Abstract
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) has multiple intracellular activities in addition to its role in gluconeogenesis. Indeed, we have reported that GAPDH is required for Rab2-mediated retrograde transport from vesicular tubular clusters (VTCs). These diverse GAPDH activities are the result of post-translational modifications that confer a new function to the enzyme. In that regard, GAPDH is tyrosine phosphorylated by Src. To establish the functional significance of this modification for GAPDH activity in Rab2-dependent events, an amino acid substitution was made at tyrosine 41 (GAPDH Y41F). The inability of Src to phosphorylate purified recombinant GAPDH Y41F was confirmed in an in vitro kinase assay. The mutant was then employed in a quantitative membrane-binding assay that measures Rab2 recruitment of soluble components to VTCs. As we observed with GAPDH wild type, Rab2 promoted GAPDH Y41F binding to membranes in a dose-dependent manner indicating that GAPDH tyrosine phosphorylation is not required for VTC association. However, GAPDH was tyrosine phosphorylated on VTCs. Importantly, GAPDH Y41F blocked VSV-G transport in an assay that reconstitutes endoplasmic reticulum (ER) to Golgi trafficking indicating that phosphorylation of tyrosine 41 is essential for GAPDH activity in the early secretory pathway. The block in transport is due to the decreased binding of atypical protein kinase C iota/lambda (aPKCι/λ) to GAPDH Y41F, which reduces β-COP association with the VTC and subsequent formation of Rab2-mediated retrograde vesicles. Our results suggest that Src plays a pivotal role in regulating the interaction of Rab2 effectors on the VTC.
Keywords: Rab2, GAPDH, aPKC ι/λ, Src, VTC, β-COP
INTRODUCTION
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is a well-characterized key enzyme in glycolysis that catalyzes the NAD-mediated oxidative phosphorylation of glyceraldehyde-3-phosphate to 1,3 diphosphoglycerate (1). The structure of GAPDH has been elucidated and studies have identified two significant regions that include the NAD+ binding domain and the catalytic domain that binds substrate (1–4). It is well recognized that GAPDH has multiple intracellular activities in addition to its role in gluconeogenesis including participation in vesicular transport, membrane-membrane fusion, modulation of the cytoskeleton, DNA repair and replication, apoptosis, and tRNA export (5–9). Since GAPDH exists as a single copy gene, the diverse GAPDH activities are most likely the result of post-translational modifications that confer a new activity to GAPDH and therefore gain of function.
Indeed, we have reported that GAPDH is phosphorylated by atypical protein kinase C iota/lambda (aPKCι/λ) on vesicular tubular clusters (VTCs) (10). VTCs are pleiomorphic structures that sort endoplasmic reticulum (ER) derived anterograde-directed cargo for delivery to the Golgi complex from the trafficking machinery that recycles to the ER for reutilization in ER export (11–13). The small GTPase Rab2 bound to VTCs stimulates the recruitment of aPKCι/λ and GAPDH where both proteins interact directly with the Rab2 amino-terminus (14, 15). Rab2 mediates retrograde-vesicle formation from the VTC and this event is dependent on aPKCι/λ kinase activity (16, 17).
Based on previous reports demonstrating that the nonreceptor tyrosine kinase Src binds directly with aPKCι and that Src activity is required for retrograde transport in the early secretory pathway, studies were initiated to determine whether Rab2 required Src function at the VTC (18, 19). We found that Src-dependent tyrosine phosphorylation is essential for aPKCι/λ association with Rab2 and GAPDH (20). Tyrosine phosphorylation has been shown to control the assembly of multi-protein signaling complexes by recruiting proteins containing phospho-specific binding domains (21). Interestingly, GAPDH has also been reported to be tyrosine phosphorylated (22–25). A GAPDH peptide corresponding to residues 31–41 was identified by tandem mass spectrometry to contain phosphotyrosine at amino acid 41 (25). However, the functional role of this GAPDH modification has not been investigated.
In this study, we sought to determine the functional significance of Src-dependent GAPDH phosphorylation. To address this question, an amino acid substitution was made at tyrosine 41 in GAPDH (GAPDH Y41F), and then the purified recombinant mutant protein was characterized in a battery of biochemical assays that assess activity in Rab2-facilitated events. As we previously observed with GAPDH wild type, Rab2 promoted GAPDH Y41F binding to membranes in a quantitative binding assay that measures Rab2-dependent recruitment of soluble components. Although GAPDH tyrosine phosphorylation is not required for VTC association, the modification is essential for GAPDH activity at the VTC since the mutant protein effectively inhibited VSV-G transport between the ER and Golgi complex in an in vitro trafficking assay indicating an important regulatory role for Src at the VTC. Moreover, GAPDH tyrosine phosphorylation is necessary for GAPDH and aPKCι/λ interaction and aPKCι/λ association with membranes. The drastic reduction of VTC associated aPKCι/λ in the presence of GAPDH Y41F results in a concomitant lose of β-COP membrane binding and production of retrograde-directed vesicles. These data confirm the importance of post-translational processing of GAPDH in promoting compartment specific protein-protein interactions and unique cellular activity.
RESULTS
Rab2-Recruited GAPDH is Tyrosine Phosphorylated after Binding to NRK Membranes
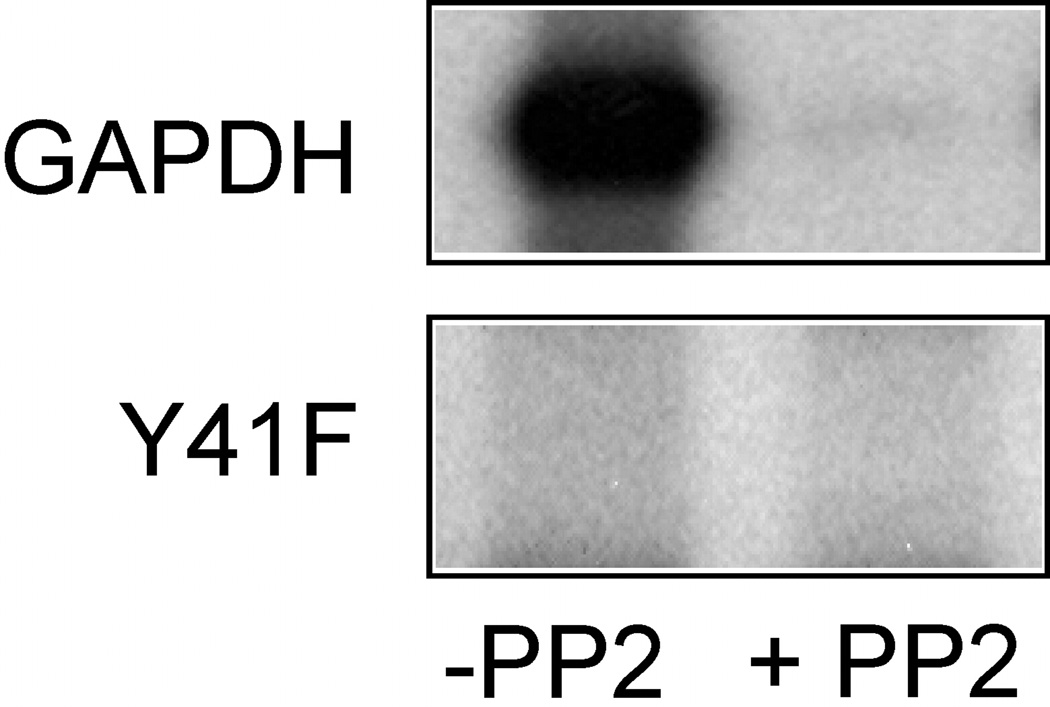
To be assured that purified recombinant GAPDH Y41F was not phosphorylated by Src, the mutant protein was introduced into an in vitro kinase assay (20). As we anticipated, recombinant GAPDH was efficiently tyrosine phosphorylated by Src and the phosphorylation was inhibited by the Src-specific kinase inhibitor 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo(3,4-d)pyrimidine (PP2) whereas no 32P-radiolabel was detected in GAPDH Y41F confirming that tyrosine 41 is modified by Src (Figure 1).
