks in the absence of staurosporine prior to re-challenge with 2μM staurosporine and live-cell imaging.
Image analysis and threshold calculations
Death times for 50-200 cells were monitored by visual inspection of IMS-RP translocation (MOMP) or by morphological changes in cells not expressing the IMS-RP reporter. The percentages of cells dying within 1h intervals, as well as the fraction surviving at the end of the movie, were calculated and plotted. Analysis of IC-RP cleavage and IMS-RP translocation for initiator caspase FRET trajectories was performed as described (Albeck et al, 2008a). Briefly, Image J was used to compute the ratio of the CFP:YFP fluorescence in single cells over time. Trajectories of this ratio were plotted for individual cells following subtraction of an average trajectory for an untreated photobleaching control. For threshold calculations, initiator caspase FRET trajectories for individual cells were normalized to the minimum and maximum intensity for each trajectory; the threshold was defined as the normalized intensity at the time of MOMP.
Statistics
Statistics, curve-fitting (for dose-response curves), and IC50 calculations were performed using GraphPad Prism Statistical Software. Time of death histograms were tested for normality using the D’Agostino Pearson test. Interquartile range (IQR) and coefficient of variation (CV) were reported as measures of variability. Mean and median death times were compared using the Mann-Whitney test for non-Gaussian distributions or using an unpaired Welch’s t-test for the death time distribution values. An F test was used to compare variances determined from Gaussian approximations. Mean threshold values were compared across treatments using an unpaired t-test for the distributions of threshold values, or using ANOVA. Significant differences were reported for p<0.05.
Supplementary Material
Acknowledgements
We thank T. Mitchison and J. Brugge for reagents; S. Spencer, J. Roux, V. Becker, A. Letai, and J. Albeck for technical assistance and helpful discussions. We also thank J. Waters and L. Petrak at the Nikon Imaging Center at Harvard Medical School. This work was supported by NIH grant P01-CA139980 to P.K.S., and by NIH pre-doctoral training grant GM07226.
REFERENCES
- Albeck JG, Burke JM, Aldridge BB, Zhang M, Lauffenburger DA, Sorger PK. Quantitative analysis of pathways controlling extrinsic apoptosis in single cells. Mol Cell. 2008a;30(1):11–25. doi: 10.1016/j.molcel.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albeck JG, Burke JM, Spencer SL, Lauffenburger DA, Sorger PK. Modeling a snap-action, variable-delay switch controlling extrinsic cell death. PLoS Biol. 2008b;6(12):2831–2852. doi: 10.1371/journal.pbio.0060299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge BB, Gaudet S, Lauffenburger DA, Sorger PK. Lyapunov exponents and phase diagrams reveal multi-factorial control over TRAIL-induced apoptosis. Mol Syst Biol. 2011;7:553. doi: 10.1038/msb.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi A. Targeting the extrinsic apoptosis pathway in cancer. Cytokine Growth Factor Rev. 2008;19(3-4):325–331. doi: 10.1016/j.cytogfr.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol. 1999;11(2):255–260. doi: 10.1016/s0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Herbst RS. To kill a tumor cell: the potential of proapoptotic receptor agonists. J Clin Invest. 2008;118(6):1979–1990. doi: 10.1172/JCI34359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagci-Onder T, Agarwal A, Flusberg D, Wanningen S, Sorger P, Shah K. Real-time imaging of the dynamics of death receptors and therapeutics that overcome TRAIL resistance in tumors. Oncogene. 2012 doi: 10.1038/onc.2012.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhart BC, Alappat EC, Peter ME. The CD95 type I/type II model. Seminars in immunology. 2003;15(3):185–193. doi: 10.1016/s1044-5323(03)00031-9. [DOI] [PubMed] [Google Scholar]
- Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehar J, Kryukov GV, Sonkin D, Reddy A, Liu M, Murray L, Berger MF, Monahan JE, Morais P, Meltzer J, Korejwa A, Jane-Valbuena J, Mapa FA, Thibault J, Bric-Furlong E, Raman P, Shipway A, Engels IH, Cheng J, Yu GK, Yu J, Aspesi P, Jr., de Silva M, Jagtap K, Jones MD, Wang L, Hatton C, Palescandolo E, Gupta S, Mahan S, Sougnez C, Onofrio RC, Liefeld T, MacConaill L, Winckler W, Reich M, Li N, Mesirov JP, Gabriel SB, Getz G, Ardlie K, Chan V, Myer VE, Weber BL, Porter J, Warmuth M, Finan P, Harris JL, Meyerson M, Golub TR, Morrissey MP, Sellers WR, Schlegel R, Garraway LA. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483(7391):603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhola PD, Simon SM. Determinism and divergence of apoptosis susceptibility in mammalian cells. J Cell Sci. 2009;122(Pt 23):4296–4302. doi: 10.1242/jcs.055590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockbrader KM, Tan M, Sun Y. A small molecule Smac-mimic compound induces apoptosis and sensitizes TRAIL- and etoposide-induced apoptosis in breast cancer cells. Oncogene. 2005;24(49):7381–7388. doi: 10.1038/sj.onc.1208888. [DOI] [PubMed] [Google Scholar]
- Breitschopf K, Zeiher AM, Dimmeler S. Ubiquitin-mediated degradation of the proapoptotic active form of bid. A functional consequence on apoptosis induction. The Journal of biological chemistry. 2000;275(28):21648–21652. doi: 10.1074/jbc.M001083200. [DOI] [PubMed] [Google Scholar]
- Broaddus VC, Dansen TB, Abayasiriwardana KS, Wilson SM, Finch AJ, Swigart LB, Hunt AE, Evan GI. Bid mediates apoptotic synergy between tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and DNA damage. The Journal of biological chemistry. 2005;280(13):12486–12493. doi: 10.1074/jbc.M408190200. [DOI] [PubMed] [Google Scholar]
- Brock A, Chang H, Huang S. Non-genetic heterogeneity--a mutation-independent driving force for the somatic evolution of tumours. Nat Rev Genet. 2009;10(5):336–342. doi: 10.1038/nrg2556. [DOI] [PubMed] [Google Scholar]
- Brooks AD, Ramirez T, Toh U, Onksen J, Elliott PJ, Murphy WJ, Sayers TJ. The proteasome inhibitor bortezomib (Velcade) sensitizes some human tumor cells to Apo2L/TRAIL-mediated apoptosis. Ann N Y Acad Sci. 2005;1059:160–167. doi: 10.1196/annals.1339.042. [DOI] [PubMed] [Google Scholar]
- Butler LM, Liapis V, Bouralexis S, Welldon K, Hay S, Thai le M, Labrinidis A, Tilley WD, Findlay DM, Evdokiou A. The histone deacetylase inhibitor, suberoylanilide hydroxamic acid, overcomes resistance of human breast cancer cells to Apo2L/TRAIL. Int J Cancer. 2006;119(4):944–954. doi: 10.1002/ijc.21939. [DOI] [PubMed] [Google Scholar]
- Callus BA, Moujallad DM, Silke J, Gerl R, Jabbour AM, Ekert PG, Vaux DL. Triggering of apoptosis by Puma is determined by the threshold set by prosurvival Bcl-2 family proteins. J Mol Biol. 2008;384(2):313–323. doi: 10.1016/j.jmb.2008.09.041. [DOI] [PubMed] [Google Scholar]
- Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, Letai A. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9(5):351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Chen KF, Tai WT, Liu TH, Huang HP, Lin YC, Shiau CW, Li PK, Chen PJ, Cheng AL. Sorafenib overcomes TRAIL resistance of hepatocellular carcinoma cells through the inhibition of STAT3. Clin Cancer Res. 2010;16(21):5189–5199. doi: 10.1158/1078-0432.CCR-09-3389. [DOI] [PubMed] [Google Scholar]
