Abstract
Sperm display remarkable morphological diversity among even closely related species, a pattern that is widely attributed to postcopulatory sexual selection. Surprisingly few studies have used phylogenetic analyses to discern the details of evolutionary diversification in ornaments and armaments subject to sexual selection, and the origins of novel sperm traits and their subsequent modification are particularly poorly understood. Here we investigate sperm evolution in diving beetles (Dytiscidae), revealing dramatic diversification in flagellum length, head shape, presence of sperm heteromorphism, and the presence/type of sperm conjugation, an unusual trait where two or more sperm unite for motility or transport. Sperm conjugation was found to be the ancestral condition in diving beetles, with subsequent diversification into three forms, each exhibiting varying degrees of evolutionary loss, convergence and recurrence. Sperm head shape, but not length or heteromorphism, was found to evolve in a significantly correlated manner with conjugation, consistent with the different mechanisms of head alignment and binding required for the different forms of conjugation. Our study reveals that sperm morphological evolution is channeled along particular evolutionary pathways (i.e., conjugate form), yet subject to considerable diversification within those pathways through modification in sperm length, head shape and heteromorphism.
Keywords: ornament evolution, postcopulatory sexual selection, reproductive traits, sperm conjugation, sperm heteromorphism
Sexually selected traits are predicted to evolve rapidly and divergently among closely related species (Andersson 1994). Their distribution across a phylogeny can be used to infer the sequence of evolutionary transitions in morphology or behavior, to identify recurrent patterns, to explore alternative models of sexual selection (Prum 1997, 2010), and to test the role of sexual selection in speciation (Kraaijeveld et al. 2011). Nevertheless, patterns of sexual trait evolution are largely unexplored (Wiens 2001), with relatively few studies examining evolutionary transitions in ornaments (Meyer et al. 1994; Prum 1997; Schulter et al. 1997; Omland and Lanyon 2000; Boughman et al. 2005; Price et al. 2007; Prager and Andersson 2010), and weapons (Emlen et al. 2005), or courtship behaviors (Prum 1997; Henry et al. 1999; Price et al. 2007; Puniamoorthy et al. 2009).
Some sperm characters are recognized as ornaments and/or armaments, evolving rapidly as a consequence of postcopulatory sexual selection (i.e. sperm competition and cryptic female choice, Keller and Reeve 1995; Pizzari and Birkhead 2002; Snook 2005; Pitnick et al. 2009a, 2009b; Pizzari and Parker 2009). For example, sperm length in Drosophila melanogaster shows a correlated response to selection for increased length of the primary female sperm-storage organ, the seminal receptacle (Miller and Pitnick 2002). Longer seminal receptacles bias sperm use in favor of longer sperm (i.e., cryptic female choice, Birkhead 2000; Pitnick and Brown 2000). Mechanistically, the interaction between sperm length and seminal receptacle morphology results in long sperm being better able to displace and resist displacement by competitor sperm (Pattarini et al. 2006). In this instance, a long sperm tail acts both as an ornament that females use to discriminate among sires and as an armament when in competition with rival sperm. Alternatively, sperm morphology can also act as a weapon in evolutionary arms races. Many species of the hermaphroditic flatworm of the genus Macrostomum participate in reciprocal matings that terminate with a sucking behavior to remove sperm and seminal fluids deposited by the mating partner (Schärer et al. 2004). Stiff lateral bristles on sperm, a trait that is hypothesized to impede sperm removal by sucking, exhibits an evolutionary association with reciprocal mating, with sperm bristles being absent in species that circumvent the sucking behavior by hypodermically inseminating sperm into the body of the mating partner (Schärer et al. 2011).
Motivated by the central role of sperm in sexual reproduction, their unusual ecology (being cast from the soma into a foreign environment to function essentially as free-living organisms), and their usefulness in systematics (Jamieson et al. 1999), biologists have described the sperm of thousands of species (Pitnick et al. 2009a). Collectively, these studies indicate that sperm are the most diverse cell type, exhibiting dramatic morphological modifications in nearly all taxa (Pitnick et al. 2009a). Despite the massive research effort describing sperm structure in myriad of species, the origins of novel sperm forms, their subsequent diversification and evolutionary loss of derived sperm traits have scarcely been investigated (Roldan et al. 1992; Breed 2005), resulting in a limited understanding of sperm phenotypic evolution. Here, we amend this deficiency by conducting detailed analyses to infer patterns of sperm evolution within a large radiation of aquatic beetles (Dytiscidae with ca. 4000 species worldwide, Nilsson 2001; Nilsson and Fery 2006) exhibiting dramatic, multivariate diversity in sperm form.
The sperm of diving beetles (Dytiscidae) first attracted attention over a century ago due to an unusual variant in morphology: conjugation, where two or more spermatozoa join together at the head for motility or transport through the female reproductive tract (Auerbach 1893; Ballowitz 1895). Sperm conjugation is rare, but has several independent origins throughout the Metazoa, occurring in relatively few species of marsupials, eutherian mammals, gastropods, annelids, myriapods, arachnids and insects (Immler 2008; Higginson and Pitnick 2011). This remarkable variant in sperm form has been of considerable interest to evolutionary biologists due to its potential role in sperm competition, implications for evolutionary cooperation, and the possibility of haploid-diploid conflict between males and the sperm they produce (Immler 2008; Pizzari and Foster 2008; Higginson and Pitnick 2011). Additionally, some species of diving beetles produce two distinct sperm morphs that vary in total length or head shape (Higginson and Pitnick 2011). Such sperm heteromorphism sometimes co-occurs with conjugation, resulting in complex conjugates constructed of both sperm morphs.
Although female mating frequency has not been determined for any species of diving beetle, several lines of evidence suggests that sexual selection has been important in shaping their behavior and morphology. First, male investment in sperm production is among the highest recorded for any taxa (up to 13% of their body mass in Dytiscus sharpi, Inoda et al. 2007). Relative testis mass is positively correlated with polyandry and is a robust indicator of the intensity of sexual selection in mammals (Harcourt et al. 1981; Gomendio and Roldan 1991), fish (Stockley et al. 1997), birds (Møller and Briskie 1995), and insects (Gage 1994). Second, sperm competition avoidance tactics of mate-guarding and copulatory plugs are observed in diving beetles (Smith 1973; Aiken and Wilkinson 1985; Aiken 1992; Simmons 2001; Inoda 2003; Cleavall 2009). Third, in some diving beetle lineages there is evidence of sexual arms races, where evolution of structures that improve females’ ability to resist mating is followed by the evolution of improved male structures used to grasp females during copulation (Miller 2003; Bergsten and Miller 2007). Lastly, sperm morphology in diving beetles is correlated with dimensions of the female reproductive tract and evolves in response to changes in the architecture of female sperm-storage organs (Higginson et al. submitted).
