Abstract
Cyclin-dependent kinase 5 (Cdk5) is associated with synaptic plasticity and cognitive function. Previous reports have demonstrated that Cdk5 is necessary for memory formation, although others have reported Cdk5 conditional knockout mouse models exhibiting enhanced learning and memory. Furthermore, how Cdk5 acts in specific cell populations to affect behavior and cognitive outcomes remains unclear. Here we conduct a behavioral characterization of a forebrain-specific Cdk5 conditional knockout mouse model under the αCaMKII promoter, in which Cdk5 is ablated in excitatory pyramidal neurons of the forebrain. The Cdk5 conditional knockouts exhibit hyperactivitiy in the open field, reduced anxiety, and reduced behavioral despair. Moreover, the Cdk5 conditional knockouts also display impaired spatial learning in the Morris water maze and are severely impaired in contextual fear memory, which correspond to deficits in synaptic transmission. Remarkably, the hyperactivity of the Cdk5 conditional knockouts can be ameliorated by the administration of lithium chloride, an inhibitor of GSK3β signaling. Collectively, our data reveal that Cdk5 ablation from forebrain excitatory neurons results in deleterious effects on emotional and cognitive behavior and highlight a key role for Cdk5 in regulating the GSK3β signaling pathway.
Keywords: Cdk5, open field, hyperactivity, transmitter release, cognitive function, lithium
1. Introduction
The neuronal cyclin-dependent kinase 5 (Cdk5) is a serine/threonine kinase with a well-established role during embryonic neuronal migration and in cytoskeletal dynamics (Dhavan et al., 2001). More recently, Cdk5 has been implicated in a variety of synaptic functions including the synaptic vesicle cycle and synapse formation (Samuels et al., 2007; Su et al., 2011). In addition to targeting NMDA receptors and postsynaptic scaffolding proteins such as PSD-95 (Li et al., 2001; Morabito et al., 2004), Cdk5 activity is critical for the formation of synaptic plasticity as measured by long-term potentiation (LTP) at hippocampal synapses (Fischer et al., 2005; Li et al., 2001). Furthermore, several intriguing reports have uncovered a prominent role for Cdk5 in regulating synaptic transmission to fine-tune homeostatic plasticity (Kim et al., 2010; Mitra et al., 2011; Seeburg et al., 2008). Despite the growing evidence highlighting Cdk5 and its influence on synaptic plasticity, recent studies on how Cdk5 loss-of-function affects behavioral outcome have reported diverging findings.
One study examining how loss of Cdk5 affects behavior reports an increase in learning and memory in a Cdk5 conditional knockout mouse line (Hawasli et al., 2007). The Cdk5 mutant animals were generated under the prion protein promoter, and they displayed enhanced spatial memory, increased memory formation during fear conditioning, and significantly enhanced LTP in synaptic plasticity experiments. However, other studies have demonstrated that Cdk5 is necessary for associative learning within the septohippocampal system, as inhibition of Cdk5 activity prevents contextual-fear memory formation (Fischer et al., 2002). Subsequent studies indicated that loss of Cdk5 in pyramidal neurons of specific hippocampal regions under the αCaMKII promoter, or pharmacological inhibition of Cdk5 activity, is detrimental to acquisition of spatial and contextual memory and results in deficits in LTP (Guan et al., 2011; Li et al., 2001). It is interesting to note that mouse models in which p35, the regulatory subunit of Cdk5, has been deleted show cortical lamination deficits, seizures, and adult lethality (Chae et al., 1997). The p35 knockout animals also exhibit hyperactivity and alterations in the dopaminergic and cholinergic systems (Drerup et al., 2010; Krapacher et al., 2010). In the p35 knockout animals, the contribution of p39, another co-activator of Cdk5, cannot be excluded from participating in a compensatory role to regulate Cdk5 function.
Given the uncertainty of how loss of Cdk5 affects the behavioral and cognitive outcomes, we have now examined a conditional knockout mouse model in which Cdk5 is selectively ablated in forebrain excitatory neurons. Through a series of behavioral tasks, we examined parameters such as motor activity, cognitive function, and synaptic plasticity after temporal, widespread Cdk5 ablation in a specific neuronal subtype. We show here that Cdk5 loss-of function in αCaMKII-positive pyramidal neurons results in strong hyperactivity, severely impaired cognitive function and marked deficits in neurotransmitter release. Remarkably, treatment of the Cdk5 conditional knockouts with lithium chloride can alleviate their hyperactivity. Further evaluation of the molecular mechanisms underlying the behavioral phenotype in these Cdk5 conditional knockouts will be useful to better understand how Cdk5 regulates behavior and cognition.
2. Materials and Methods
2.1. Animals
Cdk5 conditional knockout animals were generated by crossing the αCaMKII-Cre (CW2) line with the Cdk5 floxed/floxed (Cdk5f/f) line to generate αCaMKII-Cre;Cdk5fl/fl animals, referred to as Cdk5 conditional knockout (Cdk5f/f/CW2) animals. Mice were generated and maintained in a C57Bl/6J background and group-housed in a 12 hr light-dark cycle, with food and water ad libitum and handled according to the National Institutes of Health Care and Use of Laboratory Animals. Experiments were conducted using adult, 3–5 month old male mice.
2.2. Immunohistochemistry
Mice were transcardially perfused with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde (PFA) in PBS, and brains were postfixed in 4% PFA at 4°C overnight. Free floating coronal sections (40 μm) were prepared using a vibratome. Sections were incubated in a blocking solution of 10% normal donkey serum, 3% bovine serum albumin, 0.2% Triton-X 100, 0.02% sodium azide in PBS for 1–2 hr at room temperature (RT), followed by incubation with primary antibody (Cdk5, clone C-8, 1:100, Santa Cruz Biotechnology) in blocking solution overnight at 4°C with the appropriate Cy3-congugated (1:1000, Jackson Labs) secondary antibody for 2 hrs at RT. Hoechst dye was added to label cell nuclei. Images were acquired using high-resolution multi-channel scanning confocal microscopy (Zeiss LSM 510).
2.3. Behavioral training
2.3.1. Open Field Test
Animals were placed into 40 × 40 × 30 cm arenas with orthogonal lasers to track position and locomotor activity for 60 min (VersaMax, AccuScan Instruments). Activity was measured by movement across a grid of infrared light beams and automatically recorded. The margin was defined as the distance within 1 cm of the walls of the open field arena.
2.3.2. Light/dark exploration
Animals were placed into a light-dark chamber apparatus and allowed to explore the arenas for 10 min. Latency to enter the light for the first time, duration spent in the light chamber and the number of crossings from the dark to the light chamber were recorded.
2.3.3. Forced swim
Animals were placed in a 4000 ml Pyrex cylindrical container (10.2 × 45.7 cm) filled half-way full with water at a temperature of 24°C, and various parameters such as swim velocity, mobile duration and time during which the animal is swimming around the tank, immobile duration and time during which the animal remains almost completely still with its head above water, were automatically recorded using video tracking software for 6 min (EthovisionXT, Noldus). Immobility duration and time, parameters used to analyze learned helplessness or despair, was measured during the last 4 min of the test.
