Abstract
Objective
To determine whether the relationship between interleukin (IL)-6 and depressive symptoms is moderated by participation in moderate-intensity physical activity in a sample of primary care patients. Elevated inflammation has been associated with a number of poor health outcomes. Depressive symptoms may be related to higher levels of the inflammatory marker IL-6; however, previous findings are inconsistent, possibly due to unidentified moderating factors.
Methods
A total of 107 participants, aged ≥40 years, were recruited in Rochester, New York, in 2006 to 2007. Depressive symptoms were measured by the Center for Epidemiologic Studies Depression Scale-Revised, participation in moderate-intensity physical activity was measured using a modified version of the Community Health Activities Model Program for Seniors Activity Questionnaire for Older Adults, and serum IL-6 concentrations were determined using standard enzyme-linked immunosorbent assay protocols and high-sensitivity, anti-cytokine antibody pairs. A hierarchical multiple regression analysis was conducted.
Results
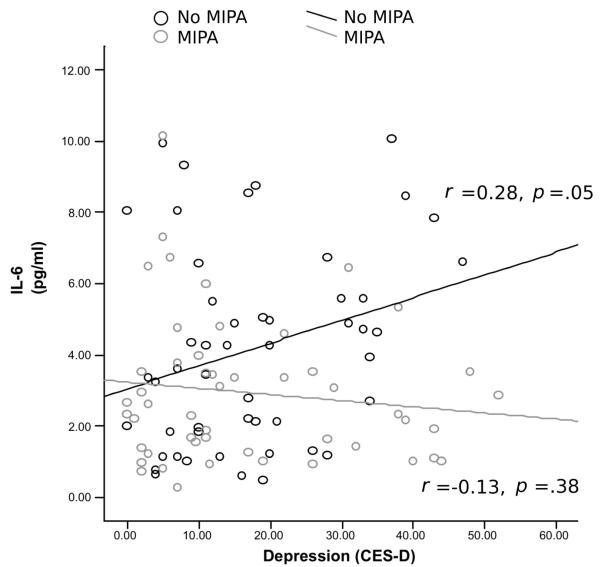
The correlation between IL-6 and depressive symptoms was nonsignificant (r = .086, p = .40). The association between IL-6 and depressive symptoms was moderated by participation in moderate-intensity physical activity (p = .02). Among those who did not engage in moderate-intensity physical activity, higher levels of depressive symptoms were significantly associated with higher levels of IL-6 (r = .28, p = .05), whereas this association was not significant among those who did participate in moderate-intensity physical activity (r = −.13, p = .38).
Conclusion
Participation in moderate-intensity physical activity may buffer the risk of higher inflammation often associated with higher levels of depressive symptoms.
Keywords: exercise, depressive symptoms, interleukin-6, inflammation, physical activity, depression
INTRODUCTION
According to the Global Burden of Disease project, depressive disorders ranked fourth in terms of global burden (1). In addition to the direct costs of care, depressive symptoms are often accompanied by a number of medical comorbidities (2) and a higher risk of mortality (3). Although the mechanisms responsible for these associations between depressive symptoms and poor physical health remain unclear, one potential factor is an altered inflammatory response among depressed individuals.
Overproduction of interleukin (IL)-6 is associated with a wide range of health conditions, including cardiovascular disease (4,5), Type 2 diabetes (6), and certain cancers (7). Furthermore, elevated IL-6 levels have been associated with higher mortality risk in older adults (8). Elevated levels of IL-6 have also frequently been associated with depressive symptoms, ranging from mild depressive symptoms to major depression (9). However, reports of the relationship between IL-6 and depressive symptoms are inconsistent. Several studies (10,11) have failed to find a significant relationship between IL-6 and depressive symptoms, whereas in other studies (12) the relationship is attenuated when controlling for potential moderating or mediating variables, such as age, gender, and body mass index. Considering the presence of these factors that influence the relationship between depressive symptoms and IL-6, it is possible that other environmental or behavioral factors may also moderate this relationship.
