Thermoregulation, or control of body temperature, has been studied as a fundamental physiological parameter defining health and disease for centuries (Ring, 2007; Ring, McEvoy, Jung, Zuber, & Machin, 2010). Multiple techniques have been used to assess thermoregulation in infants and adults, including the recent development of infrared thermography. Thermography can measure and visualize skin surface thermal patterns in the study of body temperature, and researchers have used this novel methodology to study cancer, peripheral vascular disease, trauma, and wound management (Kateb, Yamamoto, Yu, Grundfest, & Gruen, 2009; Katz et al., 2008; Mital & Scott, 2007; Nakanishi & Imai-Matsumura, 2008). We have recently developed methods to use infrared thermography to measure body temperature in neonates during their first month of life to examine relationships between control of body temperature and perfusion, as well as association between thermoregulation and clinical disease processes such as necrotizing enterocolitis. This paper will review methods for using infrared thermography, current research using this measurement tool, and use in our current neonatal research.
Historical perspective
Measurement of body temperature in humans has traditionally been done using thermistors, thermocouples or thermopiles placed on the skin in a particular location. These sensors can be slow to respond and difficult to keep attached to the skin surface. Also, this method does not allow for simultaneous measurement of adjacent skin surfaces, thus limiting the applicability of the device. Having a device attached to the skin may alter the measure by virtue of covering the skin surface.
In contrast to these techniques, infrared thermography can measure body surface temperature simultaneously over the entire body surface that is exposed in a two dimensional plane, without having physical contact with the subject. Sir William Herschel (1800) first discovered that infrared wavelengths can measure heat beyond the visible spectrum. These “calorific rays,” reflect, refract, absorb and transmit similar to visible light. This is now called infrared radiation.Sir John Herschel, William Herschel's son, produced the first heat picture in 1840 from solar radiation (Ring, 2007). He called it a “thermogram” which is the term used to describe a picture of the temperature distribution in an object and is still used today. Early thermograms were crude, slow to process and displayed in black and white. Improvements in infrared cameras have greatly improved the quality of modern day thermograms.
Prior to the 1950's, infrared thermography research and development focused on producing special sensors for troop movement and heat seeking missiles. During this time, the technology was deemed classified and top secret. Dr. Leo Massopust (1948) took the first known clinical thermogram of vascular patterns in limbs and breasts. In the early 1960s, researchers and clinicians used thermography to study thyroid diseases, peripheral vascular diseases, diseases of the chest and breast cancer.
Infrared thermography
How it works
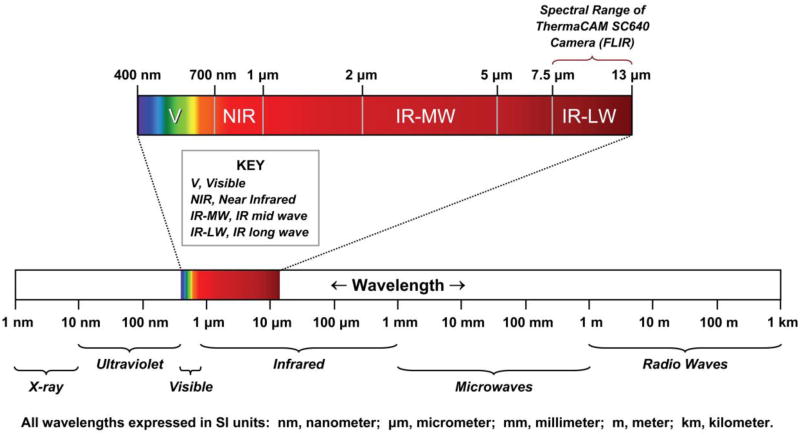
All objects having a temperature above absolute zero emit electromagnetic radiation called thermal radiation (Jones, 1998). To record an image of an object, the camera captures thermal radiation using a detector, and records the amount of emitted thermal radiation at each spatial position. The electromagnetic spectrum is divided into wavelength regions, called bands, which are distinguished by the methods used to produce and detect radiation. These regions consist of XRay, UV, Visible, Infrared, Microwave and Radiowave bands (Figure 1). Infrared thermography is used to record the temperature distribution of a surface using infrared radiation emitted by that body within the 0.8μm to 1.0 mm band (Jones, 1998). The wavelength regions of most importance for biologic imaging are the visible (400 nm-700 nm), near infrared (700 nm-1 μm), mid wave infrared (2-5 μm), and long wave infrared (8-12 μm).
Figure 1. Wavelength data for different bands in the electromagnetic spectrum.
Adapted from:
1. Level II Course Manual, FLIR Infrared Training Center, N. Billerica, MA
2. NASA Goddard Space Flight Center. Regions of the Electromagnetic Spectrum.
URL: http://imagine.gsfc.nasa.gov/docs/science/know_l1/spectrum_chart.html
(Last updated: Sep. 15, 2004; accessed Aug. 25, 2010)
The temperature of an object determines the spectral emission. Radiation is measured by the camera depending on the temperature and emissivity of that object. Emissivity is a measure of how much radiation is released from that object compared to an object with no reflectivity (ability to reflect radiation) at the same temperature. An emissivity of 1 occurs for a body with no reflectivity, which is called a blackbody. Human skin exhibits an emissivity of 0.97 to 0.98 between wavelengths 2-14 μm (Steketee, 1973). Because human skin acts like a blackbody, an infrared image records a measure of the temperature distribution of the outer surface layer of the skin (Jones, 1998). Steketee determined that there is no difference in emissivity between black, white, or burnt skin when measured in vivo or in vitro (Steketee, 1973).
