Abstract
There is a paucity of data on basal-cell carcinoma (BCC) in the United States, since most national registries do not collect information on BCC. We evaluated BCC incidence trends and associated risk factors for BCC in 140,171 participants from a US female cohort, the Nurses' Health Study (1986–2006), and a US male cohort, the Health Professionals' Follow-up Study (1988–2006). Age-adjusted BCC incidence rates increased from 519 cases per 100,000 person-years to 1,019 cases per 100,000 person years for women and increased from 606 cases per 100,000 person-years to 1,488 cases per 100,000 person-years for men during the follow-up period. Cox proportional hazards analysis identified the following phenotypic risk factors for BCC in both cohorts: family history of melanoma, blond or red hair colors, higher number of extremity moles, higher susceptibility to sunburn as a child/adolescent, and higher lifetime number of severe/blistering sunburns. The multivariate-adjusted risk ratio for the highest quintile of cumulative midrange ultraviolet B flux exposure versus the lowest quintile was 3.18 (95% confidence interval: 2.70, 3.76) in women and 1.90 (95% confidence interval: 1.57, 2.29) in men. BCC incidence was generally higher in men than in women, and BCC risk was strongly associated with several phenotypic and exposure factors, including midrange ultraviolet B radiation, in our study populations.
Keywords: basal-cell carcinoma, incidence, skin cancer, ultraviolet radiation
Basal-cell carcinoma (BCC) is the major histological type of nonmelanoma skin cancer and is the most common malignancy in fair-skinned populations all over the world. BCC incidence is expected to increase because of aging of the population and increasing exposure to solar ultraviolet (UV) radiation due to depletion of the ozone layer (1). Despite rising health-care costs (2), few population-based cancer registries monitor and report the incidence of BCC, and no active nationwide surveillance system exists for BCC in the United States.
In a previous study, Miller and Weinstock (3) estimated that there were 453,000–562,000 incident BCC cases among US men and 301,000–367,000 BCC cases among US women in 1994. However, other estimates of the trends in BCC incidence in the US population have been limited to specific states or regions. An early study in British Columbia, Canada, showed steadily increasing trends in BCC incidence rates from 1973 to 1987 in both men and women (4). The age-adjusted BCC incidence rates were 70.7 cases per 100,000 person-years for men and 61.5 cases per 100,000 person-years for women in 1973, and rates were 120.4 cases per 100,000 person-years for men and 92.2 cases per 100,000 person-years for women in 1987 (4). In a study in New Hampshire, Karagas et al. (5) estimated that BCC incidence rates increased by 80% in both men and women from 1979 to 1993 and that incidence rates in 1993 were 310 cases per 100,000 person-years for men and 166 cases per 100,000 person-years for women. A later report based on the Southeastern Arizona Skin Cancer Registry also demonstrated higher BCC incidence rates in 1996 than in 1985 (6). Recently, in a population-based descriptive analysis, Rogers et al. (7) estimated a 4.2% annual average increase in nonmelanoma skin cancer cases in the Medicare population from 1992 to 2006. However, the epidemiology of BCC in the United States is still poorly understood, because there is no systematic surveillance for BCC nationwide.
Understanding BCC incidence and associated risk factors is important for planning of prevention strategies and allocation of resources for management and treatment. In the present analysis, we investigated trends in BCC incidence over a span of 20 years and the associations between incident BCC and risk factors in a total population of 140,171 participants from 2 large US-based cohort studies: women in the Nurses' Health Study (NHS; 1986–2006) and men in the Health Professionals' Follow-up Study (HPFS; 1988–2006).
MATERIALS AND METHODS
Study population
The study population consisted of participants from 2 ongoing longitudinal cohort studies, the NHS and the HPFS. The NHS was established in 1976 when 121,701 married female registered nurses aged 30–55 years and residing in the United States at the time of enrollment responded to a baseline questionnaire that included questions about their medical history and lifestyle risk factors. The HPFS consisted of 51,529 male health professionals who were aged 40–75 years and completed a baseline questionnaire in 1986. Information on medical history and lifestyle factors was collected biennially via mailed questionnaires in the 2 cohorts. A response rate exceeding 90% had been achieved in each follow-up period. The present study was approved by the institutional review board of Brigham and Women's Hospital. We considered the participants' completion and return of the self-administered questionnaires to imply informed consent.
Identification of BCC
The biennial questionnaires mailed to all study participants included a question on diagnosis of BCC during the previous 2 years. Although medical records were not obtained for self-reported BCC, previous reports have demonstrated high validity of self-reported BCC in the 2 cohorts, with over 90% of diagnoses confirmed in women and over 80% confirmed in men through histopathological findings or medical records (8–10). Participants who reported having had any baseline cancer were excluded. In the present analysis, we included 15,673 women from the NHS and 8,270 men from the HFPS with self-reported BCC.
