Severe burn injury is associated with a profound hypermetabolic, hypercatabolic response proportional to the original size of the injury, which persists for 1 to 2 years postburn.1,2 The response is characterized by supraphysiologic metabolic rates, hyperdynamic circulation, constitutive protein fat and bone catabolism, blunted growth, insulin resistance, and increased risk for infection1–5 (Figs. 1 to 3).
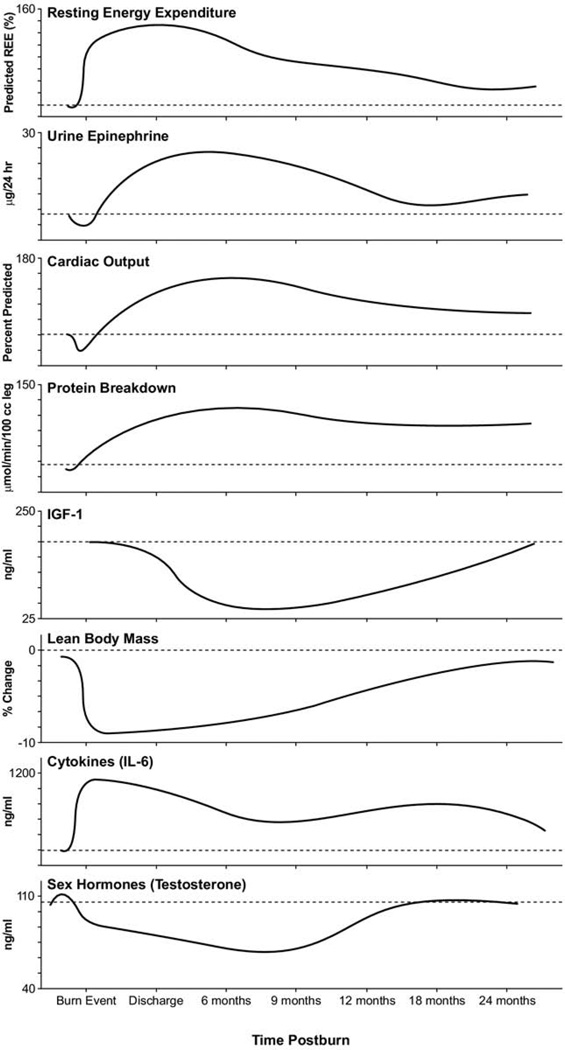
Figure 1.
Physiologic and metabolic changes post severe burn injury. Demonstrates changes in resting energy expenditure, stress hormones (epinephrine), cardiac function (cardiac output), gender hormones (testosterone), cytokines (interleukin-6) and changes in body composition (lean body mass). Data were summarized from published works from our institution.1,12 Averages for burn patients are represented by solid curves. Values from nonburned, normal patients are represented by dashed lines (—).
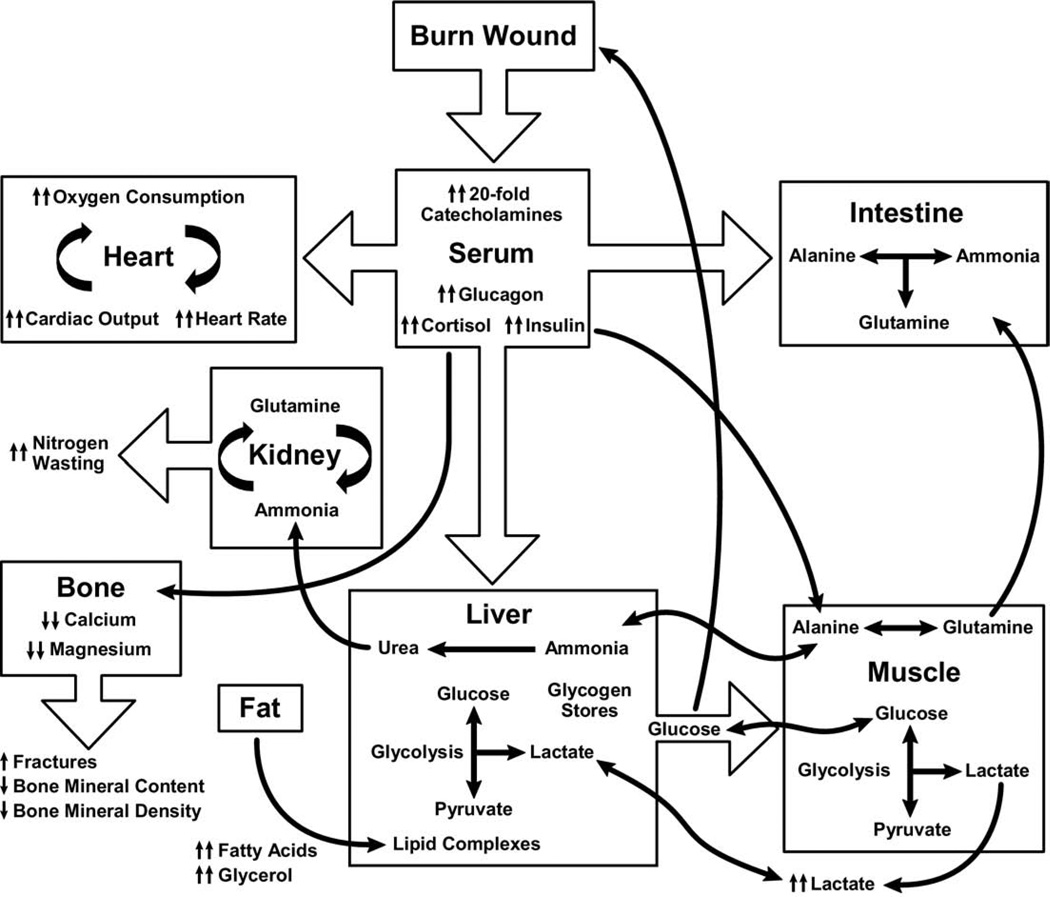
Figure 3.
Effects of metabolic dysfunction postburn.
Cuthbertson6 originally described a stress response to fractures characterized by an “ebb” phase, with a decrease in tissue perfusion and a decrease in metabolism. This is followed by a “flow” phase—starting 3 to 5 days postinjury—which is characterized by an increase in metabolic rate and hyperdynamic circulation. If left untreated, physiologic exhaustion and death can result.7–10 Severe burns exhibit the most dramatic hypermetabolic stress response of any injury.
This article reports our single institution’s experience during the past decade, in >1,000 patients with burns of >40% of their total body surface area (TBSA), delineating the magnitude of the metabolic and catabolic responses to major burn injury. Serially performed randomized prospective clinical studies using common methods are described, demonstrating the efficacy of interventions to mitigate the hypermetabolic response: the effects of early excision and grafting, environmental thermoregulation, early continuous enteral feeding with a high-carbohydrate, high-protein diet, use of anabolic agents (growth hormone, insulin-like growth factor-1 [IGF-1], with insulin-like growth factor binding protein-3 [IGFBP-3], insulin, oxandrolone), the anticatabolic agent (propranolol), and use of therapeutic exercise are compared. This article describes the destructive aspects of the hypermetabolic response and strategies implemented during the last decade to modulate this response, which have improved burn care, survival, and quality of life in burn patients. It is hoped that these strategies might also be applicable to the larger populations of patients who have undergone other forms of injury, including large elective operations.
Hypermetabolic response in severe burns
Catecholamines, corticosteroids, and inflammatory cytokines are primary mediators of the postburn hypermetabolic catabolic response.11 A 10- to 20-fold elevation of plasma catecholamines and corticosteroid levels occur in major burns, which persist up to 9 months postinjury.12,13 Burn patients have increased metabolic rates, increased cardiac work, increased myocardial oxygen consumption, marked tachycardia, severe lipolysis, liver dysfunction, muscle catabolism, increased protein degradation, insulin resistance, and growth retardation.14–17 Cytokine levels peak immediately after burn, approaching normal levels only after 4 to 5 weeks postinjury. Constitutive and acute-phase proteins are altered beginning 1 week postburn, and remain abnormal throughout acute hospital stay. Serum IGF-I, IGFBP-3, parathyroid hormone, and Osteocalcin drop 10-fold immediately after the injury, and remain substantially decreased up to 2 to 6 months postburn, compared with normal levels.12 Gender hormones and endogenous growth hormone levels decrease around 3 weeks postburn12 (Fig. 1).
For severely burned patients, the resting metabolic rate at thermal neutral temperature (30°C–33°C) exceeds 140% of normal at admission, reduces to 130% once the wounds are fully healed, then to 120% at 6 months postinjury, and 110% at 12 months postburn.1,12 Increases in catabolism result in loss of total body protein, decreased immune defenses, and decreased wound healing.1
Immediately postburn, patients have low cardiac output characteristic of early shock.6 Three to four days postburn, cardiac outputs are > 1.5 times that of nonburned, healthy volunteers.12 Heart rates of pediatric burn patients’ approach 1.6 times that of nonburned, healthy volunteers.16 Postburn, patients have increased cardiac work.18,19 Myocardial oxygen consumption surpasses that of marathon runners and are sustained well into rehabilitation.19
There is profound hepatomegaly after injury. The liver increases its size by 225% of normal by 2 weeks postburn and remains enlarged at discharge by 200%.12
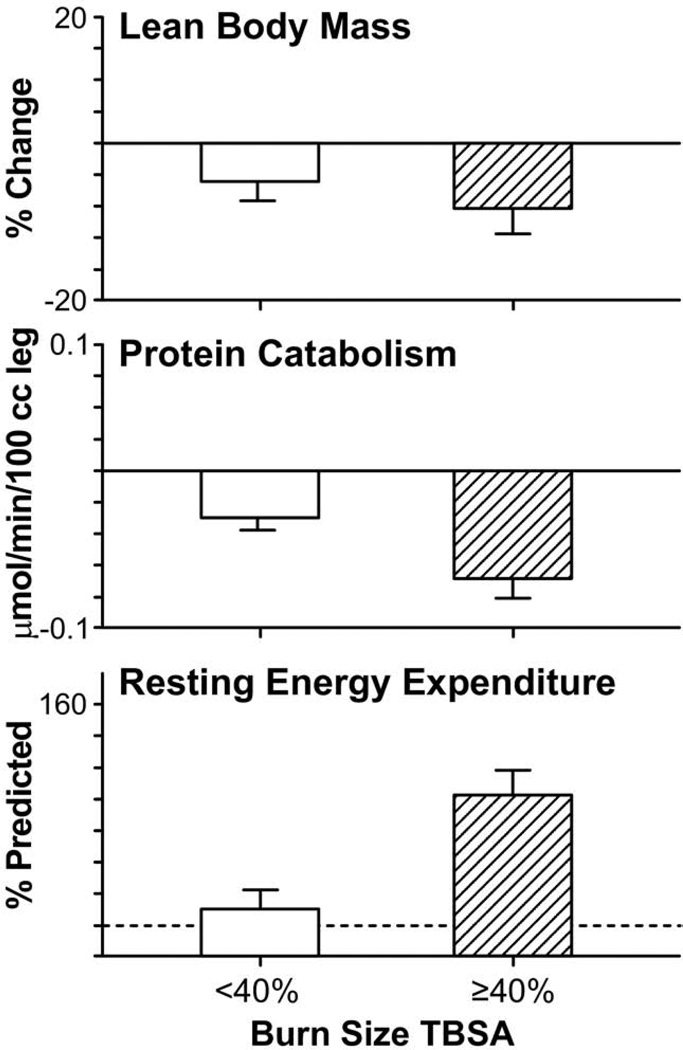
Postburn, muscle protein is degraded much faster than it is synthesized.12,16 Net protein loss leads to loss of lean body mass and severe muscle wasting, leading to decreased strength and failure to fully rehabilitate.1,20 Substantial decreases in lean body mass related to chronic illness or hypermetabolism can have dire consequences. A 10% loss of lean body mass is associated with immune dysfunction. A 20% loss of lean body mass positively correlates with decreased wound healing. A loss of 30% lean body mass leads to increased risk for pneumonia and pressure sores. A 40% loss of lean body mass has > 90% risk of death.21 Uncomplicated severely burned patients can lose up to 25% of total body mass after acute burn injury.22 Protein degradation persists up to 9 months after severe burn injury, resulting in considerable negative whole-body and cross-leg nitrogen balance18,23,24 (Fig. 2). Protein catabolism is correlated to, and can be predicted by, increases in metabolic rates.24 Severely burned patients have a daily nitrogen loss of 20 to 25 g/m2 burned skin.18,25 At this rate, a lethal cachexia can be reached in <30 days.25 Burned pediatric patients’ protein loss leads to substantial growth retardation for up to 24 months after injury.3
Figure 2.