Here we use Bayesian analyses deploying reversible-jump Markov Chain Monte Carlo (rj-MCMC) to (i) explore different models of evolution while accounting for uncertainty in phylogenetic relationships, (ii) infer the sequence of diversification in conjugate form and (iii) examine patterns of convergence, recurrence and loss of conjugation and sperm heteromorphism. We also test hypotheses of correlated evolution among sperm traits including conjugation, head shape, sperm length and heteromorphism. Head shape might affect the propensity of sperm to conjugate by modifying hydrophobic, surface-protein or glycocalyx interactions. Additionally, both theoretical and empirical studies indicate a positive relationship between sperm length and swimming speed (Lighthill 1976; Fitzpatrick et al. 2009). Conjugation might provide a mechanism for increased sperm velocity by combining the force generated by multiple flagella (Woolley et al. 2009; for empirical examples of the impact of conjugation on motility see Moore and Taggart 1995; Hayashi 1998; Moore et al. 2002; Immler et al. 2007) without the energetic costs of producing long sperm (e.g., delayed maturation and increased investment in testes; Pitnick et al. 1995; Pitnick 1996). Thus, if selection favored increased sperm velocity, long sperm would be predicted to evolve in the absence of conjugation and vice versa. Finally, conjugation might facilitate the evolution of heteromorphism by lessening the selective constraints on sperm morphology imposed by individual sperm having to affect their own transport to the site of fertilization (i.e., through functional specialization).
Methods
SPERM CHARACTERS
Beetles were dissected in phosphate-buffered saline and their sperm harvested from the seminal vesicles. The sperm were dried on a subbed microscope slide, fixed, and DNA stained (Hoechst’s or DAPI). To confirm the presence or absence of conjugation, sperm found in the female sperm-storage organs were also examined when female specimens were available (the majority of species). Sperm were visualized and imaged using darkfield and epifluorescence microscopy (100x – 400x as appropriate for sperm length; 1000x for head shape).
A species was classified as lacking sperm conjugation if there was no evidence of physical association among sperm in the samples. Species were considered to have aggregate-type conjugation when variable numbers of sperm per unit were aligned with their heads in register within the seminal vesicles of males. Sperm pairing was classified as when two sperm aligned with their heads oriented anti-parallel to each other. Rouleaux were classified as the orderly stacking of sperm, where the tip of one sperm head slips into the hooded portion of another (Higginson and Pitnick 2011), regardless of the number of sperm involved (e.g., two in Bidessonotus inconspicuous, dozens in Neoporus undulatus and hundreds in Hydroporus sp.). Typically, some single sperm were also present in the seminal vesicles of males with aggregated or paired sperm. In contrast, single sperm were never observed within the seminal vesicles of males that produced rouleaux.
Sperm length was measured from digitized images using Image J (Rasband 1997 - 2008). In some instances, mature individualized sperm could not be obtained from a species. In these instances we measured the total length of mature sperm bundles (i.e., sperm had taken on their mature head shape but had not yet individualized), providing a minimum length for the species. A species was considered to be sperm heteromorphic when two distinct (i.e., non-overlapping) sperm lengths or head shapes were produced by a single male (see Figure 1A, E, F). One to nine individuals per species (mean = 3) were examined (Table S1). Sperm characters were largely consistent within genera. There is some uncertainty to the type of conjugation present in Hygrobia. We were only able to obtain sperm from females and whereas conjugation was unambiguously present, we could not distinguish between aggregates and rouleaux (see Discussion and Table S1). Given the position of Hygrobia in the phylogeny, we chose to conservatively interpret the conjugation as aggregates. However, we explored the impact of Hygrobia’s type of conjugation on our models of trait evolution and found no substantive difference in transition rates or likelihood when Hygrobia was considered to have aggregates or rouleaux. Where species did not overlap between available sequence data and morphological data (most cases), sperm characters were mapped to the phylogenetic tree by assigning values to genera or subgenera (i.e., subgenera for Agabus and Ilybius where there has been recent and considerable taxonomic flux, Nilsson 2000, 2001; data matrix available on TreeBASE). When there was observed variation in these characters within a genus, the data were coded to reflect all observed character types (e.g., both elongate and broad sperm heads present). In total, we examined 141 species of diving beetles that provided sperm morphological data for 138 tips of our phylogeny.
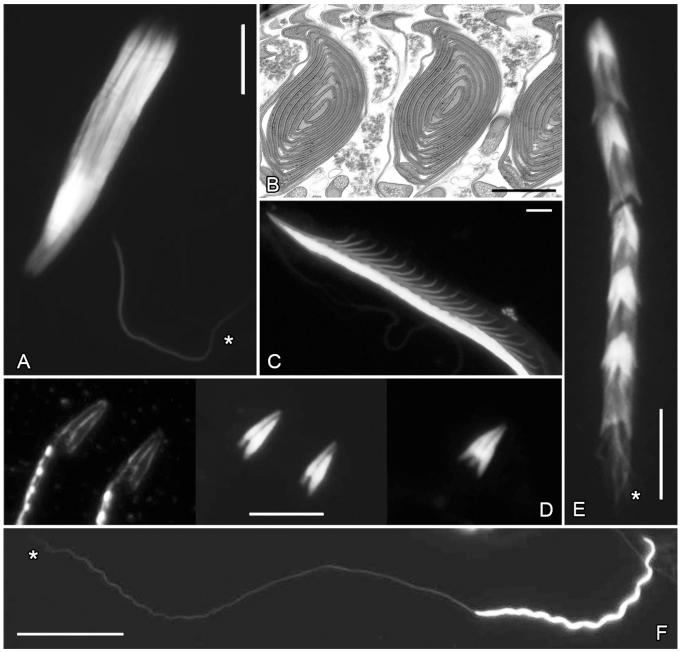
Figure 1. Head shape and conjugation in diving beetles.
(A) Elongate heads of Iybius oblitus form sperm aggregates and surround second sperm morph with broad heads (faintly visible as region of higher intensity fluorescence). (B) The cone-shaped heads of Neoporus undulatus stack together with the tip of one sperm head slips into the pocket at the base of another to form orderly stacks. In cross-section, rouleaux have an onion-like appearance with the sperm heads forming concentric circles (seven sperm heads are visible in each of the conjugates). (C) Fluorescent image of a N. undulatus rouleau. Large basal spurs of each sperm head are clearly visible projecting along one edge of the conjugate. (D) Composite darkfield and fluorescence image of the broad, flat sperm heads of Hydaticus bimarginatus. In the seminal vesicles of males, the sperm heads align anti-parallel to each other and conjugate to form pairs (far right panel). (E) Heterospermatozeugmata in Ilybius larsoni. Within the seminal vesicles of males elongate headed sperm (indicated by *) slip into the pocket of an individual broad headed sperm to form sperm aggregates. During or after transfer to the female, some of the broad headed sperm associate with each other becoming highly structurally and functionally (see Movie S1) reminiscent of rouleaux. (F) A heterospermatozeugmata of Derovatellus peruanus. Broad heads form a rouleaux with an overall helical shape. A second sperm morph, with very elongate heads, is attached to the tip of the rouleaux (indicated by *). Broadly triangular and elongate head shapes are common in diving beetles. Fluorescent images of DAPI or Hoechst’s stained sperm heads (A, C – F); transmission electron micrograph (B) and darkfield image in the left panel of (D). Flagella not visible in the fluorescent images. An asterisk indicates the elongate sperm morph in sperm heteromorphic species. Scale bars A, C – F = 10 μm, B = 1 μm.
