Abstract
OBJECTIVE
The determinants of anemia during both pregnancy and postpartum recovery remain incompletely understood in sub-Saharan African women.
SUBJECTS/METHODS
In a prospective cohort study among pregnant women, we assessed dietary, biochemical, anthropometric, infectious and sociodemographic factors at baseline. In multivariate Cox proportional hazards models, we examined predictors of incident anemia (hemoglobin <11 g/dl) and iron deficiency anemia (anemia plus mean corpuscular volume <80 fL), and recovery from anemia and iron deficiency anemia through 18 months postpartum at antenatal clinics in Dar es Salaam, Tanzania between 2001 and 2005. A total of 2364 non-anemic pregnant women and 4884 anemic women were enrolled between 12 and 27 weeks of gestation.
RESULTS
In total, 292 women developed anemia during the postpartum period and 165 developed iron deficiency anemia, whereas 2982 recovered from baseline anemia and 2044 from iron deficiency anemia. Risk factors for postpartum anemia were delivery complications (RR 1.6, 95% confidence interval (CI) 1.13, 2.22) and low postpartum CD4 cell count (RR 1.73, 95% CI 0.96, 3.17). Iron/folate supplementation during pregnancy had a protective relationship with the incidence of iron deficiency anemia. Absence of delivery complications, education status and iron/folate supplementation were positively associated with time to recovery from iron deficiency.
CONCLUSION
Maternal nutritional status during pregnancy, prenatal iron/folate supplementation, perinatal care, and prevention and management of infections, such as malaria, are modifiable risk factors for the occurrence of, and recovery from, anemia.
Keywords: anemia, postpartum, iron deficiency, pregnancy
INTRODUCTION
The World Health Organization estimates that 55.8% of pregnant women in Africa are anemic.1 In addition, 41% of women of childbearing age in Africa are anemic.2,3 It is estimated that iron deficiency, often caused by low intake of bioavailable iron, underlies about 50% of anemia in the developing world, but estimates of iron deficiency vary significantly across settings.1 Other causes of anemia include deficiencies in folate and vitamin B12, inflammatory conditions such as malaria and genetic disorders. Even though iron supplementation reduces anemia and is standard prenatal care in most countries,3,4 anemia continues to persist at relatively high rates among pregnant African women, including those receiving iron and folate supplements.5
Anemia during pregnancy is associated with adverse maternal health, possibly contributing to maternal mortality, and with poor pregnancy outcomes, including low birth weight, premature birth, neonatal and infant morbidity, and mortality, as well as reduced newborn iron stores and subsequent cognitive impairments.3,6–12
While anemia has been studied extensively in pregnant women, the extent and impact of anemia during the postpartum period has more recently become an area of interest.13–15 The mother’s health status during the postpartum period affects the potential for breastfeeding, and consequently the risks of anemia in infancy.6,16–19 This is more apparent in mothers below the poverty level, including those from developing countries. A study in the United States showed that recovery from anemia after delivery was slower for low-income women relative to those with family income above the poverty line.16,17 Other predictors that need to be considered in relation to the risk of developing anemia or recovery from it include nutritional deficiencies, infection, blood loss and delivery complications.3,20,21
We conducted a prospective study among HIV-negative Tanzania women enrolled during pregnancy to determine predictors of postpartum maternal anemia and iron deficiency anemia.
SUBJECTS/METHODS
Data for these analyses were derived from the Perinatal Study, a double-blind randomized controlled trial conducted in Dar es Salaam, Tanzania. Details of the study have been published.22 In brief, a total of 8428 HIV-negative pregnant women were randomly assigned to receive daily multivitamins (vitamin composition below) or placebo. Pregnant women who attended antenatal clinics in Dar es Salaam, Tanzania, between August 2001 and July 2004, were invited to participate in the study. Requirements for eligibility included a negative test result for HIV infection, residence in the city until delivery and for 1 year thereafter, and an estimated gestational age between 12 and 27 weeks according to the date of the last menstrual period.
Participants received standard antenatal care according to the Tanzanian Government guidelines. All women were provided daily prenatal supplemental iron (60 mg of elemental iron) and folic acid (0.25 mg), and malaria prophylaxis in the form of sulfadoxine–pyrimethamine tablets (sulfadoxine and pyrimethamine) at 20 and 30 weeks of gestation. Women received medication for any diagnosed illnesses, including treatment for malaria (additionally, women were given malaria prophylaxis) and for gastrointestinal parasites (during pregnancy all women were given de-worming medication). As part of the trial, women were randomly assigned to receive a daily oral dose of either a multivitamin supplement or placebo from the time of enrollment until the end of follow-up (12–18 months postpartum). The multivitamin supplements included vitamin B1 (20 mg), vitamin B2 (20 mg), vitamin B6 (25 mg), niacin (100 mg), vitamin B12 (50 μg), vitamin C (500 mg), vitamin E (30 mg) and folic acid (0.8 mg). At every monthly visit, a new bottle was given to each woman and the pills remaining in the used bottles were counted to measure adherence.
The Research and Publications Committee of Muhimbili University of Health and Allied Sciences, and the Institutional Review Board of the Harvard School of Public Health approved the study protocol.
Data collection
At baseline, trained research nurses collected data on sociodemographic characteristics and obstetrical history. Laboratory assessments at baseline included tests for syphilis, gonorrhea and trichomoniasis; red blood morphology; and evaluation of blood films for malaria. The total and differential white-cell counts were assayed with the use of a CBC5 counter (Coulter Corp., Miami, FL, USA) and were obtained at the first visit, which occurred between 12 and 27 weeks gestation, at 6 weeks post partum, and every 6 months thereafter. Counts of T-cell subgroups (CD4 +, CD8 + and CD3 +) were calculated in a random sample of women using the FACScount or FACScan system (Becton Dickinson, San Jose, CA, USA). Women were assessed at monthly clinic follow-up visits including presumptive diagnoses (symptomatic pending laboratory confirmation, defined as possible malaria diagnosed by a physician pending a positive blood smear), and those who did not keep their appointments were visited at home and were asked to come to the clinic if their condition allowed. Collection of hemoglobin and mean corpuscular volume (MCV) during pregnancy was at visit 1 and 2 months later or weekly, if 36 weeks gestation, at 6 weeks postpartum, and every 6 months thereafter until discharge. Additional data collected during follow-up include delivery type (spontaneous vaginal delivery, caesarian, vacuum extraction or assisted breech), survival status and malaria tests. The samples tested for T-cell counts (CD4, CD8) samples were obtained from a randomly selected subset of the entire study population.
Ascertainment of risk factors
Baseline risk factors
Baseline risk factors included malaria infection (yes/no), vaginal bleeding (yes/no), body mass index (BMI, which was calculated by dividing weight by height squared and categorized as follows: <22.5 kg/m2, 22.5–25 kg/m2, 25–30 kg/m2 and >30 kg/m2), maternal age and gestational age at enrollment. At enrollment, women were asked about their intake of alcohol, iron and soil since they became pregnant. Soil consumption, also known as pica, is common in pregnant women in this region. Pica is a craving woman have during pregnancy to eat substances such as clay soil or ash. Stillbirth was defined as the death of the fetus during labor or delivery, or intrauterine death of a fetus sometime before the onset of labor, where the fetus showed degenerative changes.
Time-varying maternal risk factors
Time-varying maternal risk factors included BMI, CD4 count, mid-upper arm circumference, malaria test results, presumptive diagnoses for malaria and dietary data ascertained by 24-h-recall questionnaires, as well as parity (continuous), malaria during this pregnancy (before enrollment) and other socio-economic factors (maternal education, daily spending for food and household items). Iron supplementation adherence during pregnancy was calculated based on adherence data (number of pills remaining from previous visit) collected at monthly follow-up visits. Presumed adherence to prenatal Fe supplements was defined as the number of days for which supplements were available divided by the total number of days between enrollment and delivery, and was included as a covariate in all multivariate analyses. Presumed adherence was calculated for every visit (current adherence), and a cumulative average of iron adherence was calculated for each woman during pregnancy. Dietary intake data were obtained from 24-h recalls completed every 3 months and nutrient intakes were classified as tertiles. Tertiles were calculated for iron, vitamin C, vitamin B12, vitamin B6, folate and heme; tertile 1 was the lowest intake (referent group) and tertile 3 was the highest intake in this population.
Ascertainment of anemia status
There were four main end points assessed in these analyses: incidence of anemia (hemoglobin <11 g/dl), incidence of iron deficiency anemia (anemia and MCV <80 g/dl), and two end points that represent recovery from these conditions (that is, recovery from anemia (hemoglobin >11 g/dl) and recovery of iron deficiency anemia (recovery from anemia and MCV >80 g/dl)).
Statistical analysis
Risk factors for the incidence of anemia and iron deficiency anemia during the postpartum period were assessed utilizing Cox proportional hazards regression methods.23 Anemia and iron deficiency anemia were assessed as the time to first occurrence of anemia and iron deficiency anemia, respectively. Follow-up started at the delivery date and was censored at the minimum date of first occurrence of anemia or last visit date that the outcome of interest was ascertained. Women who were anemic at enrollment were excluded from this analysis.
Additionally, using similar methods we assessed recovery from anemia and iron deficiency anemia. Recovery from anemia and iron deficiency anemia were assessed as the time to the first occurrence of recovery from anemia (defined by a hemoglobin concentration above 11 g/dl) and recovery from iron deficiency anemia (defined as a hemoglobin above 11 g/dl and an MCV above 80 fl). For those who did not achieve recovery during follow-up, end of follow-up was assessed at the date of their last assessment. Time zero was considered the date of delivery. All women who were non-anemic at enrollment were excluded from this analysis.
Predictors were selected from all available data based on their importance to anemia and iron deficiency, as well as prior knowledge of risk factors for anemia; all relevant variables were assessed in the univariate analyses and the most important ones are reported in the tables. Conventional cutoffs were used to categorize risk factors, where available; otherwise the medians were used to classify the variables, and for nutrient intake, tertiles were used to categorize the variables. The Wald test statistic was used to assess the significance of the association between predictors and the time to anemia, iron deficiency anemia, recovery from anemia and recovery from iron deficiency anemia to ensure maximum control for confounding. All predictors with a univariate P-value ≤0.20 were included in the multivariate model. Predictors were also examined as continuous variables and we explored non-linearity of the relationships using stepwise restricted cubic splines.24,25 Continuous variables of interest included: weight, height, mid-upper arm circumference, CD4 count and BMI. We used the counting process data structure to incorporate time-dependent covariates.26 The time interval corresponded to the interval between monthly follow-up visits after delivery. Observations with missing data were retained in the analyses with missing indicators. All analyses were done using SAS, Version 9.1, statistical software from SAS Institute Inc. (Cary, NC, USA).