Effect of burn size on body mass, resting energy expenditure, and protein degradation. Changes in net protein balance of muscle protein synthesis and breakdown induced by burn injury was measured by stable isotope studies using d5-phenyalanine infusion studies published previously.1,12,24,129 Graphs are averages ± SEM. White bars represent patients with burns <40% total body surface area (TBSA). Striped bars represent patients with burns ≥40% TBSA. Values from nonburned, normal patients are represented by dashed lines (—).
Elevated circulating levels of catecholamines, glucagon, cortisol after severe thermal injury stimulate glucose production by the liver, amino acids from muscle, and free fatty acids and glycerol from fat26 (Fig. 3). Glycolytic-gluconeogenic cycling is increased 250% during the post-burn hypermetabolic response, coupled with an increase of 450% in triglyceride fatty acid cycling.5 These changes lead to hyperglycemia and impaired insulin sensitivity related to postreceptor insulin resistance, demonstrated by elevated levels of insulin, fasting glucose, and substantial reductions in glucose clearance.4 Although glucose delivery to peripheral tissues is increased up to threefold, glucose oxidation is restricted. Increased glucose production is directed, in part, to the burn wound to support the relatively inefficient anaerobic metabolism of fibroblasts, and endothelial and inflammatory cells.27,28 The end-product of anaerobic glucose oxidation, ie, lactate, is recycled to the liver to produce more glucose through gluconeogenic pathways.18 Serum glucose and serum insulin increase postburn and remain substantially increased through the acute hospital stay.12 Insulin resistance appears during the first week postburn and persists considerably after discharge.12
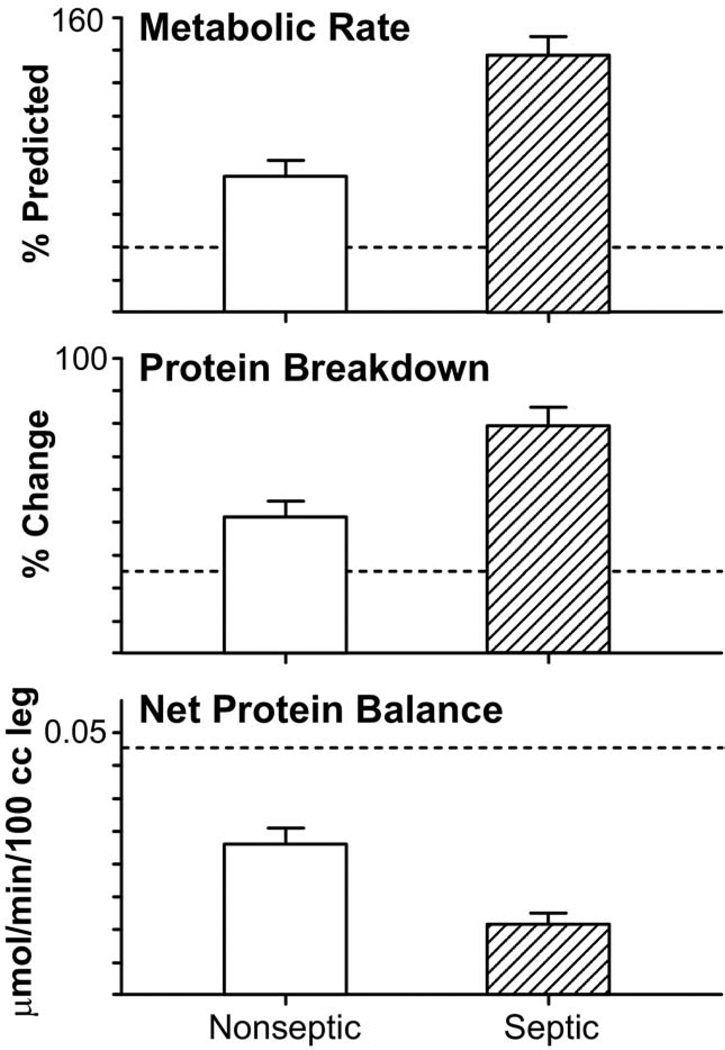
Patients who become septic have a profound increase in metabolic rates and protein catabolism, up to 40% more compared with those with like-sized burns that do not develop sepsis.1,29 A vicious cycle develops, as patients who are catabolic are more susceptible to sepsis because of changes in immune function and immune response. The emergence of multidrug-resistant organisms has led to increases in sepsis, catabolism, and mortality30,31 (Fig. 4).
Figure 4.
Effect of sepsis on resting energy expenditure, muscle protein breakdown, and fractional synthetic rate of muscle protein synthesis compared with like-sized burns. Changes in net protein balance of muscle protein synthesis and breakdown induced by burn injury was measured by stable isotope studies using d5-phenyalanine infusion studies published previously.1,12,24,129 Graphs are averages ± SEM. White bars represent nonseptic patients with burns ≥40% total body surface area (TBSA). Striped bars represent septic patients with burns ≥40% TBSA. Values from nonburned, normal patients are represented by dashed lines (—).
Modulation of hypermetabolic response in severe burns
Early excision and grafting
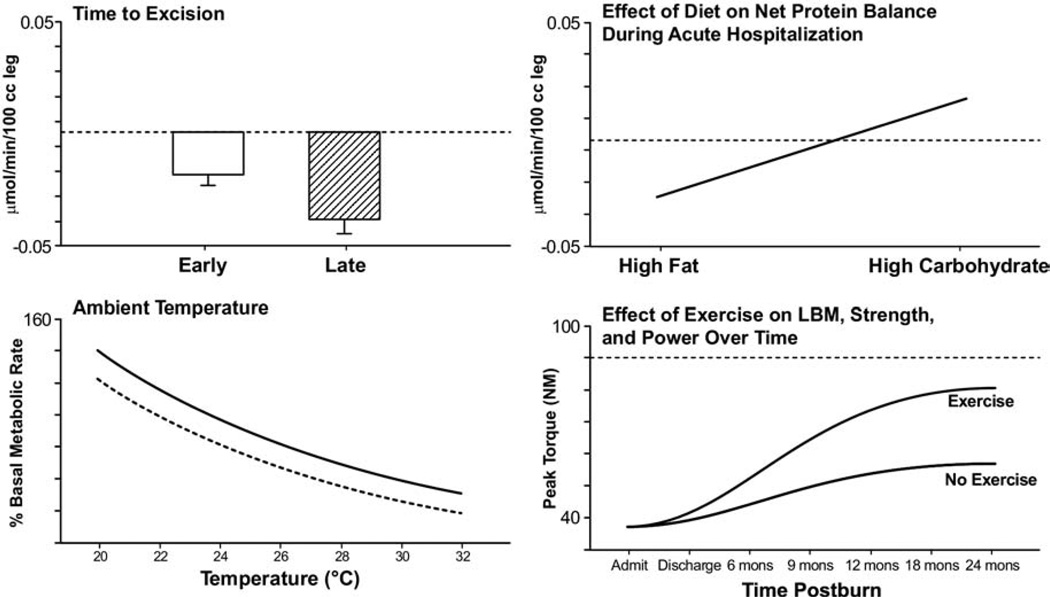
The hypermetabolic response of a severely burned patient far surpasses that of patients with any other disease state.32 One of the most revolutionary improvements in burn care that has attenuated the hypermetabolic response is the excision and grafting of the burn eschar early after injury. For burns that encompass >50% TBSA, there is a 40% decrease in metabolic rate for patients totally excised and covered within 3 days of injury compared with those patients with like-sized burns, excised and covered 1 week after injury.24 Additional benefits of early excision and grafting include less operative blood loss, fewer septic complications, decreased length of stay, and improved survival rates in children and young adults compared with patients treated with later excision.33–36 Early excision and grafting decreases net protein loss and incidence of infections (Fig. 5).37
Figure 5.
Nonpharmacologic modulations of the hypermetabolic response postburn. Demonstrates effect of early excision and grafting, environmental thermoregulation, high-carbohydrate diet and exercise on physiologic derangements postburn.12,24,68,130,131 Graphs are averages ± SEM. White bars represent patients with burns ≥40% total body surface area (TBSA) that had early excision. Striped bars represent patients with burns ≥40% TBSA that had late excision of burn eschar. Averages for burn patients are represented by solid curves. Values from non-burned, normal patients are represented by dashed lines (—).
Immediate removal of burn eschar decreases release of inflammatory mediators. Substantial reductions in interleukin (IL)-6, IL-8, tumor necrosis factor–α, and lipopolysaccharide have been seen in those patients after prompt excision and grafting of burn wounds.38 Additional studies have shown a marked reduction in C-reactive protein, and C3 complement, along with reductions in IL-6 and serum tumor necrosis–α factor, with early excision and grafting.39 IL-6, in particular, has been implicated as a potential predictor of outcomes in severely burned patients.24,40 Early excision and grafting remains a cornerstone in management of the hypermetabolic postburn response, decreasing infection rates, resting energy expenditures, and length of hospital stay.