PHYLOGENETIC TREES
To provide trees upon which to test models of character evolution, we used the large sequence data set compiled by Ribera et. al. (2008; see Appendix 1 therein for accession numbers). The data set is composed of two mitochondrial genes (COI and 16s) and two nuclear genes (H3 and 18s) with excellent taxonomic sampling that includes 222 diving beetle species, 25 of 26 tribes and 116 of 174 known genera. We truncated the 18s sequences at the 5′ end to reduce the amount of missing sequence data. Sequences were aligned with the PRANK+F algorithm (Löytynoja and Goldman 2008), which avoids over-penalization of insertion and deletion events common among distantly related sequences such as those in this study. To assess the quality of our alignments, we used the heads-or-tails (HoT) methodology that compares alignments that differ in the directionality that the sequences entered the alignment algorithm (i.e., a “heads” sequences is entered 5′ to 3′ whereas “tails” represents the identical sequence entered in the 3′ to 5′ direction, Landan and Graur 2007). If sequence alignments are unambiguous, both heads and tails alignments provide identical results. PRANK+F produced heads and tails alignments with 93.6 to 99.9% (16s and H3 respectively) identical residues within the aligned genes and resulted in phylogenetic trees that did not differ substantively (alignments available upon request). Appropriate models of sequence evolution for each of the four genes were determined using DT-ModSel (Minin et al. 2003). We used MrBAYES (Ronquist and Huelsenbeck 2003) to infer phylogenetic relationships based on our partitioned four gene, 2696 base pair data set. To encourage convergence of the MCMC chains, we provided a starting tree produced using the neighbour-joining method in PAUP (Swofford 2002). The starting tree was randomly perturbated four times prior to the starting of the chains. Four separate MCMC runs of 4 × 107 generations were performed using uninformative priors (i.e., MrBAYES default prior values, Ronquist and Huelsenbeck 2003), six chains per run with 0.15 heating. Convergence of the runs was assessed using AWTY (Nylander et al. 2008) and the first 3 × 107 generations were discarded as burnin. After the burnin period, the MCMC chains visit alternative phylogenetic trees in proportion to their probability of being true given the model, priors and data. Despite using different alignment procedures and MCMC conditions, the phylogenetic analyses produced a majority consensus tree highly similar to that of Ribera et al. (2008).
COMPARATIVE ANALYSES
All analyses were conducted with BayesTraits using reversible-jump MCMC (Pagel et al. 2004; Pagel and Meade 2006; Pagel and Meade 2007) and 1000 post-burnin trees (described above). In this context, reversible-jump MCMC explores model space by varying both the number and value of parameters in a model of trait evolution and estimates the parameters on the provided trees, in which alternative branching patterns are present in proportion to their probability, and combines the parameter estimates across the tree sample. This effectively accounts for phylogenetic and model uncertainty by combining the posterior probability across trees and set of parameters (Pagel and Meade 2006). For each analysis, we performed three separate MCMC runs to check for stability of the harmonic mean of the likelihoods.
To explore transitions between different states of conjugation and to infer probable evolutionary transitions, we used the program MULTISTATE (Pagel 2002; Pagel and Meade 2007), a hyperprior with a uniform distribution of 0 – 50 or 0 – 100 to seed the mean of our exponential rate prior and a rate deviation value resulting in the recommended value of approximately 20% of proposals being accepted (Pagel et al. 2004). The rj-MCMC chain was run for 10,050,000 iterations with the first 50,000 iterations discarded as burnin. Mean rate, standard deviation and the percentage of times a transition rate was assigned a value of zero (Z%) were calculated from posterior rate distributions. Transition rates that were rarely assigned to zero (i.e., Z% < 10) were considered probable evolutionary events.
To test for correlated evolution between conjugation and sperm head shape, presence of heteromorphism or sperm length, we used the program DISCRETE (Pagel 2000; Pagel and Meade 2006; Pagel and Meade 2007) with the conditions described above. Bayes Factors were used to evaluate alternative models, where the traits were allowed to evolve independently or constrained to evolve in a dependent manner. Bayes Factors (BF) are two times the difference in the marginal likelihoods of the best-fit and worse-fit models, as approximated by the harmonic means from the final iteration of the MCMC runs. We follow the convention that a BF > 2 is supportive of the best-fit model and that BF > 5 or 10 is considered strong or very strong support, respectively (Pagel et al. 2004; Pagel and Meade 2006).
We used the most recent common ancestor (MRCA) approach to infer ancestral states. This method identifies a node that represents the MRCA to the group species of interest for each tree the MCMC chain visits. For some trees this node will only contain the species of interest, whereas for other trees the node will additionally include other taxa. Thus, for every tree the ancestral state at this internal node can be estimated and across trees, the posterior distribution of the ancestral state can be examined (Pagel et al. 2004). This method has the advantage of combining uncertainty about the existence of a node and that of its character state (Pagel et al. 2004). To test if one ancestral state is more likely than another, we compared the likelihoods of models using Bayes Factors, where the ancestor of the species of interest was constrained to take alternative character states (i.e., the fossil command in BayesTraits, Pagel et al. 2004).
Results
GENERAL PATTERNS OF SPERM MORPHOLOGICAL DIVERSITY
Diving beetles were found to have undergone extensive and multivariate diversification in sperm form, including variation in sperm length (128 μm to 4493 μm), head shape (Figure 1A – F) and the presence of sperm heteromorphism (production of two distinct sperm morphs that differ in total length and/or head shape; Table S1; Figure 1A, E, F). In addition, sperm were found to be either single or conjugated in one of three forms: i) sperm aggregates composed of variable numbers of sperm with their heads aligned in register (Figure 1A, 2A), ii) pairs with two sperm aligned anti-parallel to each other (Figure1D, 2B), or iii) rouleaux, where the tip of one sperm head slips into the hollow, hooded portion of another sperm’s head to form orderly stacks that may be composed of a few to several hundred sperm (Figure 1B – C, F, 2D). Sperm heteromorphism and conjugation were found to sometimes co-occur, resulting in complex conjugates called heterospermatozeugmata (e.g., Figure 1A, E, F; Pitnick et al. 2009a; Higginson and Pitnick 2011).
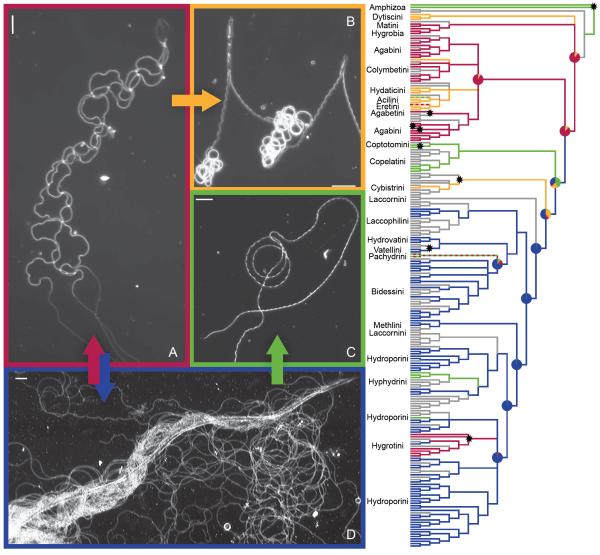
Figure 2. Transitions in sperm conjugation in diving beetles.
