Abstract
This study sought to better define the role of nitric oxide (NO) in brachial artery flow-mediated vasodilation (FMD) in young, healthy humans. Brachial artery blood velocity and diameter were determined (ultrasound Doppler) in eight volunteers (26 ± 1 yrs) before and after 5-min forearm circulatory occlusion with and without intra-arterial infusion of the endothelial nitric oxide synthase (eNOS) inhibitor L-NMMA (0.48 mg/dl/min). Control (CON) and L-NMMA trials were performed with the occlusion cuff placed in the traditional distal position, as well as proximal to the measurement site. FMD was significantly reduced, but not abolished, by L-NMMA in the distal cuff trial (8.9 ± 1.3 to 6.0 ± 0.7%, CON vs. L-NMMA, P = 0.02), with no effect of L-NMMA on FMD with proximal cuff placement (10.6 ± 1.2 to 12.4 ± 1.7%, CON vs. L-NMMA, P = 0.39). When the reduction in shear stimulus following L-NMMA was taken into account, no drug difference was observed for either distal (0.26 ± 0.02 to 0.23 ± 0.03, CON vs. L-NMMA, P = 0.40) or proximal (0.23 ± 0.08 to 0.23 ± 0.03, CON vs. L-NMMA, P = 0.89) FMD trials. These findings challenge the assertion that NO is obligatory for brachial artery FMD, and call into question the sensitivity of this procedure for non-invasive determination of NO bioavailability in young, healthy humans.
Keywords: Endothelium, Vasodilation, Blood Flow, Ultrasound Doppler
INTRODUCTION
In 1992, Celermajer et al. 1 introduced the method of measuring changes in conduit artery diameter following a period of circulatory occlusion as a non-invasive approach for in vivo determination of endothelium-dependent vasodilation in humans. In the two decades that have followed, the application of flow-mediated vasodilation (FMD) testing in clinical research has become widespread, a progression that is exemplified by adoption of this methodology into large-scale clinical trials, including recent phases of the Framingham Heart Study 2, 3. The FMD test is particularly appealing due, at least in part, to a previous report revealing a correlation between vasodilatory capacity of the peripheral and coronary arteries 4, thus presenting FMD as a potential surrogate measure of coronary endothelial health. Further, it has been clearly demonstrated that FMD provides independent predictive information for future cardiovascular events beyond traditional risk factors 5. FMD testing has thus emerged as a straight-forward, non-invasive approach for determining vascular function in health and disease.
Perhaps one of the most attractive aspects of FMD testing is that it has been purported to provide an assay of vascular endothelial nitric oxide (NO) bioavailability. The presence of this endothelially-derived vasodilator has become synonymous with vascular health 6, and thus the prospect of quantifying NO in a non-invasive manner makes FMD testing particularly appealing for both risk assessment and patient prognosis 7. The ability of endothelial nitric oxide synthase (eNOS) inhibition via NG-monomethyl-L-arginine (L-NMMA) to completely abolish radial 8, 9 and brachial 10 artery FMD provided initial evidence that FMD is governed primarily through the release of NO. However, in the face of significant technical and methodologic refinements in the field, recent studies have begun to challenge the existing dogma regarding the NO-dependent nature of the FMD response. Indeed, recent studies in the radial artery have identified only minimal (0–20%) reductions in FMD after L-NMMA administration 11, 12, leaving considerable uncertainty as to whether this test can still be relied upon to provide an index of NO bioavailability. Whether findings from these recent studies in the radial artery may be extended to the brachial artery, the vessel most often used for FMD testing, has not been determined.
In addition to this renewed controversy concerning the role of NO in traditional FMD assessment, there is also evidence that location of the occlusion cuff can impact the mechanisms responsible for vasodilation 13. Indeed, while placement of the cuff proximal to the imaged artery often results in greater vasodilation, this technique exposes the conduit artery to the ischemic milieu during occlusion, and results in a vasodilation that is only partially NO-dependent 10. Despite this finding, FMD testing with proximal cuff placement remains commonplace in the clinical literature 14–16, raising some concern with respect to whether findings from studies utilizing this approach have, in fact, provided a true assessment of NO bioavailability.
Considering these recent ambiguities in the literature, and in the face of the increasing inclusion of FMD testing as a primary outcome measure in clinical trials 17, 18, further study of the relationship between brachial artery FMD and NO is required. Thus, using up-to-date methods for FMD testing 19, the present study was undertaken to critically examine the role of NO in brachial artery FMD in young, healthy humans. Based upon the preponderance of prior data and inferences in the literature, we hypothesized that: 1) With conventional (distal) cuff placement, eNOS inhibition with L-NMMA would ablate the brachial artery FMD response; and 2) With proximal cuff placement, L-NMMA would diminish, but not abolish, brachial artery FMD.
METHODS
Subjects and General Procedures
Eight young, healthy volunteers participated in the current study. All subjects were non-smokers, normotensive (<140/90 mmHg), and free of overt cardiovascular disease, as determined by health history questionnaire and physical examination. Females were studied within the first five days of their menstrual cycle, as FMD measurements of the brachial artery have been documented to fluctuate with menstrual phase 20. Protocol approval and written informed consent were obtained according to the University of Utah and Salt Lake City Veteran Affairs Medical Center Institutional Review Board requirements. All data collection took place with subjects supine, in a thermoneutral environment.