Nutrition
Aggressive, early enteral feeding improves outcomes in the burned patient, by mitigating the degree and extent of catabolism.41,42 With oral alimentation from hospital trays alone, patients with 40% TBSA burns have lost up to one-quarter of their preadmission weight by 21 days postinjury.22 Only through aggressive, continuous parenteral or enteral, or both, nutrition with 25 kcal/kg body weight per day plus 40 kcal per percent TBSA burn per day, can body weight be maintained in adults.43,44 Children require 1,800 kcal/m2 plus 2,200 kcal/m2 of burn area per day to maintain body weight.45 Parenteral nutrition alone, or even in combination with enteral nutrition, led to overfeeding, liver failure, impaired immune response, increased infections, and mortality.46–48 Enteral nutrition reduces translocation bacteremia and sepsis, maintains gut motility, and preserves “first-pass” nutrient delivery to the liver.41 For burned patients, parenteral nutrition should be reserved for those with enteral feeding intolerance or prolonged ileus.
Milk, consisting of 44% fat, 42% carbohydrate and 14% protein, became standard of care for the pediatric burned patient.16,49 Although well-tolerated, fat did not serve as the optimal energy source for these patients. There was continued protein degradation and lean body mass acquisition was less when compared with high-carbohydrate diets consisting of 3% fat, 15% protein, and 82% carbohydrate16 (Fig. 5). The high-carbohydrate diet increased protein synthesis, increased endogenous insulin production, and improved lean body mass and muscle protein synthesis.16
By measuring resting energy expenditures with bedside carts, actual caloric requirements can be determined.50,51 Appropriate nutrient delivery can be accomplished by feeding 1.2 to 1.4 times measured resting energy expenditures (in kcal/m2 per day). Using stable isotopes we found that feeding pediatric burn patients 1.2 times measured resting energy expenditures resulted in a loss of 10% lean body mass.50 In other studies of body composition over time in burned children, we found maintenance of body weight when feeding 1.4 times the resting energy expenditure, and increases in weight when feeding 1.6 times the resting energy expenditure. The latter gains in weight were a result of fat deposition, not lean body mass accretion.51,52
There is evidence that increased protein replacement for severely burned patients might be beneficial.53,54 Healthy individuals require 1 g/kg body weight per day of protein intake.55,56 Based on in vivo kinetics studies looking at oxidation rates of essential and nonessential amino acids, burn patients have 50% higher use rates than healthy individuals in the fasting state.53,54,57 Burn patients require a minimum of 1.5 to 2 g/kg body weight per day protein intake.58–60 Higher amounts of supplementation lead to increased urea production without improvements in lean body mass or muscle protein synthesis.61
Environmental thermoregulation
Postburn, the metabolic rate increases to compensate for profound water and heat losses. Water loss approaches 4,000 mL/m2 burn area per day.62–65 The body’s natural response to this insult, partially mediated by increased ATP consumption and substrate oxidation, is to raise core and skin temperatures 2°C more than normal compared with unburned patients.66 This response is similar to the response seen during cold acclimatization. In fact, patients that do not mount this response are likely septic and or have exhausted physiologic capabilities to maintain needed body temperature.49 In 1975, Wilmore and colleagues67 showed that we can profoundly attenuate the hypermetabolic response by increasing ambient temperatures to 33°C. At this temperature, the energy required for vaporization is derived from the environment rather than from the patient.67,68 By increasing ambient temperatures from 20°C and 33°C, resting energy expenditures decrease from 2.0 to 1.4 times normal in patients with severe burn injury67,68 (Fig. 5).
Hormonal response in severe burns
Elevated levels of catecholamines, cortisol, and glucagon mediate profound changes in metabolic rates, growth, and physiology seen in the pediatric burn patient population. We have studied the therapeutic use of recombinant human growth hormone (rhGH), insulin, IGF-1 and IGFB-3 in combination, testosterone, and oxandrolone as potential anabolic agents. To combat catecholamines, we have administered the adrenergic antagonist propranolol. Use of anabolic or anticatabolic agents in severely burned children, in addition to standard of care, has led to substantial decreases in protein catabolism.
Anabolic agents
rhGH
Blunted growth lasts at least 12 to 24 months postburn in severely burned pediatric patients.3,69 Compared with age-matched controls, severely burned children have substantially reduced bone mineral density and mass, which persist indefinitely.70 Bone loss is, in part, a result of bone-marrow suppression, calcium and magnesium homeostasis dysregulation, immobilization, hypoparathyroidism, and increased endogenous glucocorticoid production.71–73 rhGH, when administered IM during acute hospitalization to patients with large burns at a dose of 0.2 mg/kg/d, significantly improved outcomes relative to blinded randomized control patients. Endogenous levels of IGF-1, which are drastically decreased in severely burned patients, were substantially increased by treatment.74,75 Donor-site healing times were decreased by 25%, as were hospital lengths of stay.75,76 Compared with patients who did not receive rhGH, scarring had improved considerably by 2 years postburn.77 By decreasing donor-site healing times, rhGH decreased hospital length of stay and cost of care for patients who received the drug during the acute hospitalization.78
The rhGH substantially decreases the acute-phase response by decreasing C-reactive protein, serum tumor necrosis factor–α, IL-1, and serum amyloid-A.79
In experimental models, growth hormone increases albumin production in the liver.79,80 rhGH treatment enhances Thelper–1 and reduces T-helper–2 cytokine production, and positively modulates the humoral immune response.81,82
At a dose of 0.05 mg/kg/d, administered to children with burns >40% of the body for 1 year postburn, there were considerable improvements in weight gain, height velocities, lean body mass, bone mineral content, and cardiac function, specifically ejection fraction, relative to blinded controls.69,83 These effects were seen up to 36 months postinjury84,85
It should be noted that rhGH treatment does not come without side effects. The most notable side effect in severely burned patients was hyperglycemia.86 Although Takala and colleagues87 found, in a double-blinded randomized controlled trial, an increased mortality rate in nonburned critically ill adults, we found no differences in mortality for severely burned pediatric patients randomized to receive the drug.80 rhGH can be used safely in pediatric burn patients to attenuate the hypermetabolic response with close monitoring to treat sequelae of therapy.
IGF-1 and IGFBP-3
Effects of growth hormone are, in part, mediated by IGF-1. Infusion of IGF-1 alone led to dramatic improvements in protein metabolism, but also episodes of hypoglycemia.88 In combination with equimolar doses of IGFBP-3 (the principal binding protein for IGF-1), severely burned catabolic patients had improved protein synthesis with fewer incidences of hyperglycemia than with use of rhGH, and fewer incidences of hypoglycemia than with use of IGF-1 alone.88–90 The combination of IGF-1 and IGFBP-3 not only decreased muscle and protein catabolism in severely burned pediatric patients, but also improved gut mucosal integrity and immune function in experimental models.90–92 In fact, the fractional synthetic rate for protein was stimulated by 2% per day for patients receiving the combination compared with standard of care.90 IGF-1 with IGFBP-3 is potentially a good drug combination to ameliorate the hypermetabolic response in pediatric burn patients. This combination is being developed for clinical use as are other effective IGF-1 mimetic agents.
Insulin
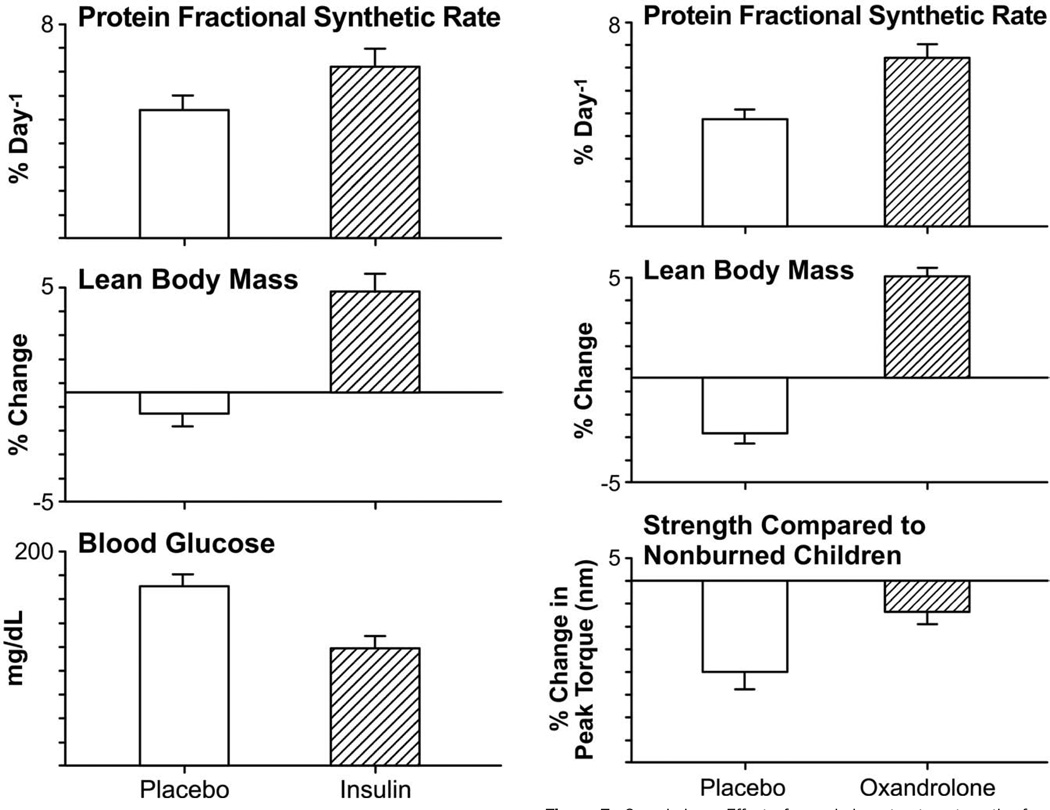
Increases in catecholamine, glucagon, and glucocorticoid production postburn led to enhanced glycogenolysis and protein breakdown in both the liver and skeletal muscle.59 There is an increase in triglycerides, glycerol, urea, and glucose production (gluconeogenesis), which leads to hyperglycemia. Hyperglycemia leads to reduced graft take and increased infections.93,94 Van den Berghe and colleagues95 showed that intensive insulin therapy to achieve blood glucose levels between 80 and 110 mg/dL in critically ill patients improves morbidity and mortality. We have found that intensive insulin therapy stimulates muscle protein synthesis and increases lean body mass without increasing hepatic triglyceride production in burn patients96,97 (Fig. 6). Severely burned patients who received continuous infusions of insulin and glucose titrated to a plasma insulin concentration of 400 to 900 μu/mL to maintain euglycemia for 7 days had improved donor-site wound healing relative to untreated controls.98
Figure 6.
