Abstract
Importance of the field
In the United States, the annual incidence of basal cell carcinoma (BCC) is close to 1 million. Ultraviolet radiation exposure is the main risk factor; however, the availability of ever more potent sunscreens and education have not prevented the rise in BCC incidence. Therefore, concerted effects to identify novel preventive and therapeutic strategies are necessary.
Areas covered in this review
This article summarizes our current understanding of the etiology and molecular mechanisms of BCC tumorigenesis and discusses the preclinical and clinical studies to identify agents with anti-BCC efficacy.
What the reader will gain
The discovery that hyperactive Hh pathway signaling causes several cancers, including BCC, has spawned the development of many pharmacologic inhibitors of Hh signaling. Early clinical testing of the most advanced, GDC-0449, demonstrated impressive efficacy in patients with advanced BCC. Other promising anti-BCC chemopreventive strategies include drugs that are already FDA-approved for treating other diseases.
Take home message
Preclinical and clinical trials with pre-existing FDA-approved drugs suggest novel uses for BCC chemoprevention and treatment. Also, new chemical entities that inhibit the Hh pathway show promise, and in combination with other drugs may provide a nonsurgical cure for this most common cancer.
Keywords: basal cell carcinoma, chemoprevention, Hedgehog pathway, NSAIDs, retinoids, smoothened antagonists, therapeutics
1. Introduction
Basal cell carcinoma (BCC) is the most common cancer in light-skinned populations, with at least 800,000 new cases diagnosed in the United States each year. It comprises 80% of all skin cancers, which include squamous cell carcinoma (SCC; ~ 16%) and melanoma (~ 4%); collectively, they have attendant yearly management costs exceeding $500 million [1,2]. Although the main etiological factor that causes skin cancer is over-exposure to solar ultraviolet radiation (UVR) [2], topical sunscreens have had a limited effect against BCCs although they have been effective at preventing SCCs [3,4]. This discrepancy may reflect different patterns of sun exposure, since SCCs occur more frequently in people who are chronically exposed to UVR for a substantial amount of time (i.e., outdoor workers), while BCCs are more frequent in people who have intermittent UVR exposure (i.e., from recreational activities) and consequently are more likely to develop severe sunburns. Patients who develop a BCC are at an increased risk of developing more BCCs [5]. With the limited effect of sunscreens on BCC prevention, it is not surprising then to observe that the annual incidence for skin cancer is increasing, which also is likely due to an ever-increasing elderly population and the trend of younger generations towards tanning by natural and artificial means [6]. Given these trends, the development of novel preventive and therapeutic strategies that reduce BCC may become crucial in the coming years to curb the alarming increase in skin cancer incidence.
2. Disease mechanisms for BCC carcinogenesis
BCC is a relatively slow-growing epithelial cancer that histologically resembles and perhaps arises from the basal cells in the epidermis and/or hair follicle. Metastasis of BCC is extremely rare; however, the tumors are locally aggressive and if left untreated or inadequately treated, they cause significant tissue destruction resulting in considerable morbidity, particularly in the head and neck regions where BCCs most commonly arise [7]. There are various types of BCCs. Nodular and superficial BCC tend to be well-defined, less aggressive tumors and therefore more straightforward to treat, while morpheaform and infiltrative BCCs have less well-defined borders and can be very difficult to remove completely with minimal tissue damage.
In the general population, BCCs arise from the fifth decade of life and in small numbers (i.e., 1 – 2 tumors). However, approximately 1 in 60,000 Americans have an autosomal dominant genetic condition, basal cell nevus syndrome (BCNS), also known as nevoid basal cell carcinoma syndrome (NBCCS) or Gorlin syndrome (OMIM #109400). These individuals are predisposed to develop tens to hundreds of BCCs from puberty and throughout their lifetime [8,9]. The discovery that these patients carry a germline mutation in the PATCHED1 (PTCH1) gene, rendering them constitutively heterozygous (+/−) for PTCH1, pointed to the Hedgehog (Hh) signaling pathway as the pivotal cause of BCC [10,11].
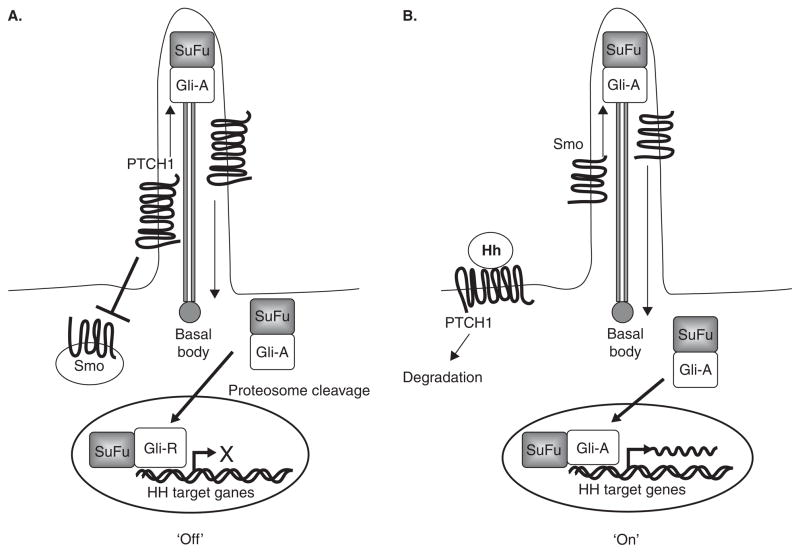
The Hh pathway is an important developmental pathway that is essential for embryogenesis. In adults, the pathway is generally dormant except in hair follicle cycling and in maintenance of some stem cell populations [12]. PTCH1 protein, a 12-transmembrane receptor, is a negative regulator of the Hh pathway (Figure 1). In the absence of Hh protein, PTCH1 inhibits the function of another transmembrane protein, Smoothened (Smo), a key, positive regulator of HH signaling. Hh binding to PTCH1 alleviates repression of Smo to allow the latter to activate the Hh pathway via protein kinases, culminating in the transcriptional activation by Gli transcription factors of Hh pathway target genes, such as Bcl2, FoxM1, Ptch1, and Gli1. There are three Gli proteins: Gli1, Gli2, and Gli3. Gli1 acts primarily as a positive regulator (Gli-A) of Hh signaling, while Gli2 and Gli3 can activate or repress the pathway depending on how these proteins are cytoplasmically processed. However, Gli2 is thought to function mainly as a transcriptional activator (Gli-A) and Gli3 as a transcriptional repressor (Gli-R) [13]. Recently, the importance of primary cilia in Hh signaling and BCC tumorigenesis was demonstrated [14]. Primary cilia are immobile organelles that require interflagellar transport (IFT) proteins, such as Kif3a and IFT88, for their structure and function. These proteins are necessary for anteroretrograde transport of Hh pathway components such as Smo and Gli for Hh signal transduction. In experimental models, genetic deletion of Kif3a or IFT88 caused the loss of Hh signaling and the inhibition of BCC carcinogenesis induced by an activated Smo transgene [14], indicating that cilia are necessary for Hh signaling and BCC carcinogenesis, at least in mice.
Figure 1. The Hedgehog (Hh) signaling pathway.
A. In the absence of Hh ligand, Ptch1 in the primary cilium represses Smo function, resulting in the proteolytic processing of Gli-activator (Gli1-A) (bound to SuFu, a negative regulator of Hh signaling) to Gli-repressor (Gli-R). The latter then binds to the promoters of Hh target genes to repress transcription. B. In the presence of Hh, Ptch1 translocates out of the cilium and is degraded, allowing Smo to enter the cilium and activate the Hh pathway by preventing cleavage of Gli proteins to its repressor form. Gli-A enters the nucleus and activates Hh-target gene (e.g., Gli1, Ptch1, Bcl2, FoxM1) transcription.
In BCNS patients, BCCs commonly develop after somatic inactivation of the remaining PTCH1 allele. Therefore PTCH1 acts as a classical tumor suppressor that inhibits Hh signaling and thereby prevents BCC carcinogenesis. Many studies confirm the pivotal role of aberrant Hh signaling in BCC carcinogenesis: all human and murine, sporadic and germline BCCs analyzed have abnormal activation of Hh signaling, commonly due to PTCH1-inactivating mutations and Smo-activating mutations (~ 90 and 10%, respectively) [15–17]. In addition, murine transgenic models that overexpress Sonic hedgehog (Shh) [18]; Gli1 [19] or Gli2 [20]; express constitutively activated Smo [21]; ‘knockout’ Ptch1 [11,22] or Suppressor of Fused (SuFu), a negative Hh pathway regulator [23,24], result in BCC carcinogenesis, indicating that hyperactivation of Hh signaling initiates BCC development. In mice in which Gli2 expression was conditionally activated in basal keratinocytes using the tetracycline-inducible (‘tet-off’) system, mice developed many BCCs. When Gli2 was ‘switched off’ by doxycycline administration, the tumors regressed almost completely except for a residual population of non-proliferating cells, which could reinitiate the BCCs once Gli2 was reactivated [20]. Therefore, Hh signaling is required for the initiation and progression of BCC. In addition to hyperactivated Hh signaling, similar to other cancers, human sporadic and familial BCCs have p53 mutations [15,25]. However, it is not clear how these mutations contribute to BCC tumorigenesis.