2.3.4 Pre-pulse inhibition
To assess sensorimotor gating, animals were habituated to the equipment for two days (Startle Monitor System, Hamilton Kinder). On the third day, the mouse was placed into the chamber, and after 5 min exposure to the white noise (65 dB), a series of trials were performed at random intervals: no acoustic stimulus, acoustic stimulus alone, or prepulse stimuli (70, 75, 80, 85 dB) followed by an acoustic startle stimulus. Percent PPI was calculated as [100 − (response amplitude for prepulse stimulus with startle stimulus/amplitude for the startle stimulus) × 100].
2.3.5. Morris water maze
Spatial memory was performed using a circular tank (1.2 m in diameter) filled with opaque water at 22°C. The walls contained spatial reference cues, and inside the tank was a fixed platform (10 cm in diameter) in a target quadrant. During pre-training (Day 1), animals were placed in the water, guided to the platform, and allowed to stay in the platform for 15 s. In the following days, animals were placed into the maze randomly and allowed to search for the platform for 60 s. Two trials a day were conducted with a 2 min interval. Mouse behavior was recorded and analyzed, and escape latency was scored (TSE Systems). On day 9, the platform was removed and a probe trial was conducted to assess spatial learning.
2.3.6. Fear conditioning
Both contextual and cued fear conditioning was conducted over the course of 3 days. On day 1, animals were placed in a fear conditioning apparatus chamber for 3 min and then presented with a 2 s foot shock delivery at 0.8 mA preceded by a 30 s, 75 dB tone (TSE Systems). After the animals were placed in their homecage for 24 hr, they were assessed for contextual learning by recording the freezing behavior for 3 min in the training context. On day 3, the animals were presented with a different context but levels of freezing were assessed after exposure to the same tone. Prior to each trial, each context was cleaned with 70% ethanol except for on day 3, where it was cleaned with acetic acid.
2.4. Electrophysiology
To record field excitatory postsynaptic potentials, transverse hippocampal slices were prepared from 8–12 week old adult mice. Briefly, the brain was rapidly removed and transferred to ice-cold, oxygenated (95 % O2 and 5 % CO2) cutting solution containing (mM) 211 sucrose, 3.3 KCl, 1.3 NaH2PO4, 0.5 CaCl2, 10 MgCl2, 26 NaHCO3 and 11 glucose. Hippocampal slices were cut with a Leica VT1000S vibratome (Leica) and transferred to a holding chamber containing oxygenated artificial cerebrospinal fluid (ACSF) consisted of (mM) 124 NaCl, 3.3 KCl, 1.3 NaH2PO4, 2.5 CaCl2, 1.5 MgCl2, 26 NaHCO3 and 11 glucose at 28–30°C for at least 1 h before recording. CA1 field potentials evoked by Schaffer collateral stimulation were measured. Recordings were performed using a MultiClamp 700B amplifier and a Digidata 1440A A-D converter and analyzed by pClamp 10 software (Axon Instruments).
For miniature recordings, whole-cell recordings were performed on CA1 pyramidal neurons. Cells were held at −70 mV and data were acquired using a MultiClamp 700B amplifier and a Digidata 1440A A-D converter (Axon Instruments). The internal solution contained (mM) 145 CsCl, 5 NaCl, 10 HEPES-CsOH, 10 EGTA, 4 MgATP and 0.3 Na2GTP. TTX (1 μM), D-APV (50 μM) and picrotoxin (50 μM) were added to ACSF for mEPSC.
2.5. Lithium administration
LiCl (Sigma) was dissolved in 0.9% NaCl saline. Injection volumes were calculated to achieve a final concentration of 85mg/kg. Intraperitoneal (IP) injections were performed 40 min prior to the experiments.
2.6. Statistical analyses
All experiments were performed blind to genotype. Statistical analyses were carried out with Student’s t-test when two groups were compared or with One-way ANOVA followed by Bonferroni correction for comparison of more than two groups (GraphPad Prism). Significance levels were set at p < 0.05 (*); p < 0.01 (**); p < 0.001(***).
3. Results
3.1. Conditional deletion of Cdk5 in excitatory neurons of the forebrain
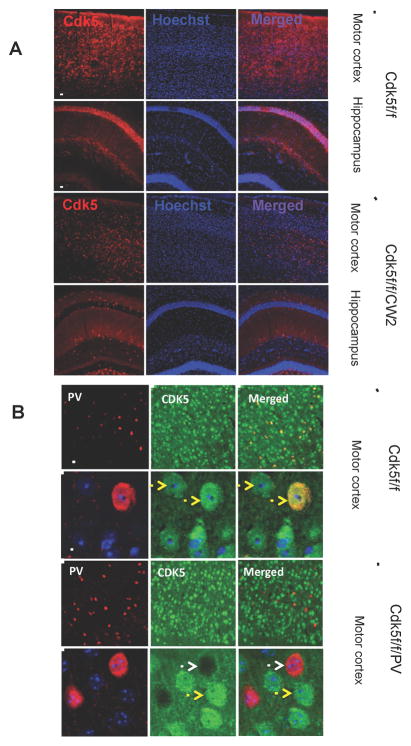
Cdk5 conditional knockout animals (Cdk5f/f/CW2) were generated by crossing floxed Cdk5 (Cdk5f/f) animals to the Cre CW2 transgenic line under the αCaMKII promoter (Guan et al., 2011; Zeng et al., 2001). The Cdk5f/f/CW2 animals do not exhibit gross developmental abnormalities and survive until 5–6 months of age, when they begin to suffer seizures and adult lethality (unpublished observations). The loss of Cdk5 specifically in forebrain pyramidal neurons was confirmed using an antibody directed towards Cdk5 in immunostaining images (Figure 1).
Figure 1. Conditional deletion of Cdk5 in excitatory neurons of the forebrain.
(A) Immunocytochemistry conducted using the Cdk5 antibody on Cdk5f/f (control) and Cdk5f/f/CW2 (Cdk5 conditional knockout) animals revealed dramatically reduced Cdk5 signal in the motor cortex and hippocampus of Cdk5f/f/CW2 animals (Scale bars, 100 μm).
(B) Positive and negative controls for CDK5 ablation in the cortical neurons. The images represent anti-CDK5 staining in the cortices of the fCdk5 mice and the littermate Cdk5f/f/PV-Cre mice in which CDK5 was ablated specifically in PV-neurons. Note that all the PV+ neurons are CDK5− while the all the other neurons show CDK5 immunoreactivity. Antibody used the immonostaining are the same anti-Cdk5 used for analysis of Cdk5f/f/CW2 mouse brains (rabbit C-8; Santa Cruz Biotech). Yellow arrows: wild type neurons expressing Cdk5; White arrows: the neurons with Cdk5 ablated. Scale bars, 15 μm.