Insulin. Effect of insulin therapy on the fractional synthetic rate of muscle protein synthesis, lean body mass, and average blood glucose levels.96,99 Changes in net protein balance of muscle protein synthesis and breakdown induced by burn injury was measured by stable isotope studies using d5-phenyalanine infusion studies published previously.90,132,133 Graphs are averages ± SEM. White bars represent patients with burns ≥40% total body surface area (TBSA) that received no anabolic agents or insulin. Striped bars represent patients with burns ≥40% TBSA who were randomized to receive insulin.
Infusing insulin at a rate of 1.5 μu/kg/min to maintain blood glucose levels between 100 to 140 mg/dL—in burn patients fed 1,500 kcal/m2 plus 1,500 kcal/m2 burned per 24 hours of high-carbohydrate, high-protein enteral feedings (Vivonex TEN; Sandoz Nutritional Corp)—substantially improved lean body mass and bone mineral density, and decreased length of hospital stay.99 Judicious control of the insulin infusion rates is paramount in the clinical management of these patients, as risk for hypoglycemia approaches 20%. There are currently no large prospective randomized controlled trials to determine which blood glucose level is most appropriate for severely burned patients.
Fenofibrate
Postburn hyperglycemia is associated with increased morbidity and mortality.93 In addition, mitochondrial oxidative function is impaired by up to 70% at 1 week after severe burn injury.100 Many animal studies have shown improvements in mitochondrial function and glucose oxidation with use of fenofibrate—a peroxisome proliferator– activated receptor [α] agonist.101 In a double-blind, prospective, placebo-controlled randomized trial, fenofibrate treatment significantly decreased plasma glucose levels without causing hypoglycemia.100 It decreased hepatic gluconeogenesis and improved mitochondrial oxidative capacity.100 Fenofibrate administration during 2 weeks improved muscle and hepatic insulin resistance in pediatric burn patients without any notable side effects, and it can be given orally.100
Oxandrolone
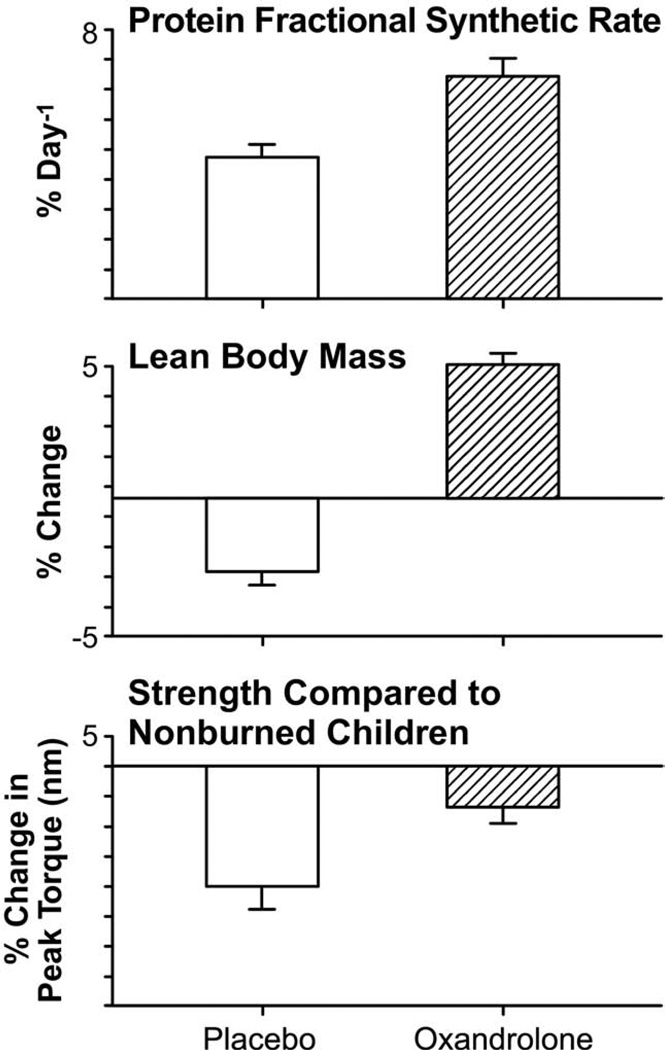
The hormonal milieu postburn is one of increased plasma catecholamines, increased cortisol and hypoandrogenemia. In severely burned male patients, testicular steroid production is substantially decreased, despite stable luteinizing hormone levels.102,103 This state of testicular dysfunction can last months postburn.103,104 Elevated levels of cortisol coupled with decreased levels of testosterone can contribute greatly to the loss of lean body mass seen in this patient population.102 Testosterone administration has been shown to ameliorate catabolism in fasting by increasing protein synthesis in normal subjects.105,106 It was also shown to ameliorate muscle protein loss after severe burn injury in male patients.102 Despite its efficacy, weekly IM administration of testosterone longterm is fraught with both expense and difficulty with compliance. In addition, it is an androgenic steroid, contraindicated for use in female patients. Oxandrolone, a synthetic analogue, administered orally, offers only 5% of the masculinizing effects of testosterone and is safe for both genders. Oxandrolone, when administered at a dose of 0.1 mg/kg twice daily, improved net muscle protein synthesis and protein metabolism in severely burned patients107,108 (Fig. 7). During the acute phase postburn and up to 1 year of treatment, oxandrolone increased lean body mass, bone mineral content, and muscle strength.107–111 In addition, it decreased length of stay by decreasing time between operations for patients randomized to receive oxandrolone plus standard of care.108 Oxandrolone results in considerable improvements in lean body mass, protein synthesis, and overall growth in burn patients, mitigating the 1% risk of hirsutism, and hepatic dysfunction that can be seen with treatment.108–111
Figure 7.
Oxandrolone. Effect of oxandrolone treatment on the fractional synthetic rate of muscle protein synthesis, lean body mass, and strength.107,108 Changes in net protein balance of muscle protein synthesis and breakdown induced by burn injury was measured by stable isotope studies using d5-phenyalanine infusion studies published previously.1,24,107 Graphs are averages ± SEM. White bars represent patients with burns ≥40% total body surface area (TBSA) who received no anabolic agents. Striped bars represent patients with burns ≥40% TBSA that were randomized to receive oxandrolone.
β-receptor-antagonists
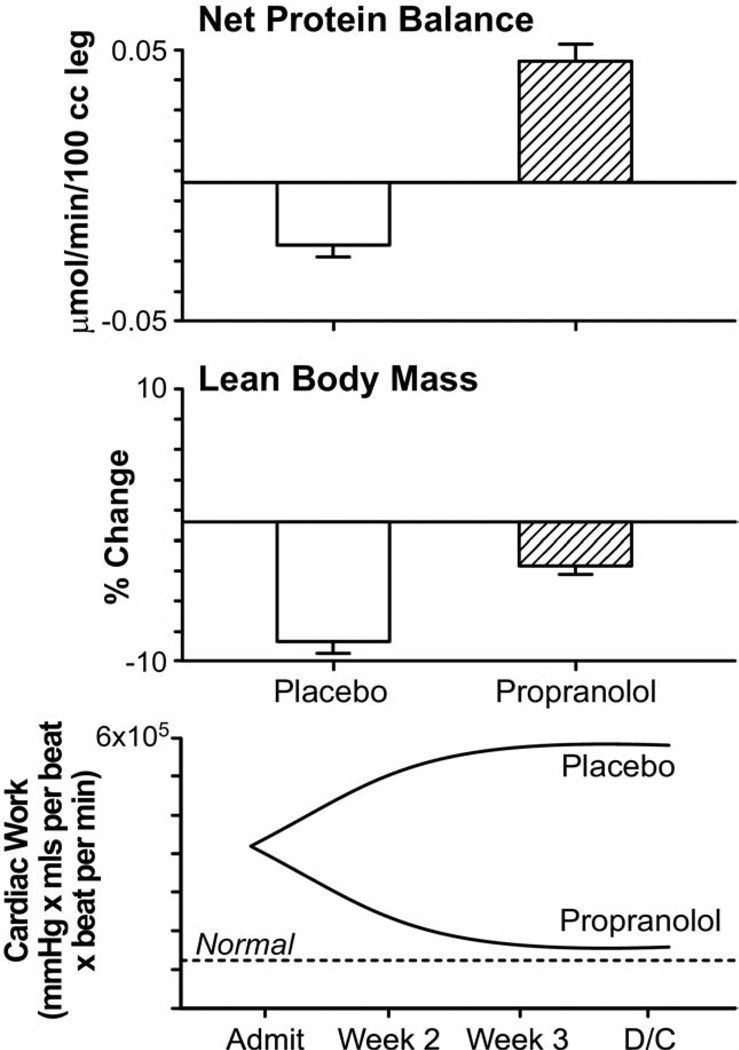
The 10-fold increase in catecholamine levels in large burns induced increased myocardial oxygen consumption, increased resting energy expenditures, and contributed greatly to the profound catabolism the body experiences after severe burn injury.11,32 One of the most effective anticatabolic strategies for the treatment of burns is to block the effects of elevated levels of plasma catecholamines. Catecholamines have been implicated in the tachycardia seen after major operations and β-receptor-antagonist treatment reduced the rate of cardiac complications and decreased mortality.112 In burns, β-receptor-antagonist treatment reduced thermogenesis, marked tachycardia, and resting energy expenditures.113,114 By reducing heart rates by 20%, there was a substantial decrease in cardiac work.19 Catecholamines trigger increased peripheral lipolysis in thermally injured patients through activation of the β2-adernergic receptor.17,113,115 Propranolol helps prevent this deleterious effect of catecholamines.113 Propranolol treatment substantially decreased fatty infiltration of the liver compared with untreated severely burned children.116,117 By inhibiting release of free fatty acids from adipose tissue, decreasing the rates of fatty acid oxidation and triacylglycerol secretion, and increasing the efficiency of the liver in excreting fatty acids, propranolol substantially reduces hepatic steatosis.117–119 Propranolol increases lean body mass and decreases skeletal muscle wasting as proved by stable isotope and body composition studies.16 It enhances the availability of free amino acids for muscle protein synthesis16,118,119 (Fig. 8). It caused no substantial bronchospasm, or cardiovascular collapse in patients studied in randomized control trials to date.
Figure 8.
Propranolol. Effect of propranolol treatment on the fractional synthetic rate of muscle protein synthesis, lean body mass, and cardiac work.16 Changes in net protein balance of muscle protein synthesis and breakdown induced by burn injury was measured by stable isotope studies using d5-phenyalanine infusion studies published previously.1,16,24 Graphs are averages ± SEM. White bars represent patients with burns ≥40% total body surface area (TBSA) that received no anabolic agents. Striped bars represent patients with burns ≥40% TBSA that were randomized to receive propranolol. Values from nonburned, normal patients are represented by dashed lines (—).