RESULTS
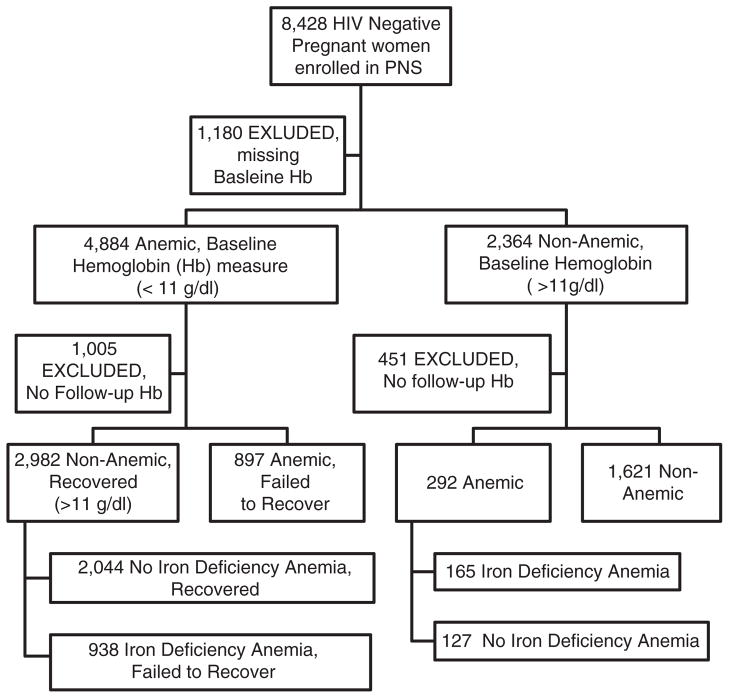
At baseline, 67.4% (4884/7248) of the population were anemic, defined by a hemoglobin concentration below 11 g/dl. Approximately 32.6% (2364/7248) of the study population were not anemic at baseline. The study flow chart can be found in Figure 1.
Figure 1.
Flow chart of all pregnant women from the Perinatal Study from 2001 to 2005 (PNS).
Among the 2364 non-anemic pregnant women at baseline, of whom 1913 had available follow-up information, 15.4% became anemic and 8.6% developed iron deficiency anemia. During the entire study period (12–27 weeks gestation through 12–18 months postpartum), only four non-anemic women became anemic during pregnancy, with the remainder developing anemia during the postpartum follow-up period. Eighty-eight percent of study participants were at 16 or more weeks gestation at enrollment and 38% were between 20 and 24 years of age (Table 1). Sixty-four percent of women delivered at a participating study clinic (compared with home deliveries and outside clinics), with 88% having a standard vaginal delivery and 96% giving birth to live babies.
Table 1.
Baseline characteristics
| Characteristics | Baseline Hb ≥11 g/dl (n =2364), n (%) | Baseline Hb <11 g/dl (n =4884), n (%) |
|---|---|---|
| Maternal age (years) | ||
| <20 | 347 (15) | 809 (17) |
| 20–24 | 892 (38) | 1978 (41) |
| 25–29 | 685 (29) | 1257 (26) |
| ≥30 | 428 (18) | 814 (17) |
| Baseline BMI (kg/m2) | ||
| <22 | 411 (20) | 1278 (30) |
| 22–24.99 | 656 (32) | 1565 (37) |
| 25–29.99 | 691 (33) | 1164 (27) |
| ≥30 | 306 (15) | 296 (6) |
| No. of previous pregnancies | ||
| 0 (primigravida) | 1038 (44) | 2235 (46) |
| 1 | 663 (28) | 1308 (27) |
| 2 | 353 (15) | 716 (15) |
| ≥3 | 297 (13) | 593 (12) |
| Weeks gestation at baseline (median) <20.9 | 1201 (51) | 2942 (60) |
| Baseline hemoglobin (g/dl)a | 11.8 (1) | 9.5 (1) |
| Height (cm)a | 155.8 (6) | 155.3 (6) |
| Baseline weight (kg)a | 62.3 (11.6) | 58.2 (10) |
| Baseline mid-upper arm circumferance (cm) (median)a | 27.5 (3.6) | 25.9 (3) |
| Mothers CD4 at baseline (cells/ml)a | 822.1 (266) | 795.6 (254) |
| Socio-economic characteristics | ||
| Per day spending for food (TShs) ≤500 | 883 (40) | 1780 (40) |
| Marital status | ||
| Cohabitating | 523 (22) | 985 (20) |
| Monogamous | 1477 (62) | 3107 (64) |
| Polygamous | 100 (4) | 172 (4) |
| Unmarried | 250 (11) | 575 (12) |
| Occupation | ||
| Housewife | 1628 (72) | 3604 (76) |
| Professional | 51 (2) | 88 (2) |
| Business | 112 (5) | 145 (3) |
| Office | 62 (3) | 65 (1) |
| Low skills | 35 (2) | 84 (2) |
| Public house | 19 (1) | 37 (1) |
| Other | 364 (16) | 689 (15) |
| Education | ||
| 0–4 years | 202 (9) | 641 (13) |
| 5–7 years | 1545 (65) | 3230 (66) |
| 8–11 years | 452 (19) | 770 (16) |
| 12 or more years | 153 (6) | 220 (5) |
| Owns/possesses | ||
| Sofa | 1796 (76.5) | 3358 (69) |
| Television | 1027 (43.8) | 1715 (51) |
| Cohabitating | 521 (22.2) | 981 (20) |
| Radio | 1969 (83.9) | 3900 (80) |
| Refrigerator | 661 (28.2) | 1029 (21) |
| Fan | 1264 (53.9) | 2192 (45) |
| Filmer–Pritchett wealth score below median <1.6 | 968 (41) | 2498 (51) |
| Dietary characteristics | ||
| Consumed alcohol at enrollment | ||
| Once a week | 1 (0.07) | 0 (0) |
| 1–3 times per month | 177 (12.2) | 334 (12) |
| Less than once per month | 80 (5.5) | 148 (5) |
| Never | 1918 (82.2) | 2370 (83) |
| History of meat consumption at enrollment | ||
| 2–4 times a week | 238 (10.1) | 434 (9) |
| Once a week | 1506 (64.2) | 2986 (61) |
| 1–3 times per month | 460 (19.6) | 1045 (21) |
| Less than once per month | 110 (4.7) | 303 (6) |
| Never | 31 (1.3) | 66 (1.4) |
| History of soil consumption at enrollment | ||
| Daily (5–7 times) | 36 (1.5) | 241 (5) |
| 2–4 times a week | 36 (1.5) | 206 (4) |
| Once a week | 38 (1.6) | 176 (4) |
| 1–3 times per month | 30 (1.3) | 203 (4) |
| Less than once per month | 92 (3.9) | 388 (8) |
| Never | 2114 (90.1) | 3617 (74) |
| Average iron supplement adherencea | 0.91 (0.22) | 0.90 (0.23) |
| Baseline iron intake (mg)a | 7.9 (3.9) | 8 (3.7) |
| Baseline vitamin C intake (mg)a | 68.6 (117.9) | 69.3 (121.7) |
| Baseline vitamin B12 intake (μg)a | 3.7 (23.7) | 3.7 (24.5) |
| Baseline vitamin B6 intake (mg)a | 0.94 (0.6) | 0.93 (0.6) |
| Baseline folate intake (mg)a | 185.8(139.6) | 186.9(145.6) |
| Baseline heme iron intake (mg)a | 0.56 (1.6) | 0.55 (1.7) |
| Medical characteristics (self report) | ||
| History of eclampsia | 22 (1) | 38 (1) |
| Fansidar this pregnancy | 406 (17) | 805 (17) |
| Diarrhea this pregnancy | 89 (4) | 169 (4) |
| Vaginal bleeding this pregnancy | 116 (5) | 205 (4) |
| Malaria this pregnancy | 716 (30) | 1314 (27) |
| Chlamydia | 7 (1.6) | 23 (3) |
| Delivery characteristics | ||
| When/how delivery information collected | ||
| Time of delivery | 1521 (77) | 3073 (76) |
| Delivery register labor ward (after delivery) | 95 (5) | 246 (5) |
| Client case notes | 18 (1) | 50 (1) |
| Family member/friend/neighbor | 43 (2) | 79 (2) |
| Medical attendant (recalled information) | 2 (0.1) | 2(0.04) |
| ANC or MCH card/medical record | 64 (3) | 122 (3) |
| Subsequent hospital admission | 2 (0.1) | 1 (0.02) |
| Mother herself/client | 213 (10) | 418 (9) |
| Other | 17 (1) | 31 (1) |
| Type of delivery | ||
| Spontaneous vaginal delivery | 2029 (88) | 4289 (90) |
| Vacuum extraction | 12 (0.5) | 43 (1) |
| Assisted breech | 38 (2) | 72 (1) |
| Emergency c-section | 189 (8) | 307 (6) |
| Elective c-section | 30 (1) | 49 (1) |
| Hemorrhage at delivery | 9 (0.4) | 19 (0.4) |
| Blood transfusion at delivery | 10 (0.4) | 20 (0.4) |
Abbreviations: ANC, antenatal care;
BMI, body mass index; Hb, hemoglobin; MCH, maternal and child health; TShs, Tanzanian shillings (US dollar is estimated at ~1250 shillings).
Mean and s.d. mean reported.
There were 4884 women with baseline hemoglobin concentrations below 11 g/dl at the enrollment, with 3879 having available follow-up information. Seventy-seven percent recovered from anemia during follow-up and 52.7% recovered from their iron deficiency anemia during follow-up. At the 6 weeks postpartum visit, 3576 (73%) of the women had a normal hemoglobin level.
Risk factors during the postpartum period
Incident anemia
Table 2 shows the univariate and multivariate analyses for anemia as defined by hemoglobin below 11 g/dl. Of the 292 women who developed anemia during the postpartum period, about half of these (n =156) presented with anemia at their 6-week-postpartum visit. The statistically significant independent risk factors for the postpartum development of anemia included postpartum CD4 cell count (RR 1.73; 95% confidence ratio (CI) 0.96, 3.17 P-value 0.007), and a delivery other than a standard vaginal delivery (RR 1.6; 95% CI 1.13, 2.22). Greater than median iron adherence, which was defined as a cumulative average during pregnancy, was marginally protective (RR 0.60; 95% CI 0.46, 0.80 P-value 0.0004).
