Abstract
Barriers to successful lung xenotransplantation appear to be even greater than for other organs. This difficulty may be related to several macro anatomic factors, such as the uniquely fragile lung parenchyma and associated blood supply that results in heightened vulnerability of graft function to segmental or lobar airway flooding caused by loss of vascular integrity (also applicable to allotransplants). There are also micro-anatomic considerations, such as the presence of large numbers of resident inflammatory cells, such as pulmonary intravascular macrophages and natural killer (NK) T cells, and the high levels of von Willebrand factor (vWF) associated with the microvasculature. We have considered what developments would be necessary to allow successful clinical lung xenotransplantation. We suggest this will only be achieved by multiple genetic modifications of the organ-source pig, in particular to render the vasculature resistant to thrombosis. The major problems that require to be overcome are multiple and include (i) the innate immune response (antibody, complement, donor pulmonary and recipient macrophages, monocytes, neutrophils, and NK cells), (ii) the adaptive immune response (T and B cells), (iii) coagulation dysregulation, and (iv) an inflammatory response (e.g., TNF-α, IL-6, HMGB1, C-reactive protein). We propose that the genetic manipulation required to provide normal thromboregulation alone may include the introduction of genes for human thrombomodulin/endothelial protein C-receptor, and/or tissue factor pathway inhibitor, and/or CD39/CD73; the problem of pig vWF may also need to be addressed. It would appear that exploration of every available therapeutic path will be required if lung xenotransplantation is to be successful. To initiate a clinical trial of lung xenotransplantation, even as a bridge to allotransplantation (with a realistic possibility of survival long enough for a human lung allograft to be obtained), significant advances and much experimental work will be required. Nevertheless, with the steadily increasing developments in techniques of genetic engineering of pigs, we are optimistic that the goal of successful clinical lung xenotransplantation can be achieved within the foreseeable future. The optimistic view would be that if experimental pig lung xenotransplantation could be successfully managed, it is likely that clinical application of this and all other forms of xenotransplantation would become more feasible.
Keywords: immune modulation; immune response; inflammation; pig, genetically engineered; thromboregulation; xenotransplantation, lung
Introduction
Many patients with end-stage lung disease (e.g., idiopathic pulmonary hypertension or that associated with congenital heart disease, interstitial pulmonary fibrosis, cystic fibrosis, sarcoidosis, emphysema, and those unfortunate individuals with destruction of the large airways) might benefit significantly from lung transplantation in terms of better quality of life and longer survival. Successful lung xenotransplantation, using pig lungs, could circumvent the huge barriers to access created by the limited number of lungs from deceased human donors that are available each year.
However, it is well-known that the barriers to successful lung xenotransplantation appear to be even greater than those of other organs, for example, heart, kidney, where there is still as yet no clinical applicability despite substantial progress over the past decade [1–24]. This may be related to several anatomic factors, such as the uniquely fragile structure of the lung parenchyma and associated blood supply that results in heightened vulnerability of organ function to segmental or lobar airway flooding caused by loss of vascular integrity, which is also pertinent to acute respiratory distress syndrome (ARDS) or non-cardiac pulmonary edema. These factors are compounded by micro-anatomic considerations, such as the presence of large numbers of resident inflammatory cells, such as pulmonary intravascular macrophages and natural killer (NK) cells [15,18,19,25], and the high levels of von Willebrand factor (vWF) associated with the microvasculature. These clearly are also important issues in human allotransplantation. Physiologic differences in characteristics of the pulmonary vascular endothelium due to rheology, expression of adhesion molecules, or nitric oxide or prostanoid metabolism [19,25–27] and susceptibility of the lung vasculature to increased resistance sufficient to precipitate right heart failure and low cardiac output are other possible contributors to the lung’s particular vulnerability to vascular injury and thrombosis. All of these mechanisms can be implicated in ARDS, ischemia-reperfusion injury, and vascular injury after allotransplantation and are substantially compounded by cross-species molecular incompatibilities in the xenograft context.
Xenotransplantation of the lungs, therefore, presents possibly the greatest challenge to those of us in this field of research. The optimistic view would be that if experimental pig lung xenotransplantation could be successfully managed, it is likely that clinical application of this and all other forms of xenotransplantation would become more feasible.
We have considered what developments would be necessary to allow successful clinical lung xenotransplantation. Our initial major conclusion is that this will only be achieved by multiple genetic modifications of the organ-source pig, in particular to render the vasculature more compatible and resistant to thrombosis. Because it may be difficult and risky to administer long-term drug therapy, for example, potent anticoagulants and anti-platelet agents, we believe that pharmacologic systemic therapies are unlikely to make the major contribution. These approaches, however, may prove to be of additional therapeutic value, particularly if their use can be limited to brief intervals, as at the time of xenograft implantation. Nevertheless, such agents will be useful in possibly determining the mechanisms involved in pig lung graft failure, for example, the coagulation factors that may be playing a major role.
If a few specific and central coagulation pathway targets can be identified, and neutralized, the information obtained will indicate what genetic modifications would be required in the pig. To some extent, because of the rapidity of the pathologic events that take place when the pig lung is perfused with human blood, pig lung xenotransplantation might provide a sensitive early-warning model to predict how genetic manipulations or exogenous agents might improve survival of pig kidney or heart xenotransplantation. However, although some genetic modifications may be important for all organs, others may need to be organ-specific.
Present status of lung xenotransplantation
This topic has been reviewed by others [17,28] and, briefly, by us [29,30], will only be summarized here. Ex vivo perfusion of the pig lung with human blood has proved a valuable method of assessing the acute effect of any therapeutic measure on pig lung function [31–33] (reviewed in Cantu et al. [17]). When survival for longer than a few hours is anticipated, orthotopic lung xenotransplantation in non-human primates has been used [7,17,34]. The longest survival of a pig lung after transplantation into a non-human primate has been <5 days [19], although survival with the recipient totally dependent on the graft has been shorter.
This situation is in marked contrast to survival of pig hearts and kidneys, where heterotopic (non-life-supporting) heart grafts have functioned for periods of up to 6–8 months [35–38], and life-supporting orthotopic heart and kidney grafts have supported the recipient’s life for <2 months [39–41] and 2–3 months [42,43], respectively. Lung xenograft performance is more comparable to survival of pig liver grafts, which has not as of yet reached 10 days [44–46].
As with other pig vascularized organs, the pig lung can be injured by antibody binding and complement activation at the endothelial cell interface [9,32,33,47,48]. The lung also appears particularly susceptible to the effects of coagulation dysregulation [49,50]. Indeed, because of the multiple pathologic processes taking place, immediate or early pig lung graft failure has been termed “hyperacute pulmonary xenograft dysfunction” rather than “hyperacute pulmonary xenograft rejection [17].” Despite depletion of antibody and/or complement, and the use of lungs from genetically modified pigs (e.g., GTKO, CD46, CD55), lungs fail rapidly, with elevated pulmonary vascular resistance and massive pulmonary edema [2,17,30,32,33]. Thrombotic injury occurs, and the associated profound systemic hypotension (which requires inotropic support to maintain recipient hemodynamics) and consumptive coagulopathy remain major problems [13,14,24,51].
Apart from the problems associated with the xenotransplantation of any pig organ, as alluded to earlier, there appear to be some hurdles specific to the inflammatory responses to the lung vasculature and to thrombotic reactions within the pulmonary venous system and at the level of alveolar capillaries. For example, unlike cardiac and renal xenografts, the vasculature of the xenografted lung releases large quantities of vWF [51]. When human vWF binds to GP1b on human platelets, platelet activation and adhesion occur, but only if the platelets are subjected to shear stress [52–54]. In contrast, pig vWF binds to human or non-human primate GP1b on quiescent platelets, leading to platelet aggregation even in the absence of shear stress [14,55–57]. Primate xenoantibodies also bind to carbohydrate epitopes on the vWF and possibly to associated heparan sulfates released from pig lungs and do not remain bound to the lung vascular endothelium [10]. The vWF-xenoantibody complex has an enhanced capacity to aggregate human and other primate platelets. When lungs from vWF-deficient pigs have been utilized, graft failure has been more typical of the hyperacute rejection seen after heart or kidney xenotransplantation; antibody has been deposited along the graft endothelium and has been accompanied by complement activation, with associated interstitial hemorrhage and edema [35,58]. These observations suggest that vWF plays a major role in the pathogenesis of pulmonary xenograft failure.
The major problems that require to be overcome are therefore multiple and include (i) the innate immune response (antibody, complement, macrophages, monocytes, neutrophils, and NK cells), (ii) the adaptive immune response (T and B cells), (iii) coagulation dysregulation, and (iv) an inflammatory response (e.g., TNF-α, IL-6, HMGB1 [59], C-reactive protein). It would appear that exploration of every available therapeutic path will be required if lung xenotransplantation is to be successful.
Currently available genetically engineered pigs
An increasing number of genetically engineered pigs are becoming available worldwide (Table 1). However, at present, very few genetically engineered pigs express more than two or three modifications, and it will take some time before pigs with multiple genetic modifications are available in sufficient numbers to test in ex vivo and in vivo lung xenotransplantation models.
Table 1.
Genetically modified pigs currently available for xenotransplantation researcha
| Gal antigen deletion or “masking”‘ |
| α1,3-galactosyltransferase gene-knockout (GTKO) |
| Human H-transferase gene expression (expression of blood type O antigen) |
| Endo-beta-galactosidase C (reduction in Gal antigen expression) |
| Complement regulation by human complement-regulatory gene expression |
| CD46 (membrane cofactor protein) |
| CD55 (decay-accelerating factor) |
| CD59 (protectin or membrane inhibitor of reactive lysis) |
| Anticoagulation and anti-inflammatory gene expression or deletion |
| Human tissue factor pathway inhibitor (TFPI) |
| Human thrombomodulin |
| Human CD39 (ectonucleoside triphosphate diphosphohydrolase-1) |
| von Willebrand factor (vWF)-deficient (natural mutant) |
| Suppression of cellular immune response by gene expression or downregulation |
| Porcine CTLA4-Ig (Cytotoxic T-Lymphocyte Antigen 4 or CD152) |
| LEA29Y (Inhibition of the B7/CD28 co-stimulatory pathway of T-cell activation) |
| CIITA-DN (MHC class II transactivator-knockdown, resulting in swine leukocyte antigen class II knockdown) |
| Human TRAIL (tumor necrosis factor-alpha-related apoptosis-inducing ligand) |
| HLA-E/human β2-microglobulin (inhibits human natural killer cell cytotoxicity) |
| Human CD47 (for species-specific CD47-signal regulatory protein-alpha natural interaction on macrophages) |
| Human FAS ligand (CD95L) |
| Human GnT-III (N-acetylglucosaminyltransferase III) gene |
| Anticoagulation, anti-inflammatory, and anti-apoptotic gene expression |
| Human A20 (tumor necrosis factor-alpha-induced protein 3) |
| Human heme oxygenase-1 (HO-1) |
| Human TNFRI-Fc (tumor necrosis factor-alpha receptor I-Fc) |
| Prevention of porcine endogenous retrovirus (PERV) activation |
| PERV siRNA |
Modified from Cooper DKC208.
Pigs with combinations of genetic modification, for example, GTKO with added transgenes are available.
Hearts from α1,3-galactosyltransferase gene-knockout (GTKO) pigs transgenic for expression of CD46 and/or CD55 appear largely resistant to hyperacute rejection in non-human primates and therefore could be the basis for further genetic manipulation (Fig. 1). When combined with potent immunosuppressive therapy, they also go a long way to resist classical acute humoral rejection, and acute cellular rejection can also be prevented or significantly delayed.