A role for combination therapy
The anticatabolic effect of propranolol and the potent anabolic effects of rhGH might represent an ideal combination for attenuating the postburn hypermetabolic response. Although improving donor site healing, growth, and the inflammatory cascade, rhGH treatment leads to hyperglycemia and increased free fatty acids and triglycerides. Propranolol treatment demonstrated improved fat metabolism and improvements in insulin sensitivity and decreasing cardiac work. In combination, there were considerable improvements in metabolic rates, serum C-reactive protein, cortisol, liver function, lipid metabolism, and cytokine profiles compared with controls, without adverse side effects.120,121 The combination of propranolol and oxandrolone is currently under trial.
Beyond acute hospitalization
The modulators mentioned previously that were used by our institution produce profound improvements in the hormonal, inflammatory, metabolic, and physiologic responses to severe burn injury during the acute hospitalization. Derangements seen in severely burned victims last well beyond acute hospitalization. Growth delay, hyperdynamic circulation, increased metabolic rates, and insulin resistance last at least 12 months after injury.1,3,16,26,122 Muscle protein and bone catabolism contribute considerably to the cachexic state of our patients up to 2 years postinjury. Administration of oxandrolone beyond the acute hospitalization, for up to 1 year postinjury, led to dramatic improvements in lean body mass and bone mineral content— essentially reversing growth retardation.111 Use of growth hormone, propranolol, and the combination of oxandrolone and propranolol longterm are currently being evaluated.
Exercise
Despite methods to abate the stress response, patients suffer growth retardation for years into the rehabilitative phase.3 Patients with severe burn injury have substantial functional limitations as they progress through rehabilitation. Exercise training is an essential adjunct to any and all metabolic treatments administered to the burn survivor. It increases lean body mass, improves strength, ability to walk distances, and overall cardiopulmonary capacity.123,124 We have reported that a 12-week resistance and aerobic exercise training program, added to standard of care after acute hospitalization, profoundly improves range of motion, muscle strength, lean body mass, and power compared with standard of care alone.125,126 An exercise training program is essential to improve body mass, decrease joint contractures, and scarring.123
Complications and cost
Most catabolic burn patients respond positively to anabolic pharmacotherapy. Subgroup analyses revealed that patients who are not catabolic fail to respond to anabolic or anti-catabolic therapy. Response to anabolic drugs was greater in victims with >40% TBSA burned than in those with <40% TBSA. In ill, catabolic patients, the incidence of pharmacologic adverse reactions in our institution is low (Table 1). The pharmacologic strategies with the least documented complications in randomized controlled trials are propranolol and oxandrolone. These agents are the most cost-effective pharmacotherapy and drug regimen with the greatest experimental efficacy. Daily cost of these agents, extrapolated to the dose for a 70-kg person is provided in Table 2. Oxandrolone is 5% of the cost of recombinant growth hormone, and its efficacy has been documented by many investigative groups in a wide array of both healthy and ill, catabolic subjects. Based on our analysis of relative efficacy, cost, and documented adverse drug reactions (at our institution), we believe that oxandrolone and propranolol hold the greatest promise as pharmacotherapies for catabolic burn victims.
Table 1.
Potential Complications and Incidence in Pediatric Burns ≥40% Total Body Surface Area
| Drug | Complications | Incidence (%) |
|---|---|---|
| rhGH | Hyperglycemia | 50* |
| Growth arrest | 0 | |
| IGF-1/IGFBP-3 | Hypoglycemia | 25* |
| Neuropathy | 23 | |
| Insulin | Hypoglycemia | 20* |
| Oxandrolone | Hirsutism | 1 |
| Hepatic dysfunction | 1 | |
| Propranolol | Bronchospasm | 0 |
| Cardiovascular collapse | 0 | |
Dose-dependent.
IGF-1, insulin-like growth factor-1; IGFBP-3, with insulin-like growth factor binding protein-3; rhGH, recombinant human growth hormone.
Table 2.
Approximate Cost of Daily Dose for 70-kg Patient
| Drug | Dose | Cost per day ($) |
|---|---|---|
| rhGH | 0.2 mg/kg/d | 490 |
| IGF-1/IGFBP-3 | 2 mg/kg/h | Off market |
| Insulin | 1–5 U/h (IV) | 3.47 |
| Oxandrolone | 15 mg/d | 16.50 |
| Propranolol | 0.5–4 mg/kg/d | 2.56 |
IGF-1, insulin-like growth factor-1; IGFBP-3, with insulin-like growth factor binding protein-3; rhGH, recombinant human growth hormone.
Relative efficacy
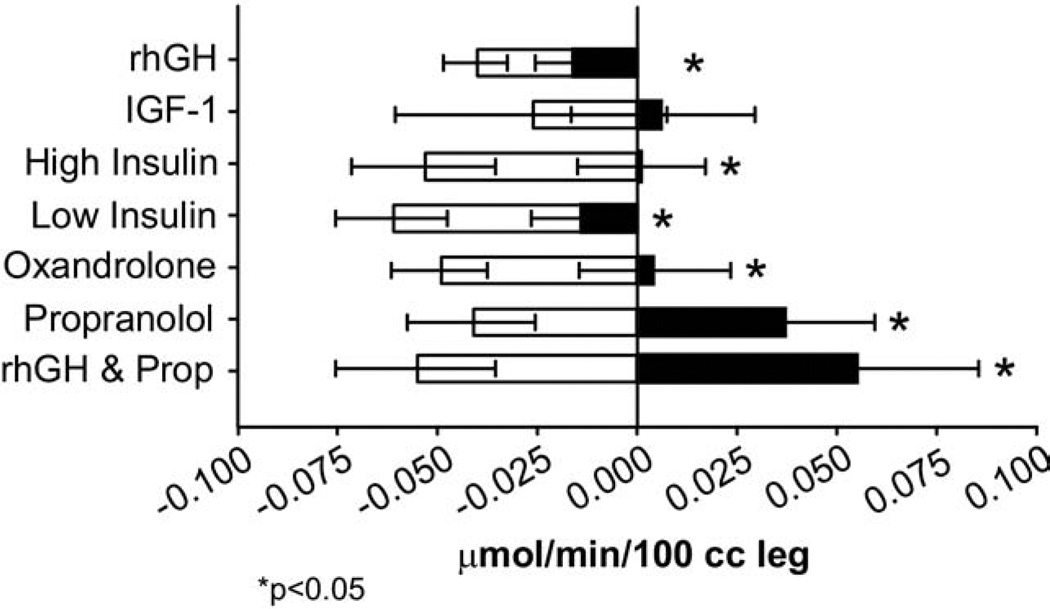
We have demonstrated the effectiveness of these pharmacologic agents in abating the hypermetabolic response, although they have different mechanisms of action. Using stable isotopes and body composition studies, we found substantial differences in outcomes with use of growth hormone, insulin, oxandrolone, propranolol, and combination therapy of growth hormone and propranolol, compared with placebo (Fig. 9). All pharmacologic treatments, specifically mentioned here, are superior to standard of care alone to curb the postburn hypermetabolic response. Currently, we recommend exercise, oxandrolone, and propranolol for the treatment of the severe hypermetabolic response in children and adults, and growth hormone for selected very large burns in pediatric patients.
Figure 9.
Relative efficacy of the different anabolic agents to improve muscle protein synthesis compared with standard of care alone. Changes in net protein balance of muscle protein synthesis and breakdown induced by burn injury was measured by stable isotope studies using d5-phenyalanine infusion studies published previously.1,12,24,90,129,132,133 *p < 0.05. Graphs are averages ± SEM. White bars represent patients with burns ≥40% total body surface area (TBSA) that received no anabolic agents. Black bars represent patients with burns ≥40% TBSA that were randomized to receive drug.
The hypermetabolic, hypercatabolic response is prolonged and persistent beyond acute hospitalization. Neither nonpharmacologic nor pharmacologic strategies are sufficient to fully abate the response to severe burn injury. The therapeutic strategies described have contributed to improvements in morbidity and mortality. In 1942, the TBSA burned that killed 50% (lethal median dose) of the youngest (younger than 4 years) and oldest victims (older than 65 years) were 49% and 10%, respectively. Today, those numbers have improved to 98% and 35%, respectively127,128 (Tables 3 to 4). Treated catabolic patients with ≥40% TBSA, on average, have a 2% to 10% loss of total body mass, down from 25% loss 20 years ago.12,129 Modulation of the hypermetabolic response is paramount in the restoration of structure and function of severely burned patients. Certain pharmacologic strategies have superior experimental efficacy than others, but each addressed and reversed, in part, the maladaptive catabolic physiologic responses to severe burn injury. Combination therapies can be superior to monotherapy interventions in attenuating the postburn response. Additional studies are necessary to determine the optimal treatment for the catabolic response in burns and to determine which of these therapies will be useful in the treatment of other injuries or elective operations.
Table 3.
Burn Mortality (LD50) Percent Total Body Surface Area Lethal Burn
| Ages 0–14 y |
Ages 15–44 y |
Ages 45–64 y |
Ages older than 65 y |
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % TBSA | n | % TBSA | n | % TBSA | n | % TBSA | n | |
| 1940–1950 | 2,807 | 49 | 1,336 | 46 | 967 | 27 | 330 | 10 | 144 |
| 1990–today | 1,722 | 98 | 1,083 | 82 | 420 | 78 | 152 | 35 | 67 |
LD50, median dose that is lethal for 50% of those tested; TBSA, total body surface area.
Table 4.
Mortality Rates for Pediatric Burn Patients in Our Institution from 1980 to 2007
| 40–60% TBSA burn (%) |
61–100% TBSA burn (%) |
|
|---|---|---|
| From 1980 to 1986 n = 1,524) | 8 | 32 |
| From 1987 to 2007 (n = 3,188) | 4 | 18 |
TBSA, total body surface area.
Acknowledgment
The authors would like to thank the Clinical Research Staff for their assistance in the performance of the studies and Eileen Figueroa and Steve Schuenke for their help in the preparation of the article.
Supported by Shriners Hospital Center grant nos. 8660, 8490, 8640, 8760, and 9145, NIH training grant no. 2T32GM0825611, NIH center grant no. 1P50GM60338-01,NIH grant no. 5RO1GM56687-03,NIH R01-GM56687, NIH grant no. R01-HD049471, National Institute on Disability and Rehabilitation Research (NIDDR) grant no. H133A020102, NIDDR grant no. H133A70019, National Institute of General Medical Sciences grant no. U54/ GM62119, American Surgical Association Foundation Grant (MJ). Presented as the IS Ravdin Lecture in the Basic Sciences at the American College of Surgeons 94th Annual Clinical Congress, San Francisco, CA, October 2008.
