Summary
Elucidating the role of the rodent hippocampus in object recognition memory is critical for establishing the appropriateness of rodents as models of human memory and for their use in the development of memory disorder treatments. In mammals, spatial memory [1–6] and non-spatial memory [7, 8] depend upon the hippocampus and associated medial temporal lobe (MTL) structures. Although well-established in humans [1, 9], the role of the rodent hippocampus in object memory remains highly debated due to conflicting findings across temporary and permanent hippocampal lesion studies [10–22] and evidence that the perirhinal cortex may support object memory [17, 23, 24]. In the current studies, we used intra-hippocampal muscimol microinfusions to transiently inactivate the male C57BL/6J mouse hippocampus at distinct stages during the novel object recognition (NOR) task: during object memory encoding and consolidation, just consolidation and/or retrieval. We also assessed the effect of temporary hippocampal inactivation when objects were presented in different contexts, thus eliminating the spatial/contextual components of the task. Lastly, we assessed extracellular dorsal hippocampal glutamate efflux and firing properties of hippocampal neurons while mice performed the NOR task. Our results reveal a clear and compelling role of the rodent hippocampus in non-spatial object memory.
Results
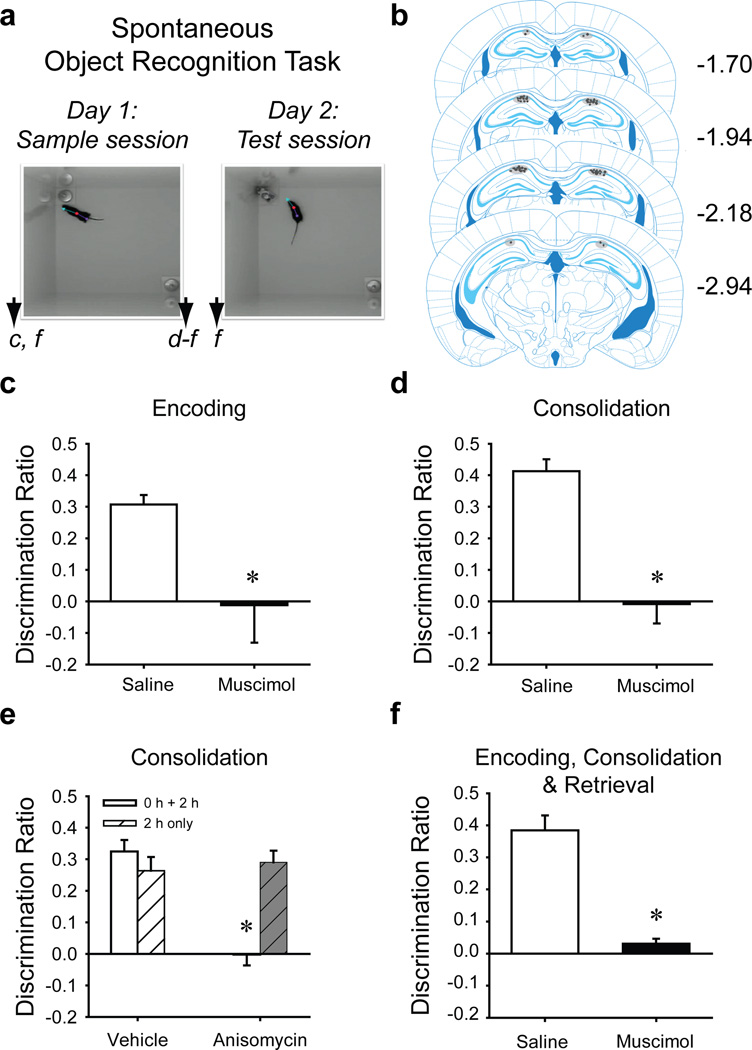
Mice were surgically implanted with intracranial infusion cannulae or recording electrodes at least one week before the onset of behavioral testing. The dorsal hippocampus was bilaterally inactivated at discrete time points relative to the NOR task: before the sample session to affect encoding and consolidation, after the sample session (consolidation) or before the test session (retrieval) (Fig. 1A). During the sample session, each mouse explored two identical objects until the object exploration criterion was reached: 30 s exploration of both objects or 38 s of either object within 10 min, except where otherwise noted. Similar latency to criterion between groups established equal motivation to explore objects. After 24 h, each mouse was given a 5-min test session with one familiar and one novel object. Preference for exploring the novel object was determined by calculating a discrimination ratio for each mouse (Discrimination Ratio=Tnovel − Tfamiliar/Tnovel+Tfamiliar). Discrimination ratios were analyzed for treatment differences in object memory. Cannula placements were verified histologically (Fig. 1B).