Table 2.
Risk factors for incidence of anemia during postpartum period (Hb <11)
| No. of anemic patients | Univariate rate ratio (95% CI)a | P-valuea | Multivariate rate ratio (95% CI)a | P-valuea | |
|---|---|---|---|---|---|
| Maternal age (years) | |||||
| <20 | 53 | 1.26 (0.91, 1.75) | 0.06b | 1.24 (0.88, 1.75) | 0.03b |
| 20–24 | 110 | 1.0 | 1.0 | ||
| 25–29 | 73 | 0.81 (0.60, 1.09) | 0.77 (0.57, 1.05) | ||
| ≥30 | 55 | 0.87 (0.63, 1.21) | 0.81 (0.57, 1.15) | ||
| Baseline BMI (kg/m2) | |||||
| <22 | 56 | 1.13 (0.80, 1.60) | 0.70b | ||
| 22–24.99 | 73 | 1.0 | |||
| 25–29.99 | 77 | 0.90 (0.64, 1.23) | |||
| ≥30 | 40 | 1.11 (0.75, 1.63) | |||
| Postpartum BMI (kg/m2)c | |||||
| <22 | 61 | 1.01 (0.70, 1.44) | 0.87b | ||
| 22–24.99 | 57 | 1.0 | |||
| 25–29.99 | 55 | 0.97 (0.67, 1.40) | |||
| ≥30 | 29 | 0.90 (0.57, 1.41) | |||
| Baseline MUAC | |||||
| >Median | 143 | 1.0 | 0.96b | ||
| ≤Median | 149 | 1.09 (0.87, 1.37) | |||
| Postpartum MUACc | |||||
| >Median | 159 | 1.0 | 0.27b | ||
| ≤Median | 133 | 1.30 (1.00, 1.69) | |||
| No. of previous pregnancies | |||||
| 0 (primigravida) | 129 | 1.0 | 0.60b | ||
| 1 | 85 | 0.98 (0.74, 1.29) | |||
| 2 | 39 | 0.79 (0.55, 1.13) | |||
| ≥3 | 38 | 1.03 (0.72, 1.48) | |||
| Weeks gestation at baseline | |||||
| ≥Median | 153 | 1.0 | 0.66b | ||
| <Median | 139 | 1.04 (0.83, 1.31) | |||
| Baseline CD4 count | |||||
| ≥500 | 282 | 1.0 | 0.48b | ||
| <500 | 10 | 1.08 (0.57, 2.06) | |||
| Postpartum CD4 countc | |||||
| ≥500 | 280 | 1.0 | 0.003d | 1.0 | 0.003b |
| <500 | 12 | 1.50 (0.83, 2.71) | 1.73 (0.96, 3.17) | ||
| Socio-economic characteristics | |||||
| Per day spending for food | |||||
| >500 | 189 | 1.0 | 0.38 | ||
| ≤500 | 103 | 1.12 (0.87, 1.43) | |||
| Marital status | |||||
| Married | 189 | 1.0 | |||
| Other | 103 | 1.04 (0.82, 1.33) | 0.73 | ||
| Education status | |||||
| 0–4 | 28 | 1.40 (0.76, 2.60) | 0.41b | ||
| 5–7 | 190 | 1.15 (0.69, 1.91) | |||
| 8–11 | 57 | 1.24 (0.71, 2.15) | |||
| 12 or More | 16 | 1.0 | |||
| Owns/possesses | |||||
| Sofa | 231 | 1.06 (0.79, 1.40) | 0.71 | ||
| No | 61 | 1.0 | |||
| Television | 129 | 1.17 (0.92, 1.47) | 0.20 | ||
| No | 163 | 1.0 | |||
| Cohabitates with partner | 53 | 0.86 (0.64, 1.16) | 0.33 | ||
| No | 239 | 1.0 | |||
| Radio | 238 | 0.76 (0.56, 1.02) | 0.07 | 0.85 (0.63, 1.16) | 0.32 |
| No | 54 | 1.0 | 1.0 | ||
| Refrigerator | 80 | 0.82 (0.64, 1.07) | 0.14 | 0.88 (0.67, 1.16) | 0.36 |
| No | 212 | 1.0 | 1.0 | ||
| Fan | 168 | 1.03 (0.81, 1.30) | 0.81 | ||
| No | 124 | 1.0 | |||
| Filmer–Pritchett wealth score | |||||
| ≥Median | 182 | 1.0 | |||
| <Median | 110 | 0.92 (0.73, 1.17) | 0.51 | ||
| Dietary characteristics | |||||
| Consumed alcohol at enrollment | 34 | 1.36 (0.91, 2.03) | 0.14 | 1.27 (0.84, 1.92) | 0.26 |
| No alcohol | 148 | 1.0 | 1.0 | ||
| History of meat use at enrollment | |||||
| At least one per month | 220 | 1.0 | |||
| Less | 72 | 1.02 (0.78, 1.33) | 0.87 | ||
| History of soil use at enrollment | |||||
| At least 1 per month | 14 | 1.0 | |||
| Less | 278 | 0.99 (0.55, 1.76) | 0.97 | ||
| Fe sups adherence during pregnancy | |||||
| ≤Median | 77 | 1.0 | 1.0 | ||
| >Median | 215 | 0.58 (0.45, 0.75) | 0.001 | 0.60 (0.46, 0.80) | 0.0004 |
| Breastfeeding since last visit | |||||
| Yes | 8 | 1.06 (0.52, 2.15) | 0.87 | ||
| No | 284 | 1.0 | |||
| Iron intakec | |||||
| Tertile 1 | 12 | 1.0 | 0.76b | ||
| Tertile 2 | 19 | 1.27 (0.61, 2.62) | |||
| Tertile 3 | 10 | 0.75 (0.33, 1.75) | |||
| Vitamin C intakec | |||||
| Tertile 1 | 12 | 1.0 | 0.73b | ||
| Tertile 2 | 16 | 1.12 (0.53, 2.36) | |||
| Tertile 3 | 13 | 1.10 (0.50, 2.40) | |||
| Vitamin B12 intakec | |||||
| Tertile 1 | 22 | 1.0 | 0.07b | 1.0 | 0.06b |
| Tertile 2 | 7 | 0.82 (0.35, 1.93) | 0.85 (0.36, 2.00) | ||
| Tertile 3 | 12 | 0.72 (0.36, 1.46) | 1.06 (0.45, 2.48) | ||
| Vitamin B6 intakec | |||||
| Tertile 1 | 15 | 1.0 | 0.32d | ||
| Tertile 2 | 15 | 0.90 (0.44, 1.84) | |||
| Tertile 3 | 11 | 0.62 (0.28, 1.35) | |||
| Folate intakec | |||||
| Tertile 1 | 13 | 1.0 | 0.73b | ||
| Tertile 2 | 14 | 1.22 (0.57, 2.60) | |||
| Tertile 3 | 14 | 1.09 (0.51, 2.31) | |||
| Heme iron intakec | |||||
| Tertile 1 | 35 | 1.0 | 0.12b | 1.0 | 0.24b |
| Tertile 2 | 6 | 0.57 (0.24, 1.37) | 0.57 (0.19, 1.65) | ||
| Tertile 3 | 0 | — | — | ||
| Medical characteristics | |||||
| History of eclampsia | 1 | 0.32 (0.05, 2.28) | 0.26 | ||
| No | 291 | 1.0 | |||
| Fansidar this pregnancy | 45 | 0.82 (0.60, 1.13) | 0.22 | ||
| No | 247 | 1.0 | |||
| Diarrhea this pregnancy | 11 | 1.10 (0.60, 2.02) | 0.75 | ||
| No | 281 | 1.0 | |||
| Vaginal bleeding this pregnancy | 13 | 0.76 (0.44, 1.33) | 0.34 | ||
| No | 279 | 1.0 | |||
| Malaria this pregnancy (prior to enroll) | 79 | 0.78 (0.60, 1.01) | 0.06 | 0.80 (0.61, 1.04) | 0.09 |
| No | 213 | 1.0 | 1.0 | ||
| Presumptive malariac | 2 | 0.68 (0.17, 2.72) | 0.58 | ||
| No | 290 | 1.0 | |||
| Postpartum malariac | 54 | 1.39 (1.01, 1.90) | 0.04 | 1.22 (0.88, 1.70) | 0.23 |
| No | 238 | 1.0 | 1.0 | ||
| Chlamydia | 3 | 2.77 (0.87, 8.80) | 0.08 | 2.70 (0.83, 8.79) | 0.10 |
| No | 289 | 1.0 | 1.0 | ||
| Delivery characteristics | |||||
| Delivery type | |||||
| Spontaneous vaginal delivery | 249 | 1.0 | 1.0 | ||
| Other | 43 | 1.51 (1.09, 2.09) | 0.001 | 1.58 (1.13, 2.22) | 0.007 |
| Blood loss (hemorrhage/transfusion at delivery) | 3 | 3.39 (1.08,10.59) | 0.04 | 2.91 (0.90, 9.37) | 0.07 |
| No | 289 | 1.0 | 1.0 | ||
Abbreviations: BMI, body mass index;
CI, confidence interval; Fe sups, iron supplements; TSh, Tanzanian shillings. Risk ratio, confidence intervals and P-values are from Cox proportional hazards models.
P-value corresponds to trend test.
Time-varying covariates. Analysis performed using Andersen–Gill data structure, with 5136 observations during follow-up period.
Association was significantly nonlinear. P-value corresponds to test of overall significance.
