Abstract
The evolutionarily conserved JNK/AP-1 (Jun N-terminal kinase/activator protein 1) and BMP (Bone Morphogenetic Protein) signaling cascades are deployed hierarchically to regulate dorsal closure in the fruit fly Drosophila melanogaster. In this developmental context, the JNK/AP-1 signaling cascade transcriptionally activates BMP signaling in leading edge epidermal cells. Here we show that the mummy (mmy) gene product, which is required for dorsal closure, functions as a BMP signaling antagonist. Genetic and biochemical tests of Mmy’s role as a BMP-antagonist indicate that its function is independent of AP-1, the transcriptional trigger of BMP signal transduction in leading edge cells. pMAD (phosphorylated Mothers Against Dpp) activity data show the mmy gene product to be a new type of epidermal BMP regulator – one which transforms a BMP ligand from a long- to a short-range signal. mmy codes for the single UDP-N-acetylglucosamine pyrophosphorylase in Drosophila, and its requirement for attenuating epidermal BMP signaling during dorsal closure points to a new role for glycosylation in defining a highly restricted BMP activity field in the fly. These findings add a new dimension to our understanding of mechanisms modulating the BMP signaling gradient.
Keywords: Dpp/BMP/TGF-β signaling, JNK signaling, Raw, dorsal closure
INTRODUCTION
The various forms of BMP (Bone Morphogenetic Protein) signaling are conserved throughout evolution. In organisms from flies to mammals, BMPs (which belong to the TGF-β [Transforming Growth Factor]-β superfamily of cytokines) function as essential patterning effectors: most notably specifying dorsoventral axis formation, maintaining stem cell niches in virtually all organisms, and directing cartilage and bone formation, fracture repair, joint maintenance and arthritic remodeling in vertebrates. The BMPs have also been implicated in pathologies ranging from neurodegeneration to fertility defects (Affolter and Basler, 2007; Dansereau and Lasko, 2008; Katsuno et al., 2011; Pogue and Lyons, 2006; Shimasaki et al., 2004). Despite these wide-ranging and essential BMP functions, many critical regulators of the pathway have yet to be elucidated. In particular, relatively little is known of BMP pathway modulation extracellularly, where the roles of proteins affecting receptor stability, ligand function, and ligand availability (such as proteoglycans, heparan and chondroitin sulfate modifying enzymes, and proteases) are only beginning to be identified and understood, likely due to their shared participation in signaling by multiple different ligands (Nishihara, 2010). The genetic and molecular studies of mummy (mmy), which we describe here, point to a role for the mmy-encoded UDP-N-acetylglucosoamine pyrophosphorylase as a BMP antagonist that acts directly in defining both the amplitude and range of the BMP signaling gradient. Within the context of dorsal closure in the fruit fly Drosophila melanogaster, the mmy gene product plays a central role in limiting embryonic epidermal BMP signaling. Moreover, Mmy function as an enzyme affecting protein modification by sugar attachment points to potential new targets for the treatment of BMP-associated developmental abnormalities and human disease pathologies.
Sequential JNK/AP-1 and BMP signaling activities direct dorsal closure
In Drosophila, dorsal closure occurs midway through embryogenesis when epidermal sheets, originally positioned ventrally and laterally, extend to the dorsal midline where they meet and fuse (reviewed in VanHook and Letsou, 2008). As the epidermis secretes the larval cuticle, dorsal-open group mutants remain uncovered by epidermis dorsally and accordingly secrete an incomplete cuticle that is distinguished by a large dorsal hole. The process of dorsal closure is dependent upon changes in cell shape but not cell number; hence not unexpectedly, mutations in several cytoarchitectural molecules give rise to defects in dorsal closure. Many other dorsal-open group loci, however, code for components of the JNK/AP-1 (Jun N-terminal kinase/activator protein 1) or BMP signaling pathways, revealing these signaling cascades as integral to and essential for this fundamental morphogenetic event.
In dorsal closure, the JNK/AP-1 and BMP pathways act sequentially. First, AP-1 functions as the transcriptional activator of dpp (decapentaplegic; the Drosophila BMP homologue) in the dorsal-most row of epidermal cells: the leading edge (LE). Later, Dpp is thought to function in an autoregulatory fashion to maintain its own expression in the LE (Johnson et al., 2003). Consistent with these molecularly defined roles, loss-of-function mutations in activating components of the JNK/AP-1 and Dpp signaling cascades disrupt signaling and consequently dorsal closure. In JNK/AP-1 and Dpp signaling mutants (Table 1A,B), epidermal sheets fail to extend to and fuse at the dorsal midline (reviewed in Xia and Karin, 2004). At the opposite end of the spectrum is a small subset of dorsal-open group loci, termed the raw-group, that lead not to the absence of dpp in LE cells but rather to ectopic dpp in epidermal cells beyond the LE (Table 1C). Whereas loss of Dpp signaling leads only to dorsal cuticle holes, ectopic signaling leads to gross defects in ventral cuticle differentiation in addition to dorsal cuticle defects (Bates et al., 2008; Byars et al., 1999). Albeit clearly present, the ventral cuticle that is secreted from raw-group mutants is hypotrophic but neither mispatterned nor transformed, and conceptually at least, raw-group genes can function as regulators of either the JNK/AP-1 or Dpp signaling pathways.
Table 1.
Dorsal-open group loci encoding signaling molecules and their hierarchical relationships
| A. JNK signaling molecules | |
| slipper/slpr | JNKKK |
| hemipterous/hep | JNKK |
| basket/bsk | JNK |
| Jun related antigen/Jra | Jun transcription factor |
| kayak/kay | Fos transcription factor |
|
| |
| B. Dpp signaling molecules | |
| decapentaplegic/dpp | TGF-β cytokine |
| thickveins/tkv | TGF-β type I receptor |
| punt/put | TGF-β type II Receptor |
| mothers against dpp/mad Smad transcription factor | |
| schnurri/shn | Zinc Finger transcription factor |
| dpp → tkv/punt → mad and shn → gene expression | |
| C. raw-group signaling antagonists | |
| raw | novel |
| puckered/puc | MKP |
| ribbon/rib | BTB/POZ-type transcription factor |
| mummy/mmy | UDP-N-acetylglucosamine pyrophosphorylase |
Antagonizing signaling in embryonic dorsal closure
While our understanding of the JNK/AP-1 and Dpp signaling activators in dorsal closure is bolstered by molecular and biochemical studies in several systems, our understanding of the raw-group signaling antagonists is not as extensive. puckered (puc) is the best characterized of the three. puc codes for a VH1-like dual specificity protein tyrosine phosphatase belonging to the mitogen-activated protein kinase (MAPK) subfamily of MAP Kinase Phosphatases (MKPs) (Martin-Blanco et al., 1998). puc is required throughout the Drosophila life-cycle; one of its earliest functions is in LE cells during dorsal closure where it is transcriptionally activated by AP-1. It is thought that the Puckered MKP functions as a negative feedback regulator, dephosphorylating and inactivating Basket (Bsk), the JNK responsible for activating AP-1 in LE cells (Martin-Blanco et al., 1998).
The action mechanisms of the two additional dorsal closure signaling antagonists - raw (encoding a novel gene product; Byars et al., 1999) and ribbon (rib; encoding a BTB/POZ-type transcription factor; Bradley and Andrew, 2001; Byars et al., 1999; Shim et al., 2001) - have yet to be defined; although as is true for puc, raw-mediated effects on dpp are secondary to its effects on JNK/AP-1 signaling (Bates et al., 2008; Bauer Huang et al., 2007). Our previously published data (Bates et al., 2008; Byars et al., 1999) indicate that the raw gene product functions broadly in the epidermis to quench permissive AP-1 activity. Moreover, raw-dependent suppression of epidermal AP-1 sets the stage for LE-specific activation of AP-1 in LE cells of the epidermis.
GlcNAc regulation of Dpp activity
In the current report, we show that mmy, originally isolated in the Heidelberg screen for embryonic lethals affecting cuticle pattern (Nusslein-Volhard et al., 1984), represents the newest member of the raw-group of signaling antagonists. Within this group, however, mmy function is unique. In contrast to the raw and puc gene products, which restrict the signaling domain of Dpp secondarily through their modulation of JNK/AP-1, mmy’s effects upon Dpp signal transduction are direct.
mmy codes for the single Drosophila UDP-N-acetylglucosamine pyrophosphorylase, a key enzyme in UDP-N-acetylglucosamine (UDP-GlcNAc) biosynthesis (Araujo et al., 2005; Schimmelpfeng et al., 2006; Tonning et al., 2006). It is clear from previous reports that mmy is required in Drosophila for the synthesis of extracellular chitin (an insoluble polymer of GlcNAc), and that this requirement manifests itself as cuticular and tracheal defects in strong loss-of-function mmy mutants (Araujo et al., 2005; Devine et al., 2005; Tonning et al., 2006). Chitin synthesis is, however, unaffected in certain mmy hypomorphs including those exhibiting defects in dorsal closure, and thus the dorsal-closure defects observed in animals homozygous for these mmy hypomorphs are thought to result from another requirement for UDP-GlcNAc (Araujo et al., 2005; Schimmelpfeng et al., 2006; Tonning et al., 2006).