Figure 1. Encoding, Consolidation and Retrieval of Object Memory by C57BL/6J Mice Requires the Hippocampus (Exp’t 1–5).
a. Depiction of the NOR task sessions. Arrowheads indicate when intra-hippocampal infusions were given for specific experiments designated by lowercase letters corresponding to the respective graph (pre-sample, c, post-sample, d and e, or pre- and post-sample and pre-test, f). b. The distribution of intra-hippocampal infusion sites within the CA1 region of dorsal hippocampus for all experiments is depicted in gray shading against respective coronal plates from the Franklin & Paxinos atlas [50] (numbers refer to mm from bregma). c. Intra-hippocampal infusion of muscimol c. pre-sample (saline, n = 8; muscimol, n = 9) or d. post-sample session (saline, n = 12; muscimol, n = 11) significantly impaired novel object preference (i.e., object memory) during the test session 24 h later. Mice exhibited similar levels of object exploration during the test session: pre-sample vehicle 45 s, muscimol 37 s.; post-sample saline 39 s, muscimol 41 s. e. Intrahippocampal anisomycin immediately and 2 h after the sample session disrupted novel object preference (vehicle, n = 12; anisomycin, n = 15), although test session object exploration was similar: vehicle 45 s, anisomycin 38 s. However, object memory was spared in mice that received intra-hippocampal anisomycin only 2 h post-sample (vehicle, n = 12; anisomycin, n = 11), and again object exploration was similar: vehicle 45 s, anisomycin 37 s. f. Novel object preference was also impaired in mice given intra-hippocampal muscimol infusions pre-sample, post-sample, and pre-test, simulating a permanent hippocampal lesion (saline, n = 8; muscimol, n = 8). Test session object exploration was equivalent between the two groups: saline 40 s, muscimol 48 s.
Exp’t 1. Hippocampus is required for object memory encoding and consolidation
Naïve mice received intra-hippocampal muscimol or the saline vehicle 20 min before the sample session, ensuring hippocampal inactivation across encoding and into the consolidation stage [25]. Both groups reached sample session exploration criterion in similar times [saline 448 s, muscimol 360 s; t(11.52)=1.489, n.s.] and spent similar total amounts of time exploring test session objects t(15)=1.147, n.s.]. However, muscimol group discrimination ratios were significantly lower than those of the saline group [t(15)=2.47, P=0.026, Fig. 1C], suggesting that inactivation of the hippocampus 20 min prior to the sample session prevents encoding and/or consolidation of object memory.
Exp’t 2–4. Hippocampus is required for object memory consolidation
Naïve mice received intra-hippocampal muscimol or saline immediately after the sample session (Exp’t 2). Sample session latency to criterion was similar between future treatment groups [saline 469 s, muscimol 459 s; t(21)=1.93, n.s]. However, discrimination ratios of the muscimol group were significantly lower than those of the saline group [t(21)=5.93, P < 0.001, Fig. 1D]. Another cohort of mice received intra-hippocampal anisomycin both immediately and 2 h after the sample session to disrupt hippocampal protein synthesis during consolidation (Exp’t 3). Discrimination ratios of the anisomycin-treated mice were also significantly lower than those of the vehicle group: t(25)=6.51, P<0.001, Fig. 1E], consistent with a prior report [26]. NOR was spared in mice that only received intra-hippocampal anisomycin 2 h post-sample (Exp’t 4, Fig. 1E). Interestingly, intra-hippocampal anisomycin given 3 h, but not 6 h, post-sample impaired NOR [26], therefore, the precise dynamics of protein synthesis-dependent consolidation of object memory remain unclear. Together our results indicate that consolidation of object memory requires a functional hippocampus and hippocampal protein synthesis occurring <2 h after the sample session.
Exp’t 5. Hippocampal inactivation during all memory stages impairs NOR performance
To test our hypothesis that the frequently reported spared NOR after permanent hippocampal lesions is due to compensatory changes within the MTL, we inactivated the hippocampus during encoding, consolidation and retrieval phases. Naïve mice received intra-hippocampal muscimol or saline 20 min before and 2 h after the sample session and 20 min before the test session. Sample session latencies to criterion were equivalent [saline 474 s, muscimol 404 s; t(14)=1.13, n.s]; however, discrimination ratios were significantly lower in muscimol-treated mice than in saline-treated mice [t(8.46)=7.241, P < 0.001, Fig. 1F]. These results suggest that spared object memory in hippocampal-lesioned rodents is likely supported by compensatory changes.