When selecting a detector to collect the radiation, it is important to look for one that responds to the strongest radiation that is needed for measurement. If the human body is the object under study, there are two transmission bands that can be used to examine radiation produced by a living body: the 2-5 μm range MWIR- Medium Wavelength Infrared or the 8-12 μm range LWIR- Long Wavelength Infrared.
An infrared camera measures and records an image called a thermogram, which is the emitted infrared radiation from an object's surface displayed as pixels arranged in a 2-dimensional array. The thermogram can be displayed in black and white or in different color palettes to aid in visual temperature differentiation). In a typical grayscale thermogram, lighter colors indicate higher temperatures, and the range is from bright white to black. Various color palettes can be used to enhance qualitative analysis.
Types of cameras
All infrared cameras have the same purpose: to convert thermal radiation into visible images (Vainer, 2009). The first generation cameras contained only one photosensitive cell and were cumbersome. The second generation cameras used a string array of about 100 cells. The newest generation cameras contain a 2-dimensional array of synchronously working cells arranged in rows and columns. Cameras of this generation use a focal-plane array (FPA) detector that enables values at all pixels on the object to be collected simultaneously (Miller & Smith, 2005). These cameras have superiority in temperature sensitivity, speed of operation and spatial resolution (Vainer, 2009).Early infrared cameras were large, cooled by liquid nitrogen and had to be kept in a horizontal position to avoid spilling the nitrogen on the subject (Jones, 1998). Cameras now available are smaller in size, easier to operate and function in any position as they are uncooled and contain no liquid nitrogen.
Infrared cameras are capable of producing video of objects as well as digital pictures. Video can capture slow or fast temperature changes on human skin to help the researcher study physiological changes.
Financial and technical aspects
Infrared cameras can range from hundreds to hundreds of thousands of dollars. The handheld microbolometer cameras, such as the one we use in our research, can be purchased from FLIR (Boston, MA) or Infrared Cameras, Inc. (Beaumont, TX) which are two of the largest producers of infrared cameras. Our FLIR SC640 handheld, camera will take infrared images, digital photos and infrared video. This camera costs approximately $50,000, and the analysis software is approximately $10,000. Most companies that sell infrared cameras offer short technical courses on use of the camera and analyzing data from images. FLIR offers a 3-day Level I Thermography training course for $1,750 in California and Massachusettes.
Analyzing measures
Thermograms provide visual pictures of temperature distribution throughout the surface of the object under study. Visual analysis can be useful in medical imaging for the diagnosis and monitoring of many pathological conditions. Analysis software provides a quantitative temperature or photon count for each pixel beneath the cursor as the user scans over the image on a computer. Software programs, such as ExaminIR® (FLIR, Billerica, MA), allow the user to draw regions of interest over areas under study using boxes, circles, cursors, or polygrams and produce statistical means, ranges and standard deviations for temperatures in the defined areas. This analysis is helpful in determining asymmetrical temperature differentials for diagnosing pathology. The researcher can analyze infrared video captured with the camera to examine longitudinal temperature change using profiling and histograms. Temporal analysis of temperature will allow the researcher to explore skin temperature under different conditions.
Human research using infrared thermography
Although infrared thermography has been extensively used for years in the aerospace, military, industry and other fields, its use in the medical setting is relatively recent. Many clinicians and researchers have recently examined new and exciting areas with a broad range of clinical implications. Fundemental biologic studies have examined asymmetrical body temperature distributions which may function in the pathophysiology of a number of diseases (Bagavathiappan et al., 2009; Bharara, Cobb, & Claremont, 2006; Mercer, Nielsen, & Hoffmann, 2008; Mital & Scott, 2007). Facial temperature distributions have been examined as measures of emotion and communication (Nakanishi & Imai-Matsumura, 2008; Nozawa & Tacano, 2009). Few studies have used infrared imaging in the pediatric population, especially in the neonatal clinical setting; however, many of the applications used in adults may be well suited for infants.
The human body core acts as a thermal reservoir and is kept at a fairly stable temperature under normal conditions. Heat is transported to the skin by blood flow through central and peripheral blood vessels. Skin surface temperature is controlled by the rate of exchange of heat transported from the core and heat lost to the surrounding environment through radiation, conduction, convection and evaporation (Hammarlund, Stomberg, & Sedin, 1986; Stolwijk, 1975). In healthy adults, skin distribution should be symmetrical, and an asymmetrical temperature differential may indicate focal perfusion abnormality (Goodman, Murphy, Siltanen, Kelley, & Rucker, 1986; Uematsu, 1986). Infrared thermography can be helpful in studying medical conditions that are associated with variations in body temperature associated with vasodilation or vasoconstriction, hypo or hyperthermia, hypo or hyperfusion, hypermetabolism, and hypervascularization resulting in an increased skin temperature (Charkoudian, 2010; Helmy, Holdmann, & Rizkalla, 2008).