Assessment of risk factors
Data on the following phenotypic and exposure characteristics were collected through questionnaires in the 2 cohorts: 1) family history of melanoma in first-degree relatives (parents and siblings), 2) natural hair color (red, blond, light brown, dark brown, or black) at a younger age (age 21 years for women and age 18 years for men), 3) number of moles with a diameter of ≥3 mm on a designated extremity (none, 1–2, 3–5, or ≥6), 4) skin reaction to sun exposure for 2 hours or more (1 hour or more for men) as a child/adolescent (“no burn or some redness,” “burn,” or “painful or blistering burn”), 5) lifetime number of severe or blistering sunburns (none, 1–2, 3–5, or ≥6), and 6) cumulative exposure to midrange ultraviolet B (UV-B) radiation (UV-B flux, classified as quintiles in each cohort). Responses for each risk factor were summarized into several representative categories across the 2 cohorts to facilitate convenient comparisons. For mole count on an extremity, the left arm from shoulder to wrist was used for women and the bilateral forearms from elbow to wrist were used for men. Annual UV-B flux is a composite measure of midrange UV radiation level based on latitude, altitude, and cloud cover (11) and was estimated according to state of residence for every 2-year period since 1986 for women and since 1988 for men. Current residence was known from the mailing addresses of participants throughout cohort follow-up, and annual UV-B flux was measured in Robertson-Berger units (12). A Robertson-Berger meter count of 440 may produce a typical sunburn reaction in untanned Caucasian skin. In energy values, this amount of biologically effective radiation (relative to 297 nm) is referred to as the minimal erythema dose and is equivalent to approximately 25–35 mJ/cm2 (13).
Statistical analysis
Updated annual UV-B flux exposure data for each questionnaire cycle were available from 1986 in women and from 1988 in men. Therefore, we chose 1986 for the NHS and 1988 for the HPFS as the starting years of follow-up. Participants were restricted to women or men who had no baseline history of any cancer, and they contributed person-time from the date of baseline questionnaire return to the date of the first report of BCC, death, or the end of follow-up (2006), whichever came first. To compare BCC incidence rates between women and men, we calculated age-adjusted incidence rates among participants aged 40–50 years at the first year of data collection in each cohort. The age-adjusted incidence rates were determined by calculating age-specific incidence rates within 1-year age categories and were weighted by the age distribution of the population for each 2-year period.
Cox proportional hazards models stratified by age and follow-up period were used to estimate the age-adjusted and multivariate-adjusted relative risks of BCC and their 95% confidence intervals. Multivariate analyses were conducted after adjusting for age, natural hair color, family history of melanoma, number of moles on an extremity, susceptibility to sunburn as a child/adolescent, lifetime number of severe/blistering sunburns, and cumulative UV-B flux exposure. Risk factors were included as dichotomous or categorical variables, except age, which was included as a continuous variable, and trend tests were carried out using median values from different categories, except cumulative UV-B flux, which was a continuous variable. Cumulative UV-B flux exposure was categorized in quintiles to examine the risk of BCC associated with midrange UV radiation in each cohort. Categories with the lowest perceived risk of BCC were used as the referents. Light brown was used as the referent category for hair color, since it was the most common hair color. For pooled analysis of data from the 2 cohorts, we tested the between-study heterogeneity and estimated the overall association from the random-effects model (weighted proportionately to the inverse of the sum of the study-specific variance plus the common between-study variance) and the fixed-effects model (weighted proportionately to the inverse of the study-specific variance) (14).
All statistical analyses were performed using SAS software, version 9.2 (SAS Institute Inc., Cary, North Carolina). All statistical tests were 2-tailed, and the significance level was set at P < 0.05.
RESULTS
We included 95,743 female nurses from the NHS and 44,428 male health professionals from the HPFS in the analysis. During follow-up, 15,673 women and 8,270 men were diagnosed with BCC.
Descriptive statistics for persons who were diagnosed with BCC during follow-up are shown in Table 1. The mean ages at diagnosis of BCC were similar among women and men (65 years and 67 years, respectively). Female BCC cases had higher proportions of a family history of melanoma (11%) and natural red hair (6%), whereas male BCC cases had higher proportions of painful or blistering reaction to sun exposure as a child/adolescent (30%) and a history of ≥6 sunburns (39%). There were 28% and 26% of BCC cases in the highest quintile of cumulative UV-B flux exposure among women and men, respectively.
Table 1.
Characteristics of Women and Men With Basal-Cell Carcinoma in the Nurses' Health Study (1986–2006; n = 95,743) and the Health Professionals' Follow-up Study (1988–2006; n = 44,428)
| NHS (1986–2006) (n = 15,673) |
HPFS (1988–2006) (n = 8,270) |
|
|---|---|---|
| Mean age at diagnosis, years | 65 (8)a | 67 (10) |
| Family history of melanoma, % | 11 | 5 |
| Natural hair color, % | ||
| Red | 6 | 3 |
| Blond | 14 | 13 |
| ≥6 moles on extremity, % | 6 | 6 |
| Painful or blistering reaction to sun exposure as a child/ adolescent, % | 18 | 30 |
| History of ≥6 severe/blistering sunburns, % | 10 | 39 |
| Quintile of cumulative UV-B flux exposure, % | ||
| 1 | 15 | 14 |
| 2 | 16 | 16 |
| 3 | 18 | 21 |
| 4 | 23 | 24 |
| 5 | 28 | 26 |
Abbreviations: HPFS, Health Professionals' Follow-up Study; NHS, Nurses' Health Study; UV-B, ultraviolet B.
a Numbers in parentheses, standard deviation.