3.2. Cdk5f/f/CW2 animals display pronounced hyperactivity in the open field arena
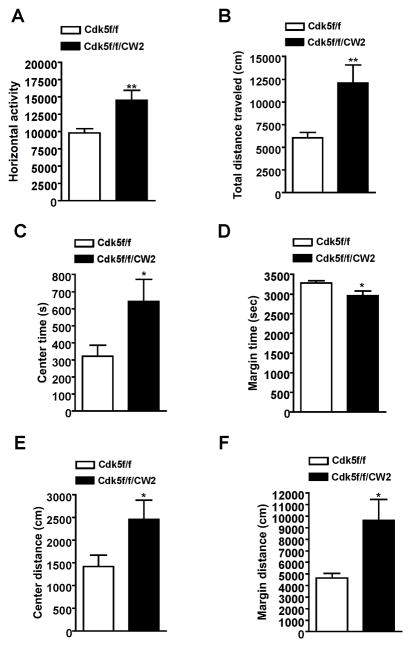
We next examined how loss of Cdk5 in excitatory pyramidal neurons might affect behavior. In an open field arena, Cdk5f/f/CW2 animals exhibited decreased anxiety as well as overall hyperactivity in the open field test as measured by the horizontal distance traveled when compared to control Cdk5f/f littermates (Figure 2A). The hyperactivity corresponded to Cdk5f/f/CW2 animals traveling a significantly greater distance overall (Figure 2B). Compared to control Cdk5f/f littermates, Cdk5f/f/CW2 animals also spent more time in the center of the open field arena and less time in the margin area (Figure 2C, 2D). Furthermore, Cdk5f/f/CW2 traveled a greater distance in both the center and margin areas compared to control Cdk5f/f, another indicator of hyperactivity along with reduced anxiety (Figure 2E, 2F).
Figure 2. Cdk5f/f/CW2 animals display pronounced hyperactivity in the open field arena.
(A) Compared to control Cdk5f/f littermates, Cdk5f/f/CW2 exhibited greater horizontal activity as measured by infrared beam breaks across the open field arena (Cdk5f/f, 9834 ± 623.7; Cdk5f/f/CW2, 14560 ± 1421; p=0.0063).
(B) Consistent with the observation that Cdk5f/f/CW2 display increased overall activity, Cdk5f/f/CW2 animals were consistently and significantly more active overall and traveled a greater distance in the open field arena over a period of 60 min compared to Cdk5f/f control littermates (Cdk5f/f, 6067 ± 598.6; Cdk5f/f/CW2, 12100 ± 1970; p=0.0076).
(C) Compared to control Cdk5f/f littermates, Cdk5f/f/CW2 animals spent more time in the center of the open field arena, indicative of reduced anxiety that is consistent with observations from the light/dark test (Cdk5f/f, 323.2 ± 63.17; Cdk5f/f/CW2, 643.6 ± 129.3; p=0.0356).
(D) Compared to control Cdk5f/f littermates, Cdk5f/f/CW2 animals spent less time in the margin of the open field arena (Cdk5f/f, 3277 ± 63.17; Cdk5f/f/CW2, 2956 ± 129.3; p=0.0356).
(E) Compared to control Cdk5f/f littermates, Cdk5f/f/CW2 traveled a greater distance in the center of the open field, indicating reduced anxiety (Cdk5f/f, 1421 ± 253.0; Cdk5f/f/CW2, 2461 ± 419.8; p=0.0458).
(F) Compared to control Cdk5f/f littermates, Cdk5f/f/CW2 animals traveled a greater distance in the margin of the open field test, reflecting a generalized hyperactivity (Cdk5f/f, 4648 ± 417.2; Cdk5f/f/CW2, 9637 ± 1808; p=0.0123). N=9 animals for Cdk5f/f; N=8 animals for Cdk5f/f/CW2 genotypes (Cdk5f/f, 4388 ± 313.0; Cdk5f/f/CW2, 5187 ± 507.0; p=0.1893). Data shown are means ± SEM. Statistical significance was calculated using Student’s t-test (*p < 0.05; **p < 0.01; ***p < 0.001).
3.3. Cdk5f/f/CW2 animals display reduced anxiety and increased acoustic startle
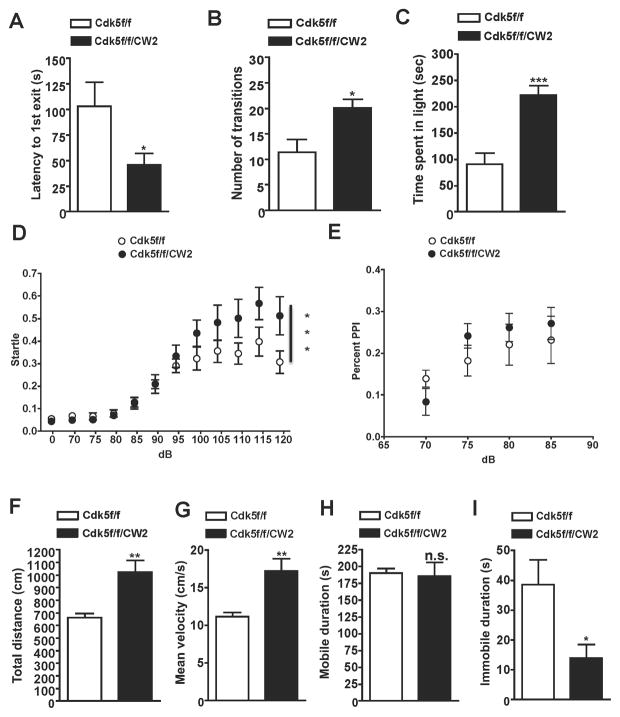
To further support our observations of reduced anxiety in Cdk5f/f/CW2 animals, we conducted a well-characterized behavioral assay for anxiety, the light-dark test. Consistent with our open field data, we observed significantly decreased anxiety in the Cdk5f/f/CW2 animals compared to control (Cdk5f/f) animals. Cdk5f/f/CW2 exhibited a decreased latency of first exit into the light (Figure 3A), made more transitions overall between the two chambers (Figure 3B), and spent more time in the brightly-lit chamber (Figure 3C). To test if Cdk5f/f/CW2 exhibited any alterations in sensorimotor gating, pre-pulse inhibition (PPI) measurements were collected. While Cdk5f/f/CW2 exhibited increased acoustic startle to the training tones (Figure 3D), they did not exhibit any differences in percentage PPI at different intervals when compared to control Cdk5f/f animals (Figure 3E). In the forced swim test, a measure of learned helplessness, the hyperactivity observed in the Cdk5f/f/CW2 animals affected their swimming behavior. Compared to control Cdk5f/f littermates, Cdk5f/f/CW2 animals exhibited greater movement as reflected in the total distance traveled (Figure 3F), and higher mean velocity (Figure 3G). In the mobile duration, there were no significant differences between Cdk5f/f and Cdk5f/f/CW2 animals (Figure 3H). However, compared to control Cdk5f/f littermates, Cdk5f/fCW2 exhibited decreased immobile duration in the forced swim test (Figure 3I), consistent with the data reflecting increased total movement in the forced swim test chamber as well as during the open field test.