Current health guidelines (13) recommended engaging in moderate-intensity physical activity for at least 30 minutes on 5 days of the week. Time spent in moderate-intensity physical activity has been associated with a lower prevalence of depressive symptoms in prospective studies of community-based samples (14,15), whereas exercise interventions have been shown to be effective in the alleviation of depressive symptoms (16). Similar exercise interventions have also been successful in reducing IL-6 levels (17). Because moderate-intensity exercise behavior affects both depressive symptoms and IL-6, the association between depressive symptoms and IL-6 may be dependent on, or moderated by, activity level.
The purpose of the current research was to examine the potential moderating effect of moderate-intensity physical activity on the relationship between depressive symptoms and IL-6 in a sample at risk for high levels of both depressive symptoms and inflammation—middle aged and older, lower income primary care patients with modest to high disease burden. We hypothesized that participation in moderate-intensity physical activity would moderate the association between depressive symptoms and IL-6. Specifically, we expected that individuals who do not participate in moderate-intensity physical activity would show the strongest positive associations among IL-6 and depressive symptoms.
METHODS
Design
Individuals, aged ≥40 years, were recruited through a university-affiliated, freestanding family medicine clinic during 2006 to 2007. Patients were approached in person during clinic visits or through flyers available at the clinic and were invited to attend a research session. After providing written informed consent, using procedures approved by the University of Rochester Research Subjects Review board, participants completed a research interview and provided blood samples via venipuncture performed by a trained phlebotomist.
Outcome Measures
IL-6
After venipuncture, the blood sample was kept on ice, centrifuged, and the serum collection was stored at −80°C. Serum IL-6 concentrations were determined via assay using standard enzyme-linked immunosorbent assay protocols and high-sensitivity, anti-cytokine antibody pairs (BD Biosciences, San Diego, California). Sensitivity of the assay showed the lowest detectable level of 0.0001 pg/mL. Intra- and interassay coefficients of variation were <5%.
Depressive Symptoms
The Center for Epidemiologic Studies Depression Scale-Revised (CESD-R) is a well-validated 20-item measure of depressive symptoms apparent in the previous week (18). In many populations, large proportions of individuals with the Center for Epidemiologic Studies Depression Scale (CES-D) scores of >15 meet the diagnostic criteria for major depression (19). Responses involve a 4-point Likert scale that ranges from 0 (“not at all”) to 3 (“nearly every day”). The Cronbach’s α for the CESD-R in the current sample was 0.93.
Physical Activity
The modified Community Health Activities Model Program for Seniors (20) included 14 items aimed to assess physical activity over the previous month. Each item asks the respondent to indicate whether they have participated in an activity (“Yes” or “No”). Ten questions included in the questionnaire assessed participation in activities assigned a value of ≥3.0 metabolic equivalents (METs) and were classified “moderate-intensity physical activity” (e.g., running, bicycling, swimming, weight lifting). This cutoff matches the current American College of Sports Medicine definition for moderate-intensity physical activity that is used in the most recent public health guidelines (13). The remaining four items assessed participation in lower-intensity activities (i.e., walking) and were excluded from further analysis.
Covariates
Several control variables were used. These were based on self-report items and validated through chart reviewers for a random subset of patients. Variables included current smoker (yes/no); age and gender; a count of the following conditions selected for their prevalence in primary care: hypertension, hypothyroidism, cardiovascular disease, cancer, and diabetes; and body weight. The latter was obtained strictly from chart reviews and was available for 80 patients.
Statistical Analysis
Linear regression analyses with robust standard errors were conducted to examine the moderating effect of physical activity on the relationship between depressive symptoms and IL-6. An interaction term tested the hypothesis that the depressive symptoms-IL-6 association varied by moderate-intensity physical activity. The statistical interaction test used product terms created from this continuous measure of activity. Graphical presentation of the interaction plotted the regression slopes in nonexercisers versus exercisers, based on the natural shape of the sample distribution (approximately half the sample had 0 MET, indicating no participation in moderate-level activity); a mixture model with two components corresponding to exercisers versus nonexercisers supported this distinction; likelihood ratio test versus 1-component distribution (χ2 = 69.99, df = 1, p < .001). Secondary analysis also controlled for medical illness using a morbidity index consisting of the sum of chronic illness reported by patients, smoking, age, and gender, as they are consistently associated with IL-6 levels. Body weight based on medical chart was available for 80 patients; so, an additional analysis controlling for this, using multiple imputation for those 17 missing body weight data, was also conducted. Those missing body weight data were demographically similar to those with the data.