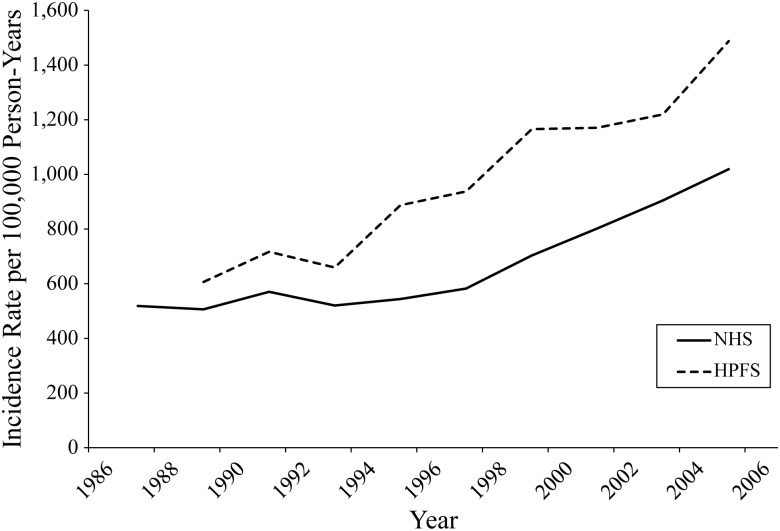
Figure 1 shows the age-adjusted BCC incidence rates for women and men aged 40–50 years at baseline. BCC incidence rates increased with advancing age, and the incidence rates in men were generally higher than those in women. Specifically, the age-adjusted BCC incidence rates increased from 519 cases per 100,000 person-years in the first 2-year period (1986–1988) to 1,019 cases per 100,000 person-years in the last 2-year period (2004–2006) among women and increased from 606 cases per 100,000 person-years in the first 2-year period (1988–1990) to 1,488 cases per 100,000 person-years in the last 2-year period (2004–2006) among men.
Figure 1.
Age-adjusted incidence rates for basal-cell carcinoma among participants aged 40–50 years in the baseline year of cohort follow-up, Nurses' Health Study (NHS; 1986–2006) and Health Professionals Follow-up Study (HPFS; 1988–2006).
Table 2 shows the associations between BCC risk and phenotypic and exposure variables. Having a family history of melanoma or a history of painful or blistering burn was associated with a higher BCC risk in both women and men. Red and blond hair colors were associated with higher BCC risk when compared with light brown hair color in women but not in men. In contrast, dark brown and black hair colors were associated with lower BCC risk when compared with light brown hair color in both women and men. The trends in BCC risk associated with hair color were significant in both cohorts (Ptrend < 0.0001). Both extremity moles and severe/blistering sunburns were significantly associated with BCC risk in women and men, and there were trends toward increased BCC risk with a greater number of extremity moles and lifetime number of severe/blistering sunburns in the 2 cohorts (Ptrend < 0.0001).
Table 2.
Risk of Basal-Cell Carcinoma According to Selected Risk Factors in Age- and Multivariate-adjusted Analyses, Nurses' Health Study (1986–2006) and Health Professionals' Follow-up Study (1988–2006)
| Nurses' Health Study |
Health Professionals' Follow-up Study |
Pooled Analysis |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Cases | Person-Years | RR1a | 95% CI | RR2b | 95% CI | No. of Cases | Person-Years | RR1a | 95% CI | RR2b | 95% CI | RR2b | 95% CI | P for Heterogeneityc | |
| Family history of melanoma | |||||||||||||||
| No | 14,023 | 1,538,273 | 1.00 | Referent | 1.00 | Referent | 7,830 | 626,925 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |
| Yes | 1,650 | 132,215 | 1.39 | 1.32, 1.46 | 1.32 | 1.26, 1.39 | 440 | 27,466 | 1.31 | 1.19, 1.45 | 1.25 | 1.14, 1.38 | 1.31 | 1.25, 1.37 | |
| P value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.34 | |||||||||
| Hair color | |||||||||||||||
| Red | 836 | 54,505 | 1.52 | 1.41, 1.63 | 1.30 | 1.20, 1.40 | 228 | 13,356 | 1.17 | 1.02, 1.34 | 1.05 | 0.91, 1.20 | 1.17 | 0.95, 1.45 | |
| Blond | 1,925 | 157,659 | 1.19 | 1.13, 1.25 | 1.13 | 1.07, 1.19 | 873 | 56,137 | 1.09 | 1.01, 1.18 | 1.05 | 0.97, 1.13 | 1.09 | 1.02, 1.18 | |
| Light brown | 5,488 | 548,261 | 1.00 | Referent | 1.00 | Referent | 2,385 | 170,277 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |
| Dark brown | 5,243 | 616,559 | 0.86 | 0.83, 0.89 | 0.90 | 0.86, 0.93 | 2,662 | 222,992 | 0.85 | 0.80, 0.90 | 0.89 | 0.84, 0.94 | 0.89 | 0.87, 0.92 | |
| Black | 331 | 44,295 | 0.70 | 0.62, 0.78 | 0.74 | 0.66, 0.83 | 597 | 48,578 | 0.83 | 0.76, 0.91 | 0.91 | 0.83, 1.00 | 0.83 | 0.67, 1.01 | |
| Ptrend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.001 | |||||||||
| No. of moles on extremity | |||||||||||||||
| 0 | 7,319 | 787,881 | 1.00 | Referent | 1.00 | Referent | 3,710 | 292,423 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |
| 1–2 | 3,180 | 299,303 | 1.18 | 1.13, 1.23 | 1.16 | 1.11, 1.21 | 1,196 | 85,071 | 1.11 | 1.04, 1.18 | 1.10 | 1.03, 1.17 | 1.13 | 1.08, 1.20 | |
| 3–5 | 1,146 | 97,628 | 1.30 | 1.22, 1.38 | 1.26 | 1.19, 1.35 | 498 | 32,513 | 1.18 | 1.07, 1.30 | 1.15 | 1.05, 1.26 | 1.21 | 1.11, 1.33 | |
| ≥6 | 704 | 55,420 | 1.44 | 1.33, 1.55 | 1.37 | 1.27, 1.49 | 344 | 22,955 | 1.15 | 1.03, 1.29 | 1.11 | 0.99, 1.23 | 1.24 | 1.00, 1.53 | |
| Ptrend | <0.0001 | <0.0001 | <0.0001 | 0.0008 | <0.0001 | 0.001 | |||||||||
| Susceptibility to sunburn as a child/adolescent | |||||||||||||||
| No burn/some redness | 7,742 | 915,653 | 1.00 | Referent | 1.00 | Referent | 1,633 | 160,724 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |
| Burn | 3,554 | 309,363 | 1.36 | 1.31, 1.42 | 1.22 | 1.17, 1.28 | 3,316 | 240,393 | 1.37 | 1.29, 1.45 | 1.31 | 1.23, 1.39 | 1.26 | 1.18, 1.35 | |
| Painful/blistering burn | 2,530 | 197,355 | 1.54 | 1.47, 1.61 | 1.27 | 1.21, 1.33 | 2,081 | 130,137 | 1.57 | 1.47, 1.68 | 1.44 | 1.34, 1.54 | 1.35 | 1.19, 1.52 | |
| Ptrend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.02 | |||||||||
| Lifetime no. of severe/ blistering sunburns | |||||||||||||||
| 0 | 7,136 | 829,350 | 1.00 | Referent | 1.00 | Referent | 941 | 85,730 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |
| 1–2 | 2,831 | 277,184 | 1.29 | 1.24, 1.35 | 1.22 | 1.17, 1.28 | 1,625 | 130,054 | 1.13 | 1.05, 1.23 | 1.08 | 1.00, 1.18 | 1.16 | 1.03, 1.30 | |
| 3–5 | 1,296 | 105,991 | 1.59 | 1.50, 1.69 | 1.44 | 1.36, 1.53 | 1,751 | 128,234 | 1.25 | 1.15, 1.35 | 1.14 | 1.05, 1.23 | 1.28 | 1.02, 1.62 | |
| ≥6 | 1,293 | 91,698 | 1.83 | 1.72, 1.94 | 1.55 | 1.46, 1.65 | 2,751 | 188,876 | 1.36 | 1.26, 1.46 | 1.16 | 1.07, 1.25 | 1.34 | 1.01, 1.78 | |
| Ptrend | <0.0001 | <0.0001 | <0.0001 | 0.0001 | <0.0001 | <0.0001 | |||||||||
Abbreviations: CI, confidence interval; RR, relative risk.
a Adjusted for age.
b Multivariate analysis controlled for age, family history of melanoma, natural hair color, number of moles on an extremity, susceptibility to sunburn as a child/adolescent, lifetime number of severe/blistering sunburns, and cumulative midrange ultraviolet B flux exposure.
c P value from a test of heterogeneity between studies.
Table 3 shows the associations between cumulative UV-B flux exposure and BCC risk. Increasing cumulative UV-B flux exposure was associated with higher BCC risk in both cohorts (Ptrend < 0.0001). Multivariate-adjusted relative risks for the highest quintile of exposure versus the lowest quintile were 3.18 (95% confidence interval (CI): 2.70, 3.76) and 1.90 (95% CI: 1.57, 2.29) for women and men, respectively, and the multivariate-adjusted relative risk was 2.46 (95% CI: 1.48, 4.09) in the combined analysis.
Table 3.