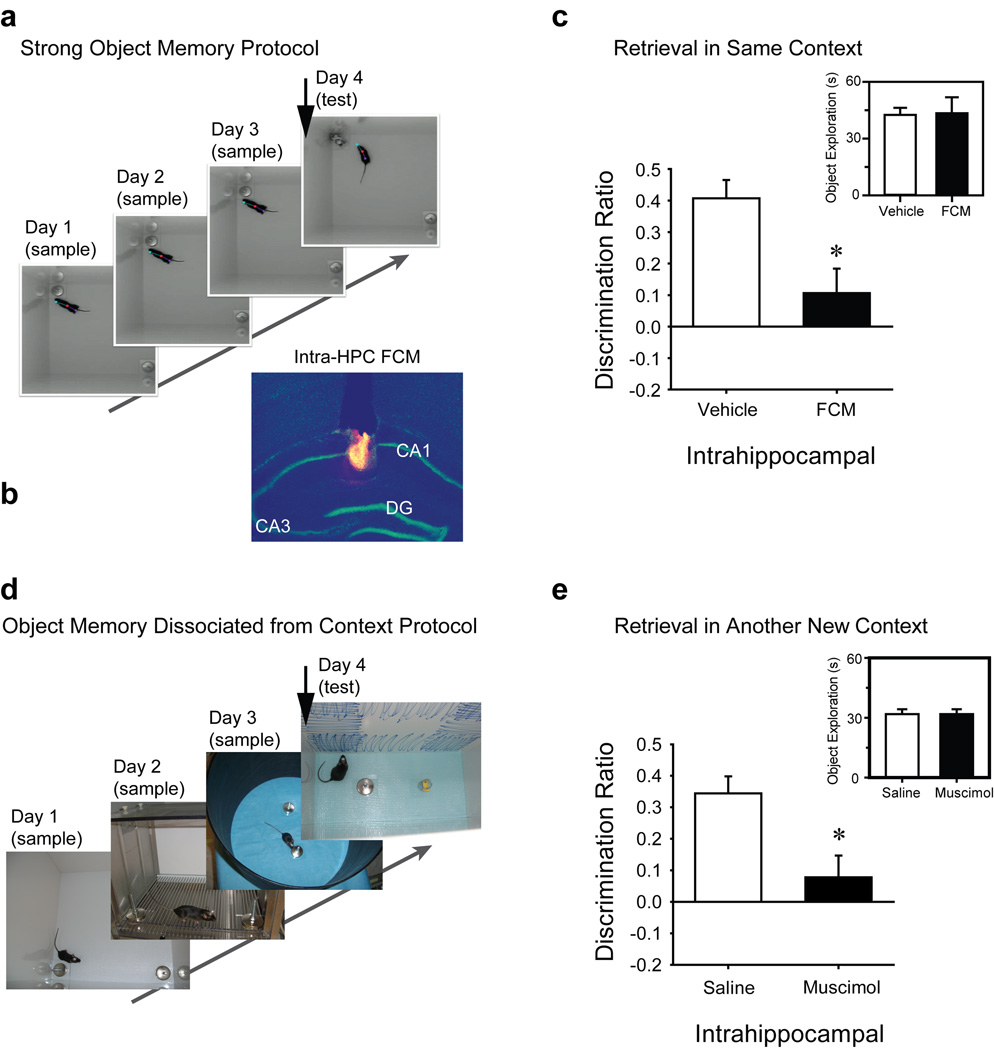
Exp’t 6 – 7. Inactivating hippocampus or changing context blocks retrieval of a strong object memory
Naïve mice received three 10-min sample sessions in the same arena (1/day, inset Fig 2A) to permit the encoding of a strong object memory. Mice received intra-hippocampal fluorophore-conjugated muscimol (Fig 2B, FCM, Molecular Probes, Eugene, OR) or vehicle 20 min before the test session (Exp’t 6). Groups exhibited similar object exploration across sample sessions [group × session, F2,28=1.46, n.s.; see Fig S1A] and in the test session [inset Fig 2C, t(14)=−0.09, n.s]; however, FCM group discrimination ratios were significantly lower than those of the vehicle group [t(14)=3.11, P = 0.008, Fig. 2C]. Examination of tissue sections revealed that at 30 min post-infusion, FCM spread in approximately a 300-µm radius from the estimated center of each infusion, not beyond the CA1 region of hippocampus (Fig. 1B). Assuming a spherical distribution at both infusion sites, FCM affected approximately 1% of the entire hippocampal volume [27]. These results indicate that the dorsal hippocampus is critical for retrieval of object memory, and very limited hippocampal inactivation is sufficient to impair NOR. To determine the significance of the context to the encoded strong object memory, a second cohort of mice received three 10-min sample sessions in the same arena on the same day (Exp’t 7, see Fig S1C and D) and a test session 24 h later in a novel arena. Neither pre-test intra-hippocampal saline or muscimol-treated mice exhibited a strong preference for exploring the novel object during the test session and there was no group difference in discrimination ratio [t(8)=0.19, n.s., Fig. S1E], presumably since rodents explore familiar objects more when presented in a novel context [15, 28, 29].
Figure 2. Hippocampal Inactivation Impairs the Retrieval of a Strong Object Memory (Exp’t 6) or Object Memory that is Independent of Context (Exp’t 8).
Modified NOR tasks were designed to test the role of the hippocampus in a. context-dependent and d. context-independent retrieval of strong object memory. Arrowhead in each montage indicates when the intra-hippocampal infusion was conducted. b. Representative spread of pretest intra-hippocampal fluorophore-conjugated muscimol (FCM) within the dorsal hippocampus. c. Pre-test session infusion of FCM (Exp’t 6) impaired object memory in mice that had received three 10-min sample sessions (1/day) in the same context (see photos in a), demonstrating hippocampal involvement in retrieving a strongly encoded object memory (saline, n = 8; FCM, n = 8). d. Modified NOR task in which mice explored two identical sample objects during three 10-min sample sessions (1/day), each in a distinct context. e. Pre-test intra-hippocampal muscimol impaired retrieval of object memory during the test session conducted in a novel context, demonstrating hippocampal involvement in retrieving object memory independent of context (saline, n = 9; muscimol, n = 8). Inset graphs of c, and e depict the test session total object exploration. *, P<0.01 vs. respective vehicle condition. Fig S1A–B depicts the total object exploration over all sessions as a function of treatment condition for both of these experiments. Fig S1C–E depicts results of Exp’t 7 in which mice received three sample sessions in the same arena (as in Exp’t 6 above); however, the test session was presented in a novel context.