Protocol
Subjects reported to the laboratory at 0800 in the fasted state, having abstained from exercise and caffeine for the previous 12 hours. Using sterile technique, an arterial catheter (Arrow, 18G, 20cm) was placed in the brachial artery approximately 10 cm distal to the axilla and advanced 6–8 cm in the retrograde direction. The catheter was placed in this region of the upper arm to ensure that L-NMMA entered the artery “upstream” (~15 cm) of the ultrasound Doppler sample volume, allowing the assessment of NOS blockade on brachial artery diameter and blood velocity. The experimental protocol consisted of four separate FMD tests; a control trial with cuff “above” (proximal to) the ultrasound probe, a control trial with cuff “below” (distal to) the ultrasound probe, a L-NMMA trial with proximal cuff placement, and a L-NMMA trial with distal cuff placement. The protocol was balanced for cuff placement, but ordered such that the control trials were always performed prior to L-NMMA infusion due to the long-lasting effects of the drug, with 60 min between control and L-NMMA trials.
Measurements
Details of the FMD procedure have been described previously 21 and were performed in accordance with recently published guidelines 19. Briefly, a blood pressure cuff was placed on the right arm, either proximal or distal to the ultrasound Doppler probe on the brachial artery. Simultaneous measurements of brachial artery blood velocity and vessel diameter were performed using a linear array transducer operating in duplex mode, with an imaging frequency of 14 MHz and Doppler frequency of 5 MHz (Logic 7, GE Medical Systems, Milwaukee, WI). All measurements were obtained with the probe appropriately positioned to maintain an insonation angle of 60° or less. The sample volume was maximized according to vessel size and was centered within the vessel on the basis of real-time ultrasound visualization. The brachial artery was insonated approximately midway between the antecubital and axillary regions, and measurements of diameter and blood velocity (Vmean) were obtained continuously at rest and for 2 minutes after cuff deflation. End-diastolic, ECG R-wave-gated images were collected via video output from the Logic 7 for off-line analysis of brachial artery vasodilation using automated edge-detection software (Medical Imaging Applications, Coralville, IA). Heart rate was monitored from a standard three-lead ECG. Arterial blood pressure measurements were collected continually from within the brachial artery, with the pressure transducer placed at the level of the catheter (Transpac IV, Abbot Laboratories).
L-NMMA
Upper and lower arm volumes were determined anthropometrically, and then used for the calculation of drug dosing, as described previously 22. L-NMMA (Bachem, Germany) was infused intra-arterially at 0.48 mg/dl (2.9–4.4 ml/min infusion rate) for 10 min before and continuously during FMD testing.
Analyses
FMD was quantified as the maximal change in brachial artery diameter after cuff release, expressed both in absolute units (i.e. Δ mm) and as a percentage increase (%Δ) above baseline. Shear rate was calculated according to the equation: shear rate (s−1)=Vmean · 8/vessel diameter. Blood flow was calculated as: Blood flow (mL · min)=(Vmean · π · [vessel diameter/2]2 · 60). For both shear rate and blood flow, cumulative area under the curve values were integrated with the trapezoidal rule and calculated as follows:
Normalized FMD was calculated by dividing FMD (percentage) by the cumulative shear rate area under the curve at the time of peak brachial artery vasodilation, multiplied by 1000. Mean arterial pressure (MAP) was calculated using the time integral of the directly measured arterial waveform.
Statistics
Statistics were performed with the use of commercially available software (SigmaPlot 11.0, Systat Software, Point Richmond, CA). Two-way repeated-measure analysis of variance was used to identify significant changes in measured variables within and between drug conditions and cuff placement, with the Bonferroni test used for post hoc analysis when a significant main effect was found. All group data are expressed as means ± SE. Significance was established at P < 0.05.
RESULTS
Subject characteristics are presented in Table 1.
TABLE 1.
Subject characteristics.
| Variable, units | Value |
|---|---|
| Age, yrs | 26 ± 1 |
| Sex, male:female | 6:2 |
| Height, cm | 178 ± 4 |
| Weight, kg | 77 ± 5 |
| Body Mass Index, kg/m2 | 24 ± 1 |
| Arm Volume, dL | 17 ± 2 |
| Heart Rate, bpm | 59 ± 1 |
| Systolic Blood Pressure, mmHg | 123 ± 4 |
| Diastolic Blood Pressure, mmHg | 72 ± 3 |
| Mean Arterial Pressure, mmHg | 90 ± 3 |
| Glucose, mg/dl | 87 ± 8 |
| Sodium, mmol/l | 140 ± 1 |
| Potassium, mmol/l | 3.9 ± 0.1 |
| Chloride, mmol/l | 103 ± 1 |
| Calcium, mg/dl | 9.1 ± 0.1 |
| Creatinine, mg/dl | 1.0 ± 0.06 |
| Cholesterol, mg/dl | 164 ± 13 |
| Triglycerides, mg/dl | 66 ± 11 |
| HDL, mg/dl | 61 ± 6 |
| LDL, mg/dl | 100 ± 10 |
Values are mean ± SE. HDL, High-Density Lipids; LDL, Low-Density Lipids.
FMD with Distal Cuff Occlusion
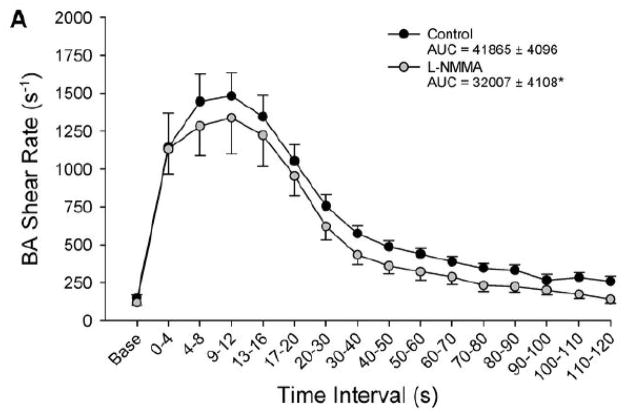
Prior to cuff occlusion, L-NMMA administration resulted in a significant reduction in brachial artery blood flow, while heart rate (HR), mean arterial blood pressure (MAP), brachial artery diameter, and brachial artery shear rate all remained unchanged (Table 2). Following 5-min suprasystolic cuff occlusion, both cumulative blood flow AUC (Table 2) and cumulative shear rate AUC (Figure 1) were significantly reduced following administration of L-NMMA. A modest (≈33%) reduction in absolute change in brachial artery diameter (Table 2) and %FMD was observed following L-NMMA; however, when FMD was normalized for the reduced shear rate following L-NMMA, the difference between L-NMMA and control trials was no longer apparent (Figure 1).