In addition to being the building block of chitin, UDP-GlcNAc is an essential precursor for the synthesis of heparin and chondroitin sulfate proteoglycans, the former having been shown to play an essential role in modulating the effects of Dpp/BMP, Wingless (Wg)/WNT, and Hedgehog (Hh) morphogen signaling in Drosophila and other eukaryotes, usually as a facilitator of long-range signaling (Akiyama et al., 2008; Beckett et al., 2008; Belenkaya et al., 2004; Capurro et al., 2008; Gallet et al., 2008; Gumienny et al., 2007). Evidence presented in the current report points to a new role for GlcNAcylation in modulating Dpp activity. Our results demonstrate that Mmy/UDP-GlcNAc constrains rather than facilitates epidermal Dpp signaling during closure, presumably by limiting the signaling capacity of the Dpp cytokine that is produced in LE epidermal cells.
MATERIALS AND METHODS
Drosophila strains
Fly lines for this study include mmy1, mmyP15133, raw1, aop1, bsk2, JraIA109, pucH246, rib1, Df(2L)BSC6, UAS-brk and 69B-gal4 (Marygold et al., 2013), as well as mmy LM1, mmy LM16, mmyLM24, mmy LM45, mmy LM47 and mmy LM51 (M. Krasnow), and pucE69 (A. Martinez-Arias).
Phenotypic analyses
Embryonic lethal cuticle phenotypes were viewed after mounting samples in One-Step Mounting Medium (30% CMCP-10, 13% lactic acid, 57% glacial acetic acid). For hybridizations in situ, we used digoxigenin-labeled RNA as described (Byars et al., 1999); with mouse anti-digoxigenin alkaline phosphatase or mouse anti-digoxigenin (Roche). For immunostains, we used rabbit anti-Jun (Santa Cruz Biotechnology), rabbit anti-Phospho-Smad1,5 Ser463/465 (Cell Signaling Technology), mouse anti-β-Gal (Promega), goat anti-mouse alkaline phosphatase (Promega), goat anti-rabbit alkaline phosphatase (Jackson ImmunoResearch), and goat anti-rabbit Alexa Fluor 488 antibodies (Invitrogen Molecular Probes).
RT-PCR
Mutant homozygotes were distinguished from wild-type siblings 4–8 and 8–12 hours AEL (after egg lay) based on the absence of a GFP (Green Fluorescent Protein)-marked balancer chromosome. RNA was isolated from wild-type and mutant embryos, and reverse transcripts generated using a dT16 primer. PCR products were generated using RA- and RB- specific 5′primers in combination with an exon two 3′primer.
Protein studies
For immunoblotting studies, proteins were prepared from experimental and control lysates 8–12 hours AEL. Mutant homozygotes were distinguished from wild-type siblings based on the absence of a GFP-marked balancer chromosome. CIP (Calf Intestine Alkaline Phosphatase) was added to half-portions of each lysate. Treated and untreated lysates were separated on SDS-acrylamide gels and analyzed by western blotting using anti-Jun antibody. The secondary antibody was HRP-conjugated goat anti-rabbit antibody (Chemicon). Jun densitometry values were calculated as ratios of experimental to control band integrated intensities (the product of mean band intensity and pixel number in an inverted image) and normalized to wild-type CIP-treated and untreated ratios. Similar methods were used to quantify pMAD in the dorsal lateral epidermis of wt and mmy1 embryos. The number of pMAD-positive nuclei in the dorsolateral epidermis were counted in columns of cells in T1, T3, A4 and A6 in wt and mmy1 embryos (n=14 and 11 embryos, respectively). pMAD-positive nuclei were counted in the middle and posterior of each segment in all embryos. Short (middle) and long (posterior) column depths were averaged for each embryo.
Yeast transformation and rescue
Rescue studies were performed in qri1 Saccharomyces cerevisiae. Diploid ura3 yeast strains harboring a heterozygous KanMX4 insertion in QRI1 were transformed with the mmy-RA+ URA3 expression plasmid (constructed by insertion of the full-length mmy RA cDNA into p426-ADH1). After transformation, yeast cells prototrophic for uracil were induced to sporulate by standard methods and tetrads dissected by micromanipulation. Rescue of qri1 lethality was determined by assessing growth on CM-ura, YPD+G418, and YPD+5-FOA agar plates.
RESULTS
Mummy functions as an N-acetylglucosamine pyrophosphorylase
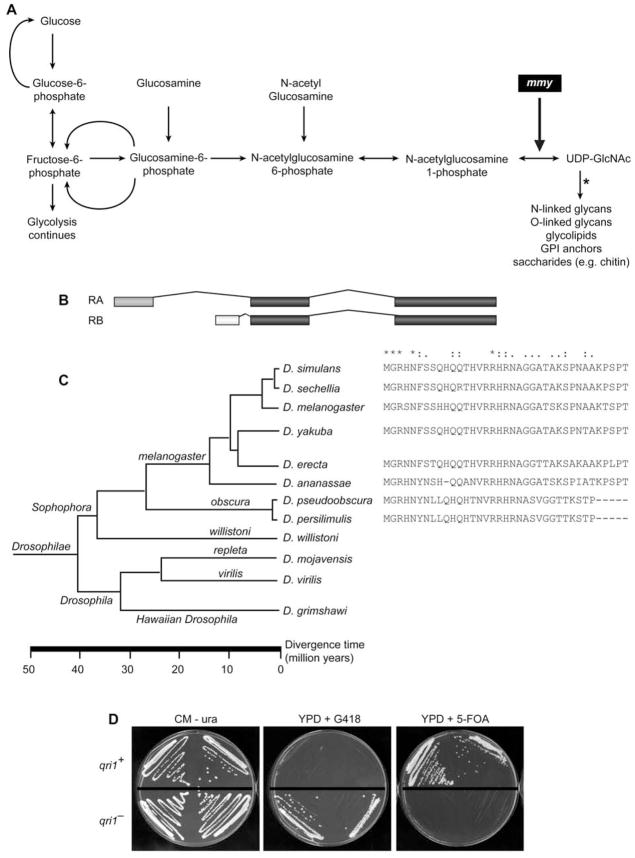
The Drosophila genome encodes a single UDP-N-acetylglucosamine pyrophosphorylase (mummy [mmy]), which potentially functions in the synthesis of saccharides as well as the modification of multiple glycosylated protein and lipid products (Fig. 1A). Two transcript isoforms (RA and RB) are derived from the mmy locus. The RB isoform is homologous along its entire length to the eukaryotic family of UDP-N-acetylglucosamine pyrophosphorylases; the RA isoform, in contrast, encodes a distinctive amino terminus, the result of a splice form variant created by use of an alternative 5′ exon (Fig. 1B). The RA amino terminus comprises a 37 amino acid stretch that has yet to be identified in genome scans of any sequenced organism other than members of the genus Drosophilidae. Except for the species willistoni, the sequence is highly conserved in all members of the subgenus Sophophora (Fig. 1C).
Figure 1. Organization and expression of the mmy gene.
(A) Schema for the generation of UDP-GlcNAc from glucose. In Drosophila there are at least 26 transferases (Correia et al., 2003), each of which can catalyze the transfer of GlcNAc from UDP-GlcNAc to a subset of acceptors. The transferase-dependent step is marked (*). (B) Intron-exon map of RA and RB mmy isoforms. (C) Cladistic analysis of the protein sequences encoded by RA exon 1. (D) A diploid ura3 yeast strain harboring a heterozygous KanMX4 insertion in QRI1 was transformed with the mmy-RA+ URA3 expression plasmid; the growth properties of four spores derived from a single tetrad are shown.