Understanding the etiology and molecular pathogenesis of this cancer and testing potentially viable drugs in preclinical systems are important approaches to the identification of novel therapeutic and preventive treatments for patients with BCC. For example, the irradiated Ptch1+/− mouse provides an accurate, practical model for studying human BCC since it phenocopies both the familial (BCNS) and somatic disease [26]. Thus, it provides an invaluable preclinical ‘tool’ for the identification and implementation of novel, effective chemopreventive and therapeutic strategies for treating human BCC. Furthermore, studies using irradiated Ptch1+/− mice have helped to gain mechanistic insights into the multistep process of BCC tumorigenesis and its chronological progression [27]. In non-irradiated Ptch1+/− mice, Ptch1 haploinsufficiency (and subsequent deregulation of Hh signaling) is sufficient to cause basaloid hyperproliferations (BCC precursor lesions) during the active hair cycling phase (anagen) when the Hh pathway is normally active. However, it is not sufficient to drive full BCC carcinogenesis, requiring additional genetic damage caused by radiation, in genes such as p53. Loss of p53 function is thought to cause genomic instability leading to the complete loss of PTCH1 function, resulting in the progression of BCC precursor lesions to clinically relevant nodular and infiltrative BCC tumors [27].
3. Current treatments for BCC
Current treatments for clinically relevant BCCs are generally invasive; not preventive of new tumor growths [28]; and in some cases, skin reconstruction is also necessary after initial treatment, thus requiring further surgery. ‘Invasive’ treatments include electrodesiccation and curettage; surgical excision; freezing (cryosurgery); Moh’s micrographic surgery (in which the BCC is removed layer by layer, examining each layer under the microscope until no abnormal cells remain); and laser surgery (which vaporizes superficial BCCs). Radiation therapy utilizing high-energy X-rays to destroy cancer cells is also used. Pharmacological therapies include the use of topical creams, including imiquimod, which induces an immune response [29], and 5-fluorouracil – an ablative agent that inhibits DNA synthesis, prevents cell proliferation, and causes tumor necrosis [30]. Both of these creams are used to treat mainly superficial BCCs and their cure rates are in the order of 80 – 95% – less than surgical excision. Photodynamic therapy (PDT) is another treatment and uses a photosensitizing agent such as 5-aminolevulinic acid (5-ALA), which accumulates in cancer cells, and is converted to porphyrins that are activated by strong light to kill the tumor cells with minimal damage to surrounding tissue. Although PDT is effective, the cure rates can vary substantially (70 – 90%).
4. Chemoprevention and therapeutic strategies with ‘established’ drugs
Although the current standard treatments for treating sporadic cases of BCCs are generally effective, patients can endure significant discomfort, scarring and/or disfigurement from these treatments. This is particularly true for those patients at high risk of developing many BCCs (i.e., BCNS patients) where surgical treatments can leave them severely scarred. Therefore, there is a real need to develop therapies that prevent BCC carcinogenesis. Currently, no FDA-approved chemoprevention strategies exist for BCC. Data from pre-clinical and clinical trials with drugs that are FDA-approved for the treatment of other diseases suggest novel uses for chemoprevention for early treatment of BCC.
4.1 Tazarotene
Retinoids are old players in skin cancer treatment. Pan-retinoic acid receptor (RAR) agonists that mimic the endogenous ligand all-trans retinoic acid (ATRA; tretinoin), such as 13-cis RA (isotretinoin), etretinate, or acitretin, given systemically at high doses to patients with BCNS, xeroderma pigmentosum (XP), or organ transplants, have shown some prophylactic effect with regards to the development of new BCCs [31–34]. However, significant systemic toxicities were associated with their long-term use (due to nonspecific activation of RARα, β, γ, and possibly retinoid X receptor (RXR)- α, -β, and -γ through isomerization of ATRA to their ligand 9-cis RA, in many organs), and preclude their widespread chemoprevention use [35]. Therefore, retinoids that are more ‘specific’ may have greater anti-BCC efficacy with reduced toxicity.
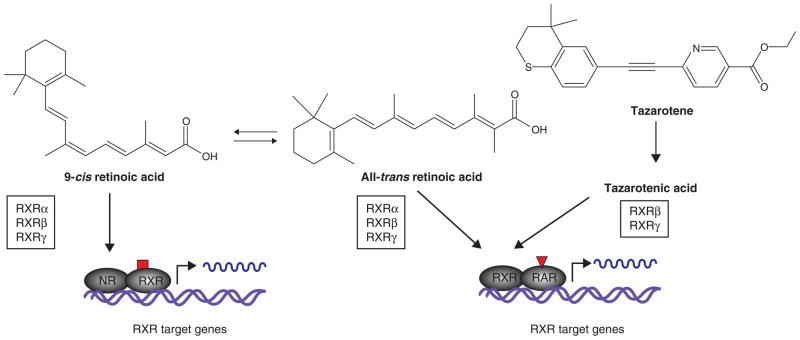
One such retinoid is tazarotene (Allergan, Irvine, CA), an FDA-approved drug for treating photoaging, psoriasis and acne [36]. Tazarotene is a third-generation synthetic acetylenic retinoid whose active metabolite, tazarotenic acid, activates only a subset of retinoid receptors, RARβ and RARγ, in contrast to ATRA (Figure 2) and 13-cis RA [37]. Also, both tazarotene and tazarotenic acid have very short half-lives, thus reducing accumulation of the drug in tissues. Only RARα and RARγ are expressed in the basal epidermis and hair follicle, and RARγ is suggested to mediate the tumor suppressor function of retinoids in the skin [38]. Therefore, a topically applied retinoid that would specifically activate RARγ in the normal skin and in BCCs may reduce the side effects associated with using systemic pan-retinoids.
Figure 2. Pan-retinoids such as all-trans retinoic acid (ATRA) activate potentially all RARs and RXRs while the acid form of tazarotene activates only RARβ and RARγ, and therefore gives greater specificity, resulting in reduced systemic toxicities.
NR: Nuclear receptor; RAR: Retinoic acid receptor; RXR: Retinoid X receptor.
In preclinical chemoprevention studies, UVR- or ionizing radiation-exposed Ptch1+/− mice treated topically with applications of tazarotene had significantly fewer and smaller microscopic BCCs than with vehicle-treated mice [39] and when the drug was withdrawn, there was no increase in BCC burden [40]. This in contrast to human chemoprevention trials with 13-cis RA [41,42]. Tazarotene also had greater efficacy than ATRA in these mice, suggesting that the difference is due to the specific activation of RARγ. This chemopreventive efficacy of tazarotene was also observed in irradiated Ptch1+/− mice with an engineered deletion of p53 in the basal epidermis (So P-L, Tang JY, Epstein EH, unpublished observations), suggesting that tazarotene’s anti-BCC effect is independent of p53 function.
These data suggest that (topically applied) tazarotene is an effective chemoprevention strategy for Hh-pathway-driven BCCs that may have additional p53 loss-of-function mutations, which are common in many cancers. However, tazarotene’s associated skin irritation, although transient and mild, and it may be reduced by a more gradual institution of treatment [43], is likely to deter some patients from its optimal use for chemoprevention. In addition, in the Ptch1+/− model of BCC, approximately 40 – 50% of untreated visible murine BCCs retain RARγ [40], suggesting that roughly half of clinically relevant BCCs (that retain RARγ expression) will respond effectively to tazarotene. This is supported by human clinical studies in which only 30 – 50% of sporadic clinically relevant BCCs treated topically with tazarotene completely regressed [44,45]. These studies suggest that ‘responding’ BCCs may be completely eradicated suggesting a curative effect of tazarotene.
Thus, tazarotene chemoprevention and therapy may a viable option for patients who develop a substantial number of BCCs (e.g., BCNS patients) for whom surgery is no longer an option and who are willing to tolerate the skin irritation that may accompany its use. It will be interesting to see whether the ongoing Phase II clinical trial for chemoprevention with tazarotene in BCNS patients (ClinicalTrials.gov: NCT00783965) will yield significant results.
4.2 NSAIDs
NSAIDS are small molecules that inhibit the function of COX1 and/or COX2 and are traditionally used to treat inflammatory disease. These enzymes normally metabolize arachidonic acid to important biological mediators called prostanoids, such as prostaglandins that generate inflammation. COX1 normally functions in the intestine, where it mediates normal physiological processes, while COX2 is inducible and is activated in macrophages and at sites of inflammation. Epidemiological studies have found that NSAID use is associated with a reduced risk for colon cancer, and thus they have been tested for chemopreventive efficacy in familial and sporadic colorectal cancer [46]. COX2 is overexpressed in many cancers, and evidence from clinical and preclinical studies indicates that COX2-derived prostaglandins participate in carcinogenesis by promoting inflammation, immune response suppression, apoptosis inhibition, angiogenesis, tumor cell invasion and metastasis [47].
In murine skin, UVR exposure induces COX2 expression, and COX2 activity has been associated with UVR-induced SCC [48]. In BCCs, COX2 protein is localized to cells that surround the tumor cells, and COX2 activity has been suggested to be antiapoptotic and pro-angiogenic in BCC [49]. In addition, COX2 gene polymorphisms are thought to modify BCC risk [50]. Collectively, these data suggest a positive role for COX2 in BCC carcinogenesis. This is further supported by murine studies in which transgenic overexpression of COX2 in basal keratinocytes of Ptch1+/− mice resulted in a twofold increase in microscopic BCC burden, while germline COX2 deletion resulted in a 75% reduction. Furthermore, the COX2-specific inhibitor celecoxib reduced BCC burden in Ptch1+/− mice, although the efficacy was mainly against tumor growth rather than tumor initiation [51].