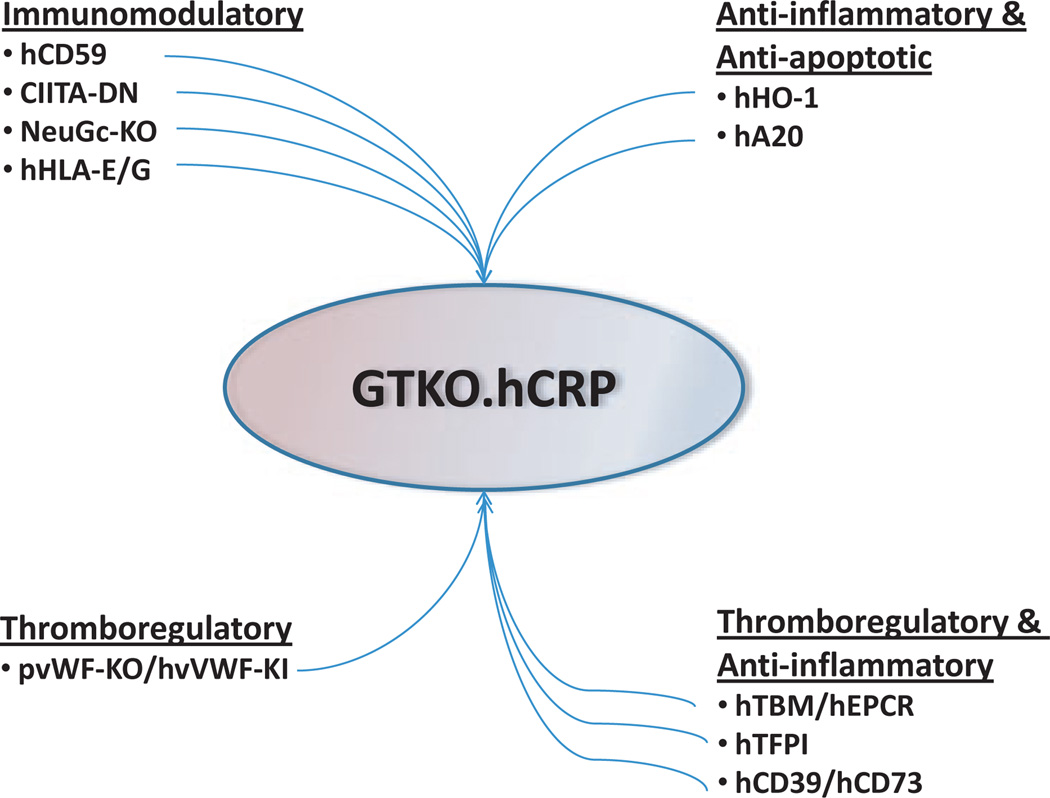
Fig. 1.
Genetic manipulations of pigs that are likely to be required to provide lungs for successful clinical xenotransplantation. hCRP = transgenic expression of one or more human complement-regulatory proteins, for example, CD46+/− CD55.
However, the currently available genetically engineered pigs whose organs have been transplanted into non-human primates do not fully prevent the activity of innate immune cells, including NK cells, macrophages, monocytes, and neutrophils [58], and neither do these genetic modifications prevent the consumptive coagulopathy that develops [60].
What pig genetic modifications will be necessary?
Immunomodulatory
Antibody and complement
In view of the lung’s susceptibility to immune and non-immune injury, in addition to GTKO, the expression in the pig of more than one human complement-regulatory protein, for example, CD46, CD55, and CD59, may prove beneficial [34,61–66], even if just to increase the overall level of complement-regulatory proteins (Fig. 1).
NonGal antigens
For clinical lung xenotransplantation (as opposed to experimental pig lung transplantation in non-human primates), the current evidence suggests that the expression of N-glycolylneuraminic acid (NeuGc) on the pig vascular endothelium will be detrimental [67]. The absence of expression of NeuGc in CMP-N-acetylneuraminic acid hydroxylase gene-knockout (NeuGc-KO) pigs will reduce anti-nonGal antibody binding, and its numerous sequelae [68–73].
The nature of other nonGal antigen targets remains elusive [74–80], although genomic and proteomic studies have identified some potential targets of anti-nonGal antibody [76,77]. It remains to be determined whether these are expressed in the lung, whether NeuGc decorates these proteins, and how GTKO impacts the high mannose or other epitopes. It is probably unrealistic, even with modern genetic engineering, to alter all or even most of the targets of anti-nonGal antibodies. However, it may be necessary and practical to pursue the most immunogenic antigens and determine the protein or carbohydrate bound by the antibody. To that end, genetic modifications could be made that may affect numerous proteins and carbohydrates that bear those epitopes. These strategies should be designed to remove the dominant antigens while preserving anti-thrombotic properties of the associated vascular proteoglycans, such as heparan sulfate.
Natural killer and related innate immune cells
Although attention has been drawn to the fact that the magnitude of the role of NK and NKT cells in xenograft rejection remains uncertain and that it may be controlled by exogenous immunosuppressive therapy [81], we suggest that, in the case of lung xenotransplantation, genetic engineering directed toward inhibiting NK cell activity may prove valuable [82–86]. The expression of HLA-E and/or G and/or Cw3, therefore, is likely to inhibit NK cell function, though, perhaps not completely across species barriers [87–93]. Pigs transgenic for overexpression of human HLA-E have been produced in combination (through breeding) with GTKO/hCD46 genetics (E. Wolf, personal communication).
The adaptive (T cell) immune response
Any genetic manipulations that allow reduction in the exogenous immunosuppressive therapy required to control the primate adaptive immune response to the pig lung will be invaluable. The evidence is that pigs with MHC class II transactivator-knockdown (CIITA-DN) will reduce the adaptive T-cell response [94]. Expression of CTLA4-Ig would also be beneficial in neutralizing the adaptive response, but pigs that express CTLA4-Ig ubiquitously have been shown not to be viable [95], and therefore, expression would be required selectively on the endothelial cells. As it may prove difficult to obtain the optimal level of immunosuppression by the endogenous expression of CTLA4-Ig, with a risk of over-immunosuppression rendering the pig liable to infectious complications [95], it may be wiser to administer exogenous CTLA4-Ig, if this is required for a limited time.
The problem of pig pulmonary macrophages
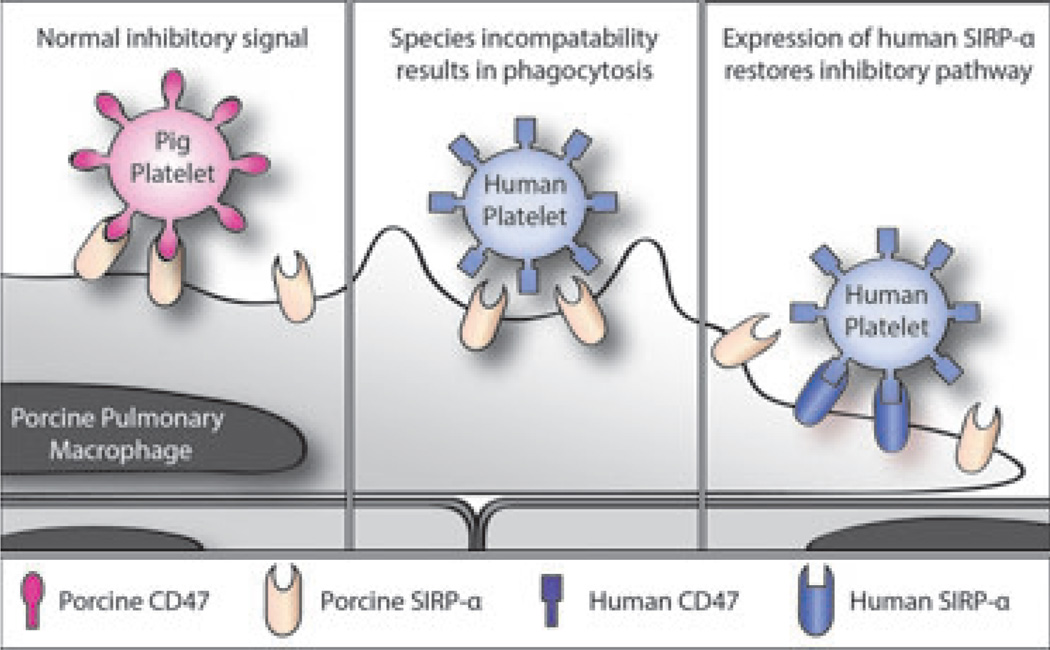
Pig lungs contain both alveolar and intravascular macrophages. The latter comprise a significant percentage (estimated at about 16%) of the endothelial surface of the microvasculature [96–98]. Pig macrophages, like hepatic Kupffer cells, can remove debris, microparticles, and primate pla telets from the circulation and are also major sources of inflammatory cytokines (e.g., TNF-α, IL-1, IL-6), metabolites (e.g., thromboxane), and procoagulant factors (e.g., tissue factor, PAI-1) [8,25,99,100]. Pig macrophages, including those resident in the lung, recognize and may phagocytose human platelets and blood cells because they are identified as foreign bodies [101]. This may be due in part to species incompatibility of the signal regulatory protein-alpha (SIRP-α) expressed on macrophages that enables the cell to identify “self” from “non-self” and control phagocytosis (Fig. 2). The “self”-confirming ligand of SIRP-α, CD47, is expressed on platelets and blood cells as well as on most other tissues.
Fig. 2.
Schematic representation of CD47-signal regulatory protein-alpha (SIRP-α) interaction in relation to natural expression of SIRP-α on pig pulmonary macrophages. Left: In the organ-source pig, there is a normal inhibitory signal of porcine CD47 (in this example expressed on platelets) that is recognized by porcine SIRP-α. Center: After pig lung xenotransplantation, there might be a lack of recognition of human CD47 by porcine SIRP-α, resulting in phagocytosis of human platelets. (Some binding between SIRP-α and CD47 occurs, but there is a deficiency in signaling that prevents signal transduction, the cause of which is uncertain.) Right: Transgenic expression of human SIRP-α on pig pulmonary macro-phages would result in recognition of human CD47 on human platelets, thus inhibiting phagocytosis.
When human SIRP-α recognizes human CD47 (e.g., on human platelets), human macrophages are not activated and phagocytosis of CD47-expressing cells does not occur [102–105]. While it is known that human SIRP-α can bind pig CD47, it is not known whether pig SIRP-α can recognize human CD47 [106]. To prevent phagocytosis of human platelets by pig macrophages, it might be necessary to express human SIRP-α on pig macrophages (Fig. 2). It is also feasible to impact these proinflammatory pathways at another site, such as in the context of CD39-mediated scavenging of extracellular nucleotides given the associated requirements in this process for chemotaxis, phagocytosis, and platelet activation, which are all dependent upon purinergic signaling [107,108].
There are several factors, however, that may complicate human SIRP-α expression. Cross-species binding of human SIRP-α to pig CD47 has a significantly higher binding affinity than to human CD47 yet does not produce an inhibitory tyrosine phosphorylation signal on SIRP-α [103,106]. Therefore, expression of human SIRP-α may compete with endogenous pig SIRP-α for binding to available pig and human CD47. Interestingly, studies with recombinant SIRP-α suggest that while human red blood cells are bound by SIRP-α, in contrast, human mesenchymal stem cells shown to express human CD47 are not bound [106]. This difference may be explained by altered binding affinity at or near the highly polymorphic domain 1 of human SIRP-α [109]. Thus, expression of human SIRP-α with appropriate affinity for CD47 ligand may be critical to inhibiting platelet phagocytosis.
Pre-transplant depletion of pig macrophages, for example, by clodronate liposomes, has been shown to be beneficial, with decreased thromboxane production, platelet sequestration, C3a levels, and TNF-α release, inhibition of rise in pulmonary vascular resistance, and prolonged graft function [22,25,110–112]. However, this approach may have potential deleterious effects on tolerance induction by impacting other antigen-presenting cells, as noted in renal xenotransplant models, that may preclude widespread application.
In addition, it has been shown that anti-GP1b Fab reduces activation and sequestration of human platelets in a xenogeneic pig lung perfusion model [113–115]. Depletion of macrophages in the pig before lung harvesting may resolve the problems associated with them, but it is uncertain whether they will be replaced by regenerating pig macrophages already resident in the graft, in which case the original problem may recur, or by human macrophages.
The problem of recipient macrophages
The primate recipient macrophages are important innate immune cells involved in pig graft rejection. The SIRP-α/CD47 interaction described earlier is also responsible for regulating pig graft rejection by primate recipient macrophages, whatever their location.