While our current understanding of the genome does not allow us to predict how the unique 5′ end of the mmy RA isoform affects its enzymatic function, we show here that the RA-encoded protein product retains N-acetylglucosamine pyrophosphorylase activity in a heterologous rescue assay. A plasmid with the Drosophila mmyRA+ gene fully restores viability to a S. cerevisiae strain with a disruption in the essential QRI1 gene (the yeast mmy homolog; Fig. 1D). A diploid ura3 yeast strain harboring a heterozygous KanMX4 insertion in QRI1 was transformed with the mmy-RA+ URA3 expression plasmid. Transformation resulted in uracil prototrophy, as indicated by qri1+ and qri1− spore survival on complete medium lacking uracil (CM-ura). Whereas transformed qri1+ spores cannot grow on medium supplemented with the drug G418 (YPD+G418), transformed qri1− spores grow well in the presence of G418, indicating that the KanMX4 insertion remains in qri1− spores after transformation. Finally, transformed qri1−, but not qri1+, spore viability is dependent upon the presence of mmy-RA+ URA3 expression plasmid as only qri1+ spores survive exposure to 5-FOA (which elicits plasmid loss). Together, these data indicate that qri1− viability is dependent upon presence of the mmy-RA+ URA3 expression plasmid and show that the Drosophila mmy RA gene product is an orthologue of the yeast N-acetylglucosamine pyrophosphorylase QRI1. Thus, as is the case for the human and Candida albicans QRI1 loci (51% and 41% identical and 70% and 60% similar to Drosophila mmy, respectively), the novel Drosophila mmy RA isoform rescues the lethal phenotype associated with the S. cerevisiae qri1 null mutation affecting N-acetylglucosamine pyrophosphorylase activity (Mio et al., 1998).
mmy expression is dynamic in Drosophila embryos
In addition to employing alternative first exons, RA and RB mmy isoforms also differ in their transcriptional regulation. Whereas RA transcript levels are invariant in wild-type embryos, RB transcript levels are low in wild-type embryos 4–8 hours AEL but greatly elevated a short time later, 8–12 hours AEL (Fig. 2A). During this period, the embryo develops trachea and undergoes dorsal closure. Despite conflicting descriptions of mmy expression in the literature (Araujo et al., 2005; Tonning et al., 2006), our strictly temporal analysis of mmy gene expression in RT-PCR studies provides clear evidence that mmy is expressed early in Drosophila embryogenesis. Complementing our temporal expression data are spatial expression data derived from in situ hybridization studies (Fig. 2B–E). These studies extend previously published reports (Araujo et al., 2005; Tonning et al., 2006) in revealing mmy expression to be dynamic spatially, as it is temporally, throughout embryogenesis. We found that mmy is expressed ubiquitously and uniformly in the cellular blastoderm. However, even though ubiquitous mmy expression persists throughout embryogenesis, transcript accrual at later developmental time points is spatially partitioned. We noted mmy accumulations in the developing mesoderm, gut primordia, and trachea. Overall, widespread mmy expression reveals its potential to function in multiple aspects of Drosophila embryonic development, consistent with its role as the single UDP-N-acetylglucosamine pyrophosphorylase in Drosophila.
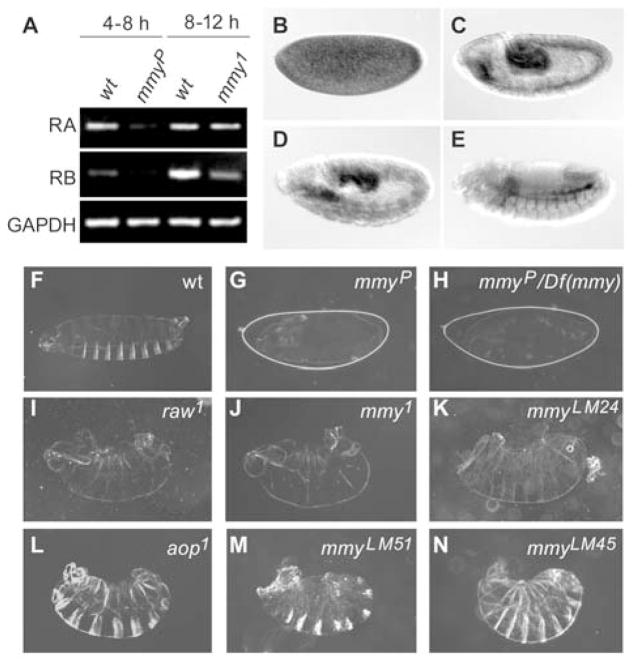
Figure 2. Pleiotropic effects of mmy.
(A) Temporal profile of mmy transcription in early embryogenesis. RA and RB transcripts were distinguished and quantified by RT-PCR using RNAs isolated from wild-type and mutant (mmy1and mmy P15133, here abbreviated mmyP) embryos 4–8 and 8–12 hours AEL. GAPDH RNA levels are shown as a control for loading. (B–E) Spatial profile of mmy transcription in hybridizations to whole mount embryos in situ using a digoxigenin-labeled mmy RNA as probe: (B) syncytial blastoderm, (C) germ band extension, (D) germ band retraction, and (E) dorsal closure. In contrast to the asymmetrically patterned cuticle that is derived from (F) wild-type embryos, (G) mmy P15133 homozygotes and (H) mmyP15133/Df (3R)345 transheterozygotes fail to secrete cuticle (and the preps have not been devitellinized). Hypomorphic mmy mutations lead to shared loss-of-function phenotypes with mutations in genes that modulate JNK and/or Dpp signaling pathways: (I) raw1, (J) mmy1, (K) mmyLM24, (L) aop1, (M) mmyLM51, and (N) mmyLM45.
mmy is essential for multiple developmental events in Drosophila
The sequence and regulatory differences that characterize the two mmy transcripts, as well as the spatially broad and dynamic mmy expression profile, suggest that mmy function is pleiotropic. Although only the mmy-dependent chitin defects have been characterized in detail (Araujo et al., 2005; Tonning et al., 2006), results from our genetic studies are consistent with the idea that the mmy-encoded N-acetylglucosamine pyrophosphorylase impacts multiple Drosophila developmental events via the action of several different downstream transferases, some of which modify proteins and lipids with GlcNAc (see Fig. 1A). Independently-derived mmy mutants exhibit a variety of highly penetrant phenotypes, ranging from cuticle defects associated with a failure to synthesize chitin (Fig. 2F–H) to cuticle defects associated with well-characterized Dpp-dependent closure abnormalities (dorsal closure and head involution; Fig. 2I–N). In particular, these mmy-associated cuticle defects are identical to those resulting from loss-of-function mutations in raw and anterior-open (aop), and point to a crucial role for Mmy in regulating embryonic Dpp signaling.
Absence of cuticle is thus far the best characterized of the mmy phenotypes and clearly results from deficiencies in chitin synthesis (Araujo et al., 2005; Schimmelpfeng et al., 2006; Tonning et al., 2006). We have used two independent strategies to show that the cuticleless phenotype defines the strongest loss of zygotic function condition. First, we confirmed that the cuticleless mmyP15133 allele harbors a transposon insertion within mmy’s second intron and demonstrated that the insertion affects transcription of the RA and RB transcripts, both being markedly reduced in mmyP15133 homozygotes in comparison to wild-type controls (Fig. 2A). More notably, we demonstrated that the mmyP15133/mmyP15133 embryonic lethal cuticular phenotype is indistinguishable from that of mmyP15133/Df(2L)BSC6 transheterozygotes and thus genetically defined the mmyP15133 allele as null (Fig. 2G,H).
While there is no redundancy in the biosynthetic pathway leading to the generation of UDP-GlcNAc, GlcNAc itself is distributed to distinct protein targets via the action of several different downstream UDP-GlcNAc transferases. Hence, even as the mmy null condition reveals much about the central role for GlcNAc in chitin synthesis, it likely masks other equally important, albeit independent, mmy-dependent GlcNAcylation processes. To help us understand one of these additional roles for mmy and GlcNAc more fully, we turned our attention to the Class III mmy mutants that as a group were characterized previously as strong loss-of-function mutants sometimes associated with dorsal closure defects (Devine et al., 2005). mmy1, a focus of the studies described here, is predicted from sequencing studies to be a regulatory mutant; indeed, the RB transcript is specifically affected, showing a 3-fold reduction 8–12 hours AEL, corresponding to the time when mmy1 mutants abort development due to defects in dorsal closure (Fig. 2A). Our sequencing studies revealed that the mmy1 coding region harbors neither missense nor nonsense mutations (data not shown). Other mmy alleles showing a high penetrance of dorsal closure and ventral cuticle defects are mmyLM1, mmyLM16, and mmyLM24. Respectively, these alleles harbor missense mutations in the mmy-encoded N-acetylglucosamine pyrophosphorylase substrate-binding site (S204L) and the diphosphorylase consensus motif (G150S; G148R) (Devine et al., 2005).