(A) Aggregate-type conjugation in Platambus semivittatus. Aggregates form the ancestral condition in Dytiscidae. (B) Paired sperm of Thermonectus marmoratus. (C) Single sperm of Pachydrus princeps. (D) A rouleaux of Neoporus undulatus. Arrows indicate probable evolutionary transitions (i.e., rate is rarely assigned to zero; z% < 5). All other possible transitions among conjugate types and single sperm were much less probable than those indicated by arrows (i.e., they were absent from 30 to 84% of the models produced by the MCMC chain; see Table 1 for mean transition rates). Sperm characters are mapped to the majority consensus tree with corresponding colors (grey indicates missing data). Two-color dashed lines indicate that both character states are present. Pie charts indicate the probability of the character states of the basal nodes. Stars indicate origins of sperm heteromorphism. For taxa and node posterior probabilities, see Figure S1. Darkfield images; scale bars = 10 μm.
TRANSITIONS AMONG TYPES OF CONJUGATION
The reconstructions of ancestral character states suggest sperm conjugation was present in the ancestor of diving beetles, with multiple subsequent losses distributed across the tree (Figure 2). Sperm aggregates are supported as the ancestral form of conjugation (models where the ancestral state is constrained to aggregates have greater likelihood than those constrained to have single sperm, paired conjugates, or sperm rouleaux (aggregates vs. single sperm Bayes Factor > 7, aggregates vs. pairs > 3). Additionally, both sperm pairing and rouleaux are identified as derived forms of conjugation originating from sperm aggregates (Figure 2; Table 1). The distribution of sperm pairing in diving beetles suggests that it has evolved three times. To test this hypothesis, we used the most recent common ancestor to reconstruct an internal node that minimally contained all of the species showing sperm pairing. We then compared the harmonic means of the likelihood of our models of evolution when this internal node was constrained to sperm pairing (indicating a single origin) or to aggregates (permitting multiple origins of pairing); multiple origins of pairing were supported (Bayes Factor > 2). Moreover, species with sperm pairing failed to form a monophyletic clade in any of the 40,000 post-burnin trees produced by MrBAYES (127 trees were consistent with only two origins of pairing). Reversions from rouleaux to the ancestral aggregates were (i) identified as probable evolutionary transitions by our rj-MCMC and (ii) observed nested well within the main lineage exhibiting rouleaux, indicating that the apparent reversions are not likely the result of incomplete lineage sorting. Loss of conjugation was observed both from lineages with paired sperm (a single event) and from those with rouleaux (multiple events), but only the transition from rouleaux to single sperm was identified as probable in our rj-MCMC analysis (see Figure 2; Table 1).
Table 1. Mean transition rate coefficients between the different forms of sperm conjugation.
Transition rates (per unit branch length) and Z-values are based on 50,000 observations from 10,000,000 iterations from each of three reversible jump-MCMC runs. Z-values indicate the percentage of times that the transition rate was assigned to zero, removing the pathway from the model of trait evolution. Evolutionary transitions are considered probable when rarely assigned to zero (e.g., the transition from rouleaux to single sperm are much more probable than those to paired sperm). If the outgroup taxa of Amphizoidae and Asidytidae are included in the analysis, a transition from single sperm to aggregates becomes probable (0.28 ± 0.20, Z% = 7.2) but other transitions are only slightly affected.
| Evolutionary transition | mean rate | SD | Z% |
|---|---|---|---|
| Single to paired | 0.14 | 0.16 | 43.7 |
| Single to aggregated | 0.13 | 0.16 | 47.5 |
| Single to rouleaux | 0.15 | 0.17 | 37.8 |
| Paired to single | 0.18 | 0.18 | 31.5 |
| Paired to aggregated | 0.17 | 0.19 | 33.8 |
| Paired to rouleaux | 0.16 | 0.18 | 35.3 |
| Aggregated to single | 0.16 | 0.14 | 29.7 |
| Aggregated to paired | 0.28 | 0.14 | 0.4 |
| Aggregated to rouleaux | 0.23 | 0.13 | 6.6 |
| Rouleaux to single | 0.23 | 0.11 | 0.6 |
| Rouleaux to paired | 0.04 | 0.08 | 75.7 |
| Rouleaux to aggregated | 0.20 | 0.10 | 3.7 |
CORRELATED EVOLUTION BETWEEN CONJUGATION AND HEAD SHAPE
We used rj-MCMC to test whether the rates of (i) gains or losses in conjugation and (ii) changes in head shape differed depending on the character state of the other trait. Sperm heads were classified as elongate (approximately the same width as the flagellum) or broad (substantively wider than the flagellum). Conjugation was characterized as present or absent. We found very strong support for correlated evolution between head shape and presence of conjugation (Bayes Factor > 13). Ancestral state reconstructions support the presence of conjugation and broad heads in the ancestor of diving beetles (Bayes Factor > 5). Examination of the transition rates indicates that evolution away from the ancestral state is likely to occur either as the loss of conjugation or the elongation of the sperm heads (Figure 3; Table 2). However, elongation of sperm heads was unlikely to result in a subsequent loss of conjugation. Interestingly, broad-head sperm in the absence of conjugation is very uncommon, with only a single observation of this character state combination among all 141 species examined. The rarity of this combination of characters is reflected in very high transition rates away from this condition (see Figure 2 lower left panel; Table S1).
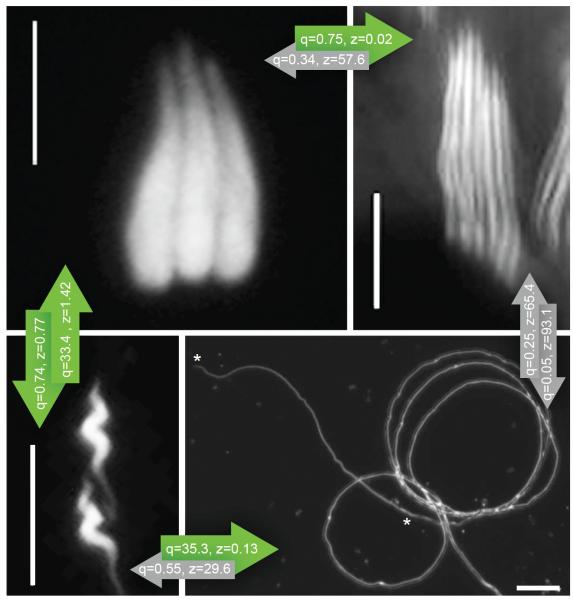
Figure 3. Evolutionary transitions in sperm head shape and conjugation.
Transition rates (q = changes per unit branch length) that are rarely assigned to zero (z% < 5 of models of trait evolution) are considered probable events (shown in green; unlikely transitions in grey). The upper left panel represents the ancestral state of broad heads and conjugation (illustrated by Rhantus consimilis). Transitions away from the ancestral state through a change in head shape or loss of conjugation occur at similar rates. The upper right panel is an example of the co-occurrence of elongate heads and conjugation (Pachydrus sp.). Single sperm may have broad (lower left; Porrhydrus sp.) or elongate heads (lower right; Desmopachria convexa). Transition rates away from broad heads in the absence of conjugation are very high, suggesting that this is an evolutionarily unstable state. Upper panels and lower left: fluorescent images of DNA-stained heads, no flagella visible. Lower right panel: darkfield image, head not visually distinguishable from flagellum. Scale bars = 10 μm.