Incident iron deficiency anemia
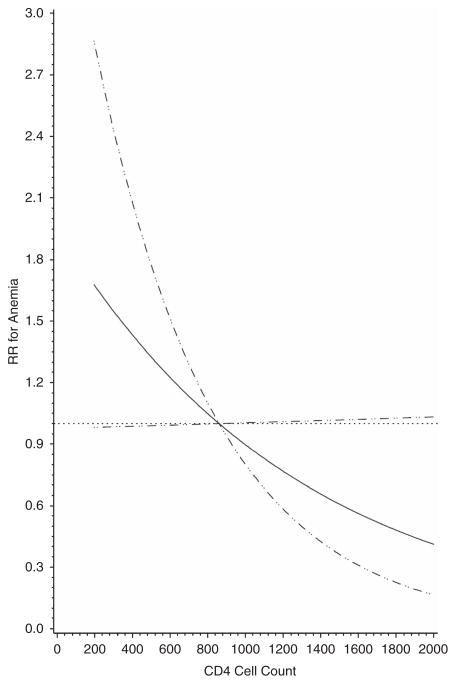
Table 3 contains the univariate and multivariate analyses for iron deficiency anemia. There were 165/1913 (8.6%) women that presented with iron deficiency anemia during the postpartum period. A total of 42% presented with anemia at their 6-week-postpartum visit (n =68). The statistically significant variables in the final multivariate model after adjusting for all other variables included adherence to iron supplements (defined as greater than median iron adherence, which was defined as a cumulative average during pregnancy) during pregnancy was significant in the multivariate model (RR 0.59; 95% CI 0.4, 0.87) and CD4 cell count was marginally significant (RR 2.16; 95% CI 0.98, 4.75). Figure 2 graphically represents the risk for iron deficiency anemia by CD4 cell count in the final adjusted model which was marginally significantly linear.
Table 3.
Risk factors for iron deficiency anemia (IDA) in the postpartum period (Hb <11 and MCV <80)
| No. of IDA patients | Univariate rate (95% CI)a | P-valuea | Multivariate rate ratio (95% CI)a | P-valuea | |
|---|---|---|---|---|---|
| Maternal age (years) <20 | 33 | 1.65 (1.07, 2.54) | 0.06b | 1.35 (0.87, 2.12) | 0.06b |
| 20–24 | 56 | 1.0 | 1.0 | ||
| 25–29 | 43 | 0.92 (0.62, 1.38) | 0.82 (0.54, 1.25) | ||
| ≥30 | 31 | 0.95 (0.61, 1.47) | 0.85 (0.52, 1.38) | ||
| Baseline BMI (kg/m2) | |||||
| <22 | 33 | 1.14 (0.72, 1.80) | 0.17b | 1.29 (0.80, 2.08) | 0.34b |
| 22–24.99 | 41 | 1.0 | 1.0 | ||
| 25–29.99 | 43 | 0.81 (0.53, 1.25) | 1.05(0.66, 1.68) | ||
| ≥30 | 18 | 0.85 (0.49, 1.48) | 1.21 (0.64, 2.30) | ||
| Postpartum BMI (kg/m2)c | |||||
| <22 | 34 | 0.97 (0.60, 1.58) | 0.37b | ||
| 22–24.99 | 31 | 1.0 | |||
| 25–29.99 | 28 | 0.89 (0.53, 1.48) | |||
| ≥30 | 16 | 0.80 (0.44, 1.47) | |||
| Baseline MUAC | |||||
| >Median | 74 | 1.0 | 0.24b | ||
| ≤Median | 90 | 0.80 (0.59, 1.10) | |||
| Postpartum MUACc | |||||
| >Median | 52 | 1.0 | 0.06b | 1.0 | 0.37b |
| ≤Median | 112 | 0.66 (0.46, 0.94) | 0.74 (0.48, 1.14) | ||
| No. of previous pregnancies | |||||
| 0 (primigravida) | 75 | 1.0 | 0.74b | ||
| 1 | 46 | 0.93 (0.64, 1.34) | |||
| 2 | 19 | 0.66 (0.40, 1.09) | |||
| ≥3 | 23 | 1.15 (0.72, 1.83) | |||
| Weeks gestation at baseline | |||||
| ≥Median | 77 | 1.05 (0.78, 1.43) | 0.80b | ||
| <Median | 87 | 1.0 | |||
| Baseline CD4 count | |||||
| ≥500 | 62 | 1.0 | 0.41b | ||
| <500 | 102 | 1.46 (0.67, 3.19) | |||
| Postpartum CD4 countc | |||||
| ≥500 | 85 | 1.0 | 1.0 | ||
| <500 | 79 | 1.40 (0.65, 3.02) | 0.05b | 2.16 (0.98, 4.75) | 0.06b |
| Socio-economic characteristics | |||||
| Per day spending for food | |||||
| >500 | 55 | 1.0 | |||
| ≤500 | 109 | 1.12 (0.80, 1.57) | 0.50 | ||
| Marital status | |||||
| Married | 64 | 1.0 | 1.0 | ||
| Other | 100 | 1.25 (0.91, 1.71) | 0.17 | 1.06 (0.75, 1.49) | 0.74 |
| Education status | |||||
| 0–4 | 15 | 1.49 (0.65, 3.42) | 0.65b | ||
| 5–7 | 104 | 1.23 (0.62, 2.44) | |||
| 8–11 | 35 | 1.50 (0.72, 3.12) | |||
| 12 or more | 9 | 1.0 | |||
| Owns/possesses | |||||
| Sofa | 124 | 0.82 (0.57, 1.18) | 0.29 | ||
| No | 40 | 1.0 | |||
| Television | 70 | 1.12 (0.82, 1.53) | 0.47 | ||
| No | 94 | 1.0 | |||
| Cohabitates with partner | 30 | 0.87 (0.58, 1.29) | 0.49 | ||
| No | 134 | 1.0 | |||
| Radio | 132 | 0.68 (0.46, 1.01) | 0.06 | 0.83(0.55, 1.26) | 0.38 |
| No | 32 | 1.0 | 1.0 | ||
| Refrigerator | 47 | 0.82 (0.58, 1.16) | 0.26 | ||
| No | 117 | 1.0 | |||
| Fan | 95 | 0.94 (0.69, 1.28) | 0.69 | ||
| No | 69 | 1.0 | |||
| Filmer–Pritchett wealth score | |||||
| ≥Median | 61 | 1.0 | |||
| <Median | 103 | 0.97 (0.71, 1.34) | 0.87 | ||
| Dietary characteristics | |||||
| Consumed alcohol at enrollment | 18 | 1.20 (0.72, 2.00) | 0.49 | ||
| No alcohol | 81 | 1.0 | |||
| History of meat use at enrollment | |||||
| At least 1 per month | 40 | 1.06 (0.74, 1.52) | 0.75 | ||
| Less | 124 | 1.0 | |||
| History of soil use at enrollment | |||||
| At least 1 per month | 158 | 1.08 (0.44, 2.65) | 0.86 | ||
| Less | 6 | 1.0 | |||
| Fe sups adherence during pregnancy | |||||
| ≤Median | 50 | 0.43 (0.31, 0.60) | <0.0001 | 0.59 (0.40, 0.87) | 0.008 |
| >Median | 114 | 1.0 | 1.0 | ||
| Breastfeeding since last visit | |||||
| Yes | 3 | 0.72 (0.23, 2.26) | 0.57 | ||
| No | 161 | 1.0 | |||
| Iron intakec | |||||
| Tertile 1 | 4 | 1.0 | 0.48b | ||
| Tertile 2 | 10 | 1.90 (0.60, 6.10) | |||
| Tertile 3 | 8 | 1.51 (0.45, 5.02) | |||
| Vitamin C intakec | |||||
| Tertile 1 | 6 | 1.0 | 0.65b | ||
| Tertile 2 | 9 | 1.34 (0.48, 3.78) | |||
| Tertile 3 | 7 | 1.12 (0.38, 3.33) | |||
| Vitamin B12 intakec | |||||
| Tertile 1 | 14 | 1.0 | 0.13b | 1.0 | 0.07b |
| Tertile 2 | 3 | 0.50 (0.14, 1.75) | 0.57 (0.16, 2.00) | ||
| Tertile 3 | 5 | 0.45 (0.16, 1.24) | 0.78 (0.23, 2.68) | ||
| Vitamin B6 intakec | |||||
| Tertile 1 | 7 | 1.0 | 0.79d | ||
| Tertile 2 | 7 | 0.88 (0.31, 2.51) | |||
| Tertile 3 | 8 | 0.83 (0.30, 2.29) | |||
| Folate intakec | |||||
| Tertile 1 | 6 | 1.0 | 0.48b | ||
| Tertile 2 | 6 | 1.00 (0.32, 3.12) | |||
| Tertile 3 | 10 | 1.43 (0.52, 3.94) | |||
| Heme iron intakec | |||||
| Tertile 1 | 19 | 1.0 | 0.07b | 1.0 | 0.31b |
| Tertile 2 | 3 | 0.48 (0.14, 1.63) | 0.47 (0.11, 2.10) | ||
| Tertile 3 | 0 | — | — | ||
| Medical characteristics | |||||
| History of eclampsia | 1 | 0.53 (0.07, 3.79) | 0.53 | ||
| No | 163 | 1.0 | |||
| Fansidar this pregnancy | 25 | 0.73 (0.48, 1.12) | 0.15 | ||
| No | 139 | 1.0 | |||
| Diarrhea this pregnancy | 6 | 1.06 (0.47, 2.40) | 0.88 | ||
| No | 158 | 1.0 | |||
| Vaginal bleeding this pregnancy | 8 | 0.82 (0.40, 1.67) | 0.58 | ||
| No | 156 | 1.0 | |||
| Malaria this pregnancy (prior to enrollment) | 47 | 0.80 (0.57, 1.12) | 0.19 | 0.76 (0.53, 1.07) | 0.12 |
| No | 117 | 1.0 | 1.0 | ||
| Presumptive malariac | 1 | 0.50 (0.07, 3.56) | 0.49 | ||
| No | 163 | 1.0 | |||
| Postpartum Malariac | 49 | 2.56 (1.80, 3.65) | <0.0001 | 1.85 (1.25, 2.74) | 0.002 |
| No | 115 | 1.0 | 1.0 | ||
| Chlamydia | 1 | 1.68 (0.23, 12.2) | 0.61 | ||
| No | 163 | 1.0 | |||
| Delivery characteristics | |||||
| Delivery type | |||||
| Spontaneous vaginal delivery | 139 | 1.0 | 1.0 | ||
| Other | 22 | 1.37 (0.88, 2.16) | 0.17 | 1.28 (0.79, 2.08) | 0.31 |
| Blood loss (hemorrhage/transfusion at delivery) | 2 | 3.54 (0.88, 14.3) | 0.08 | 4.29 (0.97, 19.01) | 0.06 |
| No | 162 | 1.0 | 1.0 | ||
Abbreviations: BMI, body mass index;
CI, confidence interval; Fe sups, iron supplements; Hb, hemoglobin; MCV, mean corpuscular volume; TSh, Tanzanian shillings. Risk ratio, confidence intervals, and P-values are from Cox proportional hazards models.
P-value corresponds to trend test.