Mmy antagonizes Dpp signaling
The mmy dorsal-open, ventral-hypotrophic cuticle phenotypes (see Fig. 2J,K) led to our speculation that Mmy-dependent glycosylation might be integral to restriction of Dpp signaling during closure. In particular, we noted that in all closure-defective mmy mutants, dorsal closure defects do not appear in isolation but rather are associated with ventral cuticular defects that we and others have shown previously to be associated with ectopic Dpp (Bates et al., 2008; Byars et al., 1999; Riesgo-Escovar and Hafen, 1997). We employed molecular and functional experimental strategies to test the hypothesis that Mmy is required to limit Dpp signaling in the embryonic epidermis of Drosophila.
First, we directly visualized embryonic epidermal Dpp activity. To do this, we used an antibody directed against pMAD (the phosphorylated [activated] form of the Dpp signal transducer Mothers against dpp) in conjunction with both DIC (differential interference contrast) and confocal imaging methods. Using DIC, we detected pMAD very broadly in the epidermis of wild-type embryos undergoing germ band extension (Fig. 3A); later in development (in germ band retracting stages of embryogenesis), we observed diminution of the pMAD immunoreactive domain (Fig. 3C). Attenuation of the Dpp signaling amplitude is most evident at dorsal closure. We detected pMAD staining at levels only modestly above background in the dorsal epidermis of 48% of dorsal-closure stage embryos and no pMAD in the dorsal epidermis of 52% of dorsal-closure stage embryos (n=219; Fig. 3E,G). Our pMAD immunoreactivity profile data point to a previously unrecognized tissue-specific Dpp signaling transition in the epidermis of wild-type, dorsal-closure stage embryos - from widespread and robust in germ band extended stages to restricted and then undetectable in dorsal closure stages.
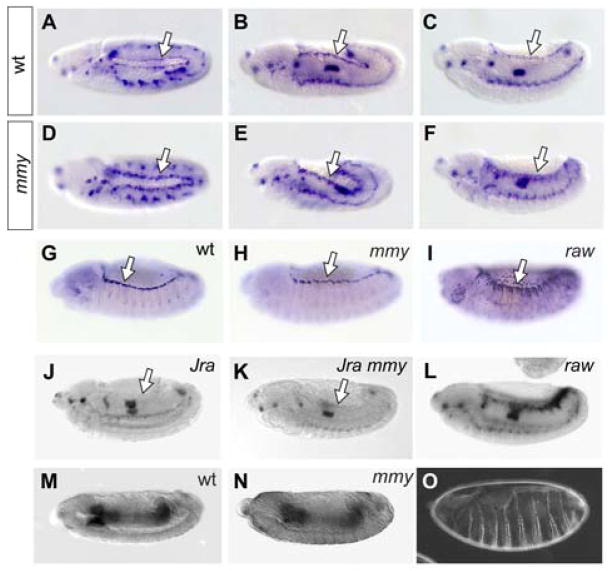
Figure 3. mmy-mediated restriction of Dpp signaling during closure.
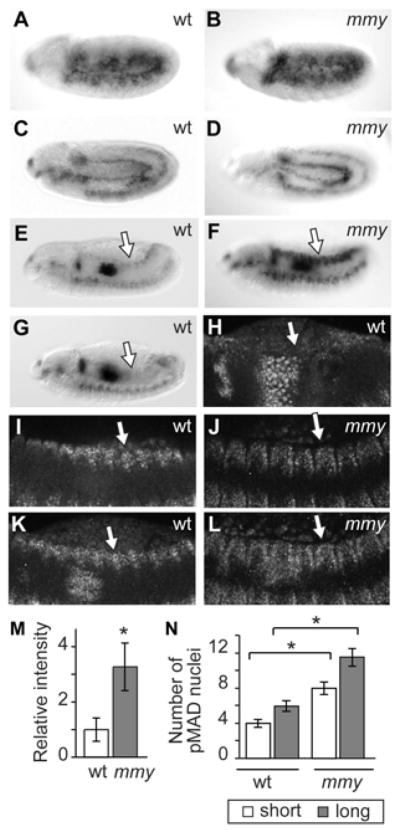
pMAD immunolocalization in whole mount wild-type (wt) and mmy1 (mmy) mutant embryos in (A,B) germ band extended, (C,D) germ band retracting, and (E-G) dorsal closure stages of embryogenesis. (H-L) Immunolocalization of pMAD in wild-type (wt), and mmy1 (mmy) embryos imaged by laser scanning microscopy with (I,J) representing single focal planes and (K,L) corresponding to compilations of multiple Z-stacks. (M) Quantitation of epidermal pMAD immunostain intensities in wt and mmy embryos at dorsal closure. Values were calculated as experimental (n=17 for wt and n=13 for mmy) to wt ratios of background-corrected integrated intensities in inverted images of whole-mount embryos. * indicates that the calculated wt and mmy values are statistically different (p = 7 × 10−6), while error bars correspond to the confidence interval of the mean (p<0.05). (N) Quantitation of spatial extent of pMAD immunostain in the epidermis of wt and mmy embryos at dorsal closure; measurements were obtained from the shortest (middle) and longest (posterior) pMAD-staining region of each segment. * indicates the calculated wt and mmy values are statistically different (p < 1.6 × 10−10), while error bars correspond to the confidence interval of the mean (p<0.05).
When we examined the epidermal Dpp signaling domain in mmy mutant embryos, we found that although germ band extended and retracted pMAD profiles are similar in wild-type and mmy mutant embryos (Fig. 3B,D), differences are evident later in development. Dpp signaling, which is attenuated in dorsal closure stages of wild-type embryogenesis, persists temporally and extends spatially in similarly staged mmy mutants (Fig. 3F). In 100% of dorsal-closure stage mmy embryos, pMAD remains robustly expressed in the dorsal epidermis at levels ~3-fold higher than that observed in the pMAD-positive fraction of wild-type embryos (Fig. 3M). Analysis of wild-type and mutant embryos by confocal microscopy not only validated the genotype-associated spatio-temporal differences in Dpp signaling that we documented previously by DIC microscopy, but also provided a platform for quantification of spatial differences (Fig. 3H–L,N). We observed that while pMAD immunoreactivity can extend to an average depth of five epidermal cells in some dorsal closure stage wild-type embryos, immunoreactivity extends to an average depth of ten epidermal cells in all similarly staged mmy mutants.
Having established that pMAD persists broadly in the epidermis of dorsal closure stage mmy embryos, we next applied functional tests to assess whether Dpp gain-of-function is causative of developmental abnormalities in mmy mutants. To this end, as epidermal Dpp can be autoregulatory (Arora et al., 1995; Johnson et al., 2003), we compared epidermal dpp expression profiles in wild-type and mutant (mmy1 and mmyLM16) whole mount embryos in situ. In wild-type embryos, from germ band extended to germ band retracted stages of development, we observed epidermal dpp only in LE cells (Fig. 4A–C). In contrast, in similarly staged dorsal-open mmy mutants we observed ectopic dpp transcription in the embryonic epidermis (Fig. 4D–F), analogous to that which we documented previously in raw and raw-group mutant embryos (Fig. 4L; see also Bates et al., 2008). Thus, as for other members of the raw-group, in mmy mutants a poorly differentiated cuticle is linked to expansion of the epidermal dpp expression domain.
Figure 4. Ectopic Dpp signaling in mmy mutants activity is causative of developmental abnormalities.
dpp mRNA transcript expression in wild-type (wt) and mutant (mmy1) whole mount embryos: (A,D) germ band extended, (B,E) germ band retracting, and (C,F) dorsal closure stages of embryogenesis. dpp expression is also shown in (J) JraIA109, (K) JraIA109 mmy1, and L) raw1, dorsal closure stage embryos. Expression of the pucE69 enhancer trap in (G) wild-type, (H) mmy1, and (I) raw1 mutant embryos during dorsal closure. (M,N) brinker expression in wild-type and mmy1 homozygotes; (O) rescued ventral cuticle in mmy1; UAS-brk/69B-Gal4 transgenics. In all panels, the LE is indicated with an arrow.
Next, we employed the UAS-GAL4 system to express the well-characterized dpp antagonist brk in the ectopic epidermal Dpp signaling domain of mmy mutants (Brand and Perrimon, 1993; Scuderi and Letsou, 2005). Cuticles derived from mmy1/mmy1; UAS-brk/69B-gal4 transgenics revealed rescue of mmy1-dependent defects; particularly clear was the restoration of ventral denticles to the cuticle (Fig. 4O; see also Fig. 2J for mmy1/mmy1 mutant comparison). It is notable that the brk and dpp domains are neither overlapping nor abutting in wild-type embryos undergoing dorsal closure (Jazwinska et al., 1999). Moreover, the brk expression domain is not altered in mmy mutants (Fig. 4M,N). Together, our Dpp/dpp localization and brk suppression data show that Dpp activity expands in mmy mutants and is causative of developmental abnormalities.