Table 2. Mean transition rate coefficients of correlated evolution between conjugation and sperm head shape.
Despite the gain of conjugation in the presence of broad heads or the loss of broad heads in the presences of single sperm rarely being removed from the models of evolution (i.e., Z < 5%) the rj-MCMC chain gave a broad range of transition rate values (indicated by large standard deviations). Maximum likelihood estimation of these rate parameters (mean ± SD: gain of conjugation = 14.68 ± 4.82, loss of broad heads = 28.78 ± 4.71) results in more precise value, but the means are of similar magnitude as those produced by rj-MCMC.
| Evolutionary transition | mean rate | SD | Z% |
|---|---|---|---|
| gain of broad heads in presence of single sperm | 0.56 | 0.81 | 29.0 |
| gain of conjugation in the presence of elongate heads | 0.26 | 0.38 | 64.5 |
| gain of conjugation in presence of broad heads | 34.79 | 28.29 | 1.9 |
| gain of broad heads in presence of conjugation | 0.30 | 0.38 | 59.0 |
| loss of broad heads in presence of single sperm | 36.78 | 27.62 | 0.4 |
| loss of conjugation in presence of elongate heads | 0.06 | 0.21 | 92.3 |
| loss of conjugation in presence of broad heads | 0.74 | 0.22 | 2.0 |
| loss of broad heads in presence of conjugation | 0.77 | 0.20 | <0.1 |
RELATIONSHIP BETWEEN CONJUGATION AND OTHER SPERM TRAITS
To test for correlated evolution between sperm length and conjugation, we classified sperm as long or short based on the bimodal distribution of the sperm length data (Figure 4; Table S1) and determined if transition rates from long to short sperm were different depending on whether conjugation was present or vice versa. We found that the dependent and independent models of trait evolution fit the data equally well (i.e., three runs of each model type resulted in overlapping harmonic means). Given motility-related functional relationships between conjugated sperm (see Movies S1 – 3; Lighthill 1976; Moore and Taggart 1995; Moore et al. 2002; Immler et al. 2007; Higginson and Pitnick 2011), the lack of significant correlated evolution between total sperm length and conjugation was surprising. It is possible that our simplistic categorization of sperm as long or short may obscure more subtle relationships. On the other hand, results of recent analyses of co-evolution of sperm and female reproductive tract morphology in diving beetles suggest that conjugation is selectively advantageous because it enhances the probability of occupying a location favorable for fertilization rather than due to any motility advantage (Higginson et al. submitted).
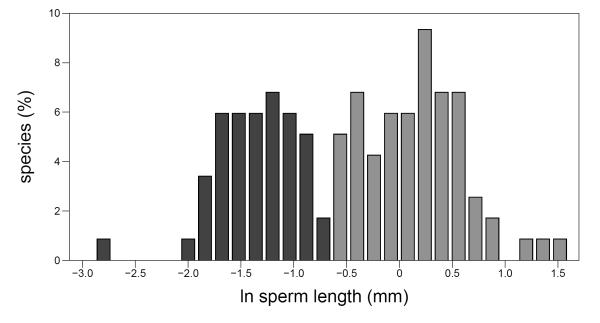
Figure 4. Distribution of sperm length.
Dark grey bars indicate mean species sperm length that was categorized as short (<−0.65. Light grey bars indicate long sperm (≥ −0.65).
Ancestral state reconstruction supports a minimum of seven origins of sperm heteromorphism within Dytiscidae; five of these origins are co-occurrences with sperm conjugation (i.e., heterospermatozeugmata; e.g., 1A, E – F). We compared the harmonic means of our model of evolution when sperm heteromorphism was constrained to have a single origin (i.e., heteromorphism present in the MRCA to sperm heteromorphic species) or permitted to evolve multiple times (i.e., the MRCA had a single sperm morph) and found that multiple origins of heteromorphism was strongly supported (Bayes Factor > 5). To test for correlated evolution between heteromorphism and conjugation, we compared the likelihood of competing models of evolution (i.e., independent or correlated) for conjugation and heteromorphism and found that independent evolution of heteromorphism and conjugation was slightly favored (Bayes Factor > 1).
Discussion
Despite long standing recognition that sperm vary not only in total length but also in the presence and organization of their constituent parts (acrosome, nucleus, mitochondria and flagellum, Pitnick et al. 2009a), we have only limited insight into the evolution of sperm characters. Particularly poorly understood is how sperm morphology can transition between discrete, alternative character states and how selection on one aspect of sperm morphology may result in correlated changes in other sperm traits. Our study of sperm evolution in diving beetles revealed that sperm morphology readily switches between three discrete forms of conjugation (aggregates, pairs and rouleaux), although not all transitions are equally likely. Sperm conjugation was found to be the ancestral condition in diving beetles, which has subsequently been lost (i.e., reverting to single sperm) at multiple times throughout the lineage. Additionally, we found that head shape, but not heteromorphism or sperm length, showed correlated evolution with conjugation. Nonetheless, the frequent co-occurrence of heteromorphism and conjugation has given rise to some of the most diverse and extravagant sperm forms observed in nature. These heterospermatozeugmata all exhibit uniquely organized structures (Figure 1A, E – F) that appear to dramatically alter the manner of sperm movement (Movies S1 – 3).
Sperm head shape was found to evolve in a significantly correlated manner with conjugation, as predicted, given that conjugation involves the conjoining of sperm heads in a manner that must accommodate functionality of the resulting multiflagellated sperm unit (Higginson and Pitnick 2011). Consistent with this result, our analysis of evolutionary transition rates between different forms of conjugation revealed non-random evolutionary trajectories (Figure 2; Table 1). Specifically, paired and rouleaux conjugates, which require different mechanisms for precise head alignment and binding (Figure 1B,D), appear never to derive from one another or from single sperm, but rather only from the aggregate condition, where variable numbers of sperm conjugate with their heads less precisely aligned. Heterochronic evolution of the timing of conjugate formation provides one possible explanation of the observed transitions between aggregates and rouleaux. Within-species comparisons of conjugates collected from males and from the sperm-storage organs of females revealed that the conjugates of some species undergo morphological transformation during or after transfer to females. For example, the sperm of Rhantus spp. (Colymbetini) form typical aggregates, with the heads aligned, within the seminal vesicles of males, but after transfer to females the conjugates elongate and appear reminiscent of rouleaux (Figure 1E). Changes in the timing of ontogenetic processes leading to conjugation might thus give rise to the different forms of conjugation, with the post-ejaculation transformations observed in Rhantus occurring within the seminal vesicles of rouleaux-producing species.