Time-varying covariates. Analysis performed using Andersen–Gill data structure with 4679 observations during follow-up period.
Association was significantly nonlinear. P-value corresponds to test of overall significance.
Figure 2.
Risk of iron deficiency anemia by CD4 cell counts.
Recovery from anemia
Table 4 contains the univariate and multivariate analyses for recovery from anemia as defined by hemoglobin above 11 g/dl. A total of 2982/3879 (77%) women recovered from anemia during the postpartum period. The significant independent risk factors for recovery from anemia were a delivery other than a standard vaginal delivery (RR 0.89; 95% CI 0.81, 1.01), history of meat consumption prior to enrollment into the study (RR 1.12; 95% CI 1.03, 1.21) and postpartum malaria (RR 0.91; 95CI 0.84, 1.00). Increasing BMI, and maternal age (P-value <0.0001 for both) both were associated with risk of recovery from anemia.
Table 4.
Risk factors for recovery from baseline anemia in the postpartum period (Hb ≥11 recovery)
| No. of patients recovered from anemia | Univariate rate ratio (95% CI)a | P-valuea | Multivariate rate ratio (95% CI)a | P-valuea | |
|---|---|---|---|---|---|
| Maternal age (years) <20 | 440 | 0.89 (0.80, 0.99) | 0.0007 | 0.86 (0.76, 0.96) | 0.004b |
| 20–24 | 1164 | 1.0 | 1.0 | ||
| 25–29 | 838 | 1.13 (1.04,1.24) | 1.15 (1.04, 1.27) | ||
| ≥30 | 519 | 1.10 (0.99, 1.22) | 1.19 (1.04, 1.37) | ||
| Baseline BMI (kg/m2) | |||||
| <22 | 775 | 1.00 (0.91, 1.10) | 0.07 | 1.21 (0.97, 1.19) | 0.02c |
| 22–24.99 | 971 | 1.0 | 1.0 | ||
| 25–29.99 | 723 | 1.04 (0.94, 1.14) | 0.90 (0.80, 1.02) | ||
| ≥30 | 175 | 1.16 (0.98, 1.36) | 0.96 (0.76, 1.20) | ||
| Postpartum BMI (kg/m2)d | |||||
| <22 | 943 | 0.91 (0.82, 0.99) | 0.0001c | 0.88 (0.78, 0.98) | <0.0001b |
| 22–24.99 | 804 | 1.0 | 1.0 | ||
| 25–29.99 | 549 | 1.13 (1.01, 1.26) | 1.20 (1.05, 1.37) | ||
| ≥30 | 115 | 0.95 (0.78, 1.16) | 1.00 (0.76, 1.30) | ||
| Baseline MUAC | |||||
| >Median | 1914 | 1.0 | 0.02b | 1.0 | 0.05b |
| ≤Median | 1068 | 0.93 (0.86, 1.00) | 0.93 (0.83, 1.03) | ||
| Postpartum MUACd | |||||
| >Median | 1889 | 1.0 | 0.003c | 1.0 | 0.61b |
| ≤Median | 1093 | 0.93 (0.85, 1.01) | 1.06 (0.94, 1.19) | ||
| No. of previous pregnancies | |||||
| 0 (primigravida) | 1285 | 1.0 | 0.02b | 1.0 | 0.34b |
| 1 | 835 | 1.11 (1.01, 1.21) | 1.10 (0.92, 1.32) | ||
| 2 | 463 | 1.16 (1.04, 1.29) | 1.02 (0.84, 1.25) | ||
| ≥3 | 379 | 1.09 (0.97, 1.22) | 0.99 (0.80, 1.23) | ||
| Weeks gestation at baseline | |||||
| ≥Median | 1152 | 1.00 (0.93, 1.08) | 0.45b | ||
| <Median | 1830 | 1.0 | |||
| Baseline CD4 count | |||||
| ≥500 | 117 | 1.0 | 1.0 | ||
| <500 | 2865 | 0.84 (0.70, 1.02) | 0.13b | 0.90 (0.74, 1.09) | 0.33b |
| Postpartum CD4 countd | |||||
| ≥500 | 99 | 1.0 | |||
| <500 | 2883 | 0.97 (0.79, 1.19) | 0.48b | ||
| Socio-economic characteristics | |||||
| Per day spending for food | |||||
| >500 | 1106 | 1.0 | |||
| ≤500 | 1876 | 1.03 (0.96, 1.12) | 0.40 | ||
| Marital status | |||||
| Married | 991 | 1.0 | |||
| Other | 1991 | 1.01 (0.94, 1.10) | 0.80 | ||
| Education status | |||||
| 0–4 | 387 | 0.97 (0.80, 1.18) | 0.28 | ||
| 5–7 | 1947 | 1.03 (0.87, 1.23) | |||
| 8–11 | 491 | 1.09 (0.90, 1.32) | |||
| 12 or more | 138 | 1.0 | |||
| Owns/possesses | |||||
| Sofa | 2072 | 1.00 (0.93, 1.09) | 0.91 | ||
| No | 910 | 1.0 | |||
| Television | 1022 | 1.02 (0.94, 1.10) | 0.65 | ||
| No | 1960 | 1.0 | |||
| Cohabitates with partner | 571 | 1.03 (0.94, 1.13) | 0.48 | ||
| No | 2411 | 1.0 | |||
| Radio | 2434 | 1.04 (0.95, 1.15) | 0.37 | ||
| No | 548 | 1.0 | |||
| Refrigerator | 629 | 0.99 (0.90, 1.08) | 0.75 | ||
| No | 2353 | 1.0 | |||
| Fan | 1381 | 0.97 (0.90, 1.04) | 0.40 | ||
| No | 1601 | 1.0 | |||
| Filmer–Pritchett wealth score | |||||
| ≥Median | 1508 | 1.0 | |||
| <Median | 1474 | 1.02 (0.95, 1.09) | 0.63 | ||
| Dietary characteristics | |||||
| Consumed alcohol at enrollment | 281 | 0.90 (0.77, 1.04) | 0.15 | 1.07 (0.89, 1.29) | 0.47 |
| No alcohol | 1528 | 1.0 | 1.0 | ||
| History of meat use at enrollment | |||||
| At least one per month | 898 | 1.12 (1.04, 1.21) | 0.004 | 1.10 (1.01, 1.19) | 0.02 |
| Less | 2084 | 1.0 | 1.0 | ||
| History of soil use at enrollment | |||||
| At least one per month | 2619 | 1.17 (1.04, 1.30) | 0.07 | 1.09 (0.98, 1.22) | 0.13 |
| Less | 363 | 1.0 | 1.0 | ||
| Fe sups adherence during pregnancy | |||||
| ≤Median | 2314 | 1.14 (1.05, 1.24) | 0.003 | 1.12 (1.02, 1.22) | 0.02 |
| >Median | 668 | 1.0 | 1.0 | ||
| Breastfeeding since last visit | |||||
| Yes | 85 | 0.82 (0.66, 1.01) | 0.07 | 0.72 (0.58, 0.90) | 0.004 |
| No | 2897 | 1.0 | 1.0 | ||
| Iron intaked | |||||
| Tertile 1 | 123 | 1.0 | 0.25b | ||
| Tertile 2 | 107 | 0.89 (0.69, 1.15) | |||
| Tertile 3 | 95 | 0.84 (0.65, 1.10) | |||
| Vitamin C intaked | |||||
| Tertile 1 | 110 | 1.0 | 0.12b | 1.0 | 0.44b |
| Tertile 2 | 114 | 1.10 (0.84, 1.43) | 1.24 (0.92, 1.66) | ||
| Tertile 3 | 101 | 1.06 (0.81, 1.39) | 1.20 (0.88, 1.64) | ||
| Vitamin B12 intaked | |||||
| Tertile 1 | 179 | 1.0 | 0.37b | ||
| Tertile 2 | 43 | 0.97 (0.69, 1.36) | |||
| Tertile 3 | 103 | 0.99 (0.78, 1.26) | |||
| Vitamin B6 intaked | |||||
| Tertile 1 | 113 | 1.0 | 0.002c | 1.0 | 0.0005c |
| Tertile 2 | 118 | 0.97 (0.75, 1.25) | 0.97 (0.73, 1.29) | ||
| Tertile 3 | 94 | 0.89 (0.68, 1.17) | 0.91 (0.65, 1.28) | ||
| Folate intaked | |||||
| Tertile 1 | 117 | 1.0 | 0.13b | 1.0 | 0.85b |
| Tertile 2 | 106 | 0.92 (0.71, 1.20) | 0.91 (0.68, 1.22) | ||
| Tertile 3 | 102 | 0.89 (0.68, 1.16) | 0.91 (0.65, 1.28) | ||
| Heme iron intaked | |||||
| Tertile 1 | 264 | 1.0 | 0.15b | 1.0 | 0.87b |
| Tertile 2 | 59 | 1.08 (0.82, 1.43) | 1.00 (0.73, 1.36) | ||
| Tertile 3 | 2 | 3.82 (0.95, 15.36) | 3.46 (0.82, 14.69) | ||
| Medical characteristics | |||||
| History of eclampsia | 20 | 1.05 (0.67, 1.62) | 0.84 | ||
| No | 2962 | 1.0 | |||
| Fansidar this pregnancy | 511 | 1.01 (0.92, 1.11) | 0.89 | ||
| No | 2471 | 1.0 | |||
| Diarrhea this pregnancy | 106 | 0.84 (0.70, 1.02) | 0.09 | 0.96 (0.79, 1.17) | 0.68 |
| No | 2876 | 1.0 | 1.0 | ||
| Vaginal bleeding this pregnancy | 119 | 0.98 (0.82, 1.18) | 0.85 | ||
| No | 2863 | 1.0 | |||
| Malaria this pregnancy (prior to enrollment) | 819 | 0.96 (0.89, 1.04) | 0.33 | ||
| No | 2163 | 1.0 | |||
| Presumptive malariad | 264 | 0.83 (0.56, 1.23) | 0.35 | ||
| No | 2956 | 1.0 | |||
| Postpartum malariad | 835 | 0.88 (0.81, 0.96) | 0.003 | 0.91 (0.84, 1.00) | 0.04 |
| No | 2147 | 1.0 | 1.0 | ||
| Chlamydia | 9 | 0.81 (0.42, 1.56) | 0.52 | ||
| No | 2973 | 1.0 | |||
| Delivery characteristics | |||||
| Delivery type | |||||
| Spontaneous vaginal delivery | 2722 | 1.0 | 1.0 | ||
| Other | 260 | 0.83 (0.73, 0.94) | 0.003 | 0.89 (0.78, 1.01) | 0.07 |
| Blood loss (hemorrhage/transfusion at delivery) | 15 | 0.57 (0.34, 0.95) | 0.03 | 0.73 (0.44, 1.22) | 0.23 |
| No | 2967 | 1.0 | 1.0 | ||
Abbreviations: BMI, body mass index; Fe sups, iron supplements; TSh, Tanzanian shillings
CI, confidence interval; risk ratio, confidence intervals and P-values are from Cox proportional hazards models.