Observational studies of NSAID chemoprevention clinical trials suggest that NSAIDs have a weak and inconsistent anti-BCC chemopreventive effect [52,53]. In a Phase II clinical trial in BCNS patients, celecoxib (a COX2-specific inhibitor) was effective as a chemopreventive agent in BCNS patients with less severe disease (< 15 BCCs at baseline), decreasing the development of new BCCs by 50% [51]. Therefore, NSAIDS may have anti-BCC chemopreventive efficacy, particularly in patients at lower risk. However, potential cardiovascular risks associated with celecoxib [54] may preclude its use as a common chemoprevention strategy in patients with BCC.
4.3 DNA repair enhancers
As previously mentioned, the main risk factor for developing BCCs and other skin cancers is over-exposure to UVR, which causes DNA damage triggering a rapid DNA damage repair response [55]. One of the ways in which the latter is activated is by the recognition of DNA damage at telomere ends, which generates 3′ overhangs [56]. T-oligos are 2- to 20-base thymidine oligonucleotides that are homologous to the 3′ telomere overhangs, and can also induce this DNA damage response without telomeric disruption [57]. Treatment with thymidine dinucleotide (pTT) has DNA-protective effects and reduces the development of SCC in UVR-irradiated mice [58]. For BCC, the preventive effect of this therapy was recently demonstrated in Ptch1+/− mice. Topical ‘t-oligo’ applications to the skin of chronically UVR-irradiated mice resulted in a significant reduction in the number and size of microscopic BCC growths [59]. This reduction was associated with an increase in apoptosis, a decrease in proliferation, and an 80% reduction of COX2 protein in the tumor-free epidermis of pTT-treated mice. Therefore, these data suggest that agents such as pTT that stimulate DNA repair mechanisms may be another viable chemoprevention strategy against BCC tumorigenesis.
4.4 α-Difluoromethylornithine (DMFO)
DMFO is an inhibitor of ornithine decarboxylase (ODC), a key enzyme in the polyamine biosynthetic pathway that converts L-ornithine to putrescine [60]. High polyamine content is associated with BCC [61], and in experimental studies in which ODC was overexpressed in Ptch1+/− mice there was a significant increase in microscopic BCC burden [62], suggesting that polyamine synthesis contributes to BCC carcinogenesis. In a 5-year, randomized, double-blind, placebo-controlled Phase III trial for non-melanoma skin cancer (NMSC), DMFO given as a chemopreventive agent did not change the overall NMSC incidence. However, when SCC and BCC incidence was assessed individually, DMFO was found to significantly reduce BCC incidence by 30%, but had no significant effect on SCC [63]. The chemopreventive dose given was well tolerated with only mild, reversible side effects (ototoxicity). DMFO may therefore be a promising chemoprevention strategy against a subset of BCCs.
5. Investigative chemoprevention strategies with chemicals found in natural products
There are numerous studies that have investigated the efficacy of ‘natural’ chemicals against skin cancer [64,65]. These include epigallocatechin-3-gallata (EGCG) (see below), the major polyphenol found in green tea; curcumin (turmeric) [66]; silibinin (a natural flavonoid) [67]; α-santalol (a phytochemical found in sandalwood oil) [68–71]; sarcophinediol (a fish toxin) [72,73]; and honokiol (plant lignan isolated from bark and seed cones of Magnolia officinalis) [74]. So far, with the exception of curcumin, these agents have demonstrated anticancer effects against cutaneous SCC. Preclinical in vivo models of SCC carcinogenesis suggest a preventive effect for EGCG/green tea [75], which is supported by human studies in which tea-drinking was associated with reduced risk of developing SCC [76]. However, for BCC the same study found no statistically significant association between reduced BCC risk and tea consumption. This observation correlates with preclinical studies in UVR-irradiated Ptch1+/− mice which showed no significant prevention of BCCs with oral doses of green and black tea preparations that had previously been shown to inhibit SCC carcinogenesis [77]. For curcumin, in vitro studies in a human BCC cell line (BCC1) suggest that curcumin can induce apoptosis [78,79]. Further studies are required to test the efficacy of curcumin in vivo against BCC and to investigate whether other ‘natural’ agents that inhibit SCC can also prevent BCC.
6. Mechanism-based therapy: Hedgehog pathway inhibitors
6.1 Smo antagonists
Perhaps the most exciting development in anti-BCC drugs is the emergence of novel, molecularly targeted drugs that inhibit the Hh pathway. The identification of cyclopamine (a naturally occurring alkaloid that causes teratogenesis in newborn lambs) as a specific Smo antagonist, which potently inhibits Hh pathway signaling [80] suggested that Hh-driven cancers like BCC potentially could be treated with molecularly targeted therapies such as small molecule Smo inhibitors. Initial experiments demonstrated that in cancer cell lines and xenograft models cyclopamine could inhibit proliferation, increase apoptosis, and reduce metastasis [81–83]. In a murine model of BCC and in BCC cell lines, cyclopamine was effective at inhibiting BCC carcinogenesis [84,85]. Furthermore, cyclopamine applied in a cream formulation to BCCs on a single patient induced rapid regression of all four BCCs with a decrease in proliferation, induction of differentiation and apoptosis, and with no adverse effects [86].
However, despite the attractive pharmacological profile of cyclopamine against a number of cancer xenografts and observations that cyclopamine can inhibit murine and human BCCs in vivo, evaluation of unmodified cyclopamine has been hampered by its poor aqueous solubility and acid lability [87], making it therapeutically nonviable. Therefore, concerted efforts have been made to identify novel natural or synthetic small-molecule Smo inhibitors [88]. Of the many Smo antagonists currently being investigated, some resemble cyclopamine in structure [89], and others bear no structural similarity to cyclopamine but inhibit Smo by other mechanisms. In addition, these antagonists may each have different cellular actions from each other: for example, cyclopamine still induces translocation of Smo to the primary cilium (similar to Hh agonists), while other Smo antagonists (e.g., SANT-2) prevent the Hh-dependent translocation of Smo to the primary cilium [90] (see below).
An early synthetic Smo antagonist Curis 61414, developed by Curis and Genentech, resembles cyclopamine and in skin explants containing murine BCC showed good efficacy [91]. However, this efficacy did not translate well in the subsequent Phase I clinical trial in patients with sporadic BCC, and the study was terminated early [92]. The reason for its clinical failure was suggested to be insufficient skin penetration of Curis 61414 in its topical formulation. Another synthetic Smo antagonist developed by Curis and Genentech is GDC-0449, which is currently the most promising and advanced Hh pathway inhibitor in clinical development. In a Phase I clinical trial to test initially its efficacy against tumor growth and toxicity in vivo, patients with advanced multiple-lesion or metastatic BCC were given systemic doses of GDC-0449 [93]. Of the 33 patients with advanced or metastatic BCC, 18 responded to the drug; 11 had stable disease up to 10.8 months; and 4 had disease progression. Analysis of BCC biopsies showed that the BCCs had significantly elevated mRNA levels of Hh target gene Gli1.
These early clinical data confirm that direct Hh pathway inhibition may be an effective therapy for those tumors driven by Hh pathway activation. Because of this initial success, GDC-0449 has now entered Phase II testing in patients with advanced BCC and BCNS (NCT00833417 and NCT00957229, respectively), as well as for metastatic colorectal and ovarian cancer (NCT00959647) (Table 1). Other Smo antagonists currently being developed for clinical application and that have entered Phase I clinical trials for treating solid tumors include IPI-926 (Infinity), a derivative of cyclopamine with significantly improved potency, solubility and metabolic stability [94]; XL139 (Exelixis/Bristol Myers Squibb), which is currently being tested in a Phase I clinical trial to determine toxicity levels and effective dose in patients with advanced or metastatic solid tumors, or in patients with uncontrolled basal cell nevoid syndrome or sporadic BCC; and LDE225 (Novartis), which is also in Phase I clinical trials for both systemic and topical use (Table 1).
Table 1.
Current clinical trials for Hh pathway inhibitors (see ClinicalTrials.gov for more information).
| Drug | Sponsor | Collaborator | ClinicalTrials.gov ID | Study |
|---|---|---|---|---|
| GDC-0449 | Genentech Children’s Hospital & Research Center, Oakland Genentech | Genentech Genentech Genentech |
NCT00833417 NCT00957229 NCT00959647 |
A study evaluating the efficacy and safety of GDC-0449 (Hedgehog pathway inhibitor) in patients with advanced basal cell carcinoma Study to determine the efficacy and safety of a systemic Hedgehog pathway antagonist (GDC-0449) in patients with basal cell nevus syndrome (BCNS) (Phase II) A study of GDC-0449 (Hedgehog pathway inhibitor) in patients treated with GDC-0449 in a previous Genentech-sponsored Phase I or II cancer study (Phase II for metastatic colorectal cancer, ovarian cancer and BCC) |
| IPI-926 | Infinity Pharmaceuticals | NCT00761696 | A Phase I study of IPI-926 in patients with advanced and/or metastatic solid tumor malignancies | |
| BMS-833923 (XL139) | Bristol-Myers Squibb | Exelixis | NCT00670189 | A Phase I study of BMS-833923 (XL139) in subjects with advanced or metastatic cancer |
| LDE225 | Novartis Pharmaceuticals | NCT00880308 | Dose finding and safety of oral LDE225 in patients with advanced solid tumors (Phase I) |
Other novel Smo inhibitors that are undergoing preclinical testing include SANT1-4 [95,96], Hh Antag [97,98]; and IPI-269609 [99,100]. In addition, naturally occurring molecules such as vitamin D3 [101,102] (see below) and drugs currently used to treat other diseases – such as itraconazole, a commonly used antifungal drug [103] (see below) – have been suggested to be Smo antagonists.