Exp’t 8. Retrieval of object memory is impaired by hippocampal inactivation even when the memory was encoded in different contexts
The above findings are consistent with the view that the NOR deficit after lesion of the hippocampus is due to impaired spatial/contextual memory [13, 17], and that successful NOR among controls is supported by conjunctive object-inplace or object-in-context memory. If so, then presenting to-be-remembered objects in a different context each session should eliminate NOR among controls. Naïve mice received three 10-min sample sessions (1/day) with the same objects; each session was presented in a novel context (Contexts A–C, Fig 2F and S1B). Twenty-four hr later, mice received intra-hippocampal muscimol or vehicle 40 min prior to a test session (Context D). Vehicle group discrimination ratios were significantly greater than chance [t(9)=6.348, P < 0.001], but those of the muscimol group were not [t(8)=1.14, P > 0.2]. Mean discrimination ratios were significantly different between the two groups [t(17)=−3.09, P=0.007; Fig 2G], yet test session object exploration was similar (inset Fig 2G). Thus, vehicle-treated mice recognized the familiar object even in a novel context, but muscimol-treated mice did not. These results strongly support the hypothesis that the mouse hippocampus is necessary for retrieval of object memory even when independent of object-in-place/context conjunctive memory.
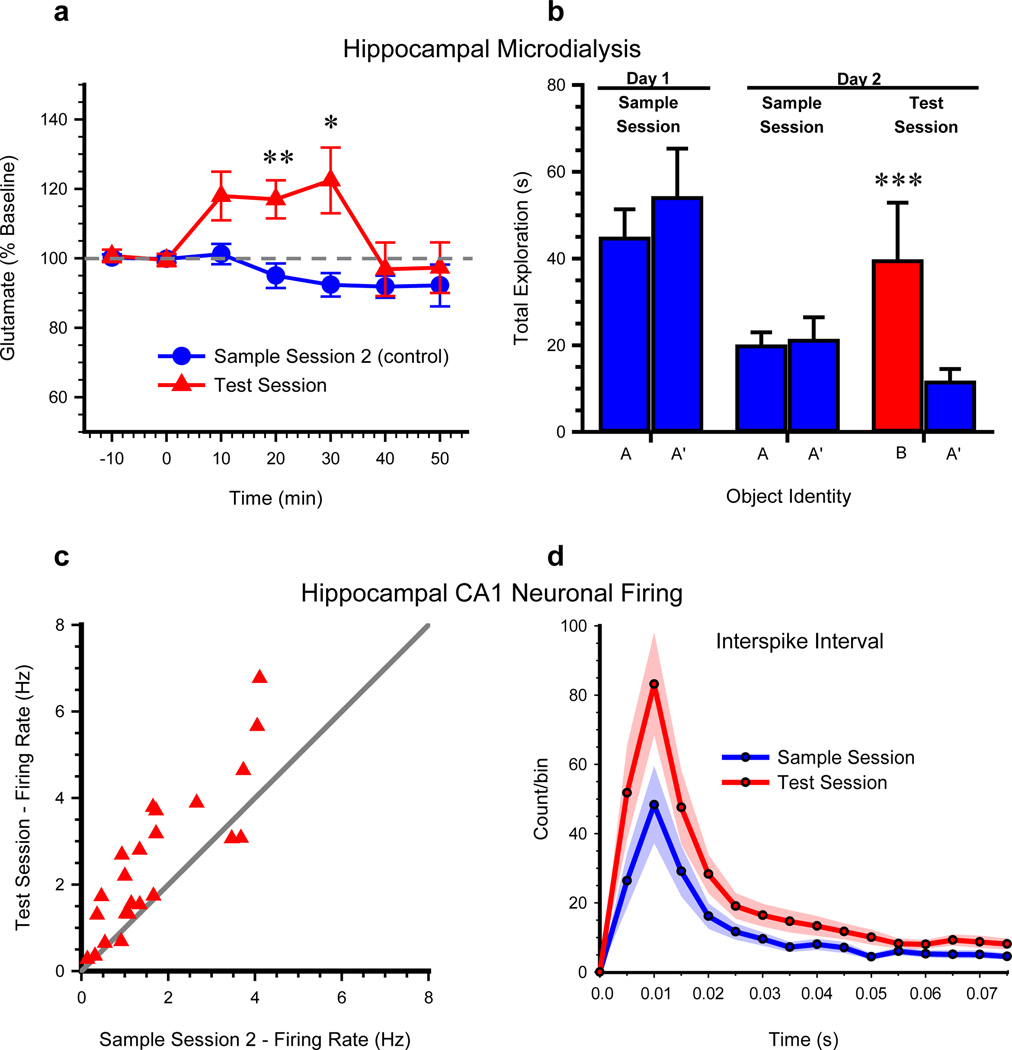
Exp’t 9. NOR task performance elevates extracellular dorsal hippocampal glutamate
To determine the degree to which this non-spatial task engaged the hippocampus physiologically, glutamate efflux was measured in dialysate samples acquired from the dorsal hippocampus during NOR. Naïve mice each received a 10-min sample session in the familiar arena. Twenty-four hr later, mice received a NOR test session or a second sample session (sample session 2) during in vivo microdialysis for hippocampal glutamate efflux. The test session and sample session 2 groups of mice exhibited similar latency to sample session criterion [t(10)=−0.56, n.s.] and equivalent basal hippocampal glutamate efflux [0.31 ± 0.06 µM and 0.34 ± 0.06 µM; t(10)=− 0.371, n.s.]. However, glutamate efflux was significantly higher in the test session mice than in the sample session 2 mice [see Fig. 3A; group, F1,10=7.12, P=0.024; time, F6, 60=3.65, P=0.004; and group × time interaction, F6, 60=3.39, P=0.006]. Locomotor activity was equivalent between groups [velocity t(10)=−0.013; n.s., distance traveled, t(10)=0.069; n.s.]. Thus, performance during an NOR test session significantly elevated hippocampal glutamate output.