RESULTS
Of 107 participants recruited and interviewed, 99 provided complete data for depressive symptoms, IL-6, and participation in moderate-intensity physical activity. Two participants had IL-6 levels of >3 standard deviations above the sample mean and were excluded from further analysis. After exclusion of the two outliers, skewness and kurtosis of IL-6 and CES-D were within an acceptable range (−1 to +1). Table 1 displays descriptive data about the sample. IL-6 levels were not significantly different between depressed and nondepressed patients (Table 1), and the Pearson’s correlation between IL-6 and depressive symptoms was nonsignificant (r = .086, p = .40). Moderate-intensity physical activity participation and depressive symptoms were also uncorrelated (r =−.082, p = .42). IL-6 was, however, significantly correlated with MET expenditure in moderate-intensity physical activity (r =−.217, p = .03).
TABLE 1.
Sample Descriptives
| Measure | Total Sample (n = 97) |
Nondepressed (n = 55) |
Depressed (n = 42) |
p |
|---|---|---|---|---|
| Age (years) | 52.4 (9.1) | 53.1 (10.5) | 51.4 (6.8) | .36 |
| Female, n (%) | 73 (75) | 44 (80) | 29 (69) | .30 |
| Medical illnessa | 0.6 (0.8) | 0.6 (0.8) | 0.7 (0.9) | .51 |
| Depressive symptoms (CES-D) | 16.5 (12.9) | 7.1 (4.3) | 28.7 (9.6) | <.001 |
| IL-6 (pg/mL) | 3.6 (2.5) | 3.5 (2.5) | 3.8 (2.5) | .47 |
| Physical activity (MET × Hours) | 9.0 (19.8) | 11.3 (24.5) | 6.0 (10.8) | .19 |
| Regular moderate-intensity physical activity, n (%) | 50 (51.5) | 31 (56.4) | 19 (45.2) | .11 |
| Weight (lb)b | 201.3 (46.7) | 194.1 (42.5) | 211.8 (51.3) | .10 |
| Current Smoker, n (%) | 41 (44) | 6 (32) | 13 (68) | .06 |
Data are reported as mean (standard deviation) values, unless otherwise indicated. The p value is based on t tests for continuous variables and χ2 test for dichotomous variables.
Dichotomization of CES-D score of >16, indicating clinically significant depressive symptoms. Regular moderate intensity exercise based on distribution of METs.
Count of hypertension, cardiovascular disease, cancer, diabetes, and hypothyroidism.
n = 80 participants who had weight data.
CES-D = Center for Epidemiological Studies Depression Scale; IL = interleukin; MET = metabolic equivalent.
Results from four linear regression analyses are presented in Table 2. The association between depressive symptoms and IL-6 was not significant in Model 1, which predicted IL-6 from depressive symptoms score. Model 2 indicated that the association between depressive symptoms and IL-6 remained nonsignificant after the addition of moderate-intensity physical activity participation to the model. However, exercisers had circulating IL-6 levels roughly 1.2 pg/mL lower than those who did not. Finally, Model 3 includes the depressive symptoms-physical activity interaction term, which was significant. Figure 1 illustrates the association between increasing depressive symptoms scores and increasing circulating IL-6 in those who did not engage in moderate-intensity physical activity (i.e., MET score of 0 for moderate activity, about half the sample), and the lack of association between depressive symptoms and IL-6 among those who engaged in moderate-intensity physical activity. Among those who did not engage in moderate-intensity physical activity, higher levels of depressive symptoms were significantly associated with higher levels of IL-6 (r = .28, p = .05). In contrast, depressive symptoms were not significantly associated with IL-6 among individuals engaging in moderate-intensity physical activity (r =−.13, p = .38). Model 4 presents the secondary analysis, replicating Model 3, at the same time controlling for disease count, smoking status, age, and gender. The interaction between depressive symptoms and participation in moderate-intensity physical activity remained significant. Further analysis also controlling for body weight and using multiple imputation for the 17 subjects missing weight data revealed similar results (interaction term B [standard error] = −0.0015 (0.0007), t =−1.99, p = .05).