6.2 Vitamin D3 (cholecalciferol)
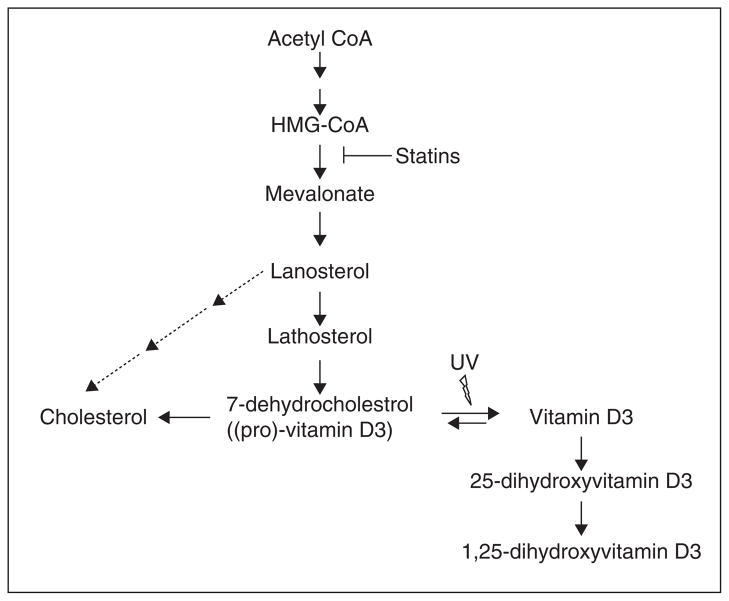
Vitamin D is a fat-soluble vitamin that is essential for bone development and the immune system. Vitamin D is mainly derived from the action of sunlight on the skin, converting 7-dehydrocholesterol to vitamin D3 (cholecalciferol) (Figure 3). Vitamin D3 from the skin and dietary vitamin D3 is further metabolized in the liver to 25-hydroxyvitamin D (25(OH)D3), the clinical indicator of vitamin D status, which is subsequently hydroxylated in the kidney and peripheral tissues to the ‘biologically active’ form of vitamin D, 1,25-dihydroxyvitamin D (1,25(OH)2D3), or calcitriol. The latter binds vitamin D receptor (VDR) transcriptional complexes to activate vitamin D target gene transcription.
Figure 3. The relationship between statins, cholesterol and vitamin D synthesis.
Statins inhibit the conversion of HMG-CoA to mevalonate, which results in the reduction of 7-dehydrocholesterol, the major precursor for cholesterol and vitamin D synthesis.
VDR transcriptional signaling is important for inhibiting proliferation and promoting differentiation and apoptosis in cancers such as breast- and colon cancer [104]. Recently, vitamin D was found to inhibit the Hh pathway by targeting Smo, and was more potent than cyclopamine in vitro [102]. Vitamin D is relatively safe even in high doses, and is effective as a Hh pathway inhibitor, at least in vitro [102]. Thus it could be a novel preventive and therapeutic approach for the prevention and of treatment of BCC and other Hh-driven cancers, since it is likely to be well-tolerated. However, some caution is necessary, since vitamin D is a precursor for 25(OH)D and 1,25(OH)D, which do not inhibit Hh signaling in vitro [102], and higher prediagnostic serum 25(OH)D levels have been associated with an increased risk of developing subsequent BCC [105]. However, further experimental studies are warranted to establish the efficacy of vitamin D in the treatment of Hh-driven cancers such as BCC.
6.3 Itraconazole
This antifungal drug was identified as a potent inhibitor of Gli reporter activity in a screen of a library of ~ 2400 FDA-approved/post-Phase I drugs using the Shh-Light2 cell line, which contains a stably integrated Gli-Luciferase reporter that responds to the active form of Shh, ShhN [103]. Itraconazole normally inhibits the enzyme 14-α-lanosterol demethylase (14LDM), which is essential for the biosynthesis of ergosterol in fungi and cholesterol in mammals [106]. However, its inhibition of Hh signaling was not due to an effect on cellular cholesterol biosynthesis but rather as a direct inhibitor of Smo protein function in a manner that was distinct from cyclopamine: itraconazole bound to a different site to cyclopamine on Smo and prevents Smo accumulation at the primary cilium [103], in contrast to cyclopamine, which induces Smo accumulation at the primary cilium [90,107]. Furthermore, systemic itraconazole dosing dramatically retarded BCC carcinogenesis in the Ptch1+/−, basal keratinocyte p53 deleted murine model, albeit with higher serum concentrations than those reached in humans at regular therapeutic doses. However, from human patient reports, these higher serum concentrations are associated with only occasional toxicities that are generally manageable.
With extensive knowledge of the safety profile and its identification as a potent Smo antagonist, itraconazole has the potential to be useful for human BCC therapy. Since itraconazole is FDA-approved and has been used for 20 years, at least its side effects are relatively well known compared with the new chemical entities that inhibit Smo (described above). Phase II clinical trials to evaluate the efficacy of itraconazole in patients with BCC and prostate cancer are currently underway (NCT01108094 and NCT00887458, respectively).
6.4 Non-Smo Hh pathway inhibitors
In addition to Smo antagonists, attempts have been made to discover novel drugs that target other Hh pathway components, such as Gli. Targeting Gli and other downstream molecules of Smo would be required to treat those cancers with Smo gain-of-function mutations, for example, which would render the tumor resistant to some Smo antagonists. Using Gli reporter assays in conjunction with the overexpression of Gli transcription factors, two molecules, GANT58 and GANT61, were found to inhibit Gli-dependent transcription downstream of Smo and SuFu [108]. In another screen for small molecules that could inhibit Hh signaling downstream of SuFu, four Hh pathway inhibitors, HPI1 – 4, were found to inhibit Hh signaling by modulating Gli processing, activation, and/or trafficking. These HPIs could also partially block Smo translocation to the cilium, reducing the extent of ciliary Smo accumulation in response to Hh ligand [109]. One of these molecules inhibited Hh signaling by blocking ciliogenesis.
Another agent that has been identified as a Hh pathway inhibitor is a imidazopyridine derivative JK184, which is thought to inhibit Hh pathway signaling by disrupting microtubules [110]. However, by the nature of its action of microtubules, JK184 also inhibits mitosis. Therefore this drug may have limited clinical potential as a specific Hh pathway inhibitor.
6.5 FOXM1 inhibitors
FOXM1 is a transcription factor belonging to the Forkhead family, the members of which are characterized by a conserved Forkhead/winged-helix DNA-binding domain that binds to consensus sequences in the promoters of target genes. FoxM1 proteins play an important role in regulating the expression of genes that are crucial for G1-S and G2-M cell cycle phase progression and mitotic spindle integrity to regulate cell growth, proliferation, differentiation, longevity, and transformation [111]. It is also one of the most overexpressed genes in many human solid tumors, including BCC (but not in SCC), and the protein is strongly localized in both nucleus and cytoplasm throughout BCC tumor islands.
FoxM1 and FoxE1 have been suggested to be a direct downstream transcriptional targets of Hh signaling [112,113], and therefore may be alternative targets for Hh pathway inhibition in BCC. Small-molecule screening of NCI libraries using cell-based assays identified the thiazole antibiotics siomycin A [114] and structurally related thiostrepton [115,116] as inhibitors of FoxM1 transcriptional activity. These drugs were also identified as proteosome inhibitors (PIs), which led to the identification of bona fide PIs MG115, MG132 and bortezomib as FoxM1 inhibitors [117]. PIs stabilize the majority of cellular proteins: it is therefore suggested that they would stabilize a negative regulator of FoxM1, NRFM, and reduce FoxM1 transcriptional activity and its expression (FoxM1 is also a target of its own activity) [118], thus reducing carcinogenesis in FoxM1-expressing cancers. Further experiments are required to test the anticancer efficacy of PIs, as well as their effects on Hh signaling in BCC.
6.6 Statins
Statins are small-molecule inhibitors of HMG-CoA reductase, the rate-limiting enzyme in the synthesis of mevalonate, the fatty acid intermediate required for cholesterol and vitamin D biosynthesis [119,120]. Currently FDA-approved to treat and prevent heart disease by lowering blood cholesterol, this group of relatively safe, well-tolerated drugs have been suggested to be a possible anticancer therapy for colorectal cancer and skin cancer [120]. Cholesterol and other oxysterols have been suggested to be positive regulators of Hh signaling. Firstly, sonic hedgehog (Shh) ligand requires extracellular sterol modification to become active and secondly, in Hh-driven medulloblastoma cells, cholesterol and specific oxysterols are required for Hh pathway signal transduction [121]. In these cells, statins reduced Hh target gene transcription and blocked Hh pathway-dependent proliferation; these effects were reversed by the addition of exogenous cholesterol or specific oxysterols, which activated Hh target gene transcription via Smo [121].