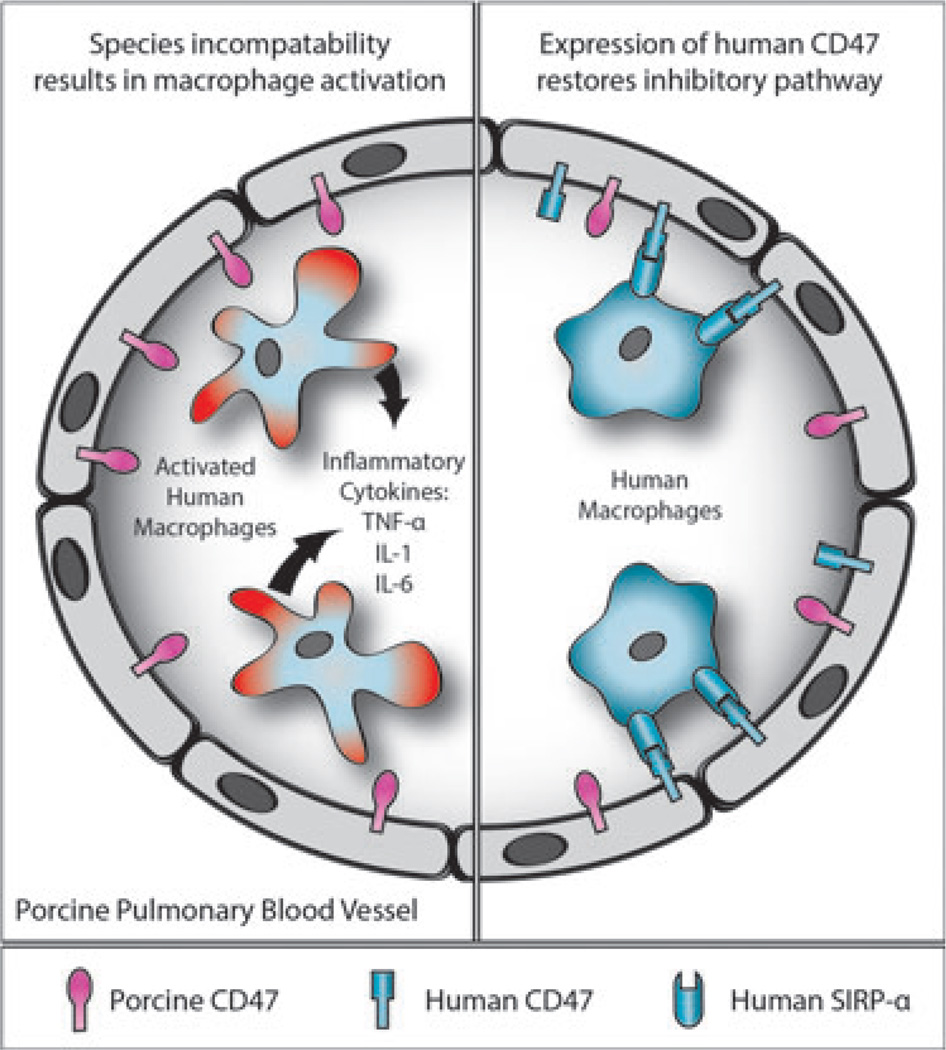
Expression of pig CD47 on pig platelets and cells does not inhibit the activation of human macrophages [103]. In the case of pig-to-human lung xenotransplantation (although, of course, phagocytosis of the pig organ does not occur), the absence of the inhibitory effect of the expression of human CD47 on the pig tissues may lead to activation of the recipient (primate) macrophages (Fig. 3). This may result in increased inflammatory responses with the production of TNF-α and other cytokines by the human macrophages.
Fig. 3.
Schematic representation of CD47-signal regulatory protein-alpha (SIRP-α) interaction in relation to natural expression of SIRP-α on human macrophages. Left: After transplantation of an unmodified pig lung into a human, the expression of pig CD47 on the endothelial cells of the pulmonary blood vessels will not be recognized by human SIRP-α-expressing macrophages, which will therefore not be inhibited but will become activated; inflammatory cytokines will be produced and graft injury will occur. Right: When a lung from a pig transgenic for human CD47 is transplanted, the human SIRP-α-expressing macrophages will recognize the pig tissues as “self,” and activation will be inhibited; cytokine production and graft injury will not occur.
Expression of human CD47 in the pig graft should inhibit human macrophage activity through its inhibitory effect on human SIRP-α (Fig. 3). But will transgenic expression of human CD47 in the same pig create problems for the pig? If pig CD47 also remains expressed in the pig, then this should continue to inhibit pig SIRP-α on pig macrophages, and no phagocytosis of the pig’s own cells and platelets should occur, although this remains uncertain. It is promising, however, that Tena et al. [116] recently announced not only several fetuses expressing human CD47, but the birth of a human CD47-expressing pig. The health and survival of this pig is currently being studied.
The generation of human CD47-expressing pigs and the recent production of viable human SIRP-α-expressing mice indicate a potential that CD47/ SIRP-α-signaling can be manipulated and still produce a viable pig that should exhibit less aberrant xenogeneic macrophage activity. It may be necessary to employ a method of inducible expression of human CD47, so that expression only occurs at the time of organ transplantation [117,118]. An alternative approach that warrants consideration is transgenic expression of both human SIRP-α and human CD47 in the pig (Dr. J. Estrada, personal communication); this might prevent phagocytosis of pig platelets and cells in the organ-source pig but should enable a human CD47-expressing lung to be transplanted into the human recipient. However, these models remain speculative.
What complicates this already complex topic further is that there is evidence that expression of CD47 on a cell is associated with an inflammatory state and an increase in vasoconstriction and pulmonary vascular resistance [119,120]. This is related to the role of CD47 as a receptor for thrombospondin-1 [121–122], which limits the angiogenic and vasodilator activities of nitric oxide. Complicating the role of CD47 is that thrombospondin-1 regulates T-cell survival via CD47 [123,124]. Of further relevance to lung xenotransplantation is the observation that thrombospondin-1-null mice are resistant to platelet aggregation by thrombin [125]. Any increase in CD47 expression (e.g., by transgenic expression of human CD47 in the pig) may therefore prove detrimental to the outcome of the graft, particularly a lung xenograft. With the many roles CD47 appears to play, knockout of the gene for CD47 would probably result in a non-viable pig. In summary, in regard to solid organ xenotransplantation, the problem of SIRP-α/CD47 remains unresolved and the exact approach to take is uncertain.
With the exception caused by doubt about what to do with regard to SIRP-α/CD47, the modifications mentioned earlier aimed at immunomodulation may be essential if successful clinical lung xenotransplantation is to be achieved (Fig. 1).
However, there may be other modifications that will provide additional or alternative benefits. For example, the expression of tumor necrosis factor (TNF)-α-related apoptosis-inducing ligand (TRAIL) should have some effect on the innate immune response [126]. GTKO/CD46/TRAIL pigs are now available [127]. When available, programmed cell death ligand-1 (PDL-1) pigs may provide additional protection against the adaptive immune cellular responses [128].
Thromboregulatory
The imbalance between human “coagulation” factors and pig ‘anticoagulation’ factors is both quantitative and qualitative. The already thrombophilic xenograft vasculature is further compromised once endothelial injury occurs as a result of the primate immune response, with acquired loss of cell-surface anticoagulant molecules resulting in a pro-thrombotic environment [129]. Despite long-term inhibition of the humoral response, in GTKO pig-to-baboon heart transplants, thrombotic microangiopathy ultimately develops in all grafts [35]. Therefore, protection from immune injury will help toward adequate thromboregulation and balanced coagulation-anticoagulation.
However, it is unlikely that this protection will be absolute, particularly in the environment of the pig lung, and so genetic manipulations to redress the thromboregulatory imbalance are likely to be essential. Once again, multiple pathways of coagulation may need to be interrupted or neutralized if coagulation dysregulation is to be overcome [57,130,131].
Pig thrombomodulin/endothelial protein C-receptor
Pig thrombomodulin is a poor activator of human thrombin, resulting in a low production of activated human protein C [132–135]. Pigs expressing human thrombomodulin have recently been produced [136–138]. Current evidence is that human thrombomodulin expression in pigs will go some way to reduce the coagulation dysfunction that is a major problem in xenotransplantation [139–141], but expression of human endothelial protein C-receptor (EPCR) may also be required for maximal effect [84,133,142–145]. Whether a genetically modified pig expressing both of these human genes will be viable is uncertain, but healthy pigs with constitutive expression of human EPCR have recently been produced (D. Ayares, personal communication).
Tissue factor pathway inhibitor (TFPI)
Even though there is evidence that pig TFPI may regulate human tissue factor pathways [146], additional expression of human TFPI may provide benefit [147–149]. However, it is likely that this will need to be expressed selectively on the endothelial cells of the vasculature, rather than ubiquitously at high levels in the pig (as previous attempts suggest constitutive-expressing TFPI-transgenic pigs are not viable [D. Ayares, unpublished]).
An alternative to expression of human TFPI may be expression of the anti-thrombin, protein, hirudin [148–150], or reduction in the expression of tissue factor in the pig, which could be achieved with siRNA technology or targeted, regulated suppression of expression [151–153].
CD39/CD73 and purinergic pathways
Expression of human CD39 (ecto-nucleoside triphosphate diphosphohydrolase [E-NTPDase], which is responsible for breakdown of ATP to ADP and then to AMP) and/or CD73 (ecto-5″-nucleotidase [E5′N], which is involved in the hydrolysis of extracellular AMP to adenosine) should prove beneficial for their thromboregulatory and anti-inflammatory effects (see below) and will also have some immunomodulatory impact [154– 166]. Expression of human CD39 alone should have a beneficial thromboregulatory effect and may be all that is required, but it may also be necessary to express human CD73, which may enhance this effect on the adenosine pathway. Further, there are data suggesting that CD73 overexpression may be beneficial in sepsis [167]. As with thrombomodulin/EPCR, the expression of both human CD39 and human CD73 at high constitutive levels in the pig might result in untoward effects.
Pig vWF
As outlined earlier, a major problem is the expression of pig vWF, and therefore, it is almost certain that a human vWF knock-in pig will be required (in which human vWF, or certain specific human domains) replaces pig vWF. (Of note, the longest pig lung graft survival in a non-human primate recorded to date involved the transplantation of a lung from a vWF-deficient pig that had been depleted of pulmonary intravascular macrophages [19]). However, due to the large size of the vWF locus (180 kb, 52 exons) and complexity of this gene, the development of a humanized vWF knock-in pig is likely to prove a major challenge to those involved in the genetic engineering of pigs and may be difficult to achieve. Selective manipulation of individual GP1b binding sites on pig vWF, to “humanize” them, may be easier to accomplish and is predicted to decrease non-physiologic interaction of pig vWF with human GP1b.
Another concern in this approach relates to potentially different levels of sialic acid expressed by platelet vWF receptors, specifically the GPIba subunit expressed by platelets and endothelium, to those in the recipient primate. These are important considerations in that relative desialylation levels may promote platelet clearance in both lung and liver xenotransplantation. Such potential incompatibilities may be further impacted by GTKO genetic manipulations in a deleterious manner.
Anti-inflammatory
There is increasing evidence that an inflammatory response does not resolve after pig organ xenotransplantation in non-human primates (M. Ezzelarab, unpublished). Furthermore, there is also increasing evidence of interaction between the coagulation–anticoagulation system and an inflammatory response and, indeed, between these two responses and the innate immune system [144,145, 168,169].
Following xenotransplantation, an inflammatory state may be more injurious to the fragile, highly vascularized lung than to organs such as the heart, as exemplified by the lung acting as the first and often principal site of injury in ARDS, as well as in the systemic inflammatory response syndrome (SIRS) and sepsis. Multiple anti-inflammatory genes may therefore be required for successful pig lung xenotransplantation. These are likely to include CD39 (+/− CD73), thrombomodulin (+/− EPCR) [140] (see above), and heme oxygenase-1 [170–172] (+/− A20) [172,173], all of which we believe may be required. Expression of several of these genes, for example, heme oxygenase-1 or A20, is likely to have the added advantage of reducing apoptosis of pig cells. Alternative or additional modifications that may need to be considered include expression of the TNF-α receptor, bcl, Lnk, and/or TRAIL [126,127,174,175].
Comment
The possible genetic modifications that may be required are summarized in Fig. 1, which illustrates the steep road ahead if we are to establish pig lung xenotransplantation in the clinical arena. Many of these genetic modifications, however, will almost certainly be beneficial if clinical heart, kidney, or liver xenotransplantation is the goal, and therefore, efforts made to genetically modify pigs will not only impact lung xenotransplantation. Success with the xenotransplantation of these other organs is likely to be achieved with fewer genetic manipulations, and progression to the clinic is likely to be earlier than envisaged for the lung.