In particular, infrared thermography has been used for the detection of cancerous tumors Mittal and Scott (2007) have used thermal imaging to develop an algorithm to determine depth and location of tumors in breast cancer. Thermal imaging can be used intraoperatively to locate the margins of primary and metastatic brain tumors to offer real time anatomical and pathophysiological clinical information (Kateb et al., 2009). Kateb et al. (2009) used a ThermaCAM P60 (TCP60, FLIR) infrared camera to map the thermal profile of a metastatic melanoma tumor in the brain suggesting that infrared thermography could be useful in intraoperative detection and treatment of metastatic brain tumors.
Another application for infrared thermography is rapid diagnosis of fever when mass screening people during times of epidemic disease spread. This technology was used during outbreaks of severe acute respiratory syndrome (SARS) (Chiang et al., 2008; Chiu et al., 2005). Because infrared cameras are compact, lightweight and accurate, these devices allow for rapid temperature measurement of a large number of people without exposure to potential contagious diseases. Researchers have used thermography to diagnose thyroid diseases (Helmy et al., 2008), confirm hydrocephalus shunt patency (Goetz, Foertsch, Schoenberger, & Uhl, 2005), examine diabetes wound management (Bharara et al., 2006) and diagnose and treat leg ulcers (Mercer et al., 2008). Researchers are using infrared thermography in the area of exercise physiology to map out temperature differentials during exercise and rest (Merla, Mattei, Di Donato, & Romani, 2010). Another new area of research using thermal imaging is in exploring tissue composition to determine oxygenation of internal tissues (Tepper, Neeman, Milstein, David, & Gannot, 2009). This work may be helpful in using infrared thermal technology using invasive endoscopy and may assist with the localization of abnormal tissue for biopsy.
Katz et al. (2008) used thermal imaging to detect acute compartment syndrome in trauma patients. This technology can be beneficial for the rapid diagnosis of compartment syndrome before the need for amputation of the affected limb or death from the vascular compromise. Patients who developed compartment syndrome had a greater difference in proximal versus distal surface temperatures of the affected limb. Similarly, researchers have used infrared themography to identify patterns related to peripheral vascular diseases (Bagavathiappan et al., 2009) which can lead to asymmetrical temperature distributions. Infrared imaging may prove to be a valuable diagnostic tool for identifying perfusion pathology in adults and pediatric patients.
Using infrared thermography in neonates
When using infrared thermal imaging to measure skin temperature in neonates, developmental physiology has to be taken into consideration. Premature infants have very little fat, thin skin and high evaporative losses (Hammarlund & Sedin, 1979). Because their skin is so thin without the fat layer for insulation, measurements taken with an infrared camera approach core temperature as do thermistors attached to their skin (Hammarlund et al., 1986). Skin temperatures measured in the abdominal and central regions should be close to core temperature and there should be a differential lower temperature in the periphery (Simbrunner, 1995). Another factor to keep in mind when conducting thermal imaging on premature infants is heat loss is prevalent due to immature thermoregulation. These infants have very little brown fat and decreased proteins to enable metabolic heat production, which means premature infants need heated environments to warm their bodies as they can not independently produce enough heat as they become cold (Houstek et al., 1993; Sauer, 1995). Thermal imaging should be conducted through a cut out opening in the top of the incubator, to decrease the amount of heat loss from the environment and to obtain a measurement closest to the infant's true body temperature inside the incubator.
Term infants have a layer of fat, which insulates the infant from heat loss and will create a barrier from the infants' internal temperature which is more reflective of core and blood temperature. These temperatures will be closer adult skin temperature and lower than skin temperature of a premature infant. Thermal imaging can be conducted on a warmer table or while the infant is resting in a bassinette, as heat loss is not as much of a problem due to their ability to generate heat through metabolic heat production.
Only two previous studies in the United States have used infrared thermal imaging with neonates. The initial use of infrared thermography in neonatal research was performed in the early 1980s by Clark and Stothers (1980) who examined temperature distribution patterns over the central and peripheral body in neonates. These researchers used a surface silvered mirror placed at an angle of 45° over a cut out in the roof of the incubator canopy so that the infrared camera would remain horizontal to avoid spillage of the liquid nitrogen coolant. The camera was an AGA Model 680 Thermovision, which when coupled with an electronic processor produced images on a television screen. This research used crude techniques that produced thermographic reproductions with much room for quality improvement.
Twenty years later, researchers were still conducting infrared thermal imaging with a liquid nitrogen cooled camera connected to a television video output signal to study calorimetry measures in premature infants (Adams, Nelson, Bell, & Egoavil, 2000). These researchers measured infrared temperatures on premature infants through the cutout in an incubator, but also used a mirror to reflect the infant due to the possibility of spilling liquid nitrogen on the infant through the cut out.