These data suggest that sterols may be critical regulators of Smo-dependent Hh pathway activation. Thus, statins may offer a novel approach to the treatment of Hh pathway driven cancers such as BCC. However, recent clinical observational studies in a relatively large cohort of patients with sporadic BCC suggests that there is no significant association between statin therapy and risk for developing new BCCs [122,123]. Perhaps a reason why the clinical data for statins on BCC are not so impressive may be because of a putative effect on vitamin D synthesis. There is a close relationship between statins, cholesterol and vitamin D. Statins inhibit HMG-CoA reductase and thereby inhibit the synthesis of 7-dehydrocholesterol, the precursor for cholesterol and vitamin D, which may have positive and negative effects, respectively, on Hh signaling (Figure 3; see above). Statins would then counteract the result of reducing cholesterol, a possible positive regulator of Hh signaling, by decreasing vitamin D levels. However, this reduction has not been reported clinically and hence remains theoretical [119].
6.7 Therapeutic use of Hh pathway antagonists
It remains to be seen whether Hh pathway antagonists like GDC-0449 can completely eradicate BCCs in patients. In experimental studies in which the Hh pathway was inactivated in visible BCCs, despite the dramatic shrinkage of the tumors, a residual population of non-proliferative cells remained, which reinitiated BCC growth when the Hh pathway (Gli2 overexpression) was reactivated [20]. This would suggest that Hh pathway antagonists are likely to be ‘growth suppressors’, and that drug withdrawal would result in the reappearance of BCC. Thus, it is likely that other agents may be required in combination with Hh pathway antagonists for the complete eradication of BCC.
GDC-0449 is currently being tested in Phase II trials in combination with erlotinib (a specific inhibitor of EGFR/ErbB1) for the treatment of metastatic pancreatic cancer (Table 1). As observed for other cancers, it is possible that in some patients with BCC, resistance to a single agent such as GDC-0449 may occur. This was observed in a patient with metastatic medulloblastoma who was treated with GDC-0449. Although the patient had a remarkable initial response to the drug (i.e., the metastatic growths diminished substantially in 2 months), unfortunately the cancer returned in a more aggressive form and the patient died shortly after [124]. Analysis of the nonresponsive growths showed a point mutation in the Smo gene that rendered the protein resistant to GDC-0449. Therefore, it is likely that other Smo inhibitors that differ in their functional abilities may also be necessary to overcome drug resistance.
7. Expert opinion
Despite significant progress in the understanding of the biology of BCC, the annual rate of BCC incidence is still increasing, and advanced BCC remains an incurable disease. There is a wealth of preclinical and clinical data to support several novel chemopreventive strategies for BCC treatment. Firstly, preclinical and clinical trials with drugs already FDA-approved for treating other nonrelated diseases have suggested novel uses of these drugs for chemo-prevention or early treatment of clinically relevant BCCs. It is likely that if clinical trials for BCC prevention indicate some ‘success’, these drugs will be approved relatively quickly by the FDA for BCC chemoprevention and therapy.
As with all non-FDA-approved investigative drugs, the optimal clinical use of the molecularly targeted novel Hh pathway inhibitors is likely to take some time to establish. However, the clinical trials that have evaluated the Smo antagonist GDC-0449 in patients with advanced BCC and medulloblastoma suggest promising prospects for the clinical use of this drug for prevention and treatment of less severe BCC. Already, clinical trials are underway to evaluate whether this drug will be a success as a chemopreventive agent against BCC in BCNS patients. However, preclinical studies suggest serious side effects for the use of Hh pathway inhibitors in children. In juvenile mice, transient inhibition of Hh signaling with a Smo inhibitor resulted in a loss of chondrocytes and premature fusion of bone growth plates leading to severe malformations [125], thus perhaps prohibiting the use of Hh inhibitors in younger BCNS or XP patients who develop skin cancers at a young age.
The clinical development of Smo inhibitors that target and inhibit Smo in different ways, or the use of another anti-cancer drug in combination, may overcome the drug resistance that is so commonly seen in cancer therapy. In addition, it is likely that a combination of Hh pathway antagonists and non Hh-pathway inhibitors will be required to eradicate BCC. It is hoped that several ongoing preclinical and clinical studies evaluating novel targeted approaches for treatment of BCC will lead to further improvement in the management of BCC.
Article highlights.
Aberrant hyperactivation of Hedgehog (Hh) signaling drives many cancers, including basal cell carcinoma (BCC), for which it is the pivotal pathway that causes the disease. Therefore, at least in BCC, the Hh pathway is an attractive target for the development of new therapeutics. The development of Ptch1+/− and Ptch1+/− epidermally deleted p53 murine models of BCC, which accurately phenocopy the human disease, allow for more rapid assessment of novel drugs for BCC chemoprevention and therapy.
In preclinical BCC studies, the ‘established’ FDA-approved drugs tazarotene and itraconazole, which are currently used to treat other diseases, show good chemopreventive and therapeutic efficacy, respectively, against murine BCC; their anti-BCC efficacy is currently being investigated in Phase II clinical trials. If successful in these trials, these drugs are likely to be approved relatively quickly by the FDA for BCC chemoprevention and therapy. Also, NSAIDs such as celecoxib may have anti-BCC efficacy in patients at lower risk and may provide another viable therapeutic option.
Mechanism-based therapies that directly target the Hh pathway are arguably the most exciting development for BCC prevention and management. The discovery of cyclopamine as a natural occurring inhibitor of Smoothened (Smo), a major Hh pathway effector, has shown that Smo inhibition by small molecules is a viable therapeutic option. This has spawned numerous efforts to design or identify viable therapeutic Smo antagonists. The most advanced Smo inhibitor currently being investigated in clinical trials is GDC-0449. In Phase I clinical trials of metastatic BCC and medulloblastoma, GDC-0449 dramatically reduced tumor growth; it has now entered Phase II testing.
The identification of Hh pathway inhibitors that are distinct from GDC-0449 and their combined use may provide enhanced efficacy against BCC tumorigenesis, and provide alternative therapies that overcome tumor resistance to a single agent.
This box summarizes key points contained in the article.
Footnotes
Declaration of interest
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Bibliography
- 1.Chen JG, Fleischer AB, Jr, Smith ED, et al. Cost of nonmelanoma skin cancer treatment in the United States. Dermatol Surg. 2001;27:1035–8. doi: 10.1046/j.1524-4725.2001.01004.x. [DOI] [PubMed] [Google Scholar]
- 2.Rigel DS. Cutaneous ultraviolet exposure and its relationship to the development of skin cancer. J Am Acad Dermatol. 2008;58:S129–32. doi: 10.1016/j.jaad.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 3.van der Pols JC, Williams GM, Pandeya N, et al. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol Biomarkers Prev. 2006;15:2546–8. doi: 10.1158/1055-9965.EPI-06-0352. [DOI] [PubMed] [Google Scholar]
- 4.Ulrich C, Jurgensen JS, Degen A, et al. Prevention of non-melanoma skin cancer in organ transplant patients by regular use of a sunscreen: a 24 months, prospective, case-control study. Br J Dermatol. 2009;161(Suppl 3):78–84. doi: 10.1111/j.1365-2133.2009.09453.x. [DOI] [PubMed] [Google Scholar]
- 5.Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30:774–8. doi: 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- 6.Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681–90. doi: 10.1001/jama.294.6.681. [DOI] [PubMed] [Google Scholar]
- 7.Rubin AI, Chen EH, Ratner D. Basal-cell carcinoma. N Engl J Med. 2005;353:2262–9. doi: 10.1056/NEJMra044151. [DOI] [PubMed] [Google Scholar]
- 8.Epstein EH. Basal cell carcinomas: attack of the hedgehog. Nat Rev Cancer. 2008;8:743–54. doi: 10.1038/nrc2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorlin RJ. Nevoid basal cell carcinoma (Gorlin) syndrome. Genet Med. 2004;6:530–9. doi: 10.1097/01.gim.0000144188.15902.c4. [DOI] [PubMed] [Google Scholar]