Figure 1. Src does not Phosphorylate GAPDH Y41F.
Src (0.1 µg) was incubated with purified recombinant GAPDH or GAPDH Y41F (0.5 µg) in 50 mM Tris (pH 7.4), 10 mM MgCl2, 3 mM MnCl2, 50 µM ATP, and 10 µCi [γ-32P]ATP for 15 min at 30°C in the absence or presence of 5 µM PP2. The reaction was stopped by the addition of 5X sample buffer, and then separated by SDS-PAGE followed by autoradiography.
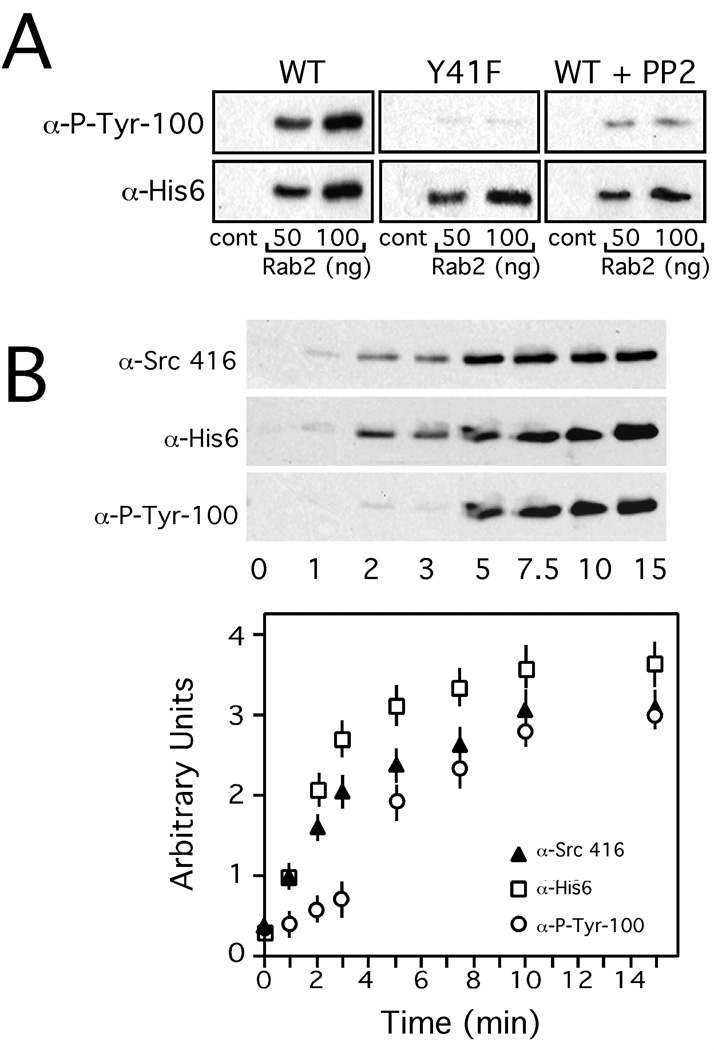
Since GAPDH is an Src substrate, a quantitative membrane binding assay was employed to determine whether Rab2 recruited GAPDH required tyrosine phosphorylation for membrane association. Salt-washed normal rat kidney (NRK) cell membranes were incubated with His6-GAPDH or His6-GAPDH Y41F, rat liver cytosol, and GTPγS in the absence or presence of increasing concentrations of purified recombinant Rab2, and then the membranes containing recruited proteins analyzed by SDS-PAGE and Western blot. As previously observed, Rab2 stimulated GAPDH membrane binding (Figure 2A). The membrane-associated His6-GAPDH is tyrosine phosphorylated as assessed by detection with a monoclonal antibody specific to phosphotyrosine that co-aligned to the chemiluminescent signal obtained with anti-His6 (Figure 2A). Likewise, Rab2 promoted GAPDH Y41F membrane binding suggesting that GAPDH association with VTCs is not dependent on tyrosine phosphorylation (Figure 2A). In further support of this interpretation, the membrane binding assay was supplemented with PP2. As shown in Figure 2A, PP2 treatment had no effect on Rab2 recruitment of His6-GAPDH and the amount of tyrosine phosphorylated His-GAPDH was negligible. It should be noted that PP2 does not interfere with Src membrane binding (20).
Figure 2. Membrane-Associated GAPDH is Tyrosine Phosphorylated.
Panel A. Salt-washed NRK membranes prepared as described under "Materials and Methods" were preincubated with recombinant GAPDH or recombinant GAPDH Y41F (75 ng) and with purified recombinant Rab2 at the concentrations indicated, and the reaction mix incubated on ice for 10 min. Rat liver cytosol and GTPγS were then added and the reactions shifted to 32°C and incubated for 12 min. To terminate the reaction, the membranes were collected by centrifugation, and the membrane pellet separated by SDS-PAGE, and then transferred to nitrocellulose. The blot was probed with an anti-phosphotyrosine monoclonal antibody (P-Tyr-100), washed, further incubated with HRP-conjugated secondary antibody, and then developed with ECL. The blot was stripped and reprobed with a monoclonal antibody to His6, and processed as above. A representative Western blot from one of three independent experiments is shown for both antibodies. Panel B. Salt-washed NRK membranes were incubated at 32°C for increasing time with His6-GAPDH (75 ng), purified recombinant Rab2 (100 ng), cytosol, and GTPγS. At the time indicated, the membranes were shifted to ice, pelleted, separated by SDS-PAGE, and then transferred to nitrocellulose. The blot was probed with anti-Src 416 (activated Src), and anti-phosphotyrosine monoclonal antibody, and processed as above. The blot was then stripped and reprobed with anti-His6 monoclonal antibody and developed as above. The developed blots were quantitated by densitometry. A representative Western blot from one of three independent experiments is shown.
We performed a time course by incubating NRK membranes with Rab2 and His6-GAPDH for increasing times to determine when activated Src is detected on membranes by a monoclonal antibody specific to phosphotyrosine 416 (26). The activated form of Src is found on membranes after ~ 2 minutes of incubation and reached saturation after ~ 10 minutes of incubation (Figure 2B). Although, His6-GAPDH can also be detected on the membranes after ~ 2 minutes of incubation, GAPDH tyrosine phosphorylation occurs at ~ 4–5 minutes after incubation suggesting that the timing of this modification may play an important regulatory role at the VTC (Figure 2B).