A few studies have examined infrared thermal imaging with infants in Europe. A group of researchers studied different pathologies in the pediatric population from 1990-2000 on 285 patients using a liquid nitrogen cooled Talytherm® camera (Saxena & Wililital, 2008). This group evaluated temperature of skin associated with hemangiomas to document the extent of the hemangioma. Regression of the hemangioma in 102 infants and children were documented through infrared imaging and following the decrease of temperature differentiation between normal skin and the hemangioma. Eighteen newborns with abdominal wall defects such as gastroschisis and omphaloceles were followed using thermal imaging to examine reperfusion of the defect post surgery using longitudinal temperature differentiations.
An Austrian group used a Thermotracer TH 3100 (NEC San-ei Instruments, Tokyo, Japan) infrared camera to determine that skin temperature in term infants immediately after birth has little variation throughout the body surface, but is significantly lower than core temperature (Christidis et al., 2003). During the first hour, peripheral temperatures begin to decrease in relationship to central temperature and bathing can lead to a decrease in peripheral – central temperature difference. This research adds to the evidence that using thermal imaging for skin surface temperature may give some indication of changes in body temperature and perfusion associated with blood flow changes. Most likely, an overall undifferentiated pattern of skin temperature immediately after birth is indicative of fetal circulation, where peripheral vascular resistance is low. Once the ductus arteriosus closes after birth and oxygenation of blood takes place in the lungs instead of the placenta, peripheral vascular resistance becomes higher and the core becomes warmer than the periphery (Emmanouilides, Moss, Duffie, & Adams, 1964).
Examples from our research
We are using a FLIR SC640 (Billerica, MA) uncooled, microbolometer infrared camera to examine temperature differentials in extremely low birth weight (ELBW) infants, who are generally housed in a controlled heated, humid environment. ELBW infants are typically less than 29 weeks gestational age and weigh less than 1 kilogram.
Abdominal surface temperature is usually monitored on a premature infant using a thermistor probe covered with an insulated reflective cover. When using these probes and covers, there should be zero heat flux and this temperature should represent deep tissue or core temperature for that infant (Simbrunner, 1995). Temperature is a measure of the energy level of tissues which can yield the amount of heat stored in that tissue. When studying heat storage or energy expenditure in infants, one needs to measure temperature profiles or as many body segments as possible. Decreases in regional skin temperature may correlate with an increase in oxygen consumption, which further complicates the homeostasis of sick premature infants. For this reason, it is necessary to study temperature profiles of premature infants.
Our research as well as that of others has traditionally studied central (abdominal) and peripheral (foot) temperatures to examine hypothermia in premature infants (Knobel, Holditch-Davis, Schwartz, & Wimmer, 2009; Lyon, Pikaar, Badger & McIntosh, 1997; Thomas, 2003). Temperature differentials between central and peripheral temperature as well as variations in central temperature can indicate abnormal clinical states (Knobel et al., 2009). Until our present studies, we measured temperature with thermistors in two locations on the body. We are now also using infrared thermal imaging technology and can measure and analyze temperatures over the entire infant body surface. Using an infrared camera for thermography to measure differentials in temperature distribution is a non-invasive, sensitive way to predict abnormal clinical states.
Our first pilot study examined the feasibility and methods of using infrared thermography to explore temperature central and peripheral body temperature in ELBW infants over their first 5 days of life. We conducted infrared imaging once a day, when the nurse was conducting morning care of the infant. Two research assistants were needed for these imaging procedures and the imaging session lasted approximately 5 minutes, once assistants were proficient in using the infrared camera. One assistant held the infant still in a prone position, using her hands to hold the infant's feet and head, while the other assistant used the infrared camera manually to image through the Drager® incubator opening (Figure 2). To minimize changes to the infants' thermal environment, thermal imaging was performed through the top of a customized incubator that had a cutout in the lid, which was covered with plastic wrap to preserve heat but to allow transmission for thermal imaging. Unlike previous neonatal studies using infrared thermography, handheld, uncooled cameras can image an infant directly over the incubator through the cut out, without the fear of spilling liquid nitrogen on the infant.
Figure 2. Research assistants imaging premature infant from over the incubator.