TABLE 2.
Baseline and FMD responses before and after L-NMMA administration with occlusion cuff placed below and above the measurement site.
| Variable, units | CUFF BELOW | CUFF ABOVE | ||
|---|---|---|---|---|
|
| ||||
| Baseline | Control | L-NMMA | Control | L-NMMA |
| Heart Rate, beats/min | 57 ± 1 | 58 ± 2 | 56 ± 1 | 59 ± 1 |
| Mean Arterial Blood Pressure, mmHg | 90 ± 3 | 94 ± 4 | 92 ± 4 | 95 ± 4 |
| Brachial artery diameter, mm | 4.2 ± 0.2 | 4.1 ± 0.2 | 4.2 ± 0.2 | 4.1 ± 0.2 |
| Brachial artery blood velocity, cm/s | 7.8 ± 0.9 | 5.9 ± 0.9 | 7.6 ± 1.3 | 5.6 ± 0.9 |
| Brachial artery blood flow, ml/min | 67 ± 11 | 46 ± 6* | 65 ± 10 | 47 ± 9* |
| Brachial artery shear rate, s−1 | 150 ± 18 | 119 ± 22 | 146 ± 26 | 110 ± 18 |
| FMD | ||||
| Change in brachial artery diameter, mm | 0.36 ± 0.04 | 0.27 ± 0.04* | 0.43 ± 0.07 | 0.50 ± 0.07 |
| Change in brachial artery diameter, % | 8.9 ± 1.3 | 6.0 ± 0.7* | 10.6 ± 1.2 | 12.4 ± 1.7† |
| Time to peak vasodilation, s | 54 ± 6 | 51 ± 8 | 88 ± 5† | 78 ± 6† |
| Blood flow AUC, ml | 336 ± 39 | 237 ± 22* | 302 ± 39 | 258 ± 27* |
| Peak Shear Rate, s−1 | 1508 ± 166 | 1387 ± 224 | 1808 ± 186† | 1640 ± 162† |
| Shear Rate AUC at peak vasodilation | 33567 ± 3191 | 29077 ± 4164 | 42681 ± 7718† | 54052 ± 4295† |
AUC, area-under-curve.
Significantly different than Control, P<0.05;
Significantly different than Cuff Below, P<0.05.
FIGURE 1. Distal cuff FMD responses.
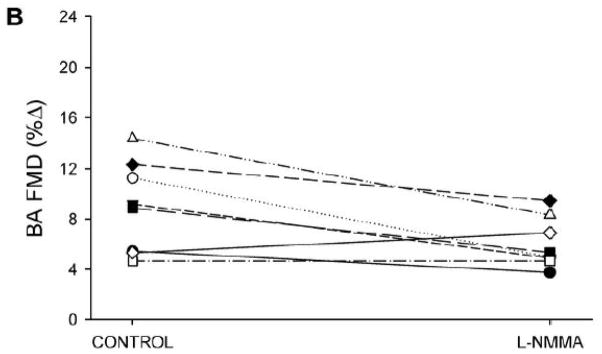
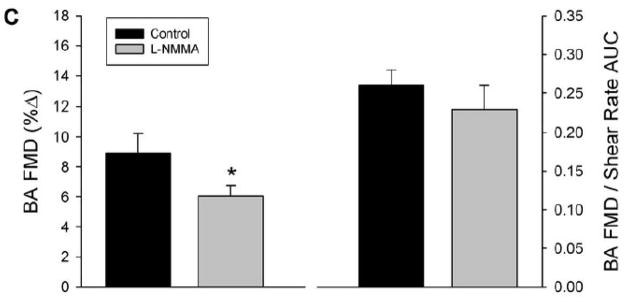
Panel A: Brachial artery (BA) shear rate following 5-min of distal cuff was reduced after eNOS inhibition with L-NMMA, as quantified by area-under-curve (AUC) for shear rate across the entire 120-sec post-occlusion time period. Panel B: Individual %FMD responses in the control and L-NMMA trials. Panel C: %FMD was reduced following L-NMMA (left); however, FMD “normalized” for shear rate AUC at peak dilation was not different between control and L-NMMA conditions (right). *Significantly different than Control, P<0.05.
FMD with Proximal Cuff Occlusion
As with the distal cuff trial, L-NMMA administration reduced resting brachial artery blood flow, while HR, MAP, brachial artery diameter, and brachial artery shear rate were similar between control and L-NMMA trials (Table 2). After cuff release, the hyperemic response was greater than with distal cuff occlusion, with augmented peak shear rate and shear rate AUC at peak vasodilation in both control and L-NMMA trials (Table 2). Similar to the distal cuff trial, L-NMMA administration blunted the post-occlusion hyperemic response, with significant reductions in both blood flow AUC (Table 2) and shear rate AUC (Figure 2). FMD was similar between control and L-NMMA trials, whether expressed as absolute change in brachial artery diameter (Table 2), %FMD, or FMD normalized for shear rate (Figure 2).
FIGURE 2. Proximal cuff FMD responses.