Src-Dependent GAPDH Phosphorylation is Required for ER to Golgi Transport
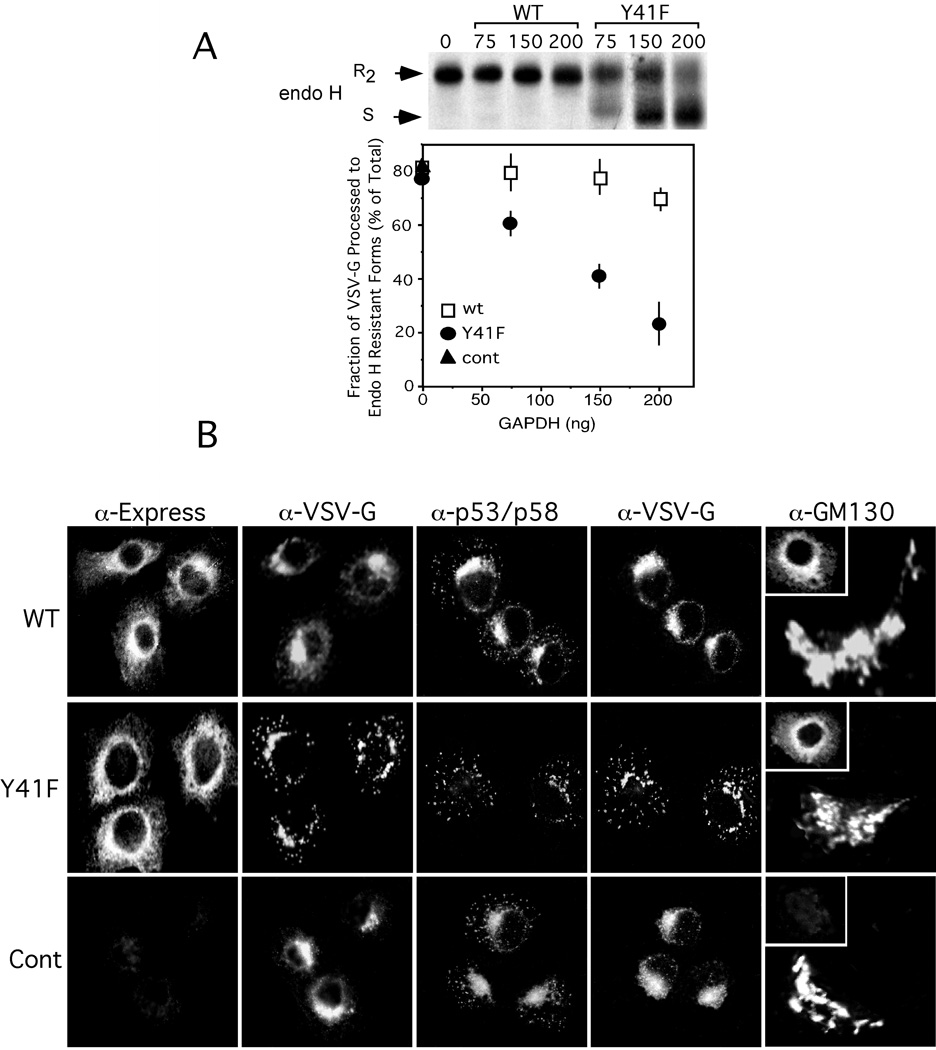
To study the effect of GAPDH Y41F on membrane trafficking in the early secretory pathway, we introduced the recombinant protein into an in vitro transport assay (27). For this assay, tissue culture cells are first infected with ts045 VSV-G, a virus that synthesizes a protein with a thermoreversible defect resulting in ER retention at 39.5°C. The plasma membrane of these cells is then perforated to release soluble content, but retain functional ER and Golgi stacks. Incubation of the semi-intact cells at the permissive temperature of 32°C, initiates export of ts045 VSV-G from the ER and transport of VSV-G protein is measured by following the processing of the two N-linked oligosaccharides to endo H-resistant forms (R2). VSV-G transport was not affected when the transport assay was performed in the presence of His6-GAPDH (Figure 3). In contrast, His6-GAPDH Y41F caused a dose dependent inhibition in VSV-G acquisition of endo H resistance (Figure 3A). VSV-G transport was arrested ~ 67% in the presence of 200 ng GAPDH Y41F.
Figure 3. Src-Dependent GAPDH Phosphorylation is Required for ts045 VSV-G Transport in the Early Secretory Pathway.
Panel A. NRK cells infected with ts045 VSV-G were metabolically labeled with [35S]-methionine at 39.5°C for 10 min. The cells were permeabilized, shifted to 32°C, and then chased in radiolabel-free media for 90 min in the absence or presence of increasing concentrations of recombinant GAPDH or GAPDH Y41F. ts045-VSV-G was immunoprecipitated from detergent-lysed cells and incubated in the absence (−) or presence (+) of endo H and then analyzed by SDS-PAGE and fluorography. The endo H resistant forms of ts045 VSV-G (R2) were quantitated using a Phosphorimager. A representative autoradiograph from one of three independent experiments is shown. Panel B. NRK cells grown on coverslips were infected with ts045 VSV-G for 2–3 h at 39.5°C. The cells were rapidly shifted to ice, permeabilized with digitonin, and then incubated in a complete transport cocktail in the presence of recombinant GAPDH (200 ng) or GAPDH Y41F (200 ng) for 40 min at 32°C. The intracellular distribution of ts045 VSV-G, recombinant GAPDH wt or mutant (inset), p53/p58, and GM130 was determined as described under "Materials and Methods."
To define the morphological site of VSV-G accumulation in response to GAPDH Y41F, NRK cells were infected with ts045 VSV-G for 2 h at the nonpermissive temperature. The cells were then permeabilized and incubated in the absence or presence of recombinant GAPDH or GAPDH Y41F for 40 min at 32°C, and the distribution of VSV-G was determined by indirect immunofluorescence. Consistent with the biochemical data, cells incubated with recombinant GAPDH efficiently transported VSV-G to the Golgi complex whereas cells incubated with GAPDH Y41F accumulated VSV-G in large perinuclear structures and vesicular elements scattered throughout the cytoplasm that co-distributed with the some of the perinuclear structures and vesicles that labeled with anti-p53/p58 (Figure 3B). The morphology of the Golgi complex in cells treated with either wild type or mutant recombinant protein was comparable to control cells as assessed by labeling with anti-GM130 (Figure 3B). These combined biochemical and morphological results are highly suggestive that GAPDH activity at the VTC is dependent on Src-tyrosine phosphorylation.