We found strong support for multiple origins of both sperm pairing and heteromorphism, but only a single origin of rouleaux formation among diving beetles (with a recurrence event within Hygrotini). There were also two independent recurrences of the aggregate sperm conjugate state within the clade of rouleaux-producing species. Convergence and recurrence implicate similar selective environments and/or evolution via regulatory changes in developmental pathways (West-Eberhard 2003). Notably, convergence of sperm form is also seen among related families of beetles. Conjugation is found in whirligig beetles (Gyrinidae), crawling water beetles (Haliplidae), burrowing water beetles (Noteridae) and the more distantly related ground beetles (Carabidae) (Higginson and Pitnick 2011). Sperm morphology of burrowing water beetles has scarcely been examined, but both sperm pairing and rouleaux have been found (D.M.H personal observations). With the exception of ground beetles, female reproductive tracts in these families characteristically form conduits, with sperm entering and exiting storage through separate ducts (as opposed to cul-de-sac type reproductive tracts where sperm enter and exit through a common duct, Miller 2001). Within Dytiscidae, dimensions of the female sperm-storage organ and exit duct are correlated with the presence of conjugation, and evidence suggests that changes in female morphology have driven the evolution of numerous aspects of sperm form (Higginson et al. submitted). Similar selective environments experienced by sperm in these families might explain the repeated evolution of such an unusual variation in sperm morphology as conjugation (Higginson et al. submitted).
The pattern of rapid but homoplastic evolution observed in diving beetle sperm appears to be generally characteristic of sexually selected traits. The few studies examining diversification of traits used in competition for and selection of mates have revealed trait distributions characterized by convergence, recurrence and loss. For example, in Onthoghagus dung beetles, horns used as weapons in male competition are highly labile with a minimum of 25 gains and losses of five discrete horn types (Emlen et al. 2005). Similarly, carotenoid-based plumage displays have evolved independently and been lost numerous times in birds including orioles (Omland and Lanyon 2000), widowbirds and bishops (Prager and Andersson 2010). Other sexual signals also appear to diverge rapidly but follow convergent evolutionary trajectories (e.g., swords in Xiphophorus fishes, (Meyer et al. 1994; Schulter et al. 1997), courtship behavior in sepsid flies (Puniamoorthy et al. 2009), and song in green lacewings (Henry et al. 1999) and orioles (Price et al. 2007)). Repeated loss of a sexually selected trait could result from ecological selection overwhelming that of female choice or a reduction in the strength of the female preference, perhaps through switching of the preference to an alternative male trait (Wiens 2001). Widespread convergence, however, suggests that whereas male traits or female preference can evolve rapidly, they are constrained or otherwise biased to evolve along particular axes of variation.
In the present study and most previous investigations of the evolution of sperm form (reviewed by, e.g., Keller and Reeve 1995; Pizzari and Birkhead 2002; Snook 2005; Pitnick et al. 2009a, 2009b; Pizzari and Parker 2009) there is an underlying assumption that postcopulatory sexual selection is the principal agent of diversification. In the case of diving beetles, the demonstrated coevolution of sperm form and female reproductive tract design (Higginson et al. submitted) supports this contention. The present analyses of macroevolutionary patterns sets the stage for future microevolutionary investigations of the relationship between variation in sperm form and fitness within species exhibiting a diversity of sperm forms across this evolutionarily dynamic lineage.
Supplementary Material
Figure S1. Majority consensus tree.
Movie S1. Heterospermatozeugmata of Ilybius larsoni.
Movie S2. Heterospermatozeugmata of Ilybius sp.
Movie S3. Heterospermatozeugmata of Hygrotus sayi.
Table S1. Sperm traits species in Dytiscidae, Amphizoidae and Paelobiidae.
ACKNOWLEDGEMENTS
We are grateful to Ignacio Ribera for making available the remarkable set of DNA sequence data set for Dytiscidae and the IBEST Bioinformatics Core at the University of Idaho for providing the computing resources for phylogenetic tree inference (NIH/NCRR P20RR16448 and P20RR-16454). We thank S. Lüpold, M.K. Manier, W.T. Starmer, J.A.C. Uy and two anonymous reviewers for thoughtful critiques on an earlier version of this manuscript. This research was supported by the National Science Foundation (DDIG-0910049 to D.M.H. and S.P.; DEB-0743101 to K.A.S; DEB-0814732 and DEB-6990357 to S.P.) and the Natural Sciences and Engineering Research Council (PGS-D 331458 to D.M.H.).
LITERATURE CITED
- Aiken RB. The mating behaviour of a boreal water beetle, Dytiscus alaskanus (Coleopotera Dytiscidae) Ethol. Ecol. Evol. 1992;4:245–254. [Google Scholar]
- Aiken RB, Wilkinson W. Bionomics of Dytiscus alaskanus J. Balfour-Browne (Coleoptera: Dytiscidae) in a central Alberta lake. Canadian Journal of Zoology. 1985;63:1316–1323. [Google Scholar]
- Andersson M. Sexual selection. Princeton University Press; Princeton, NJ: 1994. [Google Scholar]
- Auerbach L. Remarkable behaviour of the spermatozoa of Dytiscus marginalis. J. R. Micro. Soc., Ser. 1893;2:13–622. [Google Scholar]
- Badyaev AV, Hill GE. Avian sexual dichromatism in relation to phylogeny and ecology. Annu. Rev. Ecol., Evol. Syst. 2003;34:27–49. [Google Scholar]
- Ballowitz E. Die dopplespermatozoen der Dyticiden. Zeitschrift für Wissenschaftliche Zoologie. 1895;60:458–499. [Google Scholar]
- Bergsten J, Miller KB. Phylogeny of diving beetles reveals a coevolutinary arms race between the sexes. PloS One. 2007;2:e522. doi: 10.1371/journal.pone.0000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkhead TR. Defining and demonstrating postcopulatory female choice - again. Evolution. 2000;54:1057–1060. doi: 10.1111/j.0014-3820.2000.tb00108.x. [DOI] [PubMed] [Google Scholar]
- Boughman JW, Rundle HD, Schluter D. Parallel evolution of sexual isolation in sticklebacks. Evolution. 2005;59:361–373. [PubMed] [Google Scholar]
- Breed WG. Evolution of the spermatozoon in muroid rodents. J. Morphol. 2005;265:271–290. doi: 10.1002/jmor.10357. [DOI] [PubMed] [Google Scholar]
- Cleavall LM. Biology. University of New Mexico; Alburquerque: 2009. Description of Thermonectus nigrofasciatus and Rhantus binotatus (Coleoptera: Dytiscidae) mating behavior; p. 34. [Google Scholar]