Mmy modulation of Dpp signaling is Dpp-dependent and AP-1-independent
We next sought insight into the molecular basis of Mmy-mediated antagonism of Dpp signaling. Given our understanding of Dpp/dpp regulation during dorsal closure as well as in other developmental contexts, we speculated that mmy might restrict epidermal Dpp signaling: (1) in an established manner (e.g. like raw and puc), transcriptionally via JNK/AP-1-mediated restriction of dpp gene activation, or (2) in a novel manner (with respect to the LE and epidermis), post-transcriptionally via restriction of autoregulatory Dpp signaling activity.
We employed several strategies to discriminate between transcriptional and post-transcriptional models of Mmy function as a Dpp signaling antagonist. First we assayed whether ectopic epidermal gene expression in mmy mutants is limited to dpp or whether other transcriptionally regulated LE targets of JNK/AP-1 are ectopically expressed as well. In addition to dpp, there is another well-characterized transcriptionally-regulated target of JNK/AP-1 activation in the LE during closure - puc. As is true for dpp, puc transcription is abolished in LE epidermal cells in embryos harboring mutations in JNK/AP-1 signaling activators, including hep (JNKK), bsk (JNK), and Jra (Jun) (Glise and Noselli, 1997), and expanded in embryos mutant for JNK signaling antagonists raw and puc (MKP) (Byars et al., 1999; Ring and Martinez Arias, 1993).
We used the well-characterized JNK/AP-1 responsive puc enhancer trap (pucE69-lacZ) to monitor LE puc expression. As we and others have reported previously, we observed β-Gal activity that temporally and spatially mirrors LE dpp expression in wild-type animals (Byars et al., 1999; Dobens et al., 2001; Stronach and Perrimon, 2001); in this regard, we detected β-Gal initially in the LE of germ band extended embryos and we observed its persistence in LE cells throughout dorsal closure stages of development (Fig. 4G). In mmy mutants, β-Gal never expanded beyond the LE epidermal domain as it does in raw and puc mutant embryos (Fig. 4H,I; see also Byars et al., 1999). Our observation that mmy-mediated expansion of dpp gene expression does not extend to a second transcriptionally-regulated target of JNK/AP-1 in LE cells (puc) suggests that ectopic dpp transcription in mmy mutants is not a consequence of ectopic AP-1 activity.
Next, we employed biochemical methods to examine Jun in wild-type and mutant embryos directly. For initial measures of Jun/AP-1 activity in wild-type and raw-group mutant embryos, we identified an anti-Jun antibody that recognizes endogenous Drosophila Jun protein in extracts isolated from wild-type embryos (Fig. 5A). In comparisons of phosphatase-treated and untreated lysates, we identified phosphorylated (activated) and unphosphorylated (inactivated) Jun isoforms (Peverali et al., 1996). In wild-type embryos, both the phosphorylated and unphosphorylated isoforms are present (Fig. 5B). Immunoblotting studies indicated that the wild-type balance between phosphorylated and unphosphorylated Jun isoforms is unchanged in mmy mutants (Fig. 5C), demonstrating that overall embryonic phosphorylated and unphosphorylated Jun levels are not detectably altered in this quantitative assay. In contrast though, levels of phosphorylated and unphosphorylated Jun are altered considerably in raw mutants (Fig. 5D). Not only is the unphosphorylated Jun isoform undetectable in non-CIP treated extracts from raw1 (null) mutant embryos, but the more slowly migrating phosphorylated isoform accumulates to measurably higher levels in raw1 and raw1 bsk2 double mutant embryos than it does in wild-type embryos (more than 2-fold for both). This observation, as well as a parallel immunoblotting analysis of extracts from bsk2 nulls revealing a pattern of Jun phosphorylation that is indistinguishable from that of wild type, is consistent with our previous strictly genetic prediction that the majority of Jun phosphorylation in wild-type embryos is dependent upon a Jun kinase other than zygotic Basket, and that Raw antagonizes the function of this kinase (Bates et al., 2008).
Figure 5. JNK signaling is normal in mmy mutants, but not in raw mutants.
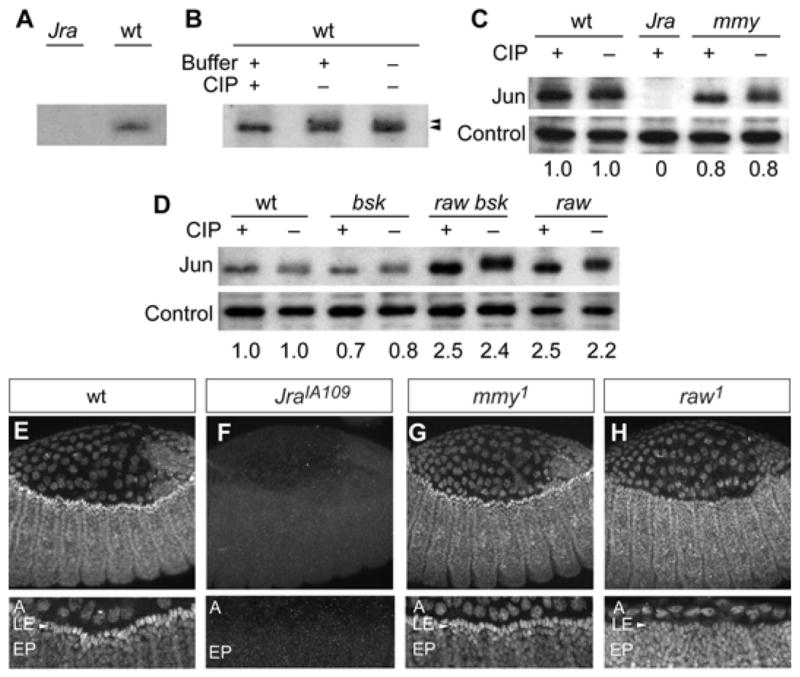
(A) Anti-Jun antibody recognizes wild-type protein but not a truncated version lacking the epitope derived from JraIA109 mutants. (B) Anti-Jun antibody recognizes a doublet in untreated extract but a singlet in extract treated with CIP. Jun modification and accumulation in (C) mmy1, and (D) raw1 and bsk2 mutant embryos. Total Jun protein was quantified, and measurements relative to wild-type are reported at the bottoms of panels C,D. Controls for protein loading equivalency are shown for all gels where protein was quantified. Immunolocalization of Jun in (E) wild-type, (F) JraIA109, (G) mmy1, and (H) raw1 embryos imaged by laser scanning microscopy with no (top row) and 2.5× (bottom row) zoom. In 2.5× zoomed images, A denotes Amnioserosa, LE denotes leading edge epidermis, and EP denotes epidermis.
Immunolocalization studies in mmy and raw mutant embryos complemented western studies and further bolstered our conclusion that Raw mediates dpp antagonism via its function upstream of Jun, while Mmy-mediated dpp antagonism likely occurs downstream. From immunolocalization studies, it is clear that: (1) Jun is expressed broadly in wild-type and mutant (mmy1 and raw1) embryos, and (2) Jun does not differentially accumulate in LE cells from raw embryos as it does in mmy and wild-type dorsal-closure stage embryos (Fig. 5E–H). Thus, biochemical markers of phenotype, in conjunction with molecular markers, indicate that mmy is different from other members of the raw group. While raw and puc antagonize Dpp indirectly through their function as Jun pathway modulators, the effects of mmy are downstream of the Jun pathway and likely target the Dpp pathway directly.
Finally, having established that mmy-dependent defects in closure effects are Jun-independent, we used mutants in the JNK/AP-1 signaling pathway to specifically ablate LE dpp and to test whether mmy-dependent defects in dorsal closure are dependent upon LE dpp. While we detect dpp ectopically in the epidermis of mmy mutant embryos, its expression is absent from the epidermis of mmy Jra and mmy bsk double mutants as it is also in Jra and bsk single mutant backgrounds (Fig. 4J,K and data not shown). Our demonstration that null mutations in either Jra or bsk prevent manifestation of the ectopic dpp phenotype associated with mmy1 indicates that Jun is the lone transcriptional trigger of epidermal dpp in both wild-type and mmy mutant embryos, and even more notably that LE JNK/AP-1-dependent dpp expression is a prerequisite for the expansion of epidermal dpp that we observe in dorsal-open mmy mutants. Thus, mmy’s effects are Dpp-dependent. Viewed from the perspective of mechanism, results from our studies point to a role for Mmy in shaping and constraining the epidermal Dpp gradient.