The infusion of mesenchymal stem cells (of human or genetically engineered pig origin [176,177]) may provide immunomodulatory and anti-inflammatory effects, and ex vivo expansion of recipient T-regulatory cells (potentially CD4+ CD39+ populations) may also contribute to suppression of the immune response [178–183].
Unfortunately, there is no suitable in vitro model of lung xenotransplantation that could provide much valuable information, and therefore, we will need to persist with ex vivo pig lung perfusions with human blood and associated in vitro assays. This model is preferable to the more expensive and time-consuming in vivo orthotopic lung transplantation in baboons. However, a demonstration at intervals that the graft can support the life of the recipient is essential to confirm that real progress toward the clinic is being made. Whenever gas transfer and other physiologic parameters of graft function remain essentially normal for >6 h in the ex vivo lung perfusion model, evaluation of the translational promise of the approach by life-supporting orthotopic lung xenotransplantation in non-human primates should be carried out.
Although the barriers to clinical lung xenotransplantation are significant, they are certainly not insurmountable. However, if sufficient progress is to be made to initiate a clinical trial within the foreseeable future, novel approaches to speed up the process of genetic engineering of pigs will likely be essential. Steps are already underway to enable multiple genes to be expressed in the pig simultaneously [184–187], and these newer techniques may allow the rapid production of pigs with multiple genetic manipulations.
Even if existing techniques are not entirely successful, the technology of genetic engineering is steadily improving and new techniques are being introduced, such as zinc finger nucleases [188–194], transcription activator-like effector nucleases (TALENS) [195], meganucleases [196], sleeping beauty transposons [197], and the use of artificial chromosomes [198], which may lead to greater efficiency [199]. Interspecific blastocyst complementation and the in vivo generation of organs derived from xenogeneic donor pluripotent stem cells is in its infancy but may also have potential [200].
The ability to direct genes to specific tissues within the pig’s body, for example, islets, neuronal cells, or endothelial cells, has already been achieved with islet-specific gene expression, using an insulin promoter [201–203], and neuron-specific gene expression, using an enolase promoter [204–206]. Gene expression in the endothelial cells alone should overcome at least some of the complications of ubiquitous expression of a transgene [137,207]. The ability to switch an inducible transgene on or off should also prove beneficial [117,118]. A transgene that is found to be detrimental to the life of the pig when expressed ubiquitously or extensively in the source animal may prove entirely safe when expressed only in a single organ or in a specific cell type, for example, endothelium, after transplantation into the recipient. Such an approach may also facilitate repopulation of a xenograft with host progenitor cells and the development of a fully functional chimeric organ rendered fully tolerant to host immune responses.
Conclusions
To initiate a clinical trial of lung xenotransplantation, even as a bridge to allotransplantation (with a realistic possibility of survival long enough for a human lung allograft to be obtained), significant advances and much experimental work will be required. We suggest that the problems are much more likely to be overcome by genetic engineering of the pig than by drug administration to the recipient. We are optimistic that the goal of successful clinical lung xenotransplantation can be achieved within the foreseeable future. However, it will require the allocation of significant resources (manpower, facilities, and funding) if success is to be assured. Work aimed at achieving this goal will inevitably be immensely beneficial to the development of pigs that are suitable as sources of the other major organs.
Acknowledgments
Research on xenotransplantation at the University of Pittsburgh, the University of Maryland, and the Beth Israel Deaconess Medical Center is funded in part by NIH grant no. IU19A1090959-01, NIH grant no. U01A1066331, and by Sponsored Research Agreements with Revivicor Inc., Blacksburg, VA. Burcin Ekser, MD is a recipient of a NIH NIAID T32 AI 074490 Training Grant. Mohamed Ezzelarab, MD is supported in part by the Shelly Patrick Research Fellowship in Transplantation of the Thomas E. Starzl Transplantation Institute. Hidetaka Hara MD, PhD is supported in part by NIH grant no. 1RO3A1096296-01.
Abbreviations
- ARDS
acute respiratory distress syndrome
- GTKO
α1,3-galactosyltransferase gene-knockout
- NeuGc-KO
CMP-N-acetylneuraminic acid hydroxylase-knockout (NeuGc-knockout)
- TFPI
tissue factor pathway inhibitor
- TRAIL
tumor necrosis factor-α-related apoptosis-inducing ligand
- vWF
von Willebrand factor
Footnotes
Disclosure of conflict of interest
David Ayares and Carol Phelps are employees of Revivicor Inc. No other author has a conflict of interest.
References
- 1.Bryant LR, Eiseman B, Avery M. Studies of the porcine lung as an oxygenator for human blood. J Thorac Cardiovasc Surg. 1968;55:255–263. [Google Scholar]
- 2.Kaplon RJ, Platt JL, Kwiatkowski PA, et al. Absence of hyperacute rejection in pig-to-primate orthotopic pulmonary xenografts. Transplantation. 1995;59:410–416. [PubMed] [Google Scholar]
- 3.Kamholz SL, Brewer RJ, Grijalva G, et al. Laboratory studies in cross-species lung transplantation. World J Surg. 1997;21:951–955. doi: 10.1007/s002689900332. [DOI] [PubMed] [Google Scholar]
- 4.Macchiarini P, Mazmanian GM, Oriol R, et al. Ex vivo lung model of pig-to-human hyperacute xenograft rejection. J Thorac Cardiovasc Surg. 1997;114:315–325. doi: 10.1016/S0022-5223(97)70175-2. [DOI] [PubMed] [Google Scholar]
- 5.Macchiarini P, Oriol R, Azimzadeh A, et al. Evidence of human non-alpha-galactosyl antibodies involved in the hyperacute rejection of pig lungs and their removal by pig organ perfusion. J Thorac Cardiovasc Surg. 1998;116:831–843. doi: 10.1016/s0022-5223(98)00447-4. [DOI] [PubMed] [Google Scholar]
- 6.Daggett CW, Yeatman M, Lodge AJ, et al. Swine lungs expressing human complement-regulatory proteins are protected against acute pulmonary dysfunction in a human plasma perfusion model. J Thorac Cardiovasc Surg. 1997;113:390–398. doi: 10.1016/S0022-5223(97)70337-4. [DOI] [PubMed] [Google Scholar]
- 7.Daggett CW, Yeatman M, Lodge AJ, et al. Total respiratory support from swine lungs in primate recipients. J Thorac Cardiovasc Surg. 1998;115:19–27. doi: 10.1016/s0022-5223(98)70438-6. [DOI] [PubMed] [Google Scholar]
- 8.Blum MG, Collins BJ, Chang AC, Zhang JP, Knaus SA, Pierson RN3rd. Complement inhibition by FUT-175 and K76-COOH in a pig-to-human lung xenotransplant model. Xenotransplantation. 1998;5:35–43. doi: 10.1111/j.1399-3089.1998.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 9.Lau CL, Daggett WC, Yeatman MF, et al. The role of antibodies in dysfunction of pig-to-baboon pulmonary transplants. J Thorac Cardiovasc Surg. 2000;120:29–38. doi: 10.1067/mtc.2000.106841. [DOI] [PubMed] [Google Scholar]
- 10.Lau CL, Cantu E, 3rd., Gonzalez-Stawinski GV, et al. The role of antibodies and von Willebrand factor in discordant pulmonary xenotransplantation. Am J Transplant. 2003;3:1065–1075. doi: 10.1034/j.1600-6143.2003.00190.x. [DOI] [PubMed] [Google Scholar]
- 11.Wiebe K, Steinhoff G, Poeling J, et al. Ex vivo perfusion of swine lungs: lung function in a pig-to-human model of xenotransplantation. Transplant Proc. 2000;32:1149–1150. doi: 10.1016/s0041-1345(00)01161-1. [DOI] [PubMed] [Google Scholar]
- 12.Gaca JG, Lee W, Aksoy O, et al. Evidence of polyreactive xenoreactive antibodies in the repertoire of human anti-swine lung antibodies: the ‘next’ humoral barrier to xenotransplantation? Transplant Immunol. 2001;9:19–27. doi: 10.1016/s0966-3274(01)00047-8. [DOI] [PubMed] [Google Scholar]
- 13.Gaca JG, Lesher A, Aksoy O, et al. Disseminated intravascular coagulation in association with pig-to-primate pulmonary xenotransplantation. Transplantation. 2002;73:1717–1723. doi: 10.1097/00007890-200206150-00005. [DOI] [PubMed] [Google Scholar]
- 14.Gaca JG, Lesher A, Aksoy O, et al. The role of porcine von Willebrand factor-baboon platelet interactions in disseminated intravascular coagulation associated with pulmonary xenotransplantation. Transplantation. 2002;74:1596–1603. doi: 10.1097/00007890-200212150-00018. [DOI] [PubMed] [Google Scholar]
- 15.Gaca JG, Palestrant D, Lukes DJ, et al. Prevention of acute lung injury in swine: depletion of pulmonary intravascular macrophages using liposomal clodronate. J Surg Res. 2003;112:19–25. doi: 10.1016/s0022-4804(03)00142-2. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Stawinski GV, Daggett CW, Lau CL, et al. Non-anti-Galα1–3Gal antibody mechanisms are sufficient to cause hyperacute lung dysfunction in pulmonary xenotransplantation. J Am Coll Surg. 2002;194:765–773. doi: 10.1016/s1072-7515(02)01162-6. [DOI] [PubMed] [Google Scholar]
- 17.Cantu E, Parker W, Platt JL, Davis RD. Pulmonary xenotransplantation: rapidly progressing into the unknown. Am J Transplant. 2004;4(Suppl. 6):25–35. doi: 10.1111/j.1600-6135.2004.0342.x. [DOI] [PubMed] [Google Scholar]
- 18.Cantu E, Gaca JG, Palestrant D, et al. Depletion of pulmonary intravascular macrophages prevents hyperacute pulmonary xenograft dysfunction. Transplantation. 2006;81:1157–1164. doi: 10.1097/01.tp.0000169758.57679.2a. [DOI] [PubMed] [Google Scholar]
- 19.Cantu E, Balsara KR, Li B, et al. Prolonged function of macrophage, von Willebrand factor-deficient porcine pulmonary xenografts. Am J Transplant. 2007;7:66–75. doi: 10.1111/j.1600-6143.2006.01603.x. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen BN, Azimzadeh AM, Schroeder C, et al. Absence of Gal epitope prolongs survival of swine lungs in an ex vivo model of hyperacute rejection. Xenotransplantation. 2011;18:94–107. doi: 10.1111/j.1399-3089.2011.00633.x. [Erratum in Xenotransplantation 2011;18:267]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen B-NH, Azimzadeh AM, Zhang T, et al. Life-supporting function of genetically modified swine lungs in baboons. J Thorac Cardiovasc Surg. 2007;133:1354–1363. doi: 10.1016/j.jtcvs.2006.11.043. [Erratum J Thorac Cardiovasc Surg. 2008;135:28A]. [DOI] [PubMed] [Google Scholar]
- 22.Stoddard TA, Kelishadi SS, Zhang T, et al. Administration of clodronate confers added protection against HALR. J Surg Res. 2008;144:447. [Google Scholar]