Figure 3. NOR Task Performance Increases Extracellular Glutamate (Exp’t 8) and Firing Rates of CA1 Neurons in the Hippocampus (Exp’t 9).
All mice received a sample session on Day 1. a. On Day 2, microdialysate samples were collected from hippocampus as mice explored a familiar arena during a baseline period (−10 to 0 min) and then while mice performed either a test session or a second sample session. *, P < 0.05, **, P < 0.01 vs. sample session 2 group. b. Object exploration during respective 10-min recordings of CA1 neuron activity from mice (n = 12) on a linear track on Day 1 and 2. ***, P < 0.001 indicates successful NOR. c. Mean firing rates of 23 simultaneously recorded CA1 pyramidal neurons were significantly greater during the NOR test session, compared to the mean firing rates of the same neurons during the second sample session. d. Averaged interspike interval histogram of the 23 CA1 pyramidal neurons during the test session or second sample session. The shaded region shows the S.E.M. for each session average. See also Figure S2 for influence of NOR on location-specific firing of CA1 pyramidal neurons.
Exp’t 10–11. NOR task performance increases hippocampal CA1 neuronal activity
The activity of CA1 pyramidal neurons (Exp’t 10; n=23) was stable between two 10-min sample sessions (1/day) in which two identical objects were positioned on opposite ends of a familiar linear track (Fig. 3B). A test session was presented 10 min after the second sample session. Overall mean firing rates were significantly greater during the test session (2.52 ± 0.35 Hz) compared to the second sample session (1.70 ± 0.26 Hz), [t(22)=−4.48, P<0.001, Fig. 3C–D]. Velocity and distance traveled were similar across sessions (P>0.05). These results provide electrophysiological support for object recognition-related neuronal activity within the rodent hippocampus.
Hippocampal place cells fire when the rodent occupies a particular location within a given environment [5, 6]. Hippocampal CA1 place cell activity was recorded as mice explored a familiar arena containing a cue card (Exp’t 11). Place fields were stable in the familiar arena and the cue card exerted typical stimulus control over the positions of place fields (Fig. S2A and C – left panel). Next, the same place cells were recorded as mice performed NOR sample and test sessions in the same familiar arena (Fig. S2B and C – right panel). Firing rate maps (Fig. S2C) indicated that place fields did not remap during the NOR task and place cell firing rates (Fig. S2D) did not change during either the sample or test session [Fig. S1E, r=−0.008, t(3)=0.656, n.s.]. These results indicate that hippocampal place fields that are already established in a familiar environment are not significantly altered when the mouse subsequently engages in a non-spatial hippocampal-dependent task in that same environment. Taken together with the observed novelty-induced increase in overall firing rates of CA1 neurons, these findings support prior reports that objects influence CA1 neuronal activity [30, 31] and suggest the presence of CA1 neurons that fire in response to objects independent of location.
Discussion
These behavioral and physiological results establish that the rodent hippocampus is obligatory in object memory, corroborating literature regarding its role in non-spatial memory [9, 32, 33]. Our finding that disruption of approximately 1% of total hippocampal volume blocked object memory processes contradicts reports that permanent lesions of <75% of hippocampus spare NOR [10, 19]. However, such studies test what a hippocampal-lesioned rodent is capable of remembering (hippocampal-independent NOR) rather than whether object memory normally recruits the hippocampus. We argue that traditional lesions provide an adequate model of human amnesia, but are ill suited for delineating the hippocampal role in healthy memory processing.