Risk of Basal-Cell Carcinoma According to Quintile of Cumulative UV-B Flux Exposure in Age- and Multivariate-adjusted Analyses, Nurses' Health Study (1986–2006) and Health Professionals' Follow-up Study (1988–2006)
| Quintile of UV-B Flux Exposure |
Median Value (Robertson-Berger Count × 104)a |
No. of Cases |
Person- Years |
Age-adjusted |
Multivariate-adjustedb |
||
|---|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | ||||
| Nurses' Health Study | |||||||
| 1 | 164 | 2,292 | 347,649 | 1.00 | Referent | 1.00 | Referent |
| 2 | 360 | 2,501 | 333,887 | 1.65 | 1.47, 1.84 | 1.63 | 1.45, 1.82 |
| 3 | 600 | 2,806 | 332,183 | 2.23 | 1.93, 2.58 | 2.19 | 1.89, 2.53 |
| 4 | 843 | 3,675 | 339,472 | 2.92 | 2.48, 3.43 | 2.84 | 2.41, 3.33 |
| 5 | 1,130 | 4,395 | 316,500 | 3.29 | 2.79, 3.88 | 3.18 | 2.70, 3.76 |
| Ptrend | <0.0001 | <0.0001 | |||||
| Health Professionals' Follow-Up Study | |||||||
| 1 | 154 | 1,107 | 131,104 | 1.00 | Referent | 1.00 | Referent |
| 2 | 350 | 1,286 | 131,938 | 1.30 | 1.13, 1.49 | 1.28 | 1.12, 1.47 |
| 3 | 565 | 1,721 | 132,572 | 1.68 | 1.42, 1.98 | 1.63 | 1.38, 1.93 |
| 4 | 791 | 1,960 | 126,775 | 1.91 | 1.60, 2.29 | 1.85 | 1.54, 2.21 |
| 5 | 1,080 | 2,163 | 128,548 | 1.99 | 1.65, 2.40 | 1.90 | 1.57, 2.29 |
| Ptrend | <0.0001 | <0.0001 | |||||
| Pooled Analysis | |||||||
| 1 | 3,399 | 478,753 | 1.00 | Referent | 1.00 | Referent | |
| 2 | 3,787 | 465,825 | 1.47 | 1.16, 1.85 | 1.45 | 1.15, 1.83 | |
| 3 | 4,527 | 464,755 | 1.94 | 1.47, 2.57 | 1.90 | 1.42, 2.53 | |
| 4 | 5,635 | 466,247 | 2.37 | 1.56, 3.58 | 2.29 | 1.51, 3.49 | |
| 5 | 6,558 | 445,048 | 2.56 | 1.56, 4.19 | 2.46 | 1.48, 4.09 | |
| Ptrend | <0.0001 | <0.0001 | |||||
| P for heterogeneityc | 0.004 | 0.002 | |||||
Abbreviations: CI, confidence interval; RR, relative risk; UV-B, ultraviolet B.
a Median cumulative midrange UV-B flux exposure for each quintile.
b Multivariate analysis controlled for age, family history of melanoma, natural hair color, number of moles on an extremity, susceptibility to sunburn as a child/adolescent, lifetime number of severe/blistering sunburns, and cumulative UV-B flux exposure.
c P value from a test of heterogeneity between studies.
We further examined potential interactions between cumulative UV-B flux exposure and phenotypic risk factors and found that only susceptibility to sunburn as a child/adolescent showed significant interactions with cumulative UV-B flux exposure in both cohorts (P for interaction < 0.05). We stratified the analysis for cumulative UV-B flux exposure and BCC risk by susceptibility to sunburn as a child/adolescent and found stronger exposure-response associations among participants with a skin reaction of “no burn or some redness” when compared with participants with a “burn” or “painful or blistering burn” reaction to sun exposure (Appendix Table 1).
DISCUSSION
In addition to previous reports which provided perspectives on BCC incidence rates at different time points or regions, we have now presented results from a continuous analysis of BCC incidence rates over the last 2 decades in the United States by using data from 2 large, prospective cohort studies. We also analyzed the associations of BCC risk with a number of phenotypic and exposure characteristics and identified several risk factors associated with BCC risk.
It has been recognized that the occurrence of cancer has increased as the population has aged, and 80% of all cancers are diagnosed above age 55 years (2). BCC incidence rates have generally shown increasing trends over time in both women and men, suggesting an increasing likelihood of developing BCC with advancing age (5, 6, 15). Several other factors may also help explain the rise in BCC incidence in addition to advancing age, which may include sun exposure habits, use of artificial UV tanning beds, ozone depletion, and increases in life expectancy (16). Furthermore, exposures to chemical carcinogens, such as air pollutants and polycyclic aromatic hydrocarbons, may also increase the risk of skin cancer (17, 18). Aside from these factors, a portion of the increased incidence rates could also be attributed to the improvements in public awareness and medical detection.
We found generally higher BCC incidence rates in men than in women over the follow-up periods, which were consistent with several previous regional studies (3–5, 15). For example, in a study in north-central New Mexico, the age-adjusted BCC incidence rates were 619 cases per 100,000 person-years in men and 399 cases per 100,000 person-years in women in 1977–1978 (15). These incidence rates increased significantly to 931 cases per 100,000 person-years in men and 486 cases per 100,000 person-years in women in 1998–1999. This gender difference in BCC incidence rates may be partly explained by gender differences in sun exposure habits and awareness of skin conditions. Men might have experienced more sun-exposure–related skin damage, as indicated by a higher proportion of a history of ≥6 sunburns, than women in the present study (33.2% vs. 8.4%; see Table 1). They might also be less likely to seek medical care, allowing the more advanced development of skin cancer in comparison with women (19, 20). It has been reported that nonmelanoma skin cancer is among the 5 most costly cancers in the Medicare population (2), and nonmelanoma skin cancer also has the potential to result in significant years of potential life lost and lost productivity (21). As the major histological type of nonmelanoma skin cancer, the already high and rising incidence of BCC may lead to a substantial disease burden on both the health-care system and society. Specifically, BCC incidence in men aged 40–50 years at baseline (1988) reached 1,000 cases per 100,000 person-years during the 1998–2000 follow-up period, after 10 years of follow-up, and was maintained above 1,000 cases per 100,000 person-years after this follow-up period. In contrast, BCC incidence in women aged 40–50 years at baseline (1986) reached only 1,000 cases per 100,000 person-years during the last follow-up period (2004–2006), after 18 years of follow-up. On the basis of these data, we recommend that BCC screening be performed for men aged 50–60 years and women aged 55–65 years in the United States in order to lower the direct and indirect costs of BCC.