Figure 3. Cdk5f/f/CW2 animals display reduced anxiety and elevated acoustic startle.
(A) The light/dark test was used to assess anxiety in Cdk5f/f and Cdk5f/f/CW2 animals. While control Cdk5f/f animals were more anxious of the light region, Cdk5f/f/CW2 animals crossed over into the brightly-lit chamber faster than their control littermates as indicated by shorter latency to enter the light (Cdk5f/f, 102.89 ± 23.63; Cdk5f/f/CW2, 45.67 ± 11.39; p=0.0389).
(B) Compared to their control Cdk5f/f littermates, Cdk5f/f/CW2 animals crossed over into the light chamber with more frequency (Cdk5f/f, 11.38 ± 2.556; Cdk5f/f/CW2, 20.11 ± 1.695; p=0.0108).
(C) Compared to their control Cdk5f/f littermates, Cdk5f/f/CW2 animals spent more time overall in the light (Cdk5f/f, 90.38 ± 21.37; Cdk5f/f/CW2, 222.2 ± 17.37; p=0.0002). N=8 for Cdk5f/f, N=9 for Cdk5f/f/CW2. Data shown are means ± SEM. Statistical significance was calculated using unpaired Student’s t-test (*p < 0.05; **p < 0.01; ***p < 0.001).
(D) Compared to control Cdk5f/f animals, Cdk5f/f/CW2 animals exhibited greater startle in the pre-pulse inhibition test at higher test decibels (100 dB: Cdk5f/f, 0.322 ± 0.051; Cdk5f/f/CW2, 0.435 ± 0.057; 105 dB: Cdk5f/f, 0.356 ± 0.050; Cdk5f/f/CW2, 0.482 ± 0.079; 110 dB: Cdk5f/f, 0.345 ± 0.047; Cdk5f/f/CW2, 0.501 ± 0.084; 115 dB: Cdk5f/f, 0.397 ± 0.064; Cdk5f/f/CW2, 0.567 ± 0.072; 120 dB: Cdk5f/f, 0.306 ± 0.049; Cdk5f/f/CW2, 0.513 ± 0.085; p<0.001 by two-way ANOVA).
(E) The percent of pre-pulse inhibition, a measurement of sensorimotor gating, did not significantly differ between Cdk5f/f and Cdk5f/f/CW2 animals (70 dB: Cdk5f/f, 0.139 ± 0.021; Cdk5f/f/CW2, 0.083 ± 0.032; 75 dB: Cdk5f/f, 0.182 ± 0.037; Cdk5f/f/CW2, 0.241 ± 0.029; 80 dB: Cdk5f/f, 0.220 ± 0.049; Cdk5f/f/CW2, 0.261 ± 0.033; 85 dB: Cdk5f/f, 0.232 ± 0.057; Cdk5f/f/CW2, 0.271 ± 0.038). N=9 animals for both Cdk5f/f and Cdk5f/f/CW2 genotypes.
(F) Compared to Cdk5f/f control littermates, Cdk5f/f/CW2 animals were more active overall during the forced swim test as indicated by the total distance traveled in the water (Cdk5f/f, 661.78 ± 33.58; Cdk5f/f/CW2, 1023.08 ± 95.32; p=0.0019).
(G) Cdk5f/f/CW2 animals were more active in the forced swim test and also exhibited a higher velocity in their swimming movements (Cdk5f/f, 11.18 ± 0.5670; Cdk5f/f/CW2, 17.29 ± 1.610; p=0.0019).
(H) The mobile durations between Cdk5f/f and Cdk5f/f/CW2 animals were not significantly different (Cdk5f/f, 190.3 ± 7.129; Cdk5f/f/CW2, 185.9 ± 20.20; p=0.8304).
(I) Compared to control Cdk5f/f animals, Cdk5f/f/CW2 animals exhibited significantly less time immobile (Cdk5f/f, 38.59 ± 8.313; Cdk5f/f/CW2, 13.93 ± 4.622; p=0.0245). N=9 for Cdk5f/f; N=8 for Cdk5f/f/CW2 genotypes. Data shown are means ± SEM. Statistical significance was calculated using Student’s t-test unless otherwise indicated (*p < 0.05; **p < 0.01; ***p < 0.001).
3.4 Cdk5f/f/CW2 animals are severely impaired in hippocampus-dependent cognitive tasks
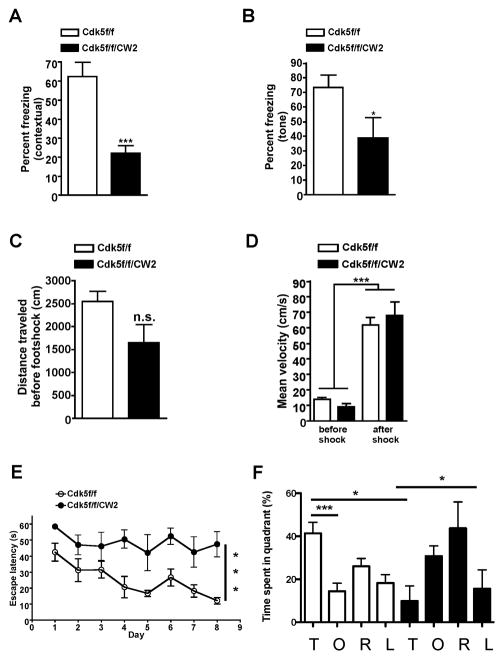
To test whether Cdk5f/f/CW2 exhibited cognitive impairments, we next conducted contextual fear conditioning, a well-established task to assess hippocampus-dependent memory formation. Animals were placed in a conditioning chamber and presented with an unconditioned stimulus (footshock). Upon testing for fear memory in the same chamber 24 hr later, Cdk5f/f/CW2 animals exhibited a dramatic reduction in freezing levels compared to control Cdk5f/f animals, indicating a severe impairment of cognitive function (Figure 4A). Moreover, Cdk5f/f/CW2 animals were also significantly impaired in tone-dependent fear memory (Figure 4B). Both Cdk5f/f and Cdk5f/f/CW2 animals displayed similar distance traveled before the footshock (Figure 4C) and increased velocity after the footshock during the training period (Figure 4D), which suggests that both groups of animals were able to respond to the footshock. To further examine the cognitive abilities of the Cdk5f/f/CW2 animals, we performed the Morris water maze task to assess hippocampus-dependent spatial learning. Contrary to several reports (Hawasli et al., 2007), but consistent with others (Guan et al., 2011), Cdk5f/f/CW2 animals displayed a severe impairment in memory acquisition and retention during training showing significantly higher escape latency then control mice at the end of the training period (Figure 4E). Moreover, control Cdk5f/f animals spent significantly more time in the target compared to the opposite quadrant (p<0.001) during probe trial indicating successful spatial learning. However, Cdk5f/f/CW2 group did not show any significant preference for a target quadrant, suggesting that Cdk5f/f/CW2 mice were unable to form spatial memory (Figure 4F).