Substitution of Tyrosine 41 in GAPDH Affects GAPDH-aPKCι/λ Interaction
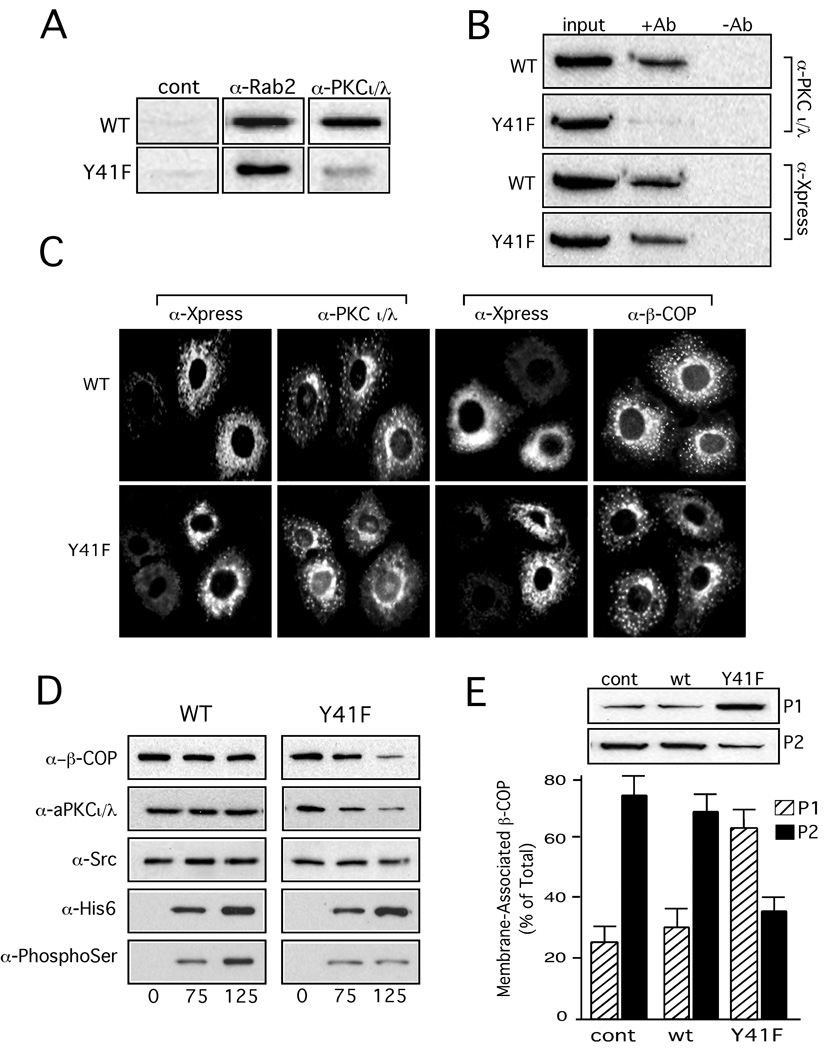
Based on our previous studies that showed GAPDH bound directly to aPKCι/λ and Rab2, a blot overlay assay was employed to determine whether GAPDH Y41F interaction with aPKCι/λ and Rab2 was compromised (10, 15). His6-GAPDH and His6-GAPDH Y41F were separated on SDS-PAGE, transferred to nitrocellulose, and then the blot incubated in overlay buffer supplemented with purified recombinant Rab2 or aPKCι/λ. The bound proteins were detected after incubation with antibodies specific to either Rab2 or aPKCι/λ followed by a secondary HRP-conjugated antibody and development with ECL. Figure 4A shows that GAPDH Y41F interacts with Rab2. This result was anticipated based on the observation that Rab2 recruited to membrane GAPDH Y41F. However, the mutant protein had negligible binding with aPKCι/λ suggesting that phosphotyrosine 41 in GAPDH is necessary for GAPDH-aPKCι/λ association. To determine whether GAPHD-aPKCι/λ interaction in vivo is compromised by the mutation, the detergent cell lysate from NRK cells overexpressing His6-GAPDH wild type or His6-GAPDH Y41F was incubated with anti-His6 antibody. We found that GAPDH and aPKCι/λ co-immunoprecipitated whereas insignificant interaction was detected between His6-GAPDH Y41F-aPKCι/λ as assessed by Western blot analysis of the immune complex (Figure 4B).
Figure 4. Tyrosine Phosphorylation of GAPDH 41 is Required for Interaction with aPKCι/λ to Promote β-COP Recruitment and Subsequent Vesicle Formation.
Panel A. Purified recombinant GAPDH and GAPDH Y41F (5 µg) were separated by SDS-PAGE and transferred to nitrocellulose, and then the blot incubated overnight at 4°C in renaturation buffer as described under "Materials and Methods." The blot was washed in TBS, and then incubated overnight at 4°C in 50 mM Tris (pH 7.4), 0.1% BSA, 200 mM NaCl, 20 µg/ml phosphatidylserine, and 10 µg/ml purified recombinant Rab2 or purified PKCι. Control was incubated in the absence of Rab2 or PKCι. After incubation, the blot was washed with TBS, and then probed with an anti-Rab2 polyclonal antibody or an anti-aPKCι/λ monoclonal antibody, washed, further incubated with an HRP-conjugated secondary antibody, and then developed with ECL. Panel B. HeLa cell lysate from vTF7-3 infected/pET15B GAPDH or GAPDH Y41F transfected cells was incubated without or with anti-His6 monoclonal antibody and Protein G plus/Protein A agarose for 4 h at RT. The immune complexes were collected by centrifugation, washed, and analyzed as described under "Methods and Materials." The blot was probed with an anti-PKCι/λ monoclonal antibody and an anti-Xpress monoclonal antibody, washed, incubated with HRP-conjugated secondary antibody, and then developed with ECL. The input lane contains one tenth of the total lysate. Panel C. NRK cells grown on coverslips were transfected with recombinant GAPDH (1 µg) or recombinant GAPDH Y41F (1 µg) using Chariot protein transfection reagent, as described under "Materials and Methods." The cells were incubated for 4 h at 37°C in a 5% CO2 incubator, shifted to 15°C for 45 min to accumulate VTCs, and then fixed in 3% formaldehyde/FBS and immunostained with anti-aPKCι antibody/Texas red anti-mouse or anti-β-COP and FITC-conjugated anti-Xpress antibody (inset). Panel D. Salt-washed NRK membranes were preincubated with the indicated concentrations of purified recombinant His6-GAPDH or His6-GAPDH Y41F and 100 ng purified recombinant Rab2 for 10 min on ice. Rat liver cytosol and GTPγS were added and the reactions shifted to 32°C and incubated for 12 min. To terminate the reaction, the membranes were collected by centrifugation, and the membrane pellet separated by SDS-PAGE, and then transferred to nitrocellulose. The blot was probed with an anti-β-COP polyclonal antibody (EAGE), anti-Src monoclonal antibody, anti-PKCι/λ monoclonal antibody, and anti-phosphoserine monoclonal antibody, washed, further incubated with HRP-conjugated secondary antibody, and then developed with ECL. The blot was then stripped and reprobed with anti-His6 monoclonal antibody and then developed, as above. Panel E. Salt-washed NRK membranes were preincubated with purified recombinant GAPDH (100 ng) or GAPDH Y41F (100 ng) and with Rab2 (300 ng) for 10 min on ice. Cytosol and GTPγS were added and the binding reaction incubated for 12 min at 32°C. Membranes were collected by centrifugation (35,000 × g for 20 min) to obtain a pellet (P1). The supernatant was re-centrifuged at 100,000 × g for 60 min and the resultant pellet (P2) and P1 were separated by SDS-PAGE, immunoblotted with an anti-β-COP polyclonal antibody, and the blot was developed with ECL. The results are representative of three independent experiments.
Since GAPDH cDNA transfected cells accumulate large GAPDH aggregates in the cytosol within 12–24 h post-transfection (data not shown) (28), our alternative approach to investigate the in vivo distribution of recombinant GAPDH-aPKCι/λ was to perform protein transfection as described under "Materials and Methods" (10, 28). Both GAPDH and GAPDH Y41F distributed to a perinuclear ring that extended into the cytoplasm and which contained some large vesicular elements. Additionally, anti-Xpress labeled vesicles were also observed scattered in the cytosol of cells transfected with either His6/Xpress-GAPDH or His6/Xpress-GAPDH Y41F (Figure 4C). The anti-aPKCι/λ monoclonal antibody also stained a perinuclear ring that appeared to overlap with anti-Xpress stained elements in cells containing GAPDH or GAPDH Y41F. However, the aPKCι/λ labeled perinuclear collar was not as prominent in cells that contained GAPDH Y41F compared to cells transfected with GAPDH wild type. Moreover, aPKCι/λ labeled peripherally located vesicles were not displayed in cells containing GAPDH Y41F suggesting that aPKCι/λ association with peripheral structures/VTCs is affected by the mutant protein (Figure 4C).