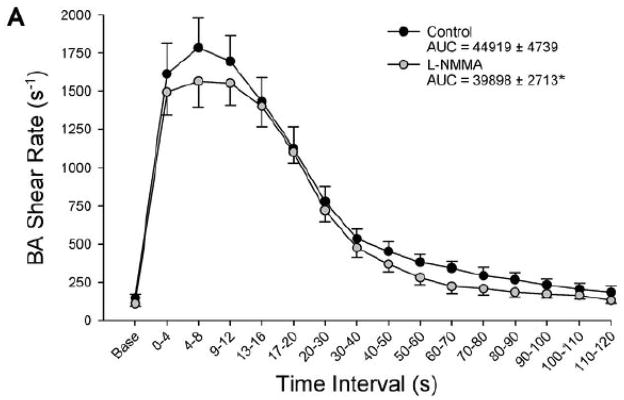
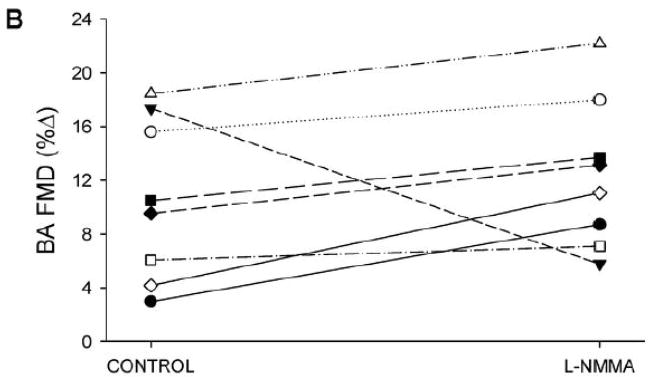
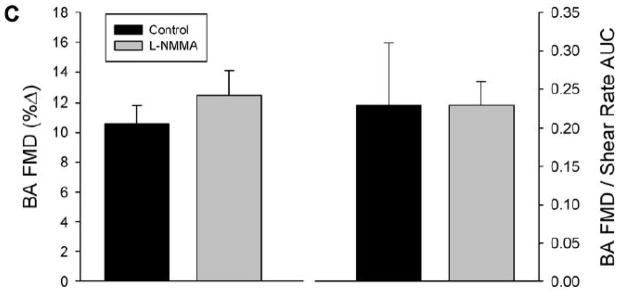
Panel A: Brachial artery (BA) shear rate following 5-min of proximal cuff was reduced after eNOS inhibition with L-NMMA, as quantified by area-under-curve (AUC) for shear rate across the entire 120-sec post-occlusion time period. Panel B: Individual %FMD responses in the control and L-NMMA trials. Panel C: Both %FMD (left) and FMD “normalized” for shear rate AUC at peak dilation (right) were similar between control and L-NMMA conditions. *Significantly different than Control, P<0.05.
DISCUSSION
Using up-to-date measurement techniques, we have identified for the first time that the majority of vasodilation provoked by brachial artery flow-mediated vasodilation (FMD) testing is not attributable to NO. Indeed, with the occlusion cuff placed in the traditional, distal position, inhibition of eNOS via intra-arterial L-NMMA resulted in only a modest (≈33%) reduction in FMD. However, L-NMMA administration also reduced the post-occlusion hyperemic response, thereby diminishing the shear stimulus for brachial artery vasodilation. When this effect was taken into account, FMD normalized for shear rate was not different between control and L-NMMA conditions. With the occlusion cuff placed proximal to the ultrasound probe, the FMD response was unchanged by eNOS inhibition, highlighting the NO-independent nature of this variant of the FMD method. Taken together, these findings challenge the assertion that NO is obligatory for brachial artery FMD, and question the role of this procedure in the non-invasive determination of NO bioavailability in young, healthy humans.
The Role of NO in FMD
In recent years, it has become widely accepted that FMD is a NO-dependent event, and therefore results from FMD testing have been thought to provide a “bioassay” for NO. Just three years after FMD testing was first described, a seminal study from Joannides et al. 8 reported complete ablation of flow-dependent dilation in the radial artery following intra-arterial L-NMMA administration, leading to the conclusion that “NO is essential for FMD of large human arteries”. This was followed by additional studies in the radial 9, 23–27 and superficial femoral 28 arteries that collectively reported a reduction or complete absence of FMD in the presence of L-NMMA (Table 3). However, it is noteworthy that even the more recent of these NO-blocking studies did not adhere to what are now standardized procedures for FMD testing 19, leaving some doubt with respect to interpretation of the findings. In fact, two very recent studies in the radial artery undertaken with strict adherence to current guidelines for FMD testing have challenged this earlier body of work, together identifying a minimal contribution of NO to FMD in young, healthy humans 11, 12. Thus, the literature concerning the ability of FMD to non-invasively assay NO bioavailability now appears somewhat equivocal (Table 3), and has become an area of increased debate 29–35.
TABLE 3.
Summary of studies examining NO-dependency of FMD in young, healthy adults. %FMD is presented as mean ± SE.
| Reference | Year | Cuff Position | Artery | %FMD Control | %FMD L-NMMA | %Δ |
|---|---|---|---|---|---|---|
| oannides et al.8 | 1995 | Distal | Radial | 3.6 ± 0.8 | −2.8 ± 1.7 | −177% |
| Lieberman et al.38 | 1996 | Proximal | Brachial | 21.1 ± 3.3 | 7.0 ± 1.4 | −67% |
| Doshi et al.10 | 2001 | Distal | Brachial | 7.3 ± 2.5 | 0.2 ± 2.2 | −97% |
| Doshi et al.10 | 2001 | Proximal | Brachial | 11.6 ± 3.2 | 7.5 ± 2.3 | −35% |
| Mullen et al.9 | 2001 | Distal | Radial | 5.3 ± 1.2 | 0.7 ± 0.7 | −87% |
| Bellien et al.27 | 2007 | Distal | Radial | 10.9 ± 0.6 | 9.3 ± 0.8 | −15% |
| Fischer et al.24 | 2007 | Distal | Radial | 11.5 ± 0.9 | 6.0 ± 0.7 | −48% |
| Kooijman et al.28 | 2008 | Distal | SF | 4.2 ± 0.3 | 1.0 ± 0.2 | −76% |
| Seddon et al.23 | 2009 | Distal | Radial | 6.5 ± 0.2 | 1.0 ± 0.3 | −85% |
| Liuni et al.26 | 2010 | Distal | Radial | 7.2 ± 0.9 | 2.7 ± 0.7 | −63% |
| Pyke et al.11 | 2010 | Distal | Radial | 5.6 ± 1.5 | 5.6 ± 1.3 | 0% |
| Parker et al.12 | 2011 | Distal | Radial | 10.0 ± 1.0 | 7.6 ± 1.2 | −24% |
| Wray et al. | 2013 | Proximal | Brachial | 10.6 ± 1.2 | 12.4 ± 1.7 | +17% |
| Wray et al. | 2013 | Distal | Brachial | 8.9 ± 1.3 | 6.0 ± 0.7 | −33% |
LNMMA, NG-monomethyl-L-arginine; SF, superficial femoral.