If rats with permanent hippocampal lesions are repeatedly exposed to the same context, then extra-hippocampal structures can support contextual memory [34, 35]. Here, object memory encoded over three 10-min sample sessions remained sensitive to pre-test hippocampal inactivation, implying that preserved NOR in permanently lesioned rodents is due to compensatory plasticity rather than to normal extra-hippocampal capabilities. This view is bolstered by findings of the hippocampal inactivation during all stages study, which also confirms that our other findings are not due to state-dependent effects.
It has been argued that the NOR task merely tests conjunctive object-in-place or object-in-context memory, known to be impaired in hippocampal-lesioned rodents [13, 17]. To limit the possibility of place/context aiding object memory retrieval, mice were presented with the objects in four distinct arenas (3 sample, 1 test). If NOR performance is supported by intact spatial/contextual memory, then the control group would have been impaired in this experiment. Alternatively, if non-spatial NOR is supported exclusively by perirhinal cortex, then intra-hippocampal muscimol would not affect performance. We found that saline-treated mice, but not muscimol-treated mice, demonstrated significant novel object preference, indicating that the perirhinal cortex alone cannot support object memory. Thus, the spatial/contextual component of the task is likely not the primary determinant of whether temporary or permanent hippocampal lesion impairs NOR.
The argument for a double dissociation of perirhinal cortex and hippocampus posits that memory for objects independent of place/context selectively engages perirhinal cortex [36], while the conjunctive memory for objects in place/context depends on hippocampus [17, 37]. Thus familiarity, or knowing that an item was recently viewed, depends on perirhinal cortex, while recollection, or remembering distinct details about an episode, depends on hippocampus [38]. This hypothesis predicts that perirhinal cortex could support NOR performance despite a hippocampal lesion, but we found that NOR performance was impaired after hippocampal inactivation. Evidently, NOR was not supported by perirhinal-dependent familiarity. Our findings substantiate previous reports that hippocampal neurons discharge differentially to novel vs. familiar items, item identity and spatial location [39–41], further weakening the familiarity/recollection double dissociation theory. Alternatively, recognition memory may exist on a continuum from weak to strong, whereby the encoding, consolidation and retrieval of only strong memory (based on familiarity or recollection) is hippocampal-dependent [9]. Considering the sensitivity to hippocampal inactivation, the probed memory reported here appears to be a strong one. If a weak counterpart was available in perirhinal cortex, then it was too weak to influence behavior and was, therefore, negligible.
Evidence supporting the role of rodent perirhinal cortex in object memory is convincing [17], but does not eliminate a role for the hippocampus. Rather, lesions of perirhinal cortex may disrupt NOR by interfering with the flow of information through the MTL circuit. Unimodal (what/item) and polymodal (where/context) information streams are routed through perirhinal and parahippocampal cortices, respectively, to hippocampus [42], and are likely both critical for spatial and non-spatial memory functions of hippocampus. Consistent with this view, perirhinal cortex lesions disrupt the stability of rodent hippocampal place cells [43]. Considering the MTL’s dense interconnectedness [9], we propose that the labor of explicit memory is carried by collective participation of hippocampus, perirhinal cortex and associated regions, but stress that normal object memory processing indeed requires the hippocampus.
We also report physiological evidence that discrimination of novel from familiar objects engages hippocampus. The significant test session increase in both hippocampal glutamate efflux and mean firing rates of CA1 neurons is consistent with prior reports of novelty-induced increases in hippocampal activity [39, 40] and in vivo recording studies, which indicate that non-spatial events are represented by rodent hippocampal neurons [44]. Whether the increased glutamate efflux observed in mice that received a test session resulted from exposure to a novel object or from object discrimination task performance is unclear. Additional research is needed to elucidate the basis for the increased glutamate efflux during the test session; however, this was beyond the scope of the current study, which aimed to demonstrate that the NOR task activates the hippocampus physiologically. Our finding that NOR test session performance increased CA1 neuron firing rates is consistent with a report that hippocampal neuronal activity represents not only object location but also object identity [31]. Associating specific objects with specific locations [5] can aid in distinguishing one place from another, providing further evidence that the hippocampus supports a global record of experience by maintaining information about the relationships between specific objects encountered in distinct locations.
While numerous reports state that the hippocampus is not involved in NOR, several studies support our findings [14, 18–20, 22, 45]. Our results elaborate on the conclusions of these supporting studies by establishing the critical and independent contribution of dorsal hippocampal neural activity to discrete stages of object memory, even when that memory is devoid of contextual components. Further, the finding that the rodent hippocampus is involved in NOR is compatible with prior studies of other species, such as those assessing visual recognition memory in primates [46–49]. Considering the known role of the human and non-human primate hippocampus in recognition memory, it is likely that the rodent hippocampus plays a similar role. Our findings support this conclusion: the rodent hippocampus isn’t just for space anymore.
Supplementary Material
Highlights.