- Clohessy JG, Zhuang J, de Boer J, Gil-Gomez G, Brady HJ. Mcl-1 interacts with truncated Bid and inhibits its induction of cytochrome c release and its role in receptor-mediated apoptosis. The Journal of biological chemistry. 2006;281(9):5750–5759. doi: 10.1074/jbc.M505688200. [DOI] [PubMed] [Google Scholar]
- Cohen AA, Geva-Zatorsky N, Eden E, Frenkel-Morgenstern M, Issaeva I, Sigal A, Milo R, Cohen-Saidon C, Liron Y, Kam Z, Cohen L, Danon T, Perzov N, Alon U. Dynamic proteomics of individual cancer cells in response to a drug. Science. 2008;322(5907):1511–1516. doi: 10.1126/science.1160165. [DOI] [PubMed] [Google Scholar]
- Cristofanon S, Fulda S. ABT-737 promotes tBid mitochondrial accumulation to enhance TRAIL-induced apoptosis in glioblastoma cells. Cell Death Dis. 2012;3:e432. doi: 10.1038/cddis.2012.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids MS, Deng J, Wiestner A, Lannutti BJ, Wang L, Wu CJ, Wilson WH, Brown JR, Letai A. Decreased mitochondrial apoptotic priming underlies stroma-mediated treatment resistance in chronic lymphocytic leukemia. Blood. 2012 doi: 10.1182/blood-2012-02-414060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30(3):256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- Deng J, Carlson N, Takeyama K, Dal Cin P, Shipp M, Letai A. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell. 2007;12(2):171–185. doi: 10.1016/j.ccr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Deng Y, Lin Y, Wu X. TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev. 2002;16(1):33–45. doi: 10.1101/gad.949602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388(6639):300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- Di Pietro R, Secchiero P, Rana R, Gibellini D, Visani G, Bemis K, Zamai L, Miscia S, Zauli G. Ionizing radiation sensitizes erythroleukemic cells but not normal erythroblasts to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)--mediated cytotoxicity by selective up-regulation of TRAIL-R1. Blood. 2001;97(9):2596–2603. doi: 10.1182/blood.v97.9.2596. [DOI] [PubMed] [Google Scholar]
- Ehrhardt H, Hacker S, Wittmann S, Maurer M, Borkhardt A, Toloczko A, Debatin KM, Fulda S, Jeremias I. Cytotoxic drug-induced, p53-mediated upregulation of caspase-8 in tumor cells. Oncogene. 2008;27(6):783–793. doi: 10.1038/sj.onc.1210666. [DOI] [PubMed] [Google Scholar]
- Eskes R, Desagher S, Antonsson B, Martinou JC. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol. 2000;20(3):929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falschlehner C, Emmerich CH, Gerlach B, Walczak H. TRAIL signalling: decisions between life and death. Int J Biochem Cell Biol. 2007;39(7-8):1462–1475. doi: 10.1016/j.biocel.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Fitzgerald JB, Schoeberl B, Nielsen UB, Sorger PK. Systems biology and combination therapy in the quest for clinical efficacy. Nat Chem Biol. 2006;2(9):458–466. doi: 10.1038/nchembio817. [DOI] [PubMed] [Google Scholar]
- Frew AJ, Lindemann RK, Martin BP, Clarke CJ, Sharkey J, Anthony DA, Banks KM, Haynes NM, Gangatirkar P, Stanley K, Bolden JE, Takeda K, Yagita H, Secrist JP, Smyth MJ, Johnstone RW. Combination therapy of established cancer using a histone deacetylase inhibitor and a TRAIL receptor agonist. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(32):11317–11322. doi: 10.1073/pnas.0801868105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker N, Beaudouin J, Richter P, Eils R, Krammer PH, Lavrik IN. Model-based dissection of CD95 signaling dynamics reveals both a pro- and antiapoptotic role of c-FLIPL. J Cell Biol. 2010;190(3):377–389. doi: 10.1083/jcb.201002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galligan L, Longley DB, McEwan M, Wilson TR, McLaughlin K, Johnston PG. Chemotherapy and TRAIL-mediated colon cancer cell death: the roles of p53, TRAIL receptors, and c-FLIP. Molecular cancer therapeutics. 2005;4(12):2026–2036. doi: 10.1158/1535-7163.MCT-05-0262. [DOI] [PubMed] [Google Scholar]