- 23.Kim YT, Lee HJ, Lee SW, et al. Pre-treatment of porcine pulmonary xenograft with desmopressin: a novel strategy to attenuate platelet activation and systemic intravascular coagulation in an ex-vivo model of swine-to-human pulmonary xenotransplantation. Xenotransplantation. 2008;15:27–35. doi: 10.1111/j.1399-3089.2008.00445.x. [DOI] [PubMed] [Google Scholar]
- 24.Bush EL, Barbas AS, Holzknecht ZE, et al. Coagulopathy in α-galactosyl transferase knockout pulmonary xenotransplants. Xenotransplantation. 2011;18:6–13. doi: 10.1111/j.1399-3089.2011.00621.x. [DOI] [PubMed] [Google Scholar]
- 25.Collins BJ, Blum MG, Parker RE, et al. Thromboxane mediates pulmonary hypertension and lung inflammation during hyperacute lung rejection. J Appl Physiol. 2001;90:2257–2268. doi: 10.1152/jappl.2001.90.6.2257. [DOI] [PubMed] [Google Scholar]
- 26.Holzknecht ZE, Coombes S, Blocher BA, Lau CL, Davis RD, Platt JL. Identification of antigens on porcine pulmonary microvascular endothelial cells recognized by human xenoreactive natural antibodies. Lab Invest. 1999;79:763–773. [PubMed] [Google Scholar]
- 27.Knosalla C, Yazawa K, Behdad A, et al. Renal and cardiac endothelial heterogeneity impact acute vascular rejection in pig-to-baboon xenotransplantation. Am J Transplant. 2009;9:1006–1016. doi: 10.1111/j.1600-6143.2009.02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Platt J, DiSesa V, Gail D, Massicot-Fisher J. Recommendations of the National Heart, Lung, and Blood Institute Heart and Lung Xenotransplantation Working Group. Circulation. 2002;106:1043–1047. doi: 10.1161/01.cir.0000031064.67525.28. [DOI] [PubMed] [Google Scholar]
- 29.Ekser B, Rigotti P, Gridelli B, Cooper DKC. Xenotransplantation of solid organs in the pig-to-primate model. Transpl Immunol. 2009;21:87–92. doi: 10.1016/j.trim.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Pierson RN, 3rd., Dorling A, Ayares D, et al. Current status of xenotransplantation and prospects for clinical application. Xenotransplantation. 2009;16:263–280. doi: 10.1111/j.1399-3089.2009.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierson RN, 3rd., Dunning JJ, Konig WK, et al. Mechanisms governing the pace and character of pig heart-lung rejection by human blood. Transplant Proc. 1994;26:2337. [PubMed] [Google Scholar]
- 32.Pierson RN, 3rd., Kasper-Konig W, Tew DN, et al. Hyperacute lung rejection in a pig-to-human transplant model: the role of anti-pig antibody and complement. Transplantation. 1997;63:594–603. doi: 10.1097/00007890-199702270-00019. [DOI] [PubMed] [Google Scholar]
- 33.Pierson RN, III., Pino-Chavez G, Young VK, Kasper-Konig W, White DJG, Wallwork J. Expression of human decay accelerating factor may protect pig lung from hyperacute rejection by human blood. J Heart Lung Transplant. 1997;16:231–239. [PubMed] [Google Scholar]
- 34.Yeatman M, Daggett CW, Lau CL, et al. Human complement regulatory proteins protect swine lungs from xenogeneic injury. Ann Thorac Surg. 1999;67:769–775. doi: 10.1016/s0003-4975(99)00049-1. [DOI] [PubMed] [Google Scholar]
- 35.Kuwaki K, Tseng YL, Dor FJ, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase geneknockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 36.McGregor CGA, Davies WR, Oi K, et al. Cardiac xenotransplantation: recent preclinical progress with 3- month median survival. J Thorac Cardiovasc Surg. 2005;130:844–851. doi: 10.1016/j.jtcvs.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 37.Mohiuddin MM, Corcoran PC, Singh AK, et al. B-cell depletion extends the survival of GTKO.hCD46Tg pig heart xenografts in baboons for up to 8 months. Am J Transplant. 2012;12:763–771. doi: 10.1111/j.1600-6143.2011.03846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ekser B, Ezzelarab M, Hara H, et al. Clinical xenotransplantation: the next medical revolution? Lancet. 2012;379:672–683. doi: 10.1016/S0140-6736(11)61091-X. [DOI] [PubMed] [Google Scholar]
- 39.Vial CM, Ostlie DJ, Bhatti FN, et al. Life supporting function for over one month of a transgenic porcine heart in a baboon. J Heart Lung Transplant. 2000;19:224–229. doi: 10.1016/s1053-2498(99)00099-6. [DOI] [PubMed] [Google Scholar]
- 40.Brenner P, Schmoeckel M, Wimmer C, et al. Mean xenograft survival of 14.6 days in a small group of hDAF-transgenic pig hearts transplanted orthotopically into baboons. Transplant Proc. 2005;37:472–476. doi: 10.1016/j.transproceed.2004.12.241. [DOI] [PubMed] [Google Scholar]
- 41.McGregor CGA, Byrne GW, Vlasin M, et al. Early cardiac function and gene expression after orthotopic cardiac xenotransplantation. Xenotransplantation. 2009;16:356. (Abstract #IXA-O-2.4). [Google Scholar]
- 42.Cozzi E, Vial C, Ostlie D, et al. Maintenance triple immunosuppression with cyclosporin A, mycophenolate sodium and steroids allows prolonged survival of primate recipients of hDAF porcine renal xenografts. Xenotransplantation. 2003;10:300–310. doi: 10.1034/j.1399-3089.2003.02014.x. [DOI] [PubMed] [Google Scholar]
- 43.Yamada K, Yazawa K, Shimizu A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11:32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 44.Ramirez P, Chavez R, Majado M, et al. Life-supporting human complement regulator decay accelerating factor transgenic pig liver xenograft maintains the metabolic function and coagulation in the nonhuman primate for up to 8 days. Transplantation. 2000;70:989–998. doi: 10.1097/00007890-200010150-00001. [DOI] [PubMed] [Google Scholar]
- 45.Ekser B, Long C, Echeverri GJ, et al. Impact of thrombocytopenia on survival of baboons with genetically-modified pig liver transplants: clinical relevance. Am J Transplant. 2010;10:273–285. doi: 10.1111/j.1600-6143.2009.02945.x. [DOI] [PubMed] [Google Scholar]
- 46.Schuetz C, Kim K, Elias N, et al. 9-day survival and control of thrombocytopenia following liver xenotransplantation in baboons using GalT-KO swine grafts. Xenotransplantation. 2011;18:314. doi: 10.1111/j.1399-3089.2012.00717.x. (Abstract #P245). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang JP, Blum MG, Chang AC, et al. Immunohistologic evaluation of mechanisms mediating hyperacute lung rejection, and the effect of treatment with K76 COOH, FUT 175, and anti Gal column immunoadsorption. Xenotransplantation. 1999;6:249–261. doi: 10.1034/j.1399-3089.1999.00029.x. [DOI] [PubMed] [Google Scholar]
- 48.Azimzadeh A, Zorn GL, III., Blair KSA, et al. Hyperacute lung rejection in the pig-to-human model. 2. Synergy between soluble and membrane complement inhibition. Xenotransplantation. 2003;10:120–131. doi: 10.1034/j.1399-3089.2003.01102.x. [DOI] [PubMed] [Google Scholar]
- 49.Zorn GL, III., Pfeiffer S, Azimzadeh A, Pierson RN., III. Thrombin inhibition protects the pulmonary xenograft from hyperacute rejection. Surg Forum. 2000;LI:338–340. [Google Scholar]
- 50.Pfeiffer S, Zorn GL, III., Kelishadi S, et al. Role of anti-Gala1,3Gal and anti-platelet antibodies in hyperacute rejection of pig lung by human blood. Ann Thorac Surg. 2001;72:1681–1689. doi: 10.1016/s0003-4975(01)03033-8. [DOI] [PubMed] [Google Scholar]
- 51.Holzknecht ZE, Coombes S, Blocher BA, et al. Immune complex formation after xenotransplantation: evidence of type III as well as type II immune reactions provide clues to pathophysiology. Am J Pathol. 2001;158:627–637. doi: 10.1016/S0002-9440(10)64004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Brien JR. Shear induced platelet aggregation. Lancet. 1990;335:711–713. doi: 10.1016/0140-6736(90)90815-m. [DOI] [PubMed] [Google Scholar]
- 53.Kroll MH, Harris TS, Moake JL, et al. Von Willebrand factor binding to platelet GPIb initiates signals for platelet activation. J Clin Invest. 1991;88:1568–1573. doi: 10.1172/JCI115468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goto S, Salomon DR, Ikeda Y, et al. Characterization of the unique mechanism mediating the shear-dependent binding of soluble von Willebrand factor to platelets. J Biol Chem. 1995;270:23352–23361. doi: 10.1074/jbc.270.40.23352. [DOI] [PubMed] [Google Scholar]
- 55.Pareti FI, Mazzucato M, Bottini E, Mannucci PM. Interaction of porcine von Willebrand factor with the platelet glycoproteins Ib and IIb/IIIa complex. Br J Haematol. 1992;82:81–86. doi: 10.1111/j.1365-2141.1992.tb04597.x. [DOI] [PubMed] [Google Scholar]
- 56.Mazzucato M, De Marco L, Pradella P, Masotti A, Pareti FI. Porcine von Willebrand factor binding to human platelets GPIb induces transmembrane calcium influx. Thromb Haemost. 1996;75:655–660. [PubMed] [Google Scholar]
- 57.Schmelzle M, Schulte am Esch J, 2nd., Robson SC. Coagulation, platelet activation and thrombosis in xenotransplantation. Curr Opin Organ Transplant. 2010;15:212–218. doi: 10.1097/MOT.0b013e3283373ccc. [DOI] [PubMed] [Google Scholar]
- 58.Ezzelarab M, Garcia B, Azimzadeh A, et al. The innate immune response and activation of coagulation in a1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 2009;87:805–812. doi: 10.1097/TP.0b013e318199c34f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schroeder C, Sangrampurkar N, Hu J, et al. Role of HMGB1 in lung xenograft injury and cytokine production. Xenotransplantation. 2009;16:431. (Abstract #P-18.6). [Google Scholar]
- 60.Lin CC, Ezzelarab M, Shapiro R, et al. Tissue factor expression on recipient platelets is associated with consumptive coagulopathy in pig-to-primate kidney xenotransplantation. Am J Transplant. 2010;10:1556–1568. doi: 10.1111/j.1600-6143.2010.03147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cozzi E, White DJG. The generation of transgenic pigs as potential organ donors for humans. Nat Med. 1995;1:964–969. doi: 10.1038/nm0995-964. [DOI] [PubMed] [Google Scholar]
- 62.Yeatman M, Daggett CW, Parker W, et al. Complement-mediated pulmonary xenograft injury: studies in swine-to-primate orthotopic single lung transplant models. Transplantation. 1998;65:1084–1093. doi: 10.1097/00007890-199804270-00013. [DOI] [PubMed] [Google Scholar]
- 63.Kulick DM, Salerno CT, Dalmasso AP, et al. Transgenic swine lungs expressing human CD59 are protected from injury in a pig-to-human model of xenotransplantation. J Thorac Cardiovasc Surg. 2000;119:690–699. doi: 10.1016/S0022-5223(00)70003-1. [DOI] [PubMed] [Google Scholar]
- 64.Miyagawa S, Yamamoto A, Matsunami K, et al. Complement regulation in the GalT KO era. Xenotransplantation. 2010;17:11–25. doi: 10.1111/j.1399-3089.2010.00569.x. [DOI] [PubMed] [Google Scholar]
- 65.Azimzadeh AM, Kelishadi S, Ezzelarab M, et al. Early graft failure of GTKO pig organs in baboons is reduced by HCPRP expression. Am J Transplant. 2010;10(Suppl. 4):186. (Abstract #499). [Google Scholar]
- 66.Morgan BP, Berg CW, Harris CL. “Homologous restriction” in complement lysis: roles of membrane complement regulators. Xenotransplantation. 2005;12:258–265. doi: 10.1111/j.1399-3089.2005.00237.x. [DOI] [PubMed] [Google Scholar]
- 67.Hurh S, Choi I, Cho B, et al. Human xenogeneic responses to HD antigen in xenotransplantation. Xenotransplantation. 2011;18:273. (Abstract #135). [Google Scholar]