Results of analyses on phenotypic and exposure variables provided further evidence for the strong risk factors associated with incident BCC. Participants with a family history of melanoma or a history of painful/blistering sunburn reaction as a child/adolescent were more likely to develop BCC in both women and men, and there were trends toward increasing BCC risk with greater numbers of extremity moles and lifetime severe/blistering sunburns. In our previous case-control study nested within the NHS, several of these factors were associated with skin cancer risk (22). However, the multivariate-adjusted associations of constitutional susceptibility and lifetime severe/blistering sunburns with BCC risk did not achieve statistical significance in that subset of NHS participants, which may have been partly due to the limited sample size. In the present analysis, we found strong associations of these factors with BCC risk when using data from the whole NHS cohort. Notably, the relationship of lifetime severe/blistering sunburns with BCC underlies the importance of sunburn prevention.
UV radiation in sunlight is an important cause of skin cancer (23). A previous animal experiment found that UV-B radiation but not UV-A radiation was able to initiate melanoma in mice (24). In our study, annual UV-B flux exposure was estimated on the basis of latitude, altitude, and cloud cover according to the participant's state of residence and could serve as a more objective measure of midrange UV radiation exposure because it is less subject to personal recall. UV-B flux was associated with melanoma risk in a previous case-control study (25), suggesting that it could be used as a reasonable proxy for sun exposure measurement. However, prospective data on the association of UV-B flux with BCC risk are not available to date. In the present analysis, we found increasing BCC risk to be associated with cumulative UV-B flux exposure in both women and men. Interestingly, the multivariate-adjusted relative risks for cumulative UV-B flux exposure in women were much higher than those in men, suggesting that women may be more susceptible to cumulative midrange UV radiation during a long-term period than men. Furthermore, we also found significant interactions between susceptibility to sunburn as a child/adolescent and cumulative UV-B flux exposure in both cohorts, and the associations between cumulative UV-B flux exposure and BCC risk were generally stronger among participants with lower susceptibility to sunburn. These results suggest that BCC risk among persons with lower susceptibility to sunburn is more likely to be UV-dependent, and it is possible that genetic determinants (26) or some other factors may play a role in the different responses to UV radiation among persons with different susceptibilities to sunburn. Further investigation is warranted to clarify the mechanisms underlying the interaction.
This study had some limitations. First, our study populations consisted of Caucasian health professionals and may not be representative of the entire US population; this may limit the generalizability of our findings. Second, the outcome disease was assessed on the basis of self-reports. However, the participants in the 2 cohorts were nurses and health professionals, who were more likely to have a better understanding and personal recall of BCC than the general population, and previous validation studies also demonstrated high validity of self-reported BCC in subsets of the cohort participants (8–10). Although misclassification of BCC cases was inevitable, it would be expected to have been nondifferential and to have biased any associations toward the null. Third, the annual UV-B flux for a particular state was calculated according to average latitude, altitude, and cloud cover over the state and then assigned to all participants residing in that state. These factors used for UV-B flux calculation may vary considerably across some states, which in turn may have reduced the accuracy of the UV-B flux level assigned to the participants residing in those states.
However, our study also had several strengths, including the prospective design, large sample size, high quality of data collected, high rates of follow-up over a considerable time span, and the homogeneity of the study populations, which could have reduced residual confounding from occupational exposure to UV radiation and socioeconomic status. In addition, most of the findings were consistent across the 2 cohorts, strengthening the validity of the results.
In summary, we have presented findings from an overall evaluation of BCC incidence rates over the last 2 decades and associated risk factors in 2 cohorts of US women and men. Our results highlight the high and rising incidence of BCC and the need to develop strategies to reduce BCC incidence in the United States. The identified risk factors include family history of melanoma, red and blond hair colors, higher number of moles on an extremity, higher susceptibility to sunburn as a child/adolescent, higher lifetime number of severe/blistering sunburns, and cumulative UV-B flux exposure. The association between BCC risk and cumulative UV-B flux is strong and consistent, providing further evidence that UV exposure intensity is essential for the development of BCC. As the most common form of skin cancer, BCC maintains high incidence and may result in substantial morbidity. Appropriate prevention targeting for the modifiable risk factors as documented herein is critical for lowering BCC incidence and its associated health impact.
ACKNOWLEDGMENTS
Author affiliations: Department of Dermatology, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts (Shaowei Wu, Jiali Han, Wen-Qing Li, Tricia Li, Abrar A. Qureshi); Channing Laboratory, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts (Shaowei Wu, Jiali Han, Tricia Li, Abrar A. Qureshi); and Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Jiali Han).
This work was supported by the Department of Dermatology, Brigham and Women's Hospital (Boston, Massachusetts), and National Institutes of Health grants to the Nurses' Health Study (grant P01 CA87969) and the Health Professionals' Follow-up Study (grant P01 CA055075).