P-value corresponds to trend test
Association was significantly nonlinear. P-value corresponds to test of overall significance.
Time-varying covariates. Analysis performed using Andersen–Gill data structure, with 5003 observations during follow-up period.
Recovery from iron deficiency anemia
Table 5 contains the univariate and multivariate analyses for recovery from iron deficiency anemia as defined by hemoglobin above 11 g/dl and a MCV about 80 fL. A total of 2044/3879 (52.7%) women recovered from anemia during the postpartum period. The statistically significant variables in the final multivariate model after adjusting for all other variables were a delivery other than a standard vaginal delivery (RR 0.76; 95% CI 0.65, 0.90), iron supplements during pregnancy (defined as greater than median iron adherence, which was defined as a cumulative average during pregnancy) (RR 1.20; 95% CI 1.07, 1.34), history of meat consumption and history of soil consumption (RR 1.14; 95% CI 1.03, 1.25; RR 1.21; 95% CI 1.05, 1.39), increased education status (P-value 0.01) and maternal age were significant. Complete results can be found in Table 5.
Table 5.
Risk factors for recovery from baseline iron deficiency anemia (IDA) in the postpartum period (Hb/MCV recovery)
| No. of recovered IDA patients | Univariate rate ratio (95% CI)a | P-valuea | Multivariate rate ratio (95% CI)a | P-valuea | |
|---|---|---|---|---|---|
| Maternal age (years) <20 | 277 | 0.81 (0.71, 0.93) | 0.001b | 0.8 (0.7, 0.92) | 0.04b |
| 20–24 | 802 | 1.0 | 1.0 | ||
| 25–29 | 583 | 1.09 (0.98, 1.21) | 1.04 (0.94, 1.16) | ||
| ≥30 | 369 | 1.10 (0.98, 1.25) | 1.11 (0.98, 1.26) | ||
| Baseline BMI (kg/m2) | |||||
| <22 | 530 | 0.97 (0.87, 1.09) | 0.71b | ||
| 22–24.99 | 685 | 1.0 | |||
| 25–29.99 | 479 | 0.97 (0.86, 1.09) | |||
| ≥30 | 114 | 1.00 (0.82, 1.22) | |||
| Postpartum BMI (kg/m2)c | |||||
| <22 | 640 | 0.91 (0.81, 1.02) | 0.02d | 0.93 (0.83, 1.05) | 0.30b |
| 22–24.99 | 535 | 1.0 | 1.0 | ||
| 25–29.99 | 353 | 1.07 (0.94, 1.22) | 1.09 (0.94, 1.27) | ||
| ≥30 | 68 | 0.80 (0.62, 1.03) | 0.82 (0.62, 1.07) | ||
| Baseline MUAC | |||||
| >Median | 672 | 1.0 | 0.47b | ||
| ≤Median | 1372 | 0.99 (0.90, 1.09) | |||
| Postpartum MUACc | |||||
| >Median | 479 | 1.0 | 0.009d | 1.0 | 0.12b |
| ≤Median | 1565 | 0.98 (0.88, 1.09) | 0.93 (0.81, 1.07) | ||
| No. of previous pregnancies | |||||
| 0 (Primigravida) | 889 | 1.0 | 0.57b | ||
| 1 | 572 | 1.07 (0.96, 1.19) | |||
| 2 | 318 | 1.10 (0.97, 1.25) | |||
| ≥3 | 253 | 0.99 (0.87, 1.14) | |||
| Weeks gestation at baseline | |||||
| ≥Median | 776 | 0.97 (0.89, 1.06) | 0.71b | ||
| <Median | 1268 | 1.0 | |||
| Baseline CD4 count | |||||
| ≥500 | 758 | 1.0 | |||
| <500 | 1286 | 0.97 (0.77, 1.21) | 0.26b | ||
| Postpartum CD4 countc | |||||
| ≥500 | 1115 | 1.0 | 1.0 | ||
| <500 | 929 | 1.20 (0.95, 1.51) | 0.002b | 1.04 (0.82, 1.31) | 0.25b |
| Socio-economic characteristics | |||||
| Per day spending for food | |||||
| >500 | 767 | 1.0 | |||
| ≤500 | 1277 | 1.05 (0.96, 1.16) | 0.27 | ||
| Marital status | |||||
| Married | 669 | 1.0 | |||
| Other | 1375 | 0.98 (0.90, 1.08) | 0.73 | ||
| Education status | |||||
| 0–4 | 256 | 0.78 (0.62, 0.98) | 0.02b | 0.76 (0.60, 0.95) | 0.01b |
| 5–7 | 1314 | 0.84 (0.69, 1.02) | 0.83 (0.68, 1.02) | ||
| 8–11 | 353 | 0.91 (0.73, 1.12) | 0.97 (0.78, 1.20) | ||
| 12 or more | 110 | 1.0 | 1.0 | ||
| Owns/possesses | |||||
| Sofa | 1442 | 1.04 (0.95, 1.15) | 0.39 | ||
| No | 602 | 1.0 | |||
| Television | 717 | 1.06 (0.97, 1.16) | 0.20 | ||
| No | 1327 | 1.0 | |||
| Cohabitates with partner | 399 | 1.11 (1.00, 1.24) | 0.07 | 1.11 (0.99, 1.24) | 0.07 |
| No | 1645 | 1.0 | 1.0 | ||
| Radio | 1677 | 1.02 (0.91, 1.14) | 0.77 | ||
| No | 367 | 1.0 | |||
| Refrigerator | 446 | 1.04 (0.93, 1.15) | 0.51 | ||
| No | 1598 | 1.0 | |||
| Fan | 973 | 1.03 (0.95, 1.13) | 0.45 | ||
| No | 1071 | 1.0 | |||
| Filmer–Pritchett wealth score | |||||
| ≥Median | 1006 | 1.0 | |||
| <Median | 1038 | 0.95 (0.87, 1.03) | 0.22 | ||
| Dietary characteristics | |||||
| Consumed alcohol at enrollment | 185 | 0.91 (0.78, 1.07) | 0.25 | ||
| No alcohol | 1034 | 1.0 | |||
| History of meat use at enrollment | |||||
| At least one per month | 611 | 1.08 (0.98, 1.18) | 0.13 | 1.14 (1.03, 1.25) | 0.009 |
| Less | 1433 | 1.0 | 1.0 | ||
| History of soil use at enrollment: | |||||
| At least one per month | 1812 | 1.29 (1.12, 1.48) | 0.0004 | 1.21 (1.05, 1.39) | 0.007 |
| Less | 232 | 1.0 | 1.0 | ||
| Fe sups adherence during pregnancy | |||||
| ≤Median | 1597 | 1.08 (0.97, 1.20) | 0.15 | 1.20 (1.07, 1.34) | 0.002 |
| >Median | 447 | 1.0 | 1.0 | ||
| Breastfeeding since last visit | |||||
| Yes | 56 | 0.90 (0.69, 1.17) | 0.43 | ||
| No | 1988 | 1.0 | |||
| Iron intakec | |||||
| Tertile 1 | 46 | 1.0 | 0.13b | 1.0 | 0.65b |
| Tertile 2 | 39 | 0.90 (0.58, 1.37) | 0.89 (0.50, 1.60) | ||
| Tertile 3 | 31 | 0.71 (0.45, 1.12) | 0.66 (0.30, 1.46) | ||
| Vitamin C intakec | |||||
| Tertile 1 | 36 | 1.0 | 0.14b | 1.0 | 0.40b |
| Tertile 2 | 32 | 0.78 (0.51, 1.20) | 0.90 (0.56, 1.45) | ||
| Tertile 3 | 48 | 0.74 (0.47, 1.16) | 0.85 (0.49, 1.48) | ||
| Vitamin B12 intakec | |||||
| Tertile 1 | 9 | 1.0 | 0.75b | ||
| Tertile 2 | 37 | 0.45 (0.22, 0.89) | |||
| Tertile 3 | 70 | 0.95 (0.64, 1.42) | |||
| Vitamin B6 intakec | |||||
| Tertile 1 | 41 | 1.0 | 0.04b | 1.0 | 0.89b |
| Tertile 2 | 31 | 0.88 (0.57, 1.34) | 1.09 (0.64, 1.87) | ||
| Tertile 3 | 44 | 0.78 (0.49, 1.23) | 1.27 (0.63, 2.58) | ||
| Folate intakec | |||||
| Tertile 1 | 31 | 1.0 | 0.03b | 1.0 | 0.17b |
| Tertile 2 | 36 | 0.69 (0.44, 1.08) | 0.81 (0.48, 1.37) | ||
| Tertile 3 | 49 | 0.76 (0.49, 1.16) | 1.02 (0.55, 1.90) | ||
| Heme iron intakec | |||||
| Tertile 1 | 96 | 1.0 | 0.50b | ||
| Tertile 2 | 19 | 1.04 (0.64, 1.70) | |||
| Tertile 3 | 1 | 4.50 (0.63, 32.37) | |||
| Medical characteristics | |||||
| History of eclampsia | 15 | 1.12 (0.68, 1.87) | 0.65 | ||
| No | 2029 | 1.0 | |||
| Fansidar this pregnancy | 355 | 1.03 (0.92, 1.15) | 0.64 | ||
| No | 1689 | 1.0 | |||
| Diarrhea this pregnancy | 72 | 0.87 (0.69, 1.10) | 0.26 | ||
| No | 1972 | 1.0 | |||
| Vaginal bleeding this pregnancy | 81 | 0.99 (0.79, 1.24) | 0.92 | ||
| No | 1963 | 1.0 | |||
| Malaria this pregnancy (prior to enrollment) | 566 | 0.99 (0.90, 1.10) | 0.92 | ||
| No | 1478 | 1.0 | |||
| Presumptive malariac | 16 | 0.76 (0.46, 1.24) | 0.27 | ||
| No | 2028 | 1.0 | |||
| Postpartum malariac | 561 | 1.20 (1.09, 1.33) | 0.0003 | 1.02 (0.92, 1.13) | 0.75 |
| No | 1483 | 1.0 | 1.0 | ||
| Chlamydia | 2 | 0.32 (0.08, 1.26) | 0.10 | 0.28 (0.07, 1.14) | 0.08 |
| No | 2042 | 1.0 | 1.0 | ||
| Delivery characteristics | |||||
| Delivery type | |||||
| Spontaneous vaginal delivery | 1867 | 1.0 | 1.0 | ||
| Other | 168 | 0.79 (0.68, 0.93) | 0.004 | 0.76 (0.65, 0.90) | 0.0009 |
| Blood loss (hemorrhage/transfusion at delivery) | 14 | 0.92 (0.54, 1.56) | 0.76 | ||
| No | 2030 | 1.0 | |||
Abbreviations: BMI, body mass index;
CI, confidence interval; Fe sups, iron supplements; Hb, hemoglobin; MCV, mean corpuscular volume; TSh, Tanzanian shillings. Risk ratio, confidence intervals and P-values are from Cox proportional hazards models.