These combined findings prompted us to investigate the effect of GAPDH Y41F on Rab2-dependent aPKCι/λ recruitment to membranes. The membrane binding assay was performed with increasing concentrations of His6-GAPDH or His6-GAPDH Y41F, and then the amount of recruited aPKCι/λ determined as described under "Materials and Methods." GAPDH Y41F caused a dose dependent inhibition in Rab2-stimulated aPKCι/λ embrane binding (Figure 4D). In contrast, the amount of membrane associated activated Src was not effected by the GAPDH mutant (Figure 4D). The inability of GAPDH Y41F to interact with aPKCι/λ suggested that GAPDH would no longer be phosphorylated by the kinase. Indeed, when the Western blot from the membrane binding assay employing GAPDH Y41F was probed with an anti-phosphoSerine monoclonal antibody, a weak signal was observed for membrane-associated phosphoSer-GAPDH Y41F (Figure 4D). As we found previously when aPKCι/λ binding is reduced, there was a concomitant decrease in membrane-associated β-COP (Figure 4D). This decrease in β-COP binding is also reflected in the reduction (~ 64% decrease) of Rab2-generated vesicles produced in the presence of GAPDH Y41F (Figure 4E). Moreover, NRK cells protein transfected with GAPDH Y41F displayed a β-COP phenotype consistent with the biochemical results in which the β-COP-labeling of perinuclear structures and peripheral elements is significantly reduced compared to cells containing GAPDH wild type (Figure 4C). These collective results indicate that GAPDH-aPKCι/λ interaction on the VTC is critical for the downstream recruitment of β-COP.
DISCUSSION
Several in vitro and in vivo studies have reported that GAPDH is tyrosine phosphorylated. Reiss et al. showed that GAPDH served as substrate for purified epidermal growth factor-receptor kinase (23). It was also demonstrated by phosphoamino acid analysis that GAPDH was tyrosine phosphorylated by tyrosine kinase related to lymphode cell kinase (29). Likewise, Sergienko et al. found that rabbit muscle GAPDH contained phosphotyrosine (24). NIH 3T3 cells overexpressing v-Src or c-Src modified GAPDH when analyzed by an immune complex kinase assay (22). The amino-terminus of GAPDH contains three tyrosine residues located with the first 50 amino acids that are potential targets for a tyrosine kinase. Indeed, recent studies using mass spectrometry to identify immunoaffinity purified tyrosine phosphorylated peptides from cancer cells reported that residue 41 in GAPDH was modified (25). However, in all these studies the functional role of this modification in GAPDH activity was not addressed.
Based on the observation that residue 41 in GAPDH was phosphorylated, we generated by site-directed mutagenesis the corresponding mutant protein. Src-dependent phosphorylation of GAPDH was abolished when GAPDH Y41F protein was employed in an in vitro kinase assay. Elimination of the phosphorylated tyrosine residue in GAPDH had no effect on recruitment and binding to membranes indicating that Src phosphorylates GAPDH on the VTC. However, tyrosine phosphorylation of GAPDH is essential for GAPDH activity in the early secretory pathway. We found that GAPDH Y41F was an effective trans-dominant inhibitor of ER to Golgi traffic when introduced into an in vitro transport assay. Unlike the results obtained by Bard et al., our observations indicate that Src activity is required for VSV-G trafficking in the early secretory pathway (19). The discrepancy may be due to the sensitivity of their assay that required subtraction of background fluorescence in the Golgi region from the ER to quantify VSV-G transport into the Golgi. In that regard, GAPDH Y41F treated cells displayed a significant amount of ts045 VSV-G that distributes to a juxtanuclear position that co-labels with antibody to p53/p58. The results of the biochemical assay indicated that VSV-G, which accumulated perinuclear in GAPDH Y41F treated cells is blocked prior to entry into the cis/medial Golgi compartments. Since anterograde and retrograde transport is coupled, it seems unlikely as reported by Bard et al., that KDEL transport to the ER would be affected by Src whereas forward transport of VSV-G to the Golgi was unaffected by Src deletion (19).
GAPDH interacts directly with the amino-terminus of Rab2 and with the regulatory domain of aPKCι/λ (14, 15). We found that Rab2-GAPDH interaction is not affected by the mutation. In contrast, GAPDH-aPKCι/λ interaction is significantly compromised when tyrosine 41 is mutated. Moreover, membranes incubated with GAPDH Y41F showed a drastic reduction in associated aPKCι/λ. We previously reported that PP2 addition to the membrane binding assay significantly reduced aPKCι/λ recruitment to membranes whereas Rab2, GAPDH, and Src membrane binding was not affected by the inhibitor (20). Therefore, we proposed that Src-mediated tyrosine phosphorylation of aPKCι/λ was a key signal in maintaining aPKCι/λ on the VTC. Based on these new studies, our results suggest that GAPDH tyrosine phosphorylation is the critical determinant that assures GAPDH-aPKCι/λ interaction and aPKCι/λ function on the VTC.
The post-translational modification of tyrosine phosphorylation has profound consequences on a protein and coordinates the assembly of signaling complexes that control various cellular activities including proliferation, differentiation, migration and apoptosis (21). Tyrosine phosphorylated GAPDH may serve as an adaptor protein that stabilizes aPKCι/λ binding to the Rab2 amino-terminus. This interpretation is consistent with our previous finding that Rab2 requires an additional cytosolic component that acts in concert to cause aPKCι/λ translocation to the membrane (17). Src-phosphorylation of aPKCι/λ on the VTC may then promote a conformational change that results in aPKCι/λ serine phosphorylation of GAPDH. Indeed, we reported that Rab2 caused a dramatic reduction of aPKCι/λ mediated GAPDH phosphorylation in an in vitro assay whereas GAPDH phosphorylation by aPKCι/λ occurs on the VTC (10, 14).
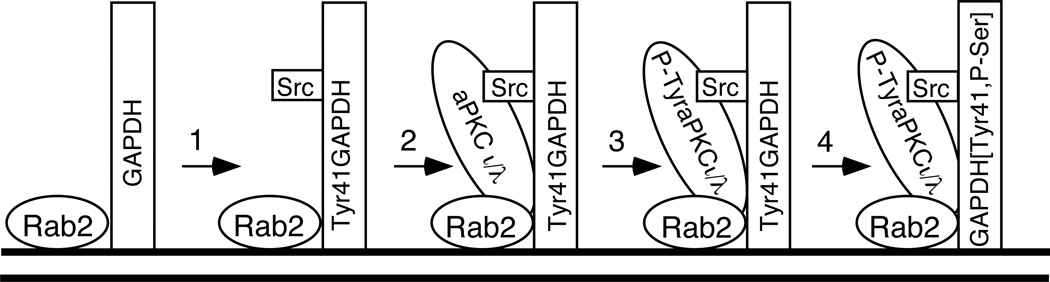
We propose that the negative regulation imposed by Rab2 on aPKCι/λ is alleviated by Src-dependent aPKCι/λ tyrosine phosphorylation. There are five Src-dependent phosphorylation sites in aPKCι/λ (18). At present, we do not know which residue(s) in aPKCι/λ is modified or is required for interaction with Rab2-GAPDH-Src on the VTC. Studies are in progress to delineate the essential tyrosine residue(s) in aPKCι/λ, which will allow us to validate our hypothesis (Figure 5).
Figure 5. Model for Rab2 Recruitment of Downstream Effectors and Subsequent Phosphorylation Events.
VTC associated Rab2-GAPDH (1) promotes recruitment of Src that phosphorylates tyrosine 41 in GAPDH, (2) aPKCι/λ binds to P-Tyr41 GAPDH and Rab2, and (3) Src phosphorylates aPKCι/λ to stabilize aPKCι/λ-GAPDH-Src-Rab2 interaction on the VTC, (4) aPKCι/λ phosphorylates GAPDH. We propose that Rab2-Src-aPKCι/λ;-GAPDH are components of a signaling complex that defines and regulates the site of vesicle budding and microtubule directed movement from the VTC.