TABLE 2.
Interleukin-6 in Relation to Depressive Symptoms and Moderate-Intensity Physical Activity Participation
| Measure | B | SE | t | p | |
|---|---|---|---|---|---|
| Model 1 | Constant | 3.617 | 0.251 | 14.37 | <.001 |
| Depressive symptoms (CES-D) | 0.016 | 0.019 | 0.82 | .41 | |
| Model 2 | Constant | 3.617 | 0.252 | 14.37 | <.001 |
| Depressive symptoms | 0.026 | 0.019 | 1.34 | .182 | |
| METs, moderate activity | −0.026 | 0.013 | −1.95 | .054 | |
| Model 3 | Constant | 3.581 | 0.250 | 14.31 | <.001 |
| Depressive symptoms | 0.031 | 0.02 | 1.58 | .11 | |
| METs, moderate activity | −0.041 | 0.011 | −2.43 | .001 | |
| Depressive symptoms × METs, moderate activity | −0.002 | 0.0007 | −2.43 | .02 | |
| Model 4 | Constant | 2.029 | 0.539 | 3.76 | <.001 |
| Depressive symptoms (CES-D) | 0.037 | 0.019 | 1.99 | .05 | |
| Age (years) | 0.029 | 0.034 | 0.87 | .39 | |
| Female | 1.54 | 0.512 | 3.02 | .003 | |
| Medical illness | 0.601 | 0.339 | 1.77 | .08 | |
| Smoker | −0.020 | 0.507 | −0.04 | .97 | |
| METs, moderate activity | −0.029 | 0.011 | −2.66 | .009 | |
| Depressive symptoms × METs, moderate activity | −0.002 | 0.0007 | −2.15 | .03 |
Depressive symptoms from CESD-R (Center for Epidemiologic Studies Depression Scale-Revised), METs from Community Health Activities Program Model for Seniors, CES-D depressive symptoms scale in raw units (range, 0 – 48; standard deviation, 13.49).
SE = standard error; CES-D = Center for Epidemiologic Studies Depression Scale; METs = metabolic equivalents; medical illness = disease count of cancer, hypertension, cardiovascular disease, hypothyroidism, and diabetes.
Figure 1.
Depressive symptoms and interleukin (IL)-6 by exercise participation. MIPA = moderate intensity physical activity; CES-D = Center for Epidemiological Studies Depression Scale.
DISCUSSION
Results support the hypothesis that participation in moderate-intensity physical activity moderated the relationship between depressive symptoms and IL-6. Among individuals who engaged in less moderate-intensity physical activity, higher depressive symptoms were associated with higher IL-6 levels, whereas virtually no association between IL-6 and depressive symptoms was observed among those who engaged in higher levels of moderate-intensity physical activity. These results suggest that, at low levels of depressive symptoms, there is no difference in IL-6 levels across physical activity levels, but as depressive symptoms increase, one begins to see an association between physical activity and IL-6. An equivalent interpretation is that depression and IL-6 seem associated only among relatively inactive people, whereas increasing levels of physical activity seem to diminish this association. Our findings may, therefore, help to explain the heterogeneity in the depressive symptoms-inflammation literature. Our findings are consistent with epidemiological (21) and intervention (17) studies that have associated exercise with lower levels of IL-6. Furthermore, our findings may help to explain the heterogeneity in the depressive symptoms-inflammation literature.