- Emlen DJ, Marangelo J, Ball B, Cunningham CW. Diversity in the weapons of sexual selection: horn evolution in the beetle genus Onthophagus (Coleoptera: Scarabaeidae) Evolution. 2005;59:1060–1084. [PubMed] [Google Scholar]
- Fitzpatrick JL, Montgomerie R, Desjardins JK, Stiver KA, Kolm N, Balshine S. Female promiscuity promotes the evolution of faster sperm in cichlid fishes. Proc. Natl. Acad. Sci. USA. 2009;106:1128–1132. doi: 10.1073/pnas.0809990106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage MJG. Associations between body size, mating patterm, testis size and sperm lengths across butterflies. Proc. R. Soc. Lond., Ser. B: Biol. Sci. 1994;258:247–254. [Google Scholar]
- Gomendio M, Roldan ERS. Sperm competition influences sperm size in mammals. Proc. R. Soc. Lond., Ser. B: Biol. Sci. 1991;243:181–185. doi: 10.1098/rspb.1991.0029. [DOI] [PubMed] [Google Scholar]
- Harcourt AH, Harvey PH, Larson DJ, Short RV. Testis weight, body weight and breeding system in primates. Nature. 1981;293:55–57. doi: 10.1038/293055a0. [DOI] [PubMed] [Google Scholar]
- Hayashi F. Sperm co-operation in the fishfly, Parachauliodes japonicus. Funct. Ecol. 1998;12:347–350. [Google Scholar]
- Henry CS, Wells MLM, Simon CM. Convergent evolution of courtship songs among cryptic species of the Carnea group of green lacewings (Neuroptera: Chrysopidae: Chrysoperla) Evolution. 1999;53:1165–1179. doi: 10.1111/j.1558-5646.1999.tb04530.x. [DOI] [PubMed] [Google Scholar]
- Higginson DM, Miller KB, Segraves KA, Pitnick S. Female reproductive tract form drives the evolution of complex sperm morphology. Proceedings of the National Academy of Science, USA. doi: 10.1073/pnas.1111474109. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginson DM, Pitnick S. Evolution of intra-ejaculate sperm interactions: do sperm cooperate? Biol. Rev. 2011;87:249–270. doi: 10.1111/j.1469-185X.2010.00147.x. [DOI] [PubMed] [Google Scholar]
- Immler S. Sperm competition and sperm cooperation: the potential role of diploid and haploid expression. Reproduction. 2008;135:275–283. doi: 10.1530/REP-07-0482. [DOI] [PubMed] [Google Scholar]
- Immler S, Moore HDM, Breed WG, Birkhead TR. By hook or by crook? Morphometry, competition and cooperation in rodent sperm. PloS One. 2007;2:e170. doi: 10.1371/journal.pone.0000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoda T. Mating and reproduction of predaceous diving beetles, Dytiscus sharpi, observed under artificial breeding conditions. Zool. Sci. 2003;20:377–382. doi: 10.2108/zsj.20.377. [DOI] [PubMed] [Google Scholar]
- Inoda T, Tajima F, Taniguchi H, Saeki M, Numakura K, Hasegawa M, Kamimura S. Temperature-dependent regulation of reproduction in the diving beetle Dytiscus sharpi (Coleoptera: Dytiscidae) Zool. Sci. 2007;24:1115–1121. doi: 10.2108/zsj.24.1115. [DOI] [PubMed] [Google Scholar]
- Jamieson BGM, Dallai R, Afzelius BA. Insects: Their Spermatozoa and Phylogeny. Science Publishers, Inc.; Enfield: 1999. [Google Scholar]
- Keller L, Reeve HK. Why do females mate with multiple males? The sexually selected sperm hypothesis. Adv. Stud. Behav. 1995;24:291–315. [Google Scholar]
- Kraaijeveld K, Kraaijeveld-Smit FJL, Maan ME. Sexual selection and speciation: the comparative evidence revisited. Biol. Rev. 2011;86:367–377. doi: 10.1111/j.1469-185X.2010.00150.x. [DOI] [PubMed] [Google Scholar]
- Landan G, Graur D. Heads or tails: a simple reliability check for multiple sequence alignments. Mol. Biol. Evol. 2007;24:1380–1383. doi: 10.1093/molbev/msm060. [DOI] [PubMed] [Google Scholar]
- Lighthill J. Flagellar hydrodynamics. SIAM Review. 1976;18:161–230. [Google Scholar]
- Löytynoja A, Goldman N. Phylogeny-aware gap placement prevents errors in sequence alignment and evolutionary analysis. Science. 2008;320:1632–1635. doi: 10.1126/science.1158395. [DOI] [PubMed] [Google Scholar]
- Meyer A, Morrissey JM, Schartl M. Recurrent origin of a sexually selected trait in Xiphophorus fishes inferred from a molecular phylogeny. Nature. 1994;368:539–542. doi: 10.1038/368539a0. [DOI] [PubMed] [Google Scholar]
- Miller GT, Pitnick S. Sperm-female coevolution in Drosophila. Science. 2002;298:1230–1233. doi: 10.1126/science.1076968. [DOI] [PubMed] [Google Scholar]
- Miller KB. On the phylogeny of the Dytiscidae (Insecta: Coleoptera) with emphasis on the morphology of the female reproductive system. Insect Syst. Evol. 2001;31:45–92. [Google Scholar]
- Miller KB. The phylogeny of diving beetles (Coleoptera: Dytiscidae) and the evolution of sexual conflict. Biol. J. Linn. Soc. 2003;79:359–388. [Google Scholar]
- Minin V, Abdo Z, Joyce P, Sullivan J. Performance-based selection of likelihood models for phylogeny estimation. Syst. Biol. 2003;52:674–683. doi: 10.1080/10635150390235494. [DOI] [PubMed] [Google Scholar]
- Møller AP, Briskie JV. Extra-pair paternity, sperm competition and the evolution of testis size in birds. Behav. Ecol. Sociobiol. 1995;36:357–365. [Google Scholar]
- Moore H, Dvořáková K, Jenkins N, Breed W. Exceptional sperm cooperation in the wood mouse. Nature. 2002;418:174–177. doi: 10.1038/nature00832. [DOI] [PubMed] [Google Scholar]
- Moore HDM, Taggart DA. Sperm pairing in the opossum increases the efficiency of sperm movement in a viscous environment. Biol. Reprod. 1995;52:947–953. doi: 10.1095/biolreprod52.4.947. [DOI] [PubMed] [Google Scholar]
- Nilsson AN. A new view on the generic classification of the Agabus-group of genera of the Agabini, aimed at solving the problem with a paraphyletic Agabus (Coleoptera: Dytiscidae) Koleopterol. Rundsch. 2000;70:17–36. [Google Scholar]
- Nilsson AN. World Catalogue of Insects. Vol. 3. Apollo Books Aps.; Stenstrup: 2001. Dytiscidae. [Google Scholar]
- Nilsson AN, Fery H. World catalogue of Dytiscidae - corrections and additions, 3. Koleopterol. Rundsch. 2006;76:55–74. [Google Scholar]
- Nylander JAA, Wilgenbusch JC, Warren DL, Swofford DL. AWTY (are we there yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics. 2008;24:581–583. doi: 10.1093/bioinformatics/btm388. [DOI] [PubMed] [Google Scholar]
- Omland KE, Lanyon SM. Reconstructing plumage evolution in Orioles (Icterus): repeated convergence and reversal in patterns. Evolution. 2000;54:2119–2133. doi: 10.1111/j.0014-3820.2000.tb01254.x. [DOI] [PubMed] [Google Scholar]
- Pagel M. Discrete. version 4.0 2000. [Google Scholar]
- Pagel M. Multistate. 2002.
- Pagel M, Meade A. Bayesian analysis of correlated evolution of discrete characters by reversible-jump Markov chain monte carlo. Am. Nat. 2006;167:808–825. doi: 10.1086/503444. [DOI] [PubMed] [Google Scholar]
- Pagel M, Meade A. BayesTraits. 2007.