MATERIALS AND METHODS
Quantitative Membrane-Binding Assay
NRK cells were washed three times with ice-cold PBS, scraped off the dish with a rubber policeman into 10 mM Hepes (pH 7.2)/250 mM mannitol, and then broken with 10 passes through a cell homogenizer using a 7.992 mm tungsten carbide ball (8 micron clearance) (Isobiotech, Heidelberg, Germany). The broken cells were centrifuged at 500 × g for 10 min at 4°C, and the supernatant removed and re-centrifuged at 35,000 × g for 30 min at 4°C in a Sorvall Discovery M120 ultracentrifuge. The pellet was washed with 1 M KCl in 10 mM Hepes (pH 7.2) for 15 min on ice to remove peripherally associated proteins, and then re-centrifugation at 35,000 × g for 30 min at 4°C. The resultant pellet was resuspended in 10 mM Hepes (pH 7.2) and 250 mM mannitol and employed in the binding reaction, as described previously (30). Membranes (~ 30 µg of total protein) were added to a reaction mixture that contained 27.5 mM Hepes (pH 7.2), 2.75 mM MgOAc, 65 mM KOAc, 5 mM EGTA, 1.8 mM CaCl2, 1 mM ATP, 5 mM creatine phosphate, and 0.2 IU rabbit muscle creatine phosphokinase. His trap HP (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) purified recombinant His6-GAPDH or His6-GAPDH Y41F and recombinant Rab2 (16) was added at the concentrations indicated under “Results” and the reaction mix incubated on ice for 10 min. Rat liver cytosol (~ 50 µg total protein) and 2.0 µM GTPγS or GTP were then added, and the reactions shifted to 32°C and incubated for 12 min. The binding reaction was terminated by transferring the samples to ice, and then centrifuged at 35,000 × g for 20 min at 4°C to obtain a pellet (P1). For some experiments, the supernatant was recentrifuged at 100,000 × g for 60 min to recover released vesicles (P2). P1 and P2 were separated by SDS-PAGE and transferred to nitrocellulose in 25 mM Tris (pH 8.3), 192 mM glycine, and 20% methanol. The membrane was blocked in Tris-buffered saline (TBS) that contained 5% nonfat dry milk or 5% bovine serum albumin and 0.5% Tween-20, and then incubated with a monoclonal antibody to Src (GD11) (Upstate, Lake Placid, NY), or activated Src (Tyr416) (Cell Signaling Technology, Inc., Beverly, MA), or a monoclonal antibody to GAPDH (Chemicon Intl., Temecula, CA), or a monoclonal antibody to aPKCι/λ (BD Biosciences, San Diego, CA), or a monoclonal antibody to His-Tag (27E8) (Cell Signaling Technology, Inc.), or a monoclonal antibody to phosphotyrosine (P-Tyr-100) (Cell Signaling Technology, Inc.), or an affinity purified polyclonal antibody to β-COP (EAGE) (30), washed, further incubated with a horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse antibody, developed with enhanced chemiluminescence (ECL) (GE Healthcare), and then quantified by densitometry.
Construction of GAPDH Mutant
The site-directed mutation was made in GAPDH by a two-step PCR procedure involving two complementary mutagenic oligonucleotides in combination with flanking 5' and 3' primers. In the first reaction, overlapping 5' and 3' fragments were produced using pDual GAPDH as the template (10). The 5' mutagenic oligonucleotide (5'-GACCCCTTCATTGACCTCAACTTCATGGTTTACATG-3') and the 3' wild-type antisense oligonucleotide that included a BamHI site (5'-GGATCCTTACTCCTTGGAGGCCATGTGGGC-3') was used to produce the 3' portion of the molecule. The 5' portion of the molecular was generated using a 5' oligonucleotide (5'-CACCATGGGGAAGGTGAAGGTCGGAGTC-3') in combination with the 3'-mutagenic oligonucleotide primer (5'-CATGTAAACCATGAAGTTGAGGTCAATGAAGGGGTC-3'). The two PCR products were combined to generate the full-length mutant in a second reaction using the respective 5' sense and 3' antisense primers. Full length GAPDH Y41F was subcloned into pET100/D-TOPO (His6/Xpress tag) (Invitrogen, Carlsbad, CA), and then the sequence verified by automated DNA sequence analysis. The transient expression and purification of GAPDH wild type and GAPDH Y41F were as previously described (20).
Overlay Binding Assay
His trap HP (GE Healthcare) purified recombinant His6-GAPDH (5 µg) and His6-GAPDH Y41F (5 µg) was separated by SDS-PAGE and transferred to nitrocellulose. The blot was incubated overnight at 4°C in 50 mM Hepes (pH 7.4), 50 mM MgOAc, 100 mM KOAc, 0.1% TX-100, 0.3% Tween 20, and 10 mg/ml bovine serum albumin to renature the proteins. The blot was washed 2X with TBS, and then incubated overnight at 4°C in 50 mM Tris (pH 7.4), 0.1% BSA, 200 mM NaCl, 20 µg/ml phosphatidylserine (Avanti Polar Lipids, Inc., Alabaster, AL) and 10 µg/ml purified recombinant Rab2 or purified aPKCι (10, 31). After incubation, the blot was washed with TBS, and then probed with an anti-Rab2 polyclonal antibody or an anti-aPKCι/λ monoclonal antibody (BD Biosciences), washed, further incubated with an HRP-conjugated secondary antibody, and then developed with ECL.
Immunoprecipitation
HeLa cells (1 × 106) infected with vaccinia T7 TNA polymerase recombinant virus (vTF7-3)(32) were transfected with pET15B-GAPDH or pET15B-GAPDH Y41F as previously described (33). 5 h post-transfection the cells were lysed in 50 mM TBS (pH 8.0) and 1% Triton X-100 for 10 min on ice, and then clarified by centrifugation at 20,000 × g for 10 min at 4°C. The soluble fraction was precleared with Protein G plus/Protein A agarose (Novagen, Madison, WI) and subjected to immunoprecipitation for 4 h at RT without or with an anti-His6 monoclonal antibody (Cell Signaling Technology, Inc.) and Protein G plus/Protein A agarose. The immune complexes were collected by centrifugation at 5,000 RPM for 5 min, washed 3X with TBS, 1% Triton X-100, and 100 mM NaCl, and then boiled in sample buffer. The immunoprecipitates were separated by SDS-PAGE and transferred to nitrocellulose. The blot was blocked in TBS that contained 5% BSA and 0.5% Tween-20, and incubated with a monoclonal antibody to aPKCι/λ (BD Biosciences), or with an anti-Xpress monoclonal antibody (Invitrogen), washed, further incubated with a horseradish peroxidase (HRP)-conjugated anti-mouse antibody, and then developed with ECL (Amersham).
Src Kinase Assay
Purified human Src (100 ng) (Upstate) was incubated with purified recombinant GAPDH or GAPDH Y41F (0.5 µg) in 50 mM Tris ( pH 7.4), 10 mM MgCl2, 3 mM MnCl2, 50 µM ATP, and 10 µCi [γ-32P]ATP (Perkin Elmer Life Sciences, Inc., Boston, MA) for 15 min at 30°C in the absence or presence of 5 µM PP2 (EMB Biosciences Inc., San Diego, CA) (20). The reaction was stopped by the addition of 5X sample buffer, and then separated by SDS-PAGE followed by autoradiography.