We are deeply indebted to the staff of the Nurses' Health Study and Health Professionals' Follow-up Study for their valuable contributions, as well as to the following state cancer registries for their help: Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, Washington, and Wyoming.
Conflict of interest: none declared.
APPENDIX
Appendix Table 1.
Risk of Basal-Cell Carcinoma According to Cumulative Midrange UV-B Flux Exposure and Susceptibility to Sunburn as a Child/Adolescent in Age- and Multivariate-adjusted Analyses, Nurses' Health Study (1986–2006) and Health Professionals' Follow-up Study (1988–2006)
| Quintile of UV-B Flux Exposure |
Susceptibility to Sunburn as a Child/Adolescent |
P for Interactionc | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Burn or Some Redness |
Burn |
Painful or Blistering Burn |
|||||||||||
| RR1a | 95% CI | RR2b | 95% CI | RR1a | 95% CI | RR2b | 95% CI | RR1a | 95% CI | RR2b | 95% CI | ||
| Nurses' Health Study | |||||||||||||
| 1 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 0.001 |
| 2 | 1.71 | 1.46, 2.00 | 1.68 | 1.44, 1.97 | 1.57 | 1.23, 2.01 | 1.54 | 1.20, 1.97 | 1.74 | 1.35, 2.25 | 1.74 | 1.35, 2.25 | |
| 3 | 2.64 | 2.14, 3.25 | 2.58 | 2.09, 3.18 | 1.80 | 1.31, 2.48 | 1.75 | 1.27, 2.40 | 2.25 | 1.61, 3.14 | 2.26 | 1.62, 3.16 | |
| 4 | 3.53 | 2.79, 4.45 | 3.43 | 2.72, 4.32 | 2.60 | 1.82, 3.71 | 2.50 | 1.75, 3.57 | 3.01 | 2.06, 4.41 | 3.03 | 2.07, 4.43 | |
| 5 | 4.14 | 3.27, 5.25 | 3.99 | 3.15, 5.06 | 2.97 | 2.07, 4.27 | 2.83 | 1.97, 4.06 | 3.12 | 2.11, 4.62 | 3.14 | 2.12, 4.65 | |
| Ptrend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||||
| Health Professionals' Follow-Up Study | |||||||||||||
| 1 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 0.01 |
| 2 | 1.26 | 0.92, 1.73 | 1.23 | 0.90, 1.69 | 1.41 | 1.13, 1.77 | 1.41 | 1.12, 1.76 | 1.17 | 0.89, 1.54 | 1.16 | 0.88, 1.52 | |
| 3 | 1.97 | 1.34, 2.90 | 1.89 | 1.28, 2.78 | 1.78 | 1.35, 2.34 | 1.76 | 1.33, 2.32 | 1.48 | 1.06, 2.07 | 1.45 | 1.04, 2.03 | |
| 4 | 2.34 | 1.54, 3.54 | 2.20 | 1.45, 3.34 | 1.86 | 1.38, 2.50 | 1.84 | 1.36, 2.47 | 1.81 | 1.26, 2.60 | 1.76 | 1.22, 2.52 | |
| 5 | 2.63 | 1.71, 4.06 | 2.44 | 1.58, 3.76 | 1.92 | 1.41, 2.61 | 1.88 | 1.38, 2.56 | 1.69 | 1.16, 2.48 | 1.63 | 1.11, 2.38 | |
| Ptrend | <0.0001 | 0.0002 | 0.03 | 0.04 | 0.04 | 0.07 | |||||||
Abbreviations: CI, confidence interval; RR, relative risk; UV-B, ultraviolet B.
a Adjusted for age.
b Multivariate analysis controlled for age, family history of melanoma, natural hair color, number of moles on an extremity, lifetime number of severe/blistering sunburns, and cumulative UV-B flux exposure.
c P value for interaction between susceptibility to sunburn as a child/adolescent and cumulative UV-B flux exposure.