- 10.Hahn H, Wicking C, Zaphiropoulous PG, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–51. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 11.Johnson RL, Rothman AL, Xie J, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–71. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 12.Jiang J, Hui CC. Hedgehog signaling in development and cancer. Dev Cell. 2008;15:801–12. doi: 10.1016/j.devcel.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruiz i Altaba A, Mas C, Stecca B. The Gli code: an information nexus regulating cell fate, stemness and cancer. Trends Cell Biol. 2007;17:438–47. doi: 10.1016/j.tcb.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong SY, Seol AD, So PL, et al. Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat Med. 2009;15:1055–61. doi: 10.1038/nm.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reifenberger J, Wolter M, Knobbe CB, et al. Somatic mutations in the PTCH, SMOH, SUFUH and TP53 genes in sporadic basal cell carcinomas. Br J Dermatol. 2005;152:43–51. doi: 10.1111/j.1365-2133.2005.06353.x. [DOI] [PubMed] [Google Scholar]
- 16.Lam CW, Xie J, To KF, et al. A frequent activated smoothened mutation in sporadic basal cell carcinomas. Oncogene. 1999;18:833–6. doi: 10.1038/sj.onc.1202360. [DOI] [PubMed] [Google Scholar]
- 17.Xie J, Murone M, Luoh SM, et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90–2. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
- 18.Oro AE, Higgins KM, Hu Z, et al. Basal cell carcinomas in mice overexpressing sonic hedgehog. Science. 1997;276:817–21. doi: 10.1126/science.276.5313.817. [DOI] [PubMed] [Google Scholar]
- 19.Nilsson M, Unden AB, Krause D, et al. Induction of basal cell carcinomas and trichoepitheliomas in mice overexpressing GLI-1. Proc Natl Acad Sci USA. 2000;97:3438–43. doi: 10.1073/pnas.050467397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutchin ME, Kariapper MS, Grachtchouk M, et al. Sustained Hedgehog signaling is required for basal cell carcinoma proliferation and survival: conditional skin tumorigenesis recapitulates the hair growth cycle. Genes Dev. 2005;19:214–23. doi: 10.1101/gad.1258705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mao J, Ligon KL, Rakhlin EY, et al. A novel somatic mouse model to survey tumorigenic potential applied to the Hedgehog pathway. Cancer Res. 2006;66:10171–8. doi: 10.1158/0008-5472.CAN-06-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hahn H, Christiansen J, Wicking C, et al. A mammalian patched homolog is expressed in target tissues of sonic hedgehog and maps to a region associated with developmental abnormalities. J Biol Chem. 1996;271:12125–8. doi: 10.1074/jbc.271.21.12125. [DOI] [PubMed] [Google Scholar]
- 23.Svard J, Heby-Henricson K, Persson-Lek M, et al. Genetic elimination of Suppressor of fused reveals an essential repressor function in the mammalian Hedgehog signaling pathway. Dev Cell. 2006;10:187–97. doi: 10.1016/j.devcel.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Svard J, Rozell B, Toftgard R, Teglund S. Tumor suppressor gene co-operativity in compound Patched1 and suppressor of fused heterozygous mutant mice. Mol Carcinog. 2009;48:408–19. doi: 10.1002/mc.20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ling G, Ahmadian A, Persson A, et al. PATCHED and p53 gene alterations in sporadic and hereditary basal cell cancer. Oncogene. 2001;20:7770–8. doi: 10.1038/sj.onc.1204946. [DOI] [PubMed] [Google Scholar]
- 26.Aszterbaum M, Epstein J, Oro A, et al. Ultraviolet and ionizing radiation enhance the growth of BCCs and trichoblastomas in patched heterozygous knockout mice. Nat Med. 1999;5:1285–91. doi: 10.1038/15242. [DOI] [PubMed] [Google Scholar]
- 27.Mancuso M, Pazzaglia S, Tanori M, et al. Basal cell carcinoma and its development: insights from radiation-induced tumors in Ptch1-deficient mice. Cancer Res. 2004;64:934–41. doi: 10.1158/0008-5472.can-03-2460. [DOI] [PubMed] [Google Scholar]
- 28.Ceilley RI, Del Rosso JQ. Current modalities and new advances in the treatment of basal cell carcinoma. Int J Dermatol. 2006;45:489–98. doi: 10.1111/j.1365-4632.2006.02673.x. [DOI] [PubMed] [Google Scholar]
- 29.Beutner KR, Geisse JK, Helman D, et al. Therapeutic response of basal cell carcinoma to the immune response modifier imiquimod 5% cream. J Am Acad Dermatol. 1999;41:1002–7. doi: 10.1016/s0190-9622(99)70261-6. [DOI] [PubMed] [Google Scholar]
- 30.Moore AY. Clinical applications for topical 5-fluorouracil in the treatment of dermatological disorders. J Dermatolog Treat. 2009;20:328–35. doi: 10.3109/09546630902789326. [DOI] [PubMed] [Google Scholar]
- 31.Kraemer KH, DiGiovanna JJ, Moshell AN, et al. Prevention of skin cancer in xeroderma pigmentosum with the use of oral isotretinoin. N Engl J Med. 1988;318:1633–7. doi: 10.1056/NEJM198806233182501. [DOI] [PubMed] [Google Scholar]
- 32.Hodak E, Ginzburg A, David M, Sandbank M. Etretinate treatment of the nevoid basal cell carcinoma syndrome. Therapeutic and chemopreventive effect. Int J Dermatol. 1987;26:606–9. doi: 10.1111/j.1365-4362.1987.tb02319.x. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez-Conejo-Mir J, Camacho F. Nevoid basal cell carcinoma syndrome: combined etretinate and surgical treatment. J Dermatol Surg Oncol. 1989;15:868–71. doi: 10.1111/j.1524-4725.1989.tb01176.x. [DOI] [PubMed] [Google Scholar]
- 34.George R, Weightman W, Russ GR, et al. Acitretin for chemoprevention of non-melanoma skin cancers in renal transplant recipients. Australas J Dermatol. 2002;43:269–73. doi: 10.1046/j.1440-0960.2002.00613.x. [DOI] [PubMed] [Google Scholar]
- 35.Wright TI, Spencer JM, Flowers FP. Chemoprevention of nonmelanoma skin cancer. J Am Acad Dermatol. 2006;54:933–46. doi: 10.1016/j.jaad.2005.08.062. quiz 947–50. [DOI] [PubMed] [Google Scholar]
- 36.Roeder A, Schaller M, Schafer-Korting M, Korting HC. Tazarotene: therapeutic strategies in the treatment of psoriasis, acne and photoaging. Skin Pharmacol Physiol. 2004;17:111–18. doi: 10.1159/000077236. [DOI] [PubMed] [Google Scholar]
- 37.Chandraratna RA. Tazarotene: the first receptor-selective topical retinoid for the treatment of psoriasis. J Am Acad Dermatol. 1997;37:S12–17. [PubMed] [Google Scholar]
- 38.Chen CF, Goyette P, Lohnes D. RAR-gamma acts as a tumor suppressor in mouse keratinocytes. Oncogene. 2004;23:5350–9. doi: 10.1038/sj.onc.1207682. [DOI] [PubMed] [Google Scholar]
- 39.So PL, Lee K, Hebert J, et al. Topical tazarotene chemoprevention reduces Basal cell carcinoma number and size in Ptch1+/− mice exposed to ultraviolet or ionizing radiation. Cancer Res. 2004;64:4385–9. doi: 10.1158/0008-5472.CAN-03-1927. [DOI] [PubMed] [Google Scholar]
- 40.So PL, Fujimoto MA, Epstein EH., Jr Pharmacologic retinoid signaling and physiologic retinoic acid receptor signaling inhibit basal cell carcinoma tumorigenesis. Mol Cancer Ther. 2008;7:1275–84. doi: 10.1158/1535-7163.MCT-07-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peck GL, DiGiovanna JJ, Sarnoff DS, et al. Treatment and prevention of basal cell carcinoma with oral isotretinoin. J Am Acad Dermatol. 1988;19:176–85. doi: 10.1016/s0190-9622(88)70162-0. [DOI] [PubMed] [Google Scholar]
- 42.Peck GL. Long-term retinoid therapy is needed for maintenance of cancer chemopreventive effect. Dermatologica. 1987;175(Suppl 1):138–44. doi: 10.1159/000248870. [DOI] [PubMed] [Google Scholar]
- 43.Ogden S, Samuel M, Griffiths CE. A review of tazarotene in the treatment of photodamaged skin. Clin Interv Aging. 2008;3:71–6. doi: 10.2147/cia.s1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bianchi L, Orlandi A, Campione E, et al. Topical treatment of basal cell carcinoma with tazarotene: a clinicopathological study on a large series of cases. Br J Dermatol. 2004;151:148–56. doi: 10.1111/j.1365-2133.2004.06044.x. [DOI] [PubMed] [Google Scholar]
- 45.Orlandi A, Bianchi L, Costanzo A, et al. Evidence of increased apoptosis and reduced proliferation in basal cell carcinomas treated with tazarotene. J Invest Dermatol. 2004;122:1037–41. doi: 10.1111/j.0022-202X.2004.22414.x. [DOI] [PubMed] [Google Scholar]
- 46.Khan MN, Lee YS. Cyclooxygenase inhibitors: Scope of their use and development in cancer chemotherapy. Med Res Rev. 2009 Dec 4; doi: 10.1002/med.20182. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 47.Zha S, Yegnasubramanian V, Nelson WG, et al. Cyclooxygenases in cancer: progress and perspective. Cancer Lett. 2004;215:1–20. doi: 10.1016/j.canlet.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 48.Athar M, An KP, Morel KD, et al. Ultraviolet B(UVB)-induced cox-2 expression in murine skin: an immunohistochemical study. Biochem Biophys Res Commun. 2001;280:1042–7. doi: 10.1006/bbrc.2000.4201. [DOI] [PubMed] [Google Scholar]