Figure 4. Cdk5f/f/CW2 animals are severely impaired in hippocampus-dependent cognitive tasks.
(A) Whereas control Cdk5f/f littermates displayed robust freezing levels upon testing 24 hr after the footshock, Cdk5f/f/CW2 animals were significantly impaired in contextual fear memory (Cdk5f/f, 62.35 ± 7.452; Cdk5f/f/CW2, 22.22 ± 3.928; p=0.0004).
(B) Compared to control Cdk5f/f littermates, tone-dependent fear conditioning was also significantly impaired in Cdk5f/f/CW2 animals (Cdk5f/f, 73.46 ± 8.475; Cdk5f/f/CW2, 38.89 ± 14.05, p=0.0471). N=9 for Cdk5f/f; N=8 for Cdk5f/f/CW2. Data shown are means ± SEM. Statistical significance was calculated using One-way ANOVA or Student’s t-test (*p < 0.05; **p < 0.01; ***p < 0.001).
(C) The distance traveled in the fear conditioning chamber did not significant differ between control Cdk5f/f and Cdk5f/f/CW2 animals in the 3 min exploration time before the footshock (Cdk5f/f, 2551 ± 216.5; Cdk5f/f/CW2, 1648 ± 393.9; p=0.0558).
(D) Both Cdk5f/f and Cdk5f/f/CW2 groups responded to the footshock as shown by their increase in mean velocity before (left) and after (right) the shock. Before footshock: Cdk5f/f, 14.17 ± 1.203; Cdk5f/f/CW2, 9.155 ± 2.188. After footshock: Cdk5f/f, 61.91 ± 4.897; Cdk5f/f/CW2, 68.00 ± 8.888.
(E) While control Cdk5f/f animals learned the spatial memory task during the 8 day training session as demonstrated by a decrease in the escape latency, Cdk5f/f/CW2 animals were consistently impaired in acquisition of the spatial memory during the training session (Escape latency on the last day of training: 12.15 +/− 2.06 s for Cdk5f/f versus 47 +/− 7.8 s for Cdk5f/f/CW2; p<0.0005 for control versus mutant groups).
(F) During the probe trial on day 9, control Cdk5f/f mice spent more time searching for the platform in the target (T) quadrant (41.3 +/− 5%; p<0.001 for the target quadrant), while Cdk5f/f/CW2 animals spent significantly less time in the target (T) quadrant (9.8 +/− 7%) and instead spent more time searching for the platform in the opposite (O) quadrant (30.78 +/− 4.7%) and the quadrant to the right (R) (43.7 +/− 12.2) of the target quadrant. N=8 for Cdk5f/f; N=5 for Cdk5f/f/CW2. Data shown are means ± SEM. Statistical significance was calculated using One-way ANOVA (*p < 0.05; **p < 0.01; ***p < 0.001).
3.5 Cdk5f/f/CW2 animals exhibit deficits in synaptic transmission
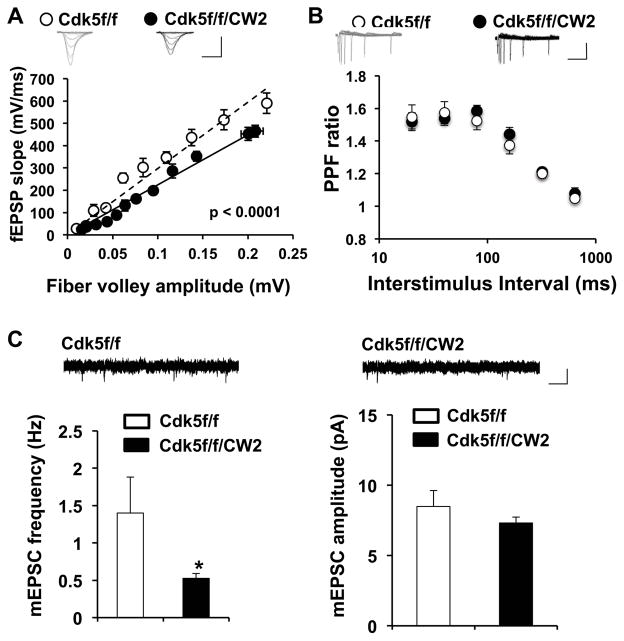
We next assessed various properties of synaptic transmission by preparing acute hippocampal slices from control Cdk5f/f and Cdk5f/f/CW2 animals. After stimulating the Schaffer collateral pathway, we noted that compared to control Cdk5f/f littermates, basal synaptic transmission was markedly impaired in slices prepared from Cdk5f/f/CW2 animals (Figure 5A). Paired-pulse facilitation ratios, a measurement of presynaptic transmitter release, did not significantly differ between control Cdk5f/f and Cdk5f/f/CW2 slices (Figure 5B). However, compared to control Cdk5f/f slices, the frequency, but not amplitude, of miniature excitatory postsynaptic current (mEPSCs) was reduced in hippocampal slices from Cdk5f/f/CW2 animals (Figure 5C), supporting the notion of reduced synaptic transmission.
Figure 5. Cdk5f/f/CW2 animals exhibit deficits in synaptic transmission.
(A) Basal synaptic transmission in the Schaffer collateral pathway is severely impaired in acute hippocampal slices prepared from Cdk5f/f/CW2 animals compared to those obtained from control Cdk5f/f animals. The data was fit by linear regression, which were determined to be significantly different by ANOVA with respect to slope (p<0.0001). Scale bars, 0.4 mV, 4 ms.
(B) Compared to slices prepared from control Cdk5f/f animals, the paired-pulse facilitation ratios at various interstimulus intervals are not significantly different from slices from Cdk5f/f/CW2 animals. Scale bars, 0.2 mV, 100 ms.
(C) Compared to slices prepared from control Cdk5f/f animals, the frequency of mEPSC is significantly decreased in slices prepared from Cdk5f/f/CW2 animals (Cdk5f/f, 1.40 ± 0.48; Cdk5f/f/CW2, 0.53 ± 0.06; p = 0.04). The mEPSC amplitude did not significantly differ between the control Cdk5f/f and Cdk5f/f/CW2 groups (Cdk5f/f, 8.49 ± 1.15; Cdk5f/f/CW2, 7.31 ± 0.42; p = 0.289). N=3 animals, 9–13 slices, for both Cdk5f/f and Cdk5f/f/CW2 genotypes. Scale bars, 10 pA, 50 ms. Data shown are means ± SEM. Statistical significance was calculated using Student’s t-test or ANOVA (*p < 0.05; **p < 0.01; ***p < 0.001).