The present study is of significance inasmuch as it extends findings from the recent radial artery studies to the brachial artery, the conduit vessel most often utilized for FMD testing. With this approach, we have demonstrated, for the first time, that NO is not obligatory for brachial artery FMD, which is in contrast to the work of Doshi et al.10. In the only other study that has examined the effect of L-NMMA on brachial artery FMD, these authors reported complete ablation of brachial artery FMD following L-NMMA, leading to the conclusion that FMD is mediated exclusively by NO. There are at least two key differences between the former and current studies that may explain these divergent results. First, the use of wrist occlusion in the previous study provoked ischemia only in the hand, a significantly smaller volume of tissue than the present study, where the entire lower arm was occluded, which has become the standardized approach 19. Indeed, while wrist occlusion evoked a 6-fold increase in peak brachial artery blood flow, this hyperemic response is substantially less than the 11–fold increase observed in the present study (Table 2, Figure 1) and may preclude a direct comparison between studies. In addition, the study from Doshi et al. 10 did not examine the time integral for the hyperemic stimulus, reporting instead a singular, peak value for blood flow that did not differ between saline and L-NMMA trials. This is an important distinction, as the preferred method of quantifying the stimulus for FMD has evolved substantially in recent years and now involves examining accumulated shear rate up to the time of peak vasodilation, a numerical value which may subsequently be used to “normalize” FMD to the shear stimulus on an individual basis 10, 19, 36. Our group has previously documented the importance of the stimulus-response relationship for proper interpretation of FMD testing in the elderly, where a diminished hyperemic response following cuff occlusion appears to partially explain the age-related decrement in FMD 37. The relevance of FMD normalization is further illustrated in the present study, where the modest but statistically significant reduction in FMD following L-NMMA is abolished when FMD is viewed in the context of the hyperemic stimulus (Figure 1). Thus, using state-of-the-art methodology and accepted standardized techniques, findings from the present study challenge the overall conclusion from this previous study 10, identifying instead only a minimal role of NO for brachial artery FMD in young, healthy humans.
Proximal Versus Distal Cuff Placement
One of the most influential methodological factors in FMD testing is the anatomic location of the occlusion cuff relative to the measurement site. Present guidelines for FMD testing recommend placement of the occlusion cuff distal to the region of the brachial artery where measurements are performed, since proximal cuff placement exposes the vessel to an ischemic environment that may provoke vasodilation which is not endothelium-dependent 13. Despite this, many clinical studies in a variety of patient populations continue to adopt this proximal cuff approach 14–16, publishing FMD findings with inference to NO bioavailability. Thus, one additional aim of the present study was to further examine the role of NO on brachial artery FMD with the occlusion cuff placed proximal to the measurement site.
As expected, brachial artery vasodilation was less affected by eNOS inhibition in the proximal cuff trial; in fact, there were no significant differences in either standard or normalized FMD between control or L-NMMA conditions (Figure 2). This is in contrast with previous studies 10, 38 that have identified a small but significant role for NO in the FMD response with proximal cuff placement. Thus, the present results with proximal cuff placement may be viewed as challenging this previous work, and lend further support to the NO-independent nature of vasodilation for the brachial artery FMD test in young, healthy humans.
Alternative Approaches for the Assessment of NO-dependent Vasodilation
Considering the ongoing clinical interest in endothelial function testing for risk assessment and cardiovascular disease prognosis 39, our group has recently begun to investigate additional experimental paradigms for non-invasive evaluation of NO bioavailability. In keeping with the established principle that NO is released in response to vascular wall shear stress 40, we have utilized small muscle mass (rhythmic handgrip) exercise to increase limb blood flow, and examined whether conduit artery vasodilation in response to this sustained increase in shear occurs via an NO-dependent mechanism. In young, healthy adults, we observed a 70% reduction in brachial artery vasodilation during handgrip exercise in the presence of L-NMMA 22, implicating NO as a major contributor to shear-induced vasodilation with this exercise modality. Similarly, we have recently developed a passive exercise paradigm that provokes a substantial rise in leg blood flow 41–44, and subsequently identified an 80% reduction in vasodilation in the passively moved limb following intra-arterial L-NMMA administration 45. Each of these experimental approaches is easily performed, provoke a robust rise in shear rate, and appear much more NO-dependent than conventional FMD testing, especially in light of the current findings. It thus appears that these alternative paradigms may represent a preferable approach for non-invasive, in vivo assessment of NO bioavailability in humans.