- Ganten TM, Haas TL, Sykora J, Stahl H, Sprick MR, Fas SC, Krueger A, Weigand MA, Grosse-Wilde A, Stremmel W, Krammer PH, Walczak H. Enhanced caspase-8 recruitment to and activation at the DISC is critical for sensitisation of human hepatocellular carcinoma cells to TRAIL-induced apoptosis by chemotherapeutic drugs. Cell death and differentiation. 2004;11(Suppl 1):S86–96. doi: 10.1038/sj.cdd.4401437. [DOI] [PubMed] [Google Scholar]
- Gascoigne KE, Taylor SS. Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell. 2008;14(2):111–122. doi: 10.1016/j.ccr.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Gaudet S, Spencer SL, Chen WW, Sorger PK. Exploring the contextual sensitivity of factors that determine cell-to-cell variability in receptor-mediated apoptosis. PLoS Comput Biol. 2012;8(4):e1002482. doi: 10.1371/journal.pcbi.1002482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JC, Waterhouse NJ, Juin P, Evan GI, Green DR. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat Cell Biol. 2000;2(3):156–162. doi: 10.1038/35004029. [DOI] [PubMed] [Google Scholar]
- Gonzalvez F, Ashkenazi A. New insights into apoptosis signaling by Apo2L/TRAIL. Oncogene. 2010;29(34):4752–4765. doi: 10.1038/onc.2010.221. [DOI] [PubMed] [Google Scholar]
- Hellwig CT, Kohler BF, Lehtivarjo AK, Dussmann H, Courtney MJ, Prehn JH, Rehm M. Real time analysis of tumor necrosis factor-related apoptosis-inducing ligand/cycloheximide-induced caspase activities during apoptosis initiation. The Journal of biological chemistry. 2008;283(31):21676–21685. doi: 10.1074/jbc.M802889200. [DOI] [PubMed] [Google Scholar]
- Hellwig CT, Rehm M. TRAIL signaling and synergy mechanisms used in TRAIL-based combination therapies. Molecular cancer therapeutics. 2012;11(1):3–13. doi: 10.1158/1535-7163.MCT-11-0434. [DOI] [PubMed] [Google Scholar]
- Henson ES, Gibson EM, Villanueva J, Bristow NA, Haney N, Gibson SB. Increased expression of Mcl-1 is responsible for the blockage of TRAIL-induced apoptosis mediated by EGF/ErbB1 signaling pathway. J Cell Biochem. 2003;89(6):1177–1192. doi: 10.1002/jcb.10597. [DOI] [PubMed] [Google Scholar]
- Hetschko H, Voss V, Horn S, Seifert V, Prehn JH, Kogel D. Pharmacological inhibition of Bcl-2 family members reactivates TRAIL-induced apoptosis in malignant glioma. J Neurooncol. 2007 doi: 10.1007/s11060-007-9472-6. [DOI] [PubMed] [Google Scholar]
- Huang S, Sinicrope FA. BH3 mimetic ABT-737 potentiates TRAIL-mediated apoptotic signaling by unsequestering Bim and Bak in human pancreatic cancer cells. Cancer research. 2008;68(8):2944–2951. doi: 10.1158/0008-5472.CAN-07-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S, MacFarlane M, Harper N, Wheat LM, Dyer MJ, Cohen GM. Histone deacetylase inhibitors potentiate TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in lymphoid malignancies. Cell death and differentiation. 2004;11(Suppl 2):S193–206. doi: 10.1038/sj.cdd.4401535. [DOI] [PubMed] [Google Scholar]
- Johnson TR, Stone K, Nikrad M, Yeh T, Zong WX, Thompson CB, Nesterov A, Kraft AS. The proteasome inhibitor PS-341 overcomes TRAIL resistance in Bax and caspase 9-negative or Bcl-xL overexpressing cells. Oncogene. 2003;22(32):4953–4963. doi: 10.1038/sj.onc.1206656. [DOI] [PubMed] [Google Scholar]
- Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8(10):782–798. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- Kabore AF, Sun J, Hu X, McCrea K, Johnston JB, Gibson SB. The TRAIL apoptotic pathway mediates proteasome inhibitor induced apoptosis in primary chronic lymphocytic leukemia cells. Apoptosis. 2006;11(7):1175–1193. doi: 10.1007/s10495-006-8048-9. [DOI] [PubMed] [Google Scholar]