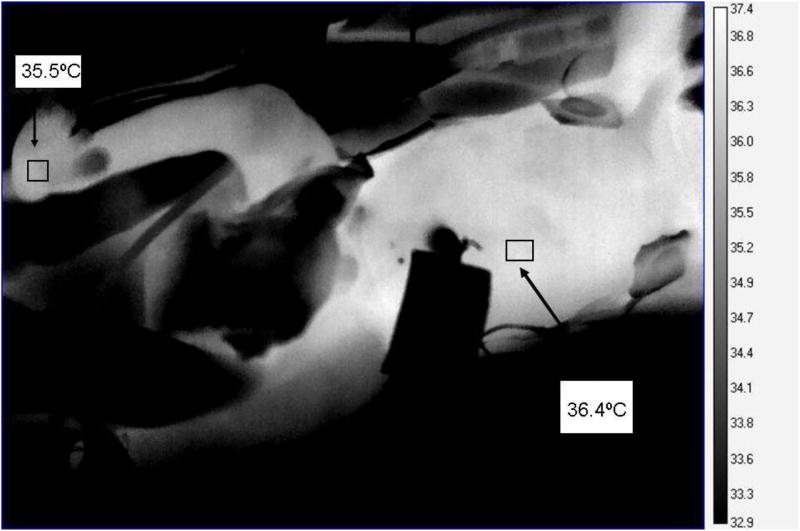
The camera weighs approximately 1.8 kilograms and was held at a 90° angle above the cutout in the incubator hood. We attempted to image with saran wrap over the cutout with the first two pilot infants; however, the saran wrap reduced the ability of the lens to focus on the infant. All remaining imaging was conducted while briefly removing the plastic wrap, then quickly returning the wrap in place. Incubators are engineered to maintain a continuous flow of warm air that envelopes the infant; when doors or portholes are opened, warm air should not escape. Therefore, even though we briefly removed plastic wrap, air temperature should not have decreased. We monitored environmental temperature inside the incubators with a data logger and found that infrared imaging did not decrease temperature near the infant. After the imaging session, the cutout covered with plastic wrap was also covered with a plexiglass cover made for Drager incubators. Our pilot study revealed that ELBW infants are relatively easy to image through non-invasive techniques and their thermograms provide a temperature diagram of the infant's body surface. Figure 3 illustrates temperature differentials of an infant's central and peripheral body areas.
Figure 3.
Thermogram of extremely low birth weight infant showing central (abdominal) temperature- 36.4°C and peripheral (foot) temperature- 35.5°C distributions. Temperature means from the box in the anatomical areas were obtained using the statistics mode of ExamIR® software. For qualitative analysis, lighter areas indicate warmer temperatures, darker areas indicate lower temperatures.
We have examined the feasibility of infrared thermal imaging for assessment of abdominal skin temperature in ELBW infants and to explore any association between abdominal skin temperature and necrotizing enterocolitis (NEC)(Rice et al., 2010). One of the most common neonatal gastrointestinal emergencies in ELBW infants is NEC, a disease of the intestinal tissue causing necrosis of the bowel (Blakely et al., 2005). The etiology of NEC is not clear; however it likely involves a combination of immaturity of the intestinal epithelial barrier and mucosal immune system, predisposing these infants to bacterial invasion, particularly in the context of poor intestinal perfusion (Nankervis, Giannone, & Reber, 2008). Because abnormal perfusion may be critical both in the development of NEC as well as for its diagnosis, we used infrared thermography to examine temperature differentials between each infants chest and abdomen over their postnatal age for the development of NEC in 13 ELBW infants. We defined infants as having NEC based on radiographic findings of suspected or definitive pneumatosis intestinalis, portal venous gas, or pneumoperitoneum. Our comparison between abdominal skin temperature and NEC was confined to 10 infants who had infrared thermal imaging as well as abdominal radiographs performed on the same day of each other.
We used the same FLIR® SC640 infrared camera (FLIR, North Ballerica MA) as in the previous study. Each thermography pixel of area could be analyzed separately, with measurement of each area of skin temperature precise to 0.1°C. Images were obtained over the incubator hood with the cutout. To facilitate temperature analysis, we adopted published techniques to measure the mean skin temperature over a desired region of interest (ROI). For our analysis, we used a segmentation tool to develop oval ROIs over the abdomen or thorax. Within each ROI, the ExaminIR® software calculated the mean skin temperature using every pixel in the ROI. We compared the mean abdominal skin temperature (Tabdomen) to the mean thoracic skin temperature (Tnon-abdomen) within each ROI.
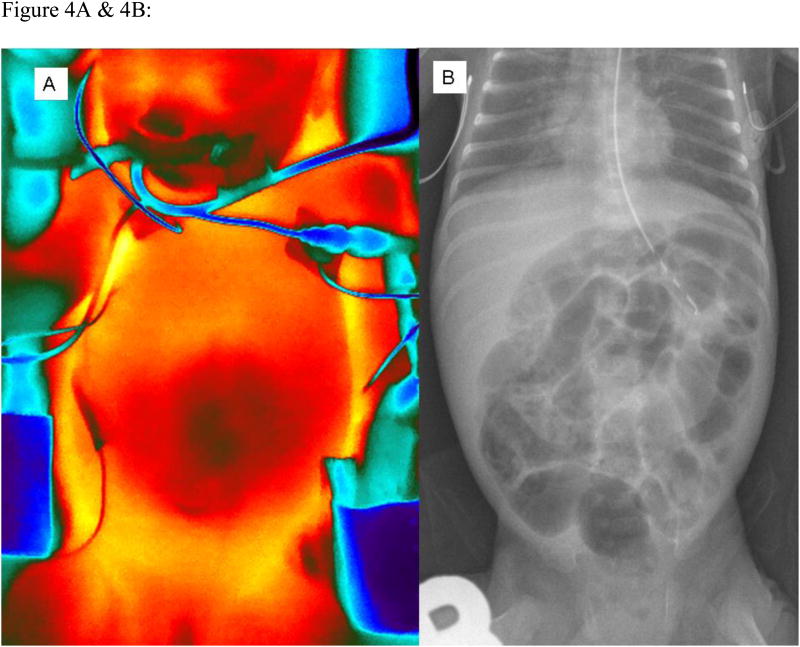
The scale and intensity of thermal images were controlled by software analysis tools (Fig 4A), and thermal images were compared to abdominal radiographs (Fig 4B). The infant presented in Figures 6A& 6B had the diagnosis of NEC. We had concurrent thermal images and abdominal radiographs in 10 infants, yielding 25 measurements. In these 10 infants, we found that those with the radiographic diagnosis of NEC (n=3) had lower abdominal skin temperature (35.3 ± 0.8 °C, 6 measurements) compared to those without evidence of NEC (n=7) (36.6 ± 0.9 °C, 19 measurements) (p < 0.05 by unpaired Student t-test)(Rice et al., 2010).