Perspectives
It has been clearly demonstrated that FMD provides independent, predictive information for future cardiovascular events beyond traditional risk factors 5, and based on previous studies identifying the role of NO in FMD testing 9 it has been assumed that the prognostic value of FMD is related to the ability of this test to estimate NO bioavailability. While the current findings challenge the role of NO in the FMD response, it should be noted that this does not contradict the large body of evidence supporting FMD testing as a valid assessment of disease risk and progression. Rather, it suggests that the FMD response should be viewed as endothelium-dependent vasodilation, but not necessarily NO-dependent vasodilation. This concept is supported by a recent brief review in Hypertension which discussed FMD testing and cardiovascular event prediction 5, and concluded that “FMD derived using a proximal cuff is at least as predictive (of future cardiovascular events) as that derived using distal cuff placement, despite the latter being more NO dependent”.
The robust endothelium-dependent vasodilation observed in the presence of L-NMMA does raise the question of what other, non-NO pathways may contribute to the FMD response. While identifying such vasodilatory mechanisms is beyond the scope of the present study, it seems reasonable to speculate that prostaglandins and/or endothelium-derived hyperpolarizing factor (EDHF) may play an important compensatory role in the FMD response, particularly in the absence of NO. Regarding the former, Parker et al. 12 recently examined radial artery FMD during independent and combined inhibition of NO and prostaglandins via intra-arterial L-NMMA and Ketorolac, respectively, in young, healthy adults. While this study identified a small reduction in radial artery FMD following L-NMMA, no additional change was observed in the L-NMMA + Ketorolac trial, suggesting a minimal role for prostaglandins in the FMD response. With respect to EDHF, it has been reported that maximal radial artery dilation during sustained shear stress was substantially reduced when both L-NMMA and tetraethylammonium chloride (an EDHF inhibitor) were infused concomitantly compared to L-NMMA alone, implicating EDHF as an important contributor to FMD in the absence of NO. Whether the EDHF pathway is involved in BA FMD remains unknown, and is certainly deserving of further study.
Experimental Considerations
As we did not pharmacologically challenge the eNOS blockade, we cannot exclude the possibility that the lack of a drug effect on FMD was due to incomplete inhibition of eNOS. However, the dose of L-NMMA utilized in this study (0.48 mg/dl/min, ≈8mg/min) is equivalent to that used previously by our group 22, 45, and is among the highest intra-arterial doses ever administered in humans, almost 3-fold higher than previous work demonstrating complete abolition of brachial artery FMD in the presence of L-NMMA 10. This, in combination with our published evidence of a plateau in brachial artery blood flow during L-NMMA dose-response experiments 22, increase confidence that local eNOS inhibition was accomplished, and that the observed responses are, therefore, not attributable to incomplete eNOS blockade. By design, in the present study the control trials always precede the L-NMMA trials, and thus we acknowledge the possibility that a drug ordering effect may exist.
NOVELTY AND SIGNIFICANCE.
What is New?
Using up-to-date measurement techniques, we have identified for the first time that the majority of vasodilation provoked by brachial artery flow-mediated vasodilation (FMD) testing is not attributable to nitric oxide (NO).
What is Relevant?
FMD testing has been purported to provide an assay of vascular endothelial nitric oxide (NO) bioavailability, making it particularly appealing for both risk assessment and patient prognosis. Results from the present study indicate that NO is not obligatory for brachial artery FMD, and question the role of this procedure in the non-invasive determination of NO bioavailability in young, healthy humans.
Summary
Using both proximal and distal cuff placement, we have identified a negligible role of NO in the brachial artery FMD test in young, healthy humans. These findings challenge the convention that FMD testing provides a specific “bioassay” for NO, and emphasize the need for alternative experimental approaches for the non-invasive assessment of NO bioavailability in humans.
Acknowledgments
Sources of Funding. Funded in part by NIH PO1 HL-091830 (R.S.R.), VA RR&D E6910R (R.S.R.), AHA 0835209N (D.W.W.), and VA RR&D CDA2 E7560W (J.M.).
Footnotes
Disclosures:
There are no financial disclosures to report.
References
- 1.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Larson MG, Keyes MJ, Mitchell GF, Vasan RS, Keaney JF, Jr, Lehman BT, Fan S, Osypiuk E, Vita JA. Clinical correlates and heritability of flow-mediated dilation in the community: The framingham heart study. Circ. 2004;109:613–619. doi: 10.1161/01.CIR.0000112565.60887.1E. [DOI] [PubMed] [Google Scholar]
- 3.Thanassoulis G, Lyass A, Benjamin EJ, Larson MG, Vita JA, Levy D, Hamburg NM, Widlansky ME, O’Donnell CJ, Mitchell GF, Vasan RS. Relations of exercise blood pressure response to cardiovascular risk factors and vascular function in the framingham heart study. Circ. 2012;125:2836–2843. doi: 10.1161/CIRCULATIONAHA.111.063933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC, Selwyn AP. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 5.Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow-mediated dilation and cardiovascular event prediction: Does nitric oxide matter? Hyper. 2011;57:363–369. doi: 10.1161/HYPERTENSIONAHA.110.167015. [DOI] [PubMed] [Google Scholar]