- Kang J, Kisenge RR, Toyoda H, Tanaka S, Bu J, Azuma E, Komada Y. Chemical sensitization and regulation of TRAIL-induced apoptosis in a panel of B-lymphocytic leukaemia cell lines. Br J Haematol. 2003;123(5):921–932. doi: 10.1046/j.1365-2141.2003.04699.x. [DOI] [PubMed] [Google Scholar]
- Karikari CA, Roy I, Tryggestad E, Feldmann G, Pinilla C, Welsh K, Reed JC, Armour EP, Wong J, Herman J, Rakheja D, Maitra A. Targeting the apoptotic machinery in pancreatic cancers using small-molecule antagonists of the X-linked inhibitor of apoptosis protein. Molecular cancer therapeutics. 2007;6(3):957–966. doi: 10.1158/1535-7163.MCT-06-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane MM, Ettenberg SA, Nau MM, Russell EK, Lipkowitz S. Chemotherapy augments TRAIL-induced apoptosis in breast cell lines. Cancer research. 1999;59(3):734–741. [PubMed] [Google Scholar]
- Kim SH, Ricci MS, El-Deiry WS. Mcl-1: a gateway to TRAIL sensitization. Cancer research. 2008;68(7):2062–2064. doi: 10.1158/0008-5472.CAN-07-6278. [DOI] [PubMed] [Google Scholar]
- Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, Peter ME. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. Embo J. 1995;14(22):5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuz S, Siegmund D, Scheurich P, Wajant H. NF-kappaB inducers upregulate cFLIP, a cycloheximide-sensitive inhibitor of death receptor signaling. Mol Cell Biol. 2001;21(12):3964–3973. doi: 10.1128/MCB.21.12.3964-3973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D, Cote M, Grondin R, Couture MC, Piche A. Acquired resistance to TRAIL-induced apoptosis in human ovarian cancer cells is conferred by increased turnover of mature caspase-3. Molecular cancer therapeutics. 2006;5(3):509–521. doi: 10.1158/1535-7163.MCT-05-0362. [DOI] [PubMed] [Google Scholar]
- Laussmann MA, Passante E, Dussmann H, Rauen JA, Wurstle ML, Delgado ME, Devocelle M, Prehn JH, Rehm M. Proteasome inhibition can induce an autophagy-dependent apical activation of caspase-8. Cell death and differentiation. 2011;18(10):1584–1597. doi: 10.1038/cdd.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laussmann MA, Passante E, Hellwig CT, Tomiczek B, Flanagan L, Prehn JH, Huber HJ, Rehm M. Proteasome inhibition can impair caspase-8 activation upon submaximal stimulation of apoptotic tumor necrosis factor-related apoptosis inducing ligand (TRAIL) signaling. The Journal of biological chemistry. 2012;287(18):14402–14411. doi: 10.1074/jbc.M111.304378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Shin MS, Kim HS, Lee HK, Park WS, Kim SY, Lee JH, Han SY, Park JY, Oh RR, Kang CS, Kim KM, Jang JJ, Nam SW, Lee JY, Yoo NJ. Somatic mutations of TRAIL-receptor 1 and TRAIL-receptor 2 genes in non-Hodgkin’s lymphoma. Oncogene. 2001;20(3):399–403. doi: 10.1038/sj.onc.1204103. [DOI] [PubMed] [Google Scholar]
- Letai AG. Diagnosing and exploiting cancer’s addiction to blocks in apoptosis. Nat Rev Cancer. 2008;8(2):121–132. doi: 10.1038/nrc2297. [DOI] [PubMed] [Google Scholar]
- Li S, Zhao Y, He X, Kim TH, Kuharsky DK, Rabinowich H, Chen J, Du C, Yin XM. Relief of extrinsic pathway inhibition by the Bid-dependent mitochondrial release of Smac in Fas-mediated hepatocyte apoptosis. The Journal of biological chemistry. 2002;277(30):26912–26920. doi: 10.1074/jbc.M200726200. [DOI] [PubMed] [Google Scholar]