DISCUSSION
In the current report, we provide significant new insights into the mechanisms by which morphogen activity domains are spatially constrained in development. Although secreted Dpp/BMP is a potent and largely unconstrained morphogen in most developmental contexts, in the Drosophila embryonic epidermis dpp/Dpp expression and function are precisely regulated in time and space. Results from studies reported here and elsewhere reveal that at least two tiers of signaling antagonism contribute to this spatio-temporal restriction. In particular, we show that JNK/AP-1 and Dpp regulatory machineries function independently to limit bioactive Dpp signaling fields in the Drosophila embryonic epidermis. Whereas widespread transcriptional activation of epidermal dpp is suppressed by Raw, long-range Dpp signaling is suppressed by Mmy. Thus, Mmy and Raw, despite their striking shared loss-of-function phenotypes, independently constrain the amplitude and range of Dpp activity during dorsal closure. Moreover, the Mmy and Raw pathways are not redundant, as loss of either leads to ectopic Dpp activity.
mmy, which encodes the Drosophila UDP-N-acetylglucosamine pyrophosphorylase, represents the fourth and newest member of the raw-group of dorsal-open mutants. As we showed previously for the three defining members of this group (raw, puc, and rib; Bates et al., 2008), mmy mutants exhibit: (1) defects in two Dpp-dependent embryonic processes - dorsal closure and ventral cuticle differentiation, as well as (2) expansion of epidermal Dpp activity. mmy is, however, distinct from the two members of the raw-group whose modes of action have been defined previously. Specifically, we have demonstrated that unlike the novel Raw protein and the Puckered MKP, which mediate their effects on Dpp via their modulation of a JNK/AP-1 signaling cascade, Mmy mediates restriction of Dpp activity directly. Moreover, our data provide strong evidence that a target of the mmy-encoded UDP-N-acetylglucosamine pyrophosphorylase functions as a developmental switch in the Drosophila epidermis, eliminating long-range Dpp signaling.
Modulation of Dpp signaling activity by Mmy and UDP-GlcNAc
Dpp signaling has been exceptionally well characterized in patterning Drosophila embryos and imaginal discs (O’Connor et al., 2006). A key feature of Dpp action in these two developmental contexts is that Dpp exerts its effects on immediate neighbors, as well as on more distant cells, via an extracellular morphogen gradient. Based on these clear and strong precedents, it has been widely assumed that during dorsal closure Dpp is secreted from its LE cell source and subsequently functions non-cell autonomously to direct cell changes in the lateral epidermis that are essential for Drosophila morphogenesis during dorsal closure (Fernandez et al., 2007). Evidence from studies presented here and elsewhere (Wang et al., 2008) have, however, led us to a new model of Dpp function in LE cells during closure. As discussed below, it is now apparent that during dorsal closure LE Dpp does not function exclusively as a long-range epidermal signal. Moreover, long-range epidermal Dpp signaling is constrained during dorsal closure, and the mmy gene product plays a central role in refining this vital epidermal Dpp activity profile.
Our studies have their foundation in the observation that embryos homozygous for several mmy alleles (mmy1, mmyLM1, mmy LM16, mmy LM24) suffer a fully penetrant embryonic lethality associated with a cuticle phenotype that we have shown here and elsewhere (Byars et al., 1999) is due to misregulated Dpp signaling - spatially, temporally, and quantitatively. Moreover, our data lead us to suggest that Dpp secreted from LE epidermal cells encounters an as yet unidentified sugar-modified sink. For example, Mmy-dependent modification of either an ECM or Dpp receptor component might constrain Dpp activity (by either degradation or titration). In contrast, we suspect that in the absence of mmy, Dpp sequestration is down-regulated and Dpp is consequently free to: (1) move away from its source, and (2) generate the expanded and more robust signaling field that we visualize in epidermal dpp/Dpp expression and activity profiles (Fig. 6).
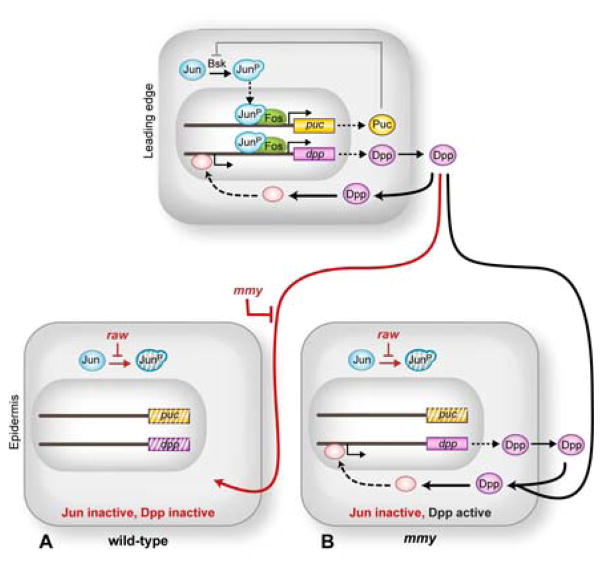
Figure 6. Modeling LE dpp restriction.
JNK/AP-1 and Dpp signaling in LE cells is shown at top. Dorsolaterally-positioned epidermal cells are shown below: (A) In wild-type cells, raw prevents accumulation of the bioactive, phosphorylated form of Jun in the dorsolateral epidermis, while mmy prevents Dpp from signaling between LE and neighboring epidermal cells. (B) In mmy mutants, LE Dpp activates signaling in neighboring epidermal cells, where autoregulatory dpp feeds forward to activate its own transcription.
Somewhat contrary to the established paradigm of Glc-NAc/glypican function in modulating Dpp signaling in the wing disc of Drosophila, we found that Mmy limits rather than augments the Dpp signaling field in the embryonic epidermis. There is precedence for opposing effects of glypicans in Hedgehog signaling, where the sugar modification has been shown to augment signaling in Drosophila while down-regulating signaling in mouse (Beckett et al., 2008). In addition, and although not previously recognized as such, our current studies, along with previous studies focusing on LE dpp transcription (Arora et al., 1995; Johnson et al., 2003; Letsou et al., 1995), indicate that the embryonic epidermis is competent to regulate dpp in an autoregulatory fashion as it is in several other developmental contexts (Capovilla et al., 1994; Hursh et al., 1993; Panganiban et al., 1990; Staehling-Hampton and Hoffmann, 1994). Thus, epidermal signaling abnormalities in mmy mutants can be amplified via a feed forward mechanism of intercellular communication. Moreover, in this context at least, cytokine diffusion represents the default state. Finally, it is notable that while pMAD activates dpp transcription beyond the leading edge in mmy mutant embryos, the more modest amounts of pMAD visualized in dorsal-closure stage wild-type embryos are insufficient to activate dpp transcription in these same cells. Quantitation of this difference in wild-type and mmy mutant embryos defined a three-fold threshold for dpp-activation by pMAD.
Future experiments will require identification of the Mmy-dependent glycosylated protein product(s) essential for Dpp restriction to LE cells. Certainly there are several potential candidates; very high among these are Dpp receptors (and co-receptors). Both Tkv and Punt, the type I and type II receptors functioning in dorsal closure, harbor multiple potential glycosylation sites; modification of any of these might enhance ligand affinity for its receptor. The Type I Dpp receptor Tkv has been shown previously to be a means for Dpp signal down-regulation (Lecuit and Cohen, 1998), and the type II Dpp receptor has been shown previously to be a dosage sensitive component of the signaling pathway (Simin et al., 1998). Collagen might also be modified by GlcNAc as it has been suggested that the Drosophila collagen Viking sequesters and limits the Dpp signaling range in the germarium (Wang et al., 2008). The Dally or Dally-like glypicans, although augmenting Dpp diffusion rates at previously defined sites of Dpp action in Drosophila and also play limiting roles in restricting the movement of a morphogen signal in other organisms (Gumienny et al., 2007), are not likely to function downstream of Mmy in regulating epidermal signal transduction. Loss-of-function dally or dally-like mutations are associated with neither dorsal closure defects nor embryonic lethality more generally. In addition, dally and dally-like double mutant studies with mmy show no genetic interactions (GH, unpublished). Finally, it is possible that Mmy contributes to the modification of intracellular proteins or chromatin through O-linked glycosylation. We do not favor this mechanism of action as the effect that we see is dependent upon leading edge dpp expression, and this strongly implicates signaling itself as the Mmy-dependent step in Dpp regulation. Moreover, as for dally and dally-like, loss of function mutations of the single O-linked transferase in Drosophila are not associated with defects in dorsal closure (Ingham, 1984).