REFERENCES
- 1.Slaper H, Velders GJM, Daniel JS, et al. Estimates of ozone depletion and skin cancer incidence to examine the Vienna convention achievements. Nature. 1996;384(6606):256–258. doi: 10.1038/384256a0. [DOI] [PubMed] [Google Scholar]
- 2.Housman TS, Feldman SR, Williford PM, et al. Skin cancer is among the most costly of all cancers to treat for the Medicare population. J Am Acad Dermatol. 2003;48(3):425–429. doi: 10.1067/mjd.2003.186. [DOI] [PubMed] [Google Scholar]
- 3.Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30(5):774–778. doi: 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- 4.Gallagher RP, Becky M, McLean DI, et al. Trends in basal cell carcinoma, squamous cell carcinoma, and melanoma of the skin from 1973 through 1987. J Am Acad Dermatol. 1990;23(3):413–421. doi: 10.1016/0190-9622(90)70234-9. [DOI] [PubMed] [Google Scholar]
- 5.Karagas MR, Greenberg ER, Spencer SK, et al. Increase in incidence rates of basal cell and squamous cell skin cancer in New Hampshire, USA. Int J Cancer. 1999;81(4):555–559. doi: 10.1002/(sici)1097-0215(19990517)81:4<555::aid-ijc9>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 6.Harris RB, Griffith K, Moon TE. Trends in the incidence of nonmelanoma skin cancers in southeastern Arizona, 1985–1996. J Am Acad Dermatol. 2001;45(4):528–536. doi: 10.1067/mjd.2001.114742. [DOI] [PubMed] [Google Scholar]
- 7.Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146(3):283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 8.Colditz GA, Martin P, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123(5):894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 9.Hunter DJ, Colditz GA, Stampfer MJ, et al. Diet and risk of basal cell carcinoma of the skin in a prospective cohort of women. Ann Epidemiol. 1992;2(3):231–239. doi: 10.1016/1047-2797(92)90055-u. [DOI] [PubMed] [Google Scholar]
- 10.van Dam RM, Huang Z, Giovannucci E, et al. Diet and basal cell carcinoma of the skin in a prospective cohort of men. Am J Clin Nutr. 2000;71(1):135–141. doi: 10.1093/ajcn/71.1.135. [DOI] [PubMed] [Google Scholar]
- 11.Scotto J, Fears TR, Fraumeni JF., Jr . Solar radiation. In: Schottenfeld D, Fraumeni JF Jr, editors. 2nd ed. New York, NY: Oxford University Press; 1996. pp. 355–372. [Google Scholar]
- 12.Scotto J, Cotton G, Urbach F, et al. Biologically effective ultraviolet radiation: surface measurements in the United States, 1974 to 1985. Science. 1988;239(4841):762–764. doi: 10.1126/science.3340857. [DOI] [PubMed] [Google Scholar]
- 13.DeLuisi JJ, Harris JM. A determination of the absolute radiant energy of a Robertson-Berger meter sunburn unit. Atmos Environ. 1983;17(4):751–758. [Google Scholar]
- 14.Smith-Warner SA, Spiegelman D, Ritz J, et al. Methods for pooling results of epidemiologic studies: the Pooling Project of Prospective Studies of Diet and Cancer. Am J Epidemiol. 2006;163(11):1053–1064. doi: 10.1093/aje/kwj127. [DOI] [PubMed] [Google Scholar]
- 15.Athas WF, Hunt WC, Key CR, et al. Changes in nonmelanoma skin cancer incidence between 1977–1978 and 1998–1999 in northcentral New Mexico. Cancer Epidemiol Biomarkers Prev. 2003;12(10):1105–1108. [PubMed] [Google Scholar]
- 16.Diepgen TL, Mahler V. The epidemiology of skin cancer. Br J Dermatol. 2002;146(suppl 61):1–6. doi: 10.1046/j.1365-2133.146.s61.2.x. [DOI] [PubMed] [Google Scholar]
- 17.Chen MR, Tsai PJ, Wang YF. Assessing inhalatory and dermal exposures and their resultant health-risks for workers exposed to polycyclic aromatic hydrocarbons (PAHs) contained in oil mists in a fastener manufacturing industry. Environ Int. 2008;34(7):971–975. doi: 10.1016/j.envint.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Goldsmith LA. Skin effects of air pollution. Otolaryngol Head Neck Surg. 1996;114(2):217–219. doi: 10.1016/S0194-59989670169-9. [DOI] [PubMed] [Google Scholar]
- 19.Geller AC, Miller DR, Annas GD, et al. Melanoma incidence and mortality among US whites, 1969–1999. JAMA. 288(14):1719–1720. doi: 10.1001/jama.288.14.1719. [DOI] [PubMed] [Google Scholar]
- 20.Miller DR, Geller AC, Wyatt SW, et al. Melanoma awareness and self-examination practices: results of a United States survey. J Am Acad Dermatol. 1996;34(6):962–970. doi: 10.1016/s0190-9622(96)90273-x. [DOI] [PubMed] [Google Scholar]
- 21.Guy GP, Ekwueme DU. Years of potential life lost and indirect costs of melanoma and non-melanoma skin cancer: a systematic review of the literature. Pharmacoeconomics. 2011;29(10):863–874. doi: 10.2165/11589300-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.Han J, Colditz GA, Hunter DJ. Risk factors for skin cancers: a nested case-control study within the Nurses’ Health Study. Int J Epidemiol. 2006;35(6):1514–1521. doi: 10.1093/ije/dyl197. [DOI] [PubMed] [Google Scholar]
- 23.Mackie RM, Elwood JM, Hawk JLM. Links between exposure to ultraviolet radiation and skin cancer. J R Coll Physicians Lond. 1987;21(2):91–96. [PMC free article] [PubMed] [Google Scholar]
- 24.De Fabo E, Noonan FP, Fears T, et al. Ultraviolet B but not ultraviolet A radiation initiates melanoma. Cancer Res. 2004;64(18):6372–6376. doi: 10.1158/0008-5472.CAN-04-1454. [DOI] [PubMed] [Google Scholar]
- 25.Fears TR, Bird CC, Guerry D IV, et al. Average midrange ultraviolet radiation flux and time outdoors predict melanoma risk. Cancer Res. 2002;62(14):3992–3996. [PubMed] [Google Scholar]
- 26.Nan H, Xu M, Kraft P, et al. Genome-wide association study identifies novel alleles associated with risk of cutaneous basal cell carcinoma and squamous cell carcinoma. Hum Mol Genet. 2011;20(18):3718–3724. doi: 10.1093/hmg/ddr287. [DOI] [PMC free article] [PubMed] [Google Scholar]