- Li Z, Xu X, Bai L, Chen W, Lin Y. EGFR-mediated tissue transglutaminase overexpression couples acquired TRAIL-resistance and migration through c-FLIP and MMP-9 in lung cancer cells. The Journal of biological chemistry. 2011 doi: 10.1074/jbc.M110.207571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lickliter JD, Cox J, McCarron J, Martinez NR, Schmidt CW, Lin H, Nieda M, Nicol AJ. Small-molecule Bcl-2 inhibitors sensitise tumour cells to immune-mediated destruction. Br J Cancer. 2007;96(4):600–608. doi: 10.1038/sj.bjc.6603599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94(4):481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- MacFarlane M, Kohlhaas SL, Sutcliffe MJ, Dyer MJ, Cohen GM. TRAIL receptor-selective mutants signal to apoptosis via TRAIL-R1 in primary lymphoid malignancies. Cancer research. 2005;65(24):11265–11270. doi: 10.1158/0008-5472.CAN-05-2801. [DOI] [PubMed] [Google Scholar]
- Martin DA, Siegel RM, Zheng L, Lenardo MJ. Membrane oligomerization and cleavage activates the caspase-8 (FLICE/MACHalpha1) death signal. The Journal of biological chemistry. 1998;273(8):4345–4349. doi: 10.1074/jbc.273.8.4345. [DOI] [PubMed] [Google Scholar]
- Mazurek N, Byrd JC, Sun Y, Hafley M, Ramirez K, Burks J, Bresalier RS. Cell-surface galectin-3 confers resistance to TRAIL by impeding trafficking of death receptors in metastatic colon adenocarcinoma cells. Cell death and differentiation. 2011 doi: 10.1038/cdd.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XW, Lee SH, Dai H, Loegering D, Yu C, Flatten K, Schneider P, Dai NT, Kumar SK, Smith BD, Karp JE, Adjei AA, Kaufmann SH. MCL-1 as a Buffer for Proapoptotic BCL-2 Family Members during TRAIL-induced Apoptosis: A MECHANISTIC BASIS FOR SORAFENIB (BAY 43-9006)-INDUCED TRAIL SENSITIZATION. The Journal of biological chemistry. 2007;282(41):29831–29846. doi: 10.1074/jbc.M706110200. [DOI] [PubMed] [Google Scholar]
- Morizot A, Merino D, Lalaoui N, Jacquemin G, Granci V, Iessi E, Lanneau D, Bouyer F, Solary E, Chauffert B, Saas P, Garrido C, Micheau O. Chemotherapy overcomes TRAIL-R4-mediated TRAIL resistance at the DISC level. Cell death and differentiation. 2010 doi: 10.1038/cdd.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann L, Pforr C, Beaudouin J, Pappa A, Fricker N, Krammer PH, Lavrik IN, Eils R. Dynamics within the CD95 death-inducing signaling complex decide life and death of cells. Mol Syst Biol. 2010;6:352. doi: 10.1038/msb.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, Speed T, Spellman PT, DeVries S, Lapuk A, Wang NJ, Kuo WL, Stilwell JL, Pinkel D, Albertson DG, Waldman FM, McCormick F, Dickson RB, Johnson MD, Lippman M, Ethier S, Gazdar A, Gray JW. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10(6):515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmanapalli R, Perkins CL, Orlando M, O’Bryan E, Nguyen D, Bhalla KN. Pretreatment with paclitaxel enhances apo-2 ligand/tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis of prostate cancer cells by inducing death receptors 4 and 5 protein levels. Cancer research. 2001;61(2):759–763. [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, Kitada S, Korsmeyer SJ, Kunzer AR, Letai A, Li C, Mitten MJ, Nettesheim DG, Ng S, Nimmer PM, O’Connor JM, Oleksijew A, Petros AM, Reed JC, Shen W, Tahir SK, Thompson CB, Tomaselli KJ, Wang B, Wendt MD, Zhang H, Fesik SW, Rosenberg SH. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435(7042):677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- Rahman M, Pumphrey JG, Lipkowitz S. The TRAIL to targeted therapy of breast cancer. Adv Cancer Res. 2009;103:43–73. doi: 10.1016/S0065-230X(09)03003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm M, Dussmann H, Prehn JH. Real-time single cell analysis of Smac/DIABLO release during apoptosis. J Cell Biol. 2003;162(6):1031–1043. doi: 10.1083/jcb.200303123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm M, Huber HJ, Dussmann H, Prehn JH. Systems analysis of effector caspase activation and its control by X-linked inhibitor of apoptosis protein. EMBO J. 2006;25(18):4338–4349. doi: 10.1038/sj.emboj.7601295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci MS, Kim SH, Ogi K, Plastaras JP, Ling J, Wang W, Jin Z, Liu YY, Dicker DT, Chiao PJ, Flaherty KT, Smith CD, El-Deiry WS. Reduction of TRAIL-induced Mcl-1 and cIAP2 by c-Myc or sorafenib sensitizes resistant human cancer cells to TRAIL-induced death. Cancer Cell. 2007;12(1):66–80. doi: 10.1016/j.ccr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Riedl SJ, Salvesen GS. The apoptosome: signalling platform of cell death. Nature reviews. 2007;8(5):405–413. doi: 10.1038/nrm2153. [DOI] [PubMed] [Google Scholar]