As a final point, Mmy’s role as a Dpp signaling antagonist likely extends beyond the embryonic epidermis and dorsal closure, as Schimmelpfeng and coworkers have reported ectopic Dpp activity in a mmy7 background in the Drosophila eye (Schimmelpfeng et al., 2006). The failure in this study to detect ectopic dpp in the epidermis of mutants likely reflects the low penetrance of dorsal closure defects in the mmy7 background. We too are unable to detect ectopic dpp in alleles with low penetrance of dorsal closure defects. For raw mutants as well, there is a gradient of dpp expansion that parallels the strength of molecularly characterized loss-of-function alleles (Bates et al., 2008). Furthermore, this result highlights the fact, that in terms of signaling, less gylcosyolation does not necessarily correlate with more signaling; i.e. specific thresholds of decreased gylcosylation/modifications might either augment or limit signaling.
Conclusions
During development, a surprisingly small number of signaling cascades are used again and again to mediate communication within and between cells, and to regulate a variety of cellular responses, including proliferation, differentiation, survival, and death. Among these essential signaling pathways are two (Dpp and JNK) that we have studied here in the context of Drosophila dorsal closure, and for which conserved functions have been repeatedly demonstrated. In virtually all animal models, the Dpp (TGF-β/BMP) pathways mediate both short- and long-range intercellular communication in response to the eponymous, diffusible extracellular cytokine. Similarly, the conserved JNK (MAPK) pathways activate transcription of gene suites in virtually all animal models in response to a variety of both extracellular and intracellular stimuli, including peptide growth factors, cytokines, and hormones, as well as diverse cellular stressors including oxidative and endoplasmic reticulum stress. Deviation from the strict control of any of these signaling pathways has been implicated in the development of countless human developmental abnormalities, degenerative diseases, and cancer pathologies. The fact that health and development consequences of misregulated signaling are so far-reaching has prompted numerous research programs to seek a better understanding of how these complex regulatory circuits are controlled - at the levels of both activation and repression.
Approaching this problem by dissecting signaling circuitry in the model genetic system of Drosophila dorsal closure, we have made considerable progress in unraveling the complex circuitry that links JNK to Dpp, and both to epithelial morphogenesis. Our previous studies of raw have revealed its role as a master regulator in the complex circuitry of the developing Drosophila embryo. Our current studies of mmy reveal that UDP-N-acetylglucosamine pyrophosphorylase activity is required to spatially limit Dpp activity in a JNK/AP-1-independent fashion. Together, our studies of the Drosophila Mmy and Raw signaling antagonists lead us to a fuller understanding of the molecular mechanisms governing coordinated signaling pathways, which throughout the animal kingdom control a variety of biologically essential cell growth, proliferation, and differentiation pathways.
Acknowledgments
We are indebted to Dr. Judith Lengyel for pointing us to the striking shared loss-of-function phenotypes in mmy and raw. We also thank Drs. Mark Krasnow, and Alfonso Martinez-Arias for providing flies and reagents, Dr. Alice Schmid and the microscopy core for help with confocal imaging, and Diana Lim for help with figure preparation. This work was supported by grants from the NIH (R01-GM068083) and NSF (IOS-0922757) to AL. GBH was supported in part by NIH T32-GM007464.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Affolter M, Basler K. The Decapentaplegic morphogen gradient: from pattern formation to growth regulation. Nat Rev Genet. 2007;8:663–74. doi: 10.1038/nrg2166. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Kamimura K, Firkus C, Takeo S, Shimmi O, Nakato H. Dally regulates Dpp morphogen gradient formation by stabilizing Dpp on the cell surface. Dev Biol. 2008;313:408–19. doi: 10.1016/j.ydbio.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo SJ, Aslam H, Tear G, Casanova J. mummy/cystic encodes an enzyme required for chitin and glycan synthesis, involved in trachea, embryonic cuticle and CNS development--analysis of its role in Drosophila tracheal morphogenesis. Dev Biol. 2005;288:179–93. doi: 10.1016/j.ydbio.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Arora K, Dai H, Kazuko S, Jamal J, O’Connor M, Letsou A, Warrior R. The Drosophila schnurri gene acts in the Dpp/TGF beta signaling pathway and encodes a transcription factor homologous to the human MBP family. Cell. 1995;81:781–90. doi: 10.1016/0092-8674(95)90539-1. [DOI] [PubMed] [Google Scholar]
- Bates KL, Higley M, Letsou A. Raw Mediates Antagonism of AP-1 Activity in Drosophila. Genetics. 2008;178:1989–2002. doi: 10.1534/genetics.107.086298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer Huang SL, Saheki Y, VanHoven MK, Torayama I, Ishihara T, Katsura I, van der Linden A, Sengupta P, Bargmann CI. Left-right olfactory asymmetry results from antagonistic functions of voltage-activated calcium channels and the Raw repeat protein OLRN-1 in C. elegans. Neural Dev. 2007;2:24. doi: 10.1186/1749-8104-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett K, Franch-Marro X, Vincent JP. Glypican-mediated endocytosis of Hedgehog has opposite effects in flies and mice. Trends Cell Biol. 2008;18:360–3. doi: 10.1016/j.tcb.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Belenkaya TY, Han C, Yan D, Opoka RJ, Khodoun M, Liu H, Lin X. Drosophila Dpp morphogen movement is independent of dynamin-mediated endocytosis but regulated by the glypican members of heparan sulfate proteoglycans. Cell. 2004;119:231–44. doi: 10.1016/j.cell.2004.09.031. [DOI] [PubMed] [Google Scholar]
- Bradley PL, Andrew DJ. ribbon encodes a novel BTB/POZ protein required for directed cell migration in Drosophila melanogaster. Development. 2001;128:3001–15. doi: 10.1242/dev.128.15.3001. [DOI] [PubMed] [Google Scholar]
- Brand A, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–15. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Byars CL, Bates KL, Letsou A. The dorsal-open group gene raw is required for restricted DJNK signaling during closure. Development. 1999;126:4913–4923. doi: 10.1242/dev.126.21.4913. [DOI] [PubMed] [Google Scholar]
- Capovilla M, Brandt M, Botas J. Direct regulation of decapentaplegic by Ultrabithorax and its role in Drosophila midgut morphogenesis. Cell. 1994;76:461–75. doi: 10.1016/0092-8674(94)90111-2. [DOI] [PubMed] [Google Scholar]
- Capurro MI, Xu P, Shi W, Li F, Jia A, Filmus J. Glypican-3 inhibits Hedgehog signaling during development by competing with patched for Hedgehog binding. Dev Cell. 2008;14:700–11. doi: 10.1016/j.devcel.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Correia T, Papayannopoulos V, Panin V, Woronoff P, Jiang J, Vogt TF, Irvine KD. Molecular genetic analysis of the glycosyltransferase Fringe in Drosophila. Proc Natl Acad Sci U S A. 2003;100:6404–9. doi: 10.1073/pnas.1131007100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansereau DA, Lasko P. The development of germline stem cells in Drosophila. Methods Mol Biol. 2008;450:3–26. doi: 10.1007/978-1-60327-214-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine WP, Lubarsky B, Shaw K, Luschnig S, Messina L, Krasnow MA. Requirement for chitin biosynthesis in epithelial tube morphogenesis. Proc Natl Acad Sci U S A. 2005;102:17014–9. doi: 10.1073/pnas.0506676102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobens LL, Martin-Blanco E, Martinez-Arias A, Kafatos FC, Raftery LA. Drosophila puckered regulates Fos/Jun levels during follicle cell morphogenesis. Development. 2001;128:1845–56. doi: 10.1242/dev.128.10.1845. [DOI] [PubMed] [Google Scholar]