Figure 4.
A & 4B:Comparison of concurrent infrared thermal image (A) and abdominal radiograph (B) of a single ELBW infant with necrotizing enterocolitis. Images were taken on same day with head superior. Thermogram is taken with FLIR SC640 camera (FLIR, North Ballerica MA) and expressed using color palette. Bright, red-yellow areas (i.e. over thorax) correlate with higher temperature compared to darker, blue areas (ie. central area of abdomen, periphery around infant) which correlate with cooler temperature. Abdominal radiograph shows fixed featureless bowel segments, pneumatosis intestinalis and portal venous gas, consistent with NEC.
This pilot study confirmed the feasibility and safety of thermal imaging for study of skin temperature in ELBW infants. This technology could enhance ongoing surveillance of infants for diagnosis of NEC, by comparing daily images of abdominal and chest temperatures in infants that are suspect for signs and symptomss of NEC such as feeding intolerance, distended abdomens or stooling problems.
Future directions
Infrared thermal imaging is a novel technique to examine body temperature and tissue perfusion of adults, children and infants in research and the clinical setting. This technology is non-invasive, inexpensive and does not expose infants to harmful radiation effects. Research using infrared imaging technology is rapidly expanding, with many opportunities for research directions. Nurse researchers may be able to use thermal imaging for wound surveillance or recognition of skin temperature changes in association with decubitus ulcers. Temperature differentials may be obtained using infrared thermography between normal skin and skin that is beginning to be compromised from malpositioning or infection. These temperature differentials may be seen long before color changes identify compromised skin integrity.
Neonatal researchers may be able to use this technology in several areas of neonatal physiology, including the study of kangaroo care, which is heat transfer between the mother's chest and the swaddled infant, body cooling for the treatment of hypoxic-ischemic encephalopathy, and necrotizing enterocolitis. We are just beginning to use infrared thermal imaging to explore identification of intestinal necrosis in the operating room as more diagnostic tools are needed to help surgeons differentiate between ischemic and viable bowel. Researchers of the future may design studies to use infrared thermography in surveillance of perfusion after gastroschisis or omphalocele repair as was done by (Saxena & Wililital, 2008). Infrared thermography technology may decrease medical costs and length of stay for neonates by allowing clinicians early surveillance of skin temperature differentials associated with pathology such as NEC or poor perfusion or with postoperative healing after repair of abdominal defects and subsequent early treatment or feeding progression. Many more research and clinical uses will be revealed as researchers advance this methodology. This technology is in its infancy and research opportunities are endless.
Acknowledgments
Partially Funded By: AWHONN/March of Dimes: Saving Babies, Together® Grant; Children's Miracle Network and the Jean and George Brumley, Jr. Neonatal-Perinatal Research Institute (NPRI); National Institute of Health, NINR: R15: 1R15NR012157-01; & Robert Wood Johnson Foundation Nurse Faculty Scholars Grant
The authors would like to thank research assistants Elizabeth Bradsher, RN and Stephanie Crosby, RN for their involvement in the neonatal studies using infrared thermography. We would also like to thank Diane Holditch-Davis, PhD, RN, FAAN and the Research Development Group at Duke School of Nursing for their thoughtful review of this manuscript.
Contributor Information
Robin B. Knobel, Duke University School of Nursing and Jean and George Brumley, Jr. Neonatal-Perinatal, Research Institute.
Bob D. Guenther, Email: Bob.guenther@duke.edu, Duke University, Physics, Fizpatrick Photonics Center.
Henry E. Rice, Email: Rice0017@mc.duke.edu, Paul H. Sherman Associate Professor of Surgery; Chief, Division of Pediatric Surgery, Duke University Medical Center, Department of Surgery and George Brumley, Jr. Neonatal-Perinatal Research Institute.