The observations of the current research should be interpreted with caution and require further replication. However, the presence of a significant interaction in a relatively small sample potentially speaks to the strong effect of moderate-intensity physical activity on the relationship between depressive symptoms and IL-6. The cross-sectional nature of the data, while allowing determination of moderators, precludes the examination of causality. Additionally, retrospective reports of exercise behaviors may not provide the most accurate information on actual physical activity behavior. A subset of morning blood collections occurred. However, this likely introduced random variation into IL-6 levels, as time of blood collection was not a function of depressive symptoms or participation in moderate-intensity physical activity, making it more rather than less difficult to detect systematic associations. Thus, our estimates of associations may be conservative. Nevertheless, we note this as a caveat. Also related to our measurement of inflammation was our choice to focus solely on IL-6 as a marker for inflammation. Future studies might consider using additional markers of inflammation, such as C-reactive protein, which has been associated with depression (22); the observations of a sample of urban primary care patients may not generalize to other segments of the population. Although the mean CES-D score in our sample was above the current cutoff for major depression, it is lower than what would be expected in a sample of participants with diagnosable depressive disorders. Also, our sample was highly inactive (50% participating in no moderate-intensity physical activity), which is not surprising given the demographics of the sample, as older age, lower socioeconomic status, and racial minorities are all associated with lower levels of physical activity (23). Future studies should aim to replicate these findings across broader segments of the population.
In conclusion, we found that participation in moderate-intensity physical activity moderated the relationship between IL-6 and depressive symptoms. Continued research will help clarify the potential mechanisms underlying these associations and may lead to improved, more specifically targeted treatment strategies. At the present time, clinicians should be aware that the health benefits of physical activity may be of particular importance to individuals with elevated depressive symptoms.
Acknowledgments
We thank Paul Duberstein, PhD, Nancy Talbot, PhD, Ayesha Khan, MD, Mary Harper, and the staff, patients, and providers of Highland Family Medicine Clinic for their assistance.
This work was supported, in part, by Grants K24MH71509 and T32MH073452 (J.M.L.) R21AG023956 (J.M.), 1R24AG031089-01 (J.M.), and K08AG031328 (B.P.C.) from the National Institutes of Health (NIH). The study was also supported, in part, by General Clinical Research Center Grant 5 MO1 RR00044 from the National Center for Research Resources, NIH.
Glossary
- IL
interleukin
- CESD-R
Center for Epidemiologic Studies Depression Scale-Revised
REFERENCES
- 1.Murray CJL, Lopez AD. The global burden of disease: a comprehensive assessment of mortality and disability from diseases, injuries and risk factors in 1990 and projected to 2020. Global Burden of Disease and Injury Series. 1996;1:1–900. [Google Scholar]
- 2.Lyness JM, Niculescu A, Tu X, Reynolds CF, 3rd, Caine ED. The relationship of medical comorbidity and depression in older, primary care patients. Psychosomatics. 2006;47:435–9. doi: 10.1176/appi.psy.47.5.435. [DOI] [PubMed] [Google Scholar]
- 3.Wulsin LR, Vaillant GE, Wells VE. A systematic review of the mortality of depression. Psychosom Med. 1999;61:6–17. doi: 10.1097/00006842-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–72. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 6.Pradhan AD, Manson JAE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 7.Il’yasova D, Colbert LH, Harris TB, Newman AB, Bauer DC, Satterfield S, Kritchevsky SB. Circulating levels of inflammatory markers andcancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:2413–8. doi: 10.1158/1055-9965.EPI-05-0316. [DOI] [PubMed] [Google Scholar]