- Pagel M, Meade A, Barker D. Bayesian estimation of ancestral character states on phylogenies. Syst. Biol. 2004;53:673–684. doi: 10.1080/10635150490522232. [DOI] [PubMed] [Google Scholar]
- Pattarini JM, Starmer WT, Bjork A, Pitnick S. Mechanisms underlying the sperm quality advantage in Drosophila melanogaster. Evolution. 2006;60:2064–2080. [PubMed] [Google Scholar]
- Pitnick S. Investment in testes and the cost of making long sperm in Drosophila. Am. Nat. 1996;148:57–90. [Google Scholar]
- Pitnick S, Brown WD. Criteria for demonstrating female sperm choice. Evolution. 2000;54:1052–1056. doi: 10.1111/j.0014-3820.2000.tb00107.x. [DOI] [PubMed] [Google Scholar]
- Pitnick S, Markow TA, Spicer GS. Delayed male maturity is a cost of producing large sperm in Drosophila. Proc. Natl. Acad. Sci. USA. 1995;92:10614–10618. doi: 10.1073/pnas.92.23.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitnick S, Hosken DJ, Birkhead TR. Sperm Morphological Diversity. In: Birkhead TR, Hosken DJ, Pitnick S, editors. Sperm Biology, an Evolutionary Perspective. Academic Press; San Diego: 2009a. pp. 69–149. [Google Scholar]
- Pitnick S, Wolfner MF, Suarez SS. Ejaculate-female and Sperm-Female Interactions. In: Birkhead TR, Hosken DJ, Pitnick S, editors. Sperm Biology, an Evolutionary Perspective. Academic Press; San Diego: 2009b. pp. 247–304. [Google Scholar]
- Pizzari T, Birkhead TR. The sexually-selected sperm hypothesis: sex-biased inheritance and sexual antagonism. Biol. Rev. 2002;77:183–209. doi: 10.1017/s1464793101005863. [DOI] [PubMed] [Google Scholar]
- Pizzari T, Foster KR. Sperm sociality: Cooperation, altruism, and spite. PLoS Biol. 2008;6:e130. doi: 10.1371/journal.pbio.0060130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzari T, Parker GA. Sperm competition and sperm phenotype. In: Birkhead TR, Hosken DJ, Pitnick S, editors. Sperm Biology, an Evolutionary Perspective. Elsevier; London: 2009. pp. 205–244. [Google Scholar]
- Prager M, Andersson S. Convergent evolution of red carotenoid coloration in widowbirds and bishops (Euplectes spp.) Evolution. 2010;64:3609–3619. doi: 10.1111/j.1558-5646.2010.01081.x. [DOI] [PubMed] [Google Scholar]
- Price JJ, Friedman NR, Omland KE. Song and plumage evolution in the new world orioles (Icterus) show similar lability and convergence in patterns. Evolution. 2007;61:850–863. doi: 10.1111/j.1558-5646.2007.00082.x. [DOI] [PubMed] [Google Scholar]
- Prum RO. Phylogenetic tests of alternative intersexual selection mechanisms: trait macroevolution in a polygynous clade (Aves: Pipridae) Am. Nat. 1997;149:668–692. [Google Scholar]
- Prum RO. The Lande-Kirkpatrick mechanism is the null model of evolution by intersexual selection: implications for the meaning, honesty, and design in intersexual signals. Evolution. 2010;64:3085–3100. doi: 10.1111/j.1558-5646.2010.01054.x. [DOI] [PubMed] [Google Scholar]
- Puniamoorthy N, Ismail MRB, Tan DSH, Meier R. From kissing to belly stridulation: comparative analysis reveals surprising diversity, rapid evolution, and much homoplasy in the mating behaviour of 27 species of sepsid flies (Diptera: Sepsidae) J. Evol. Biol. 2009;22:2146–2156. doi: 10.1111/j.1420-9101.2009.01826.x. [DOI] [PubMed] [Google Scholar]
- Rasband WS. ImageJ. U.S. National Institutes of Health; Bethesda, Maryland, USA: 1997 - 2008. [Google Scholar]
- Ribera I, Vogler AP, Balke M. Phylogeny and diversification of diving beetles (Coleoptera: Dytiscidae) Cladistics. 2008;23:1–28. doi: 10.1111/j.1096-0031.2007.00192.x. [DOI] [PubMed] [Google Scholar]
- Roldan ERS, Gomendio M, Vitullo AD. The evolution of eutherian spermatozoa and underlying selective forces: female selection and sperm competition. Biol. Rev. 1992;67:551–593. doi: 10.1111/j.1469-185x.1992.tb01193.x. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Schärer L, Littlewood DTJ, Waeschenbach A, Yoshida W, Vizoso DB. Mating behavior and the evolution of sperm design. Proc. Natl. Acad. Sci. USA. 2011;108:1490–1495. doi: 10.1073/pnas.1013892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulter D, Price T, Mooers AØ, Ludwig D. Likelihood of ancestor states in adaptive radiation. Evolution. 1997;51:1699–1711. doi: 10.1111/j.1558-5646.1997.tb05095.x. [DOI] [PubMed] [Google Scholar]
- Schärer L, Joss G, Sandner P. Mating behaviour of the marine turbellarian Macrostomum sp.: these worms suck. Mar. Biol. 2004;145:373–380. [Google Scholar]
- Simmons LW. Sperm competition and its evolutionary consequences in the insects. Princeton University Press; Princeton: 2001. [Google Scholar]
- Smith RL. Aspects of the biology of three species of the genus Rhantus (Coleoptera: Dytiscidae) with special reference to the acoustical behavior of two. Can. Entomol. 1973;105:909–919. [Google Scholar]
- Snook RR. Sperm in competition: not playing by the numbers. Trends Ecol. Evol. 2005;20:46–53. doi: 10.1016/j.tree.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Stockley P, Gage MJG, Parker GA, Møller AP. Sperm competition in fishes: the evolution of testis size and ejaculate characteristics. Am. Nat. 1997;149:933–954. doi: 10.1086/286031. [DOI] [PubMed] [Google Scholar]
- Swofford DL. PAUP*. Phylogenetic analysis using parsimony (*and other methods) Version 4 Sinauer Associates; Sunderland, Massachusetts: 2002. [Google Scholar]
- West-Eberhard MJ. Developmental Plasticity and Evolution. Oxford University Press; New York: 2003. [Google Scholar]
- Wiens JJ. Widespread loss of sexually selected traits: how the peacock lost its spots. Trends Ecol. Evol. 2001;16:517–523. [Google Scholar]
- Woolley DM, Crockett RF, Groom WDI, Revell SG. A study of synchronisation between the flagella of bull spermatozoa, with related observations. J. Exp. Biol. 2009;212:2215–2223. doi: 10.1242/jeb.028266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Majority consensus tree.
Movie S1. Heterospermatozeugmata of Ilybius larsoni.
Movie S2. Heterospermatozeugmata of Ilybius sp.
Movie S3. Heterospermatozeugmata of Hygrotus sayi.
Table S1. Sperm traits species in Dytiscidae, Amphizoidae and Paelobiidae.