P-value corresponds to trend test.
Time-varying covariates. Analysis performed using Andersen–Gill data structure with 7127 observations during follow-up period.
Association was significantly nonlinear. P-value corresponds to the test of overall significance.
DISCUSSION
In the United States, women below the poverty level recovered more slowly from anemia, slower than non-poor women.17 The dearth of research globally on postpartum anemia has been formally recognized,16 and the focus of postpartum anemia and more specifically iron deficiency have only recently been studied in more detail.13–15 These studies of postpartum anemia have limited follow-up and only considered risk factors present during pregnancy. We examined incidence of anemia starting at 6 weeks postpartum, after which about 40% of non-anemic women developed anemia. Inversely, almost two-thirds of women who were anemic during the prepartum period recovered during the follow-up period. This was expected because women were no longer receiving iron supplements after delivery and DHS surveys report 56% prevalence of anemia in women of childbearing age in Tanzania.27 In comparison with other previous studies that only focused on baseline risk factors (assessments done during pregnancy), our study includes these risk factors, but additionally has a number of time-varying risk factors assessed throughout the study period up to 12–18 months postpartum.
The results show associations with iron intake and specifically heme iron intake in the univariate analyses, and iron supplementation in the multivariate.3 Iron or heme intake and B12 intake from the 24-h recalls were not significantly associated with anemia, iron deficiency anemia or recovery from anemia in the multivariate analysis; this is most likely due in part to the fact that a 24-h recall of iron intake prior to the outcome may not fully describe intake over a given period of time. This can also be said of histories of meat intake and soil intake, as availability of meat or craving of soil may have changed during pregnancy and is likely different in the postpartum period. Adherence to iron supplements during pregnancy was found to be beneficial in all analyses (anemia, recovery from anemia and recovery from iron deficiency anemia), and was significantly protective for iron deficiency anemia. It is well documented that iron supplementation during pregnancy is important for proper development of the fetus and for maternal health. In addition, it is well known that iron intake, especially heme iron from meats, is protective against anemia and iron deficiency anemia.28–32 Breastfeeding was not a risk factor for anemia, as noted in previous studies, due to its limited impact on iron stores.33–36 However, breastfeeding since the last visit was a statistically significant predictor of the recovery from anemia. Thus, breastfeeding delayed recovery from anemia that could be due to maternal depletion syndrome, defined as a broad pattern of maternal malnutrition resulting from the combined effects of dietary inadequacy, heavy workloads and energetic costs of repeated rounds of reproduction.37 Maternal depletion syndrome can possibly lead to additional nutritional requirements of breastfeeding women, leading to a delay in recovery from anemia in the postpartum period. Our results are consistent with previous studies and demonstrate the importance of assessing nutritional status within the pre- and postpartum periods, as elsewhere in the world.38–40
CD4 cell count <500, malaria, delivery complications, blood loss at delivery, and lower supplemental intake during pregnancy and dietary iron intake were associated with postpartum maternal anemia and iron deficiency anemia. Additionally, normal BMI, mid-upper arm circumference (MUAC), CD4 cell count <500 and absence of delivery complications were associated with recovery of postpartum anemia and iron deficiency anemia.
Malaria was significantly independently associated with an increased risk for iron deficiency anemia, and presumptive malaria reduced the recovery from anemia. CD4 cell count was assessed because of its relationship with immune status and was, therefore, used as a marker for disease. Malaria is a potential risk factor for anemia due to the anemia of chronic disease and our results being consistent with other studies.41–44 In anemia of chronic disease, the immune response releases hepcidin that blocks the release of iron stores, which lower hemoglobin during illnesses.5,21,43,45 This mechanism is consistent with the inverse effect we saw with presumptive malaria in relation to recovery from anemia. The same effects were seen with lower CD4 cell counts, which are markers for immune status.46
Complications during delivery, including blood loss and a delivery other than standard vaginal delivery, were related to an increased risk of anemia and iron deficiency anemia, and were limiting factors in recovery from anemia and iron deficiency anemia. This is as expected, as the main cause of anemia other than insufficient iron intake is blood loss.33,47,48 This is consistent with other studies that showed blood loss and delivery complications were the risk factors for anemia.18,49
Educational status was associated with a recovery from iron deficiency anemia, and heme iron intake was protective of both anemia and iron deficiency anemia risk, pointing to high meat and fish intake that is correlated with income levels. These studies have shown low socioeconomic status (SES) to be a risk for anemia or for the association to be null, but none have provided multivariate adjusted results or considered other factors that can cause anemia during the postpartum period. A couple of studies have examined the impact of anemia during the postpartum period, where socio-economic status was shown to decrease the time for a woman to return to a non-anemic hemoglobin concentration and also as a risk factor for anemia in the postpartum period.16,17,50
Our study had a few limitations. We focused on the postpartum period. The first postpartum period assessment was at 6 weeks, therefore, it is possible that they may have become anemic between delivery and 6 weeks. The fact that a very small number of non-anemic women became anemic during pregnancy supports the importance of supplementing pregnant women with both iron/folate supplements, as well as preventive measures for infections such as malaria and intestinal parasites by preventive treatments. We cannot exclude the possibility that women may have become anemic between delivery and 6 weeks and inversely, for the end point of recovery from anemia, some may have recovered during pregnancy up to 6 weeks, but were detected instead in the period past 6 weeks postpartum. MCV and hemoglobin are easy and convenient tests to assess iron deficiency anemia, although there are more sensitive tests available such as serum ferritin, they are costly and not always available in this setting.
The risk factors are consistent with those found in other studies. Our results show that risk factors observed during pregnancy are relevant to the risk of developing anemia 6 weeks postpartum. Although hemoglobin concentrations and hypochromatic micro-cytosis (MCV concentrations) are used to detect iron deficiency anemia in the developing world, which have been utilized here, there is the potential bias that we have not comprehensively assessed all competing risks such as anemia of infection (anemia of chronic disease) or other genetic causes of anemia that are properly assessed with additional tests using iron stores (ferritin) or other techniques. As anemia of chronic disease does not preclude iron deficiency anemia or anemia, and as other genetic factors for anemia are rare in this setting, it is unlikely that the inability to assess these types of anemia would have an appreciable impact on our analysis.
A major strength of our study is that it is one of very few that has examined risk factors for anemia in the postpartum period. We had a large sample compared with similar studies, providing substantial statistical power to detect a broad range of risk factors, even after detailed multivariate analysis. Our results are consistent with previous studies and are supported by various known and hypothesized mechanisms for the development of and recovery from anemia. Our findings illustrate the importance of providing iron–folate during pregnancy, and the need to improve access to and compliance with this intervention. Women who have complications during childbirth that potentially cause blood loss and stress may need to be given an additional supply of iron supplements to assist in the recovery of their iron status after discharge. Furthermore, it is important to further evaluate the significance of iron deficiency anemia in the postpartum period, especially among those with low iron intake and with infections, including malaria, intervention such as supplementation of all women to their 6-week-postpartum visit to assist with replenishing the iron stores after pregnancy, or testing hemoglobin levels so that women with slight anemia or borderline hemoglobin levels can be given iron supplements should be evaluated in randomized trials. All women should be counseled at delivery and at their postpartum visit on the importance of iron-rich foods in their diets to assist with replenishing their stores.