Abbreviations and Acronyms
- IGF-1
insulin-like growth factor-1
- IGFBP-3
insulin-like growth factor binding protein-3
- IL
interleukin
- rhGH
recombinant human growth hormone
- TBSA
total body surface area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
REFERENCES
- 1.Hart DW, Wolf SE, Mlcak R, et al. Persistence of muscle catabolism after severe burn. Surgery. 2000;128:312–319. doi: 10.1067/msy.2000.108059. [DOI] [PubMed] [Google Scholar]
- 2.Reiss E, Pearson E, Artz CP. The metabolic response to burns. J Clin Invest. 1956;35:62–77. doi: 10.1172/JCI103253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rutan RL, Herndon DN. Growth delay in postburn pediatric patients. Arch Surg. 1990;125:392–395. doi: 10.1001/archsurg.1990.01410150114021. [DOI] [PubMed] [Google Scholar]
- 4.Wilmore DW, Mason AD, Jr., Pruitt BA., Jr. Insulin response to glucose in hypermetabolic burn patients. Ann Surg. 1976;183:314–320. doi: 10.1097/00000658-197603000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu YM, Tompkins RG, Ryan CM, Young VR. The metabolic basis of the increase of the increase in energy expenditure in severely burned patients. JPEN J Parenter Enteral Nutr. 1999;23:160–168. doi: 10.1177/0148607199023003160. [DOI] [PubMed] [Google Scholar]
- 6.Cuthbertson D. Post-shock metabolic response. Lancet. 1942:433–436. [PubMed] [Google Scholar]
- 7.Goldstein DS, Kopin IJ. Evolution of concepts of stress. Stress. 2007;10:109–120. doi: 10.1080/10253890701288935. [DOI] [PubMed] [Google Scholar]
- 8.Selye H. Stress and the general adaptation syndrome. Br Med J. 1950;1:1383–1392. doi: 10.1136/bmj.1.4667.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selye H. An extra-adrenal action of adrenotropic hormone. Nature. 1951;168:149–150. doi: 10.1038/168149a0. [DOI] [PubMed] [Google Scholar]
- 10.Selye H, Fortier C. Adaptive reaction to stress. Psychosom Med. 1950;12:149–157. doi: 10.1097/00006842-195005000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Wilmore DW, Long JM, Mason AD, Jr., et al. Catecholamines: mediator of the hypermetabolic response to thermal injury. Ann Surg. 1974;180:653–669. doi: 10.1097/00000658-197410000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeschke MG, Chinkes DL, Finnerty CC, et al. Pathophysiologic response to severe burn injury. Ann Surg. 2008;248:387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilmore DW, Aulick LH. Metabolic changes in burned patients. Surg Clin North Am. 1978;58:1173–1187. doi: 10.1016/s0039-6109(16)41685-3. [DOI] [PubMed] [Google Scholar]
- 14.Barrow RE, Hawkins HK, Aarsland A, et al. Identification of factors contributing to hepatomegaly in severely burned children. Shock. 2005;24:523–528. doi: 10.1097/01.shk.0000187981.78901.ee. [DOI] [PubMed] [Google Scholar]
- 15.Barrow RE, Wolfe RR, Dasu MR, et al. The use of beta-adrenergic blockade in preventing trauma-induced hepatomegaly. Ann Surg. 2006;243:115–120. doi: 10.1097/01.sla.0000193834.07413.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223–1229. doi: 10.1056/NEJMoa010342. [DOI] [PubMed] [Google Scholar]
- 17.Wolfe RR, Herndon DN, Peters EJ, et al. Regulation of lipolysis in severely burned children. Ann Surg. 1987;206:214–221. doi: 10.1097/00000658-198708000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004;363:1895–1902. doi: 10.1016/S0140-6736(04)16360-5. [DOI] [PubMed] [Google Scholar]
- 19.Baron PW, Barrow RE, Pierre EJ, Herndon DN. Prolonged use of propranolol safely decreases cardiac work in burned children. J Burn Care Rehabil. 1997;18:223–237. doi: 10.1097/00004630-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Bessey PQ, Jiang ZM, Johnson DJ, et al. Posttraumatic skeletal muscle proteolysis: the role of the hormonal environment. World J Surg. 1989;13:465–470. doi: 10.1007/BF01660758. discussion 471. [DOI] [PubMed] [Google Scholar]
- 21.Chang DW, DeSanti L, Demling RH. Anticatabolic and anabolic strategies in critical illness: a review of current treatment modalities. Shock. 1998;10:155–160. doi: 10.1097/00024382-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Newsome TW, Mason AD, Jr., Pruitt BA., Jr. Weight loss following thermal injury. Ann Surg. 1973;178:215–217. doi: 10.1097/00000658-197308000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jahoor F, Desai M, Herndon DN, Wolfe RR. Dynamics of the protein metabolic response to burn injury. Metabolism. 1988;37:330–337. doi: 10.1016/0026-0495(88)90132-1. [DOI] [PubMed] [Google Scholar]
- 24.Hart DW, Wolf SE, Chinkes DL, et al. Determinants of skeletal muscle catabolism after severe burn. Ann Surg. 2000;232:455–465. doi: 10.1097/00000658-200010000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinney JM, Long CL, Gump FE, Duke JH., Jr. Tissue composition of weight loss in surgical patients. I Elective operation. Ann Surg. 1968;168:459–474. doi: 10.1097/00000658-196809000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolfe RR. Review: acute versus chronic response to burn injury. Circ Shock. 1981;8:105–115. [PubMed] [Google Scholar]
- 27.Wilmore DW, Aulick LH, Mason AD, Pruitt BA., Jr. Influence of the burn wound on local and systemic responses to injury. Ann Surg. 1977;186:444–458. doi: 10.1097/00000658-197710000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carter EA, Tompkins RG, Babich JW, et al. Thermal injury in rats alters glucose utilization by skin, wound, and small intestine, but not by skeletal muscle. Metabolism. 1996;45:1161–1167. doi: 10.1016/s0026-0495(96)90017-7. [DOI] [PubMed] [Google Scholar]
- 29.Greenhalgh DG, Saffle JR, Holmes JHIV, et al. American Burn Association consensus conference to define sepsis and infection in burns. J Burn Care Res. 2007;28:776–790. doi: 10.1097/BCR.0b013e3181599bc9. [DOI] [PubMed] [Google Scholar]
- 30.Murray CK, Loo FL, Hospenthal DR, et al. Incidence of systemic fungal infection and related mortality following severe burns. Burns. 2008;34:1108–1112. doi: 10.1016/j.burns.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Pruitt BA, Jr., McManus AT, Kim SH, Goodwin CW. Burn wound infections: current status. World J Surg. 1998;22:135–145. doi: 10.1007/s002689900361. [DOI] [PubMed] [Google Scholar]
- 32.Goodall M, Stone C, Haynes BW., Jr. Urinary output of adrenaline and noradrenaline in severe thermal burns. Ann Surg. 1957;145:479–487. doi: 10.1097/00000658-195704000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desai MH, Herndon DN, Broemeling L, et al. Early burn wound excision significantly reduces blood loss. Ann Surg. 1990;211:753–759. doi: 10.1097/00000658-199006000-00015. discussion 9–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herndon DN, Barrow RE, Rutan RL, et al. A comparison of conservative versus early excision. Therapies in severely burned patients. Ann Surg. 1989;209:547–552. doi: 10.1097/00000658-198905000-00006. discussion 52–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gray DT, Pine RW, Harnar TJ, et al. Early surgical excision versus conventional therapy in patients with 20 to 40 percent burns. A comparative study. Am J Surg. 1982;144:76–80. doi: 10.1016/0002-9610(82)90605-5. [DOI] [PubMed] [Google Scholar]
- 36.Merrell SW, Saffle JR, Sullivan JJ, et al. Increased survival after major thermal injury. A nine year review. Am J Surg. 1987;154:623–627. doi: 10.1016/0002-9610(87)90229-7. [DOI] [PubMed] [Google Scholar]
- 37.Hart DW, Wolf SE, Chinkes DL, et al. Effects of early excision and aggressive enteral feeding on hypermetabolism, catabolism, and sepsis after severe burn. J Trauma. 2003;54:755–761. doi: 10.1097/01.TA.0000060260.61478.A7. discussion 761–764. [DOI] [PubMed] [Google Scholar]
- 38.Chai J, Sheng Z, Diao L, et al. Effect of extensive excision of burn wound with invasive infection on hypermetabolism in burn patients with sepsis. Zhonghua Wai Ke Za Zhi. 2000;38:405–408. [PubMed] [Google Scholar]
- 39.Barret JP, Herndon DN. Modulation of inflammatory and catabolic responses in severely burned children by early burn wound excision in the first 24 hours. Arch Surg. 2003;138:127–132. doi: 10.1001/archsurg.138.2.127. [DOI] [PubMed] [Google Scholar]
- 40.Gauglitz GG, Finnerty CC, Herndon DN, et al. Are serum cytokines early predictors for the outcome of burn patients with inhalation injuries who do not survive? Crit Care. 2008;12:R81. doi: 10.1186/cc6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mochizuki H, Trocki O, Dominioni L, et al. Mechanism of prevention of postburn hypermetabolism and catabolism by early enteral feeding. Ann Surg. 1984;200:297–310. doi: 10.1097/00000658-198409000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dominioni L, Trocki O, Fang CH, et al. Enteral feeding in burn hypermetabolism: nutritional and metabolic effects of different levels of calorie and protein intake. JPEN J Parenter Enteral Nutr. 1985;9:269–279. doi: 10.1177/0148607185009003269. [DOI] [PubMed] [Google Scholar]
- 43.Curreri PW, Richmond D, Marvin J, Baxter CR. Dietary requirements of patients with major burns. J Am Diet Assoc. 1974;65:415–417. [PubMed] [Google Scholar]
- 44.Wilmore DW, Curreri PW, Spitzer KW, et al. Supranormal dietary intake in thermally injured hypermetabolic patients. Surg Gynecol Obstet. 1971;132:881–886. [PubMed] [Google Scholar]
- 45.Hildreth MA, Herndon DN, Desai MH, Duke MA. Reassessing caloric requirements in pediatric burn patients. J Burn Care Rehabil. 1988;9:616–68. doi: 10.1097/00004630-198811000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Herndon DN, Barrow RE, Stein M, et al. Increased mortality with intravenous supplemental feeding in severely burned patients. J Burn Care Rehabil. 1989;10:309–313. doi: 10.1097/00004630-198907000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Herndon DN, Stein MD, Rutan TC, et al. Failure of TPN supplementation to improve liver function, immunity, and mortality in thermally injured patients. J Trauma. 1987;27:195–204. doi: 10.1097/00005373-198702000-00018. [DOI] [PubMed] [Google Scholar]
- 48.Jeejeebhoy KN. Total parenteral nutrition: potion or poison? Am J Clin Nutr. 2001;74:160–163. doi: 10.1093/ajcn/74.2.160. [DOI] [PubMed] [Google Scholar]