References
- Adams AK, Nelson RA, Bell EF, Egoavil CA. Use of infrared thermographic calorimetry to determine energy expenditure in preterm infants. American Journal of Clinical Nutrition. 2000;71(4):969–977. doi: 10.1093/ajcn/71.4.969. [DOI] [PubMed] [Google Scholar]
- Bagavathiappan S, Saravanan T, John Philip T, Baldev Raj R, Karunanithi R, Panicker T, et al. Infared thermal imaging for detection of peripheral vascular disorders. Journal of Medical Physics. 2009;34(1):43–47. doi: 10.4103/0971-6203.48720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharara M, Cobb J, Claremont D. Thermography and thermometry in the assessment of diabetic neuropathic foot: A case for furthering the role of thermal techniques. The International Journal of Lower Extremity Wounds. 2006;5:250–260. doi: 10.1177/1534734606293481. [DOI] [PubMed] [Google Scholar]
- Blakely M, Lally K, McDonald S, Brown R, Barnhart D, Ricketts R. Postoperative outcomes of extremely low birth weight infants with necrotizing enterocolitis or isolated intestinal perforation. Anals of Surgery. 2005;241:984–994. doi: 10.1097/01.sla.0000164181.67862.7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charkoudian N. Mechanisms and modifiers of reflex induced cutaneous vasodilation and vasoconstriction in humans. Journal of Applied Physiology. 2010 doi: 10.1152/japplphysiol.00298.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang M, Lin P, Lin L, Chiou H, Chien C, Chu S, et al. Mass screening of suspected febrile patients with remote-sensing infrared thermography: Alarm temperature and optimal distance. Journal of the Formosan Medical Association. 2008;107:937–944. doi: 10.1016/S0929-6646(09)60017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu W, Lin P, Chiou H, Lee W, Lee C, Yang Y, et al. Infrared thermography to mass-screen suspected Sars patients with fever. Asia-Pacific Journal of Public Health. 2005;17:26–28. doi: 10.1177/101053950501700107. [DOI] [PubMed] [Google Scholar]
- Christidis I, Zotter H, Rosegger H, Engele H, Kurz R, Kerbl R. Infrared thermography in newborns: The first hour after birth. Gynakol Geburtshilfliche Rundsch. 2003;43:31–35. doi: 10.1159/000067168. [DOI] [PubMed] [Google Scholar]
- Clark R, Stothers J. Neonatal skin temperature distribution using infra-red coulour thermography. Journal of Physiology. 1980;302:323–333. doi: 10.1113/jphysiol.1980.sp013245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanouilides G, Moss A, Duffie EJ, Adams F. Pulmonary arterial pressure changes in human newborn infants from birth to 3 days of age. Journal of Pediatrics. 1964;65:327–333. doi: 10.1016/s0022-3476(64)80395-4. [DOI] [PubMed] [Google Scholar]
- Goetz C, Foertsch D, Schoenberger J, Uhl E. Thermography- a valuable tool to test hydrocephalus shunt patency. Acta Neurochirurgica. 2005;147:1167–1173. doi: 10.1007/s00701-005-0608-1. [DOI] [PubMed] [Google Scholar]
- Goodman P, Murphy M, Siltanen M, Kelley M, Rucker L. Normal temperature asymmetry of the back and extremeties by computer-assissted infrared imaging. Thermology. 1986;1:195–202. [Google Scholar]
- Hammarlund K, Sedin G. Transepidermal water loss in newborn infants: III. Relation to gestation age. Acta Paediatrica. 1979;68:795–801. doi: 10.1111/j.1651-2227.1979.tb08214.x. [DOI] [PubMed] [Google Scholar]
- Hammarlund K, Stomberg B, Sedin G. Heat loss from the skin of preterm and fullterm newborn infants during the first weeks after birth. Biology of the Neonate. 1986;50(1):1–10. doi: 10.1159/000242554. [DOI] [PubMed] [Google Scholar]
- Helmy A, Holdmann M, Rizkalla M. Application of thermography of non-invasive diagnosis of thyroid gland disease. IEEE Transactions on Biomedical Engineering. 2008;55:11681175. doi: 10.1109/TBME.2008.915731. [DOI] [PubMed] [Google Scholar]
- Herschel W. Investigation of the powers of the prismatic colours to heat and illuminate objects; with remarks that prove the different refrangibility of radiant heat. Philosophical Transactions. 1800;90:255–283. [Google Scholar]
- Houstek J, Vizek K, Pavelka S, Kopecky J, Krejcova E, Hermanska J, et al. Type II iodothyronine 5′-deiodinase and uncoupling protein in brown adipose tissue of human newborns. Journal of Clinical Endocrinology Metabolism. 1993;77(2):382–387. doi: 10.1210/jcem.77.2.8393883. [DOI] [PubMed] [Google Scholar]
- Jones BF. A reappraisal of the use of infrared thermal image analysis in medicine. IEEE Transactions on Medical Imaging. 1998;17(6):1019–1027. doi: 10.1109/42.746635. [DOI] [PubMed] [Google Scholar]
- Kateb B, Yamamoto V, Yu C, Grundfest W, Gruen J. Infrared thermal imaging: A review of the literature and case report. Neuroimage. 2009;47:T154–T162. doi: 10.1016/j.neuroimage.2009.03.043. [DOI] [PubMed] [Google Scholar]