In mice the hippocampus is required for distinct stages of object memory processing
Retrieval of object memory independent of context also requires the hippocampus
Glutamate efflux measurements during NOR support hippocampal involvement in mice
NOR test session performance increased firing rates of hippocampal pyramidal neurons
Acknowledgements
We thank Dr. Rui Tao and Dr. Kathleen Guthrie for facilitating the microdialysis and epifluorescence work, respectively, and Dr. K. Matt Lattal for advising on the preparation of anisomycin. This work was supported by a grant from the NIH/NIMH (MH086591) to RWS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
S.J.C., L.M.R. and G.Z. designed and performed the experiments and analyzed data; R.W.S. designed the experiments and analyzed data; H.N.A. and A.H.M. performed experiments and analyzed data. All authors wrote the paper.
References
- 1.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 3.Morris RGM, Garrud P, Rawlins JNP, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 4.Riedel G, Micheau J, Lam AG, Roloff EL, Martin SJ, Bridge H, de Hoz L, Poeschel B, McCulloch J, Morris RG. Reversible neural inactivation reveals hippocampal participation in several memory processes. Nat Neurosci. 1999;2:898–905. doi: 10.1038/13202. [DOI] [PubMed] [Google Scholar]
- 5.O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Clarendon Press; 1978. [Google Scholar]
- 6.Moser EI, Kropff E, Moser MB. Place cells, grid cells, and the brain's spatial representation system. Annu Rev Neurosci. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- 7.Clark RE, West AN, Zola SM, Squire LR. Rats with lesions of the hippocampus are impaired on the delayed nonmatching-to-sample task. Hippocampus. 2001;11:176–186. doi: 10.1002/hipo.1035. [DOI] [PubMed] [Google Scholar]
- 8.Ross RS, Eichenbaum H. Dynamics of hippocampal and cortical activation during consolidation of a nonspatial memory. J Neurosci. 2006;26:4852–4859. doi: 10.1523/JNEUROSCI.0659-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ainge JA, Heron-Maxwell C, Theofilas P, Wright P, de Hoz L, Wood ER. The role of the hippocampus in object recognition in rats: examination of the influence of task parameters and lesion size. Behav Brain Res. 2006;167:183–195. doi: 10.1016/j.bbr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Barker GR, Warburton EC. When is the hippocampus involved in recognition memory? J Neurosci. 2011;31:10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duva CA, Floresco SB, Wunderlich GR, Lao TL, Pinel JP, Phillips AG. Disruption of spatial but not object-recognition memory by neurotoxic lesions of the dorsal hippocampus in rats. Behav Neurosci. 1997;111:1184–1196. doi: 10.1037//0735-7044.111.6.1184. [DOI] [PubMed] [Google Scholar]
- 13.Forwood SE, Winters BD, Bussey TJ. Hippocampal lesions that abolish spatial maze performance spare object recognition memory at delays of up to 48 hours. Hippocampus. 2005;15:347–355. doi: 10.1002/hipo.20059. [DOI] [PubMed] [Google Scholar]
- 14.Gaskin S, Tremblay A, Mumby DG. Retrograde and anterograde object recognition in rats with hippocampal lesions. Hippocampus. 2003;13:962–969. doi: 10.1002/hipo.10154. [DOI] [PubMed] [Google Scholar]
- 15.Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn. Mem. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mumby DG, Tremblay A, Lecluse V, Lehmann H. Hippocampal damage and anterograde object-recognition in rats after long retention intervals. Hippocampus. 2005;15:1050–1056. doi: 10.1002/hipo.20122. [DOI] [PubMed] [Google Scholar]
- 17.Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J. Neurosci. 2004;24:5901–5908. doi: 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broadbent NJ, Gaskin S, Squire LR, Clark RE. Object recognition memory and the rodent hippocampus. Learn. Mem. 2010;17:794–800. doi: 10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc. Natl. Acad. Sci. U.S.A. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J. Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliveira AM, Hawk JD, Abel T, Havekes R. Post-training reversible inactivation of the hippocampus enhances novel object recognition memory. Learn Mem. 2010;17:155–160. doi: 10.1101/lm.1625310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammond RS, Tull LE, Stackman RW. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol. Learn. Mem. 2004;82:26–34. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Winters BD, Bussey TJ. Transient inactivation of perirhinal cortex disrupts encoding, retrieval, and consolidation of object recognition memory. J Neurosci. 2005;25:52–61. doi: 10.1523/JNEUROSCI.3827-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Good MA, Barnes P, Staal V, McGregor A, Honey RC. Context- but not familiarity-dependent forms of object recognition are impaired following excitotoxic hippocampal lesions in rats. Behav Neurosci. 2007;121:218–223. doi: 10.1037/0735-7044.121.1.218. [DOI] [PubMed] [Google Scholar]
- 25.Bonnevie T, Dunn B, Fyhn M, Hafting T, Derdikman D, Kubie JL, Roudi Y, Moser EI, Moser MB. Grid cells require excitatory drive from the hippocampus. Nat Neurosci. 2013;16:309–317. doi: 10.1038/nn.3311. [DOI] [PubMed] [Google Scholar]