- 49.Lee J. The pediatric burned patient. In: Herndon DN, editor. Total burn care. 3rd ed. Philadelphia: Saunders Elsevier; 2007. pp. 485–493. [Google Scholar]
- 50.Goran MI, Peters EJ, Herndon DN, Wolfe RR. Total energy expenditure in burned children using the doubly labeled water technique. Am J Physiol. 1990;259:E576–E585. doi: 10.1152/ajpendo.1990.259.4.E576. [DOI] [PubMed] [Google Scholar]
- 51.Gore DC, Rutan RL, Hildreth M, et al. Comparison of resting energy expenditures and caloric intake in children with severe burns. J Burn Care Rehabil. 1990;11:400–404. doi: 10.1097/00004630-199009000-00005. [DOI] [PubMed] [Google Scholar]
- 52.Hart DW, Wolf SE, Herndon DN, et al. Energy expenditure and caloric balance after burn: increased feeding leads to fat rather than lean mass accretion. Ann Surg. 2002;235:152–161. doi: 10.1097/00000658-200201000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolfe RR, Goodenough RD, Burke JF, Wolfe MH. Response of protein and urea kinetics in burn patients to different levels of protein intake. Ann Surg. 1983;197:163–171. doi: 10.1097/00000658-198302000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu YM, Young VR, Castillo L, et al. Plasma arginine and leucine kinetics and urea production rates in burn patients. Metabolism. 1995;44:659–666. doi: 10.1016/0026-0495(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 55.Melville S, McNurlan MA, McHardy KC, et al. The role of degradation in the acute control of protein balance in adult man: failure of feeding to stimulate protein synthesis as assessed by L-[1–13C]leucin infusion. Metabolism. 1989;38:248–255. doi: 10.1016/0026-0495(89)90083-8. [DOI] [PubMed] [Google Scholar]
- 56.Hoerr RA, Matthews DE, Bier DM, Young VR. Effects of protein restriction and acute refeeding on leucine and lysine kinetics in young men. Am J Physiol. 1993;264:E567E75. doi: 10.1152/ajpendo.1993.264.4.E567. [DOI] [PubMed] [Google Scholar]
- 57.Yu YM, Ryan CM, Burke JF, et al. Relations among arginine, citrulline, ornithine, and leucine kinetics in adult burn patients. Am J Clin Nutr. 1995;62:960–968. doi: 10.1093/ajcn/62.5.960. [DOI] [PubMed] [Google Scholar]
- 58.Matthews DE, Marano MA, Campbell RG. Splanchnic bed utilization of leucine and phenylalanine in humans. Am J Physiol. 1993;264:E109–E118. doi: 10.1152/ajpendo.1993.264.1.E109. [DOI] [PubMed] [Google Scholar]
- 59.Norbury WB. Modulation of the hypermetabolic response after burn injury. In: Herndon DN, editor. Total burn care. 3rd ed. Philadelphia: Saunders Elsevier; 2007. pp. 420–433. [Google Scholar]
- 60.Saffle JR. Nutritional support of the burned patient. In: Herndon DN, editor. Total burn care. 3rd ed. Philadelphia: Saunders Elsevier; 2007. pp. 398–419. [Google Scholar]
- 61.Patterson BW, Nguyen T, Pierre E, et al. Urea and protein metabolism in burned children: effect of dietary protein intake. Metabolism. 1997;46:573–578. doi: 10.1016/s0026-0495(97)90196-7. [DOI] [PubMed] [Google Scholar]
- 62.Barr PO, Birke G, Liliedal SO. [The treatment of thermal burns with dry, warm air] Eksp Khir Anesteziol. 1968;13:39–43. [PubMed] [Google Scholar]
- 63.Barr PO, Birke G, Liljedahl SO, Plantin LO. Studies on burns. X Changes in BMR and evaporative water loss in the treatment of severe burns with warm dry air. Scand J Plast Reconstr Surg. 1969;3:30–38. doi: 10.3109/02844316909036692. [DOI] [PubMed] [Google Scholar]
- 64.Caldwell FT. Metabolic response to thermal trauma: II. Nutritional studies with rats at two environmental temperatures. Ann Surg. 1962;155:119–126. doi: 10.1097/00000658-196201000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zawacki BE, Spitzer KW, Mason AD, Jr., Johns LA. Does increased evaporative water loss cause hypermetabolism in burned patients? Ann Surg. 1970;171:236–240. doi: 10.1097/00000658-197002000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wolfe RR, Herndon DN, Jahoor F, et al. Effect of severe burn injury on substrate cycling by glucose and fatty acids. N Engl J Med. 1987;317:403–408. doi: 10.1056/NEJM198708133170702. [DOI] [PubMed] [Google Scholar]
- 67.Wilmore DW, Mason AD, Jr., Johnson DW, Pruitt BA., Jr. Effect of ambient temperature on heat production and heat loss in burn patients. J Appl Physiol. 1975;38:593–597. doi: 10.1152/jappl.1975.38.4.593. [DOI] [PubMed] [Google Scholar]
- 68.Herndon DN. Mediators of metabolism. J Trauma. 1981;21(Suppl):701–705. [Google Scholar]
- 69.Hart DW, Herndon DN, Klein G, et al. Attenuation of post-traumatic muscle catabolism and osteopenia by long-term growth hormone therapy. Ann Surg. 2001;233:827–834. doi: 10.1097/00000658-200106000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klein GL, Herndon DN, Langman CB, et al. Long-term reduction in bone mass after severe burn injury in children. J Pediatr. 1995;126:252–256. doi: 10.1016/s0022-3476(95)70553-8. [DOI] [PubMed] [Google Scholar]
- 71.Klein GL, Herndon DN. Magnesium deficit in major burns: role in hypoparathyroidism and end-organ parathyroid hormone resistance. Magnes Res. 1998;11:103–109. [PubMed] [Google Scholar]
- 72.Klein GL, Langman CB, Herndon DN. Persistent hypoparathyroidism following magnesium repletion in burn-injured children. Pediatr Nephrol. 2000;14:301–304. doi: 10.1007/s004670050763. [DOI] [PubMed] [Google Scholar]
- 73.Klein GL, Nicolai M, Langman CB, et al. Dysregulation of calcium homeostasis after severe burn injury in children: possible role of magnesium depletion. J Pediatr. 1997;131:246–251. doi: 10.1016/s0022-3476(97)70161-6. [DOI] [PubMed] [Google Scholar]
- 74.Klein GL, Wolf SE, Langman CB, et al. Effects of therapy with recombinant human growth hormone on insulin-like growth factor system components and serum levels of biochemical markers of bone formation in children after severe burn injury. J Clin Endocrinol Metab. 1998;83:21–24. doi: 10.1210/jcem.83.1.4518. [DOI] [PubMed] [Google Scholar]
- 75.Herndon DN, Hawkins HK, Nguyen TT, et al. Characterization of growth hormone enhanced donor site healing in patients with large cutaneous burns. Ann Surg. 1995;221:649–656. doi: 10.1097/00000658-199506000-00004. discussion 656–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Herndon DN, Barrow RE, Kunkel KR, et al. Effects of recombinant human growth hormone on donor-site healing in severely burned children. Ann Surg. 1990;212:424–429. doi: 10.1097/00000658-199010000-00005. discussion 430–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barret JP, Dziewulski P, Jeschke MG, et al. Effects of recombinant human growth hormone on the development of burn scarring. Plast Reconstr Surg. 1999;104:726–729. doi: 10.1097/00006534-199909030-00017. [DOI] [PubMed] [Google Scholar]
- 78.Herndon DN. Nutritional and pharmacological support of the metabolic response to injury. Minerva Anestesiol. 2003;69:264–274. [PubMed] [Google Scholar]
- 79.Jeschke MG, Barrow RE, Herndon DN. Recombinant human growth hormone treatment in pediatric burn patients and its role during the hepatic acute phase response. Crit Care Med. 2000;28:1578–1584. doi: 10.1097/00003246-200005000-00053. [DOI] [PubMed] [Google Scholar]
- 80.Ramirez RJ, Wolf SE, Barrow RE, Herndon DN. Growth hormone treatment in pediatric burns: a safe therapeutic approach. Ann Surg. 1998;228:439–448. doi: 10.1097/00000658-199810000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takagi K, Suzuki F, Barrow RE, et al. Recombinant human growth hormone modulates Th1 andTh2 cytokine response in burned mice. Ann Surg. 1998;228:106–111. doi: 10.1097/00000658-199807000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Takagi K, Suzuki F, Barrow RE, et al. Growth hormone improves immune function and survival in burned mice infected with herpes simplex virus type 1. J Surg Res. 1997;69:166–170. doi: 10.1006/jsre.1997.5066. [DOI] [PubMed] [Google Scholar]
- 83.Mlcak RP, Suman OE, Murphy K, Herndon DN. Effects of growth hormone on anthropometric measurements and cardiac function in children with thermal injury. Burns. 2005;31:60–66. doi: 10.1016/j.burns.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 84.Low JF, Herndon DN, Barrow RE. Effect of growth hormone on growth delay in burned children: a 3-year follow-up study. Lancet. 1999;354:1789. doi: 10.1016/s0140-6736(99)02741-5. [DOI] [PubMed] [Google Scholar]
- 85.Aili Low JF, Barrow RE, Mittendorfer B, et al. The effect of short-term growth hormone treatment on growth and energy expenditure in burned children. Burns. 2001;27:447–452. doi: 10.1016/s0305-4179(00)00164-9. [DOI] [PubMed] [Google Scholar]
- 86.Singh KP, Prasad R, Chari PS, Dash RJ. Effect of growth hormone therapy in burn patients on conservative treatment. Burns. 1998;24:733–738. doi: 10.1016/s0305-4179(98)00113-2. [DOI] [PubMed] [Google Scholar]
- 87.Takala J, Ruokonen E, Webster NR, et al. Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med. 1999;341:785–792. doi: 10.1056/NEJM199909093411102. [DOI] [PubMed] [Google Scholar]
- 88.Cioffi WG, Gore DC, Rue LW, 3rd., et al. Insulin-like growth factor-1 lowers protein oxidation in patients with thermal injury. Ann Surg. 1994;220:310–316. doi: 10.1097/00000658-199409000-00007. discussion 316–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moller S, Jensen M, Svensson P, Skakkebaek NE. Insulin-like growth factor 1 (IGF-1) in burn patients. Burns. 1991;17:279–281. doi: 10.1016/0305-4179(91)90039-j. [DOI] [PubMed] [Google Scholar]
- 90.Herndon DN, Ramzy PI, DebRoy MA, et al. Muscle protein catabolism after severe burn: effects of IGF-1/IGFBP-3 treatment. Ann Surg. 1999;229:713–720. doi: 10.1097/00000658-199905000-00014. discussion 20–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Spies M, Wolf SE, Barrow RE, et al. Modulation of types I and II acute phase reactants with insulin-like growth factor-1/ binding protein-3 complex in severely burned children. Crit Care Med. 2002;30:83–88. doi: 10.1097/00003246-200201000-00013. [DOI] [PubMed] [Google Scholar]