- Katz LM, Nauriyal V, Nagaraj S, Finch A, Pearlstein K, Szymanowski A, et al. Infrared imaging of trauma patients for detection of acute compartment syndrome of the leg. Critical Care Medicine. 2008;36(6):1756–1761. doi: 10.1097/CCM.0b013e318174d800. [DOI] [PubMed] [Google Scholar]
- Knobel R, Holditch-Davis D, Schwartz T, Wimmer JE. Extremely low birth weight preterm infants lack vasomotor response in relationship to cold body temperatures at birth. Journal of Perinatology. 2009 doi: 10.1038/jp.2009.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon AJ, Pikaar ME, Badger P, McIntosh N. Temperature control in very low birthweight infants during first five days of life. Archives in Diseases of Childhood: Fetal/Neonatal Edition. 1997;76(1):F47–50. doi: 10.1136/fn.76.1.f47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massopust L. Infrared photographic study of the superficial veins of the thorax in relation to breast tumors; a preliminary report. Surgery, Gynecology & Obstetrics. 1948;86(1):54–58. [PubMed] [Google Scholar]
- Mercer J, Nielsen S, Hoffmann G. Improvement of wound healing by water-filtered infrared-A (wIRA) in patients with chronic venous stasis ulcers of the lower legs including evaluation using infrared thermography. German Medical Science. 2008;6(11) [PMC free article] [PubMed] [Google Scholar]
- Merla A, Mattei P, Di Donato L, Romani G. Thermal imaging of cutaneous temperature modifications in runners during graded exercise. Annals of Biomedical Engineering. 2010;38:158–163. doi: 10.1007/s10439-009-9809-8. [DOI] [PubMed] [Google Scholar]
- Miller L, Smith R. Synchronous versus globars, point-detectors versus focal plane arrays: Selecting the best source and detector for specific infrared microspectroscopy and imaging applications. Vibrational Spectroscopy. 2005;38:237–240. [Google Scholar]
- Mital M, Scott E. Thermal detection of embedded tumors using infrared imaging. Journal of Biomechanical Engineering. 2007;129:33–39. doi: 10.1115/1.2401181. [DOI] [PubMed] [Google Scholar]
- Nakanishi R, Imai-Matsumura K. Facial skin temperature decreases in infants with joyful expression. Infant Behavior & Development. 2008;31:137–144. doi: 10.1016/j.infbeh.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Nankervis C, Giannone P, Reber K. The neonatal intestinal vasculature: Contributing factors to necrotizing entercolitis. Seminars in Perinatology. 2008;32:83–91. doi: 10.1053/j.semperi.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Nozawa A, Tacano M. Correlation analysis on alpha attenuation and nasal skin temperauture. Journal of Statistical Mechanics: Theory and Experiment. 2009:1–10. [Google Scholar]
- Rice H, Hollingsworth C, Bradsher E, Danko M, Crosby S, Goldberg R, et al. Infrared thermal imaging (thermography) of the abdomen in extremely low birthweight infants. Journal of Surgical Radiology. 2010;1:82–89. [Google Scholar]
- Ring E. The historical development of temperature measurement in medicine. Infrared Physics & Technology. 2007;49:297–301. [Google Scholar]
- Ring E, McEvoy H, Jung A, Zuber J, Machin G. New standards for devices used for the measurement of human body temperature. Journal of Medical Engineering & Technology. 2010;34:249–253. doi: 10.3109/03091901003663836. [DOI] [PubMed] [Google Scholar]
- Sauer P. Thermoregulation of sick and low birth weight neonates. Germany: Springer-Verlag Berlin; 1995. [Google Scholar]
- Saxena A, Wililital G. Infrared thermography: experience from a decade of pediatric imaging. European Journal of Pediatrics. 2008;167(7):757–764. doi: 10.1007/s00431-007-0583-z. [DOI] [PubMed] [Google Scholar]
- Simbrunner G. Thermoregulation of sick and low birth weight neonates. Germany: Spring-Verlag; 1995. [Google Scholar]
- Steketee J. Spectral emissivity of skin and pericardium. Physics in Medicine and Biology. 1973;18(5):686–694. doi: 10.1088/0031-9155/18/5/307. [DOI] [PubMed] [Google Scholar]
- Stolwijk J. Heat exchangers between body and environment. Bibliotheca Radiologica. 1975;6:144–150. [PubMed] [Google Scholar]
- Tepper M, Neeman R, Milstein Y, David M, Gannot I. Thermal imaging method for estimating oxygen saturation. Journal of Biomedical Optics. 2009;14(5):1–12. doi: 10.1117/1.3251036. [DOI] [PubMed] [Google Scholar]
- Thomas KA. Preterm infant thermal responses to caregiving differ by incubator control mode. Journal of Perinatology. 2003;23(8):640–645. doi: 10.1038/sj.jp.7211002. [DOI] [PubMed] [Google Scholar]
- Uematsu S. Symmetry of skin temperature comparing one side of the body to the other. Thermology. 1986;1:4–7. [Google Scholar]
- Vainer B. The use of infrared thermography for investigation of thermoregulation in humans. In: Cisneros A, Goins B, editors. Body Temperature Regulation. New York: Nova Science Publishers, Inc; 2009. [Google Scholar]