Analysis of Transport, in vitro
NRK cells were infected for 4 h with the temperature sensitive vesicular stomatitis virus (VSV) strain ts045, and then biosynthetically radiolabeled with 100 µCi Expre35S35S (spec act. 1175 Ci/mmol, PerkinElmer Life Sciences) for 10 min at the restrictive temperature (39.5°C) to maintain the VSV-G mutant protein in the ER. The cells were perforated by swelling and scraping, and then employed in the ER to cis/medial Golgi transport assay as described (27). Transport reactions were performed in a final volume of 40 µl in a buffer which contains 25 mM Hepes-KOH (pH 7.2), 75 mM KOAc, 2.5 mM MgOAc, 5 mM EGTA, 1.8 mM CaCl2, 1 mM N-acetylglucosamine, an ATP-regeneration system (1 mM ATP, 5 mM creatine phosphate and 0.2 IU rabbit muscle creatine phosphokinase), 5 µl rat liver cytosol, and 5 µl of semi-intact cells (~5×107 cells/ml, ~25–30 µg/ml total protein), resuspended in 50 mM Hepes-KOH, 90 mM KOAc (pH 7.2). The reactions were supplemented without or with the indicated concentration of purified recombinant GAPDH or GAPDH Y41F, incubated at 32°C for 90 min, and then transferred to ice to terminate transport. The cells were pelleted by centrifugation, solubilized in buffer and digested with endoglycosidase H (endo H) (New England Biolabs, Beverly, MA). The samples were analyzed by SDS-PAGE and the fraction of ts045 VSV-G protein processed to the endo H resistant forms quantitated by a Storm PhosphorImager.
Indirect Immunofluorescence
NRK cells plated on coverslips were infected with ts045 VSV-G at 39.5°C for 2–3 h, and then shifted to ice and permeabilized with digitonin (20 µg/ml) as outlined previously (16, 34). Coverslips with permeabilized cells were inverted and placed in tissue culture wells that contained the transport cocktail described above, supplemented with purified recombinant GAPDH (200 ng) or with GAPDH Y41F (200 ng), and then incubated for 40 min at 32°C. To terminate transport, the coverslips were transported to ice and fixed in 3% formaldehyde/PBS for 10 min. Intracellular ts045-VSV-G was detected by re-permeabilization of the fixed cells with 0.05% saponin in PBS and 5% normal goat serum for 10 min, washed twice with PBS, and than incubated for 30 min with a monoclonal antibody to VSV-G (P5D4) (Sigma-Aldrich, St. Louis, MO), or to anti-p53/p58 (35) or with anti-GM130 (BD Sciences). The cells were washed 3X with PBS, incubated with Texas Red anti-mouse antibody and FITC-conjugated anti-Xpress antibody (Invitrogen) for 1 h, washed, mounted in Prolong antifade (Molecular Probes, Eugene, OR), and then viewed under a Zeiss Axiovert fluorescence microscope. Alternatively, NRK cells were plated overnight on coverslips, and then transfected with purified recombinant GAPDH (1 µg) or purified recombinant GAPDH Y41F (1 µg) using Chariot protein transfection reagent (Active Motif, Carlsbad, CA). Briefly, GAPDH or GAPDH Y41F was diluted in PBS (100 µl total volume), then added to the Chariot reagent pre-diluted 1:100 in H2O, (total volume 100 µl), and incubated for 30 min at RT to allow the Chariot-GAPDH complexes to form. The cells were washed 3X with PBS, and the coverslips containing the cells inverted and placed over a drop containing the macromolecular complex. The transfected cells were incubated for 4 h at 37°C in a 5% CO2 incubator, and then shifted to 15°C for 45 min. The cells were fixed and washed as above, and then incubated for 30 min with a monoclonal antibody to aPKCι/λ (BD Biosciences) or anti-β-COP (EAGE peptide) (30), and then incubated with the secondary reagents as described above. The transfection efficiency was ~ 52% and in 46% of the cells the input protein induced a phenotypic change.
ACKNOWLEDGEMENTS
This work was supported by National Institute of Health grants GM068813 (to EJT), DK58921, and DK57637 (to CRA).
Abbreviations
- ER
endoplasmic reticulum
- VTC
vesicular tubular cluster
- aPKCι/λ
atypical protein kinase C ι/λ
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- NRK
normal rat kidney
- VSV-G
vesicular stomatitis virus glycoprotein
- β-COP
β-coat protein
- GTPγS
guanosine 5'-O-(thiotriphosphate)
- TBS
Tris-buffered saline
- PP2
4-amino-5-(4-chlorophenyl)-7-(t-butyl) pyrazolo (3,4-d) pyrimidine
REFERENCES
- 1.Harris J, Waters MG. Glyceraldehyde-3-phosphate dehydrogenase. New York: Academic Press; 1976. [Google Scholar]
- 2.Skarzynski T, Moody P, Wonacott A. Structure of holo-glyceraldehyde-3-phosphate dehydrogenase from bacillus st earothermophilus at 1.8 A Resolution. J. Mol. Biol. 1987;193:171–187. doi: 10.1016/0022-2836(87)90635-8. [DOI] [PubMed] [Google Scholar]
- 3.Yun M, Park C-G, Kim J-Y, Park H-W. Structural analysis of glyceraldehyde-3-phosphate dehydrogenase from e. coli: Direct evidence of substrate binding and cofactor-induced conformationl changes. Biochem. 2000;39:10702–10710. doi: 10.1021/bi9927080. [DOI] [PubMed] [Google Scholar]
- 4.Nagy E, Henics T, Eckert M, Miseta A, Lightowlers R, Kellermayer M. Identification of the NAD+-binding fold of glyceraldehyde-3-phosphate dehydrogenase as a novel RNA-binding domain. Bioch. Biophys. Res. Comm. 2000;275:253–260. doi: 10.1006/bbrc.2000.3246. [DOI] [PubMed] [Google Scholar]
- 5.Robbins A, Ward R, Oliver C. A mutation in glyceraldehyde-3-phosphate dehydrogenase alters endocytosis in CHO cells. J. Cell Biol. 1995;130:1093–1104. doi: 10.1083/jcb.130.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tisdale EJ. Glyceraldehyde-3-phosphate dehydrogenase is required for vesicular transport in the early secretory pathway. J. Biol. Chem. 2001;276:2480–2486. doi: 10.1074/jbc.M007567200. [DOI] [PubMed] [Google Scholar]
- 7.Chuang DM, Hough C, Senatorov VV. Glyceraldehyde-3-phosphate dehydrogenase, apoptosis, and neurodegenerative diseases. Ann. Rev. Pharmacol. Toxicol. 2005;45:269–290. doi: 10.1146/annurev.pharmtox.45.120403.095902. [DOI] [PubMed] [Google Scholar]
- 8.Sirover M. New nuclear functions of the glycolytic protein, glyceraldehyde-3-phosphate dehydrogenase, in mammalian cells. J. Cell. Biochem. 2005;95:45–52. doi: 10.1002/jcb.20399. [DOI] [PubMed] [Google Scholar]
- 9.Sirover MA. New insights into an old protein: the functional diversity of mammalian glyceraldehyde-3-phosphate dehydrogenase. Biochim Biophys Acta. 1999;1432:159–184. doi: 10.1016/s0167-4838(99)00119-3. [DOI] [PubMed] [Google Scholar]