- Rosato RR, Almenara JA, Coe S, Grant S. The multikinase inhibitor sorafenib potentiates TRAIL lethality in human leukemia cells in association with Mcl-1 and cFLIPL down-regulation. Cancer research. 2007;67(19):9490–9500. doi: 10.1158/0008-5472.CAN-07-0598. [DOI] [PubMed] [Google Scholar]
- Sah NK, Munshi A, Kurland JF, McDonnell TJ, Su B, Meyn RE. Translation inhibitors sensitize prostate cancer cells to apoptosis induced by tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) by activating c-Jun N-terminal kinase. The Journal of biological chemistry. 2003;278(23):20593–20602. doi: 10.1074/jbc.M211010200. [DOI] [PubMed] [Google Scholar]
- Sanchez-Perez T, Ortiz-Ferron G, Lopez-Rivas A. Mitotic arrest and JNK-induced proteasomal degradation of FLIP and Mcl-1 are key events in the sensitization of breast tumor cells to TRAIL by antimicrotubule agents. Cell death and differentiation. 2009;17(5):883–894. doi: 10.1038/cdd.2009.176. [DOI] [PubMed] [Google Scholar]
- Sayers TJ, Brooks AD, Koh CY, Ma W, Seki N, Raziuddin A, Blazar BR, Zhang X, Elliott PJ, Murphy WJ. The proteasome inhibitor PS-341 sensitizes neoplastic cells to TRAIL-mediated apoptosis by reducing levels of c-FLIP. Blood. 2003;102(1):303–310. doi: 10.1182/blood-2002-09-2975. [DOI] [PubMed] [Google Scholar]
- Sayers TJ, Murphy WJ. Combining proteasome inhibition with TNF-related apoptosis-inducing ligand (Apo2L/TRAIL) for cancer therapy. Cancer Immunol Immunother. 2006;55(1):76–84. doi: 10.1007/s00262-005-0676-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secchiero P, Gonelli A, Carnevale E, Milani D, Pandolfi A, Zella D, Zauli G. TRAIL promotes the survival and proliferation of primary human vascular endothelial cells by activating the Akt and ERK pathways. Circulation. 2003;107(17):2250–2256. doi: 10.1161/01.CIR.0000062702.60708.C4. [DOI] [PubMed] [Google Scholar]
- Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, McDermott U, Azizian N, Zou L, Fischbach MA, Wong KK, Brandstetter K, Wittner B, Ramaswamy S, Classon M, Settleman J. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141(1):69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh MS, Burns TF, Huang Y, Wu GS, Amundson S, Brooks KS, Fornace AJ, Jr., el-Deiry WS. p53-dependent and -independent regulation of the death receptor KILLER/DR5 gene expression in response to genotoxic stress and tumor necrosis factor alpha. Cancer research. 1998;58(8):1593–1598. [PubMed] [Google Scholar]
- Shrader M, Pino MS, Lashinger L, Bar-Eli M, Adam L, Dinney CP, McConkey DJ. Gefitinib reverses TRAIL resistance in human bladder cancer cell lines via inhibition of AKT-mediated X-linked inhibitor of apoptosis protein expression. Cancer research. 2007;67(4):1430–1435. doi: 10.1158/0008-5472.CAN-06-1224. [DOI] [PubMed] [Google Scholar]
- Singh TR, Shankar S, Chen X, Asim M, Srivastava RK. Synergistic interactions of chemotherapeutic drugs and tumor necrosis factor-related apoptosis-inducing ligand/Apo-2 ligand on apoptosis and on regression of breast carcinoma in vivo. Cancer research. 2003;63(17):5390–5400. [PubMed] [Google Scholar]