- 68.Varki A. Loss of N-glycolylneuraminic acid in humans: mechanisms, consequences, and implications for hominid evolution. Am J Phys Anthropol. 2001;(Suppl. 33):54–69. doi: 10.1002/ajpa.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu A, Hurst R. Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation. 2002;9:376–381. doi: 10.1034/j.1399-3089.2002.02138.x. [DOI] [PubMed] [Google Scholar]
- 70.Tangvoranuntakul P, Gagneux P, Diaz S, et al. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci U S A. 2003;100:12045–12050. doi: 10.1073/pnas.2131556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miwa Y, Kobayashi T, Nagasaka T, et al. Are N-glycolylneuraminic acid (Hanganutziu-Deicher) antigens important in pig-to-human xenotransplantation. Xenotransplantation. 2004;11:247–253. doi: 10.1111/j.1399-3089.2004.00126.x. [DOI] [PubMed] [Google Scholar]
- 72.Tahara H, Ide K, Basnet NB, et al. Immunological property of antibodies against N-glycolylneuraminic acid epitopes in cytidine monophospho-N-acetylneuraminic acid hydroxylase-deficient mice. J Immunol. 2010;184:3269–3275. doi: 10.4049/jimmunol.0902857. [DOI] [PubMed] [Google Scholar]
- 73.Padler-Karavani V, Varki A. Potential impact of the non-human sialic acid N-glycolylneuraminic acid on transplant rejection risk. Xenotransplantation. 2011;18:1–5. doi: 10.1111/j.1399-3089.2011.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cooper DKC. Xenoantigens and xenoantibodies. Xenotransplantation. 1998;5:6–17. doi: 10.1111/j.1399-3089.1998.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 75.Ezzelarab M, Ayares D, Cooper DKC. Carbohydrates in xenotransplantation. Immunol Cell Biol. 2005;83:396–404. doi: 10.1111/j.1440-1711.2005.01344.x. [DOI] [PubMed] [Google Scholar]
- 76.Byrne GW, Stalboerger PG, Davila E, et al. Proteomic identification of non-Gal antibody targets after pig-to-primate cardiac xenotransplantation. Xenotransplantation. 2008;15:268–276. doi: 10.1111/j.1399-3089.2008.00480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Byrne GW, Stalboerger PG, Du Z, Davis TR, McGregor CG. Identification of new carbohydrate and membrane protein antigens in cardiac xenotransplantation. Transplantation. 2011;91:287–292. doi: 10.1097/TP.0b013e318203c27d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yeh P, Ezzelarab M, Bovin N, et al. Investigation of potential carbohydrate antigen targets for human and baboon antibodies. Xenotransplantation. 2010;17:197–206. doi: 10.1111/j.1399-3089.2010.00579.x. [DOI] [PubMed] [Google Scholar]
- 79.Breimer ME. Gal/non-Gal antigens in pig tissues and human non-Gal antibodies in the GalT-KO era. Xenotransplantation. 2011;18:215–228. doi: 10.1111/j.1399-3089.2011.00644.x. [DOI] [PubMed] [Google Scholar]
- 80.Burlak C, Wang ZY, Chihara RK, et al. Identification of human preformed antibody targets in GTKO pigs. Xenotransplantation. 2012;19:92–101. doi: 10.1111/j.1399-3089.2012.00695.x. [DOI] [PubMed] [Google Scholar]
- 81.d’Apice AJ, Cowan PJ. Xenotransplantation: the next generation of engineered animals. Transpl Immunol. 2009;21:111–115. doi: 10.1016/j.trim.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Inverardi L, Clissi B, Stolzer AL, et al. Human natural killer lymphocytes directly recognize evolutionarily conserved oligosaccharide ligands expressed by xenogeneic tissues. Transplantation. 1997;63:1318–1330. doi: 10.1097/00007890-199705150-00021. [DOI] [PubMed] [Google Scholar]
- 83.Baumann BC, Forte P, Hawley RJ, Rieben R, Schneider MK, Seebach JD. Lack of galactose-a1, 3-galactose expression on porcine endothelial cells prevents complement-induced lysis but not direct xenogeneic NK cytotoxicity. J Immunol. 2004;172:6460–6467. doi: 10.4049/jimmunol.172.10.6460. [DOI] [PubMed] [Google Scholar]
- 84.Rieben R, Seebach JD. Xenograft rejection: IgG1, complement and NK cells team up to activate and destroy the endothelium. Trends Immunol. 2005;26:2–5. doi: 10.1016/j.it.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 85.Horvath-Arcidiacono JA, Porter CM, Bloom ET. Human NK cells can lyse porcine endothelial cells independent of their expression of Galalpha(1,3)-Gal and killing is enhanced by activation of either effector or target cells. Xenotransplantation. 2006;13:318–327. doi: 10.1111/j.1399-3089.2006.00316.x. [DOI] [PubMed] [Google Scholar]
- 86.Kennett SB, Porter CM, Horvath-Arcidiacono JA, Bloom ET. Characterization of baboon NK cells and their xenogeneic activity. Xenotransplantation. 2010;17:288–299. doi: 10.1111/j.1399-3089.2010.00591.x. [DOI] [PubMed] [Google Scholar]
- 87.Seebach JD, Comrack C, Germana S, LeGuern C, Sachs DH, DerSimonian H. HLA-Cw3 expression on porcine endothelial cells protects against xenogeneic cytotoxicity mediated by a subset of human NK cells. J Immunol. 1997;159:3655–3661. [PubMed] [Google Scholar]
- 88.Dorling A, Monk NJ, Lechler RI. HLA-G inhibits the transendothelial migration of human NK cells. Eur J Immunol. 2000;30:586–593. doi: 10.1002/1521-4141(200002)30:2<586::AID-IMMU586>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 89.Sharland A, Patel A, Lee JH, et al. Genetically modified HLA class I molecules able to inhibit human NK cells without provoking alloreactive CD8+ CTLs. J Immunol. 2002;168:3266–3274. doi: 10.4049/jimmunol.168.7.3266. [DOI] [PubMed] [Google Scholar]
- 90.Forte P, Baumann BC, Weiss EH, Seebach JD. HLA-E expression on porcine cells: protection from human NK cytotoxicity depends on peptide loading. Am J Transplant. 2005;5:2085–2093. doi: 10.1111/j.1600-6143.2005.00987.x. [DOI] [PubMed] [Google Scholar]
- 91.Crew MD, Cannon MJ, Phanavanh B, Garcia-Borges CN. An HLA-E single chain trimer inhibits human NK cell reactivity towards porcine cells. Mol Immunol. 2005;42:1205–1214. doi: 10.1016/j.molimm.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 92.Crew MD. Play it in E or G: utilization of HLA-E and - G in xenotransplantation. Xenotransplantation. 2007;14:198–207. doi: 10.1111/j.1399-3089.2007.00395.x. [DOI] [PubMed] [Google Scholar]
- 93.Weiss EH, Lilienfeld BG, Müller S, et al. HLA-E/ human beta2-microglobulin transgenic pigs: protection against xenogeneic human anti-pig natural killer cell cytotoxicity. Transplantation. 2009;87:35–43. doi: 10.1097/TP.0b013e318191c784. [DOI] [PubMed] [Google Scholar]
- 94.Hara H, Crossley T, Witt W, et al. Dominant-negative CIITA transgenic pigs – effect on the human anti-pig T cell immune response and immune status. Am J Transplant. 2010;10(Suppl. 4):187. (Abstract #503). [Google Scholar]
- 95.Phelps C, Ball S, Vaught T, et al. Production and characterization of transgenic pigs expressing porcine CTLA4-Ig. Xenotransplantation. 2009;16:477–485. doi: 10.1111/j.1399-3089.2009.00533.x. [DOI] [PubMed] [Google Scholar]
- 96.Brain JD, Molina RM, DeCamp MM, Warner AE. Pulmonary intravascular macrophages: their contribution to the mononuclear phagocyte system in 13 species. Am J Physiol. 1999;276:L146–L154. doi: 10.1152/ajplung.1999.276.1.L146. [DOI] [PubMed] [Google Scholar]
- 97.Winkler GC, Cheville NF. Monocytic origin and postnatal mitosis of intravascular macrophages in the porcine lung. J Leukoc Biol. 1985;38:471–480. doi: 10.1002/jlb.38.4.471. [DOI] [PubMed] [Google Scholar]
- 98.Winkler GC, Cheville NF. Postnatal colonization of porcine lung capillaries by intravascular macrophages: an ultrastructural, morphometric analysis. Microvasc Res. 1987;33:224–232. doi: 10.1016/0026-2862(87)90019-7. [DOI] [PubMed] [Google Scholar]
- 99.Miyamoto K, Schultz E, Heath T, et al. Pulmonary intravascular macrophages and hemodynamic effects of liposomes in sheep. J Appl Physiol. 1988;64:1143–1152. doi: 10.1152/jappl.1988.64.3.1143. [DOI] [PubMed] [Google Scholar]
- 100.Chitko-McKown CG, Chapes SK, Brown RE, et al. Porcine alveolar and pulmonary intravascular macrophages: comparison of immune functions. J Leukocyte Biol. 1991;50:364–372. doi: 10.1002/jlb.50.4.364. [DOI] [PubMed] [Google Scholar]
- 101.Paris L, Reyes LM, Tector AJ, et al. SIRPA interspecies incompatibilities lead to xenogeneic phagocytosis of platelets by liver cells. Xenotransplantation. 2011;18:293. (Abstract #411). [Google Scholar]
- 102.Ide K, Ohdan H, Kobayashi T, Hara H, Ishiyama K, Asahara T. Antibody- and complement-independent phagocytotic and cytolytic activities of human macrophages toward porcine cells. Xenotransplantation. 2005;12:181–188. doi: 10.1111/j.1399-3089.2005.00222.x. [DOI] [PubMed] [Google Scholar]
- 103.Ide K, Wang H, Tahara H, et al. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc Natl Acad Sci U S A. 2007;14:5062–5066. doi: 10.1073/pnas.0609661104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang YG. CD47 in xenograft rejection and tolerance induction. Xenotransplantation. 2010;17:267–273. doi: 10.1111/j.1399-3089.2010.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Navarro-Alvarez N, Yang YG. CD47: a new player in phagocytosis and xenograft rejection. Cell Mol Immunol. 2011;8:285–288. doi: 10.1038/cmi.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Subramanian S, Tsai R, Discher DE. The ‘metabolon,’ CD47, and the ‘phagocytic synapse’: molecular co-localization and species divergence. Transfus Clin Biol. 2006;13:31–38. doi: 10.1016/j.tracli.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 107.Robson SC, Wu Y, Sun X, Knosalla C, Dwyer K, Enjyoji K. Ectonucleotidases of CD39 family modulate vascular inflammation and thrombosis in transplantation. Semin Thromb Hemost. 2005;31:217–233. doi: 10.1055/s-2005-869527. [DOI] [PubMed] [Google Scholar]
- 108.Deaglio S, Robson SC. Ectonucleotidases as regulators of purinergic signaling in thrombosis, inflammation, and immunity. Adv Pharmacol. 2011;61:301–332. doi: 10.1016/B978-0-12-385526-8.00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Takenaka K, Prasolava TK, Wang JC, et al. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol. 2007;8:1313–1323. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- 110.Staub NC. Pulmonary intravascular macrophages. Ann Rev Physiol. 1994;56:47–67. doi: 10.1146/annurev.ph.56.030194.000403. [DOI] [PubMed] [Google Scholar]
- 111.Sone Y, Nicolaysen A, Staub NC. Effect of particles on sheep lung hemodynamics parallels depletion and recovery of intravascular macrophages. J Appl Physiol. 1997;83:1499–1507. doi: 10.1152/jappl.1997.83.5.1499. [DOI] [PubMed] [Google Scholar]
- 112.Sone Y, Serikov KB, Staub NC. Intravascular macrophage depletion attenuates endotoxin in lung injury in anesthetized sheep. J Appl Physiol. 1999;87:1354–1359. doi: 10.1152/jappl.1999.87.4.1354. [DOI] [PubMed] [Google Scholar]
- 113.Burdorf L, Zhang T, Rybak E, et al. Anti-GP1b Fab reduces activation and sequestration of platelets in a xenogeneic pig lung perfusion model. Transplantation. 2010;90:422. (Abstract #223.3). [Google Scholar]