- 10.Tisdale EJ. Glyceraldehyde-3-phosphate dehydrogenase is phosphorylated by PKCι/λ and plays a role in microtubule dynamics in the early secretory pathway. J. Biol. Chem. 2002;277:3334–3341. doi: 10.1074/jbc.M109744200. [DOI] [PubMed] [Google Scholar]
- 11.Balch WE, McCaffery JM, Plutner H, Farquhar MG. Vesicular stomatitis virus glycoprotein is sorted and concentrated during export from the endoplasmic reticulum. Cell. 1994;76:841–852. doi: 10.1016/0092-8674(94)90359-x. [DOI] [PubMed] [Google Scholar]
- 12.Altan-Bonnet N, Sougrat R, Lippincott-Schwartz J. Molecular basis for Golgi maintenance and biogenesis. Curr. Op. Cell Biol. 2004;16:364–372. doi: 10.1016/j.ceb.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Lee MCS, Miller EA, Goldberg J, Orci L, Schekman R. Bi-directional protein transport between the ER and Golgi. Annu. Rev. Cell Dev. Biol. 2004;20:87–123. doi: 10.1146/annurev.cellbio.20.010403.105307. [DOI] [PubMed] [Google Scholar]
- 14.Tisdale EJ. Rab2 interacts directly with atypical protein kinase C (aPKCι/λ) ι/λ and inhibits aPKCι/λ-dependent glyceraldehyde-3-phosphate dehydrogenase phosphorylation. J. Biol. Chem. 2003;278:52524–52530. doi: 10.1074/jbc.M309343200. [DOI] [PubMed] [Google Scholar]
- 15.Tisdale EJ, Kelly C, Artalejo CR. Glyceraldehyde-3-phosphate dehydrogenase interacts with Rab2 and plays an essential role in endoplasmic reticulum to Golgi transport exclusive of its glycolytic activity. J. Biol. Chem. 2004;279:54046–54052. doi: 10.1074/jbc.M409472200. [DOI] [PubMed] [Google Scholar]
- 16.Tisdale EJ. A Rab2 mutant with impaired GTPase activity stimulates vesicle formation from pre-Golgi intermediates. Mol. Biol. Cell. 1999;10:1837–1849. doi: 10.1091/mbc.10.6.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tisdale EJ. Rab2 requires PKCι/λ to recruit β-COP for vesicle formation. Traffic. 2000;1:702–712. doi: 10.1034/j.1600-0854.2000.010903.x. [DOI] [PubMed] [Google Scholar]
- 18.Wooten M, Vandenplas M, Seibenhener ML, Geetha T, Diaz-Meco M. Nerve growth factor stimulates multisite tyrosine phosphorylation and activation of the atypical protein kinase C's via a Src kinase pathway. Mol. Cell Biol. 2001;21:8414–8427. doi: 10.1128/MCB.21.24.8414-8427.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bard F, Mazelin L, Pechoux-Longin C, Malhotra V, Jurdic P. Src regulates Golgi structure and KDEL receptor-dependent retrograde transport to the endoplasmic reticulum. J. Biol. Chem. 2003;278:46601–46606. doi: 10.1074/jbc.M302221200. [DOI] [PubMed] [Google Scholar]
- 20.Tisdale EJ, Artalejo CR. Src-dependent aprotein kinase C ι/λ (aPKC ι/λ) tyrosine phosphorylation is required for a PKCι/λ association with Rab2 and glyceraldehyde-3-phosphate dehydrogenase on pre-Golgi intermediates. J. Biol. Chem. 2006;281:8436–8442. doi: 10.1074/jbc.M513031200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yaffe M. Phosphotyrosine-binding domains in signal transduction. Nat. Rev. 2002;3:177–186. doi: 10.1038/nrm759. [DOI] [PubMed] [Google Scholar]
- 22.Coussens P, Cooper JA, Hunter T, Shalloway D. Restriction of the in vitro and in vivo tyrosine protein kinase activities of pp60 c-Src relative to pp60 v-Src. Mol. Cell Biol. 1985;5:2753–2763. doi: 10.1128/mcb.5.10.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reiss N, Kanety H, Schlessinger J. Five enzymes of the glycolytic pathway serve as substrates for purified epidermal-growth-factor-receptor kinase. Biochem. J. 1986;239:691–697. doi: 10.1042/bj2390691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sergienko E, Kharitonenkov A, Bulargina T, Muronetz V, Nadradova N. D-Glyceraldehyde-3-phosphate dehydrogenase purified from rabbit muscle contains phosphotyrosine. FEBS Lett. 1992;304:21–23. doi: 10.1016/0014-5793(92)80580-a. [DOI] [PubMed] [Google Scholar]
- 25.Rush J, Moritz A, Lee K, Guo A, Goss V, Spek E, Zhang H, Zha X-M, Polakiewicz R, Comb MJ. Immunoaffinity profiling of tyrosine phosphorylation in cancer cells. Nature Biotech. 2005;23:94–101. doi: 10.1038/nbt1046. [DOI] [PubMed] [Google Scholar]
- 26.Bjorge J, Jakymiw A, Fujita D. Selected glimpses into the activation and function of Src kinase. Oncogene. 2000;19:5620–5635. doi: 10.1038/sj.onc.1203923. [DOI] [PubMed] [Google Scholar]
- 27.Beckers CJM, Keller DS, Balch WE. Semi-intact cells permeable to macromolecules: use in reconstitution of protein transport from the endoplasmic reticulum to the Golgi complex. Cell. 1987;50:523–534. doi: 10.1016/0092-8674(87)90025-0. [DOI] [PubMed] [Google Scholar]
- 28.Ishitani R, Tajima H, Takata H, Tsuchiya K, Kuwae T, Yamada M, Takahashi h, Tatton N, Katsube N. Proapoptotic protein glyceraldehyde-3-phosphate dehydrogenase: a possible site of action of antiapoptotic drugs. Prog. in Neuro-Psychopharm. 2003;27:291–301. doi: 10.1016/S0278-5846(03)00024-1. [DOI] [PubMed] [Google Scholar]
- 29.Gartner T, Kuhnel H, Raab G, Raab M, Strebhardt K, Rubsamen-Waigmann H. A strong protein-tyrosine kinase activity is associated with a baculovirus-expressed chicken tkl gene. Eur. J. Biochem. 1992;208:91–100. doi: 10.1111/j.1432-1033.1992.tb17162.x. [DOI] [PubMed] [Google Scholar]
- 30.Tisdale EJ, Jackson MR. Rab2 protein enhances coatomer recruitment to pre-Golgi intermediates. J. Biol. Chem. 1998;273:17269–17277. doi: 10.1074/jbc.273.27.17269. [DOI] [PubMed] [Google Scholar]
- 31.Chapline C, Cottom J, Tobin H, Hulmes J, Crabb J, Jaken S. A major, transformation-sensitive PKC binding protein is also a PKC substrate involved in cytoskeletal remodeling. J. Biol. Chem. 1998;273:19482–19489. doi: 10.1074/jbc.273.31.19482. [DOI] [PubMed] [Google Scholar]
- 32.Fuerst T, Niles EG, Studier FW. Eukaryotic Transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc. Natl. Acad. Sci. USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tisdale EJ, Bourne JR, Khosravi-Far R, Der CJ, Balch WE. GTP-binding mutants of Rab1 and Rab2 are potent inhibitors of vesicular transport from the endoplasmic reticulum to the Golgi complex. J. Cell Biol. 1992;119:749–761. doi: 10.1083/jcb.119.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tisdale EJ, Balch WE. Rab2 is essential for the maturation of pre-Golgi intermediates. J. Biol. Chem. 1996;271:29372–29379. doi: 10.1074/jbc.271.46.29372. [DOI] [PubMed] [Google Scholar]
- 35.Tisdale EJ, Plutner H, Matteson J, Balch WE. p53/58 binds COPI and is required for selective transport through the early secretory pathway. J. Cell Biol. 1997;137:581–593. doi: 10.1083/jcb.137.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]