- Song JH, Kandasamy K, Kraft AS. ABT-737 induces expression of the death receptor 5 and sensitizes human cancer cells to TRAIL-induced apoptosis. The Journal of biological chemistry. 2008;283(36):25003–25013. doi: 10.1074/jbc.M802511200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, Tse MC, Bellail A, Phuphanich S, Khuri F, Kneteman NM, Hao C. Lipid rafts and nonrafts mediate tumor necrosis factor related apoptosis-inducing ligand induced apoptotic and nonapoptotic signals in non small cell lung carcinoma cells. Cancer research. 2007;67(14):6946–6955. doi: 10.1158/0008-5472.CAN-06-3896. [DOI] [PubMed] [Google Scholar]
- Spencer SL, Gaudet S, Albeck JG, Burke JM, Sorger PK. Non-genetic origins of cell-to-cell variability in TRAIL-induced apoptosis. Nature. 2009 doi: 10.1038/nature08012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SL, Sorger PK. Measuring and modeling apoptosis in single cells. Cell. 2011;144(6):926–939. doi: 10.1016/j.cell.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagni V, Mingardi M, Santini S, Giaccari D, Barila D. ATM kinase activity modulates cFLIP protein levels: potential interplay between DNA damage signalling and TRAIL-induced apoptosis. Carcinogenesis. 2010;31(11):1956–1963. doi: 10.1093/carcin/bgq193. [DOI] [PubMed] [Google Scholar]
- Sun XM, Bratton SB, Butterworth M, MacFarlane M, Cohen GM. Bcl-2 and Bcl-xL inhibit CD95-mediated apoptosis by preventing mitochondrial release of Smac/DIABLO and subsequent inactivation of X-linked inhibitor-of-apoptosis protein. The Journal of biological chemistry. 2002;277(13):11345–11351. doi: 10.1074/jbc.M109893200. [DOI] [PubMed] [Google Scholar]
- van Geelen CM, de Vries EG, Le TK, van Weeghel RP, de Jong S. Differential modulation of the TRAIL receptors and the CD95 receptor in colon carcinoma cell lines. Br J Cancer. 2003;89(2):363–373. doi: 10.1038/sj.bjc.6601065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanoosten RL, Moore JM, Ludwig AT, Griffith TS. Depsipeptide (FR901228) enhances the cytotoxic activity of TRAIL by redistributing TRAIL receptor to membrane lipid rafts. Mol Ther. 2005;11(4):542–552. doi: 10.1016/j.ymthe.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Wagner KW, Punnoose EA, Januario T, Lawrence DA, Pitti RM, Lancaster K, Lee D, von Goetz M, Yee SF, Totpal K, Huw L, Katta V, Cavet G, Hymowitz SG, Amler L, Ashkenazi A. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nature medicine. 2007;13(9):1070–1077. doi: 10.1038/nm1627. [DOI] [PubMed] [Google Scholar]
- Wajant H, Haas E, Schwenzer R, Muhlenbeck F, Kreuz S, Schubert G, Grell M, Smith C, Scheurich P. Inhibition of death receptor-mediated gene induction by a cycloheximide-sensitive factor occurs at the level of or upstream of Fas-associated death domain protein (FADD) The Journal of biological chemistry. 2000;275(32):24357–24366. doi: 10.1074/jbc.M000811200. [DOI] [PubMed] [Google Scholar]
- Wertz IE, Kusam S, Lam C, Okamoto T, Sandoval W, Anderson DJ, Helgason E, Ernst JA, Eby M, Liu J, Belmont LD, Kaminker JS, O’Rourke KM, Pujara K, Kohli PB, Johnson AR, Chiu ML, Lill JR, Jackson PK, Fairbrother WJ, Seshagiri S, Ludlam MJ, Leong KG, Dueber EC, Maecker H, Huang DC, Dixit VM. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature. 2011;471(7336):110–114. doi: 10.1038/nature09779. [DOI] [PubMed] [Google Scholar]
- Wu GS, Burns TF, McDonald ER, 3rd, Jiang W, Meng R, Krantz ID, Kao G, Gan DD, Zhou JY, Muschel R, Hamilton SR, Spinner NB, Markowitz S, Wu G, el-Deiry WS. KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nature genetics. 1997;17(2):141–143. doi: 10.1038/ng1097-141. [DOI] [PubMed] [Google Scholar]
- Zhang L, Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther. 2005;12(3):228–237. doi: 10.1038/sj.cgt.7700792. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.