3.6 Rescue of hyperactivity in Cdk5f/f/CW2 animals by lithium administration
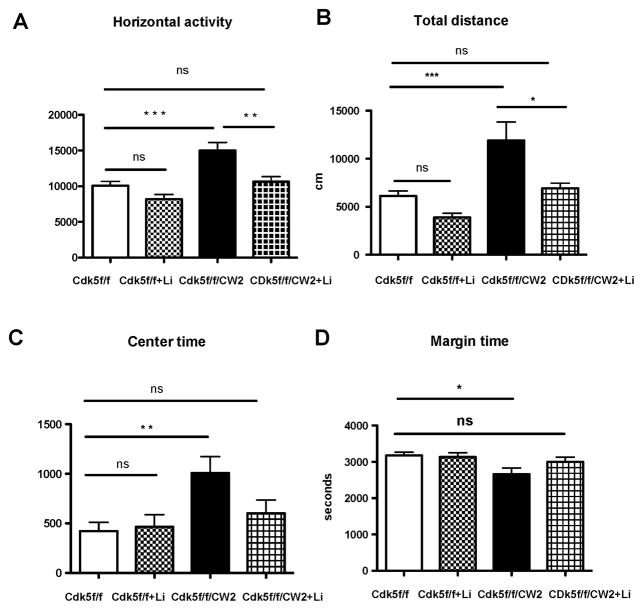
Since our data indicate that Cdk5f/f/CW2 animals exhibit reduced anxiety along with mania-like hyperactivity, we sought to address whether pharmacological administration might alleviate several of these behaviors. A previous mouse model exhibiting mania-like behavior including reduced anxiety, hyperactivity, and reduced depressive-like symptoms has been reported in a kainate receptor (GluR6) knockout, and successful amelioration of the symptoms was achieved by lithium administration (Shaltiel et al., 2008). Therefore, we asked whether lithium could improve some of the notable behavioral deficits, such as hyperactivity and reduced anxiety that are observed in the Cdk5f/f/CW2 animals during the open field. To test this prediction, a new cohort of Cdk5f/f and Cdk5f/f/CW2 animals were subjected to the first trial of the open field task, and after acute lithium chloride administration (85 mg/kg) they were subjected to a second trial. Consistent with the previous cohort in the open field test, untreated Cdk5f/f/CW2 animals exhibited severe hyperactivity in the increased horizontal activity, total distance traveled, center time, and decreased margin time compared to control, untreated Cdk5f/f littermates. Remarkably, the hyperactivity of Cdk5f/f/CW2 animals treated with acute lithium was restored to levels of untreated, control Cdk5f/f animals as measured by horizontal activity (Figure 6A) and total distance traveled (Figure 6B). Moreover, after lithium treatment, Cdk5f/f/CW2 spent less time in the center of the open field arena and greater time in the margin, similar to control, untreated Cdk5f/f littermates (Figure 6C, 6D). Importantly, there were no significant differences in the locomotor activity of control Cdk5f/f animals before and after lithium treatment, indicating that lithium has a specific effect on Cdk5f/f/CW2 animals (Figure 6A–D).
Figure 6. Rescue of hyperactivity in Cdk5f/f/CW2 animals by lithium administration.
(A) Lithium chloride administration prior to the open field test is able to restore the elevated horizontal activity displayed by the Cdk5f/f/CW2 animals to control Cdk5f/f levels (Cdk5f/f, 10068 ± 611.8; Cdk5f/f + LiCl, 8196 ± 642.9; Cdk5f/f/CW2, 14996 ± 1122.5; Cdk5f/f/CW2 + LiCl, 10639 ± 698.8).
(B) Lithium administration is able to reduce the total distance traveled by the Cdk5f/f/CW2 animals to control Cdk5f/f littermates, whereas lithium treatment did not significantly alter the activity of Cdk5f/f animals (Cdk5f/f, 6135 ± 438.8; Cdk5f/f + LiCl, 3888 ± 438.7; Cdk5f/f/CW2, 11912 ± 1923.8; Cdk5f/f/CW2 + LiCl, 6935 ± 527.2).
(C) While the Cdk5f/f/CW2 animals spent more time in the center of the open field arena before treatment, lithium administration decreased the amount of time Cdk5f/f/CW2 animals spent in the center back to control Cdk5f/f levels (Cdk5f/f, 422 ± 88.8; Cdk5f/f + LiCl, 466 ± 121.6; Cdk5f/f/CW2, 1007 ± 166.5; Cdk5f/f/CW2 + LiCl, 601 ± 166.5).
(D) Conversely, while Cdk5f/f/CW2 animals initially sent less time in the margin of the open field arena before treatment, lithium administration ameliorated this effect and restored time spent in the margin to control Cdk5f/f levels (Cdk5f/f, 3178 ± 88.8; Cdk5f/f + LiCl, 3134 ± 121.6; Cdk5f/f/CW2, 2662 ± 169; Cdk5f/f/CW2 + LiCl, 2999 ± 133.3). Importantly, lithium treatment on control Cdk5f/f littermates did not significantly impact their exploratory activity in the open field (p>0.05 when comparing Cdk5f/f to Cdk5f/f + LiCl).
N=11 for Cdk5f/f; N=9 for Cdk5f/f/CW2. Data shown are means ± SEM. Statistical significance was calculated using One-way ANOVA (Tukey’s multiple comparison test) (*p < 0.05; **p < 0.01; ***p < 0.001).
3.7 Proteomic data reveals activity-dependent alterations in GSK3 and protein phosphatases in synaptosomal fractions of Cdk5f/f/CW2 animals
To examine the potential mechanisms underlying the mania-like behavioral features and cognitive deficits in Cdk5f/f/CW2 animals, we collated data from our proteomics study in which synaptosomal preparations from Cdk5f/f and Cdk5f/f/CW2 were subjected to analysis as previously described (Guan et al., 2011). In addition to baseline abundances, a separate group of animals were subjected to contextual fear conditioning and brains were harvested one hour later for synaptosomal enrichment. Consistent with the loss of Cdk5 in Cdk5f/f/CW2 animals, the proteomics data indicated a 2.5-fold decrease in Cdk5 protein in the postsynaptically enriched fraction (Figure 7). Because mania-like behaviors, as well as the effects of lithium, have been associated with PP2A and GSK3β signaling pathways (Beaulieu et al., 2008; Prickaerts et al., 2006), we examined potential alterations in the amounts of GSK3β and PP2A subunits in the synaptosomal fractions. Interestingly, we observed an increase in GSK3β, in addition to changes in the multiple PP2A regulatory subunit abundances, before and after activity-dependent stimulation (Figure 7).
Figure 7. Baseline and activity-dependent expression of Cdk5, GSK3β and PP1/PP2A subunits in Cdk5f/f/CW2 versus control Cdk5f/f forebrain synaptosomes.