- Fernandez BG, Arias AM, Jacinto A. Dpp signalling orchestrates dorsal closure by regulating cell shape changes both in the amnioserosa and in the epidermis. Mech Dev. 2007;124:884–97. doi: 10.1016/j.mod.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Gallet A, Staccini-Lavenant L, Therond PP. Cellular trafficking of the glypican Dally-like is required for full-strength Hedgehog signaling and wingless transcytosis. Dev Cell. 2008;14:712–25. doi: 10.1016/j.devcel.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Glise B, Noselli S. Coupling of Jun amino-terminal kinase and Decapentaplegic signaling pathways in Drosophila morphogenesis. Genes Dev. 1997;11:1738–47. doi: 10.1101/gad.11.13.1738. [DOI] [PubMed] [Google Scholar]
- Gumienny TL, MacNeil LT, Wang H, de Bono M, Wrana JL, Padgett RW. Glypican LON-2 is a conserved negative regulator of BMP-like signaling in Caenorhabditis elegans. Curr Biol. 2007;17:159–64. doi: 10.1016/j.cub.2006.11.065. [DOI] [PubMed] [Google Scholar]
- Hursh D, Padgett R, Gelbart W. Cross regulation of decapentaplegic and Ultrabithorax transcription in the embryonic visceral mesoderm of Drosophila. Development. 1993;117:1211–22. doi: 10.1242/dev.117.4.1211. [DOI] [PubMed] [Google Scholar]
- Ingham PW. A gene that regulates the bithorax complex differentially in larval and adult cells of Drosophila. Cell. 1984;37:815–23. doi: 10.1016/0092-8674(84)90416-1. [DOI] [PubMed] [Google Scholar]
- Jazwinska A, Kirov N, Wieschaus E, Roth S, Rushlow C. The Drosophila gene brinker reveals a novel mechanism of Dpp target gene regulation. Cell. 1999;96:563–73. doi: 10.1016/s0092-8674(00)80660-1. [DOI] [PubMed] [Google Scholar]
- Johnson AN, Bergman CM, Kreitman M, Newfeld SJ. Embryonic enhancers in the dpp disk region regulate a second round of Dpp signaling from the dorsal ectoderm to the mesoderm that represses Zfh-1 expression in a subset of pericardial cells. Dev Biol. 2003;262:137–51. doi: 10.1016/s0012-1606(03)00350-6. [DOI] [PubMed] [Google Scholar]
- Katsuno M, Adachi H, Banno H, Suzuki K, Tanaka F, Sobue G. Transforming growth factor-beta signaling in motor neuron diseases. Curr Mol Med. 2011;11:48–56. doi: 10.2174/156652411794474356. [DOI] [PubMed] [Google Scholar]
- Lecuit T, Cohen SM. Dpp receptor levels contribute to shaping the Dpp morphogen gradient in the Drosophila wing imaginal disc. Development. 1998;125:4901–7. doi: 10.1242/dev.125.24.4901. [DOI] [PubMed] [Google Scholar]
- Letsou A, Arora K, Wrana J, Simin K, Twombly V, Jamal J, Staehling-Hampton K, Hoffmann F, Gelbart W, Massague J, et al. Drosophila Dpp signaling is mediated by the punt gene product: a dual ligand-binding type II receptor of the TGF beta receptor family. Cell. 1995;80:899–908. doi: 10.1016/0092-8674(95)90293-7. [DOI] [PubMed] [Google Scholar]
- Martin-Blanco E, Gampel A, Ring J, Virdee K, Kirov N, Tolkovsky A, Martinez-Arias A. puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev. 1998;12:557–70. doi: 10.1101/gad.12.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marygold SJ, Leyland PC, Seal RL, Goodman JL, Thurmond J, Strelets VB, Wilson RJ. FlyBase: improvements to the bibliography. Nucleic Acids Res. 2013;41:D751–7. doi: 10.1093/nar/gks1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mio T, Yabe T, Arisawa M, Yamada-Okabe H. The eukaryotic UDP-N-acetylglucosamine pyrophosphorylases. Gene cloning, protein expression, and catalytic mechanism. J Biol Chem. 1998;273:14392–7. doi: 10.1074/jbc.273.23.14392. [DOI] [PubMed] [Google Scholar]
- Nishihara S. Glycosyltransferases and transporters that contribute to proteoglycan synthesis in Drosophila: Identification and functional analyses using the heritable and inducible RNAi system. Methods Enzymol. 2010;480:323–51. doi: 10.1016/S0076-6879(10)80015-1. [DOI] [PubMed] [Google Scholar]
- Nusslein-Volhard C, Wieschaus E, Kluding H. Mutations affecting the pattern of the larval cuticl in Drosophila melanogaster. I. Zygotic loci on the second chromosome. Roux’s Archives of Developmental Biology. 1984;183:267–282. doi: 10.1007/BF00848156. [DOI] [PubMed] [Google Scholar]
- O’Connor MB, Umulis D, Othmer HG, Blair SS. Shaping BMP morphogen gradients in the Drosophila embryo and pupal wing. Development. 2006;133:183–93. doi: 10.1242/dev.02214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panganiban G, Reuter R, Scott M, Hoffmann F. A Drosophila growth factor homolog, decapentaplegic, regulates homeotic gene expression within and across germ layers during midgut morphogenesis. Development. 1990;110:1041–50. doi: 10.1242/dev.110.4.1041. [DOI] [PubMed] [Google Scholar]
- Peverali FA, Isaksson A, Papavassiliou AG, Plastina P, Staszewski LM, Mlodzik M, Bohmann D. Phosphorylation of Drosophila Jun by the MAP kinase rolled regulates photoreceptor differentiation. Embo J. 1996;15:3943–50. [PMC free article] [PubMed] [Google Scholar]
- Pogue R, Lyons K. BMP signaling in the cartilage growth plate. Curr Top Dev Biol. 2006;76:1–48. doi: 10.1016/S0070-2153(06)76001-X. [DOI] [PubMed] [Google Scholar]
- Riesgo-Escovar JR, Hafen E. Common and distinct roles of DFos and DJun during Drosophila development. Science. 1997;278:669–672. doi: 10.1126/science.278.5338.669. [DOI] [PubMed] [Google Scholar]
- Ring JM, Martinez Arias A. puckered, a gene involved in position-specific cell differentiation in the dorsal epidermis of the Drosophila larva. Dev Suppl. 1993;1993:251–259. [PubMed] [Google Scholar]
- Schimmelpfeng K, Strunk M, Stork T, Klambt C. Mummy encodes an UDP-N-acetylglucosamine-dipohosphorylase and is required during Drosophila dorsal closure and nervous system development. Mech Dev. 2006;123:487–99. doi: 10.1016/j.mod.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Scuderi A, Letsou A. Amnioserosa is required for dorsal closure in Drosophila. Dev Dyn. 2005;232:791–800. doi: 10.1002/dvdy.20306. [DOI] [PubMed] [Google Scholar]
- Shim K, Blake KJ, Jack J, Krasnow MA. The Drosophila ribbon gene encodes a nuclear BTB domain protein that promotes epithelial migration and morphogenesis. Development (Cambridge, England) 2001;128:4923–33. doi: 10.1242/dev.128.23.4923. [DOI] [PubMed] [Google Scholar]
- Shimasaki S, Moore RK, Otsuka F, Erickson GF. The bone morphogenetic protein system in mammalian reproduction. Endocr Rev. 2004;25:72–101. doi: 10.1210/er.2003-0007. [DOI] [PubMed] [Google Scholar]
- Simin K, Bates E, Horner M, Letsou A. Genetic analysis of punt, a type II Dpp receptor that functions throughout the Drosophila melanogaster life cycle. Genetics. 1998;148:801–13. doi: 10.1093/genetics/148.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehling-Hampton K, Hoffmann F. Ectopic decapentaplegic in the Drosophila midgut alters the expression of five homeotic genes, dpp, and wingless, causing specific morphological defects. Dev Biol. 1994;164:502–12. doi: 10.1006/dbio.1994.1219. [DOI] [PubMed] [Google Scholar]
- Stronach BE, Perrimon N. Investigation of leading edge formation at the interface of amnioserosa and dorsal ectoderm in the Drosophila embryo. Development. 2001;128:2905–13. doi: 10.1242/dev.128.15.2905. [DOI] [PubMed] [Google Scholar]
- Tonning A, Helms S, Schwarz H, Uv AE, Moussian B. Hormonal regulation of mummy is needed for apical extracellular matrix formation and epithelial morphogenesis in Drosophila. Development. 2006;133:331–41. doi: 10.1242/dev.02206. [DOI] [PubMed] [Google Scholar]
- VanHook A, Letsou A. Head involution in Drosophila: genetic and morphogenetic connections to dorsal closure. Dev Dyn. 2008;237:28–38. doi: 10.1002/dvdy.21405. [DOI] [PubMed] [Google Scholar]
- Wang X, Harris RE, Bayston LJ, Ashe HL. Type IV collagens regulate BMP signalling in Drosophila. Nature. 2008;455:72–7. doi: 10.1038/nature07214. [DOI] [PubMed] [Google Scholar]
- Xia Y, Karin M. The control of cell motility and epithelial morphogenesis by Jun kinases. Trends Cell Biol. 2004;14:94–101. doi: 10.1016/j.tcb.2003.12.005. [DOI] [PubMed] [Google Scholar]