- 92.Jeschke MG, Herndon DN, Barrow RE. Insulin-like growth factor I in combination with insulin-like growth factor binding protein 3 affects the hepatic acute phase response and hepatic morphology in thermally injured rats. Ann Surg. 2000;231:408–416. doi: 10.1097/00000658-200003000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gore DC, Chinkes D, Heggers J, et al. Association of hyperglycemia with increased mortality after severe burn injury. J Trauma. 2001;51:540–544. doi: 10.1097/00005373-200109000-00021. [DOI] [PubMed] [Google Scholar]
- 94.Gore DC, Chinkes DL, Hart DW, et al. Hyperglycemia exacerbates muscle protein catabolism in burn-injured patients. Crit Care Med. 2002;30:2438–2442. doi: 10.1097/00003246-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 95.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 96.Sakurai Y, Aarsland A, Herndon DN, et al. Stimulation of muscle protein synthesis by long-term insulin infusion in severely burned patients. Ann Surg. 1995;222:283–294. doi: 10.1097/00000658-199509000-00007. 294–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aarsland A, Chinkes DL, Sakurai Y, et al. Insulin therapy in burn patients does not contribute to hepatic triglyceride production. J Clin Invest. 1998;101:2233–2239. doi: 10.1172/JCI200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pierre EJ, Barrow RE, Hawkins HK, et al. Effects of insulin on wound healing. J Trauma. 1998;44:342–345. doi: 10.1097/00005373-199802000-00019. [DOI] [PubMed] [Google Scholar]
- 99.Thomas SJ, Morimoto K, Herndon DN, et al. The effect of prolonged euglycemic hyperinsulinemia on lean body mass after severe burn. Surgery. 2002;132:341–347. doi: 10.1067/msy.2002.126871. [DOI] [PubMed] [Google Scholar]
- 100.Cree MG, Zwetsloot JJ, Herndon DN, et al. Insulin sensitivity and mitochondrial function are improved in children with burn injury during a randomized controlled trial of fenofibrate. Ann Surg. 2007;245:214–221. doi: 10.1097/01.sla.0000250409.51289.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Minnich A, Tian N, Byan L, Bilder G. A potent PPARalpha agonist stimulates mitochondrial fatty acid beta-oxidation in liver and skeletal muscle. Am J Physiol Endocrinol Metab. 2001;280:E270–E279. doi: 10.1152/ajpendo.2001.280.2.E270. [DOI] [PubMed] [Google Scholar]
- 102.Ferrando AA, Sheffield-Moore M, Wolf SE, et al. Testosterone administration in severe burns ameliorates muscle catabolism. Crit Care Med. 2001;29:1936–1942. doi: 10.1097/00003246-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 103.Lephart ED, Baxter CR, Parker CR., Jr. Effect of burn trauma on adrenal and testicular steroid hormone production. J Clin Endocrinol Metab. 1987;64:842–848. doi: 10.1210/jcem-64-4-842. [DOI] [PubMed] [Google Scholar]
- 104.Woolf PD. Hormonal responses to trauma. Crit Care Med. 1992;20:216–226. doi: 10.1097/00003246-199202000-00011. [DOI] [PubMed] [Google Scholar]
- 105.Ferrando AA, Tipton KD, Doyle D, et al. Testosterone injection stimulates net protein synthesis but not tissue amino acid transport. Am J Physiol. 1998;275:E864–E8671. doi: 10.1152/ajpendo.1998.275.5.E864. [DOI] [PubMed] [Google Scholar]
- 106.Sheffield-Moore M, Urban RJ, Wolf SE, et al. Short-term oxandrolone administration stimulates net muscle protein synthesis in young men. J Clin Endocrinol Metab. 1999;84:2705–2711. doi: 10.1210/jcem.84.8.5923. [DOI] [PubMed] [Google Scholar]
- 107.Hart DW, Wolf SE, Ramzy PI, et al. Anabolic effects of oxandrolone after severe burn. Ann Surg. 2001;233:556–564. doi: 10.1097/00000658-200104000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jeschke MG, Finnerty CC, Suman OE, et al. The effect of oxandrolone on the endocrinologic, inflammatory, and hypermetabolic responses during the acute phase postburn. Ann Surg. 2007;246:351–360. doi: 10.1097/SLA.0b013e318146980e. discussion 360–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Demling RH, DeSanti L. Oxandrolone, an anabolic steroid, significantly increases the rate of weight gain in the recovery phase after major burns. J Trauma. 1997;43:47–51. doi: 10.1097/00005373-199707000-00012. [DOI] [PubMed] [Google Scholar]
- 110.Barrow RE, Dasu MR, Ferrando AA, et al. Gene expression patterns in skeletal muscle of thermally injured children treated with oxandrolone. Ann Surg. 2003;237:422–428. doi: 10.1097/01.SLA.0000055276.10357.FB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Przkora R, Jeschke MG, Barrow RE, et al. Metabolic and hormonal changes of severely burned children receiving long-term oxandrolone treatment. Ann Surg. 2005;242:384–389. doi: 10.1097/01.sla.0000180398.70103.24. discussion 390–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med. 1996;335:1713–1720. doi: 10.1056/NEJM199612053352301. [DOI] [PubMed] [Google Scholar]
- 113.Herndon DN, Barrow RE, Rutan TC, et al. Effect of propranolol administration on hemodynamic and metabolic responses of burned pediatric patients. Ann Surg. 1988;208:484–492. doi: 10.1097/00000658-198810000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Minifee PK, Barrow RE, Abston S, et al. Improved myocardial oxygen utilization following propranolol infusion in adolescents with postburn hypermetabolism. J Pediatr Surg. 1989;24:806–810. doi: 10.1016/s0022-3468(89)80541-x. discussion 810–811. [DOI] [PubMed] [Google Scholar]
- 115.Herndon DN, Nguyen TT, Wolfe RR, et al. Lipolysis in burned patients is stimulated by the beta 2-receptor for catecholamines. Arch Surg. 1994;129:1301–1304. doi: 10.1001/archsurg.1994.01420360091012. discussion 1304–1305. [DOI] [PubMed] [Google Scholar]
- 116.Barret JP, Jeschke MG, Herndon DN. Fatty infiltration of the liver in severely burned pediatric patients: autopsy findings and clinical implications. J Trauma. 2001;51:736–739. doi: 10.1097/00005373-200110000-00019. [DOI] [PubMed] [Google Scholar]
- 117.Aarsland A, Chinkes D, Wolfe RR, et al. Beta-blockade lowers peripheral lipolysis in burn patients receiving growth hormone. Rate of hepatic very low density lipoprotein triglyceride secretion remains unchanged. Ann Surg. 1996;223:777–787. doi: 10.1097/00000658-199606000-00016. discussion 787–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gore DC, Honeycutt D, Jahoor F, et al. Propranolol diminishes extremity blood flow in burned patients. Ann Surg. 1991;213:568–573. doi: 10.1097/00000658-199106000-00006. discussion 573–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Morio B, Irtun O, Herndon DN, Wolfe RR. Propranolol decreases splanchnic triacylglycerol storage in burn patients receiving a high-carbohydrate diet. Ann Surg. 2002;236:218–225. doi: 10.1097/00000658-200208000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jeschke MG, Finnerty CC, Kulp GA, et al. Combination of recombinant human growth hormone and propranolol decreases hypermetabolism and inflammation in severely burned children. Pediatr Crit Care Med. 2008;9:209–216. doi: 10.1097/PCC.0b013e318166d414. [DOI] [PubMed] [Google Scholar]
- 121.Hart DW, Wolf SE, Chinkes DL, et al. Beta-blockade and growth hormone after burn. Ann Surg. 2002;236:450–456. doi: 10.1097/00000658-200210000-00007. discussion 456–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ferrando AA, Lane HW, Stuart CA, et al. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol. 1996;270:E627–E633. doi: 10.1152/ajpendo.1996.270.4.E627. [DOI] [PubMed] [Google Scholar]
- 123.Celis MM, Suman OE, Huang TT, et al. Effect of a supervised exercise and physiotherapy program on surgical interventions in children with thermal injury. J Burn Care Rehabil. 2003;24:57–61. doi: 10.1097/00004630-200301000-00014. discussion 56. [DOI] [PubMed] [Google Scholar]
- 124.Cucuzzo NA, Ferrando A, Herndon DN. The effects of exercise programming vs traditional outpatient therapy in the rehabilitation of severely burned children. J Burn Care Rehabil. 2001;22:214–220. doi: 10.1097/00004630-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 125.Neugebauer CT, Serghiou M, Herndon DN, Suman OE. Effects of a 12-week rehabilitation program with music & exercise groups on range of motion in young children with severe burns. J Burn Care Res. 2008;29:939–948. doi: 10.1097/BCR.0b013e31818b9e0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Suman OE, Spies RJ, Celis MM, et al. Effects of a 12-wk resistance exercise program on skeletal muscle strength in children with burn injuries. J Appl Physiol. 2001;91:1168–1175. doi: 10.1152/jappl.2001.91.3.1168. [DOI] [PubMed] [Google Scholar]
- 127.Bull JP, Fisher AJ. A study of mortality in a burns unit: a revised estimate. Ann Surg. 1954;139:269–274. doi: 10.1097/00000658-195403000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Herndon DN, Blakeney PE. Teamwork for total burn care: achievements, directions, and hopes. In: Herndon DN, editor. Total burn care. 3rd ed. Philadelphia: Saunders Elsevier; 2007. pp. 9–13. [Google Scholar]
- 129.Jeschke MG, Mlcak RP, Finnerty CC, et al. Burn size determines the inflammatory and hypermetabolic response. Crit Care. 2007;11:R90. doi: 10.1186/cc6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hart DW, Wolf SE, Zhang XJ, et al. Efficacy of a high-carbohydrate diet in catabolic illness. Crit Care Med. 2001;29:1318–1324. doi: 10.1097/00003246-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 131.Hildreth MA, Herndon DN, Desai MH, Duke MA. Caloric needs of adolescent patients with burns. J Burn Care Rehabil. 1989;10:523–526. doi: 10.1097/00004630-198911000-00012. [DOI] [PubMed] [Google Scholar]
- 132.Debroy MA, Wolf SE, Zhang XJ, et al. Anabolic effects of insulin-like growth factor in combination with insulin-like growth factor binding protein-3 in severely burned adults. J Trauma. 1999;47:904–910. doi: 10.1097/00005373-199911000-00015. discussion 910–911. [DOI] [PubMed] [Google Scholar]
- 133.Ferrando AA, Chinkes DL, Wolf SE, et al. A submaximal dose of insulin promotes net skeletal muscle protein synthesis in patients with severe burns. Ann Surg. 1999;229:11–18. doi: 10.1097/00000658-199901000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]