- 6.Flammer AJ, Luscher TF. Human endothelial dysfunction: Edrfs. Pflugers Arch. 2010;459:1005–1013. doi: 10.1007/s00424-010-0822-4. [DOI] [PubMed] [Google Scholar]
- 7.Moens AL, Goovaerts I, Claeys MJ, Vrints CJ. Flow-mediated vasodilation: A diagnostic instrument, or an experimental tool? Chest. 2005;127:2254–2263. doi: 10.1378/chest.127.6.2254. [DOI] [PubMed] [Google Scholar]
- 8.Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circ. 1995;91:1314–1319. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 9.Mullen MJ, Kharbanda RK, Cross J, Donald AE, Taylor M, Vallance P, Deanfield JE, MacAllister RJ. Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo: Relevance to endothelial dysfunction in hypercholesterolemia. Circ Res. 2001;88:145–151. doi: 10.1161/01.res.88.2.145. [DOI] [PubMed] [Google Scholar]
- 10.Doshi SN, Naka KK, Payne N, Jones CJ, Ashton M, Lewis MJ, Goodfellow J. Flow-mediated dilatation following wrist and upper arm occlusion in humans: The contribution of nitric oxide. Clin Sci (Lond) 2001;101:629–635. [PubMed] [Google Scholar]
- 11.Pyke K, Green DJ, Weisbrod C, Best M, Dembo L, O’Driscoll G, Tschakovsky M. Nitric oxide is not obligatory for radial artery flow-mediated dilation following release of 5 or 10 min distal occlusion. Am J Physiol Heart Circ Physiol. 2010;298:H119–126. doi: 10.1152/ajpheart.00571.2009. [DOI] [PubMed] [Google Scholar]
- 12.Parker BA, Tschakovsky ME, Augeri AL, Polk DM, Thompson PD, Kiernan FJ. Heterogenous vasodilator pathways underlie flow-mediated dilation in men and women. Am J Physiol Heart Circ Physiol. 2011;301:H1118–1126. doi: 10.1152/ajpheart.00400.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agewall S, Doughty RN, Bagg W, Whalley GA, Braatvedt G, Sharpe N. Comparison of ultrasound assessment of flow-mediated dilatation in the radial and brachial artery with upper and forearm cuff positions. Clin Physiol. 2001;21:9–14. doi: 10.1046/j.1365-2281.2001.00302.x. [DOI] [PubMed] [Google Scholar]
- 14.Wisloff U, Stoylen A, Loennechen JP, Bruvold M, Rognmo O, Haram PM, Tjonna AE, Helgerud J, Slordahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen O, Skjaerpe T. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: A randomized study. Circ. 2007;115:3086–3094. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 15.Ades PA, Savage PD, Lischke S, Toth MJ, Harvey-Berino J, Bunn JY, Ludlow M, Schneider DJ. The effect of weight loss and exercise training on flow-mediated dilatation in coronary heart disease: A randomized trial. Chest. 2011;140:1420–1427. doi: 10.1378/chest.10-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golledge J, Leicht AS, Crowther RG, Glanville S, Clancy P, Sangla KS, Spinks WL, Quigley F. Determinants of endothelial function in a cohort of patients with peripheral artery disease. Cardiology. 2008;111:51–56. doi: 10.1159/000113428. [DOI] [PubMed] [Google Scholar]
- 17.Korkmaz H, Karaca I, Koc M, Onalan O, Yilmaz M, Bilen MN. Early effects of treatment with nebivolol and quinapril on endothelial function in patients with hypertension. Endothelium. 2008;15:149–155. doi: 10.1080/10623320802125565. [DOI] [PubMed] [Google Scholar]
- 18.Balletshofer BM, Goebbel S, Rittig K, Enderle M, Schmolzer I, Wascher TC, Ferenc Pap A, Westermeier T, Petzinna D, Matthaei S, Haring HU. Intense cholesterol lowering therapy with a hmg-coa reductase inhibitor does not improve nitric oxide dependent endothelial function in type-2-diabetes--a multicenter, randomised, double-blind, three-arm placebo-controlled clinical trial. Exp Clin Endocrinol Diabetes. 2005;113:324–330. doi: 10.1055/s-2005-865642. [DOI] [PubMed] [Google Scholar]
- 19.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55:1075–1085. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams MR, Westerman RA, Kingwell BA, Paige J, Blombery PA, Sudhir K, Komesaroff PA. Variations in endothelial function and arterial compliance during the menstrual cycle. J Clin Endocrinol Metab. 2001;86:5389–5395. doi: 10.1210/jcem.86.11.8013. [DOI] [PubMed] [Google Scholar]
- 21.Harris RA, Nishiyama SK, Wray DW, Tedjasaputra V, Bailey DM, Richardson RS. The effect of oral antioxidants on brachial artery flow-mediated dilation following 5 and 10 min of ischemia. Eur J Appl Physiol. 2009;107:445–453. doi: 10.1007/s00421-009-1147-x. [DOI] [PubMed] [Google Scholar]
- 22.Wray DW, Witman MA, Ives SJ, McDaniel J, Fjeldstad AS, Trinity JD, Conklin JD, Supiano MA, Richardson RS. Progressive handgrip exercise: Evidence of nitric oxide-dependent vasodilation and blood flow regulation in humans. Am J Physiol Heart Circ Physiol. 2011;300:H1101–1107. doi: 10.1152/ajpheart.01115.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seddon M, Melikian N, Dworakowski R, Shabeeh H, Jiang B, Byrne J, Casadei B, Chowienczyk P, Shah AM. Effects of neuronal nitric oxide synthase on human coronary artery diameter and blood flow in vivo. Circ. 2009;119:2656–2662. doi: 10.1161/CIRCULATIONAHA.108.822205. [DOI] [PubMed] [Google Scholar]
- 24.Fischer D, Landmesser U, Spiekermann S, Hilfiker-Kleiner D, Hospely M, Muller M, Busse R, Fleming I, Drexler H. Cytochrome p450 2c9 is involved in flow-dependent vasodilation of peripheral conduit arteries in healthy subjects and in patients with chronic heart failure. Eur J Heart Fail. 2007;9:770–775. doi: 10.1016/j.ejheart.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Ghiadoni L, Versari D, Magagna A, Kardasz I, Plantinga Y, Giannarelli C, Taddei S, Salvetti A. Ramipril dose-dependently increases nitric oxide availability in the radial artery of essential hypertension patients. J Hypertens. 2007;25:361–366. doi: 10.1097/HJH.0b013e3280115901. [DOI] [PubMed] [Google Scholar]