- 49.Tjiu JW, Liao YH, Lin SJ, et al. Cyclooxygenase-2 overexpression in human basal cell carcinoma cell line increases antiapoptosis, angiogenesis, and tumorigenesis. J Invest Dermatol. 2006;126:1143–51. doi: 10.1038/sj.jid.5700191. [DOI] [PubMed] [Google Scholar]
- 50.Vogel U, Christensen J, Wallin H, et al. Polymorphisms in COX-2, NSAID use and risk of basal cell carcinoma in a prospective study of Danes. Mutat Res. 2007;617:138–46. doi: 10.1016/j.mrfmmm.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 51.Tang JY, Aszterbaum M, Athar M, et al. Basal cell carcinoma chemoprevention with nonsteroidal anti-inflammatory drugs in genetically predisposed PTCH1 +/− humans and mice. Cancer Prev Res (Phila Pa) 2010;3:25–34. doi: 10.1158/1940-6207.CAPR-09-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clouser MC, Roe DJ, Foote JA, Harris RB. Effect of non-steroidal anti-inflammatory drugs on non-melanoma skin cancer incidence in the SKICAP-AK trial. Pharmacoepidemiol Drug Saf. 2009;18:276–83. doi: 10.1002/pds.1718. [DOI] [PubMed] [Google Scholar]
- 53.Grau MV, Baron JA, Langholz B, et al. Effect of NSAIDs on the recurrence of nonmelanoma skin cancer. Int J Cancer. 2006;119:682–6. doi: 10.1002/ijc.21878. [DOI] [PubMed] [Google Scholar]
- 54.Bertagnolli MM, Eagle CJ, Zauber AG, et al. Five-year efficacy and safety analysis of the Adenoma Prevention with Celecoxib Trial. Cancer Prev Res (Phila Pa) 2009;2:310–21. doi: 10.1158/1940-6207.CAPR-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakanishi M, Niida H, Murakami H, Shimada M. DNA damage responses in skin biology – implications in tumor prevention and aging acceleration. J Dermatol Sci. 2009;56:76–81. doi: 10.1016/j.jdermsci.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 56.De Lange T. Telomere-related genome instability in cancer. Cold Spring Harb Symp Quant Biol. 2005;70:197–204. doi: 10.1101/sqb.2005.70.032. [DOI] [PubMed] [Google Scholar]
- 57.Li GZ, Eller MS, Hanna K, Gilchrest BA. Signaling pathway requirements for induction of senescence by telomere homolog oligonucleotides. Exp Cell Res. 2004;301:189–200. doi: 10.1016/j.yexcr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 58.Goukassian DA, Helms E, van Steeg H, et al. Topical DNA oligonucleotide therapy reduces UV-induced mutations and photocarcinogenesis in hairless mice. Proc Natl Acad Sci USA. 2004;101:3933–8. doi: 10.1073/pnas.0306389101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arad S, Zattra E, Hebert J, et al. Topical thymidine dinucleotide treatment reduces development of ultraviolet-induced basal cell carcinoma in Ptch-1+/− mice. Am J Pathol. 2008;172:1248–55. doi: 10.2353/ajpath.2008.071117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gilmour SK. Polyamines and nonmelanoma skin cancer. Toxicol Appl Pharmacol. 2007;224:249–56. doi: 10.1016/j.taap.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elmets CA, Athar M. Targeting ornithine decarboxylase for the prevention of nonmelanoma skin cancer in humans. Cancer Prev Res (Phila Pa) 2010;3:8–11. doi: 10.1158/1940-6207.CAPR-09-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tang X, Kim AL, Feith DJ, et al. Ornithine decarboxylase is a target for chemoprevention of basal and squamous cell carcinomas in Ptch1+/− mice. J Clin Invest. 2004;113:867–75. doi: 10.1172/JCI20732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bailey HH, Kim K, Verma AK, et al. A randomized, double-blind, placebo-controlled phase 3 skin cancer prevention study of {alpha}-difluoromethylornithine in subjects with previous history of skin cancer. Cancer Prev Res (Phila Pa) 2010;3:35–47. doi: 10.1158/1940-6207.CAPR-09-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Filip A, Clichici S, Daicoviciu D, et al. Photochemoprevention of cutaneous neoplasia through natural products. Exp Oncol. 2009;31:9–15. [PubMed] [Google Scholar]
- 65.Clifford JL, DiGiovanni J. The promise of natural products for blocking early events in skin carcinogenesis. Cancer Prev Res (Phila Pa) 2010;3:132–5. doi: 10.1158/1940-6207.CAPR-09-0267. [DOI] [PubMed] [Google Scholar]
- 66.Limtrakul P, Lipigorngoson S, Namwong O, et al. Inhibitory effect of dietary curcumin on skin carcinogenesis in mice. Cancer Lett. 1997;116:197–203. doi: 10.1016/s0304-3835(97)00187-0. [DOI] [PubMed] [Google Scholar]
- 67.Gu M, Singh RP, Dhanalakshmi S, et al. Silibinin inhibits inflammatory and angiogenic attributes in photocarcinogenesis in SKH-1 hairless mice. Cancer Res. 2007;67:3483–91. doi: 10.1158/0008-5472.CAN-06-3955. [DOI] [PubMed] [Google Scholar]
- 68.Bommareddy A, Hora J, Cornish B, Dwivedi C. Chemoprevention by alpha-santalol on UVB radiation-induced skin tumor development in mice. Anticancer Res. 2007;27:2185–8. [PubMed] [Google Scholar]
- 69.Dwivedi C, Valluri HB, Guan X, Agarwal R. Chemopreventive effects of alpha-santalol on ultraviolet B radiation-induced skin tumor development in SKH-1 hairless mice. Carcinogenesis. 2006;27:1917–22. doi: 10.1093/carcin/bgl058. [DOI] [PubMed] [Google Scholar]
- 70.Dwivedi C, Maydew ER, Hora JJ, et al. Chemopreventive effects of various concentrations of alpha-santalol on skin cancer development in CD-1 mice. Eur J Cancer Prev. 2005;14:473–6. doi: 10.1097/01.cej.0000178075.20124.2a. [DOI] [PubMed] [Google Scholar]
- 71.Dwivedi C, Guan X, Harmsen WL, et al. Chemopreventive effects of alpha-santalol on skin tumor development in CD-1 and SENCAR mice. Cancer Epidemiol Biomarkers Prev. 2003;12:151–6. [PubMed] [Google Scholar]
- 72.Zhang X, Bommareddy A, Chen W, et al. Chemopreventive effects of sarcophine-diol on ultraviolet B-induced skin tumor development in SKH-1 hairless mice. Mar Drugs. 2009;7:153–65. doi: 10.3390/md7020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang X, Kundoor V, Khalifa S, et al. Chemopreventive effects of sarcophine-diol on skin tumor development in CD-1 mice. Cancer Lett. 2007;253:53–9. doi: 10.1016/j.canlet.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 74.Chilampalli S, Zhang X, Fahmy H, et al. Chemopreventive effects of honokiol on UVB-induced skin cancer development. Anticancer Res. 2010;30:777–83. [PubMed] [Google Scholar]
- 75.Katiyar S, Elmets CA, Katiyar SK. Green tea and skin cancer: photoimmunology, angiogenesis and DNA repair. J Nutr Biochem. 2007;18:287–96. doi: 10.1016/j.jnutbio.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 76.Rees JR, Stukel TA, Perry AE, et al. Tea consumption and basal cell and squamous cell skin cancer: results of a case-control study. J Am Acad Dermatol. 2007;56:781–5. doi: 10.1016/j.jaad.2006.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hebert JL, Khugyani F, Athar M, et al. Chemoprevention of basal cell carcinomas in the ptc1+/− mouse – green and black tea. Skin Pharmacol Appl Skin Physiol. 2001;14:358–62. doi: 10.1159/000056369. [DOI] [PubMed] [Google Scholar]
- 78.Park K, Lee JH. Bcl-XL protein is markedly decreased in UVB-irradiated basal cell carcinoma cell lines through proteasome-mediated degradation. Oncol Rep. 2009;21:689–92. [PubMed] [Google Scholar]
- 79.Jee SH, Shen SC, Tseng CR, et al. Curcumin induces a p53-dependent apoptosis in human basal cell carcinoma cells. J Invest Dermatol. 1998;111:656–61. doi: 10.1046/j.1523-1747.1998.00352.x. [DOI] [PubMed] [Google Scholar]
- 80.Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002;16:2743–8. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Berman DM, Karhadkar SS, Hallahan AR, et al. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science. 2002;297:1559–61. doi: 10.1126/science.1073733. [DOI] [PubMed] [Google Scholar]
- 82.Dahmane N, Sanchez P, Gitton Y, et al. The Sonic Hedgehog-Gli pathway regulates dorsal brain growth and tumorigenesis. Development. 2001;128:5201–12. doi: 10.1242/dev.128.24.5201. [DOI] [PubMed] [Google Scholar]
- 83.Stecca B, Mas C, Clement V, et al. Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proc Natl Acad Sci USA. 2007;104:5895–900. doi: 10.1073/pnas.0700776104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Athar M, Li C, Tang X, et al. Inhibition of smoothened signaling prevents ultraviolet B-induced basal cell carcinomas through regulation of Fas expression and apoptosis. Cancer Res. 2004;64:7545–52. doi: 10.1158/0008-5472.CAN-04-1393. [DOI] [PubMed] [Google Scholar]