- 26.Rossato JI, Bevilaqua LR, Myskiw JC, Medina JH, Izquierdo I, Cammarota M. On the role of hippocampal protein synthesis in the consolidation and reconsolidation of object recognition memory. Learn Mem. 2007;14:36–46. doi: 10.1101/lm.422607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peirce JL, Chesler EJ, Williams RW, Lu L. Genetic architecture of the mouse hippocampus: identification of gene loci with selective regional effects. Genes Brain Behav. 2003;2:238–252. doi: 10.1034/j.1601-183x.2003.00030.x. [DOI] [PubMed] [Google Scholar]
- 28.Dix SL, Aggleton JP. Extending the spontaneous preference test of recognition: evidence of object-location and object-context recognition. Behav Brain Res. 1999;99:191–200. doi: 10.1016/s0166-4328(98)00079-5. [DOI] [PubMed] [Google Scholar]
- 29.Rudy JW. Context representations, context functions, and the parahippocampal-hippocampal system. Learn Mem. 2009;16:573–585. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cressant A, Muller RU, Poucet B. Further study of the control of place cell firing by intra-apparatus objects. Hippocampus. 1999;9:423–431. doi: 10.1002/(SICI)1098-1063(1999)9:4<423::AID-HIPO8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 31.Manns JR, Eichenbaum H. A cognitive map for object memory in the hippocampus. Learn Mem. 2009;16:616–624. doi: 10.1101/lm.1484509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fortin NJ, Agster KL, Eichenbaum HB. Critical role of the hippocampus in memory for sequences of events. Nat Neurosci. 2002;5:458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fortin NJ, Wright SP, Eichenbaum H. Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature. 2004;431:188–191. doi: 10.1038/nature02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piterkin P, Cole E, Cossette MP, Gaskin S, Mumby DG. A limited role for the hippocampus in the modulation of novel-object preference by contextual cues. Learn Mem. 2008;15:785–791. doi: 10.1101/lm.1035508. [DOI] [PubMed] [Google Scholar]
- 35.Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS. Context fear learning in the absence of the hippocampus. J Neurosci. 2006;26:5484–5491. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winters BD, Saksida LM, Bussey TJ. Object recognition memory: neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci Biobehav Rev. 2008;32:1055–1070. doi: 10.1016/j.neubiorev.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Bussey TJ, Duck J, Muir JL, Aggleton JP. Distinct patterns of behavioural impairments resulting from fornix transection or neurotoxic lesions of the perirhinal and postrhinal cortices in the rat. Behav Brain Res. 2000;111:187–202. doi: 10.1016/s0166-4328(00)00155-8. [DOI] [PubMed] [Google Scholar]
- 38.Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- 39.Otto T, Eichenbaum H. Neuronal activity in the hippocampus during delayed non-match to sample performance in rats: evidence for hippocampal processing in recognition memory. Hippocampus. 1992;2:323–334. doi: 10.1002/hipo.450020310. [DOI] [PubMed] [Google Scholar]
- 40.Rutishauser U, Schuman EM, Mamelak AN. Activity of human hippocampal and amygdala neurons during retrieval of declarative memories. Proc Natl Acad Sci USA. 2008;105:329–334. doi: 10.1073/pnas.0706015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wood ER, Dudchenko PA, Eichenbaum H. The global record of memory in hippocampal neuronal activity. Nature. 1999;397:613–616. doi: 10.1038/17605. [DOI] [PubMed] [Google Scholar]
- 42.Burwell RD. The parahippocampal region: corticocortical connectivity. Annals NY Acad Sci. 2000;911:25–42. doi: 10.1111/j.1749-6632.2000.tb06717.x. [DOI] [PubMed] [Google Scholar]
- 43.Muir GM, Bilkey DK. Instability in the place field location of hippocampal place cells after lesions centered on the perirhinal cortex. J Neurosci. 2001;21:4016–4025. doi: 10.1523/JNEUROSCI.21-11-04016.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 45.de Lima MN, Luft T, Roesler R, Schroder N. Temporary inactivation reveals an essential role of the dorsal hippocampus in consolidation of object recognition memory. Neurosci Lett. 2006;405:142–146. doi: 10.1016/j.neulet.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 46.Nemanic S, Alvarado MC, Bachevalier J. The hippocampal/parahippocampal regions and recognition memory: insights from visual paired comparison versus object-delayed nonmatching in monkeys. J Neurosci. 2004;24:2013–2026. doi: 10.1523/JNEUROSCI.3763-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeamer A, Meunier M, Bachevalier J. Stimulus similarity and encoding time influence incidental recognition memory in adult monkeys with selective hippocampal lesions. Learn Mem. 2011;18:170–180. doi: 10.1101/lm.2076811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pascalis O, Bachevalier J. Neonatal aspiration lesions of the hippocampal formation impair visual recognition memory when assessed by paired-comparison task but not by delayed nonmatching-to-sample task. Hippocampus. 1999;9:609–616. doi: 10.1002/(SICI)1098-1063(1999)9:6<609::AID-HIPO1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 49.Zola SM, Squire LR, Teng E, Stefanacci L, Buffalo EA, Clark RE. Impaired recognition memory in monkeys after damage limited to the hippocampal region. J Neurosci. 2000;20:451–463. doi: 10.1523/JNEUROSCI.20-01-00451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. 3rd Edition. San Diego, CA: Academic Press; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.