Acknowledgments
This work was supported by the National Institute of Child Health and Human Development (NICHD R01 37701), (clinicaltrials.gov #NCT00197548). CD was supported in part by NICHD K24HD058795. Research reported in this publication (AM) was supported by the Fogarty International Center of the National Institutes of Health under Award Number U2RTW008254. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.WHO. Worldwide prevalence of anemia 1993–2005. 2008. [accessed March 30, 2013]. http://www.who.int/vmnis/publications/anaemia_prevalence/en/
- 2.Stoltzfus RJ. Iron deficiency: global prevalence and consequences. Food Nutr Bull. 2003;24(4 Suppl):S99–103. doi: 10.1177/15648265030244S206. [DOI] [PubMed] [Google Scholar]
- 3.Pena-Rosas JP, Viteri FE. Effects and safety of preventive oral iron or iron +folic acid supplementation for women during pregnancy. Cochrane Database Syst Rev. 2009:CD004736. doi: 10.1002/14651858.CD004736.pub3. [DOI] [PubMed] [Google Scholar]
- 4.Mungen E. Iron supplementation in pregnancy. J Perinat Med. 2003;31:420–426. doi: 10.1515/JPM.2003.065. [DOI] [PubMed] [Google Scholar]
- 5.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 6.Brabin BJ, Premji Z, Verhoeff F. An analysis of anemia and child mortality. J Nutr. 2001;131:636S–645SS. doi: 10.1093/jn/131.2.636S. discussion 46S–48S. [DOI] [PubMed] [Google Scholar]
- 7.Marchant T, Schellenberg JA, Nathan R, Abdulla S, Mukasa O, Mshinda H, et al. Anaemia in pregnancy and infant mortality in Tanzania. Trop Med Int Health. 2004;9:262–266. doi: 10.1046/j.1365-3156.2003.01178.x. [DOI] [PubMed] [Google Scholar]
- 8.Ngnie-Teta I, Kuate-Defo B, Receveur O. Multilevel modelling of socio-demographic predictors of various levels of anaemia among women in Mali. Public Health Nutr. 2009;12:1462–1469. doi: 10.1017/S1368980008004400. [DOI] [PubMed] [Google Scholar]
- 9.Scholl TO, Hediger ML, Fischer RL, Shearer JW. Anemia vs iron deficiency: increased risk of preterm delivery in a prospective study. Am J Clin Nutr. 1992;55:985–988. doi: 10.1093/ajcn/55.5.985. [DOI] [PubMed] [Google Scholar]
- 10.Lopez de Romana G, Cusirramos S, Lopez de Romana D, Gross R. Efficacy of multiple micronutrient supplementation for improving anemia, micronutrient status, growth, and morbidity of Peruvian infants. J Nutr. 2005;135:646S–652SS. doi: 10.1093/jn/135.3.646S. [DOI] [PubMed] [Google Scholar]
- 11.Massawe SN, Urassa EN, Mmari M, Ronquist G, Lindmark G, Nystrom L. The complexity of pregnancy anemia in Dar-es-Salaam. Gynecol Obstet Invest. 1999;47:76–82. doi: 10.1159/000010067. [DOI] [PubMed] [Google Scholar]
- 12.Watson-Jones D, Weiss HA, Changalucha JM, Todd J, Gumodoka B, Bulmer J, et al. Adverse birth outcomes in United Republic of Tanzania–impact and prevention of maternal risk factors. Bull World Health Organ. 2007;85:9–18. doi: 10.2471/BLT.06.033258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christian P, Stewart CP, LeClerq SC, Wu L, Katz J, West KP, Jr, et al. Antenatal and postnatal iron supplementation and childhood mortality in rural Nepal: a prospective follow-up in a randomized, controlled community trial. Am J Epidemiol. 2009;170:1127–1136. doi: 10.1093/aje/kwp253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dibley MJ, Titaley CR, d’Este C, Agho K. Iron and folic acid supplements in pregnancy improve child survival in Indonesia. Am J Clin Nutr. 2012;95:220–230. doi: 10.3945/ajcn.111.022699. [DOI] [PubMed] [Google Scholar]
- 15.Titaley CR, Dibley MJ, Roberts CL, Agho K. Combined iron/folic acid supplements and malaria prophylaxis reduce neonatal mortality in 19 sub-Saharan African countries. Am J Clin Nutr. 2010;92:235–243. doi: 10.3945/ajcn.2009.29093. [DOI] [PubMed] [Google Scholar]
- 16.Bodnar LM, Cogswell ME, McDonald T. Have we forgotten the significance of postpartum iron deficiency? Am J Obstet Gynecol. 2005;193:36–44. doi: 10.1016/j.ajog.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Bodnar LM, Scanlon KS, Freedman DS, Siega-Riz AM, Cogswell ME. High prevalence of postpartum anemia among low-income women in the United States. Am J Obstet Gynecol. 2001;185:438–443. doi: 10.1067/mob.2001.115996. [DOI] [PubMed] [Google Scholar]
- 18.Chersich MF, Luchters SM, Yard E, Othigo JM, Kley N, Temmerman M. Morbidity in the first year postpartum among HIV-infected women in Kenya. Int J Gynaecol Obstet. 2008;100:45–51. doi: 10.1016/j.ijgo.2007.06.053. [DOI] [PubMed] [Google Scholar]
- 19.Isanaka S, Duggan C, Fawzi WW. Patterns of postnatal growth in HIV-infected and HIV-exposed children. Nutr Rev. 2009;67:343–359. doi: 10.1111/j.1753-4887.2009.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antelman G, Msamanga GI, Spiegelman D, Urassa EJ, Narh R, Hunter DJ, et al. Nutritional factors and infectious disease contribute to anemia among pregnant women with human immunodeficiency virus in Tanzania. J Nutr. 2000;130:1950–1957. doi: 10.1093/jn/130.8.1950. [DOI] [PubMed] [Google Scholar]
- 21.Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102:783–788. doi: 10.1182/blood-2003-03-0672. [DOI] [PubMed] [Google Scholar]
- 22.Fawzi WW, Msamanga GI, Urassa W, Hertzmark E, Petraro P, Willett WC, et al. Vitamins and perinatal outcomes among HIV-negative women in Tanzania. N Engl J Med. 2007;356:1423–1431. doi: 10.1056/NEJMoa064868. [DOI] [PubMed] [Google Scholar]
- 23.Cox DR. Regression models and life tables. J R Stat Soc Series B. 1972:187–220. [Google Scholar]
- 24.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 25.Govindarajulu US, Spiegelman D, Thurston SW, Ganguli B, Eisen EA. Comparing smoothing techniques in Cox models for exposure-response relationships. Stat Med. 2007;26:3735–3752. doi: 10.1002/sim.2848. [DOI] [PubMed] [Google Scholar]
- 26.Andersen PKaGRD. Cox’s regression model counting process: a large sample study. Ann Stat. 1982;10:1100–1120. [Google Scholar]
- 27.Tanzania Demographic and Health Survey 2004–2005. DHS; 2005. [Google Scholar]
- 28.Isanaka S, Mora-Plazas M, Lopez-Arana S, Baylin A, Villamor E. Food insecurity is highly prevalent and predicts underweight but not overweight in adults and school children from Bogota, Colombia. J Nutr. 2007;137:2747–2755. doi: 10.1093/jn/137.12.2747. [DOI] [PubMed] [Google Scholar]
- 29.Krummel D, Kris-Etherton P. Nutrition in Women’s Health. Aspen Publishers, Inc; Gaithersburg: 1996. p. 582. [Google Scholar]
- 30.Lartey A. Maternal and child nutrition in Sub-Saharan Africa: challenges and interventions. Proc Nutr Soc. 2008;67:105–108. doi: 10.1017/S0029665108006083. [DOI] [PubMed] [Google Scholar]
- 31.Makola D, Ash DM, Tatala SR, Latham MC, Ndossi G, Mehansho H. A micronutrient-fortified beverage prevents iron deficiency, reduces anemia and improves the hemoglobin concentration of pregnant Tanzanian women. J Nutr. 2003;133:1339–1346. doi: 10.1093/jn/133.5.1339. [DOI] [PubMed] [Google Scholar]
- 32.Ramakrishnam U, editor. Nutritional Anemias. CRC Press; 2001. [Google Scholar]
- 33.Ramakrishnam U, Manjrekrar R, Rivera J, Gonzales-Cossio T, Martorell R. Micro-nutrients and pregnancy outcomes: a review of the literature. Nutr Res. 1999;19:103–159. [Google Scholar]
- 34.Rozenberg G. In: Microscopic Haematology: A Practical Guide for the Laboratory. Dunitz M, editor. Martin Dunitz; London: 2003. p. 230. [Google Scholar]
- 35.Shashiraj, Faridi MM, Singh O, Rusia U. Mother’s iron status, breastmilk iron and lactoferrin—are they related? Eur J Clin Nutr. 2006;60:903–908. doi: 10.1038/sj.ejcn.1602398. [DOI] [PubMed] [Google Scholar]
- 36.de Teixeira ML, Lira PI, Coutinho SB, Eickmann SH, Lima MC. Influence of breastfeeding type and maternal anemia on hemoglobin concentration in 6-month-old infants. J Pediatr. 2010;86:65–72. doi: 10.2223/JPED.1959. [DOI] [PubMed] [Google Scholar]
- 37.Shell-Duncan B, Yung SA. The maternal depletion transition in northern Kenya: the effects of settlement, development and disparity. Soc Sci Med. 2004;58:2485–2498. doi: 10.1016/j.socscimed.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 38.Maruti SS, Feskanich D, Rockett HR, Colditz GA, Sampson LA, Willett WC. Validation of adolescent diet recalled by adults. Epidemiology. 2006;17:226–229. doi: 10.1097/01.ede.0000198181.86685.49. [DOI] [PubMed] [Google Scholar]
- 39.Hernandez-Avila M, Romieu I, Parra S, Hernandez-Avila J, Madrigal H, Willett W. Validity and reproducibility of a food frequency questionnaire to assess dietary intake of women living in Mexico City. Salud Publica Mex. 1998;40:133–140. doi: 10.1590/s0036-36341998000200005. [DOI] [PubMed] [Google Scholar]
- 40.Suitor CJ, Gardner J, Willett WC. A comparison of food frequency and diet recall methods in studies of nutrient intake of low-income pregnant women. J Am Diet Assoc. 1989;89:1786–1794. [PubMed] [Google Scholar]
- 41.Abrams ET, Kwiek JJ, Mwapasa V, Kamwendo DD, Tadesse E, Lema VM, et al. Malaria during pregnancy and foetal haematological status in Blantyre, Malawi. Malar J. 2005;4:39. doi: 10.1186/1475-2875-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brabin B. Malaria in pregnancy: current issues. Afr Health. 1997;19:19–20. [PubMed] [Google Scholar]
- 43.Ekvall H. Malaria and anemia. Curr Opin Hematol. 2003;10:108–114. doi: 10.1097/00062752-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Ratledge C. Iron metabolism and infection. Food Nutr Bull. 2007;28(4 Suppl):S515–S523. doi: 10.1177/15648265070284S405. [DOI] [PubMed] [Google Scholar]
- 45.Uneke CJ. Impact of placental Plasmodium falciparum malaria on pregnancy and perinatal outcome in sub-Saharan Africa: I: introduction to placental malaria. Yale J Biol Med. 2007;80:39–50. [PMC free article] [PubMed] [Google Scholar]
- 46.Isanaka S, Mugusi F, Urassa W, Willett WC, Bosch RJ, Villamor E, et al. Iron deficiency and anemia predict mortality in patients with tuberculosis. J Nutr. 2012;142:350–357. doi: 10.3945/jn.111.144287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolinsky I, Klimis-Tavantzis D, editors. Nutritional Concerns of Women. CRC Press; Boca Raton, FL, USA: 1996. Nutritional Concerns of Women; p. 335. [Google Scholar]
- 48.Dacie JV. Practical Hematology 1991. Longman Group; UK: 1991. [Google Scholar]
- 49.Dim CC, Onah HE. The prevalence of anemia among pregnant women at booking in Enugu, South Eastern Nigeria. Med Gen Med. 2007;9:11. [PMC free article] [PubMed] [Google Scholar]
- 50.Dijkhuizen MA, Wieringa FT, West CE, Muherdiyantiningsih, Muhilal Concurrent micronutrient deficiencies in lactating mothers and their infants in Indonesia. Am J Clin Nutr. 2001;73:786–791. doi: 10.1093/ajcn/73.4.786. [DOI] [PubMed] [Google Scholar]