- 114.Burdorf L, Barth R, Zhang T, et al. Pilot evaluation of anti-GP1b effects on platelet sequestration in an ex-vivo xenogeneic pig liver perfusion model. Transplantation. 2010;90:327. (Abstract #202.3). [Google Scholar]
- 115.Burdorf L, Zhang T, Rybak E, et al. Combined GPIb and GPIIb/IIIa blockade prevents sequestration of platelets in a pig-to-human lung perfusion model. Xenotransplantation. 2011;18:287. (Abstract #392). [Google Scholar]
- 116.Tena A, Turcotte N, Barone AAL, et al. Miniature swine expressing human CD47 to enhance bone marrow engraftment in non-human primates. Xenotransplantation. 2011;18:271. (Abstract #127). [Google Scholar]
- 117.Kues WA, Schwinzer R, Wirth D, et al. Epigenetic silencing and tissue independent expression of a novel tetracycline inducible system in double- transgenic pigs. FASEB J. 2006;20:1200–1202. doi: 10.1096/fj.05-5415fje. [DOI] [PubMed] [Google Scholar]
- 118.Li L, Pang D, Wang T, et al. Production of a reporter transgenic pig for monitoring Cre recombinase activity. Biochem Biophys Res Commun. 2009;382:232–235. doi: 10.1016/j.bbrc.2009.02.146. [DOI] [PubMed] [Google Scholar]
- 119.Isenberg JS, Romeo MJ, Abu-Asab M, et al. Increasing survival of ischemic tissue by targeting CD47. Circ Res. 2007;100:712–720. doi: 10.1161/01.RES.0000259579.35787.4e. [DOI] [PubMed] [Google Scholar]
- 120.Roberts DD, Miller TW, Rogers NM, Yao M, Isenberg JS. The matricellular protein thrombospondin-1 globally regulates cardiovascular function and responses to stress. 2012;31:162–169. doi: 10.1016/j.matbio.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Isenberg JS, Ridnour LA, Dimitry J, Frazier WA, Wink DA, Roberts DD. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J Biol Chem. 2006;281:26069–26080. doi: 10.1074/jbc.M605040200. [DOI] [PubMed] [Google Scholar]
- 122.Isenberg JS, Annis DS, Pendrak ML, et al. Differential interactions of thrombospondin-1, −2, and −4 with CD47 and effects on cGMP signaling and ischemic injury responses. J Biol Chem. 2009;284:1116–1125. doi: 10.1074/jbc.M804860200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lamy L, Ticchioni M, Rouquette-Jazdanian AK, et al. CD47 and the 19 kDa interacting protein-3 (BNIP3) in T cell apoptosis. J Biol Chem. 2003;278:23915–23921. doi: 10.1074/jbc.M301869200. [DOI] [PubMed] [Google Scholar]
- 124.Lamy L, Foussat A, brown EJ, Bornstein P, Ticchioni M, Bernard A. Interactions between CD47 and thrombospondin reduce inflammation. J Immunol. 2007;178:5930–5939. doi: 10.4049/jimmunol.178.9.5930. [DOI] [PubMed] [Google Scholar]
- 125.Isenberg JS, Romeo MJ, Yu C, et al. Thrombospondin-1 stimulates platelet aggregation by blocking the anti-thrombotic activity of nitric oxide/cGMP signaling. Blood. 2008;111:613–623. doi: 10.1182/blood-2007-06-098392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Klose R, Kemter E, Bedke T, et al. Expression of biologically active human TRAIL in transgenic pigs. Transplantation. 2005;80:222–230. doi: 10.1097/01.tp.0000164817.59006.c2. [DOI] [PubMed] [Google Scholar]
- 127.Kemter E, Lieke T, Kessler B, et al. Human TNF-related apoptosis-inducing ligand-expressing dendritic cells from transgenic pigs attenuate human xenogeneic T cell responses. Xenotransplantation. 2012;19:40–51. doi: 10.1111/j.1399-3089.2011.00688.x. [DOI] [PubMed] [Google Scholar]
- 128.Bozeman EN, Hood JD, Shashidharamurthy R, Selvaraj P. ‘Exhaustive’ look at PD-1/PDL-1 blockade in vivo. Immunotherapy. 2009;1:525–527. doi: 10.2217/imt.09.37. [DOI] [PubMed] [Google Scholar]
- 129.Siegel JB, Grey ST, Lesnikosko BA, et al. Xenogeneic endothelial cells activate human prothrombin. Transplantation. 1997;64:888–896. doi: 10.1097/00007890-199709270-00017. [DOI] [PubMed] [Google Scholar]
- 130.Schulte am Esch J, Rogiers X, Robson SC. Molecular incompatibilities in hemostasis between swine and men—impact on xenografting. Ann Transplant. 2001;6:12–16. [PubMed] [Google Scholar]
- 131.Cowan PJ, Robson SC, d’Apice AJF. Controlling coagulation dysregulation in xenotransplantation. Curr Opin Organ Transplant. 2011;16:214–221. doi: 10.1097/MOT.0b013e3283446c65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lawson JH, Daniels LJ, Platt JL. The evaluation of thrombomodulin activity in porcine to human xenotransplantation. Transplant Proc. 1997;29:884–885. doi: 10.1016/s0041-1345(96)00192-3. [DOI] [PubMed] [Google Scholar]
- 133.Esmon CT. The protein C pathway. Chest. 2003;124:26S–32S. doi: 10.1378/chest.124.3_suppl.26s. [DOI] [PubMed] [Google Scholar]
- 134.Esmon CT, Owen WG. The discovery of thrombomodulin. J Thromb Haemost. 2004;2:209–213. doi: 10.1046/j.1538-7933.2003.00537.x. [DOI] [PubMed] [Google Scholar]
- 135.Roussel JC, Moran CJ, Salvaris EJ, et al. Pig thrombomodulin binds human thrombin but is a poor cofactor for activation of human protein C and TAFI. Am J Transplant. 2008;8:1101–1112. doi: 10.1111/j.1600-6143.2008.02210.x. [DOI] [PubMed] [Google Scholar]
- 136.Iwamoto M, Yazaki S, Onishi A, et al. Successful production and breeding of cloned pigs expressing human thrombomodulin in endothelial cells. Xenotransplantation. 2011;18:270. doi: 10.1111/j.1399-3089.2012.00696.x. (Abstract 125). [DOI] [PubMed] [Google Scholar]
- 137.Klymiuk N, Wuensch A, Kurome M, et al. GalT-KO/ CD46/hTM triple-transgenic donor animals for pig-to-baboon heart transplantation. Xenotransplantation. 2011;18:271. (Abstract #126). [Google Scholar]
- 138.Salvaris E, Fisicaro N, Harrison S, et al. Expression of human thrombomodulin in GTKO/hCD55-CD59-HTF pigs. Xenotransplantation. 2011;18:296. (Abstract #513). [Google Scholar]
- 139.Petersen B, Ramackers W, Tiede A, et al. Pigs transgenic for human thrombomodulin have elevated production of activated protein C. Xenotransplantation. 2009;16:486–495. doi: 10.1111/j.1399-3089.2009.00537.x. [DOI] [PubMed] [Google Scholar]
- 140.Crikis S, Zhang XM, Dezfouli S, et al. Anti-inflammatory and anticoagulant effects of transgenic expression of human thrombomodulin in mice. Am J Transplant. 2010;10:242–250. doi: 10.1111/j.1600-6143.2009.02939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Miwa Y, Yamamoto K, Onishi A, et al. Potential value of human thrombomodulin and DAF expression for coagulation control in pig-to-humanxenotransplantation. Xenotransplantation. 2010;17:26–37. doi: 10.1111/j.1399-3089.2009.00555.x. [DOI] [PubMed] [Google Scholar]
- 142.Esmon CT. Structure and functions of the endothelial cell protein C receptor. Crit Care Med. 2004;32:S298–S301. doi: 10.1097/01.ccm.0000126128.64614.81. [DOI] [PubMed] [Google Scholar]
- 143.Esmon CT. The endothelial protein C receptor. Curr Opin Hematol. 2006;13:382–385. doi: 10.1097/01.moh.0000239712.93662.35. [DOI] [PubMed] [Google Scholar]
- 144.Esmon CT. Inflammation and the activated protein C anticoagulant pathway. Semin Thromb Hemost. 2006;32:49–60. doi: 10.1055/s-2006-939554. [DOI] [PubMed] [Google Scholar]
- 145.Esmon CT. The discovery of the endothelial cell protein C receptor. J Thromb Haemost. 2010;8:2–5. doi: 10.1111/j.1538-7836.2009.03660.x. [DOI] [PubMed] [Google Scholar]
- 146.Lee KF, Salvaris EJ, Roussel JC, et al. Recombinant pig TFPI efficiently regulates human tissue factor. Xenotransplantation. 2008;15:191–197. doi: 10.1111/j.1399-3089.2008.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kopp CW, Siegel JB, Hancock WW, et al. Effect of porcine endothelial tissue factor pathway inhibitor on human coagulation factors. Transplantation. 1997;63:749–758. doi: 10.1097/00007890-199703150-00023. [DOI] [PubMed] [Google Scholar]
- 148.Chen D, Giannopoulos K, Shiels PG, et al. Inhibition of intravascular thrombosis in murine endotoxemia by targeted expression of hirudin and tissue factor pathway inhibitor analogs to activated endothelium. Blood. 2004;104:1344–1349. doi: 10.1182/blood-2003-12-4365. [DOI] [PubMed] [Google Scholar]
- 149.Chen D, Weber M, McVey JH, et al. Complete inhibition of acute humoral rejection using regulated expression of membrane-tethered anticoagulants on xenograft endothelium. Am J Transplant. 2004;4:1958–1963. doi: 10.1111/j.1600-6143.2004.00625.x. [DOI] [PubMed] [Google Scholar]
- 150.Schelzig H, Vogel A, Krischer C, Simon F, Abendroth D. Role of recombinant hirudin in a pig-to-human lung transplantation model. Transplant Proc. 2002;34:2384–2386. doi: 10.1016/s0041-1345(02)03288-8. [DOI] [PubMed] [Google Scholar]
- 151.Brummelkamp TR, Bernards R, Agami R. A system for stable expression of small interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 152.Couzin J. Breakthrough of the year. Small RNAs make big splash. Science. 2002;298:2296–2297. doi: 10.1126/science.298.5602.2296. [DOI] [PubMed] [Google Scholar]
- 153.Dallas S, Vlassov AV. RNAi: a novel antisense technology and its therapeutic potential. Med Sci Monit. 2006;12:RA67–RA74. [PubMed] [Google Scholar]
- 154.Le Hir M, Kaissling B. Distribution and regulation of renal ecto-5¢-nucleosidase: implications for physiological functions of adenosine. Am J Physiol. 1993;264:F377–F387. doi: 10.1152/ajprenal.1993.264.3.F377. [DOI] [PubMed] [Google Scholar]
- 155.Kaczmarek E, Koziak K, Sevigny J, et al. Identification and characterization of CD39/vascular ATP diphosphohydrolase. J Biol Chem. 1996;271:33116–33122. doi: 10.1074/jbc.271.51.33116. [DOI] [PubMed] [Google Scholar]
- 156.Smolenski RT, Kochan Z, Karbowska J, et al. Low expression of ecto-5′- nucleosidase in the pig heart – a potential barrier to xenotransplantation. J Heart Lung Transplant. 2001;20:214–215. doi: 10.1016/s1053-2498(00)00467-8. [DOI] [PubMed] [Google Scholar]
- 157.Smolenski RT, Khalpey Z, Yuen AC, et al. Purine metabolism in pigs and humans and implications for xenotransplantation. Nucleosides, Nucleotides Nucleic Acids. 2005;24:263–266. doi: 10.1081/NCN-59701. [DOI] [PubMed] [Google Scholar]
- 158.Smolenski RT, Khalpey Z, Osborne FN, et al. Species differences of endothelial extracellular nucleotide metabolism and its implications for xenotransplantation. Pharmacol Rep. 2006;58(Suppl):118–125. [PubMed] [Google Scholar]
- 159.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dwyer KM, Robson SC, Nandurkar HH, et al. Thromboregulatory manifestations in human CD39 transgenic mice and the implications for thrombotic disease and transplantation. J Clin Invest. 2004;113:1440–1446. doi: 10.1172/JCI19560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dwyer KM, Mysore TB, Crikis S, et al. The transgenic expression of human CD39 on murine islets inhibits clotting of human blood. Transplantation. 2006;82:428–432. doi: 10.1097/01.tp.0000229023.38873.c0. [DOI] [PubMed] [Google Scholar]