- 8.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–12. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 9.Tiemeier H, Hofman A, van Tuijl HR, Kiliaan AJ, Meijer J, Breteler MMB. Inflammatory proteins and depression in the elderly. Epidemiology. 2003:103–7. doi: 10.1097/00001648-200301000-00025. [DOI] [PubMed] [Google Scholar]
- 10.Steptoe A, Kunz-Ebrecht SR, Owen N. Lack of association between depressive symptoms and markers of immune and vascular inflammation in middle-aged men and women. Psychol Med. 2003;33:667–74. doi: 10.1017/s0033291702007250. [DOI] [PubMed] [Google Scholar]
- 11.Lesperance F, Frasure-Smith N, Theroux P, Irwin M. The association between major depression and levels of soluble intercellular adhesion molecule 1, interleukin-6, and C-reactive protein in patients with recent acute coronary syndromes. Am J Psychiatry. 2004;161:271–7. doi: 10.1176/appi.ajp.161.2.271. [DOI] [PubMed] [Google Scholar]
- 12.Haack M, Hinze-Selch D, Fenzel T, Kraus T, Kühn M, Schuld A, Pollmacher T. Plasma levels of cytokines and soluble cytokine receptors in psychiatric patients upon hospital admission: effects of confounding factors and diagnosis. J Psychiatr Res. 1999;33:407–18. doi: 10.1016/s0022-3956(99)00021-7. [DOI] [PubMed] [Google Scholar]
- 13.Haskell WL, Lee IM, Pate RR, Powell K, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–93. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 14.Farmer ME, Locke BEN, Moscicki EVEK, Dannenberg AL, Larson DB, Radloff LS. Physical activity and depressive symptoms: the NHANES I epidemiologic follow-up study. Am J Epidemiol. 1988;128:1340–51. doi: 10.1093/oxfordjournals.aje.a115087. [DOI] [PubMed] [Google Scholar]
- 15.Camacho TC, Roberts RE, Lazarus NB, Kaplan GA, Cohen RD. Physical activity and depression: evidence from the Alameda County study. Am J Epidemiol. 1991;134:220–31. doi: 10.1093/oxfordjournals.aje.a116074. [DOI] [PubMed] [Google Scholar]
- 16.Rethorst CD, Wipfli BM, Landers DM. The antidepressive effects of exercise: a meta-analysis of randomized trials. Sports Med. 2009;39:491–511. doi: 10.2165/00007256-200939060-00004. [DOI] [PubMed] [Google Scholar]
- 17.Kohut M, McCann D, Russell D, Konopka D, Cunnick J, Franke W, Catillo MC, Reighard AE, Vanderah E. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of β-blockers, BMI, and psychosocial factors in older adults. Brain Behav Immun. 2006;20:201–9. doi: 10.1016/j.bbi.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Eaton WW, Smith C, Ybarra M, Muntaner C, Tien A. Center for Epidemiologic Studies Depression Scale: review and revision (CESD and CESD-R) In: Maruish M, editor. Use of Psychological Testing for Treatment Planning and Outcomes Assessment. Lawrence Erlbaum Associates; Princeton, NJ: 2004. [Google Scholar]
- 19.Schulberg HC, Saul M, McClelland M, Ganguli M, Christy W, Frank R. Assessing depression in primary medical and psychiatric practices. Arch Gen Psychiatry. 1985;42:1164–70. doi: 10.1001/archpsyc.1985.01790350038008. [DOI] [PubMed] [Google Scholar]
- 20.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33:1126–41. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Colbert LH, Visser M, Simonsick EM, Tracy RP, Newman AB, Kritchevsky SB, Pahor M, Taaffe DR, Brach J, Subin S, Harris TB. Physical activity, exercise, and inflammatory markers in older adults: findings from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2004;52:1098–104. doi: 10.1111/j.1532-5415.2004.52307.x. [DOI] [PubMed] [Google Scholar]
- 22.Ford DE, Erlinger TP. Depression and C-reactive protein in US adults: data from the third National Health and Nutrition Examination Survey. Arch Intern Med. 2004;164:1010–4. doi: 10.1001/archinte.164.9.1010. [DOI] [PubMed] [Google Scholar]
- 23.Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, Buchner D, Ettinger W, Heath GW, King AC, Kriska A, Leon AS, Marcus BH, Morris J, Paffenbarger RS, Patrick K, Pollock ML, Rippe JM, Sallis J, Wilmore JH. Physical activity and public health: a recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–7. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]