- 85.So PL, Langston AW, Daniallinia N, et al. Long-term establishment, characterization and manipulation of cell lines from mouse basal cell carcinoma tumors. Exp Dermatol. 2006;15:742–50. doi: 10.1111/j.1600-0625.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 86.Tabs S, Avci O. Induction of the differentiation and apoptosis of tumor cells in vivo with efficiency and selectivity. Eur J Dermatol. 2004;14:96–102. [PubMed] [Google Scholar]
- 87.Tremblay MR, Nevalainen M, Nair SJ, et al. Semisynthetic cyclopamine analogues as potent and orally bioavailable hedgehog pathway antagonists. J Med Chem. 2008;51:6646–9. doi: 10.1021/jm8008508. [DOI] [PubMed] [Google Scholar]
- 88.Stanton BZ, Peng LF. Small-molecule modulators of the Sonic Hedgehog signaling pathway. Mol Biosyst. 2010;6:44–54. doi: 10.1039/b910196a. [DOI] [PubMed] [Google Scholar]
- 89.Winkler JD, Isaacs A, Holderbaum L, et al. Design and synthesis of inhibitors of Hedgehog signaling based on the alkaloid cyclopamine. Org Lett. 2009;11:2824–7. doi: 10.1021/ol900974u. [DOI] [PubMed] [Google Scholar]
- 90.Wang Y, Zhou Z, Walsh CT, McMahon AP. Selective translocation of intracellular Smoothened to the primary cilium in response to Hedgehog pathway modulation. Proc Natl Acad Sci USA. 2009;106:2623–8. doi: 10.1073/pnas.0812110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Williams JA, Guicherit OM, Zaharian BI, et al. Identification of a small molecule inhibitor of the hedgehog signaling pathway: effects on basal cell carcinoma-like lesions. Proc Natl Acad Sci USA. 2003;100:4616–21. doi: 10.1073/pnas.0732813100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Scales SJ, de Sauvage FJ. Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol Sci. 2009;30:303–12. doi: 10.1016/j.tips.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 93.Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164–72. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 94.Tremblay MR, Nesler M, Weatherhead R, Castro AC. Recent patents for Hedgehog pathway inhibitors for the treatment of malignancy. Expert Opin Ther Pat. 2009;19:1039–56. doi: 10.1517/13543770903008551. [DOI] [PubMed] [Google Scholar]
- 95.Buttner A, Seifert K, Cottin T, et al. Synthesis and biological evaluation of SANT-2 and analogues as inhibitors of the hedgehog signaling pathway. Bioorg Med Chem. 2009;17:4943–54. doi: 10.1016/j.bmc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 96.Chen JK, Taipale J, Young KE, et al. Small molecule modulation of Smoothened activity. Proc Natl Acad Sci USA. 2002;99:14071–6. doi: 10.1073/pnas.182542899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yauch RL, Gould SE, Scales SJ, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–10. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 98.Romer JT, Kimura H, Magdaleno S, et al. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/−) p53(−/−) mice. Cancer Cell. 2004;6:229–40. doi: 10.1016/j.ccr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 99.Tremblay MR, Lescarbeau A, Grogan MJ, et al. Discovery of a potent and orally active hedgehog pathway antagonist (IPI-926) J Med Chem. 2009;52:4400–18. doi: 10.1021/jm900305z. [DOI] [PubMed] [Google Scholar]
- 100.Feldmann G, Fendrich V, McGovern K, et al. An orally bioavailable small-molecule inhibitor of Hedgehog signaling inhibits tumor initiation and metastasis in pancreatic cancer. Mol Cancer Ther. 2008;7:2725–35. doi: 10.1158/1535-7163.MCT-08-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bijlsma MF, Peppelenbosch MP, Spek CA. (Pro-)vitamin D as treatment option for hedgehog-related malignancies. Med Hypotheses. 2008;70:202–3. doi: 10.1016/j.mehy.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 102.Bijlsma MF, Spek CA, Zivkovic D, et al. Repression of smoothened by patched-dependent (pro-)vitamin D3 secretion. PLoS Biol. 2006;4:e232. doi: 10.1371/journal.pbio.0040232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim J, Tang JY, Gong R, et al. Itraconazole, a commonly used antifungal that inhibits Hedgehog pathway activity and cancer growth. Cancer Cell. 2010;17:388–99. doi: 10.1016/j.ccr.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Samuel S, Sitrin MD. Vitamin D’s role in cell proliferation and differentiation. Nutr Rev. 2008;66:S116–124. doi: 10.1111/j.1753-4887.2008.00094.x. [DOI] [PubMed] [Google Scholar]
- 105.Asgari MM, Tang J, Warton ME, et al. Association of prediagnostic serum vitamin D levels with the development of basal cell carcinoma. J Invest Dermatol. 2010;130(5):1438–43. doi: 10.1038/jid.2009.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lepesheva GI, Waterman MR. Sterol 14alpha-demethylase cytochrome P450 (CYP51), a P450 in all biological kingdoms. Biochim Biophys Acta. 2007;1770:467–77. doi: 10.1016/j.bbagen.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rohatgi R, Milenkovic L, Corcoran RB, Scott MP. Hedgehog signal transduction by Smoothened: pharmacologic evidence for a 2-step activation process. Proc Natl Acad Sci USA. 2009;106:3196–201. doi: 10.1073/pnas.0813373106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lauth M, Bergstrom A, Shimokawa T, Toftgard R. Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proc Natl Acad Sci USA. 2007;104:8455–60. doi: 10.1073/pnas.0609699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hyman JM, Firestone AJ, Heine VM, et al. Small-molecule inhibitors reveal multiple strategies for Hedgehog pathway blockade. Proc Natl Acad Sci USA. 2009;106:14132–7. doi: 10.1073/pnas.0907134106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cupido T, Rack PG, Firestone AJ, et al. The imidazopyridine derivative JK184 reveals dual roles for microtubules in Hedgehog signaling. Angew Chem Int Ed Engl. 2009;48:2321–4. doi: 10.1002/anie.200805666. [DOI] [PubMed] [Google Scholar]
- 111.Myatt SS, Lam EW. Targeting FOXM1. Nat Rev Cancer. 2008;8:242. doi: 10.1038/nrc2223-c2. [DOI] [PubMed] [Google Scholar]
- 112.Brancaccio A, Minichiello A, Grachtchouk M, et al. Requirement of the forkhead gene Foxe1, a target of sonic hedgehog signaling, in hair follicle morphogenesis. Hum Mol Genet. 2004;13:2595–606. doi: 10.1093/hmg/ddh292. [DOI] [PubMed] [Google Scholar]
- 113.Teh MT, Wong ST, Neill GW, et al. FOXM1 is a downstream target of Gli1 in basal cell carcinomas. Cancer Res. 2002;62:4773–80. [PubMed] [Google Scholar]
- 114.Radhakrishnan SK, Bhat UG, Hughes DE, et al. Identification of a chemical inhibitor of the oncogenic transcription factor forkhead box M1. Cancer Res. 2006;66:9731–5. doi: 10.1158/0008-5472.CAN-06-1576. [DOI] [PubMed] [Google Scholar]
- 115.Bhat UG, Halasi M, Gartel AL. Thiazole antibiotics target FoxM1 and induce apoptosis in human cancer cells. PLoS One. 2009;4:e5592. doi: 10.1371/journal.pone.0005592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kwok JM, Myatt SS, Marson CM, et al. Thiostrepton selectively targets breast cancer cells through inhibition of forkhead box M1 expression. Mol Cancer Ther. 2008;7:2022–32. doi: 10.1158/1535-7163.MCT-08-0188. [DOI] [PubMed] [Google Scholar]
- 117.Bhat UG, Halasi M, Gartel AL. FoxM1 is a general target for proteasome inhibitors. PLoS One. 2009;4:e6593. doi: 10.1371/journal.pone.0006593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Halasi M, Gartel AL. A novel mode of FoxM1 regulation: positive auto-regulatory loop. Cell Cycle. 2009;8:1966–7. doi: 10.4161/cc.8.12.8708. [DOI] [PubMed] [Google Scholar]
- 119.Grimes DS. Statins and vitamin D. Cardiovasc Drugs Ther. 2009;23:261–2. doi: 10.1007/s10557-009-6182-7. [DOI] [PubMed] [Google Scholar]
- 120.Demierre MF, Higgins PD, Gruber SB, et al. Statins and cancer prevention. Nat Rev Cancer. 2005;5:930–42. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- 121.Corcoran RB, Scott MP. Oxysterols stimulate Sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proc Natl Acad Sci USA. 2006;103:8408–13. doi: 10.1073/pnas.0602852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dore DD, Lapane KL, Trivedi AN, et al. Association between statin use and risk for keratinocyte carcinoma in the veterans affairs topical tretinoin chemoprevention trial. Ann Intern Med. 2009;150:9–18. doi: 10.7326/0003-4819-150-1-200901060-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Asgari MM, Tang J, Epstein EH, Jr, et al. Statin use and risk of basal cell carcinoma. J Am Acad Dermatol. 2009;61:66–72. doi: 10.1016/j.jaad.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yauch RL, Dijkgraaf GJ, Alicke B, et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science. 2009;326:572–4. doi: 10.1126/science.1179386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kimura H, Ng JM, Curran T. Transient inhibition of the Hedgehog pathway in young mice causes permanent defects in bone structure. Cancer Cell. 2008;13:249–60. doi: 10.1016/j.ccr.2008.01.027. [DOI] [PubMed] [Google Scholar]