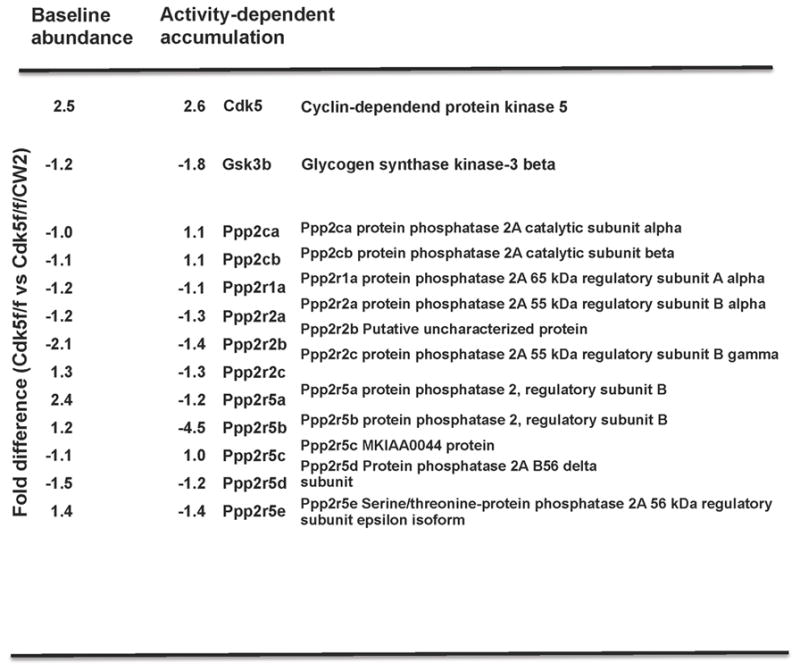
Proteomic data followed by mass spec analysis revealed an approximately two-and-a-half fold reduction in Cdk5 protein in Cdk5f/f/CW2 synaptosome-enriched fraction, which is consistent with the gradual ablation of Cdk5 in a subset of neurons in the forebrain. Unexpectedly, abundances of GSK3β and various subunits from the protein phosphatase 2A (PP2A) family are also altered at baseline conditions and after activity-dependent paradigm, suggesting their involvement in the hyperactivity and cognitive deficits observed in the Cdk5f/f/CW2 animals and the subsequent improvement in hyperactivity following lithium chloride administration.
4. Discussion
Here we have described a mouse model in which Cdk5 deletion from αCaMKII-positive forebrain-specific pyramidal neurons results in a distinct phenotype characterized by reduced anxiety, hyperactivity, increased acoustic startle, and a decrease in learned helplessness. Additionally, Cdk5f/f/CW2 animals displayed impaired cognitive performance, reduced basal synaptic transmission, and altered spontaneous neurotransmitter release. Our observations that treating Cdk5f/f/CW2 animals with lithium can alleviate the hyperactivity are further supported by proteomic data suggesting alterations in activity-dependent GSK3β and protein-phosphatase levels. Taken together, our Cdk5 conditional knockout mouse model together with our proteomic data serve as a useful tool to examine signaling cascades underlying mania-like behavior in animals with cognitive deficits.
4.1 Cdk5 and homeostatic plasticity
An increasing number of studies have identified Cdk5 as a critical regulator of synaptic transmission and homeostatic plasticity. For example, Cdk5 can dampen synaptic activity in response to heightened activity (Seeburg et al., 2008), influence vesicle pool size during neurotransmitter release (Kim et al., 2010), and silence recurrent networks during prolonged inactivity (Mitra et al., 2011). While Cdk5 activity is important as a mediator of homeostatic plasticity, how chronic Cdk5 loss of-function affects behavior and cognitive functions is unclear and has been limited to conditional Cdk5 knockout studies due to the embryonic lethality of Cdk5 null mice. Earlier studies from p35 knockout animals found that loss of one copy of p35 leads to pre-pulse inhibition deficits in female mice and impaired reverse learning in the water maze task (Engmann et al., 2011). However, the authors found that only male mice exhibited cognitive deficits in a spatial memory task, along with impaired social interaction. In these animals, treatment with a chromatin modifying enzyme inhibitor restored the behavioral deficits, which suggests a distinct pathway that is affected in the p35 mutant mice or an additional mechanism to consider in which chromatin factors are implicated in Cdk5-dependent cognition.
4.2 Differences between Cdk5 conditional knockouts
Our results differ from previous reports that Cdk5 conditional knockout animals exhibit enhanced learning and memory in the water maze task and fear conditioning, and increased NR2B surface expression levels due to a reduction in calpain-mediated cleavage and reduction of NR2B (Hawasli et al., 2007). One notable difference was the use of a prion promoter Cre mouse line, which provided temporal deletion of Cdk5 but ablated Cdk5 in a wider range of different cell types and brain regions including the retina, olfactory bulb, and cerebellum (Weber et al., 2001). However, both Cdk5 mutant mouse models exhibit seizures. Hawasli et al. proposed that an acute increase in p25 levels in their mouse model during seizure activity is a compensatory mechanism, and long-term loss of Cdk5 also results in a reduction in p25 (Hawasli et al., 2009). While we have not yet examined the relationship of p35 to p25 levels in our Cdk5f/f/CW2 animals, we cannot exclude the possibility that cleavage of p35 to p25 is altered under neurotoxic conditions. Here we also observed decreased basal synaptic transmission, corresponding with a reduction in mEPSC frequency. Based on the deficits in cognitive functions and long-term potentiation (LTP) deficits in another αCaMKII-specific line in which Cdk5 ablation occurs in the hippocampus (Guan et al., 2011), we predict deficits in LTP in the Cdk5f/f/CW2 animals.
4.3 The involvement of Cdk5 in mania-like behavior
Our behavioral rescue of hyperactivity using lithium treatment suggests that GSK3β signaling is impaired in Cdk5f/f/CW2 animals, as lithium is a well-known inhibitor of GSK3 β, In a previous study, young p25 transgenic animals expressing hyperactive Cdk5 have increased GSK3αβ phosphorylation (S21/9), whereas in older p25 transgenics, phospho-GSKβ (S21/9) levels do not differ from control animals, and GSK3β activity is increased overall (Plattner et al., 2006). Because of the widespread loss of Cdk5 throughout the forebrain, we are able to utilize proteomics to further probe for signaling pathways that were potentially altered in these Cdk5f/f/CW2 animals. When we examined data specifically related to a mania-like phenotype and to GSK3β signaling, we determined that protein conditions were dynamically regulated by activity, including GSK3β, which was upregulated in Cdk5f/f/CW2 after fear conditioning. In addition to changes in GSK3β, we observed alterations in amounts of various subunits of PP2A, an enzyme critical for mania-like phenotype and affect of lithium on behavior (Beaulieu et al., 2008). Here we cannot exclude the possibility that prolonged ablation of Cdk5 throughout the mouse lifespan may result in long-term compensatory changes that were observed. Nevertheless, our data suggests that multiple pathways, including PP2A and GSK3β, target multiple substrates to produce a mania-like phenotype and impair learning and memory in our Cdk5f/f/CW2 animals. Therefore, our mouse model, together with a proteomics approach, can serve as a powerful tool allowing us to simultaneously examine multiple molecular components underlying cognitive dysfunction and mania-related behaviors.