- 26.Liuni A, Luca MC, Lisi M, Dragoni S, di Stolfo G, Mariani JA, Uxa A, Gori T, Parker JD. Observations of time-based measures of flow-mediated dilation of forearm conduit arteries: Implications for the accurate assessment of endothelial function. Am J Physiol Heart Circ Physiol. 2010;299:H939–945. doi: 10.1152/ajpheart.00271.2010. [DOI] [PubMed] [Google Scholar]
- 27.Bellien J, Iacob M, Eltchaninoff H, Bourkaib R, Thuillez C, Joannides R. At1 receptor blockade prevents the decrease in conduit artery flow-mediated dilatation during nos inhibition in humans. Clin Sci (Lond) 2007;112:393–401. doi: 10.1042/CS20060236. [DOI] [PubMed] [Google Scholar]
- 28.Kooijman M, Thijssen DH, de Groot PC, Bleeker MW, van Kuppevelt HJ, Green DJ, Rongen GA, Smits P, Hopman MT. Flow-mediated dilatation in the superficial femoral artery is nitric oxide mediated in humans. J Physiol. 2008;586:1137–1145. doi: 10.1113/jphysiol.2007.145722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casey DP, Beck DT, Braith RW. Systemic plasma levels of nitrite/nitrate (nox) reflect brachial flow-mediated dilation responses in young men and women. Clin Exp Pharmacol Physiol. 2007;34:1291–1293. doi: 10.1111/j.1440-1681.2007.04715.x. [DOI] [PubMed] [Google Scholar]
- 30.Geny B, Rouyer O, Doutreleau S, Piquard F. Comments on point-counterpoint “flow-mediated dilation does/does not reflect nitric oxide-mediated endothelial function”. J Appl Physiol. 2006;100:362. [PubMed] [Google Scholar]
- 31.Torgrimson BN, Meendering JR, Minson CT. Comments on point-counterpoint “flow-mediated dilation does/does not reflect nitric oxide-mediated endothelial function”. J Appl Physiol. 2006;100:362. doi: 10.1152/japplphysiol.01313.2005. [DOI] [PubMed] [Google Scholar]
- 32.Joyner MJ. Comment on point:Counterpoint “flow-mediated dilation does/does not reflect nitric oxide-mediated endothelial function”. J Appl Physiol. 2005;99:2452. doi: 10.1152/japplphysiol.00841.2005. [DOI] [PubMed] [Google Scholar]
- 33.Austin CE. Flow-mediated dilation does/does not reflect nitric oxide-mediated endothelial function. J Appl Physiol. 2005;99:1621. doi: 10.1152/japplphysiol.00817.2005. [DOI] [PubMed] [Google Scholar]
- 34.Tschakovsky ME, Pyke KE. Counterpoint: Flow-mediated dilation does not reflect nitric oxide-mediated endothelial function. J Appl Physiol. 2005;99:1235–1237. doi: 10.1152/japplphysiol.00607.2005. discussion 1237–1238. [DOI] [PubMed] [Google Scholar]
- 35.Green D. Point: Flow-mediated dilation does reflect nitric oxide-mediated endothelial function. J Appl Physiol. 2005;99:1233–1234. doi: 10.1152/japplphysiol.00601.2005. discussion 1237–1238. [DOI] [PubMed] [Google Scholar]
- 36.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: Implications for the assessment of endothelial function. J Physiol. 2005;568:357–369. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wray DW, Uberoi A, Lawrenson L, Richardson RS. Evidence of preserved endothelial function and vascular plasticity with age. Am J Physiol Heart Circ Physiol. 2006;290:H1271–1277. doi: 10.1152/ajpheart.00883.2005. [DOI] [PubMed] [Google Scholar]
- 38.Lieberman EH, Gerhard MD, Uehata A, Selwyn AP, Ganz P, Yeung AC, Creager MA. Flow-induced vasodilation of the human brachial artery is impaired in patients <40 years of age with coronary artery disease. Am J Cardiol. 1996;78:1210–1214. doi: 10.1016/s0002-9149(96)00597-8. [DOI] [PubMed] [Google Scholar]
- 39.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Luscher TF, Shechter M, Taddei S, Vita JA, Lerman A. The assessment of endothelial function: From research into clinical practice. Circ. 2012;126:753–767. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sessa WC. Enos at a glance. J Cell Sci. 2004;117:2427–2429. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- 41.Trinity JD, McDaniel J, Venturelli M, Fjeldstad AS, Ives SJ, Witman MA, Barrett-O’Keefe Z, Amann M, Wray DW, Richardson RS. Impact of body position on central and peripheral hemodynamic contributions to movement-induced hyperemia: Implications for rehabilitative medicine. Am J Physiol Heart Circ Physiol. 2011;300:H1885–1891. doi: 10.1152/ajpheart.00038.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDaniel J, Hayman MA, Ives S, Fjeldstad AS, Trinity JD, Wray DW, Richardson RS. Attenuated exercise induced hyperaemia with age: Mechanistic insight from passive limb movement. J Physiol. 2010;588:4507–4517. doi: 10.1113/jphysiol.2010.198770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trinity JD, Amann M, McDaniel J, Fjeldstad AS, Barrett-O’Keefe Z, Runnels S, Morgan DE, Wray DW, Richardson RS. Limb movement-induced hyperemia has a central hemodynamic component: Evidence from a neural blockade study. Am J Physiol Heart Circ Physiol. 2010;299:H1693–1700. doi: 10.1152/ajpheart.00482.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wray DW, Donato AJ, Uberoi A, Merlone JP, Richardson RS. Onset exercise hyperaemia in humans: Partitioning the contributors. J Physiol. 2005;565:1053–1060. doi: 10.1113/jphysiol.2005.084327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Runnels S, Gmelch B, Bledsoe A, Richardson RS. Nitric oxide and passive limb movement: A new approach to assess vascular function. J Physiol. 2012;590:1413–1425. doi: 10.1113/jphysiol.2011.224741. [DOI] [PMC free article] [PubMed] [Google Scholar]