- 162.Dwyer KM, Deaglio S, Gao W, et al. CD39 and control of cellular immune responses. Purinergic Signal. 2007;3:171–180. doi: 10.1007/s11302-006-9050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Di Virgilio F, Boeynaems JM, Robson SC. Extracellular nucleotides as negative modulators of immunity. Curr Opin Pharmacol. 2009;9:507–513. doi: 10.1016/j.coph.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Crikis S, Lu B, Murray-Segal LM, et al. Transgenic overexpression of CD39 protects against renal ischemia-reperfusion and transplant vascular injury. Am J Transplant. 2010;10:2586–2595. doi: 10.1111/j.1600-6143.2010.03257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Le Bas-Bernardet S, Branchereau J, Tillou X, et al. Porcine galactosyl- transferase knock-out transgenic for human CD55, CD59, CD39 kidneys are actually rejected by baboons despite plasma/B cells and complement blockade. Xenotransplantation. 2011;18:298. (Abstract #556). [Google Scholar]
- 166.Le Bas-Bernardet S, Poirier N, Tillou X, et al. Preformed non-Gal antibodies are highly cytotoxic even against Galactosyl-transferase knockout, human CD55, CD59, CD39 transgenic pig organs in a model of kidney transplantation into baboons. Xenotransplantation. 2011;18:298. doi: 10.1016/j.transproceed.2011.09.024. (Abstract #557). [DOI] [PubMed] [Google Scholar]
- 167.Haskó G, Csóka B, Koscsó B, et al. Ecto-5′-nucleotidase (CD73) decreases mortality and organ injury in sepsis. J Immunol. 2011;187:4256–4267. doi: 10.4049/jimmunol.1003379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Esmon CT. Protein C anticoagulant system-anti-inflammatory effects. Semin Immunopathol. 2012;34:127–132. doi: 10.1007/s00281-011-0284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Esmon CT, Xu J, Lupu F. Innate immunity and coagulation. J Thromb Haemost. 2011;9:182–188. doi: 10.1111/j.1538-7836.2011.04323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Peterson B, Lucas-Harn A, Lemme E, et al. Generation and characterization of pigs transgenic for human hemeoxygenase-1 (hHO-1) Xenotransplantation. 2010;17:102–103. [Google Scholar]
- 171.Peterson B, Ramackers W, Lucas-Hahn A, et al. Transgenic expression of human heme oxygenase-1 in pigs confers resistance against xenograft rejection during ex vivo perfusion of porcine kidneys. Xenotransplantation. 2011;18:355–368. doi: 10.1111/j.1399-3089.2011.00674.x. [DOI] [PubMed] [Google Scholar]
- 172.Schwinzer R, Baars W, Borns K, Petersen B, Kues W, Niemann H. Over- expression of of human HO-1 and A20 in porcine cells: effects on susceptibility to cell-mediated lysis and inflammatory cytokines. Xenotransplantation. 2011;18:269. (Abstract #122). [Google Scholar]
- 173.Oropeza M, Petersen B, Carnwath JW, et al. Transgenic expression of the human A20 gene in cloned pigs provides protection against apoptotic and inflammatory stimuli. Xenotransplantation. 2009;16:522–534. doi: 10.1111/j.1399-3089.2009.00556.x. [DOI] [PubMed] [Google Scholar]
- 174.Cho B, Koo OJ, Hwang JI, et al. Generation of soluble human tumor necrosis factor-a receptor 1-Fc transgenic pig. Transplantation. 2011;92:139–147. doi: 10.1097/TP.0b013e3182215e7e. [DOI] [PubMed] [Google Scholar]
- 175.Campbell PD, Weinberg A, Chee J, et al. Expression of pro- and anti-apoptotic molecules of the Bcl-2 family in human islets post-isolation. Cell Transplant. 2012;21:49–60. doi: 10.3727/096368911X566262. [DOI] [PubMed] [Google Scholar]
- 176.Ezzelarab M, Ayares D, Cooper DKC. The potential of genetically-modified pig mesenchymal stromal cells in xenotransplantation. Xenotransplantation. 2010;17:3–5. doi: 10.1111/j.1399-3089.2009.00567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ezzelarab M, Ezzelarab C, Wilhite T, et al. Genetically-modified pig mesenchymal stromal cells: xenoantigenicity and effect on human T-cell xenoresponses. Xenotransplantation. 2011;18:183–195. doi: 10.1111/j.1399-3089.2011.00635.x. [DOI] [PubMed] [Google Scholar]
- 178.Porter CM, Horvath-Arcidiacono JA, Singh AK, Horvath KA, Bloom ET, Mohiuddin MM. Characterization and expansion of baboon CD4+CD25+ Treg cells for potential use in a non-human primate xenotransplantation model. Xenotransplantation. 2007;14:298–308. doi: 10.1111/j.1399-3089.2007.00416.x. [DOI] [PubMed] [Google Scholar]
- 179.Fu Y, Yi S, Wu J, et al. In vitro suppression of xenoimmune-mediated macrophage activation by human CD4+CD25+ regulatory T cells. Transplantation. 2008;86:865–874. doi: 10.1097/TP.0b013e31818530fd. [DOI] [PubMed] [Google Scholar]
- 180.Wu J, Yi S, Ouyang L, et al. In vitro expanded human CD4+CD25+ regulatory T cells are potent suppressors of T-cell-mediated xenogeneic responses. Transplantation. 2008;85:1841–1848. doi: 10.1097/TP.0b013e3181734793. [DOI] [PubMed] [Google Scholar]
- 181.Sun L, Yi S, O’Connell PJ. Foxp3 regulates human natural CD4+CD25+ regulatory T-cell-mediated suppression of xenogeneic response. Xenotransplantation. 2010;17:121–130. doi: 10.1111/j.1399-3089.2010.00571.x. [DOI] [PubMed] [Google Scholar]
- 182.O’Connell PJ, Yi S, Carrington EM, Lew AM. Role of regulatory T cells in xenotransplantation. Curr Opin Organ Transplant. 2010;15:224–229. doi: 10.1097/MOT.0b013e3283373c27. [DOI] [PubMed] [Google Scholar]
- 183.Dons EM, Raimondi G, Cooper DKC, Thomson AW. Non-human primate regulatory T cells: current biology and implications for transplantation. Transplantation. 2010;90:811–816. doi: 10.1097/TP.0b013e3181ebf782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Webster NL, Forni M, Bacci ML, et al. Multi-transgenic pigs expressing three fluorescent proteins produced with high efficiency by sperm mediated gene transfer. Mol Reprod Dev. 2005;72:68–76. doi: 10.1002/mrd.20316. [DOI] [PubMed] [Google Scholar]
- 185.Nottle MB, Vassiliev I, O’Connell PJ, d’Apice AJ, Cowan PJ. On the need for porcine embryonic stem cells to produce Gal KO pigs expressing multiple transgenes to advance xenotransplantation. Xenotransplantation. 2010;17:411–412. doi: 10.1111/j.1399-3089.2010.00611.x. [DOI] [PubMed] [Google Scholar]
- 186.Deng W, Yang D, Zhao B, et al. Use of the 2A peptide for generation of multi- transgenic pigs through a single round od nuclear transfer. PLoS ONE. 2011;6:e19986. doi: 10.1371/journal.pone.0019986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fisicaro N, Londrigan SL, Brady JL, et al. Versatile co-expression of graft- protective proteins using 2A-linked cassettes. Xenotransplantation. 2011;18:121–130. doi: 10.1111/j.1399-3089.2011.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bibikova M, Golic M, Golic KG, Carroll D. Targeted chromosomal cleavage and mutagenesis in Drosophila using zinc-finger nucleases. Genetics. 2002;161:1169–1175. doi: 10.1093/genetics/161.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Carroll D. Progress and prospects: zinc-finger nucleases as gene therapy agents. Gene Ther. 2008;15:1463–1468. doi: 10.1038/gt.2008.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Klug A. The discovery of zinc fingers and their applications in gene regulation and genome manipulation. Annu Rev Biochem. 2010;79:213–231. doi: 10.1146/annurev-biochem-010909-095056. [DOI] [PubMed] [Google Scholar]
- 191.Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD. Genome editing with engineered zinc finger nucleases. Nature. 2010;11:636–646. doi: 10.1038/nrg2842. [DOI] [PubMed] [Google Scholar]
- 192.Watanabe M, Umeyama K, Matsunari H, et al. Knockout of exogenous EGFP gene in porcine somatic cells using zinc-finger nucleases. Biochem Biophys Res Commun. 2010;402:14–18. doi: 10.1016/j.bbrc.2010.09.092. [DOI] [PubMed] [Google Scholar]
- 193.Hauschild J, Petersen B, Santiago Y, et al. Efficient generation of a biallelic knockout in pigs using zinc-finger nucleases. Proc Natl Acad Sci U S A. 2011;108:12013–12017. doi: 10.1073/pnas.1106422108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hauschild J, Petersen B, Santiago Y, et al. Zinc-finger nuclease mediated knockout of the porcine alpha1, 3-galactosyltransferase gene. Xenotransplantation. 2011;18:270. (Abstract #124). [Google Scholar]
- 195.Tesson L, Usal C, Ménoret S, et al. Knockout rats generated by embryo microinjection of TALENs. Nat Biotechnol. 2011;29:695–696. doi: 10.1038/nbt.1940. [DOI] [PubMed] [Google Scholar]
- 196.Izmiryan A, Basmaciogullari S, Henry A, et al. Efficient gene targeting mediated by a lentiviral vector-associated meganuclease. Nucleic Acids Res. 2011;39:7610–7619. doi: 10.1093/nar/gkr524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Garrels W, Mátés L, Holler S, et al. Germline transgenic pigs by sleeping beauty transposition in porcine zygotes and targeted integration in the pig genome. PLoS ONE. 2011;6:e23573. doi: 10.1371/journal.pone.0023573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kraner S, Merkl C, Schnieke A. Genes, BACs, and artificial chromosomes. Xenotransplantation. 2011;18:56–72. [Google Scholar]
- 199.Galli C, Perota A, Brunetti D, Lagutina I, Lazzari G, Lucchini F. Genetic engineering including superseding microinjection: new ways to make GM pigs. Xenotransplantation. 2010;17:397–410. doi: 10.1111/j.1399-3089.2010.00590.x. [DOI] [PubMed] [Google Scholar]
- 200.Kobayashi T, Yamaguchi T, Hamanaka S, et al. Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell. 2010;142:787–799. doi: 10.1016/j.cell.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 201.Phelps C, Vaught T, Ball S, et al. Multi-transgenic pigs designed for xenoislet transplants. Xenotransplantation. 2009;16:374. (Abstract # IXA-O-7.3). [Google Scholar]
- 202.Ayares D, Vaught T, Ball S, et al. Islet-specific expression of TFPI, CD39, and CTLA4Ig in transgenic pigs designed for xenoislet transplantation. Xenotransplantation. 2011;18:269. (Abstract #119). [Google Scholar]
- 203.Baehr A, van Buerck L, Kurome M, et al. LEA29Y transgenic pigs for overcoming cellular rejection in xenotransplantation. Xenotransplantation. 2011;18:271. (Abstract #128). [Google Scholar]
- 204.Martin C, Plat M, Nerriére-Daguin V, et al. Transgenic expression of CTLA4-Ig by fetal pig neurons for xenotransplantation. Transgenic Res. 2005;14:373–384. doi: 10.1007/s11248-004-7268-4. [DOI] [PubMed] [Google Scholar]
- 205.Badin RA, Padoan A, Vadori M, et al. Long-term clinical recovery in parkinsonian monkey recipients of CTLA4-Ig transgenic porcine neural precursors. Transplantation. 2010;90(Suppl. 2):47. (Abstract #LB-3288). [Google Scholar]
- 206.Lévéque X, Cozzi E, Naveilhan P, Neveu I. Intracerebral xenotransplantation: recent findings and perspectives for local immunosuppression. Curr Opin Organ Transplant. 2011;16:190–194. doi: 10.1097/MOT.0b013e32834494b5. [DOI] [PubMed] [Google Scholar]
- 207.Ayares D, Phelps C, Vaught T, et al. Multi-transgenic pigs for vascularized pig organ xenografts. Xenotransplantation. 2011;18:269. (Abstract #119). [Google Scholar]
- 208.Cooper DKC. A brief history of cross-species organ transplantation. Proc (Bayl Univ Med Cent) 2012;25:49–57. doi: 10.1080/08998280.2012.11928783. [DOI] [PMC free article] [PubMed] [Google Scholar]