Highlights.
Cdk5 conditional knockouts display hyperactivity and reduced anxiety
Cdk5 conditional knockouts exhibit cognitive impairments
Hyperactivity in Cdk5 conditional knockouts can be ameliorated by lithium treatment
Acknowledgments
We thank Dr. Susumu Tonegawa for αCaMKII-Cre (CW2) line. S.C.S. was supported by NIH T32 MH074249 and a Norman B. Leventhal fellow ship. A.R. is a recipient of the NARSAD Young Investigator Award. This work is supported by an NIH R01 NS051874 to L.-H. T. L.-H. T. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beaulieu JM, Marion S, Rodriguiz RM, Medvedev IO, Sotnikova TD, Ghisi V, et al. A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell. 2008;132:125–136. doi: 10.1016/j.cell.2007.11.041. [DOI] [PubMed] [Google Scholar]
- Chae T, Kwon YT, Bronson R, Dikkes P, Li E, Tsai LH. Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron. 1997;18:29–42. doi: 10.1016/s0896-6273(01)80044-1. [DOI] [PubMed] [Google Scholar]
- Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- Drerup JM, Hayashi K, Cui H, Mettlach GL, Long MA, Marvin M, et al. Attention-deficit/hyperactivity phenotype in mice lacking the cyclin-dependent kinase 5 cofactor p35. Biol Psychiatry. 2010;68:1163–1171. doi: 10.1016/j.biopsych.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engmann O, Hortobagyi T, Pidsley R, Troakes C, Bernstein HG, Kreutz MR, et al. Schizophrenia is associated with dysregulation of a Cdk5 activator that regulates synaptic protein expression and cognition. Brain. 2011;134:2408–2421. doi: 10.1093/brain/awr155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Schrick C, Spiess J, Radulovic J. Cyclin-dependent kinase 5 is required for associative learning. J Neurosci. 2002;22:3700–3707. doi: 10.1523/JNEUROSCI.22-09-03700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Pang PT, Lu B, Tsai LH. Opposing roles of transient and prolonged expression of p25 in synaptic plasticity and hippocampus-dependent memory. Neuron. 2005;48:825–838. doi: 10.1016/j.neuron.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Guan JS, Su SC, Gao J, Joseph N, Xie Z, Zhou Y, et al. Cdk5 Is Required for Memory Function and Hippocampal Plasticity via the cAMP Signaling Pathway. PLoS One. 2011;6:e25735. doi: 10.1371/journal.pone.0025735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawasli AH, Benavides DR, Nguyen C, Kansy JW, Hayashi K, Chambon P, et al. Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nat Neurosci. 2007;10:880–886. doi: 10.1038/nn1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawasli AH, Koovakkattu D, Hayashi K, Anderson AE, Powell CM, Sinton CM, et al. Regulation of hippocampal and behavioral excitability by cyclin-dependent kinase 5. PLoS One. 2009;4:e5808. doi: 10.1371/journal.pone.0005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Ryan TA. CDK5 serves as a major control point in neurotransmitter release. Neuron. 2010;67:797–809. doi: 10.1016/j.neuron.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapacher FA, Mlewski EC, Ferreras S, Pisano V, Paolorossi M, Hansen C, et al. Mice lacking p35 display hyperactivity and paradoxical response to psychostimulants. J Neurochem. 2010;114:203–214. doi: 10.1111/j.1471-4159.2010.06748.x. [DOI] [PubMed] [Google Scholar]
- Li BS, Sun MK, Zhang L, Takahashi S, Ma W, Vinade L, et al. Regulation of NMDA receptors by cyclin-dependent kinase-5. Proc Natl Acad Sci U S A. 2001;98:12742–12747. doi: 10.1073/pnas.211428098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra A, Mitra SS, Tsien RW. Heterogeneous reallocation of presynaptic efficacy in recurrent excitatory circuits adapting to inactivity. Nat Neurosci. 2011:250–257. doi: 10.1038/nn.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morabito MA, Sheng M, Tsai LH. Cyclin-dependent kinase 5 phosphorylates the N-terminal domain of the postsynaptic density protein PSD-95 in neurons. J Neurosci. 2004;24:865–876. doi: 10.1523/JNEUROSCI.4582-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plattner F, Angelo M, Giese KP. The roles of cyclin-dependent kinase 5 and glycogen synthase kinase 3 in tau hyperphosphorylation. J Biol Chem. 2006;281:25457–25465. doi: 10.1074/jbc.M603469200. [DOI] [PubMed] [Google Scholar]
- Prickaerts J, Moechars D, Cryns K, Lenaerts I, van Craenendonck H, Goris I, et al. Transgenic mice overexpressing glycogen synthase kinase 3beta: a putative model of hyperactivity and mania. J Neurosci. 2006;26:9022–9029. doi: 10.1523/JNEUROSCI.5216-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels BA, Hsueh YP, Shu T, Liang H, Tseng HC, Hong CJ, et al. Cdk5 promotes synaptogenesis by regulating the subcellular distribution of the MAGUK family member CASK. Neuron. 2007;56:823–837. doi: 10.1016/j.neuron.2007.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg DP, Feliu-Mojer M, Gaiottino J, Pak DT, Sheng M. Critical role of CDK5 and Polo-like kinase 2 in homeostatic synaptic plasticity during elevated activity. Neuron. 2008;58:571–583. doi: 10.1016/j.neuron.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaltiel G, Maeng S, Malkesman O, Pearson B, Schloesser RJ, Tragon T, et al. Evidence for the involvement of the kainate receptor subunit GluR6 (GRIK2) in mediating behavioral displays related to behavioral symptoms of mania. Mol Psychiatry. 2008;13:858–872. doi: 10.1038/mp.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su SC, Tsai LH. Cyclin-dependent kinases in brain development and disease. Annu Rev Cell Dev Biol. 2011;27:465–491. doi: 10.1146/annurev-cellbio-092910-154023. [DOI] [PubMed] [Google Scholar]
- Weber P, Metzger D, Chambon P. Temporally controlled targeted somatic mutagenesis in the mouse brain. Eur J Neurosci. 2001;14:1777–1783. doi: 10.1046/j.0953-816x.2001.01803.x. [DOI] [PubMed] [Google Scholar]
- Zeng H, Chattarji S, Barbarosie M, Rondi-Reig L, Philpot BD, Miyakawa T, et al. Forebrain-specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic-like memory. Cell. 2001;107:617–629. doi: 10.1016/s0092-8674(01)00585-2. [DOI] [PubMed] [Google Scholar]